Abstract
This study identifies a dendritic cell (DC) subset that constitutively transports apoptotic intestinal epithelial cell remnants to T cell areas of mesenteric lymph nodes in vivo. Rat intestinal lymph contains two DC populations. Both populations have typical DC morphology, are major histocompatibility complex class IIhi, and express OX62, CD11c, and B7. CD4+/OX41+ DCs are strong antigen-presenting cells (APCs). CD4−/OX41− DCs are weak APCs and contain cytoplasmic apoptotic DNA, epithelial cell–restricted cytokeratins, and nonspecific esterase (NSE)+ inclusions, not seen in OX41+ DCs. Identical patterns of NSE electrophoretic variants exist in CD4−/OX41− DCs, intestinal epithelial cells, and mesenteric node DCs but not in other DC populations, macrophages, or tissues. Terminal deoxynucleotidyl transferase–mediated dUTP-biotin nick-end labeling (TUNEL)-positive DCs and strongly NSE+ DCs are present in intestinal lamina propria. Peyer's patches and mesenteric but not other lymph nodes contain many strongly NSE+ DCs in interfollicular and T cell areas. Similar DCs are seen in the ileum and in T cell areas of mesenteric nodes in gnotobiotic rats. These results show that a distinct DC subset constitutively endocytoses and transports apoptotic cells to T cell areas and suggest a role for these DCs in inducing and maintaining peripheral self-tolerance.
Keywords: rat, lymph, esterase, self tolerance, oral tolerance
Introduction
Dendritic cells (DCs) have a central role in the activation of resting T cells and the initiation of primary responses. They acquire Ag in peripheral tissues and transport it to LNs for presentation to lymphocytes 1. DCs can acquire Ag via multiple mechanisms 2, and recently, attention has focussed on interactions between DCs and apoptotic cells. DCs can endocytose apoptotic cells and can present peptides derived from these on MHC class I and II 3 4 5 6 7. Some current thinking suggests that DCs only migrate from peripheral tissues when they have acquired “foreign” Ag, perhaps as a result of inflammatory stimuli 8 9. Many studies, however, show that DCs migrate constitutively from peripheral tissues in the absence of any overt antigenic or inflammatory stimuli 10 11, although migration is enhanced by such stimuli 12 13 14 15. We have shown previously that in specific pathogen–free (SPF) rats, DCs continually migrate from the intestine to mesenteric LNs (MLNs) in the absence of overt antigenic stimulation 16. Recently, we have shown that these DCs comprise two distinct subpopulations 17. DCs that coexpress CD4 and OX41, a member of the SIRP (signal regulatory protein) family (references 18 and 19; referred to here as OX41+ lymph-borne dendritic cell [L-DC]), have functional properties typical of mature DCs. In contrast, CD4−/OX41− L-DCs (referred to as OX41− L-DCs) are weak APCs for specific Ag and in the allogeneic MLR, survive poorly in culture, contain large cytoplasmic inclusions, and are very strongly nonspecific esterase (NSE)+. We now show that these OX41− L-DCs transport apoptotic intestinal epithelial cells (IECs) to T cell areas of MLNs and that this traffic exists in gnotobiotic rats.
Whereas presentation of Ag by DCs in vitro generally leads to T cell activation, the same may not apply in vivo, and there is circumstantial evidence that DCs may be able to present Ag in a tolerogenic manner 20 21 22 23. To maintain peripheral tolerance, Ag has to be exported from the periphery to secondary lymphoid tissues for presentation to T cells, and some models of peripheral tolerance require bone marrow–derived APCs, DCs being prime candidates 24 25. The continual transport of self-Ag to MLNs by a distinct subpopulation of DCs suggests one mechanism for the maintenance of self-tolerance.
Materials and Methods
Animals
Conventional rats were PVG (RT1c) or DA (RT1a) bred and maintained under SPF conditions in the Medical Research Council Cellular Immunology Unit, Sir William Dunn School of Pathology. Gnotobiotic inbred rats (strain AVN, F89; Prague) were reared in plastic isolators for 10 generations under germ-free conditions. All animal experiments were carried out under the authority of a licence issued by the Home Office, UK.
Surgical Procedures
Mesenteric lymphadenectomy and thoracic duct cannulation were carried out as described previously 11.
Abs and Other Reagents
“OX” and “W3” series mAbs were supplied by the MRC Cellular Immunology Unit, Sir William Dunn School of Pathology. Anti–rat cytokeratin mAbs were supplied by Prof. A. Quaroni (Cornell University, Ithaca, NY). Anti–rat CD11c mAb was from Serotec Ltd. Secondary Abs included rabbit or goat anti–mouse IgG coupled to horseradish peroxidase or alkaline phosphatase, respectively (DAKO Corp.) and biotinylated horse anti–mouse IgG (H+L chain) absorbed against rat serum proteins (Vector Labs.). MicroBeads™ conjugated to goat anti–mouse IgG (H+L chain) F(ab′)2 fragments (Miltenyi Biotec), streptavidin R–PE conjugate (Serotec Ltd.), streptavidin Quantum Red conjugate (Sigma Chemical Co.), and NycoPrep™ solution (NP 1.068; NycoMed) were used according to manufacturers' instructions. Avidin/biotin blocking kit, VECTASTAIN® ABC kits, and the substrates for horseradish peroxidase or alkaline phosphatase were from Vector Labs. NSE substrates, α-naphthyl butyrate or α-naphthyl acetate, were from Sigma Chemical Co.
Cell Preparation and Purification
L-DCs.
L-DCs were enriched from lymph cells collected from the thoracic ducts of mesenteric lymphadenectomized rats (XTDLs) by single step density separation. XTDLs resuspended in washing medium (at a concentration of 1–2 × 107/cells ml) were overlaid over NP 1.068 solution and centrifuged at 400 g for 20 min at room temperature. The interface cells contained 20–40% L-DCs. The major contaminating cells were B lymphocytes. There was <1% macrophages present, as identified by morphology and the ability to phagocytose opsonized sheep RBCs. To further purify L-DCs, the interface cells were labeled with a cocktail of antilymphocyte mAbs: OX52 (pan-T cell), OX19 (CD5), OX8 (CD8), OX12 (Ig L chain), and OX33 (B cell CD45) and separated using magnetic beads (MACS; Miltenyi Biotec) coated with goat anti–mouse Ig according to the manufacturer's instructions. Importantly, the positive selection column was used for negative selection, as this greatly decreased nonspecific DC loss. L-DCs were separated into subpopulations by MACS using W3/25 (anti-CD4) and/or OX41 mAbs 18.
