Abstract
Immunization with T cell–dependent antigens generates long-lived memory B cells and antibody-forming cells (AFCs). Both populations originate in germinal centers and, predominantly, produce antibodies with high affinity for antigen. The means by which germinal center B cells are recruited into these populations remains unclear. We have examined affinity maturation of antigen-specific B cells in mice expressing the cell death inhibitor bcl-2 as a transgene. Such mice had reduced apoptosis in germinal centers and an excessive number of memory B cells with a low frequency of V gene somatic mutation, including those mutations encoding amino acid exchanges known to enhance affinity. Despite the frequency of AFCs being increased in bcl-2–transgenic mice, the fraction secreting high-affinity antibody in the bone marrow at day 42 remained unchanged compared with controls. The inability of BCL-2 to alter selection of bone marrow AFCs is consistent with these cells being selected within the germinal center on the basis of their affinity being above some threshold rather than their survival being due to a selective competition for an antigen-based signal. Continuous competition for antigen does, however, explain formation of the memory compartment.
Keywords: affinity maturation, B cell, immunologic memory, plasma cell, somatic mutation
Introduction
During primary T cell–dependent immune responses, somatic mutation of Ig V region genes in germinal center B cells generates variants expressing Ig with altered affinity for antigen 1 2 3. Variants with improved affinity are positively selected so as to eventually comprise the majority of the antigen-specific memory and antibody-forming cell (AFC) B cell populations 4 5 6 7. The increasing frequency of high-affinity B cells within these two populations is referred to as affinity maturation, a phenomenon originally observed in the improvement in the average affinity of serum antibodies 8. Although both memory B cells 2 3 and high-affinity AFCs located in the bone marrow 5 6 9 originate in the germinal center, the mechanism underlying the recruitment and maintenance of high-affinity germinal center variants into these populations remains obscure. Although it is generally regarded that memory B cells require antigen for their continued survival 10, the means by which bone marrow AFCs are selected and maintained is less clear. A number of reports indicate that once in the bone marrow, these AFCs are unresponsive to antigen 9 11 12, arguing against antigen-dependent selection in the bone marrow. This is consistent with the downregulation of surface Ig on AFCs as they mature 5. Takahashi and colleagues have proposed that, after the cells leave the germinal center, selection for high-affinity bone marrow AFCs continues in an antigen-dependent manner 6 13. If selection of memory B cells and bone marrow AFCs utilizes similar criteria, it would follow that circumstances that alter selection of one cell type would affect the other in the same manner. Differences in outcome, however, would indicate differences in selection criteria.
In this study, we have assessed the relative contribution of cell survival mechanisms regulated by BCL-2 in the affinity maturation of antigen-specific memory B cell and AFC compartments. This was done by analysis of the immune response in bcl-2–transgenic mice in which, to a large degree, B cell survival is independent of B cell antigen receptor (BCR)-mediated stimuli 14 15 16. A previous analysis of the immune response of these transgenic mice revealed an amplification of both the antigen-specific germinal center/memory and splenic AFC compartments 5, although affinity maturation among the memory and bone marrow AFC compartments was not measured. Although other groups have examined aspects of immunity in bcl-2–transgenic mice 17 18, the effects of blocking cell death in this way on affinity maturation in the memory and AFC compartments has yet to be assessed.
Our results lead us to conclude that constitutive expression of the bcl-2 cell survival gene distorts selection of the memory B cell compartment while leaving the proportion of high-affinity cells in the bone marrow AFC compartment essentially unchanged. B cells with no affinity-enhancing VH gene somatic mutations persist in abnormally large numbers in the germinal center/memory B cell pathway of the transgenic mice. Similarly, low-affinity AFCs persist in the spleens of these mice. The preferential appearance of high-affinity AFCs in the bone marrow, however, is not altered despite an overall increase in the number of AFCs in both the spleen and bone marrow. The ability of a bcl-2 transgene to alter the composition of the memory population but not that of the bone marrow AFCs implies that fundamental differences exist in the manner in which germinal center B cells are selected and/or maintained in these compartments. The basis of this difference is discussed.
Materials and Methods
Mice and Immunization.
Hemizygous transgenic mice of the Eμ-bcl-2-36 strain 19, backcrossed with inbred C57BL/6 mice for more than 15 generations, were provided by Drs. A.W. Harris and S. Cory (The Walter and Eliza Hall Institute). Transgene-bearing mice were identified as described 19. Nontransgenic littermates were used as controls throughout. In most experiments, mice were immunized by intraperitoneal injection of 100 μg of alum-precipitated NP ([4-hydroxy-3-nitrophenyl]acetyl) conjugated to keyhole limpet hemocyanin (KLH) (NP/KLH conjugation ratio 17:1), prepared as described previously 20. For the bromodeoxyuridine (BrdU) incorporation experiments, mice were immunized subcutaneously at the base of the tail with 100 μg of alum-precipitated NP17–KLH.
Immunofluorescent Staining, Flow Cytometric Analysis, and Cell Sorting.
NP-specific memory B cells, defined as IgM−IgD− IgG1+CD38+ and NP binding, were resolved from other splenocytes as previously described 21. The so-called “dump” channel comprised biotin conjugates of the rat mAbs 281.2 (antisyndecan), 331.12 (anti-IgM), and 11-26C (anti-IgD), all revealed with streptavidin–PE (Caltag Labs.), plus propidium iodide to exclude AFCs, naive B cells, and dead cells, respectively, from the analysis. Antigen-specific B cells were identified by simultaneously binding NP coupled to allophycocyanin and Texas Red–conjugated goat anti–mouse IgG1 (Southern Biotechnology Associates). CD38 expression levels, used to resolve NP-specific germinal center (CD38−) and memory (CD38+) B cells 21, were determined using FITC-conjugated NIMR5/18 (a gift from Dr. M. Howard, DNAX Research Institute, Palo Alto, CA). Cells were sorted with a dual laser FACStarPLUS™ (Becton Dickinson) and an associated automated cell deposition unit (ACDU).
Cell Culture.
Cells were sorted into flat-bottomed 96-well plates using the ACDU and cultured in 200 μl of RPMI culture medium supplemented with 50 μM 2-ME, 10% FCS, IL-4, IL-5, and an optimal concentration of CD40 ligand (CD40L) expressed from a baculovirus construct (a gift from Dr. P. Hodgkin, Centenary Institute, Sydney, Australia). After 7 d, culture supernatants were assayed for high-affinity and total anti-NP IgG1 as described below. Purified NP-specific monoclonal IgG1 was used as a standard.
