What makes a protein immunogenic, particularly for strong T cell–mediated immunity? To a first approximation, this determination seems to be made by dendritic cells (DCs). Immature DCs, as in skin 1 2 3 4, lung 5, blood 6 7, and spleen 7 8, take up proteins, immune complexes, microbes, and dying cells. However, in order to use these antigens to stimulate a T cell response, the DCs must undergo a characteristic process of terminal differentiation called “maturation.” The known stimuli for DC maturation are numerous and include inflammatory cytokines, CD40 ligand (CD40L), viral and microbial constituents such as double-stranded RNA and LPS, and certain CpG oligonucleotides.
DC Maturation as a Control Point in the Initiation of Immunity
Maturation changes DCs in many ways that help explain their potent immunogenicity. Examples include de novo expression of T cell costimulatory molecules like CD86 9 10; the capacity to produce IL-12 11 12 and resist immunosuppression by IL-10 13; the development of a new repertoire of chemokine receptors, especially CCR7 14 15 16 17, that guide entry into lymphatics and migration to the T cell areas 18; the production of DC survival and stimulatory molecules like CD40 and TNF-related activation-induced cytokine receptor (TRANCE-R) 19 20; and a redistribution of MHC class II molecules from lysosomes to the cell surface 21 22. Recently, it has been found (Inaba, K., S. Turley, T. Iyoda, F. Yamaide, S. Shimoyama, C. Reis e Sousa, R.N. Germain, J. Mellman, and R.M. Steinman, manuscript submitted for publication) that immature DCs endocytose proteins into MHC class II–rich intracellular compartments, but the cells must also mature to form MHC–peptide complexes there and to export the complexes to the cell surface together with B7 costimulators. DC maturation is understandably a key control point in converting an antigen into an immunogen.
The role for DCs in determining immunogenicity seems well established, but many are now pursuing their role in other contexts: immune deviation, i.e., skewing T cells to the Th2 phenotype; immune regulation, i.e., inducing Tr1 cells that make IL-10; and bona fide tolerance, i.e., deletion and anergy. We will comment on the idea that the uptake of dying cells by immature DCs is critical for the maintenance of peripheral tolerance, including the findings in two papers in this issue. Huang et al. demonstrate that DCs in afferent lymph carry apoptotic bodies derived from the intestinal epithelium 23. They present evidence, using an isoform of intestinal nonspecific esterase, that DCs continually deliver samples of this tissue to the lymph node. Sauter et al. find that DCs phagocytose apoptotic and necrotic cell lines, but only the latter cause DCs to mature into strong stimulators of T cell immunity 24. Both papers suggest that the uptake of apoptotic cells allows DCs to induce peripheral tolerance to self. We will first outline why it makes sense for DCs, such potent agents of immunity, to also ensure tolerance to cell-associated self-antigens that unavoidably are present at sites of foreign antigen deposition.
The Value of Peripheral Tolerance Induction by DCs
Central or thymic tolerance is likely to be mediated by thymic DCs 25 26, but these DCs may not be able to delete T cells that react with many self-antigens in peripheral tissues. Many proteins may not have access to the thymus during development, especially antigens that are expressed after the thymus has generated a T cell repertoire, e.g., breast constituents that are first expressed at puberty 27. Therefore, autoreactive T cells that are not deleted in the thymus need to be silenced in the periphery to prevent immune responses to self-tissues.
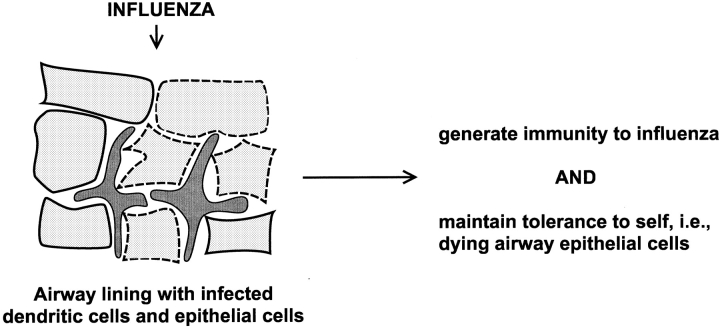
We would argue that it is essential for DCs to play a role in the induction of peripheral tolerance. The reasoning is as follows. Maturing DCs have the capacity to process and present peptides from dying cells to CD4 and CD8 T lymphocytes 28 29 30 31. In fact, DCs may be the principal cells that present antigens from dying cells (“cross presentation”; see below). In this light, consider what might occur during influenza infection of the airway (Fig. 1). During infection, there is extensive death of virus-infected, airway epithelial cells or “self.” How do DCs focus immunity on the virus, when they also should be presenting self-antigens from the infected, apoptotic, airway epithelial cells (29 32; Fig. 1)? Because cell death is a feature of many infections, the danger of what Ehrlich rightly termed “horror autotoxicus” is hardly limited to influenza.
