Abstract
Germinal centers are critical for affinity maturation of antibody (Ab) responses. This process allows the production of high-efficiency neutralizing Ab that protects against virus infection and bacterial exotoxins. In germinal centers, responding B cells selectively mutate the genes that encode their receptors for antigen. This process can change Ab affinity and specificity. The mutated cells that produce high-affinity Ab are selected to become Ab-forming or memory B cells, whereas cells that have lost affinity or acquired autoreactivity are eliminated. Normally, T cells are critical for germinal center formation and subsequent B cell selection. Both processes involve engagement of CD40 on B cells by T cells. This report describes how high-affinity B cells can be induced to form large germinal centers in response to (4-hydroxy-3-nitrophenyl) acetyl (NP)-Ficoll in the absence of T cells or signaling through CD40 or CD28. This requires extensive cross-linking of the B cell receptors, and a frequency of antigen-specific B cells of at least 1 in 1,000. These germinal centers abort dramatically at the time when mutated high-affinity B cells are normally selected by T cells. Thus, there is a fail-safe mechanism against autoreactivity, even in the event of thymus-independent germinal center formation.
Keywords: quasimonoclonal mice, (4-hydroxy-3-nitrophenyl)acetyl–Ficoll, germinal centers, CD40 ligation, thymus independent
Introduction
Germinal centers are characteristically formed in response to protein-based antigens, and this process is dependent on T cell help and does not occur in athymic nude mice 1. Thymus-independent antigens, such as the antigen formed by haptenating the polysaccharide Ficoll, elicit strong Ab responses in both nude and euthymic mice 2, but typically do not induce germinal centers. Sporadic germinal centers do appear in congenitally athymic rodents and mice lacking all T cells 3 4, but the basis for their production is unclear. In addition, some polysaccharide antigens have been associated with germinal centers, but these have been found to depend on T cells for their formation 5 6.
Ligation of the B cell receptor (BCR) by antigen and T cell help provides distinct signals that are critical in determining B cell fate and differentiation during immune responses. When B cells bind protein-based antigen, they become highly efficient at seeking out and interacting with primed T cells 7 8. After receiving T cell help, B cells proliferate in either follicles or extrafollicular sites 8 9. In extrafollicular foci, B cells express syndecan-1 (CD138 10) and secrete Ab as they grow; they do not mutate their Ig genes 11. In follicles, small numbers of precursors upregulate expression of a ligand for peanut agglutinin (PNA [12]) and undergo massive rapid clonal expansion in forming germinal centers 7. When the center of the follicle is filled, the B blasts differentiate into centroblasts, which remain as a nonexpanding population as their progeny differentiate into nonproliferating centrocytes 7. The latter appear to be the target for selection. The factors that promote exponential growth, hypermutation, and selection are still not well understood. Blocking CD40 ligation with protein antigens around the time of immunization inhibits germinal center formation 13 14, and blockade applied to established germinal centers causes their dissolution 14. The finding that B cells in certain circumstances may express CD40 ligand 15 16 has raised the possibility that this effect is not due to interference in T cell–B cell interactions. In this study, we show that in exceptional circumstances germinal centers can be formed reproducibly in the absence of T cells. Although this does not represent the normal way germinal centers are induced, it does allow us to determine if T cell–derived signals are indispensable at particular stages.
The model for T cell–independent germinal center formation described in this report uses mice with transgenic BCRs that have high affinity for the hapten (4-hydroxy-3-nitrophenyl)acetyl (NP), quasimonoclonal (QM) mice 17, and their response to the thymus-independent type 2 antigen, NP-Ficoll. NP-Ficoll characteristically does not induce germinal centers in normal mice 18 19 20 21. QM mice carry a rearranged VDJ IgH chain V region in the J4 H chain locus 17. Expression of this transgene in combination with λ L chains results in BCRs with high affinity for NP. 60% of peripheral B cells in these mice are specific for NP, and other specificities result from diversification of the repertoire by somatic mutation and V gene replacements 22. Consequently, the mice remain immunocompetent 23 and form small non–NP-specific germinal centers in the absence of immunization, presumably in response to environmental antigens. Immunization of these mice intraperitoneally with NP-Ficoll causes an impressive extrafollicular Ab response, and also induces large germinal centers in all splenic follicles. This report investigates the way these germinal centers are induced, and shows that T cells are not required until the stage when centrocytes are normally selected.
