Abstract
In many cases, induction of CD8+ CTL responses requires CD4+ T cell help. Recently, it has been shown that a dominant pathway of CD4+ help is via antigen-presenting cell (APC) activation through engagement of CD40 by CD40 ligand on CD4+ T cells. To further study this three cell interaction, we established an in vitro system using dendritic cells (DCs) as APCs and influenza hemagglutinin (HA) class I and II peptide–specific T cell antigen receptor transgenic T cells as cytotoxic T lymphocyte precursors and CD4+ T helper cells, respectively. We found that CD4+ T cells can provide potent help for DCs to activate CD8+ T cells when antigen is provided in the form of either cell lysate, recombinant protein, or synthetic peptides. Surprisingly, this help is completely independent of CD40. Moreover, CD40-independent CD4+ help can be documented in vivo. Finally, we show that CD40-independent T cell help is delivered through both sensitization of DCs and direct CD4+–CD8+ T cell communication via lymphokines. Therefore, we conclude that CD4+ help comprises at least three components: CD40-dependent DC sensitization, CD40-independent DC sensitization, and direct lymphokine-dependent CD4+–CD8+ T cell communication.
Keywords: cross-priming, dendritic cells, CD40, CD4+ help, CD8+ cytotoxic T lymphocytes
Introduction
CD4+ T cells are essential for induction of CD8+ CTL responses against many cell-based antigens, including male-specific antigen, model antigens loaded into splenocytes, or tumor antigen transfected into cell lines 1 2 3 4. Many cell-based antigens have been proven to activate CD8+ CTLs through the cross-priming pathway, which involves bone marrow–derived APCs that take up exogenous antigens and present them via the MHC class I pathway 5 6. Thus, CD4+ T cells are also essential for in vivo cross-priming 3 4. However, only some viruses require CD4+ T cells to induce CD8+ CTL responses 7 8 9; others do not 10 11 12. Viruses that illicit helper-independent CTL responses likely produce high epitope densities, probably via infection of APCs leading to direct access of antigen to the MHC class I processing pathway.
The role of CD4+ T cells was originally thought to be the provision of cytokines such as IL-2 to facilitate activation, survival, and clonal expansion of CD8+ T cells 1. Recent reports have suggested an alternative model involving APC sensitization by CD4+ T cells, demonstrating that ligation of the TNFR family member, CD40, on APCs can completely replace the necessity for CD4+ help 4 13 14 15. Ridge et al. 14 showed that either ligation of CD40 with an agonist anti-CD40 antibody or incubation with antigen-specific CD4+ T cells can activate DCs to a state in which they are able to prime naive CD8+ T cells and activate memory CD8+ T cells. Schoenberger et al. 4 and Bennett et al. 15 used in vivo cross-priming models to demonstrate that ligation of CD40 can completely restore CD8+ CTL activities in CD4+ T cell–depleted mice. These results suggest that the dominant pathway by which CD4+ T cells assist in CD8+ CTLs is via the engagement of CD40 on the surface of APCs. To further dissect the mechanism by which CD4+ cells provide help for CD8+ T cell activation, we developed an in vitro system using dendritic cells (DCs) as APCs and influenza hemagglutinin (HA) class I and II peptide–specific TCR transgenic (Tg) T cells as CTL precursors (CTLs) and CD4+ helper cells, respectively. We also examined the pathways of CD4+ T cell help for priming CD8+ CTLs in vivo. Our findings demonstrate that in addition to CD40-dependent DC sensitization, CD40-independent DC sensitization and direct, lymphokine-dependent CD4+–CD8+ T cell communication represent important components of CD4+ help for priming of CD8+ CTLs.
Materials and Methods
Mice.
6–8-wk-old inbred BALB/c mice were obtained from the National Cancer Institute, National Institutes of Health (Frederick, Maryland). TCR Tg mice expressing an α/β TCR specific for amino acids 110–120 from influenza HA presented by I-Ed (6.5) were the gift of Dr. Harald von Boehmer, Harvard University, Boston, MA 16. TCR Tg mice expressing an α/β TCR specific for amino acids 518–527 from influenza HA presented by Kd (clone 4) were the gift of Dr. Linda Sherman, Scripps Research Institute, La Jolla, CA 17. Clone 4 mice were also crossed with Thy1.1+/+ BALB/c mice to obtain Thy1.1+/−TCR+/− mice. Tg mice used in these experiments were heterozygous for the transgene. CD40-deficient (CD40−/−) mice in BALB/c background were purchased from The Jackson Laboratory. All experiments involving the use of mice were performed in accordance with protocols approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
DC Preparation.
Bone marrow–derived DCs were prepared according to a previously described protocol 18. In brief, bone marrow was obtained from tibia and femurs by flushing media through. After lysis of red blood cells, cells were resuspended at 106 cells in RPMI 1640 supplemented with 5% FBS, l-glutamine (2 mM), penicillin/streptomycin (50 U/ml), 10% nonessential amino acids, and 50 mM 2-ME plus 1,000 U/ml of recombinant GM-CSF (provided by Immunex). 1 ml of cells was then plated per well in 24-well plates and incubated at 37°C, 5% CO2. Fresh GM-CSF–supplemented medium was added on days 2 and 4, and loosely adherent cells on day 6 were transferred from tightly adherent cells to 10-cm petri dishes. A second transfer was done on day 7, and cells were collected on day 8. To enrich day 6 DCs, these loosely adherent cells (some of them forming clumps) were loaded on the top of a 50% FBS gradient column in 15-ml conical tubes to allow cell clumps to sediment. After a 30-min incubation at 4°C, the top half of the column was removed and cells at the bottom part of the column were collected by centrifugation and used as day 6 DCs. About 50–70% of day 8 DCs express MHC IIhighB7-2high, and 20–30% express MHC IIlowB7-2low. The expression levels of these molecules are lower in day 6 DCs. Splenic DCs were isolated according to a standard protocol 19. In brief, spleens were digested with collagenase D (Boehringer Mannheim), splenocytes were centrifuged on BSA column (3220-75; Intergen), and low density splenocytes were obtained. 107 cells were plated into 60-mm tissue culture dishes and incubated at 37°C for 90 min. Nonadherent cells were thoroughly washed away, and remaining adherent cells were incubated at 37°C overnight. Detached nonadherent cells were then collected as splenic DCs. Almost all of the cells expressed MHC IIhigh, B7-2high, B220−.
Antigens.
The CT26 mouse colon carcinoma cell line was transfected with the full-length HA gene encoding HA protein of influenza virus A/PR/8/34 (CT26-HA). CT26-HA was grown as an adherent population under selection of G418 (400 μg/ml). About 75% of the cells express HA protein on the surface as shown by FACS® analysis. CT26-HA lysate was prepared according to a previously described protocol 20. In brief, cells were scraped off the flask and hypotonically lysed in 5P8 buffer (5 mM sodium phosphate, pH 8) for 10 min. Cells were sonicated and centrifuged at 5,000 rpm for 15 min to remove nuclei from the low-speed spin. The supernatant was collected and recentrifuged at 15,000 rpm for 1 h. The supernatant from this high-speed spin was discarded, and the pellet was resuspended in a volume equivalent to ∼3–4 × 107 cells/ml. To prepare recombinant HA protein, the full-length HA DNA was cloned into the pTrcHis vector (Invitrogen), and protein expression was induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG, 2 mM). HA protein was then purified using Qiagen Ni-NTA agarose kit and dialyzed in 1× PBS. HA110–120 peptide (class II) and HA518–527 peptide (class I) were synthesized by Macromolecular Resources and dissolved in 1× HBSS at a concentration of 1 mg/ml.
Viruses.