Figure 1.
An illustration of the self-nonself problem from the perspective of DCs. During influenza infection of the airway, a site rich in DCs (reference 59), there is extensive apoptotic death of infected airway epithelial cells, which the DCs would likely process simultaneously with the influenza virus (references 29, 30).
Thus, peripheral tolerance to those peptides that can be processed from dying cells seems critical for preventing autoreactivity, but when and how does this occur? It would be valuable for DCs to induce peripheral tolerance to dying noninfected tissues, since this would inactivate the key self-reactive T cells before the DCs are called upon to initiate immunity to microbial antigens. In effect, peripheral tolerance should share with central thymic tolerance the capacity to self-tolerize before foreign antigen exposure and to use the same APC that will later be called upon to initiate immunity.
Peripheral Tolerance to Tissue Antigens Via Bone Marrow–derived Cells in Draining Lymph Nodes
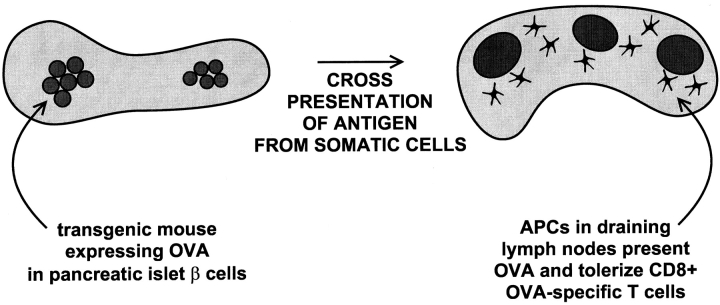
Precise tools have been developed to study peripheral tolerance. Neo self-antigens are expressed as transgenes in peripheral tissues, and then the animal is injected with the corresponding antigen-reactive, TCR transgenic T cells. Adler et al. 33 expressed the influenza hemagglutinin (HA) in many tissues. When HA-reactive CD4+ T cells were injected, the T cells were anergized, and when bone marrow chimeras were examined, the marrow-derived cells had to express the MHC that was recognized by the anergized, HA-reactive, TCR transgenic T cells 33. Anergy did not develop if only nonhematopoietic tissue cells expressed the appropriate MHC. In similarly elegant studies, Kurts et al. 34 35 expressed OVA sequences in insulin-producing β cells of pancreatic islets (Fig. 2). The OVA antigen in tissue cells was again presented to T cells by marrow-derived cells 34, and the TCR transgenic CD8+ T cells seemed to be tolerized by deletion after a series of cell divisions 35. Kurts et al. showed that the tolerizing, marrow-derived cells were confined to the draining lymph nodes (Fig. 2), i.e., the nodes that received afferent lymphatics from the tissue expressing the OVA antigen (the pancreas or kidney in their studies). Analogous results have been reported by others 36 37 38.
Figure 2.
Diagram of the model of Kurts et al. (references 34, 35). Self-peptides in tissue cells, here the insulin-producing β cells of the pancreatic islets, are presented in a tolerogenic way by bone marrow–derived cells in the draining pancreatic lymph nodes.
Somehow then, self-antigens in peripheral tissues are transferred to marrow-derived cells in a lymph node, and this can tolerize adult T cells. Although the marrow-derived cells have yet to be pinpointed, DCs are a likely candidate since they comprise a link between the peripheral tissues and the lymph node, the latter being the site where the tolerizing self-signals appear to be presented.
