Abstract
Chemokines are a family of small proteins that interact with seven-transmembrane domain receptors and modulate the migration of immune cells into sites of inflammation and infection. The murine gammaherpesvirus 68 M3 gene encodes a secreted 44-kD protein with no sequence similarity to known chemokine receptors. We show that M3 binds a broad range of chemokines, including CC, CXC, C, and CX3C chemokines, but does not bind human B cell–specific nor mouse neutrophil–specific CXC chemokines. This herpesvirus chemokine binding protein (hvCKBP) blocks the interaction of chemokines with high-affinity cellular receptors and inhibits chemokine-induced elevation of intracellular calcium levels. hvCKBP is the first soluble chemokine receptor identified in herpesviruses; it represents a novel protein structure with the ability to bind all subfamilies of chemokines in solution and has potential therapeutic applications.
Keywords: chemokine, cytokine receptor, virus, viral immune evasion, anti-inflammatory protein
Introduction
The migration of leukocytes from blood vessels to sites of infection and inflammation is an important phenomenon in host defense. Chemokines are chemotactic cytokines that interact with G protein–coupled chemokine receptors (CKRs) and play a key role in leukocyte recruitment; they have also been implicated in hematopoiesis, angiogenesis, and development 1 2. Chemokines are divided into four structural subfamilies based on the number and arrangement of conserved cysteines: CC chemokines such as RANTES (regulated upon activation, normal T cell expressed and secreted), macrophage inflammatory protein (MIP)-1α, and monocyte chemoattractant protein (MCP)-1; CXC chemokines such as IL-8 and growth-related oncogene (GRO)-α; the C chemokine lymphotactin; and the CX3C chemokine fractalkine. Chemokines form a chemical gradient via electrostatic interactions with negatively charged glycosaminoglycans (GAGs). The binding sites of chemokines for GAGs and specific CKRs are distinct.
Chemokines are tightly regulated to prevent excessive inflammation that can cause disease and represent targets for therapeutic intervention. The production of soluble versions of cytokine receptors represents a strategy to block cytokine activity 3. However, the seven-transmembrane domain structure of CKRs makes the construction of soluble, inhibitory CKRs difficult, and thus antagonists based on mutated chemokines, blocking peptides, or antibodies are under evaluation as chemokine inhibitors 1 2.
It has been established that molecular mimicry of cytokines and cytokine receptors is a strategy adopted by large DNA viruses to modulate the host immune response 4 5 6 7. Poxviruses encode a unique set of secreted proteins that bind cytokines such as TNF, IL-1β, IFN-α/β, IFN-γ, and chemokines with high affinity and block their activity by preventing interaction with cellular receptors. Herpesviruses encode a number of cytokine homologues, including IL-6, IL-10, and IL-17 4, and proteins with sequence similarity to chemokines and seven-transmembrane domain CKRs, which modulate or utilize the chemokine network for the benefit of the virus 7.
Members of the gammaherpesvirus subfamily are widespread in nature and are characterized by their ability to establish latency in lymphocytes and by an association with lymphoproliferative disorders and a variety of tumors. Murine gammaherpesvirus (MHV)-68 is a natural pathogen of murid rodents 8 genetically related to the primate gammaherpesviruses herpesvirus saimiri, human herpesvirus (HHV)-8 (also known as Kaposi's sarcoma–associated herpesvirus), and EBV 9 10 11. Interest in MHV-68 stems from its ability to establish both acute and persistent infection within laboratory mice, therefore offering a unique opportunity to investigate immunological and virological aspects of gammaherpesvirus pathogenesis 12. During primary infection of mice, acute phase virus replication is detected in multiple organs, with latency being largely restricted to B lymphocytes and macrophages 13 14 15 16.
Previous in situ hybridization studies have shown that the unique MHV-68–encoded open reading frame (ORF) M3 is transcribed during acute infection and at early stages of latency establishment in the spleen 17. However, the precise function of this ORF is unclear. M3 encodes a 44-kD secreted protein translated from an abundant 1.4-kb early–late lytic transcript, and it has been proposed that this protein may interact with host cellular receptors or cytokines 18.