A recombinant vaccinia virus encoding HA (vac-HA) or nuclear protein (vac-NP, V69) from influenza virus A/PR/8/34 was a gift of Dr. Jack Bennick and Jonathan Yewdell, NIAID, National Institutes of Health. Viruses were expanded on Hu-TK− cells in the presence of 5-bromo-2′-deoxyuridine (Sigma Chemical Co.) at 25 μg/ml, purified from the cellular lysate by sucrose banding, and titered by plaque assay on B-SC-1 cells. 107 PFU of vac-HA or vac-NP were used for in vivo priming.
Enrichment of CD4+ and CD8+ T Cells.
Spleen and lymph nodes from either naive CD4+ TCR Tg mice (6.5), CD8+ TCR Tg mice (clone 4), or wild-type mice were collected, and single cell suspensions were prepared through nylon mesh. After lysis of red blood cells, cells were resuspended in 10 ml of 1× HBSS containing 2% FBS. To purify clone 4 CD8+ T cells, a mixture of biotinylated antibodies (5–10 μg/ml) against MHC class II (14.4.4; American Type Culture Collection), B220 (B220b; PharMingen), and CD4 (L3T4/MR4-4; PharMingen) was added to deplete MHC class II+ cells, B cells, and CD4+ T cells, respectively. To purify 6.5 CD4+ T cells, the biotinylated 14.4.4b, B220b, and anti-CD8 (ly-2; PharMingen) antibodies were added to deplete MHC class II+ cells, B cells, and CD8+ T cells. Cells were then incubated on a rocker at 4°C for 30 min and washed three times with 1× HBSS (2% FBS) at the end of the incubation. Cells were then mixed with 3–4 × 108 prewashed streptavidin beads (Dynal) in 50-ml conical tubes to a final volume of 10 ml, tubes were then rocked at 4°C for 1 h. At the end of the incubation, the tube was placed onto the magnet rack (Dynal) for 15 min at 4°C. This procedure was repeated for a second time on nonmagnetized cells. Twice-enriched CD4+ or CD8+ T cells were 85–94% pure, containing 33% (6.5+CD4+) and 100% (Vβ8.2+CD8+) clonotypic T cells, respectively, as shown by FACS® analysis.
Proliferation Assay.
3–5 × 104 DCs were placed in triplicate into 96-well U-bottomed plates, and cells were loaded with a serial dilution of tumor lysate or synthetic HA class I or II peptide for 6–12 h. After two to three washes with CTL medium (RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 50 U/ml penicillin/streptomycin, 10% nonessential amino acids, and 50 mM 2-ME), 3 × 104 purified naive Tg CD4+ T cells were added. 48 h after adding T cells, [3H]thymidine (1 μCi/well; Amersham Pharmacia Biotech) was added to each well. Cells were harvested 10–12 h later with a Packard Micromate cell harvester. [3H]Thymidine incorporation into DNA was measured as counts per minute on a Packard Matrix 96 direct beta counter.
Enzyme-linked Immunospot Assay.
A 96-well filtration plate (Millipore) was coated with 10 μg/ml of anti–IFN-γ antibody (PharMingen) overnight. The wells were washed with PBST (PBS containing 0.05% Tween 20) and blocked with culture medium for 1 h. 3 × 105 splenocytes from viral infected mice were plated into each well in the presence or absence of 1 μg/ml NP-Kd peptide and incubated at 37°C overnight. The plate was then washed with PBST to thoroughly remove splenocytes and incubated with 5 μg/ml biotinylated IFN-γ antibody (PharMingen) at 4°C overnight. After washing with PBST, 1 μg/ml of alkaline phosphatase–conjugated streptavidin (Jackson ImmunoResearch Labs) was added to each well and the plate was incubated at room temperature for 2 h. Spots were developed by adding 100 μl of BCIP/NBT solution (Sigma Chemical Co.) and incubating at room temperature for 10–20 min. Images of the spots were captured by CCD video camera and counted. The number of peptide-specific IFN-γ–positive spots was obtained by subtracting the number of spots in the no peptide group from the number of spots in the peptide group.
In Vitro CD4+ Helper System.
3–5 × 104 day 6–8 bone marrow–derived DCs or splenic DCs were first plated in triplicate in 96-well U-bottomed plates and then loaded with serial dilutions of either CT26-HA tumor lysate, recombinant HA protein, or synthetic HA class I peptide together with a constant amount of HA class II peptide (3.3 μg/ml). 3–5 × 104 cell equivalents of lysate were used for 1:1 dilution. After 6–12 h incubation, cells were washed two to three times with CTL medium, and 3–5 × 104 purified naive HA-specific Tg CD8+ T cells plus either 3–5 × 104 naive HA-specific Tg or control non-Tg CD4+ T cells were added to each well. After 3 d incubation at 37°C, 5% CO2, cells from each well were split into two V-bottomed wells. 51Cr-labeled P815 target cells, pulsed with HA class I peptide or unpulsed, were added to each of the two wells and incubated at 37°C for 4 h. Released 51Cr activity was then measured by gamma counter. Percent specific lysis was calculated as (sample cpm – spontaneous cpm)/(maximum cpm – spontaneous cpm) × 100. Percent peptide-specific lysis was calculated as (percent specific lysis of P815 cells pulsed with peptide) − (percent specific lysis of unpulsed P815 cells).
In Vivo CD4+ Helper System.
3 × 106 purified clonotypic CD8+ T cells (Thy1.1+/−) were transferred intravenously with or without 2.5 × 106 purified clonotypic 6.5 CD4+ T cells to BALB/c wild-type or CD40−/− BALB/c mice. 24 h later, mice were infected intraperitoneally with 107 PFU of vac-HA. 4 d after infection, spleens were removed and single cell suspensions were prepared. Cells were stained with PE-conjugated Thy1.1 and FITC-conjugated CD8 antibodies and analyzed by FACS®. Double-positive populations represent the clonotypic CD8+ T cells. The second approach involved in vivo depletion of CD4+ T cells in BALB/c wild-type or CD40−/− mice with 100 μg GK1.5 given intraperitoneally every other day for 1 wk followed by intraperitoneal infection with 107 PFU vac-NP. 7 d later, enzyme-linked immunospot (ELISPOT) assays for activated CD8+ T cells were performed as described above, and T cells were restimulated with NP-Kd peptide for an additional 6–7 d followed by 51Cr-release assay.
In Vitro Sensitization of DCs and Activation of CD8+ T Cells.
1–2 × 106 day 6 enriched DCs were plated in 24-well plates, and CT26-HA lysate was added (1–2 × 106 cell equivalents). 6 h later, 2 × 106 purified HA-specific or nonspecific CD4+ T cells were added and incubated for an additional 24–36 h. To remove CD4+ T cells from these cultures, cells were recovered from the 24-well plate and washed twice with 1× HBSS, and 10 μg/ml of biotinylated antibody against CD4 (L3T4 MR44-4; PharMingen) was added. Cells were incubated on a rocker at 4°C for 30 min, washed three times with 1× HBSS buffer, and mixed with streptavidin-conjugated beads (Dynal) previously washed with 1× HBSS. Cells were incubated on the rocker for 1 h and recovered by placing the tube on the magnet rack twice for 15 min each. Cells were stained with the same biotinylated antibody followed by PE-conjugated streptavidin to verify complete removal. Separated DCs were then incubated with 3 × 104 naive Tg HA-specific CD8+ T cells with or without supernatant from activated Tg CD4+ T cells for 3 d. Percent peptide-specific lysis was determined from a 4-h 51Cr-release assay. In parallel, 5 × 104 DCs were directly plated in triplicate into 96-well U-bottomed plates and loaded with CT26-HA lysate (5 × 104 cell equivalents) for 6 h. 5 × 104 purified naive Tg CD4+ T cells or control non-Tg CD4+ T cells were added to each well. After a 24-h culture, cells were washed and 3 × 104 HA-specific Tg CD8+ T cells were added and incubated for 3 d. Percent peptide-specific lysis was determined as above.
Flow Cytometric Analysis.