LN DCs.
These were prepared as described previously 26 27 28. In brief, organs or tissues were teased apart and then digested with collagenase D and DNAse (Boehringer Mannheim). DCs were enriched by density gradient centrifugation and negative selection using a cocktail of mAbs as above and rosetting or MACS bead separation. To avoid maturing the DCs, an overnight adherence step was omitted.
Bone Marrow–derived DCs.
Rat femurs were dissected out, and both ends were cut off. Bone marrow cells were flushed out with PBS, and a single-cell suspension was made by pipetting. Erythrocytes were lysed in Gey's solution, and bone marrow cells were washed and passed through a cell strainer (70-μm pore size; Becton Dickinson). Lymphocytes and MHC class II+ cells were removed by rosetting using mAbs OX52 (pan-T), OX12 (Ig L chain), and OX6 (MHC class II). The remaining cells were cultured at 106 cells/ml in RPMI 1640 containing 2 mM l-glutamine, 1 mM sodium pyruvate, 40 ng/ml murine GM-CSF, and 1,500 U/ml rat IL-4. After 7–12 d of culture, proliferating non- and semiadherent cells were harvested. At day 12, 90% of cells were MHC class II+ and CD11c+ with irregular outlines.
Alveolar and Peritoneal Macrophages.
Animals were killed by CO2 and cervical dislocation. Alveolar macrophages (aMΦs) and peritoneal macrophages (pMΦs) were obtained by repeated flushing of the trachea and bronchi and peritoneal cavity, respectively, with ice cold PBS containing 25 mM EDTA. Nonadherent cells were removed after 1-h culture in complete culture medium containing 10% FCS.
IECs.
10–15-cm lengths of rat small intestine were flushed with PBS, inverted, and tightened onto a glass rod, which was attached to a vibratory mixer. After brief vibrations (5–10-s intervals) in ice cold PBS containing 25 mM EDTA, IECs were released and collected.
Immunocytochemistry
For light microscopy, tissue specimens were snap frozen in liquid nitrogen, and cryostat sections were fixed with cold ethanol or 2% paraformaldehyde (for NSE detection). To inhibit endogenous peroxidase, sections were quenched at 37°C for 15 min in 0.1 M phosphate buffer containing glucose oxidase (0.5 U/ml; Sigma Chemical Co.), glucose (0.18%), and sodium azide (10 mM). When alkaline phosphatase–conjugated Abs were used for detection, levamisole was added to block endogenous enzyme. Nonspecific binding sites were blocked with 2% BSA, 0.1% Tween 20, appropriate normal serum, and the avidin/biotin blocking kit when biotinylated Abs were used. First layer Abs were neat tissue culture supernatants or purified Ig at 10–20 μg/ml. Second layer Abs were coupled to horseradish peroxidase or alkaline phosphatase (DAKO Corp.) or to biotin and followed by streptavidin conjugated to the enzymes (ABC kits; Vector Labs.). All substrates were freshly prepared before use. For electron microscopic immunocytochemistry, anesthetized rats were perfuse fixed via the abdominal aorta with 0.5% glutaraldehyde in phosphate buffer. 0.5-mm slices were cut on a tissue slicer (Polaron Instruments, Inc.). The slices were incubated with OX6 (anti–MHC class II), followed by horseradish peroxidase–coupled rabbit anti–mouse IgG (DAKO Corp.), in 96-well plates for 24 h at 4°C on a rocker. Sections were washed for 24 h between incubations. The reaction product was developed using 0.005% H2O2 in 0.1% 3,3′-diaminobenzidine tetrahydrochloride (Polysciences, Inc.) in 50 mM Tris/HCl. Sections were embedded in epoxy resin, and thin sections were examined on a Phillips 3000 electron microscope.
Detection of NSE
Cytospin preparations or cryostat sections were fixed in formaldehyde vapor or 2% paraformaldehyde. After washing, NSE reactivity was developed using α-naphthyl butyrate or α-naphthyl acetate (Sigma Chemical Co.) as substrate 29. For simultaneous detection of NSE reactivity and Ab binding, cells or sections were fixed with 2% paraformaldehyde, reacted for NSE, washed extensively, and then labeled with Abs as above. To identify NSE variants, zymograms were prepared according to von Deimling et al. 30. In brief, cells or tissues were lysed in lysis buffer containing 0.1% Triton X-100 and 15 mM EDTA and sonicated on ice for 10–30 s using an MSE sonicator. Debris was removed by centrifugation, and the supernatant was electrophoresed on 7.5% polyacrylamide gels under nondenaturing conditions. Gels were then incubated with α-naphthyl butyrate or α-naphthyl acetate to reveal the enzymes, washed in PBS, and dried between two sheets of cellophane in a Bio-Rad GelAir drying system.
Labeling for Apoptotic DNA
Cryostat sections or cytospin preparations were processed for apoptotic DNA by the TUNEL (terminal deoxynucleotidyl transferase–mediated dUTP-biotin nick-end labeling) method using an Apoptag-Fluorescein kit (Oncor Inc.) according to the manufacturer's instructions and subsequently labeled with OX6, OX62, or CD11c and appropriate secondary reagents. Images were collected on a Bio-Rad MRC-1024 confocal microscope.
Results
OX41− Intestinal L-DCs Contain Apoptotic DNA.