Here we report that the MHV-68–encoded M3 protein binds chemokines from all four known subfamilies and demonstrate that the inhibitory mechanism of the herpesvirus chemokine binding protein (hvCKBP) is to block the interaction of chemokines with cellular receptors. This represents the first protein identified in herpesviruses that is secreted from infected cells and sequesters chemokines.
Materials and Methods
Reagents.
Radioiodinated recombinant human IL-8, RANTES, MIP-1α, and fractalkine (2,000 Ci/mmol) were obtained from Amersham. Recombinant human RANTES and IL-18 and murine B cell–attracting chemokine (BCA)-1 (B lymphocyte chemoattractant [BLC]-1) were obtained from R & D Systems, Inc. Recombinant human MIP-1α, MCP-1, MCP-4, secondary lymphoid tissue chemokine (SLC), IL-8, GRO-α, IFN-γ–inducible protein (IP)-10, granulocyte chemotactic protein (GCP)-2, BCA-1, stromal cell–derived factor (SDF)-1α, lymphotactin, fractalkine, and murine RANTES, MIP-1α, KC, MIP-2, and LPS-induced CXC chemokine (LIX) were obtained from PeproTech, Inc. Recombinant human and murine IL-1β, IFN-γ, and TNF-α were obtained from PeproTech, Inc.
Growth of MHV-68 and Generation of Recombinant Viruses.
MHV-68 was grown and assayed in baby hamster kidney (BHK)-21 cells and purified from cell supernatants by Ficoll gradient centrifugation 19. Recombinant viruses were generated by cotransfection of the relevant plasmid with MHV-68 virion DNA into BHK-21 cells 19. For the generation of a recombinant MHV-68 with a disrupted M3 gene, two primers were used (5′-GGACTCTTGAGGAGCTCGAG-3′ and 5′-TAGGTGGCTGCTGAGTGATT-3′) to PCR amplify a 2,602-bp fragment (genomic coordinates 5,347–7,929) 10 with Taq polymerase (Perkin-Elmer Corp.) using MHV-68 genomic DNA as template. The PCR fragment was cloned into pCR2.1 (Invitrogen Corp.) to yield pM3. A 4.1-kbp HindIII cassette containing the LacZ gene driven by the human CMV immediate early promoter derived from pMV10 20 was inserted at the HincII site (coordinate 7,156) in an orientation opposite to the direction of M3 transcription to yield pM3MV10. The virus mutant with a disrupted M3 ORF was named MHV-M3LacZ. For the generation of an M3 revertant, named MHV-M3R, linearized pM3 was cotransfected with M3 mutant virus DNA. Recombinant viruses were identified by X-Gal staining and plaque purified three times, and their genetic structures were confirmed by Southern blot hybridization.
Growth and Construction of Recombinant Baculoviruses.
The MHV-68 M3 ORF was amplified from infected cell DNA by PCR using oligonucleotides 5′-CGCGAATTCATGGCCTTCCTATCCACATCG-3′, inserting an EcoRI site, and 5′-GGTGCGGCCGCATGATCCCCAAAATACTCCAGC-3′, which inserts a NotI site. The 1,238-bp product was cloned into EcoRI/NotI-digested pBAC-1 (Novagen Corp.), creating pBACM3. The nucleotide sequence of the cloned ORF was confirmed by sequencing before the recombinant baculovirus, named AcM3, was constructed as described 21. Recombinant M3 protein containing a COOH-terminal 6-histidine tag was produced in Spodoptera frugiperda (Sf)21 insect cells infected with AcM3. The recombinant baculovirus AcB15R has been described 22.
Binding Assays.