DCs were stained with antibodies against MHC class I (28-84S; American Type Culture Collection), MHC class II (14.44; American Type Culture Collection) followed by FITC-conjugated goat anti–mouse IgG2a (Southern Biotechnology), CD80/86 (CTLA-4Ig) followed by PE-conjugated goat anti–human IgG, CD40 (3/23; PharMingen) followed by PE-conjugated goat anti–rat IgG, or CD11c (biotinylated N418; PharMingen) followed by PE-conjugated streptavidin (Caltag). HA-specific CD4+ TCR Tg T cells (6.5) were double-stained with FITC-conjugated goat anti–mouse CD4 (Caltag) and biotinylated rat anti-clonotypic TCR antibody mAb 6.5 followed by PE-conjugated streptavidin (Caltag). HA class I–specific CD8+ TCR Tg T cells were stained with PE-conjugated Thy1.1 (PharMingen) and FITC-conjugated CD8 (PharMingen). Cells were collected on a FACScan™ (Becton Dickinson) and analyzed using CELLQuest™ software (Becton Dickinson).
Measurement of Cytokines.
IL-2 and IFN-γ were measured using an ELISA kit from R&D Systems.
Statistical Analysis.
Two-way analysis of variance (Student's t test) was used to evaluate the difference between clonal expansion of Thy1.1+CD8+ T cells in wild-type and CD40−/− mice transferred with or without 6.5 CD4+ Tg T cells.
Results
CD4+ T Cells Can Provide Potent Help for DCs to Cross-Prime and Direct-Prime CD8 T Cells.
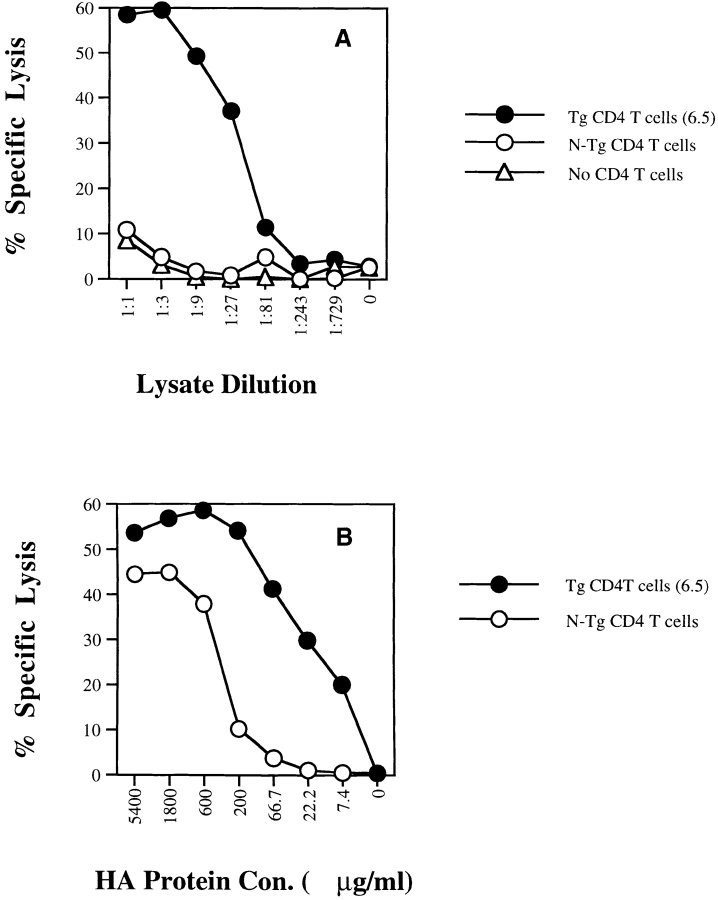
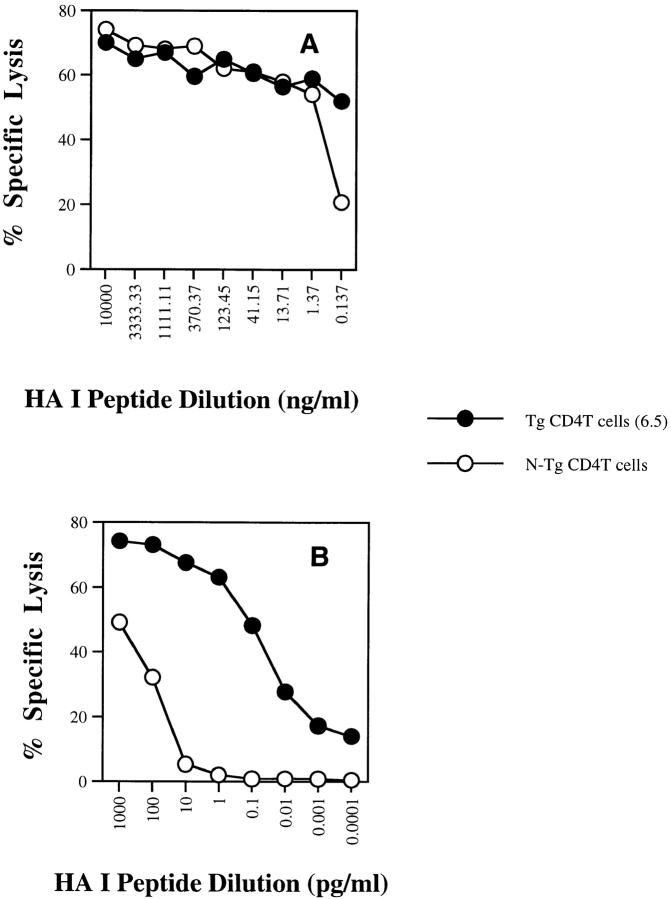
CD4+ T cells have been shown to be important for in vivo cross-priming 10. In vitro studies using antigens such as OVA have demonstrated CD4-independent cross-priming but only at excessive concentrations of antigen 21 22 23 24 25, although the efficiency of cross-priming may be increased by incorporation of antigen into apoptotic bodies 26 or complexing with heat shock proteins 27. To further study the role of CD4+ T cells in cross-priming, we established an in vitro system in which bone marrow–derived DCs were used as APCs and CD4+ and CD8+ TCR Tg T cells specific to influenza HA were used as T helper cells and CD8+ CTLs, respectively. When DCs were loaded with different concentrations of lysate from a tumor expressing HA (CT26-HA), antigen-specific CD4+ T cells (6.5) provided potent help activating CD8+ CTLs, even at relatively low antigen doses, whereas non-Tg CD4+ T cells failed to do so (Fig. 1 A). At high doses of HA in the form of lysate (>10:1 lysate/DC ratio by cell equivalents), CD4-independent CD8+ T cell activation was observed (data not shown), indicating that antigen-specific CD4+ T cell help shifted the antigen dose–response curve for CD8+ T cell activation ∼100-fold. Similar results were obtained when recombinant HA protein was used (Fig. 1 B). Similar phenomena can be demonstrated using splenic DCs (data not shown). To determine whether this effect is unique to cross-priming, we pulsed splenic DCs with serial dilutions of the minimal cognate MHC class I–restricted peptide (thereby bypassing antigen processing) and a constant amount of the cognate HA class II peptide in the presence or absence of antigen-specific CD4+ T cells (Fig. 2A and Fig. B). We found that in the range of 10 μg/ml to 1 ng/ml, DCs activate CD8+ CTLs at equivalent plateau levels with or without CD4+ help. However, at HA class I peptide concentrations <1 ng/ml, HA-specific T cell help significantly enhanced CD8+ T cell activation, shifting the dose–response curve 3 logs and allowing priming of naive CD8+ T cell activation in the femtomolar range of antigen. Not unexpectedly, direct infection of DCs with recombinant vac-HA, which produces high levels of MHC class I loading directly through the endogenous pathway, efficiently stimulated CD8+ T cells in the absence of CD4+ T helper cells (data not shown).
Figure 1.