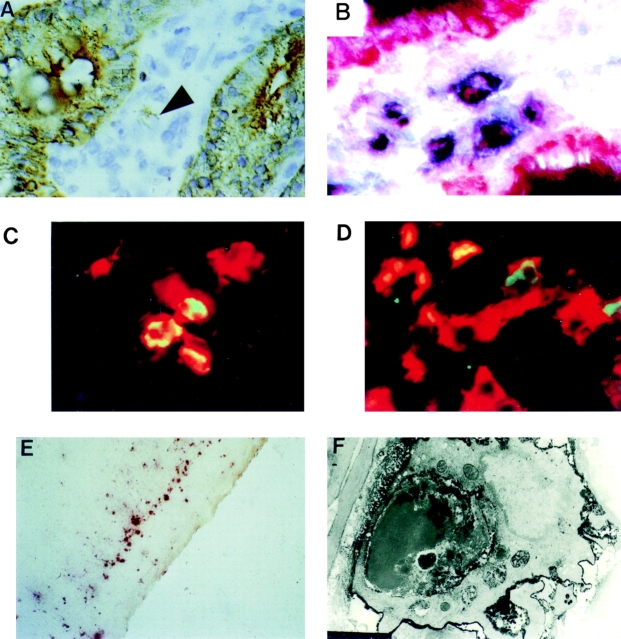
OX41− L-DCs (65–75% of total L-DCs) possess large cytoplasmic inclusions, some of which contain DNA 11 17. To characterize these inclusions, L-DCs were separated into OX41+ and OX41− subpopulations. Cytospins were labeled for MHC class II, OX62, or CD11c and for apoptotic DNA by the TUNEL method (Fig. 1A–C) and examined by confocal microscopy. Up to 30% of OX41− L-DCs contained TUNEL-positive inclusions of different sizes (Fig. 1A and Fig. C). In many cases, these inclusions appeared not to colocalize with MHC class II+ or OX62+ vesicles within the DC (Fig. 1 C). Rare inclusions in OX41+ L-DCs (<1%) probably represent contaminating OX41− L-DCs (3–5% of the separated population).
Figure 1.
OX41− L-DCs contain apoptotic epithelial cell remnants. (A–C) L-DCs, partially enriched over NycoPrep™, were separated into OX41+ and OX41− populations on a MACS column. Cytospin preparations were labeled for apoptotic DNA by the TUNEL method (green) and for MHC class II (red) and examined on a confocal microscope. (A) OX41− DCs. 20–30% of cells contain TUNEL-positive inclusions. (B) OX41+ DCs. No cells contain TUNEL-positive inclusions. (C) TUNEL-positive inclusions are present within the cytoplasm of a DC. There is little overlap between apoptotic DNA and MHC class II (×80 objective). (D) OX41− L-DCs, separated as above, were labeled for the epithelial cell cytokeratins 4 and 21 by mAbs RK4 and RK5. 4–7% of OX41− DCs contain cytokeratin-positive (RK4) inclusions (>, brown). (E) Cytospins of OX41− DCs were stained for NSE using α-naphthyl butyrate as substrate. More than 80% of DCs are very strongly positive. (F) OX41+ DCs reacted as in E. Esterase reactivity is negative or weak. One positive cell probably represents an OX41− contaminant. (G) OX41− DCs reacted for 1 min for NSE. Esterase reactivity is present as large, irregular, cytoplasmic inclusions.
OX41− Intestinal L-DCs Contain IEC Remnants.
Two approaches show that OX41− L-DCs contain IEC remnants. Cytospins of separated L-DCs were labeled for the IEC-restricted cytokeratins RK4 and RK5 31 32. 3–5% of OX41− L-DCs contained cytokeratin-positive inclusions (Fig. 1 D). These varied in size from small dots to large masses; all were discrete, and no diffuse labeling was seen. No such inclusions were seen in OX41+ L-DCs.
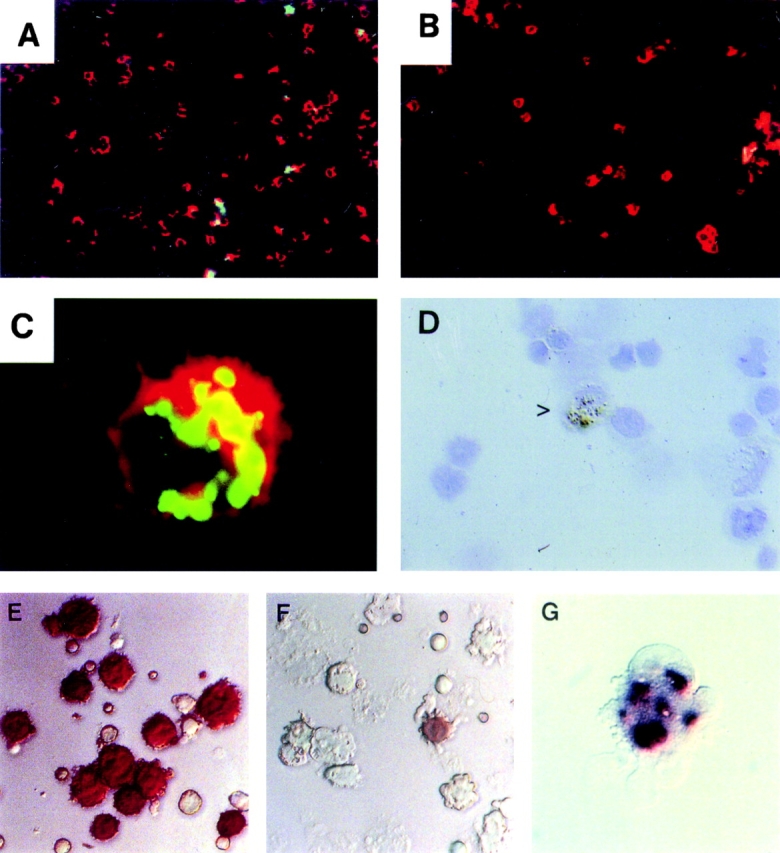
More than 80% of OX41− L-DCs but <5% of OX41+ L-DCs (probably OX41− contaminants; 3–5%) react intensely for NSE (reference 17 and Fig. 1E and Fig. F). In contrast to monocytes and macrophages, where staining is diffuse and perinuclear, OX41− L-DC reactivity in cells stained for short periods is granular or vesicular (Fig. 1 G). NSE exists as different isoforms with different electrophoretic mobilities 30 33 34. To identify the origin of the NSE in OX41− L-DCs, we prepared NSE zymograms from IECs, L-DCs, and other cells and tissues (Fig. 2A–C). Multiple variants of NSE were identified, but IECs and OX41− L-DCs contained at least two variants with identical mobilities (Fig. 2, arrowheads). These were not seen in OX41+ L-DCs, cervical LN (CLN) DCs, bone marrow–derived DCs, aMΦs, resident or elicited pMΦs, or many tissues. Unseparated CLN and MLN cells did contain low levels of NSE, but the variants were similar to those from macrophages and different from those from OX41− L-DCs, probably representing endogenous NSE. Only isolated MLN DCs showed a pattern similar to OX41− L-DCs.