Supernatants from MHV-68–infected BHK-21 cells or baculovirus-infected Sf21 cells, infected at high multiplicity of infection, were harvested 2 or 3 d after infection, respectively, and prepared as described 22. Infectious virus present in supernatants was inactivated with 4,5′,8-trimethylpsoralen and exposure to UV light 21. Binding medium was RPMI 1640 containing 20 mM Hepes, pH 7.4, and 0.1% BSA. Cross-linking experiments with bis(sulfosuccinimidyl) suberate (BS3; 5 mM; Pierce Chemical Co.) to 125I-labeled chemokines (0.4 nM) were performed in 25 μl as described 21. The amount of medium used was equivalent to 5 × 102 cells. Samples were analyzed by 12% acrylamide SDS-PAGE. In the competition assays with U937 cells, supernatants were preincubated with 100 pM 125I-labeled chemokine in 100 μl for 1 h at 4°C. Subsequently, 2.5 × 106 U937 cells were added in 50 μl and incubated for 2 h at 4°C. Bound 125I-labeled chemokine was determined by phthalate oil centrifugation 21.
Measurement of Calcium Mobilization.
PBMCs were separated from human whole blood by centrifugation over Lymphoprep™ reagent (Nycomed) and maintained in RPMI 1640 supplemented with 20% FCS. Cells were incubated at a density of 2 × 106 cells/ml in RPMI 1640, 1% FCS, and 5 μM Indo-1 for 1 h before being washed twice in HBSS and resuspended at 105 cells/ml in HBSS/0.2% FCS. Calcium influx was monitored after the addition of 20 nM RANTES, preincubated for 2 h at room temperature with baculovirus-expressed proteins, by flow cytometry at excitation wavelengths of 480 and 400 nm using a Becton Dickinson FACS VantageSE™ cytometer.
Results
MHV-68 Encodes a Soluble CKBP with Broad Specificity.
To investigate the expression of soluble CKBPs encoded by MHV-68, binding assays with human 125I-labeled IL-8 (CXC chemokine), 125I-labeled RANTES, and 125I-labeled MIP-1α (CC chemokines) and the radioiodinated soluble chemokine domain of fractalkine (CX3C chemokine) were carried out with MHV-68–infected cell supernatants, followed by chemical cross-linking with BS3. Complexes of chemokines with a soluble protein were detected with all three subfamilies of chemokines tested but not with the control supernatant from mock-infected cultures (Fig. 1). The size of the complex was ∼45 kD, suggesting a viral protein of 38 kD after subtraction of the 8-kD mass of the monomeric 125I-labeled chemokines. The higher molecular size complex observed was probably the result of protein aggregation and was not observed when samples were not boiled before the electrophoretic analysis (data not shown).
Figure 1.