Effect of CD4+ T cell help for cross-priming CD8+ CTLs in vitro. Bone marrow–derived DCs (day 6–8) were incubated with (A) tumor lysate of CT26 expressing influenza HA antigen (CT26-HA) or (B) recombinant HA protein for 6–12 h and then washed. Purified naive HA-specific CD4+ TCR Tg T cells (6.5) or non-Tg (N-Tg) CD4+ T cells were then added to DCs together with the same number of purified naive HA-specific CD8+ TCR Tg T cells. After a 3-d incubation, cells in each plate were split into two 96-well V-bottomed plates to which 51Cr-labeled target cells (P815+/− HA518–527 peptide) were added. After a 4-h incubation, 51Cr release was measured and percent peptide-specific lysis was determined as described in Materials and Methods.
Figure 2.
Effect of CD4+ T cell help for direct priming CD8+ CTLs in vitro. Splenic DCs were incubated with a constant amount of the cognate HA class II peptide and with serial dilutions of HA class I peptide over (A) a high dose range or (B) a low dose range for 6–12 h and then washed. Purified naive CD4+ TCR Tg T cells (6.5) or non-Tg (N-Tg) CD4+ T cells were then added to DCs together with the same number of purified naive CD8+ TCR Tg T cells. After a 3-d incubation, a 51Cr-release assay was performed and percent peptide-specific lysis was determined as in the legend to Fig. 1.
In Vitro CD4+ Help for CTL Activation Is CD40 Independent.
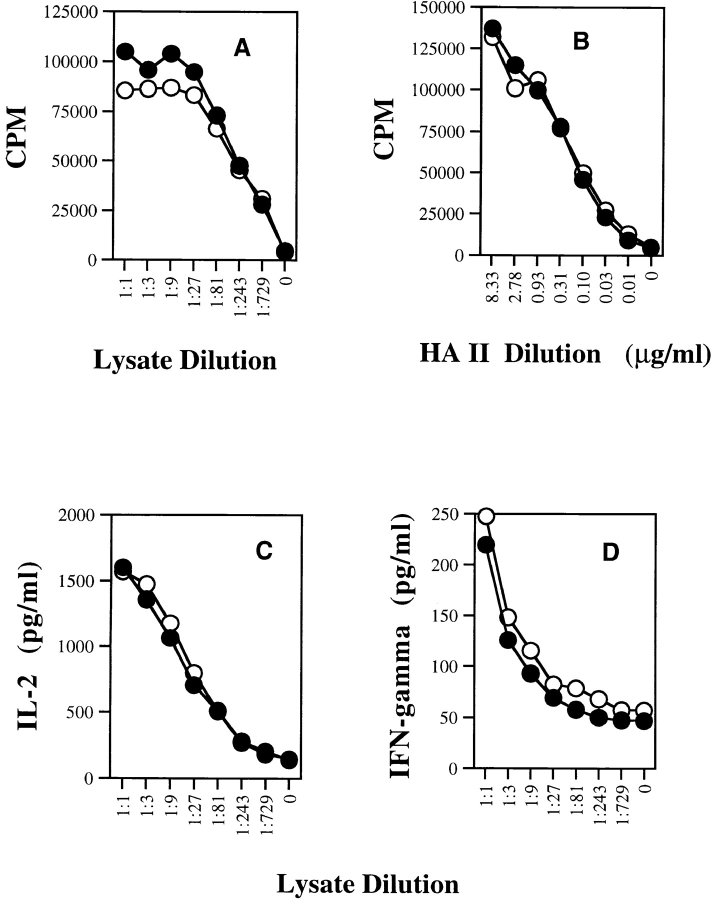
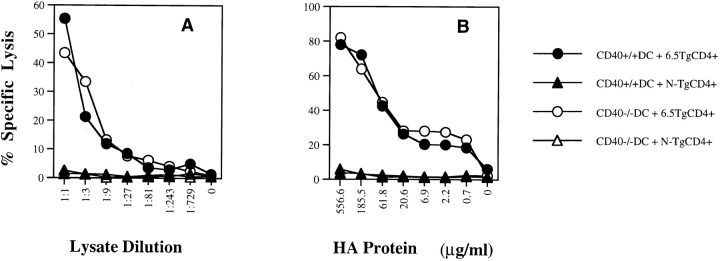
To address whether CD40 is important for in vitro CD4+ help, we first evaluated its necessity for activating naive Tg CD4+ T cells. We found that naive Tg CD4+ T cells were activated equivalently by wild-type and CD40−/− DCs as measured by antigen-specific proliferation or lymphokine release (Fig. 3). Naive Tg CD8+ T cells were also equivalently activated by wild-type and CD40−/− DCs pulsed with synthetic HA class I peptide (data not shown). Most importantly, CD4+ help for CTL activation was mediated equivalently by wild-type and CD40−/− DCs (Fig. 4). Furthermore, agonist anti-CD40 antibody failed to replace CD4+ T cells (data not shown). Therefore, the help for CD8+ CTL priming in this in vitro system was completely CD40 independent.
Figure 3.
Comparison of the ability of wild-type DCs and CD40−/− DCs to activate naive CD4+ T cells. Bone marrow–derived DCs from either BALB/c wild-type or BALB/c CD40−/− mice were incubated with (A) serial dilutions of CT26-HA lysate or (B) synthetic HA class II peptide for 6–12 h and then washed. Purified naive Tg HA-specific CD4+ T cells were then added to the DCs. Supernatant was collected after 48 h for lymphokine measurement, and cells were pulsed with [3H]thymidine (1 μCi/well) and harvested at 72 h. Thymidine incorporation was measured as counts per minute. The levels of (C) IL-2 and (D) IFN-γ in the supernatant were measured by ELISA.
Figure 4.
Comparison of wild-type DCs and CD40−/− DCs for their ability to cross-prime naive CD8+ T cells in vitro. Bone marrow–derived DCs (day 6) from either BALB/c wild-type or BALB/c CD40−/− mice were incubated with (A) serial dilutions of CT26-HA lysate or (B) recombinant HA protein for 6–12 h and then washed. Purified naive HA-specific CD4+ TCR Tg T cells (6.5) or non-Tg (N-Tg) CD4+ T cells were added to the DCs together with the same number of purified naive HA-specific CD8+ TCR Tg T cells. After a 3-d incubation, percent peptide-specific lysis was determined as in the legend to Fig. 1.
Evidence for CD40-independent CD4+ T Cell Help In Vivo.
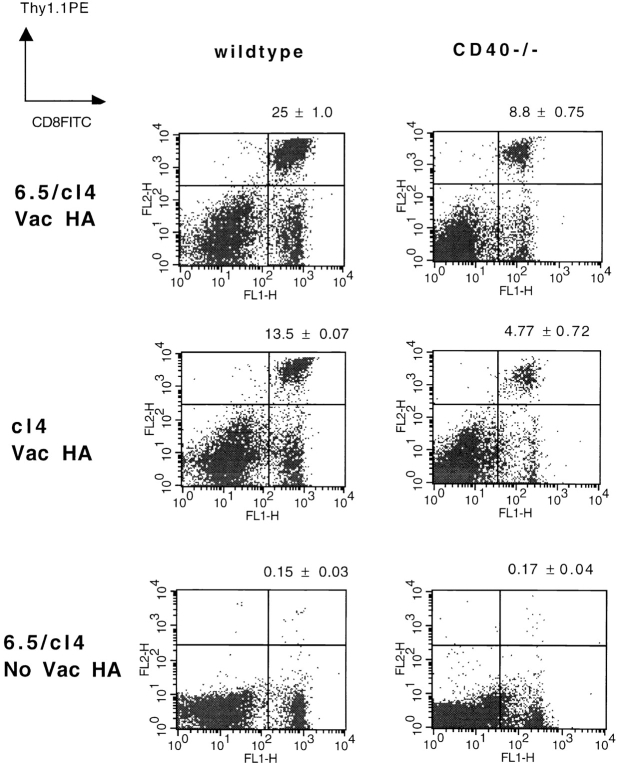
Given the clear in vitro evidence of CD40-independent help for CD8+ CTLs, we determined whether this could be demonstrated in vivo. We initially examined the response of adoptively transferred Tg T cells to infection with vac-HA (Fig. 5). The clonal expansion of HA-specific TCR Tg CD8+ T cells was analyzed in the presence or absence of adoptively transferred HA-specific Tg CD4+ T cells. In wild-type BALB/c mice, the presence of Tg HA-specific CD4+ T cells significantly augmented the clonal expansion of Tg HA-specific CD8+ T cells, indicating that CD4+ help for CD8+ T cell activation was indeed operative in this system. The same adoptive transfer experiments into CD40−/− BALB/c mice demonstrated two important outcomes. First, consistent with earlier reports, expansion of HA-specific CD8+ T cells was significantly reduced in CD40−/− animals relative to wild-type 9. Nonetheless, a significant CD4-dependent enhancement of HA-specific CD8+ T cell expansion was observed in the CD40−/− mice.