ELISA and ELISPOT Assays.
Total and high-affinity NP-specific IgG1 was detected by ELISA using 96-well plates (Costar Corp.) coated with NP13– and NP2–BSA, respectively, as described 20. ELISPOT assays to enumerate AFCs were performed by titrating bone marrow or spleen cells into replicate wells of 96-well cellulose ester–based plates (Millipore Corp.) coated with NP13– or NP2–BSA, followed by 16-h culture in RPMI containing 50 μM 2-ME and 10% FCS. Plates were washed, and bound NP-specific IgG1 was revealed with goat anti–mouse IgG1 conjugated to horseradish peroxidase (Southern Biotechnology Associates), visualized by the addition of 3-aminoethyl carbazole. Spots, each representing a single AFC, were counted using a dissecting microscope.
VH Gene Sequence Analysis of NP-specific B Cells.
Sequences of VH186.2 genes were obtained from single NP-specific memory B cells as described 5. Two rounds of PCR were performed on cDNA derived from single B cells using nested primers specific for Cγ1 22, together with a single proximal 5′ primer for the J558 VH gene family 23. Products with bands of the expected size were purified and sequenced, with VH186.2-containing sequences positively identified at this stage. The efficiency of each step in this procedure is shown in Table . The improved efficiency of cDNA synthesis from bcl-2–transgenic single B cells (80 vs. 40%) is presumably due to the enhanced viability of these cells during the ex vivo manipulations. The entire PCR product was sequenced, and the region encoding amino acids 10–96 was compared in detail with the germline VH186.2 sequence 24. No clonal repeats were found, as assessed by comparison of complementarity determining region (CDR)3 sequences.
Table 1.
Summary of VH186.2 Sequences from NP-specific IgG1+ B Cells at Day 42 after Immunization
| Control CD38+ | bcl-2 CD38+ | |
|---|---|---|
| Single cells | 74 | 38 |
| PCR+ | 31 | 32 |
| VH186.2+ | 26 | 24 |
| R/S ratio | ||
| CDR1+2 | 4.0 | 2.9 |
| FW1−3 | 2.7 | 1.8 |
| Position 33 W→L | 62% | 13% |
| Mutation average | 4.6 | 2.7 |
| Range | 0–14 | 0–12 |
R/S ratio, ratio of replacement to silent mutations.
BrdU Incorporation and Detection.
To identify proliferating cells within LN germinal centers, mice immunized 14 d previously were injected with a single dose of BrdU (100 μg per gram body weight; Sigma Chemical Co.) dissolved in 0.007 N NaOH in normal saline. After 6 h, the mice were killed, and LNs were fixed in Bouin's fluid and processed for paraffin embedding. Immunostaining was performed as described 25. In brief, sections of LN were treated with xylene to remove paraffin and rehydrated in solutions with graded ethanol concentrations. Excessive aldehydes in the fixed sections were quenched by incubation in 0.2 M glycine for 30 min. Sections were then treated for 30 min with 0.3% hydrogen peroxide to block endogenous peroxidase, followed by a 30-min incubation in 10% normal mouse serum to block nonspecific binding sites. All antibody incubation steps were for 30 min, followed by washings with PBS/1% BSA. To make BrdU accessible to antibody, sections were pretreated with 1.4 N HCl for 2 h, followed by staining with rat anti-BrdU antibody (Sera-Lab Ltd.). Bound antibody was detected by the secondary reagent, biotinylated MAR 18.5 (mouse anti–rat Igκ). The sections were then incubated for 30 min in avidin–peroxidase complex (ABC Elite kit; Vector Labs., Inc.), followed by detection with diaminobenzidene in 0.07% hydrogen peroxide as the substrate. Sections were counterstained with hematoxylin, dehydrated in alcohol and xylene, and mounted in DPX. All germinal centers in two nonsequential sections from each of two LNs from each control and bcl-2–transgenic animal were scored and the percentage of BrdU-containing cells within each germinal center calculated. The average BrdU+ percentage from all individual germinal centers was then calculated for each mouse.
TUNEL Staining.
LN sections, initially treated as above, were incubated with proteinase K (20 μg/ml) for 15 min and then washed three times in PBS. Endogenous peroxidases were blocked by incubating the sections with 0.3% hydrogen peroxide in methanol for 5 min, followed by washing. Sections were then incubated for 60 min in a humidified incubator with terminal deoxynucleotidyltransferase (TdT; Promega Corp.) in the presence of biotinylated dUTP (Pharmacia). Incorporated biotinylated dUTP was revealed with avidin–peroxidase complex (ABC Elite kit; Vector Labs., Inc.), followed by detection with diaminobenzidene in 0.07% hydrogen peroxide as the substrate. Sections were counterstained with hematoxylin, dehydrated in alcohol and xylene, and mounted in DPX. TUNEL+ clusters within germinal centers were scored from two nonsequential sections from each of two LNs per mouse. Thus, a total of 12 sections were analyzed for each genotype. Sections were photographed using a Zeiss Axiaphot (Carl Zeiss, Inc.) with Kodak 64T film at the degrees of magnification indicated.
Results
bcl-2 Promotes the Accumulation of Memory B Cells with Few Ig V Gene Mutations.