The Capture of Tissue Cells by DCs
Phagocytic inclusions have been described previously in DCs that traffic from tissues to lymph nodes in afferent lymph 39 40 41. Huang et al. now show that these inclusions are apoptotic bodies 23. Furthermore, their new data indicate that the apoptotic bodies derive from intestinal epithelium, presumably picked up from epithelial cells undergoing normal cell turnover.
The only comparable description of phagocytic inclusions in DCs in situ is a report involving NK cell–mediated clearance of allogeneic leukocytes 42. DCs may also take up apoptotic bodies during negative selection in the thymic medulla 43, but one cannot visualize this, presumably because the digestion of apoptotic cells is so rapid 28. Likewise, it is difficult to identify macrophages with phagocytosed dying cells in situ. For example, many developing thymocytes undergo apoptosis if they fail to be positively selected. The thymocytes are likely to be scavenged by macrophages in the cortex, but this is only evident histologically if massive thymocyte death is induced with steroids or irradiation 43.
Therefore, the sighting by Huang et al. 23 of apoptotic epithelial cells in mesenteric lymph DCs suggests a major flux of tissue antigens via DCs that are heading to lymph nodes. Immature DCs or their precursors may always be trafficking through tissues 44 45, picking up apoptotic material from cells undergoing the turnover that is characteristic of most tissues. If the events described by Huang et al. 23 silence reactivity to the intestinal peptides that are processed from dying cells, then DCs maturing during a subsequent intestinal infection would only stimulate a response to foreign antigen, thus alleviating the problem posed in Fig. 1.
Processing of Apoptotic Cells onto MHC Class I and II Products
Formation of MHC class I–peptide complexes from antigens in endocytosed dying cells 29 30 illustrates phenomena termed the “exogenous pathway” and the “cross-presentation” of antigens. In the exogenous pathway, MHC class I molecules present peptides derived from endocytosed proteins, rather than newly synthesized (“endogenous”) proteins in the cytoplasm. One example of the exogenous pathway is cross-presentation, since exogenous peptides from cells of one MHC, or even xenogeneic MHC, are presented by DCs of a different MHC.
DCs efficiently carry out the exogenous pathway for MHC class I. This applies to peptides derived from immune complexes 46, bacteria 47, and apoptotic cells dying because of viral 29 30 or bacterial 31 infection. Rodriguez et al. have shown that molecules with molecular masses of 3–20 kD somehow can escape the endocytic system of DCs into the cytoplasm 48. They postulate that the endocytic vacuoles of DCs have a transporter or pore whereby substrates gain access to TAP molecules in the endoplasmic reticulum, followed by presentation on MHC class I. The recent results from the Bhardwaj and Amigorena laboratories also provide evidence that the exogenous pathway is expressed much more efficiently in DCs than in macrophages and B cells 30 46 48.
Effects of Apoptotic Cells on DC Maturation
The paper by Sauter et al. in this issue introduces the critical events of DC maturation to this topic. The uptake of apoptotic cells in the steady state must not mature the DCs if these cells are to induce tolerance rather than immunity, and indeed this is what Sauter et al. 24 and Gallucci et al. 49 now report. Immature DCs selectively carry out phagocytosis of apoptotic cells 28 30, as is also the case for the uptake of microbes, latex, and immune complexes 4 46 50. For one thing, relevant phagocytic receptors are better expressed on immature DCs, e.g., αVβ5 integrin for apoptotic bodies and FcγR for immune complexes 30 46.
If DCs only take up apoptotic cells when immature 28 30, if apoptotic cells do not mature the DCs 24, and if immature DCs are poor stimulators of immunity 1, then what are the immunological consequences to the carriage of large numbers of dying somatic cells by DCs in lymph 23? Is uptake immunologically “null,” like the clearance of apoptotic bodies by macrophages, or might peripheral tolerance ensue?