A soluble chemokine binding activity is encoded by MHV-68. Media from BHK-21 cells uninfected (Mock) or infected with MHV-68 were incubated with 125I-labeled chemokines and treated with the cross-linker BS3. An autoradiograph of the SDS-PAGE analysis, with molecular masses in kD, is shown. The positions of the chemokines and ligand–receptor complexes are indicated.
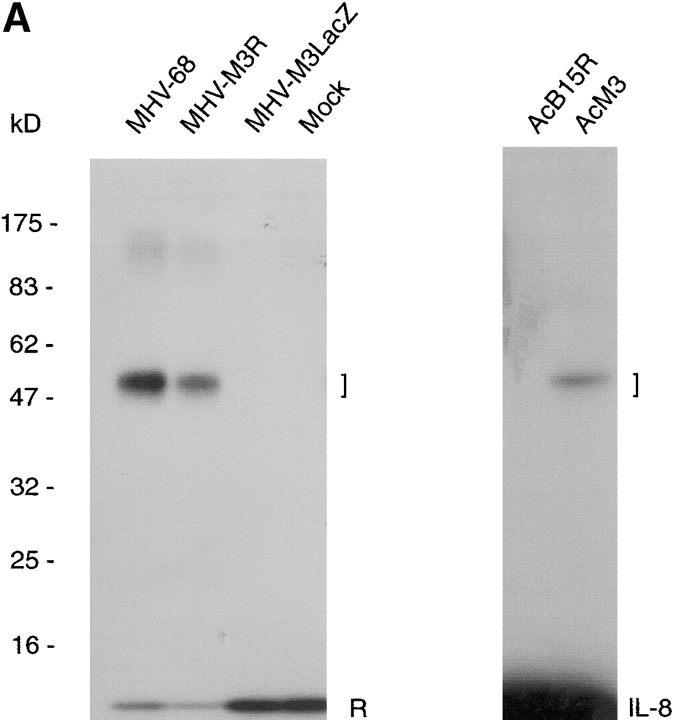
Analysis of the MHV-68 genomic sequence failed to identify a secreted protein with sequence similarity to CKRs 10. However, a possible candidate was the 44-kD secreted protein encoded by ORF M3 18. On this basis, the M3 ORF was inactivated by insertion of a LacZ expression cassette and the mutant virus designated MHV-M3LacZ (Fig. 2 B). A revertant virus named MHV-M3R in which the M3 ORF was reinserted into the virus genome was also constructed to control for possible mutations elsewhere in the viral genome. Fig. 2 A shows that supernatants from wild-type MHV-68 and revertant virus infections formed a complex with 125I-labeled RANTES after cross-linking, whereas supernatants from M3 mutant and mock infections did not produce a complex. To further demonstrate that the M3 protein binds chemokines, a recombinant baculovirus named AcM3 was constructed that expressed the M3 ORF. Medium from insect cells infected with AcM3 formed a complex with 125I-labeled IL-8 of size similar to that observed with MHV-68–infected cell supernatants (Fig. 2 A). This complex was not found with the control recombinant baculovirus AcB15R expressing the vaccinia virus soluble IL-1βR.
Figure 2.
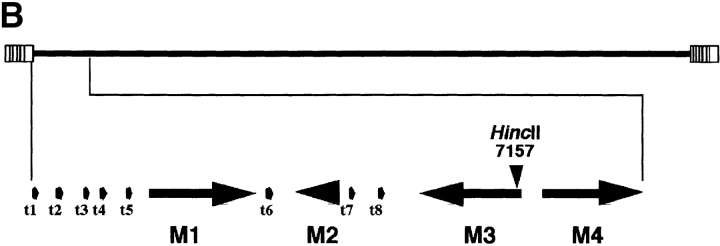
The M3 ORF encodes the soluble chemokine binding activity expressed by MHV-68. (A) Media from BHK-21 cells uninfected (Mock) or infected with wild-type MHV-68, the M3 mutant MHV-M3LacZ, or the revertant virus MHV-M3R were incubated with 125I-labeled RANTES. Media from Sf21 cells infected with the recombinant baculoviruses AcM3 or AcB15R were incubated with 125I-labeled IL-8. Samples were treated with the cross-linker BS3. Autoradiographs of the SDS-PAGE analysis, with molecular masses in kD, are shown. The positions of RANTES (R), IL-8, and ligand–receptor complexes (square brackets) are indicated. (B) Diagram showing the site of insertion of LacZ into the M3 gene of MHV-68 to yield a recombinant virus with the M3 gene disrupted, designated MHV-M3LacZ. The MHV-68 genome contains 118,237 bp of unique sequence flanked by variable copies of a 1,213-bp terminal repeat 10. The leftmost end of the virus genome is expanded, depicting the first ORFs, M1 to M4, interspersed by the eight viral tRNA–like molecules 25. The HincII site at position 7,157 indicates the site of insertion of MV10 containing the LacZ gene.
Chemokine Binding Specificity of hvCKBP.