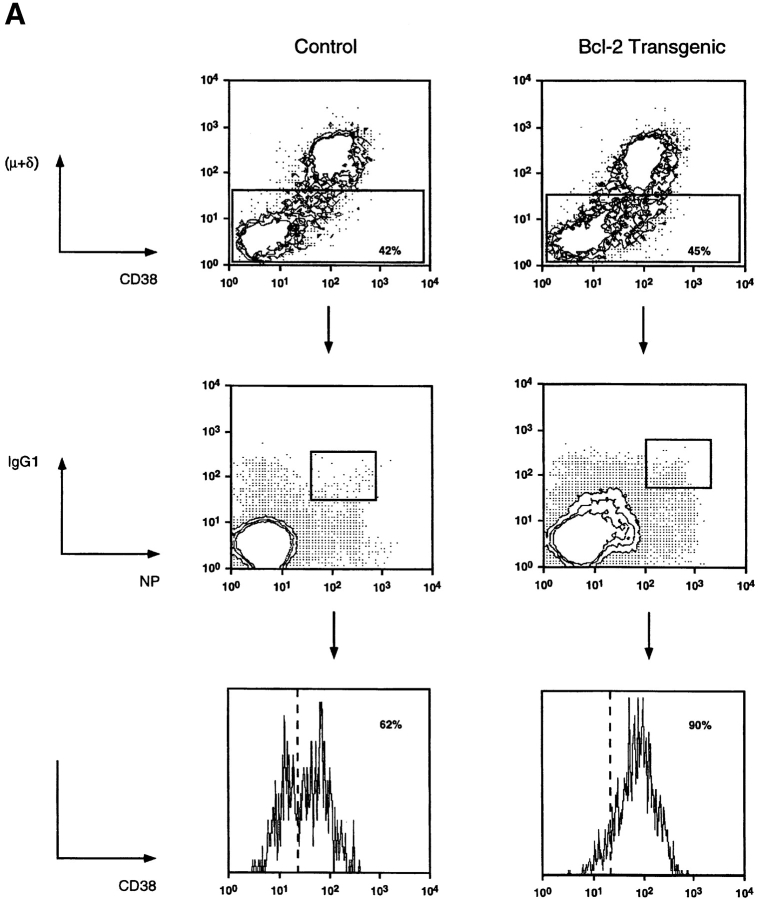
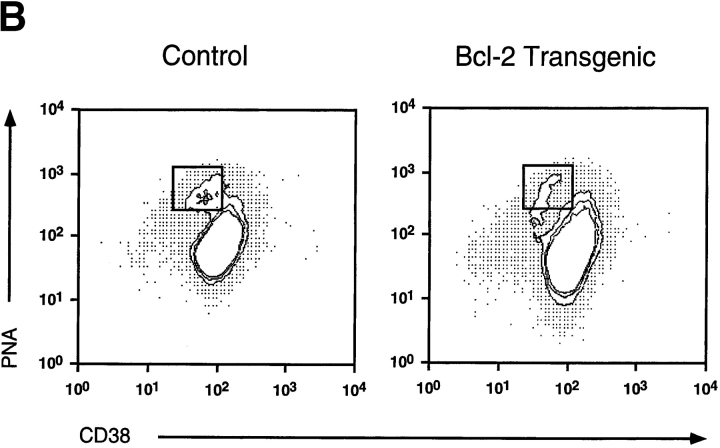
To investigate the role of apoptotic pathways blocked by BCL-2 in B cell selection in the germinal center, we examined this process in mice expressing a bcl-2 transgene. We have shown previously that a bcl-2 transgene expressed in the B cell lineage increases the cellularity of the germinal center/memory compartment by 10- to 20-fold 20. We sought to determine the nature of the B cell expansion with respect to cell subset composition and affinity-enhancing VH gene mutations to gain insight into selection in the germinal center. To evaluate which population of B cells was expanded in the transgenic mice, we determined CD38 expression on antigen-specific IgG1+ B cells late in the primary immune response. Germinal center B cells can be resolved from naive and memory B cells by reduced levels of CD38 21. Mice were immunized by intraperitoneal injection of 100 μg of alum-precipitated NP–KLH, and 42 d later they were examined for the frequency of isotype-switched NP-binding B cells. Approximately 50% of NP-binding IgG1+ B cells in the spleens of control mice were CD38+, whereas the remainder retained the CD38lo germinal center B cell phenotype. More than 80% of NP+IgG1+ B cells in the bcl-2–transgenic mice had the CD38+ memory phenotype (Fig. 1 A). That the expanded CD38+ antigen-specific B cell population in the spleens of bcl-2–transgenic mice actually constituted the memory population and was not due to altered CD38 regulation in the transgenic B cells was confirmed by the following observations. First, these B cells bound low levels of peanut agglutinin and did not have the light scatter characteristics of blast cells (data not shown). Second, germinal center B cells within the mesenteric LNs of bcl-2–transgenic mice reduced CD38 expression to the same extent as control mice (Fig. 1 B), indicating that CD38 expression is regulated appropriately in these mice.
Figure 1.
Levels of CD38 on antigen-specific IgG1 B cells at day 42 of the primary response. (A) Spleens from mice immunized 42 d previously with 100 μg i.p. of NP–KLH were stained with the indicated antibodies and analyzed by flow cytometry. Viable cells having the phenotype IgM−IgD− were electronically gated on (rectangle) and examined for expression of IgG1 and the ability to bind the immunizing hapten NP coupled to a fluorescent protein. Such double-positive cells were gated on (rectangle), and the level of CD38 was determined. This result is depicted as the solid histogram in this figure. Negative control staining was less than the fluorescence level marked by the dashed vertical line. The percentages of NP-binding IgG1+ cells expressing high levels of CD38 are indicated. Cells used in this experiment were pooled from three mice, and the data shown are representative of three experiments. (B) Germinal center cells in bcl-2–transgenic mice downregulate CD38. Mesenteric LN B cells were gated on by expression of CD45R. These cells were then examined for CD38 levels and binding of the lectin peanut agglutinin; such B cells are boxed.