Hypothesis: Immature DCs Phagocytose Tissue Cells Undergoing Normal Cell Turnover by Apoptosis; This Leads to Tolerance or Regulation of Self-reactive, Adult T Cells in the Draining Lymph Node
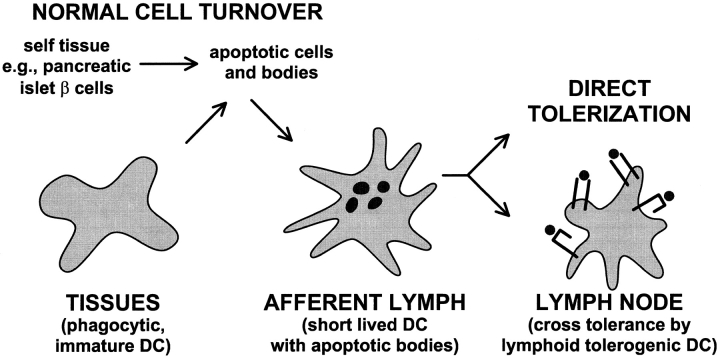
In the steady state, i.e., in the absence of inflammation, infection, and necrosis, DCs are always found in afferent lymph, where they are also called “veiled cells.” Veiled cells might derive from precursors in the blood 6 51 including monocytes 44 45. The idea is that circulating immature DCs and monocytes can traffic through tissues, picking up cells that die by apoptosis 28 30, and then enter the afferent lymph (Fig. 3). In the steady state, these DCs will not receive maturation stimuli and therefore will be unable to stimulate immunity to the self-antigens they have captured.
Figure 3.
Pathways whereby DCs might induce peripheral tolerance to antigens within tissue cells undergoing normal cell turnover by apoptosis. DC precursors traffic through tissues, phagocytose dying cells or apoptotic bodies, and then enter the lymph. Upon reaching the lymph node, the DCs tolerize naive, self-reactive T cells either directly, or indirectly (as diagrammed here) after reprocessing by different regulatory or tolerogenic DCs in the lymph node.
How might immature DCs induce tolerance to self-antigens in phagocytosed apoptotic cells? One view is that migratory immature DCs tolerize T cells directly because of a lack of costimulators (Fig. 3). There are potential difficulties with this idea. For example, we have just found that immature DCs do not process endocytosed antigens well to form MHC–peptide complexes (Inaba, K., S. Turley, T. Iyoda, F. Yamaide, S. Shimoyama, C. Reis e Sousa, R.N. Germain, J. Mellman, and R.M. Steinman, manuscript submitted for publication). Therefore, self-reactive T cells would not be able to recognize their ligand on immature DCs. Also, the immature DCs may lack the CD40 and TRANCE-R that sustain DC viability for the 3–4 d needed before T cell tolerance becomes evident 35 37. In contrast, as summarized above, mature DCs express high levels of MHC–peptide, as well as the CD40 and TRANCE-R that sustain DC viability during the interaction with activated T cells 52. Possibly the migratory immature DCs overcome some of these potential shortcomings and develop their tolerizing function upon encountering T cells or other stimuli after reaching the node.
A second mechanism is that there will be subsets of DCs, as first proposed by Suss and Shortman 53, that are somehow specialized to regulate immunity or to induce tolerance. Direct evidence for this DC subset remains elusive. However, the idea is that immature DCs capture apoptotic bodies peripherally and transfer tissue-derived peptides to tolerogenic DCs upon reaching the lymph node. We are intrigued by this possibility because of the information that migratory DCs in lymph are short lived and appear to be processed by longer-lived, resident DCs in lymph node 28. When migratory DCs are injected into mice, the cells leave the injection site 54, presumably via the afferent lymph, but <1% of the injected cells can be recovered 2 d later in a lymph node according to new data from Josien et al. 55. The dying, injected DCs are not totally destroyed by some “big Mac,” but instead can be processed and presented by DCs in the lymph node 28. The lymph node DCs can express high levels of MHC–peptide and interestingly, relatively low levels of surface CD86 costimulator 56. If these lymph node DCs efficiently form MHC–peptide complexes from incoming DCs and their contents, the former subset may be the best candidate to present self-antigens from apoptotic cells in a tolerogenic way. We postulate that there are resident, lymph node DCs which in the steady state induce tolerance to antigens in apoptotic bodies carried by migratory lymph DCs (Fig. 3).