To determine the binding specificity of M3, cross-linking experiments were carried out with radiolabeled CC and CXC chemokines in the presence of various amounts of unlabeled chemokine competitors. Fig. 3 shows that the binding to 125I-labeled IL-8 and 125I-labeled MIP-1α was inhibited by all of the unlabeled CC chemokines tested. In addition, viral (v)MIP-2 encoded by HHV-8 bound to hvCKBP in a similar competition experiment (data not shown). CXC chemokines such as human GRO-α and IP-10 and murine BCA-1 also inhibited binding to both 125I-labeled IL-8 and 125I-labeled MIP-1α. Other CXC chemokines, including murine KC (murine GRO-α), MIP-2, and LIX and human BCA-1 and SDF-1α were poor inhibitors of the binding to 125I-labeled chemokines. Human lymphotactin and the soluble chemokine domain of human fractalkine, the single members of the C and CX3C chemokine subfamilies, respectively, also inhibited binding to both 125I-labeled IL-8 and 125I-labeled MIP-1α. The competition profiles of the chemokines tested suggested different affinities of hvCKBP for various chemokines. The interaction between hvCKBP and 125I-labeled chemokines was not inhibited by a 500-fold excess of a variety of cytokines, including human and mouse IL-1β, IL-18, IFN-γ, and TNF-α, indicating binding specificity for chemokines (data not shown). The recombinant M3 protein produced in the baculovirus system showed the same chemokine binding specificity in similar competition experiments with 125I-labeled MIP-1α and 125I-labeled IL-8 in the presence of excess unlabeled chemokines (data not shown).
Figure 3.
Binding specificity of hvCKBP encoded by MHV-68. Cross-linking of 0.4 nM human 125I-labeled MIP-1α or 125I-labeled IL-8 with BS3 to medium from uninfected (Mock) and MHV-68–infected cultures, in the absence (NC) or presence of increasing doses of the indicated unlabeled human or murine chemokines. The concentrations of unlabeled chemokines were 10-, 100-, 500-, and 2,000-fold excess. In the cross-linking performed in the presence of GAGs, radiolabeled MIP-1α and IL-8 were preincubated with heparin or heparan sulfate for 1 h at room temperature before addition of supernatants. The doses of heparin and heparan sulfate added were 1,000, 100, 10, 1, 0.1, 0.01, and 0.001 μg/ml. An autoradiograph of the SDS-PAGE analysis showing the ligand–hvCKBP complexes is shown.
To demonstrate that hvCKBP does not bind to the chemokine GAG binding domain, radiolabeled ligands were preincubated with different doses of heparin and heparan sulfate before the addition of MHV-68 supernatants. Preincubation with heparin or heparan sulfate, representing a 3 × 106 molar excess over the chemokines in the case of heparin, had no effect on the binding of 125I-labeled IL-8 or 125I-labeled MIP-1α to hvCKBP (Fig. 3).
hvCKBP Blocks the Binding of Chemokines to Cell Surface Receptors.
Specific binding of 125I-labeled MIP-1α and 125I-labeled IL-8 to U937 cells was inhibited in a dose-dependent manner by supernatants from cultures infected with wild-type MHV-68 or recombinant baculovirus AcM3 but not by the virus mutant MHV-M3LacZ or the recombinant baculovirus AcB15R expressing the vaccinia virus soluble IL-1βR (Fig. 4). The partial inhibitory effect with high doses of MHV-M3LacZ supernatants was also observed with mock-infected BHK-21 supernatants (data not shown). Inhibition of chemokine binding to cells was achieved by very low doses of supernatant containing the natural or recombinant M3 protein and was more efficient for IL-8 (0.1 μl, equivalent to supernatant from 250 infected cells) than MIP-1α (1 μl, equivalent to supernatant from 3,000 infected cells).
Figure 4.
Natural and recombinant hvCKBP block the specific binding of MIP-1α and IL-8 to U937 cells. Binding assay of 125I-labeled MIP-1α and 125I-labeled IL-8 to U937 cells in the presence of supernatants from cells infected with MHV-68 or MHV-M3LacZ or with recombinant baculovirus AcM3 or AcB15R. The dose of supernatant corresponding to a number of cells (cell equivalents) is indicated. The binding specificity was determined in the presence of a 1,000-fold excess of unlabeled MIP-1α or IL-8. Means (±SEM) from duplicate samples are expressed as the percentage of counts binding in the absence of competitor.
hvCKBP Blocks the Biological Activity of Chemokines.
Recombinant hvCKBP specifically inhibited, in a dose-dependent manner, the transient increase in intracellular calcium induced by RANTES in human PBMCs (Fig. 5).
Figure 5.