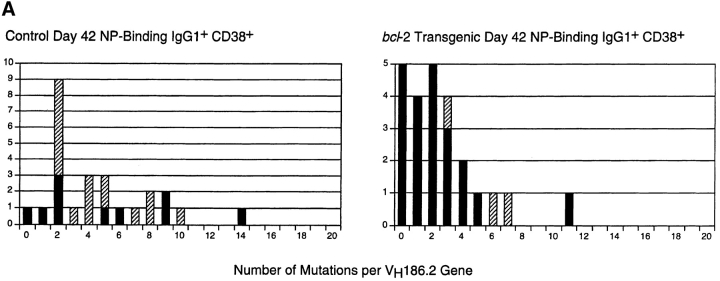
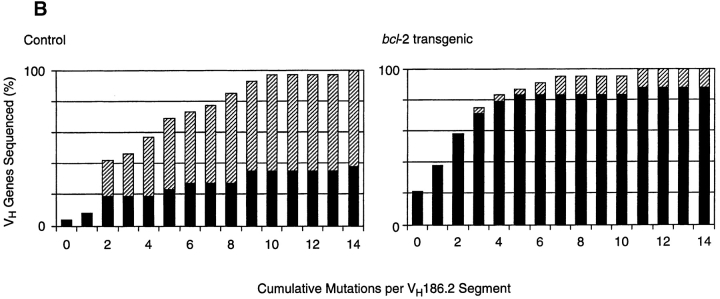
We next compared the distribution of VH gene somatic mutations in purified NP-binding B cells from transgenic and control mice. To ensure that the same B cell populations were compared, we focused on the antigen-specific CD38+ subset. Single IgG1+ NP-specific CD38+ B cells were sorted from the spleens of bcl-2–transgenic and control mice immunized 42 d previously. IgG1 rearrangements involving the VH186.2 gene segment were amplified by PCR from cDNA templates and sequenced. Analysis of these sequences from bcl-2 mice revealed a number of differences in the pattern of somatic mutation seen in the equivalent control B cell population (Table and Fig. 2 A). First, 25% of the VH186.2 sequences from bcl-2–transgenic cells contained zero mutations, compared with 4% in controls. Second, 36% of VH186.2 gene sequences from bcl-2–transgenic mice contained either one or two mutations and lacked the affinity-enhancing tryptophan→leucine exchange at amino acid 33. The equivalent group comprised 16% of control sequences. Thus, some 60% of memory phenotype B cells in the bcl-2–transgenic mice showed no evidence of affinity maturation of their VH gene sequences, compared with only 20% in controls. The affinity-enhancing exchange at VH position 33 was present in 13% of bcl-2–transgenic memory B cells, compared with 64% in control mice (Table and Fig. 2), which indicated that BCL-2 did not prevent the appearance of high-affinity cells. The cumulative distribution of somatic mutations in the VH gene sequences is depicted in Fig. 2 B. It should also be noted that the proportion of recovered PCR products using the VH186.2 gene segment was the same in antigen-specific B cells from both types of mice. That is, there was no bias toward related VH genes in the bcl-2–transgenic memory B cells. Finally, when VH genes were sequenced from populations of IgG1+ antigen-specific B cells irrespective of their CD38 levels (i.e., all B220+IgG1+ NP-binding cells) from both bcl-2 and control mice, the observed pattern of mutations was very similar to that observed when CD38 was used as a marker (not shown).
Figure 2.
Reduced frequency of VH gene somatic mutations in NP-specific memory B cells from bcl-2–transgenic mice. The frequency of mutations in VH186.2 genes from single, antigen-specific CD38+IgG1+ B cells was determined by nucleotide sequencing. Single cells were sorted from a pooled spleen preparation from each mouse strain using the criteria depicted in Fig. 1. All recovered sequences showed unique CDR3 junctions, indicating clonality. (B) Cumulative distribution of somatic mutations in control and bcl-2 NP-specific B cells. In both components of the figure, the number of sequences containing mutations giving rise to a tryptophan→leucine exchange at amino acid 33 (numbered according to reference 24) is depicted by the hatched segment of each column. Details of the sequences are summarized in Table . These sequence data are available from EMBL/GenBank/DDBJ under accession no. AF210258-AF210307.
From these results, it should follow that NP-specific Ig produced by the memory B cell population of bcl-2–transgenic mice should correlate with a lower degree of affinity maturation than Ig produced by the equivalent population from control mice. To determine whether this was the case, IgM−IgD−CD38+IgG1+ NP-binding B cells were sorted from spleens of bcl-2–transgenic and control mice at day 42 after immunization and stimulated in vitro with CD40L plus cytokines. The fraction of total NP-specific IgG1 able to bind to NP2-conjugated plate coats from bcl-2–transgenic mice was 30%, compared with 100% in control cultures. This confirms a lower representation of high-affinity B cells in the bcl-2 memory B cell population. Collectively, these data demonstrate the persistence of excessive numbers of low-affinity B cells in the memory populations of bcl-2–transgenic mice.
Transgenic expression of bcl-2 therefore results in the persistence of cells with a memory phenotype and a pattern of VH gene mutations suggesting low affinity, an observation confirmed by measurement of the affinity of antibody produced by these same cells after sorting and culture in vitro. These results indicate that constitutive expression of BCL-2 allows for and may even enhance the differentiation of germinal center cells into memory cells, despite their being of low affinity.
bcl-2 Inhibits Apoptosis and Proliferation in Germinal Centers to Varying Degrees.
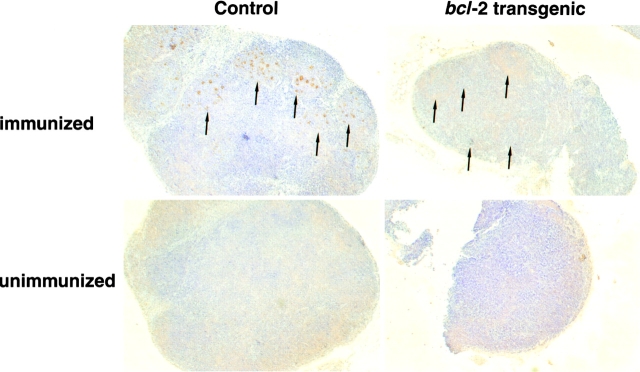
BCL-2, defined as an inhibitor of apoptosis 26, is also able to delay entry of mitogen-stimulated lymphocytes into the cell cycle 27 28 29. To better define the basis of the effect of constitutive bcl-2 expression on selection of antigen-specific B cells, we sought to quantify the extent of apoptosis and proliferation in the germinal centers of bcl-2–transgenic mice. For these experiments, mice were immunized at the base of the tail, and 14 d later the paraaortic LNs were taken, fixed in paraformaldehyde, and sectioned. Paraaortic LNs were chosen because without deliberate immunization, this tissue contained no germinal centers and we could therefore be certain that all of the germinal centers that developed had developed in a synchronized manner. Sections from immunized and unimmunized transgenic and control animals were stained using the TUNEL (TdT-mediated dUTP-biotin nick-end labeling) technique to reveal the frequency of apoptotic cells (Fig. 3). The results show that BCL-2 reduced apoptosis by a factor of ∼10-fold (Table ). This implies that the major form of cell death in germinal centers is due to apoptotic pathways that can be blocked by BCL-2 and that BCL-2–insensitive pathways of apoptosis, such as those activated by death receptors, play only a minor role 30 31.
Figure 3.
The frequency of apoptotic cells in germinal centers is reduced in bcl-2–transgenic mice. 2 wk after immunization, LNs were sectioned and stained for the presence of apoptotic cells using the TUNEL technique. An example of one such experiment using LNs from immunized and unimmunized control and bcl-2–transgenic mice is shown. Arrows indicate the location of germinal centers. No germinal centers were observed in the LNs of unimmunized mice. Magnification is 100, and one representative section is shown. Quantification of this data is presented in Table .