Figure 5.
Adoptively transferred antigen-specific CD4+ T cells can deliver help through the CD40-independent pathway in vivo. BALB/c wild-type or CD40−/− mice (Thy1.2+/+) were injected intravenously with 3 × 106 clonotypic HA-specific CD8+ T cells (Thy1.1+/−, cl4) with or without 2.5 × 106 HA-specific clonotypic CD4+ T cells (6.5). 24 h later, mice were infected intraperitoneally with 107 PFU of vac-HA or were left untreated. 4 d after infection, spleens were removed and cells were double-stained with PE-conjugated anti-Thy1.1 antibody and FITC-conjugated anti-CD8 antibody. The double-positive population represents expansion of transferred clonotypic CD8+ T cells. The numbers shown above each graph represent the mean ± SD of three independent determinations of percentages of Thy1.1+CD8+ cells. P < 0.01, 25 ± 1.0 vs. 13.5 ± 0.07% (wild-type); P < 0.005, 8.8 ± 0.75 vs. 4.77 ± 0.72% (CD40−/−).
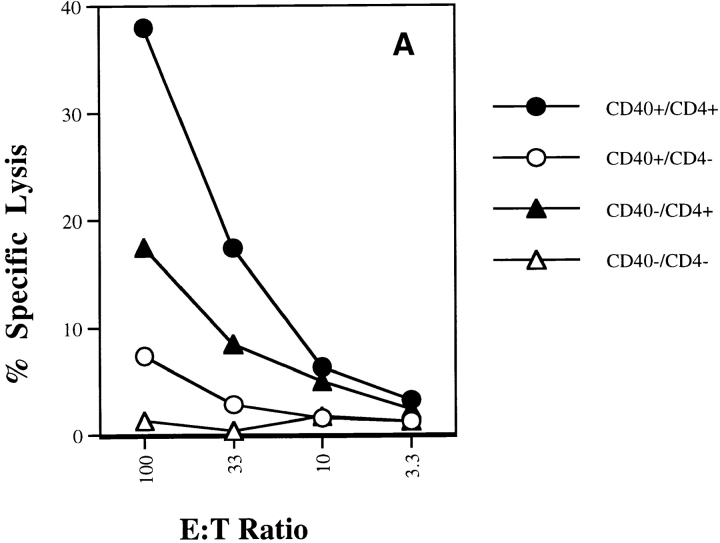
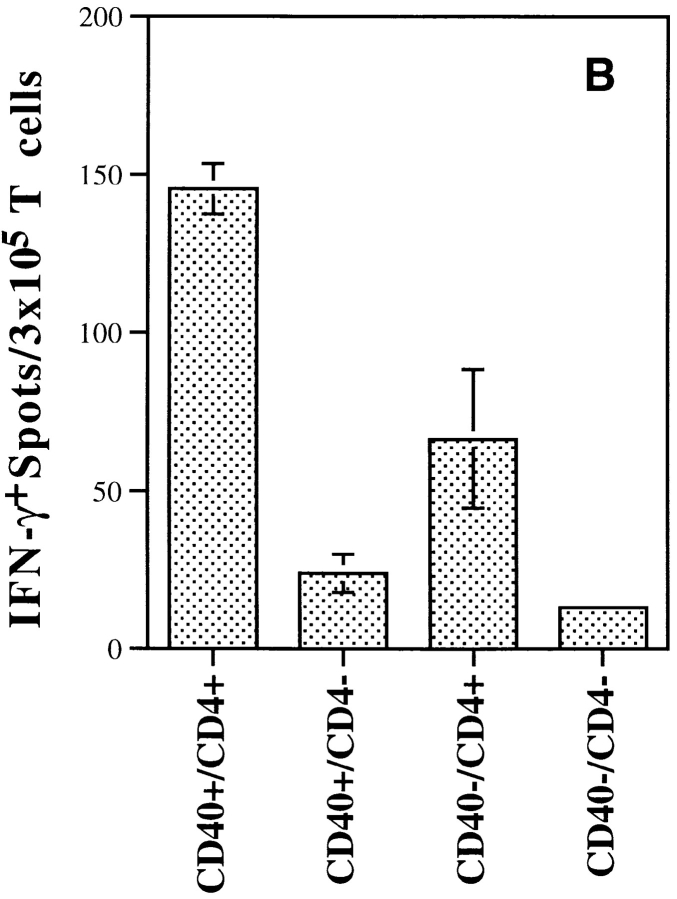
Similar results were observed in an examination of the endogenous T cell repertoire having normal antigen-specific T cell precursor frequencies. For these experiments, we switched to the influenza NP system because this antigen generates more easily measurable CTL responses. By using antibody depletion, we examined the contribution of CD4+ T cells to CTL generation in vac-NP–infected wild-type and CD40−/− mice. As shown in Fig. 6 A, we found that while wild-type mice (CD40+CD4+) produced the strongest Kd-restricted anti-NP CTL response, the presence of CD4+ T cells in CD40−/− mice (CD40−CD4+) still provided significant help for CTL induction. The same pattern was also seen using IFN-γ ELISPOT assay, which provides a more quantitative measure of antigen-specific CD8+ T cell frequencies (Fig. 6 B). Taken together, these data strongly indicate that CD4+ T cells provide significant help for induction of CTLs in vivo in the absence of CD40.
Figure 6.
Endogenous CD4+ T cells can also deliver help through a CD40-independent pathway in vivo. BALB/c wild-type or CD40−/− mice were either injected intraperitoneally with antibody to CD4 (GK1.5) every other day for 1 wk or left untreated. After complete depletion of CD4+ T cells was confirmed by FACS®, the mice were infected with 107 PFU of vac-NP. (A) NP-specific CTL analysis. 7 d after infection, spleens were removed and splenocytes were restimulated with NP-Kd peptide for 5–6 d. A standard 4-h 51Cr-release assay was performed. Percent specific lysis is shown. Background lysis in the absence of peptide was <1% for all E/T ratios shown. Data shown are from an experiment representative of three independent experiments. (B) NP-specific IFN-γ ELISPOT analysis. Splenocytes were restimulated with or without 1 μg/ml of NP-Kd peptide in a 96-well filtration plate previously coated with anti–IFN-γ antibody. After overnight stimulation, the wells were washed and biotinylated IFN-γ was added. After overnight incubation at 4°C, streptavidin-conjugated alkaline phosphatase was plated into each well and followed by addition of the substrate solution BCIP/NBT. Peptide-specific IFN-γ–positive spots are presented with the background subtracted.
CD4+ T Cells Deliver CD40-independent Help through Sensitizing DCs and through Direct Lymphokine-mediated CD4–CD8 Communication.