Recombinant hvCKBP blocks chemokine biological activity in vitro. Elevation of intracellular calcium levels in human PBMCs by 20 nM RANTES, preincubated with or without supernatants from cultures infected with recombinant baculovirus AcM3 or AcB15R. The dose of supernatant corresponding to a number of cells (cell equivalents) is indicated. Cell signaling is expressed as the percentage of cells with elevated calcium levels in the presence of RANTES alone.
Discussion
This paper describes the identification and characterization of a novel soluble CKBP encoded by the MHV-68 M3 ORF. The secreted M3 protein shows no amino acid sequence similarity to seven-transmembrane domain CKRs, which are hydrophobic molecules that cannot be engineered as soluble chemokine inhibitors 1 2. A number of her-pesviruses encode homologues of membrane-bound cellular CKRs 7, but hvCKBP is the first example of a soluble inhibitor encoded by a herpesvirus that sequesters chemokines. A soluble colony-stimulating factor 1 receptor encoded by EBV is the only soluble cytokine receptor identified in the herpesvirus family to date 23, and several soluble cytokine receptors are expressed by poxviruses 5 6 24. hvCKBP may be a unique herpesvirus molecule or may represent a viral version of an unidentified cellular molecule.
The M3 gene product is unique to MHV-68, with no identifiable homology to known cellular or viral proteins, but displays significant sequence similarity to the MHV-68 ORF M1 17 18, which is dispensable for the establishment of and reactivation from latency 19. The M1 translation product, but not the M3 protein, has amino acid sequence similarity to members of the poxvirus serpin family, although the functionally important serpin hinge region is not conserved 10 25. The absence of chemokine binding activity in the M3 mutant MHV-M3LacZ suggests that M3 is the only MHV-68 CKBP. However, M1 transcription has not been detected in BHK-21 cells 17, and thus the M1 ORF may also encode chemokine binding activity.
We show that hvCKBP interacts with the receptor binding domain of chemokines, at a site different to the GAG binding domain. Consistent with this observation, hvCKBP blocks at very low doses the binding of IL-8 and MIP-1α to high-affinity cellular CKRs and RANTES-induced signal transduction in vitro. This indicates that the affinity of hvCKBP for these chemokines is similar to or higher than their affinity for cellular receptors. Furthermore, it establishes the mechanism of action of hvCKBP and shows that hvCKBP is a potent chemokine inhibitor.
hvCKBP exhibits a broad binding specificity, extending to all subfamilies of chemokines and vMIP-2 encoded by HHV-8. Interestingly, hvCKBP does not bind to human B cell–specific or murine neutrophil–specific CXC chemokines, which may be of relevance in viral pathogenesis. The broad binding specificity of hvCKBP is unique amongst known CKRs. A soluble 35-kD CKBP encoded by poxviruses that binds a broad range of CC chemokines but not CXC or C chemokines has been described 6. The poxvirus CKBP has no sequence similarity to the M3 protein and is likely to interact with chemokines in a different way. Future structural analysis of these viral CKBPs will establish the molecular interaction of soluble chemokine inhibitors with their ligands and may help to design small soluble inhibitors of chemokines.
Expression of a broad range and potent soluble CKBP by MHV-68–infected cells indicates an immune modulatory role for the M3 protein. M3 is abundantly expressed during lytic infection in the lungs of infected mice 11, and this may delay leukocyte influx into infected areas. Notably, M3 transcription has also been detected in spleens of latently infected mice 17 26 and is localized to periarteriolar lymphoid sheets early during the establishment of latency 17. The M3 protein does not bind human BCA-1 but binds the mouse homologue. Further investigations to determine whether the binding affinity of hvCKBP for mouse BCA-1 is sufficient to block its biological activity will be important, as BCA-1 is required for formation of germinal centers 27 28, where MHV-68 latency is confined within the spleen 17 25.