Table 2.
Apoptosis in the Germinal Centers of bcl-2–transgenic and Control Mice
| Mouse | TUNEL+ clusters per germinal center in immunized mice | |
|---|---|---|
| bcl-2 | Control | |
| 1 | 0.8 ± 1.7 | 8.2 ± 6.2 |
| 2 | 0.8 ± 1.0 | 8.1 ± 3.8 |
| 3 | 2.7 ± 2.1 | 9.7 ± 3.7 |
| Mean | 1.4 | 8.7 |
The frequency of proliferating cells in LN germinal centers of bcl-2–transgenic and control mice was determined by scoring the number of cells per germinal center that had incorporated BrdU during a 6-h pulse. Histological sections were first scored for the frequency and size of germinal centers, and no significant difference was observed between bcl-2–transgenic mice and controls (Table ). The similarity in germinal center size and frequency in the two strains implies that the additional antigen-specific B cells that accumulate during the immune response in the bcl-2–transgenic mice reside in a postgerminal center compartment. This is consistent with the increased frequency of B cells with a memory phenotype defined above (Fig. 1). The fraction of cells synthesizing DNA during the 6-h pulse differed somewhat between the two groups, with transgenic mice showing a decrease of ∼25% (Table ). As the size distribution of germinal centers in the two strains was the same (Table ), the decrease in the proportion of BrdU-labeled germinal center cells in the bcl-2–transgenic mice is equivalent to a reduced number of proliferating cells. Thus, constitutive bcl-2 expression does not affect germinal center formation but does reduce both apoptosis and proliferation within the germinal center, although the effect on the former is much greater.
Table 3.
Proliferation in the Germinal Centers of bcl-2–transgenic and Control Mice
| Mouse | Percent BrdU+ cells per GC | GC size (cells per germinal center) | Total | |||
|---|---|---|---|---|---|---|
| Small (1–99) | Medium (100–299) | Large (>300) | ||||
| Control | 1 | 41.6 ± 10.6 | 0 | 16 | 2 | 18 |
| 2 | 43.0 ± 13.6 | 1 | 21 | 6 | 28 | |
| 3 | 35.9 ± 14.8 | 4 | 12 | 1 | 15 | |
| Mean | 40.8 | 1.7 | 16.0 | 3.0 | — | |
| bcl-2 | 1 | 27.3 ± 11.3 | 3 | 17 | 3 | 23 |
| 2 | 19.2 ± 9.3 | 0 | 11 | 5 | 16 | |
| 3 | 31.1 ± 13.7 | 3 | 18 | 11 | 32 | |
| Mean | 27.2 | 2.0 | 15.3 | 6.3 | — | |
GC, germinal center.
High-Affinity AFCs Are Selectively Recruited into the Bone Marrow of bcl-2–transgenic Mice.
Having observed an enhanced preservation of low-affinity B cells within the memory compartment of bcl-2–transgenic mice, we next examined whether the AFC compartments in the spleen and bone marrow were similarly affected. We measured the frequency of high-affinity NP-specific IgG1 AFCs in spleens and bone marrow of bcl-2–transgenic and control animals (Table ). At day 42 after immunization, the average frequency of NP-specific IgG1 AFCs in the spleens of control mice was 1.5 per 105 splenocytes, of which 90% were high affinity, as determined by binding to the low conjugation plate coat, NP2–BSA. The frequency of antigen-specific IgG1 AFCs in the bone marrow of control animals at day 42 was 5 per 105 cells, of which 80% were high affinity, a proportion not significantly different from that in the spleens of the same animals (Table ). Thus, in control mice at this time after primary immunization, the vast majority of antigen-specific AFCs secreted high-affinity antibody, irrespective of their location. The situation in bcl-2–transgenic animals differed in two respects: first, the frequency of NP-specific IgG1 AFC was higher, and second, the degree of affinity maturation differed between the tissues examined (Table ). The frequency of NP-specific IgG1 AFCs in the spleens of bcl-2–transgenic mice was ∼50-fold higher than in controls—corresponding to a 150-fold increase in absolute numbers—but only 30% of these AFC secreted high-affinity antibody (Table ). This result confirms the preservation of splenic AFCs in bcl-2–transgenic mice 19 20 and demonstrates that most of these cells secrete low-affinity Ig. Although the frequency of NP-specific IgG1 AFCs in the bone marrow of bcl-2–transgenic mice was approximately threefold higher than in controls, the proportion of AFCs secreting high-affinity antibody was close to normal at 70%. Importantly, the degree of affinity maturation amongst the bone marrow AFCs of bcl-2–transgenic mice differed significantly from that in the spleen of these mice (Table ). That high-affinity cells accumulate normally in the bone marrow AFC compartment of bcl-2–transgenic mice but fail to do so in either the memory or splenic AFC compartments demonstrates a significant difference in the selective processes operating to establish and/or maintain these populations of antigen-specific B cells.
Table 4.
Frequency and Affinity of AFCs at Day 42 of the Primary Response
| Anti-NP IgG1 AFCs per 105 Input Cells at Day 42 | |||||||
|---|---|---|---|---|---|---|---|
| Spleen | Bone marrow | ||||||
| NP2 | NP13 | Ratio | NP2 | NP13 | Ratio | P value | |
| Control | 1.3 ± 1.1 | 1.4 ± 0.9 | 0.9 ± 0.04 | 7.9 ± 6.6 | 10.2 ± 9.3 | 0.8 ± 0.03 | 0.46 |
| bcl-2 | 18.0 ± 9.0 | 64.8 ± 33.0 | 0.3 ± 0.01 | 21.3 ± 12 | 31.0 ± 17.0 | 0.7 ± 0.01 | <0.001 |
| P value | <0.001 | 0.43 | |||||
Discussion
It is widely accepted that both the memory B cell and high-affinity AFC populations are recruited in the germinal center on the basis of their affinity for antigen 2 3 5 6. Although little is known regarding the molecular signals underpinning germinal center activity, a model to explain affinity maturation in the germinal center 2 3 32 33 may be summarized as follows. Somatic hypermutation of Ig V genes randomizes the affinity for antigen of B cells within germinal centers. Some variants will have their affinity increased, some left unchanged, and others decreased. As germinal center B cells require an antigen-dependent signal for their continued survival and participation in the processes of affinity maturation, those with improved affinity will have a competitive advantage in accessing antigen, localized in the germinal center as immune complexes on follicular dendritic cells. Such high-affinity B cells will therefore preferentially survive and thus will come to dominate the germinal center and, eventually, the memory population. Germinal center B cells that fail to receive antigen-dependent survival signals will undergo apoptosis, reinforcing the ascendancy of the high-affinity variants. In such a model, preventing the death of low-affinity variants should distort the process of affinity maturation. This model of germinal center activity in the generation of B cell memory is well supported by data from our current analysis of bcl-2–transgenic mice. By blocking apoptosis in the germinal center, a population of low-affinity B cells has been preserved inappropriately, and these cells have assumed the phenotype of memory B cells (Fig. 1). This result implies that cell survival is a key determinant for entry into the memory B cell compartment. Thus, a constitutive cell survival signal has uncoupled affinity maturation in the germinal center from entry into the memory B cell compartment. This provides the first direct evidence that formation of a normally selected memory B cell compartment requires apoptosis in the germinal center.