Figure 2.

OX41− DCs and IECs display similar NSE variants. Cells and tissues were lysed, and the lysates were electrophoresed in native form (zymograms). The gels were reacted for NSE using α-naphthyl acetate or α-naphthyl butyrate as substrates. (A) Different numbers of cell equivalents from OX41+ and OX41− DCs and purified IECs were loaded onto the gel. IECs and OX41− L-DCs show a similar pattern of bands, although IECs contain larger amounts of enzyme. OX41+ DCs express very little NSE, that seen probably being due to endogenous NSE variants and/or to contamination with small numbers of OX41− DCs. (B) Zymograms were prepared from OX41+ and OX41− L-DCs, IECs, and total (unseparated) MLN or CLN cells and other tissues. OX41− DCs and IECs show similar isoforms (arrowheads). This pattern is not seen in any other tissue tested. Protein extracts equivalent to 8 × 105 OX41− DCs, 104 IECs, 5 × 105 MLN or CLN cells, 1 mg wet weight (spleen, thymus), or 0.1 mg wet weight (lung, liver, kidney) were loaded onto each lane. (C) Zymograms were prepared from OX41− L-DCs, IECs, DCs isolated from MLN and CLN, bone marrow–derived DCs, aMΦs (Alv. MΦ), and resident and thioglycollate (Thio.)-elicited pMΦs (Pe. MΦ). MLN DCs, but not CLN DCs, contain NSE variants similar to those present in OX41− DCs and IECs (arrowheads). These are not seen in zymograms from any other cell type. Material equivalent to 106 cells was loaded onto each lane, except for IECs (104 cells).
IEC-bearing L-DCs Derive from Lamina Propria and Peyer's Patch.
L-DCs derive from the small intestine 11. To identify the origins of IEC-containing L-DCs, cryostat sections of lamina propria (LP) and Peyer's patch (PP) were labeled for NSE and DC markers. Some large, irregular LP cells contained IEC-restricted cytokeratin (Fig. 3 A). Many NSE+ cells in the LP were negative for DC markers and are probably macrophages. However, some NSE+, large, irregular cells were CD11c+ (Fig. 3 B) or OX62+ (not shown). Some MHC class II+ or OX62+ cells in the LP contained apoptotic DNA (Fig. 3C and Fig. D). Thus, cells similar to OX41− L-DCs are present in the LP. In PP, similar cells were present at the base (Fig. 3 E) and underlying dome epithelium (not shown). Electron microscopic immunocytochemistry showed that some MHC class II+, irregular cells at the base contained apoptotic cells (Fig. 3 F) or large, electron dense granules similar to those in L-DCs and interdigitating DCs in MLNs (not shown; see Fig. 4 E).
Figure 3.
DCs containing IEC markers are present in LP and PP. Cryostat sections of small intestine from SPF rats were labeled for IEC markers, apoptotic DNA, or NSE, with or without DC markers. (A) Intestinal villi labeled for epithelial cell cytokeratin 4 with mAb RK4. The IECs react strongly for cytoplasmic cytokeratin. Occasional large irregular cells in the LP contain granular, cytokeratin-positive inclusions (arrowhead). (B) Intestinal villi labeled for OX62 (blue) and NSE (red). The epithelial cells react strongly for NSE. Several cells in the LP are positive for both NSE and OX62. (C) Intestinal villi labeled for MHC class II and apoptotic DNA. Several cells in the LP are positive for MHC class II and apoptotic DNA. (D) As C, but labeled for OX62 and apoptotic DNA. Some cells express both markers. (E) PP; base, NSE. Many positive cells are in close apposition to the muscle layers. (F) PP. Electron micrograph of basal layers labeled for MHC class II. An MHC class II+, irregular cell contains an apoptotic cell within its cytoplasm.
Figure 4.
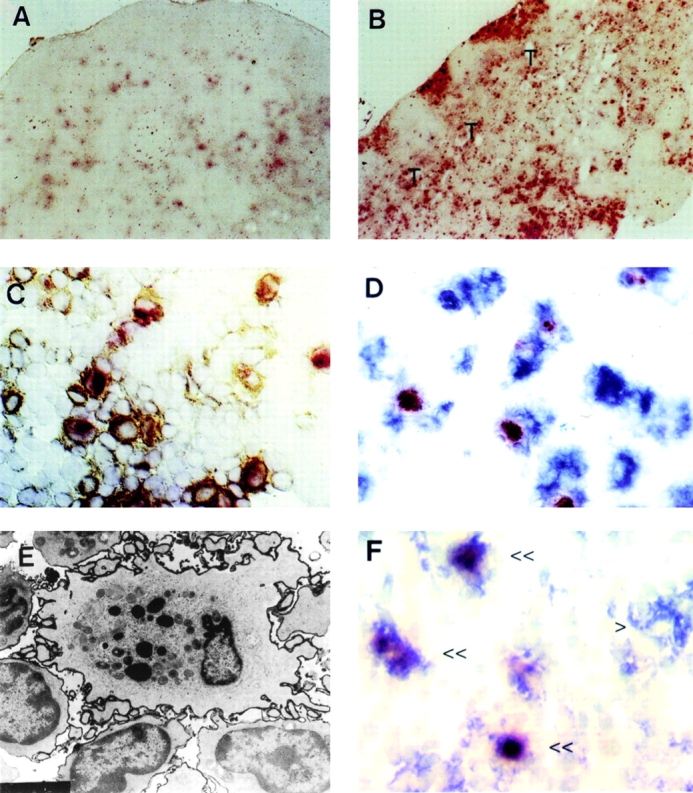
Cells with the characteristics of OX41−NSE+ L-DCs are present in PP and MLNs but not other nodes. (A) Cryosection of CLN reacted for NSE. Weakly positive cells are present, particularly in the follicles. (B) Cryosection of MLN reacted for NSE. Large numbers of large, irregular, strongly positive cells are present in the subcapsular sinus, interfollicular traffic areas, and the paracortical T cell area (T). (C) Cryosection of MLN reacted for NSE and labeled for MHC class II. The paracortex contains large numbers of large, irregular cells positive for both markers. (D) Cryosection of MLN labeled for NSE and OX62. The paracortex contains large numbers of large irregular cells positive for both markers. (E) Electron micrograph of MLN labeled for MHC class II. An MHC class II+ interdigitating cell in the paracortex contains large, dense cytoplasmic inclusions, similar to those seen in L-DCs. (F) Cryosection of PP labeled for NSE (red) and CD11c (blue). Several double-positive cells (<<) and one CD11c+NSE− cell (>) is present in the T cell area of the patch.