The important role of chemokines and CKRs in antiviral defense is emphasized by the various strategies encoded by large DNA viruses to modulate chemokine activity. These include the expression of secreted CKBPs that block interaction of chemokines with cellular receptors or GAGs 5 6 24, chemokine-like proteins that may block or activate specific chemokine pathways 7, and seven-transmembrane domain CKR homologues that may downregulate the local concentration of chemokines or hijack chemokine-induced signals 7. Some of these viral proteins, such as the HHV-8 vMIP-2 that has angiogenic properties 29, may also contribute directly to pathology.
Viral proteins that counteract the immune system have been optimized during evolution and represent potential sources of immunomodulatory proteins 4 5 6 24. As chemokines have been implicated in many disease processes, such as rheumatoid arthritis, transplant rejection, HIV progression, and atherosclerosis, the novel soluble chemokine inhibitor encoded by MHV-68 has potential therapeutic applications. The existence of a large number of chemokines suggests redundancy, and thus the therapeutic use of a broad chemokine antagonist such as hvCKBP may be advantageous. In addition, hvCKBP may provide the structural scaffolding needed to design soluble chemokine inhibitors with a narrower specificity.
The MHV-68 chemokine inhibitor reported here represents a novel virus immune evasion strategy that provides insights into herpesvirus–host interactions and the mechanisms of herpesvirus pathogenesis.
Acknowledgments
We thank Phillip Stevenson for helpful discussions, Nigel Miller (Babraham Institute, Cambridge) for help with calcium mobilization assays, and Debbie Swann for technical assistance.
This work was funded by grants from the Wellcome Trust (051087/Z/97/Z, 036076/Z/96/A, and 054458/Z/98/Z) and the Medical Research Council. A. Alcami is a Wellcome Trust Senior Research Fellow.
Footnotes
J.P. Simas' present address is Gulbenkian Institute for Science, Rua da Quinta Grande 6, 2780-156 Oeiras, Portugal.
Antonio Alcami, Div. of Virology, Dept. of Pathology, University of Cambridge, Tennis Court Rd., Cambridge CB2 1QP, UK. Phone: 44-1223-336922; Fax: 44-1223-336926; E-mail: aa258@mole.bio.cam.ac.uk
References
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568 . doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Rollins B.J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Heaney M.L., Golde D.W. Soluble cytokine receptors. Blood. 1996;87:847–857. [PubMed] [Google Scholar]
- Spriggs M.K. One step ahead of the gameviral immunomodulatory molecules. Annu. Rev. Immunol. 1996;14:101–130. doi: 10.1146/annurev.immunol.14.1.101. [DOI] [PubMed] [Google Scholar]
- Smith G.L., Symons J.A., Khanna A., Vanderplasschen A., Alcami A. Vaccinia virus immune evasion. Immunol. Rev. 1997;159:137–154. doi: 10.1111/j.1600-065x.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Alcami A., Symons J.A., Khanna A., Smith G.L. Poxvirusescapturing cytokines and chemokines. Semin. Virol. 1998;8:419–427. [Google Scholar]
- Dairaghi D.J., Greaves D.R., Schall T.J. Abduction of chemokine elements by herpesviruses. Semin. Virol. 1998;8:377–385. [Google Scholar]
- Blaskovic D., Stancekova M., Svobodova J., Mistrikova J. Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol. 1980;24:468. [PubMed] [Google Scholar]
- Efstathiou S., Ho Y.M., Hall S., Styles C.J., Scott S.D., Gompels U.A. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J. Gen. Virol. 1990;71:1365–1372. doi: 10.1099/0022-1317-71-6-1365. [DOI] [PubMed] [Google Scholar]
- Virgin H.W., 4th, Latreille P., Wamsley P., Hallsworth K., Weck K.E., Dal Canto A.J., Speck S.H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simas J.P., Efstathiou S. Murine gammaherpesvirus 68a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 1998;6:276–282. doi: 10.1016/s0966-842x(98)01306-7. [DOI] [PubMed] [Google Scholar]