A model of survival-dependent differentiation, however, does not explain the formation of the high-affinity bone marrow AFC compartment. Transgenic bcl-2 clearly promoted the survival of AFCs, as demonstrated by the 50-fold increase in antigen-specific IgG1 AFCs in the spleen and threefold increase in bone marrow AFCs (Table ). Recruitment and persistence of AFCs in the bone marrow, however, was not random, as the fraction of such cells secreting high-affinity antibody remained the same as in controls (Table ). That is, germinal center–derived cells were recruited into the bone marrow AFC compartment on the basis of affinity and not solely on their potential to survive. Thus, the ability of BCL-2 to distort affinity maturation of memory B cells but not bone marrow AFCs reveals a fundamental difference in the means by which these populations are formed (or possibly maintained) from germinal center precursors.
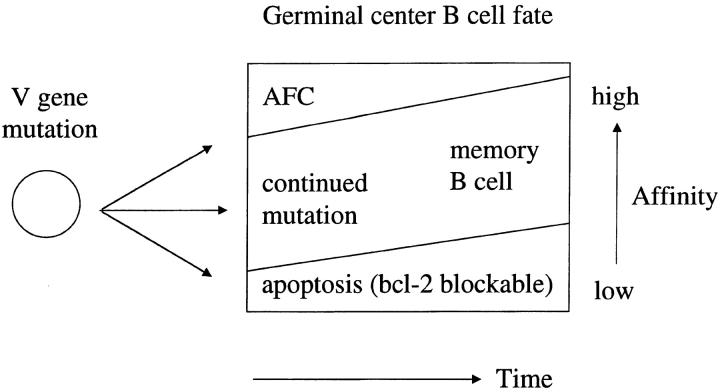
One explanation of how transgenic bcl-2 alters the composition of the memory B cell population but not that of bone marrow AFCs is that the differentiation of germinal center B cells is determined by the strength of the BCR interaction with antigen. B cells that bind antigen avidly will have a greater degree of receptor occupancy and cross-link more of their surface Ig than B cells with low-affinity Ig. This strength of signal could then be translated into commitment to differentiate into an AFC at the high end of the spectrum, survival in the germinal center at a central level, or apoptosis at the low end of the spectrum. Thus, differentiation into an AFC requires a particular threshold of signal strength to be reached, a signal that cannot be provided by BCL-2. Current data do not allow determination of where or over what time frame this BCR signal would be delivered, although recent data suggest a period of postgerminal center selection in bone marrow AFC formation 6. Indeed, an extended period of selection may distinguish the developmental pathway of bone marrow AFCs from that of the splenic foci, which are very sensitive to bcl-2 (Table ). The proposed model of the germinal center (Fig. 4) accounts for several aspects of germinal center activity: (a) the early appearance of sparsely mutated, high-affinity AFCs in the bone marrow 5 and their corresponding absence from the germinal center 34; (b) the particular ability of BCL-2 to rescue low-affinity B cells in the germinal center; and (c) the continued improvement of AFC affinity during the first weeks of the response 5 6. This last observation would reflect the continued heightening of the affinity threshold necessary to enter the AFC population caused by germinal center–derived B cells having to compete with increasing titers of high-affinity serum Ig. Such competition would also require B cells within the germinal center to improve their affinity for antigen to receive a signal of sufficient strength to ensure their survival. The affinity of the memory B cell population would therefore be improved but would lag behind that of the cells destined to become bone marrow AFCs.
Figure 4.
Selection in the germinal center during the development of memory and AFCs in a primary immune response. A schematic representation of the forces acting to influence the outcome of the germinal center reaction during the primary immune response. Indicted are the fact that (a) high-affinity cells selectively become AFCs; (b) low-affinity cells apoptose, an event that can be blocked by BCL-2; and (c) intermediate affinity cells remain in the germinal center. As the response progresses, the affinity threshold for each differentiation pathway will increase as the cells compete with antibody in serum. It is predicted that this reaches a level where memory B cell production will be the favored outcome. This change may well involve FcγRII.
The concept of lymphocyte differentiation being influenced by the degree of receptor occupancy has been proposed to explain early stages of the B cell response to both self- and foreign antigen 35 36. Regulating differentiation by the same mechanism in the germinal center would obviate the need for unique signaling pathways for different developmental stages. A prediction of this model is that mutations that alter the threshold of BCR-mediated activation should have an inverse effect on affinity maturation. Thus, mice with hyperresponsive B cells should show diminished affinity maturation, whereas those with hyporesponsive B cells should show more stringent selection. This principle may, for example, underlie the poor survival in germinal centers of B cells rendered hyporesponsive by ablation of CD21 37 and CD19 38. A definitive answer requires a more detailed analysis of affinity maturation in these mice.
A fundamental question remaining to be answered is, What changes occur to allow the germinal center output to shift from AFC production to that of memory B cell production? The memory B cell population is formed over a longer period than the bone marrow AFC population 21 39 40, although analysis of V gene somatic mutation indicates that the bulk of the memory population is generated late in the response 4 5 41. As this corresponds to the period of the response when titers of antibody are highest, it may be that B cell Fc receptors are of prime importance. Cross-linking FcγRIIb with the BCR inhibits B cell proliferation 42 and differentiation into AFCs 43 and may thus trigger germinal center B cells to assume a memory phenotype. Interestingly, in the absence of FcγRII, antibody production is elevated in response to immunization 44, consistent with FcγRII being involved in cessation of AFC production.