Tolerogenic DCs may constitute a separate differentiation pathway, as suggested by Suss and Shortman 53. Perhaps these DCs derive from the distinct plasmacytoid precursor termed DC2 by Liu and colleagues 57 58. The tolerizing function of DCs may be quite sophisticated. For example, tolerance could ensue by deletion or anergy of the self-reactive T cell, as suggested by the work of Adler et al. 33 and Kurts et al. 34 35, discussed above. Alternatively, DCs might expand regulatory T cells. The latter may be needed for self-antigens at body surfaces like the intestine and airway, which are unlikely to be devoid of DC maturation stimuli.
Conclusion
DCs are specialized to control immunity, to trigger immune responses, and also, it appears, to maintain tolerance. These two spheres become intimately linked when one appreciates that cell death often accompanies infection and that DCs can present self-antigens from dying cells. The maturation of peripheral DCs, which is often triggered by infectious agents, should allow at least some phagocytosed self-antigens to become immunogenic. We develop the hypothesis that immature DCs in the steady state are inducing tolerance to self-antigens within phagocytosed apoptotic bodies, derived from the normal turnover of tissues. This occurs well before the entry of a foreign antigen, so when infection and DC maturation take place, the immune system can focus on the foreign peptides that the DCs have processed.
Sauter et al. 24 report that the uptake of apoptotic cells does not directly mature DCs. Also Huang et al. 23 find that intestinal lymph DCs normally carry phagocytosed, apoptotic, intestinal epithelial cells towards the lymph node, presumably without inducing intestinal autoimmunity. It is known that marrow-derived cells within lymph nodes can tolerize T cells to peptides synthesized in other tissues. Thus, DCs may traffic through tissues, pick up apoptotic cells arising from normal cell turnover, and then, upon migration to lymph nodes in afferent lymph, silence T cells to self-antigens in the phagocytosed apoptotic bodies. Tolerance to self-antigens in the steady state need not be direct. It may instead involve transport of apoptotic bodies in short-lived migratory DCs to longer-lived, tolerizing DCs in the lymph node. The latter are able in the steady state to form high levels of MHC–peptide complexes but either lack key costimulators for immunity or have unique products for inducing tolerance.
References
- Schuler G., Steinman R.M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J. Exp. Med. 1985;161:526–546 . doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Inaba K., Pure E., Crowley M., Witmer-Pack M., Steinman R.M. A small number of anti-CD3 molecules on dendritic cells stimulate DNA synthesis in mouse T lymphocytes. J. Exp. Med. 1989;169:1153–1168. doi: 10.1084/jem.169.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Koide S., Crowley M., Witmer-Pack M., Livingstone A.M., Fathman C.G., Inaba K., Steinman R.M. Presentation of exogenous protein antigens by dendritic cells to T cell clonesintact protein is presented best by immature, epidermal Langerhans cells. J. Exp. Med. 1989;169:1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C., Stahl P.D., Austyn J.M. Phagocytosis of antigens by Langerhans cells in vitro. J. Exp. Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumbles P.A., Thomas J.A., Pimm C.L., Lee P.T., Venaille T.J., Proksch S., Holt P.G. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med. 1998;188:2019–2031. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U., Steinman R.M., Peng M., Cameron P.U., Gezelter S., Kopeloff I., Swiggard W.J., Pope M., Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J. Exp. Med. 1993;178:1067–1078. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijman H.W., Kleijmeer M.J., Ossevoort M.A., Oorschot V.M.J., Vierboom M.P.M., van de Keur M., Kenemans P., Kast W.M., Geuze H.J., Melief C.J.M. Antigen capture and major histocompatibility complex class II compartments of freshly isolated and cultured human blood dendritic cells. J. Exp. Med. 1995;182:163–174. doi: 10.1084/jem.182.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt T., Pajak B., Muraille E., Lespagnard L., Heinen E., De Baetselier P., Urbain J., Leo O., Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Witmer-Pack M., Inaba M., Hathcock K.S., Sakuta H., Azuma M., Yagita H., Okumura K., Linsley P.S., Ikehara S. The tissue distribution of the B7-2 costimulator in miceabundant expression on dendritic cells in situ and during maturation in vitro. J. Exp. Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C., Vanbervliet B., Massacrier C., Azuma M., Okumura K., Lanier L.L., Banchereau J. B70/B7-2 is identical to CD86 and is the major functional ligand for CD28 expressed on human dendritic cells. J. Exp. Med. 1994;180:1841–1847. doi: 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Scheidegger D., Palmer-Lehmann K., Lane P., Lanzavecchia A., Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacityT–T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F., Stanzl U., Jennewien P., Janke K., Heufler C., Kampgen E., Romani N., Schuler G. High level IL-12 production by murine dendritic cellsupregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner B., Roder C., Dieckmann D., Heuer M., Kruse M., Glaser A., Keikavoussi P., Kampgen E., Bender A., Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukopheresis products for clinical application. J. Immunol. Methods. 1999;223:1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- Dieu M.-C., Vanbervliet B., Vicari A., Bridon J.-M., Oldham E., Ait-Yahia S., Briere F., Zlotnik A., Lebecque S., Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Schaerli P., Loetscher P., Schaniel C., Lenig D., Mackay C.R., Qin S., Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Yanagihara S., Komura E., Nagafune J., Watarai H., Yamaguchi Y. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J. Immunol. 1998;161:3096–3102. [PubMed] [Google Scholar]
- Gunn M.D., Kyuwa S., Tam C., Kakiuchi T., Matsuzawa A., Williams L.T., Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R., Schubel A., Breitfeld D., Kremmer E., Renner-Muller I., Wolf E., Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Caux C., Massacrier C., Vanbervliet B., Dubois B., Van Kooten C., Durand I., Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B.R., Josien R., Lee S.Y., Sauter B., Li H.-L., Steinman R.M., Choi Y. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell–specific survival factor. J. Exp. Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Engering A., Pinet V., Pieters J., Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- Pierre P., Turley S.J., Gatti E., Hull M., Meltzer J., Mirza A., Inaba K., Steinman R.M., Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- Huang F.-P., Platt N., Wykes M., Major J.R., Powell T.J., Jenkins C.D., MacPherson G.G. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 2000;191:435–443. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter B., Albert M.L., Francisco L., Larsson M., Somersan S., Bhardwaj N. Consequences of cell deathexposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 2000;191:423–433. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P., Guerder S. Does T-cell tolerance require a dedicated antigen-presenting cell? Nature. 1989;338:74–76. doi: 10.1038/338074a0. [DOI] [PubMed] [Google Scholar]
- Zal T., Volkmann A., Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II–restricted T cells specific for a blood-borne self-antigen. J. Exp. Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Inaba K., Turley S., Yamaide F., Iyoda T., Mahnke K., Inaba M., Pack M., Subklewe M., Sauter B., Sheff D. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Albert M.L., Pearce S.F.A., Francisco L.M., Sauter B., Roy P., Silverstein R.L., Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrlid U., Wick M.J. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J. Exp. Med. 2000;In press doi: 10.1084/jem.191.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N., Bender A., Gonzalez N., Bui L.K., Garrett M.C., Steinman R.M. Influenza virus–infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J. Clin. Invest. 1994;94:797–807. doi: 10.1172/JCI117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler A.J., Marsh D.W., Yochum G.S., Guzzo J.L., Nigam A., Nelson W.G., Pardoll D.M. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow–derived antigen-presenting cells. J. Exp. Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Heath W.R., Carbone F.R., Allison J., Miller J.F.A.P., Kosaka H. Constitutive class I–restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Kosaka H., Carbone F.R., Miller J.F.A.P., Heath W.R. Class I–restricted cross-presentation of exogenous self antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster I., Lieberam I. Peripheral tolerance of CD4 T cells following local activation in adolescent mice. Eur. J. Immunol. 1998;26:3194–3202. doi: 10.1002/eji.1830261253. [DOI] [PubMed] [Google Scholar]
- Morgan D.J., Kreuwel H.T., Sherman L.A. Antigen concentration and precursor frequency determine the rate of CD8+ T cell tolerance to peripherally expressed antigens. J. Immunol. 1999;163:723–727. [PubMed] [Google Scholar]