OX41− L-DCs Migrate to T Cell Areas of PPs and MLNs.
To determine the fate of L-DCs within nodes, cryosections of MLNs and other nodes were labeled for NSE and DC markers. All nodes contain NSE+ cells in the medulla and follicles. However, the interfollicular traffic areas and T cell areas of MLNs (Fig. 4 B), but not CLNs (Fig. 4 A) or popliteal nodes (not shown), contained large irregular cells that were strongly NSE+ and were also MHC class II+ (Fig. 4 C), OX62+ (Fig. 4 D), or CD11c+ (not shown; see Fig. 5 B). Follicular and medullary NSE+ cells were negative for these markers (not shown). Electron microscopic immunocytochemistry showed large, irregular MHC class II+ cells in the interfollicular areas and paracortex. Many of these cells contained electron dense cytoplasmic inclusions similar to those in L-DCs (Fig. 4 E). Zymograms prepared from purified MLN DCs showed the same two NSE variants identified in IECs and CD4−/OX41− L-DCs but not in other cells or tissues (Fig. 2 C). In PP, strongly NSE+ CD11c+ DCs were present in T cell areas (Fig. 4 F). Thus, some DCs in T cell areas of PPs and MLNs represent migrant OX41− L-DCs containing apoptotic IECs.
Figure 5.
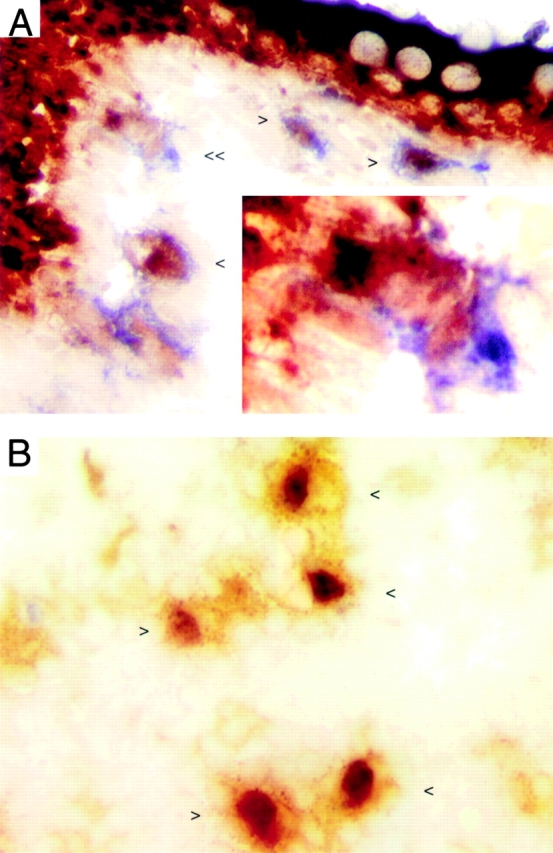
Strongly NSE+ DCs are present in the LP and T cell areas of MLNs from germ-free rats. LNs and ileum from germ-free rats were snap frozen. Cryosections of ileum (A) and MLN (B) were fixed in 2% paraformaldehyde, reacted for NSE (red), and labeled for OX62 (blue in A, brown in B). (A) Double-positive cells (<) are present in apposition to the epithelium. One cell (<<, enlarged in inset) appears to be engulfing an epithelial cell. (B) Several large, irregular, double-positive cells are present in the T cell area of the node (<, >).
NSE+ DCs Are Present in LP and MLNs of Gnotobiotic Rats.
To determine if migration of IEC-containing L-DCs into T cell areas of MLNs depends on intestinal bacterial flora, cryosections of small intestine, MLN, and CLN from gnotobiotic rats were double labeled for NSE and DC markers. Cells that coexpress strong NSE reactivity and OX62 (Fig. 5 A), CD11c (not shown), or MHC class II (not shown) are present in small numbers in the LP and also in interfollicular and T cell areas of MLNs (Fig. 5 B).
Discussion
The best understood role of DCs is as sentinel cells that transport Ag from peripheral tissues to secondary lymphoid tissues and present peptides to T cells 1. It has been suggested that DCs only leave peripheral tissues when they receive an inflammatory or “danger” signal, but many observations strongly suggest that this is not true. DCs are present in intestinal lymph from SPF rats 11 17, and strongly NSE+ DCs are present in lymph obtained by direct cannulation of intestinal afferent lymphatics (lacteals; reference 11). It could be argued that the presence of “normal” bowel flora might be sufficient to generate local low grade inflammatory stimuli that are responsible for DC migration. The observation that strongly NSE+ cells expressing DC markers are present in T cell areas of MLNs from gnotobiotic rats suggests otherwise. In sheep, veiled DCs are present in all peripheral lymph examined, including renal and hepatic lymph (reference 10 and MacPherson, G.G., unpublished observations). Thus, although DC migration from peripheral tissues and entry of DCs into intestinal lymph can be stimulated by proinflammatory stimuli 13 14 15 35, some DC traffic is constitutive.