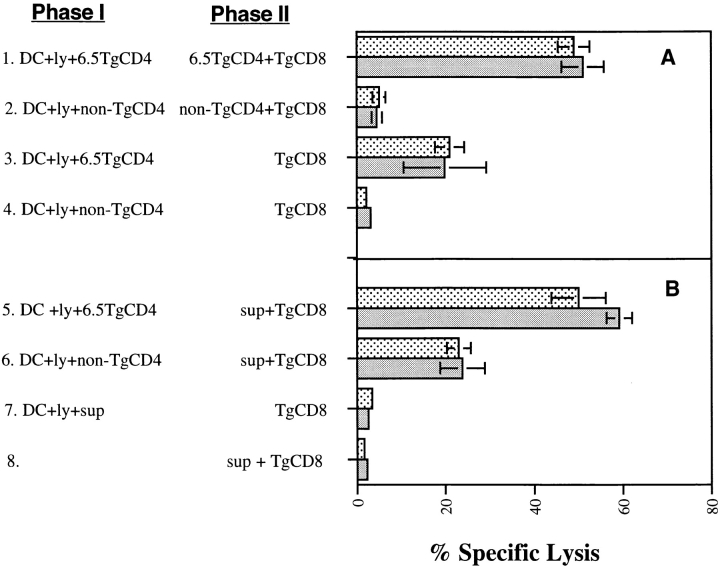
The results obtained so far demonstrate a CD40-independent component of T cell help for CD8+ CTL activation. We wished to examine whether this pathway was mediated through an enhanced capacity of DCs to activate CD8+ T cells (“sensitization”), a direct effect of the products of CD4+ T cell activation on the CD8+ T cell response, or a combination of the two. This distinction was achieved by modifying our in vitro system to separate the CD4+ T cell activation phase (phase I) from the CD8+ T cell activation phase (phase II). Enriched day 6 bone marrow–derived DCs were pulsed with a cell lysate of CT26-HA and incubated with HA-specific CD4+ T cells or with an equal number of control non-Tg CD4+ T cells. 24–36 h later, CD4+ T cells were either removed (by magnetic separation; see Materials and Methods) or left in place. After magnetic separation, contaminating CD4+ T cells were undetectable by FACS® analysis or by proliferation ([3H]thymidine incorporation) in response to pulsing DCs with excess HA class II peptide (data not shown). After CD4+ T cell removal, DCs were washed free of lymphokines released during the phase I culture period, and were then incubated with purified HA-specific CD8+ T cells for 3 d, at which time HA-specific lysis was measured. We found that only those DCs cultured with antigen-specific CD4+ T cells were able to activate CD8+ T cells, even after CD4+ T cells were removed (Fig. 7 A, groups 3 and 4). Thus, a major component of CD40-independent help for CTL priming involves DC sensitization. However, the magnitude of CTL activity induced by those DCs was greatly reduced compared with that generated by DCs left in the presence of HA-specific CD4+ T cells (Fig. 7 A, groups 1 and 2). This suggests either that DC sensitization wanes if CD4+ T cell help is removed, or that signals provided by activated CD4+ T cells directly augment CTL activation as reflected in the maximal CTL response observed when antigen-specific CD4+ and CD8+ T cells are present simultaneously.
Figure 7.
Dissecting routes of CD4+ T cell help for priming CD8+ T cells. (A) Bone marrow DCs (day 6, enriched) were incubated with CT26-HA lysate in either a 24-well plate or a 96-well U-bottomed plate for 6 h and washed. Purified 6.5 Tg CD4+ T cells or non-Tg CD4+ T cells were then added to the DCs. After a 24–36-h incubation, supernatant in the 24-well plate was saved and CD4+ T cells were removed from DCs by Dynal magnetic beads (see Materials and Methods). Cells in the 96-well plate were washed, and CD4+ T cells were left in place. Nonseparated DCs (groups 1 and 2) or separated DCs (groups 3 and 4) were then cultured with purified naive HA-specific Tg CD8+ T cells in a 96-well U-bottomed plate for 3 d. Percent peptide-specific lysis was determined as in the legend to Fig. 1. (B) Separated DCs from CD4+ T cells were cultured with HA-specific Tg CD8+ T cells for 3 d in the presence of supernatant (100 μl) from activated CD4+ T cell culture (groups 5 and 6). Alternatively, supernatant was also added to DC cultures instead of CD4+ T cells in phase I, and then washed twice before being cultured with naive CD8+ Tg T cells (group 7). Supernatant was also incubated with naive Tg CD8+ T cells (group 8). After a 3-d incubation, percent peptide-specific lysis was determined as in the legend to Fig. 1.
We next examined the extent to which supernatant from activated HA-specific CD4+ T cells could replace the CD4+ T cells themselves in providing help for CTL activation (Fig. 7 B). We first measured lymphokine profile in the supernatant collected from overnight culture of HA antigen–pulsed DCs and 6.5 Tg CD4+ T cells. A significant amount of IFN-γ (150–200 pg/ml) and IL-2 (900–1,500 pg/ml) was detected, whereas the levels of TNF-α, IL-4, and IL-12 were not measurable (data not shown). The supernatant was added either to DCs in the DC sensitization phase (phase I) or to the DCs after separation from CD4+ T cells in the CD8+ T cell activation phase (phase II). Although supernatant could not substitute for CD4+ T cells in the sensitization of DCs (Fig. 7 B, group 7), its addition to the culture during the period of CD8+ T cell activation restored the level of CTL killing to that observed when CD4+ T cells were present throughout (Fig. 7 B, groups 5 and 6). Interestingly, supernatant also enhanced the extent of CTL activation achieved by unconditioned DCs (Fig. 7 B, group 6). There was no effect on CD8+ T cells alone (Fig. 7 B, group 8), indicating that the lymphokine effect represented a true TCR (signal 1)–linked costimulatory signal. Notably, CD40−/− DCs responded similarly to wild-type DCs. Therefore, these data indicate that lymphokine-mediated CD4+–CD8+ T cell communication represents a component of CD40-independent help for CTL activation that acts independently of the DC sensitization component.
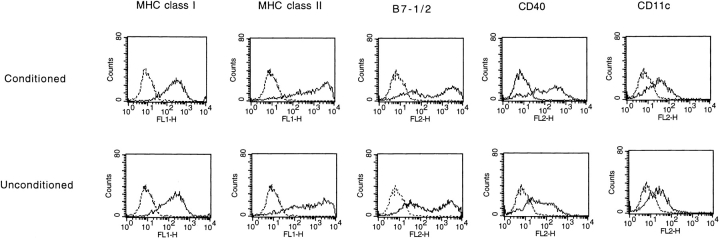
Characteristics of Surface Molecules of Conditioned DCs.
Since CD4+ T cell–conditioned DCs demonstrate an enhanced ability to activate antigen-specific CD8+ T cells, it was of interest to characterize expression of cell surface molecules known to be involved in T cell activation. Compared with unconditioned DCs, the levels of MHC class I, class II, B7-1/2, and CD11c were not significantly changed on DCs conditioned for 24 h with CD4+ T cells (Fig. 8). There was a modest upregulation of CD40. Given that CD4+ T cell conditioning of DCs in vitro is CD40 independent (see above), it is therefore likely that other molecules account for the enhanced ability of CD4+ conditioned DCs to activate CD8+ CTLs.
Figure 8.
Characteristics of cell surface markers of conditioned versus unconditioned DCs. DCs separated from a 24-h coculture with HA-specific CD4+ T cells (Conditioned) or HA-nonspecific CD4 T cells (Unconditioned) were stained for the indicated cell surface molecules: MHC class I, MHC class II, CD80/CD86, CD40, and CD11c. Solid line, primary antibody plus FITC- or PE-labeled secondary antibody. Broken line, isotype control primary antibody plus FITC- or PE-labeled secondary antibody.