Our analysis of the immune response of bcl-2–transgenic mice is not the first to address the impact of apoptosis on affinity maturation. Transgenic mice expressing in their B cells the bcl-2 homologue bcl-xL have been similarly analyzed 13. Despite the similarity of mechanism by which these two antiapoptotic genes block cell death 45, some differences are evident in the results obtained. First, bcl-xL mice show no increase in the frequency or longevity of AFCs in either the spleen or bone marrow compared with bcl-2 mice in which both of these compartments are amplified, raising the question of whether the bcl-xL transgene is expressed in terminally differentiated B cells. Second, bcl-xL mice show reduced affinity maturation of both serum Ig and bone marrow AFCs 13, again unlike bcl-2 mice (Table and reference 20). Third, there was an abnormal persistence of B cells containing noncanonical VDJ gene rearrangements in bcl-xL mice despite V186.2 rearrangements being normally mutated and selected 13. That is, the effect of the bcl-xL transgene appeared to be restricted to B cells bearing non-VH186.2 rearrangements. The effects of the bcl-2 transgene reported here, on the other hand, are distributed irrespective of VH gene content, as there is no distortion of VH gene usage in any of the compartments examined (Table ). Thus, without knowing the full extent of bcl-xL transgene expression, we must assume that the differences in results obtained are due to the bcl-xL transgene not being expressed in all B cell compartments. This may also explain the variance observed between these two strains in a model system of self-, anti-self B cell tolerance 46.
In summary, we have used bcl-2–transgenic mice to demonstrate that apoptosis of low-affinity germinal center B cells is necessary for the formation of a normally selected memory B cell population. Moreover, this increase in germinal center cell survival revealed marked differences in the nature of selection of memory B cells and bone marrow AFCs. Selection of the memory compartment was perturbed by constitutive bcl-2 expression in the germinal center, consistent with a model of selection where competition of B cells for an antigen-mediated survival signal is required for entry to the memory compartment. In contrast, the stringent selection of high-affinity bone marrow AFCs is not influenced by the bcl-2 transgene, consistent with a selective process requiring the germinal center B cell to exceed an “affinity threshold.”
Acknowledgments
We thank the WEHI Flow Cytometry facility for help with cell sorting and Drs. Alan Harris and Suzanne Cory for providing bcl-2–transgenic mice.
This work was supported primarily by the National Health and Medical Research Council (NH & MRC), Canberra, Australia. Additional support was provided by the Wellcome Trust through a Biomedical Research Collaboration Grant (to K.G.C. Smith and D. Tarlinton), an MRC Career Establishment Grant (to K.G.C. Smith), and by grants and fellowships from the Dr. Josef Steiner Cancer Research Foundation (Bern, Switzerland), the Anti-Cancer Council of Victoria, the Cancer Research Institute (New York), and the Leukemia Society of America (New York) (to A. Strasser and L. O'Reilly).
Footnotes
Abbreviations used in this paper: AFC, antibody-forming cell; BCR, B cell antigen receptor; BrdU, bromodeoxyuridine; KLH, keyhole limpet hemocyanin; TUNEL, terminal deoxynucleotidyltransferase–mediated dUTP-biotin nick-end labeling.
References
- Nossal G.J. The molecular and cellular basis of affinity maturation in the antibody response. Cell. 1992;68:1–2 . doi: 10.1016/0092-8674(92)90198-l. [DOI] [PubMed] [Google Scholar]
- MacLennan I.C. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Kelsoe G. Life and death in germinal centers (redux) Immunity. 1996;4:107–111. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- Weiss U., Zoebelein R., Rajewsky K. Accumulation of somatic mutants in the B cell compartment after primary immunization with a T cell-dependent antigen. Eur. J. Immunol. 1992;22:511–517. doi: 10.1002/eji.1830220233. [DOI] [PubMed] [Google Scholar]
- Smith K.G., Light A., Nossal G.J., Tarlinton D.M. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Dutta P.R., Cerasoli D.M., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Przylepa J., Miller C., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J. Exp. Med. 1993;178:1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen H.N., Siskind G.N. Variations in affinities of antibodies during the immune response. Biochem. 1964;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Tew J.G., DiLosa R.M., Burton G.F., Kosco M.H., Kupp L.I., Masuda A., Szakal A.K. Germinal centers and antibody production in bone marrow. Immunol. Rev. 1992;126:99–112. doi: 10.1111/j.1600-065x.1992.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Gray D., Skarvall H. B-cell memory is short-lived in the absence of antigen. Nature. 1988;336:70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- Manz R.A., Cassese G., Thiel A., Radbruch A. Long-lived plasma cells survive independent of antigen. Curr. Top. Microbiol. Immunol. 1999;246:71–74. doi: 10.1007/978-3-642-60162-0_9. [DOI] [PubMed] [Google Scholar]
- Slifka M.K., Ahmed R. Long-lived plasma cellsa mechanism for maintaining persistent antibody production. Curr. Opin. Immunol. 1998;10:252–258. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Cerasoli D.M., Dal Porto J.M., Shimoda M., Freund R., Fang W., Telander D.G., Malvey E.N., Mueller D.L., Behrens T.W. Relaxed negative selection in germinal centers and impaired affinity maturation in bcl-xL transgenic mice. J. Exp. Med. 1999;190:399–410. doi: 10.1084/jem.190.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A.W., Corcoran L.M., Cory S. Bcl-2 expression promotes B- but not T-lymphoid development in scid mice. Nature. 1994;368:457–460. doi: 10.1038/368457a0. [DOI] [PubMed] [Google Scholar]
- Tarlinton D.M., Corcoran L.M., Strasser A. Continued differentiation during B lymphopoiesis requires signals in addition to cell survival. Int. Immunol. 1997;9:1481–1494. doi: 10.1093/intimm/9.10.1481. [DOI] [PubMed] [Google Scholar]
- Young F., Mizoguchi E., Bhan A.K., Alt F.W. Constitutive Bcl-2 expression during immunoglobulin heavy chain-promoted B cell differentiation expands novel precursor B cells. Immunity. 1997;6:23–33. doi: 10.1016/s1074-7613(00)80239-3. [DOI] [PubMed] [Google Scholar]