Rat pseudoafferent intestinal lymph DCs arise from the small intestine 11 and comprise two subpopulations, one of which coexpresses OX41, a SIRP family member 18 19, and CD4 17. OX41+ L-DCs are typical mature CD11c+ DCs expressing high levels of surface MHC class II. They are weakly NSE+ and are strong APCs. OX41− L-DCs also express high levels of MHC class II and are CD11c+ but are weak APCs despite expressing levels of B7 similar to those of OX41+ L-DCs 17. OX41− L-DCs do not express any typical macrophage properties 17. More than 80% of OX41− L-DCs contain large, very strongly NSE+ inclusions, some of which represent recognizable cellular remains 11. Here, we present strong evidence that these inclusions derive at least in part from apoptotic IECs.
IECs are formed in the crypts of intestinal villi, rapidly migrate to the tips of villi 36, and undergo apoptosis 37. Most IECs are shed into the intestinal lumen, but our results suggest that some are endocytosed by OX41− DCs in the LP and PP and that these DCs enter afferent lymph. Thus, TUNEL of DCs in LP and lymph shows that some contain cytoplasmic apoptotic DNA. Two lines of evidence suggest that this DNA derives from IECs. Some OX41− DCs contain inclusions that stain specifically for cytokeratins only expressed in IECs 31 32. Splenic DCs are negative for these markers. Also, the very strong NSE reactivity in OX41− L-DCs, much stronger than in any other DC or macrophage population yet described, most probably derives from IECs, which also stain strongly for NSE. In macrophages and other DCs (including OX41+ DCs), NSE is diffuse and perinuclear, but brief incubation of OX41− L-DCs with substrate shows NSE in large cytoplasmic inclusions. Positive identification of NSE origins is possible, because NSE represents different enzymes existing in different isoforms 30 33 38 39 that can be distinguished by their electrophoretic mobility. Expression patterns of variants differ in different cells and tissues. IECs and OX41− L-DCs express some NSE bands with identical mobility. In contrast, out of all other cells and tissues examined, including other DC-containing populations and macrophages, similar patterns were seen only in MLN DCs, which include OX41− L-DCs that have entered the LNs. Thus, we conclude that NSE reactivity in OX41− L-DCs derives from endocytosed apoptotic IECs. NSE reactivity is present in >80% of OX41− L-DCs, whereas a much smaller proportion expresses cytokeratin epitopes or apoptotic DNA. This may represent differential sensitivity of the molecules to degradation after endocytosis, as some IEC NSE is lysosomal 40 and would resist lysosomal degradation.
DCs, especially when immature, can endocytose apoptotic cells in vitro 3 5 6 7 41, and the endocytosis of apoptotic vaginal keratinocytes by Langerhans cells has been described in mice during the estrus cycle 42. The endocytosis of epithelial cells in the LP by cells identified as macrophages has been described in several species but, interestingly, was not observed in rats 37 43 44 45 46. Endocytosis and transport of apoptotic cells by DCs in vivo has not, however, been described previously. Our findings suggest that immature DCs continually endocytose apoptotic cells dying in peripheral tissues; however, the frequency of this event would depend on the rate of turnover of cells in different tissues. In most tissues, where turnover of cells is slower than in the intestine, such endocytosis would be relatively rare and hence difficult to detect. As cells expressing DC markers and containing apoptotic DNA or NSE are present in intestinal LP and PP, this strongly suggests that these are the sites where DCs endocytose IECs. It is also clear that these DCs migrate to T cell areas of MLNs. DCs in T cell areas have a rapid turnover similar to that of L-DCs 47. Strongly NSE+ cells are present in T cell areas of MLNs but not other nodes. NSE+ cells in T areas of MLNs are likely to be DCs because they are also MHC class II+, OX62+, or CD11c+. The NSE they express is very likely to be derived from IECs, because purified MLN DCs, but not DCs purified from CLNs, express NSE variants identical to those from OX41− L-DCs and IECs.
The function of this continual transport of IECs to MLNs may relate to peripheral tolerance. It is essential for the prevention of autoimmunity that potentially self-reactive T cells emerging from the thymus do not mount active immune responses. As lymphocyte migration through peripheral tissues in adult animals is largely limited to cells with an activated/memory phenotype 48, active tolerization to peripheral self-Ag must occur at the sites of naive lymphocyte recirculation, the nodes, spleen, and mucosal lymphoid tissues. One model of peripheral self-tolerance requires T cell activation and a bone marrow–derived APC that presents Ag to naive T cells in secondary lymphoid tissues 24 25. The nature of the APC involved and details of its physiology are unclear, but DCs are strong candidates 25 49. Although DCs are primarily considered to be activators of naive T cells, acquisition of Ag by DCs in vivo may lead to tolerance. Thus, murine splenic DCs and L-DCs can acquire Ag given intravenously or orally, respectively 20 21, but these routes of Ag administration may lead to tolerance. The rat antimurine DC mAb 33D1 50, given intravenously and thus targeted to splenic DCs, can induce tolerance to rat IgG 22. We have shown previously that oral Ag can be acquired by DCs in the intestinal wall and that these DCs rapidly migrate into lymph 21 and can present the contained Ag. This may represent one mechanism for oral tolerance induction.
Two other implications of our observations are that, first, they suggest a mechanism by which a protective immune response may be stimulated by pathogens whose tropism is confined to epithelial cells. Second, they suggest a mechanism by which pathogens that primarily infect epithelial cells may be able to cross the epithelial barrier, the DCs acting as a “Trojan horse.”
We conclude that a distinct subpopulation of DCs is specialized to acquire self-Ag in the form of apoptotic epithelial cells in the intestine and to transport them to draining LNs. The DC-contained self-Ag may then be available either directly or indirectly 51 for the tolerization of naive T cells within the node.
Acknowledgments
We are grateful for help with photography from Lance Tomlinson, with histology from Liz Darley, and with confocal microscopy from Emma Turnbull. Tissues from gnotobiotic rats were very kindly supplied by Drs. R. Stepkanova and H. Tlaskalova-Hogenova, Academy of Sciences of the Czech Republic, Prague.