Discussion
The importance of CD4+ help for CTL priming in vivo has been well established 1 2. Recent work has further documented the importance of CD4+ T cells for in vivo cross-priming 3, antitumor immunity 28, and tolerance induction 29. Evidence points to bone marrow–derived APCs as the critical cells that process and present antigen to naive CD4 and CD8 T cells in lymphatic tissue such as spleen and draining lymph nodes 6 30. Among bone marrow–derived APCs, DCs have proven to be the most potent APCs because of their unique characteristic features: very high MHC class II expression, costimulatory molecules B7-1/2, and the ability to capture antigen at an immature stage and efficiently present to T cells at a mature stage 31 32.
Despite the availability of such potent APCs in vivo, it is not well understood why generation of a CD8+ CTL response against many cell-based antigens requires additional help from CD4+ T cells. In vitro isolation of DCs from spleen or bone marrow in the presence of GM-CSF makes it possible to study these unique APCs in vitro 18 19. Early work showed that bone marrow–derived DCs or macrophages did not need CD4+ T cell help to cross-present model antigens such as OVA 21 22 23 24, but only in the presence of excessive concentration of antigen (>0.5 mg/ml). Indeed, we were able to confirm this CD4-independent CD8+ T cell activation in our current in vitro system, which employs DCs as APCs and TCR Tg CD4+ and CD8+ T cells as helper and CTL responder cells, respectively (Fig. 1). Most importantly, we observed potent help from antigen-specific CD4+ T cells for CTL activation when antigen is limiting. Similar enhancement by CD4+ help can also be seen when minimal class I peptide is presented by DCs together with a constant amount of HA class II peptide. Thus, it appears that help for CD8+ T cell activation is not an all-or-none phenomenon but rather shifts the antigen dose–response curve. Taking this into account in a viral infection, it is possible that the need for CD4+ T cell help for CTL activation depends on the level of the particular viral antigen expressed in vivo. Thus, viral infections that lead to a high epitope density on bone marrow–derived APCs can lead to helper-independent activation of CD8+ CTLs analogous to the high end of the antigen dose–response curves in our in vitro system.
The mechanism of CD4+ help for CTL activation was originally felt to occur via provision of lymphokines 1. This model was recently revised by studies that demonstrated that the dominant T component of CD4+ T cell help is mediated through CD40 engagement on APCs 4 13 14 15. CD40 ligation has been shown to result in activation and maturation of APCs 33 34, which are then thought to be responsible for their ability to drive a full activation of naive CD8+ T cells without need for direct CD4+–CD8+ T cell communication 13. Development of in vitro systems to study complex cell–cell interactions such as the CD4–DC–CD8 interaction involved in helper cell facilitation of CTL priming is critical in dissecting the molecular mechanisms that mediate cellular functions. The in vitro system we describe here indeed confirmed the importance of helper cell sensitization of DCs central to the “temporal bridge” model, but revealed a surprisingly important role for a CD40-independent pathway. Thus, DCs from CD40−/− mice were equally capable of being sensitized by CD4+ helper cells as wild-type DCs, as assayed by their ability to activate naive CD8+ CTLs. The inability of agonist anti-CD40 antibodies to replace any of this helper cell function further suggests that CD40-independent helper cell sensitization for DCs represents a major pathway. This in vitro finding led us to develop systems to directly evaluate the role of CD40-dependent versus CD40-independent pathways of help for CTL activation in vivo. Two different in vivo systems, one using adoptively transferred marked Tg T cells and one evaluating responses mediated by the endogenous repertoire, revealed a significant CD40-independent pathway of help for CTL activation in addition to the CD40-dependent pathway. Therefore, we believe that dissection of the CD40-independent pathways in the in vitro system will yield important information relevant to in vivo CTL priming.
That there are multiple CD4 signals for DC activation is not surprising, given the precedent set by CD4 activation of other MHC class II+ immune cells such as B cells and macrophages. B cells receive both CD40-dependent and lymphokine-mediated signals from CD4+ T cells during antigen-dependent differentiation 35. In our preliminary experiments, supernatants from activated CD4+ cells failed to replace CD4+ help in the DC activation phase of the in vitro system, suggesting that other membrane–membrane receptor ligand interactions may constitute the CD40-independent component. Recent in vivo experiments on the importance of the TNF-related activation induced cytokine (TRANCE)–TRANCE receptor interaction in mediating CD40-independent immune responses to certain viral infections 36 37 indeed suggest that this novel TNF–TNFR family pair may be a promising candidate for the CD40-independent pathway of CD4-dependent DC activation revealed in our in vitro system. It is important to note that the failure to replace CD4+ help by supernatant from activated CD4+ T cells does not absolutely rule out the involvement of lymphokines in the DC activation process. It is quite possible that the lymphokine concentrations in supernatant collected from DC–CD4+ T cell cultures may not be high enough to mediate effects generated by directed secretion from a CD4 cell that is engaged with a DC.
An as yet unresolved question raised by our experiments is whether the CD4+ T cell effect on DC activation of CD8+ CTLs occurs at the level of antigen processing, costimulation, or both. The 2-log dose–response curve shift when antigen was provided to DCs as cell lysate initially suggested that CD4 sensitization of DCs may enhance processing through the cross-priming pathway. However, the significant dose–response curve shift seen when antigen is provided as minimal class I peptide (thereby bypassing antigen processing), together with the absence of an increase in overall surface MHC class I levels, indicates that an important component of DC sensitization involves expression of costimulatory molecules capable of enhancing CD8+ T cell priming. Importantly, the finding that CD4+ T cell activation of DCs fails to increase B7 expression and IL-12 but is important for the overall enhanced CTL activity indicates that another as yet unidentified costimulatory molecule(s) plays an important role in DC activation of CD8 CTLs.
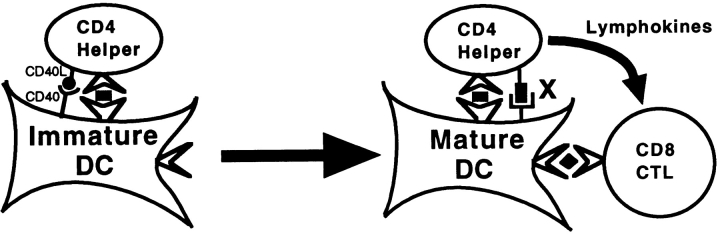
Another component of CD4+ help for CTLs revealed by the in vitro system represents direct CD4+–CD8+ T cell communication as demonstrated by a decrease in help for CD8+ T cell priming observed when the CD4–DC and DC–CD8 phases were separated. In contrast to the DC activation process, this component could be replaced by supernatant from DC-activated CD4+ T cells. Based on these results, we propose an integrated model of CD4+ help for CD8+ CTL activation as shown in Fig. 9. In addition to the signal of CD40-dependent DC sensitization, our data indicate two additional signals: CD40-independent DC sensitization and direct CD4+–CD8+ T cell communication via lymphokines. However, it is not clear how these three signaling components are interacting with each other. Based on the fact that viral infection can sensitize DCs without CD4+ T cells 14, the findings that CD4+ T cells still provide significant help for the viral-induced CTL responses in the absence of CD40 reported here and elsewhere 9 suggest that there are two different pathways of CD4+ help. One is the CD40-dependent pathway in which CD4+ T cells sensitize and mature DCs through engagement of CD40 (primary CD4+ help), and another is the CD40-independent pathway, which takes place after DC activation (secondary CD4+ help). While virus can bypass the primary CD4+ help, cell-based antigen requires CD4+ T cells or anti-CD40 agonist antibody to cross-link CD40 on DCs for the initial activation 4 15. The complete replacement of CD4+ T cells for induction of CTLs in vivo by cross-linking of CD40 on APCs seems to exclude requirement of any secondary CD4+ help such as secreting lymphokines 4 15. However, it is quite possible that these systems used antigen at a high antigen dose range, which may allow CD40-activated DCs to sufficiently drive CD8+ T cell activation without secondary CD4+ help, similar to what was observed in vitro when a high antigen dose was provided. The effect of antigen dose on the antigen presentation to CD8+ T cells was also documented in other in vivo systems 38 39. Interestingly, our in vitro system demonstrated complete independence of CD40 signaling, suggesting that in vitro–cultured DCs may have differentiated past the initial CD40-dependent activation stage and are therefore subject to CD40-independent secondary CD4+ help.