- Marzo A.L., Lake R.A., Lo D., Sherman L., McWilliam A., Nelson D., Robinson B.W.S., Scott B. Tumor antigens are constitutively presented in the draining lymph nodes. J. Immunol. 1999;162:5838–5845. [PubMed] [Google Scholar]
- Kelly R.H., Balfour B.M., Armstrong J.A., Griffiths S. Functional anatomy of lymph nodes. II. Peripheral lymph-borne mononuclear cells. Anat. Rec. 1978;190:5–21. doi: 10.1002/ar.1091900103. [DOI] [PubMed] [Google Scholar]
- Sokolowski J., Jakobsen E., Johannessen J.V. Cells in peripheral leg lymph of normal men. Lymphology. 1978;11:202–207. [PubMed] [Google Scholar]
- Pugh C.W., MacPherson G.G., Steer H.W. Characterization of nonlymphoid cells derived from rat peripheral lymph. J. Exp. Med. 1983;157:1758–1779. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossum S., Rolstad B. The roles of interdigitating cells and natural killer cells in the rapid rejection of allogeneic lymphocytes. Eur. J. Immunol. 1986;16:440–450. doi: 10.1002/eji.1830160422. [DOI] [PubMed] [Google Scholar]
- Surh C.D., Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- Randolph G.J., Beaulieu S., Steinman R.M., Muller W.A. Differentiation of monocytes into dendritic cells in a model that mimics entry of cells into afferent lymph. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- Randolph G.J., Inaba K., Robbiani D.F., Steinman R.M., Muller W.A. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- Regnault A., Lankar D., Lacabanne V., Rodriguez A., Thery C., Rescigno M., Saito T., Verbeek S., Bonnerot C., Ricciardi-Castagnoli P., Amigorena S. Fc gamma receptor–mediated induction of dendritic cell maturation and major histocompatibility complex class I–restricted antigen presentation after immune complex internalization. J. Exp. Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M., Stockinger B., Wick M.J. Bone marrow-derived dendritic cells can process bacteria for MHC-I and MHC-II presentation to T cells. J. Immunol. 1997;158:4229–4236. [PubMed] [Google Scholar]
- Rodriquez A., Regnault A., Kleijmeer M., Ricciardi-Castagnoli P., Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat. Cell Biolog. 1999;1:362–368. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- Gallucci S., Lolkema M., Matzinger P. Natural adjuvantsendogenous activators of dendritic cells. Nat. Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Naito M., Steinman R.M. Dendritic cell progenitors phagocytose particulates, including Bacillus Calmette-Guérin organisms, and sensitize mice to mycobacterial antigens in vivo. J. Exp. Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Fuhlbrigge R.C., Kieffer J.D., Ayehunie S., Hynes R.O., Cheng G., Grabbe S., von Andrian U.H., Kupper T.S. Interaction of dendritic cells with skin endotheliuma new perspective on immunosurveillance. J. Exp. Med. 1999;189:627–636. doi: 10.1084/jem.189.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josien R., Wong B.R., Li H.-L., Steinman R.M., Choi Y. TRANCE, a TNF-family member is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J. Immunol. 1999;162:2562–2568. [PubMed] [Google Scholar]
- Suss G., Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand–induced apoptosis. J. Exp. Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austyn J.M., Kupiec-Weglinski J.W., Hankins D.F., Morris P.J. Migration patterns of dendritic cells in the mouse. Homing to T cell–dependent areas of spleen, and binding within marginal zone. J. Exp. Med. 1988;167:646–651. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josien R., Li H.-L., Ingulli E., Sarma S., Wong B.R., Vologodskaia M., Steinman R.M., Choi Y. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J. Exp. Med. 1999;191:495–501. doi: 10.1084/jem.191.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Pack M., Inaba M., Sakuta H., Isdell F., Steinman R.M. High levels of a major histocompatibility complex II–self peptide complex on dendritic cells from the T cell areas of lymph nodes. J. Exp. Med. 1997;186:665–672. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouard G., Rissoan M.-C., Filgueira L., Durand I., Banchereau J., Liu Y.-J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissoan M.C., Soumelis V., Kadowaki N., Grouard G., Briere F., de Waal Malefyt R., Liu Y.J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- Holt P.G., Schon-Hegrad M.A., Oliver J. MHC class II antigen–bearing dendritic cells in pulmonary tissues of the rat. Regulation of antigen presentation activity by endogenous macrophage populations. J. Exp. Med. 1988;167:262–274. doi: 10.1084/jem.167.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]