- Doherty P.C., Tripp R.A., Hamilton-Easton A.M., Cardin R.D., Woodland D.L., Blackman M.A. Tuning into immunological dissonancean experimental model for infectious mononucleosis. Curr. Opin. Immunol. 1997;9:477–483. doi: 10.1016/s0952-7915(97)80098-2. [DOI] [PubMed] [Google Scholar]
- Sunil-Chandra N.P., Efstathiou S., Nash A.A. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 1992;73:3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- Usherwood E.J., Stewart J.P., Robertson K., Allen D.J., Nash A.A. Absence of splenic latency in murine gammaherpesvirus 68-infected B cell-deficient mice. J. Gen. Virol. 1996;77:2819–2825. doi: 10.1099/0022-1317-77-11-2819. [DOI] [PubMed] [Google Scholar]
- Weck K.E., Kim S.S., Virgin H.I., Speck S.H. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 1999;73:4651–4661. doi: 10.1128/jvi.73.6.4651-4661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck K.E., Kim S.S., Virgin H.I., Speck S.H. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 1999;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simas J.P., Swann D., Bowden R., Efstathiou S. Analysis of murine gammaherpesvirus-68 transcription during lytic and latent infection. J. Gen. Virol. 1999;80:75–82. doi: 10.1099/0022-1317-80-1-75. [DOI] [PubMed] [Google Scholar]
- van Berkel V., Preiter K., Virgin H.W., 4th, Speck S.H. Identification and initial characterization of the murine gammaherpesvirus 68 gene M3, encoding an abundantly secreted protein. J. Virol. 1999;73:4524–4529. doi: 10.1128/jvi.73.5.4524-4529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simas J.P., Bowden R.J., Paige V., Efstathiou S. Four tRNA-like sequences and a serpin homologue encoded by murine gammaherpesvirus 68 are dispensable for lytic replication in vitro and latency in vivo. J. Gen. Virol. 1998;79:149–153. doi: 10.1099/0022-1317-79-1-149. [DOI] [PubMed] [Google Scholar]
- Wilkinson G.W., Akrigg A. Constitutive and enhanced expression from the CMV major IE promoter in a defective adenovirus vector. Nucleic Acids Res. 1992;20:2233–2239. doi: 10.1093/nar/20.9.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcami A., Symons J.A., Collins P.D., Williams T.J., Smith G.L. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J. Immunol. 1998;160:624–633. [PubMed] [Google Scholar]
- Alcami A., Smith G.L. A soluble receptor for interleukin-1β encoded by vaccinia virusa novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Strockbine L.D., Cohen J.I., Farrah T., Lyman S.D., Wagener F., DuBose R.F., Armitage R.J., Spriggs M.K. The Epstein-Barr virus BARF1 gene encodes a novel, soluble colony-stimulating factor-1 receptor. J. Virol. 1998;72:4015–4021. doi: 10.1128/jvi.72.5.4015-4021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash P., Barrett J., Cao J.X., Hota-Mitchell S., Lalani A.S., Everett H., Xu X.M., Robichaud J., Hnatiuk S., Ainslie C. Immunomodulation by virusesthe myxoma virus story. Immunol. Rev. 1999;168:103–120. doi: 10.1111/j.1600-065x.1999.tb01286.x. [DOI] [PubMed] [Google Scholar]
- Bowden R.J., Simas J.P., Davis A.J., Efstathiou S. Murine gammaherpesvirus 68 encodes tRNA-like sequences which are expressed during latency. J. Gen. Virol. 1997;78:1675–1687. doi: 10.1099/0022-1317-78-7-1675. [DOI] [PubMed] [Google Scholar]
- Virgin H.W., 4th, Presti R.M., Li X.Y., Liu C., Speck S.H. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J. Virol. 1999;73:2321–2332. doi: 10.1128/jvi.73.3.2321-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R., Mattis A.E., Kremmer E., Wolf E., Brem G., Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Legler D.F., Loetscher M., Roos R.S., Clark-Lewis I., Baggiolini M., Moser B. B cell–attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshoff C., Endo Y., Collins P.D., Takeuchi Y., Reeves J.D., Schweickart V.L., Siani M.A., Sasaki T., Williams T.J., Gray P.W. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]