Figure 9.
Integrated model of the delivery of CD4+ help to CD8+ CTLs. The multistep model shows activated CD4+ T cells interacting first with immature DCs via the CD40–CD40L pathway, thereby inducing maturation to a state of enhanced ability to present to CTLs. Mature DCs can be further acted upon by CD4 T cells through a CD40-independent pathway (designated X) to boost their ability to activate CD8 T cells. At this stage, direct CD4–CD8 communication via lymphokines further enhances CD8 activation.
Ridge et al. 14 have argued that a direct CD4–CD8 signaling pathway of CD4+ help for CTL activation would be inefficient, since it would require that CD4+ and CD8+ cells contact the same APC at the same time. However, given recent evidence that DC–T cell interactions can be extremely long-lasting (hours to days), the idea that antigen-specific CD4+ T cells and CD8+ T cells may engage the same DC for an overlapping segment of time is in fact plausible. Ultimately, direct demonstration of the temporal proximity of this three cell interaction in vivo must be demonstrated to verify this notion experimentally. The ability to adoptively transfer marked antigen-specific CD4+ and CD8+ T cells, together with new techniques of in vivo imaging, makes this an experimentally tractable question.
Acknowledgments
We would like to thank Dr. Ephriam Fuchs and Dr. Antony Rosen for their critical review of this manuscript. H.I. Levitsky is a Scholar of the Leukemia Society of America.
This work was supported by gifts from the Hanford family, the Topercer family, Dorothy Needle, and the Janney fund, and by National Institutes of Health, National Cancer Institute grant R01 CA57842 and National Institutes of Health Public Health Service grants CA78658 and CA15396.
Footnotes
Abbreviations used in this paper: DC, dendritic cell; ELISPOT, enzyme-linked immunospot; HA, hemagglutinin; NP, nuclear protein; Tg, transgenic; vac, vaccinia virus.
E. Sotomayor's current address is H. Lee Moffit Cancer Center and Research Institute, University of South Florida, Tampa, FL 33612.
References
- Keene J.A., Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J. Exp. Med. 1982;155:768–782 . doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husmann L.A., Bevan M.J. Cooperation between helper T cells and cytotoxic T lymphocyte precursors. Ann. NY Acad. Sci. 1988;532:158–162. doi: 10.1111/j.1749-6632.1988.tb36335.x. [DOI] [PubMed] [Google Scholar]
- Bennett S.R.M., Carbone F.R., Karamalis F., Miller J.F.A.P., Heath W.R. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberger S.P., Toes R.E.M., van der Voort E.I.H., Offringa R., Melief C.J.M. T cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Bevan M.J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A.Y., Golumbek P., Ahmadzadeh M., Jaffee E., Pardoll D., Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- Cardin R.D., Brooks J.W., Sarawar S.R., Doherty P.C. Progressive loss of CD8+ T cell–mediated control of a γ-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp R.A., Sarawar S.R., Doherty P.C. Characteristics of the influenza virus-specific CD8+ T cell response in mice homozygous for disruption of H-2I-Ab gene. J. Immunol. 1995;155:2955–2959. [PubMed] [Google Scholar]
- Ruedl C., Kopf M., Bachmann M.F. CD8 T cells mediate CD40-independent maturation of dendritic cells in vivo. J. Exp. Med. 1999;189:1875–1884. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Mo X.Y., Hyland L., Doherty P.C. Host response to Sendai virus in mice lacking class II major histocompatibility complex glycoproteins. J. Virol. 1995;69:1429–1434. doi: 10.1128/jvi.69.3.1429-1434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R.M., Holmes K.L., Hugin A., Frederickson T.N., Morse H.C., III. Induction of cytotoxic T cell responses in vivo in the absence of CD4 helper cells. Nature. 1987;328:77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- Rahemtulla A., Fung-Leung W.P., Schilham M.W., Kundig T.M., Sambhara S.R., Narendran A., Arabian A., Wakeham A., Paige C.J., Zinkernagel R.M. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Immunology. Licence to kill. Nature. 1998;393:413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- Ridge J.P., Di Rosa F., Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Bennett S.R., Carbone F.R., Karamalis F., Flavell R.A., Miller J.F., Heath W.R. Help for cytotoxic-T cell responses is mediated by CD40 signaling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Kirberg J., Baron A., Jakob S., Rolink A., Karjalainen K., von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex–restricted receptor. J. Exp. Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.J., Liblau R., Scott B., Fleck S., McDevitt H.O., Sarvetnick N., Lo D., Sherman L.A. CD8+ cell-mediated spontaneous diabetes in neonatal mice. J. Immunol. 1996;157:978–984. [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;173:889–897. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiggard W.J., Nonacs R.M., Witmer-Pack M.D., Steinman R.M., Inaba K. Enrichment of dendritic cells by plastic adherence and EA rosetting Coligan J.E., Kruisbeek A.M., Marguiles D.H., Shevach E.M., Strober W. Current Protocols in Immunology 1998. John Wiley & Sons; New York: 3.7.1. [Google Scholar]
- Tse C.M., Levine S.A., Yun C.H., Khurana S.M., Donowitz M. Na+/H+ exchanger-2 is an O-linked but not an N-linked sialoglycoprotein. Biochemistry. 1994;33:12954–12961. doi: 10.1021/bi00248a003. [DOI] [PubMed] [Google Scholar]
- Moore M.W., Carbone F.R. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M., Rock K.L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- Norbury C.C., Hewlett L.J., Prescott A.R., Shastri N., Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Shen Z., Reznikoff G., Dranoff G., Rock K.L. Cloned dendritic cells can present exogenous antigen on both MHC class I and class II molecules. J. Immunol. 1997;158:2723–2730. [PubMed] [Google Scholar]
- Pfeifer J.D., Wick M.J., Roberts R.L., Findlay K., Normark S.J., Harding C.V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Suzue K., Zhou X., Eisen H.N., Young R.A. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc. Natl. Acad. Sci. USA. 1997;94:13146–13151. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung K., Hayashi R., Lafond-Walker A., Lowenstein C., Pardoll D.M., Levitsky H. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerder S., Matzinger P. A fail-safe mechanism for maintaining self-tolerance. J. Exp. Med. 1992;176:553–564. doi: 10.1084/jem.176.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler A.J., Marsh D.W., Yochum G.S., Guzzo J.L., Nigam A., Nelson W.G., Pardoll D.M. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow–derived antigen-presenting cells. J. Exp. Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Sallusto F., Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Caux C., Massacrier C., Vanbervliet B., Dubois B., Kooten C.V., Durrand I., Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Scheidegger D., Palmer-Lehmann K., Lane P., Lanzavecchia A., Albert G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacityT–T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway, C., and P. Travers. 1997. The humoral immune response. In Immunobiology. Garland Publishing, Inc., New York. 2-1–2-32.
- Green E.A., Flavell R.A. TRANCE-RANK, a new signal pathway involved in lymphocyte development and T cell activation. J. Exp. Med. 1999;189:1017–1020. doi: 10.1084/jem.189.7.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Wong E.R., Josien R., Steinman R.M., Oxenius A., Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand–independent T helper cell activation. J. Exp. Med. 1999;189:1025–1031. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Miller J.F.A.P., Subramaniam R.M., Carbone F.R., Heath W.R. Major histocompatibility complex class I–restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J. Exp. Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.J., Kreuwel H.T.C., Sherman L.A. Antigen concentration and precursor frequency determine the rate of CD8+ T cell tolerance to peripherally expressed antigens. J. Immunol. 1999;163:723–727. [PubMed] [Google Scholar]