This work was supported in part by grants from the Biotechnology and Biosciences Research Council, the Wellcome Trust, and the Medical Research Council.
Footnotes
M. Wykes' current address is Mater Medical Research Institute, South Brisbane, Queensland, Australia.
T.J. Powell's current address is Institute for Animal Health, Compton, Berks, UK.
Abbreviations used in this paper: aMΦs, alveolar macrophages; CLN, cervical lymph node; DCs, dendritic cells; IECs, intestinal epithelial cells; L-DC, lymph-borne dendritic cell; LP, lamina propria; MLNs, mesenteric lymph nodes; NSE, nonspecific esterase; pMΦs, peritoneal macrophages; PP, Peyer's patch; SPF, specific pathogen–free; TUNEL, terminal deoxynucleotidyl transferase–mediated dUTP-biotin nick-end labeling.
References
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252 . doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Mechanisms of antigen uptake for presentation. Curr. Opin. Immunol. 1996;8:348–354. doi: 10.1016/s0952-7915(96)80124-5. [DOI] [PubMed] [Google Scholar]
- Zocchi M.R., Poggi A., Rubartelli A. The RGD-containing domain of exogenous HIV-1 Tat inhibits the engulfment of apoptotic bodies by dendritic cells. AIDS. 1997;11:1227–1235. doi: 10.1097/00002030-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Rovere P., Manfredi A.A., Vallinoto C., Zimmermann V.S., Fascio U., Balestrieri G., Ricciardi Castagnoli P., Rugarli C., Tincani A. Dendritic cells preferentially internalize apoptotic cells opsonized by anti-β2-glycoprotein I antibodies. J. Autoimmun. 1998;11:403–411. doi: 10.1006/jaut.1998.0224. [DOI] [PubMed] [Google Scholar]
- Albert M.L., Pearce S.F., Francisco L.M., Sauter B., Roy P., Silverstein R.L., Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Rubartelli A., Poggi A., Zocchi M.R. The selective engulfment of apoptotic bodies by dendritic cells is mediated by the alpha(v)beta3 integrin and requires intracellular and extracellular calcium. Eur. J. Immunol. 1997;27:1893–1900. doi: 10.1002/eji.1830270812. [DOI] [PubMed] [Google Scholar]
- Janeway C.A., Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Smith J.B., McIntosh G.H., Morris B. The traffic of cells through tissuesa study of peripheral lymph in sheep. J. Anat. 1970;107:87–100. [PMC free article] [PubMed] [Google Scholar]
- Pugh C.W., MacPherson G.G., Steer H.W. Characterization of nonlymphoid cells derived from rat peripheral lymph. J. Exp. Med. 1983;157:1758–1779. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinniard A., Peters S.W., Foster J.R., Kimber I. Dendritic cell accumulation in draining lymph nodes during the induction phase of contact allergy in mice. Int. Arch. Allergy Appl. Immunol. 1989;89:202–210. doi: 10.1159/000234947. [DOI] [PubMed] [Google Scholar]
- Hill S., Edwards A., Kimber I., Knight S. Systemic migration of dendritic cells during contact sensitization. Immunology. 1990;71:277–281. [PMC free article] [PubMed] [Google Scholar]
- MacPherson G.G., Jenkins C.D., Stein M.J., Edwards C. Endotoxin-mediated dendritic cell release from the intestinecharacterization of released dendritic cells and TNF dependence. J. Immunol. 1995;154:1317–1322. [PubMed] [Google Scholar]
- Roake J.A., Rao A.S., Morris P.J., Larsen C.P., Hankins D.F., Austyn J.M. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 1995;181:2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh C.W., MacPherson G.G., Steer H.W. Characterization of nonlymphoid cells derived from rat peripheral lymph. J. Exp. Med. 1983;157:1758–1779. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.M., Zhang M., Jenkins C., MacPherson G.G. Dendritic cell heterogeneity in vivotwo functionally different dendritic cell populations in rat intestinal lymph can be distinguished by CD4 expression. J. Immunol. 1998;161:1146–1155. [PubMed] [Google Scholar]
- Robinson A.P., White T.M., Mason D.W. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986;57:239–247. [PMC free article] [PubMed] [Google Scholar]
- Adams S., van der Laan L.J., Vernon Wilson E., Renardel de Lavalette C., Dopp E.A., Dijkstra C.D., Simmons D.L., van den Berg T.K. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J. Immunol. 1998;161:1853–1859. [PubMed] [Google Scholar]
- Crowley M., Inaba K., Steinman R. Dendritic cells are the principal cells in mouse spleen bearing immunogenic fragments of foreign proteins. J. Exp. Med. 1990;172:383–386. doi: 10.1084/jem.172.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.M., MacPherson G.G. Antigen acquisition by dendritic cellsintestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J. Exp. Med. 1993;177:1299–1307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F.D., Lees A., Birnbaum R., Gause W.C., Morris S.C. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J. Immunol. 1996;157:1406–1414. [PubMed] [Google Scholar]
- Viney J.L., Mowat A.M., Omalley J.M., Williamson E., Fanger N.A. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J. Immunol. 1998;160:5815–5825. [PubMed] [Google Scholar]
- Adler A., Marsh D.W., Yochum G.S., Guzzo J., Nigam A., Nelson W.G., Pardoll D.M. T cell tolerance to parenchymal self-antigens requires presentation by bone marrow–derived antigen-presenting cells. J. Exp. Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath W.R., Kurts C., Miller J.F.A.P., Carbone F.R. Cross-tolerancea pathway for inducing tolerance to peripheral tissue antigens. J. Exp. Med. 1998;187:1549–1553. doi: 10.1084/jem.187.10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.-M., MacPherson G.G. Rat intestinal dendritic cellsimmunostimulatory potency and phenotypic characterisation. Immunology. 1995;85:88–93. [PMC free article] [PubMed] [Google Scholar]