- Hande S., Notidis E., Manser T. Bcl-2 obstructs negative selection of autoreactive, hypermutated antibody V regions during memory B cell development. Immunity. 1998;8:189–198. doi: 10.1016/s1074-7613(00)80471-9. [DOI] [PubMed] [Google Scholar]
- Kuo P., Alban A., Gebhard D., Diamond B. Overexpression of bcl-2 alters usage of mutational hot spots in germinal center B cells. Mol. Immunol. 1997;34:1011–1018. doi: 10.1016/s0161-5890(97)00117-x. [DOI] [PubMed] [Google Scholar]
- Strasser A., Whittingham S., Vaux D.L., Bath M.L., Adams J.M., Cory S., Harris A.W. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl. Acad. Sci. USA. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.G., Weiss U., Rajewsky K., Nossal G.J., Tarlinton D.M. Bcl-2 increases memory B cell recruitment but does not perturb selection in germinal centers. Immunity. 1994;1:803–813. doi: 10.1016/s1074-7613(94)80022-7. [DOI] [PubMed] [Google Scholar]
- Ridderstad A., Tarlinton D.M. Kinetics of establishing the memory B cell population as revealed by CD38 expression. J. Immunol. 1998;160:4688–4695. [PubMed] [Google Scholar]
- McHeyzer-Williams M.G., Nossal G.J., Lalor P.A. Molecular characterization of single memory B cells. Nature. 1991;350:502–505. doi: 10.1038/350502a0. [DOI] [PubMed] [Google Scholar]
- Ehlich A., Martin V., Muller W., Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr. Biol. 1994;4:573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Kabat E.A., Wu T.T., Perry H.M., Gottesman K.S., Foeller C. Sequences of proteins of immunological interest, Vol. 2 1991. US National Institutes of Health; Bethesda, MD: pp. 1394 [Google Scholar]
- O'Reilly L.A., Gu D., Sarvetnick N., Edlund H., Phillips J.M., Fulford T., Cooke A. α-cell neogenesis in an animal model of IDDM. Diabetes. 1997;46:599–606. doi: 10.2337/diab.46.4.599. [DOI] [PubMed] [Google Scholar]
- Vaux D.L., Cory S., Adams J.M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Mazel S., Burtrum D., Petrie H.T. Regulation of cell division cycle progression by bcl-2 expressiona potential mechanism for inhibition of programmed cell death. J. Exp. Med. 1996;183:2219–2226. doi: 10.1084/jem.183.5.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linette G.P., Li Y., Roth K., Korsmeyer S.J. Cross talk between cell death and cell cycle progressionBCL-2 regulates NFAT-mediated activation. Proc. Natl. Acad. Sci. USA. 1996;93:9545–9552. doi: 10.1073/pnas.93.18.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly L.A., Huang D.C., Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6979–6990. [PMC free article] [PubMed] [Google Scholar]
- Smith K.G., Nossal G.J., Tarlinton D.M. FAS is highly expressed in the germinal center but is not required for regulation of the B-cell response to antigen. Proc. Natl. Acad. Sci. USA. 1995;92:11628–11632. doi: 10.1073/pnas.92.25.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A.W., Huang D.C., Krammer P.H., Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- Tarlinton D. Germinal centersform and function. Curr. Opin. Immunol. 1998;10:245–251. doi: 10.1016/s0952-7915(98)80161-1. [DOI] [PubMed] [Google Scholar]
- Radmacher M.D., Kelsoe G., Kepler T.B. Predicted and inferred waiting times for key mutations in the germinal centre reactionevidence for stochasticity in selection. Immunol. Cell. Biol. 1998;76:373–381. doi: 10.1046/j.1440-1711.1998.00753.x. [DOI] [PubMed] [Google Scholar]
- Fulcher D.A., Lyons A.B., Korn S.L., Cook M.C., Koleda C., Parish C., Fazekas de St B., Groth, Basten A. The fate of self-reactive B cells depends primarily on the degree of antigen receptor engagement and availability of T cell help. J. Exp. Med. 1996;183:2313–2328. doi: 10.1084/jem.183.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M.C., Basten A., Fazekas de St. Groth B. Outer periarteriolar lymphoid sheath arrest and subsequent differentiation of both naive and tolerant immunoglobulin transgenic B cells is determined by B cell receptor occupancy. J. Exp. Med. 1997;186:631–643. doi: 10.1084/jem.186.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M.B., Goerg S., Shen L., Prodeus A.P., Goodnow C.C., Kelsoe G., Carroll M.C. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science. 1998;280:582–585. doi: 10.1126/science.280.5363.582. [DOI] [PubMed] [Google Scholar]
- Fehr T., Rickert R.C., Odermatt B., Roes J., Rajewsky K., Hengartner H., Zinkernagel R.M. Antiviral protection and germinal center formation, but impaired B cell memory in the absence of CD19. J. Exp. Med. 1998;188:145–155. doi: 10.1084/jem.188.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Zhang J., Lane P.J., Chan E.Y., MacLennan I.C. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Odermatt B., Hengartner H., Zinkernagel R.M. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J. Exp. Med. 1996;183:2259–2269. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Kocks C., Rajewsky K., Dildrop R. Analysis of somatic mutation and class switching in naive and memory B cells generating adoptive primary and secondary responses. Cell. 1987;48:757–770. doi: 10.1016/0092-8674(87)90073-0. [DOI] [PubMed] [Google Scholar]
- Sinclair N.R., Lees R.K., Elliott E.V. Role of the Fc fragment in the regulation of the primary immune response. Nature. 1968;220:1048–1049. doi: 10.1038/2201048a0. [DOI] [PubMed] [Google Scholar]
- Uher F., Lamers M.C., Dickler H.B. Antigen-antibody complexes bound to B-lymphocyte Fc gamma receptors regulate B-lymphocyte differentiation. Cell. Immunol. 1985;95:368–379. doi: 10.1016/0008-8749(85)90324-7. [DOI] [PubMed] [Google Scholar]
- Takai T., Ono M., Hikida M., Ohmori H., Ravetch J.V. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- Huang D.C., Cory S., Strasser A. Bcl-2, Bcl-XL and adenovirus protein E1B19kD are functionally equivalent in their ability to inhibit cell death. Oncogene. 1997;14:405–414. doi: 10.1038/sj.onc.1200848. [DOI] [PubMed] [Google Scholar]
- Fang W., Weintraub B.C., Dunlap B., Garside P., Pape K.A., Jenkins M.K., Goodnow C.C., Mueller D.L., Behrens T.W. Self-reactive B lymphocytes overexpressing Bcl-xL escape negative selection and are tolerized by clonal anergy and receptor editing. Immunity. 1998;9:35–45. doi: 10.1016/s1074-7613(00)80586-5. [DOI] [PubMed] [Google Scholar]