- Kushnir N., Liu L., MacPherson G.G. Dendritic cells and resting B cells form clusters in vitro and in vivoT cell independence, partial LFA-1 dependence, and regulation by cross-linking surface molecules. J. Immunol. 1998;160:1774–1781. [PubMed] [Google Scholar]
- Wykes M., Pombo A., Jenkins C., MacPherson G.G. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- Goud T.J., Schotte C., van Furth R. Identification and characterization of the monoblast in mononuclear phagocyte colonies grown in vitro. J. Exp. Med. 1975;142:1180–1199. doi: 10.1084/jem.142.5.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Deimling O., Muller M., Eisenhardt E. The non-specific esterases of mouse lung. Histochemistry. 1983;78:271–284. doi: 10.1007/BF00489504. [DOI] [PubMed] [Google Scholar]
- Chandler J.S., Calnek D., Quaroni A. Identification and characterization of rat intestinal keratins. Molecular cloning of cDNAs encoding cytokeratins 8, 19, and a new 49-kDa type I cytokeratin (cytokeratin 21) expressed by differentiated intestinal epithelial cells. J. Biol. Chem. 1991;266:11932–11938. [PubMed] [Google Scholar]
- Quaroni A., Calnek D., Quaroni E., Chandler J.S. Keratin expression in rat intestinal crypt and villus cells. Analysis with a panel of monoclonal antibodies. J. Biol. Chem. 1991;266:11923–11931. [PubMed] [Google Scholar]
- de Looze S.M., Ronai A., von Deimling O. Organ specific expression of esterase-6 in the house mouse, Mus musculus . Histochemistry. 1982;74:553–561. doi: 10.1007/BF00496669. [DOI] [PubMed] [Google Scholar]
- Bender K., Nagel M., Gunther E. Es-6, a further polymorphic esterase in the rat. Biochem. Genet. 1982;20:221–228. doi: 10.1007/BF00484420. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M., Kimber I. Dermal tumour necrosis factor-alpha induces dendritic cell migration to draining lymph nodes, and possibly provides one stimulus for Langerhans' cell migration. Immunology. 1992;75:257–263. [PMC free article] [PubMed] [Google Scholar]
- Eastwood G.L. Gastrointestinal epithelial renewal. Gastroenterology. 1977;72:962–975. [PubMed] [Google Scholar]
- Iwanaga T. The involvement of macrophages and lymphocytes in the apoptosis of enterocytes. Arch. Histol. Cytol. 1995;58:151–159. doi: 10.1679/aohc.58.151. [DOI] [PubMed] [Google Scholar]
- Hermes B., Riebschlager M., Deimling O. Esterase XX. Disc-electrophoretic investigations on the polymorphism of the esterases of the house mouse. Histochemistry. 1975;43:81–96. doi: 10.1007/BF00490157. [DOI] [PubMed] [Google Scholar]
- Lindena G., Gartner K. Identification and genetic monitoring of rat inbred strains using esterase mobility by polyacrylamide disc electrophoresis. Folia Biol. (Praha). 1978;24:400–401. [PubMed] [Google Scholar]
- Bocking A., Grossarth C., Deimling O.V. Esterase. XXIII. Electron microscopical demonstration of non-specific esterases in the jejunum of the mouse (Mus musc.) with two quinoline derivatives. Histochemistry. 1976;50:65–76. doi: 10.1007/BF00492787. [DOI] [PubMed] [Google Scholar]
- Rovere P., Vallinoto C., Bondanza A., Crosti M.C., Rescigno M., Ricciardi Castagnoli P., Rugarli C., Manfredi A.A. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J. Immunol. 1998;161:4467–4471. [PubMed] [Google Scholar]
- Parr M.B., Kepple L., Parr E.L. Langerhans cells phagocytose vaginal epithelial cells undergoing apoptosis during the murine estrous cycle. Biol. Reprod. 1991;45:252–260. doi: 10.1095/biolreprod45.2.252. [DOI] [PubMed] [Google Scholar]
- Han H., Iwanaga T., Uchiyama Y., Fujita T. Aggregation of macrophages in the tips of intestinal villi in guinea pigstheir possible role in the phagocytosis of effete epithelial cells. Cell. Tissue Res. 1993;271:407–416. doi: 10.1007/BF02913723. [DOI] [PubMed] [Google Scholar]
- Han H., Iwanaga T., Fujita T. Species-differences in the process of apoptosis in epithelial cells of the small intestinean ultrastructural and cytochemical study of luminal cell elements. Arch. Histol. Cytol. 1993;56:83–90. doi: 10.1679/aohc.56.83. [DOI] [PubMed] [Google Scholar]
- Iwanaga T., Han H., Adachi K., Fujita T. A novel mechanism for disposing of effete epithelial cells in the small intestine of guinea pigs. Gastroenterology. 1993;105:1089–1097. doi: 10.1016/0016-5085(93)90953-a. [DOI] [PubMed] [Google Scholar]
- Iwanaga T., Hoshi O., Han H., Takahashi Iwanaga H., Uchiyama Y., Fujita T. Lamina propria macrophages involved in cell death (apoptosis) of enterocytes in the small intestine of rats. Arch. Histol. Cytol. 1994;57:267–276. doi: 10.1679/aohc.57.267. [DOI] [PubMed] [Google Scholar]
- Fossum S. Dendritic leukocytesfeatures of their in vivo physiology. Res. Immunol. 1989;140:877–926. doi: 10.1016/0923-2494(89)90048-5. [DOI] [PubMed] [Google Scholar]
- Mackay C.R., Kimpton W.G., Brandon M.R., Cahill N.P. Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J. Exp. Med. 1988;167:1755–1765. doi: 10.1084/jem.167.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de St. Groth B.F. The evolution of self-tolerancea new cell arises to meet the challenge of self-reactivity. Immunol. Today. 1998;19:448–454. doi: 10.1016/s0167-5699(98)01328-0. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M.C., Steinman R.M., Witmer M.D., Gutchinov B., Cohn Z.A. A monoclonal antibody specific for mouse dendritic cells. Proc. Natl. Acad. Sci. USA. 1982;79:161–165. doi: 10.1073/pnas.79.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Turley S., Yamaide F., Iyoda T., Mahnke K., Inaba M., Pack M., Subklewe M., Sauter B., Sheff D. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]