Materials and Methods
Animals and Experimental Procedures.
All mice were bred and maintained in specific pathogen-free conditions at the University of Birmingham. QM mice were generated on a 129 background 17 and backcrossed onto C57BL/6. To study the effects of CD80/CD86 signaling on germinal center formation, QM mice were crossed with CTL antigen 4 (CTLA-4)–human γ1 (Hγ1) mice with the same C57BL/6 background 24. Approximately 50% of the F1 mice from this mating have the transgene as assessed by hemagglutination for Hγ1 in the serum. Nontransgenic littermates were used as controls. These transgenic mice were provided by Dr. Peter Lane of the Center for Immune Regulation (Birmingham, UK).
C57BL/6 mice were obtained from Harlan, Ltd. (QM × C57BL/6)F1 recipients and congenic littermate controls that lacked the VDJ 17.2.25 insertion (JH +/−, κ+/−, λ+/+) were used for cell transfer experiments. Mice were immunized between 6 and 16 wk of age. Unless otherwise stated, immunizations were by intraperitoneal injection with 30 μg NP-Ficoll or 50 μg alum-precipitated chicken γ-globulin (Sigma Chemical Co.), plus 109 heat-inactivated Bordetella pertussis bacteria. In the CD40 blocking experiments, mice received either 250 μg of the anti–CD40 ligand mAb MR1 (American Type Culture Collection [13]) intraperitoneally, or hamster IgG as a control (Pierce Chemical Co.), on days 0.5, 1, and 3 after immunization. To label cells in S phase of the cell cycle, mice were given 2 mg of 5′-bromo-2-deoxyuridine (BrdU; Sigma Chemical Co.) intraperitoneally 2 h before the spleens were removed.
Cell Transfers.
Single-cell suspensions were prepared from donor spleens by gently sieving spleens through nylon mesh. QM mouse–derived donor splenocytes were analyzed by flow cytometry before transfer to determine the proportion of NP-specific B lymphocytes in the cell suspension. Donor splenocytes, in the numbers specified in Results, were suspended in 0.2 ml of PBS and injected into the lateral tail vein of nonirradiated recipients. Recipients were immunized with 30 μg NP-Ficoll 24 h after the cells were transferred.
Generation of QM Fetal Liver Irradiation Chimeras.
CBA/c nude recipient mice were irradiated (400 cGy), then reconstituted 6 h later by intravenous injection of 3 × 106 fetal liver cells obtained from day 17 QM mouse embryos. Mice were immunized 6 wk after reconstitution.
Abs and Reagents Used for Immunohistology and Flow Cytometry.
The following antibodies and reagents were used: rat anti–mouse monoclonal IgM (LO-MM-9; Serotec), sheep anti–mouse IgD (The Binding Site), syndecan-1 (CD138; PharMingen), rat anti–mouse CD3 (PharMingen), biotinylated PNA (Vector Labs), biotinylated mouse anti–mouse anti–Bcl-6 (Santa Cruz Biotechnology), NP conjugated to rabbit or sheep IgG (produced in our laboratory), rat Ab against the QM IgH idiotype R2.248 (gift from Dr. T. Imanishi-Kari, Tufts University School of Medicine, Boston, MA), mouse anti-BrdU (Dako), biotinylated or peroxidase-conjugated rabbit anti–rat IgG, rabbit anti–sheep IgG-biotin, goat anti–mouse IgG-biotin, pig anti–rabbit IgG-biotin (Dako), donkey anti–sheep IgG–horseradish peroxidase (The Binding Site), streptavidin-CyChrome (PharMingen), NP-PE (conjugated in our laboratory), and B220-FITC (PharMingen). The succinimide ester of 3-nitro-4-hydroxy-3-phenyl acetate was conjugated to Ficoll (Biosearch Technologies).
Flow Cytometry.
Cells were maintained in the dark at 4°C throughout the staining procedure. Red cells were lysed using Gey's solution. Data were acquired on a Coulter EPICS® XL-MCL flow cytometer, and analyzed using WinMDI 2.7 software.
Immunohistology.
Immunohistology was performed as described previously 8 21. In brief, frozen sections of spleen were air dried; primary Abs were added to sections, followed after washing by secondary reagents. When biotin-conjugated reagents were used, StreptABComplex/alkaline phosphatase (AP; Dako) was added after another wash. Horseradish peroxidase activity was detected using diaminobenzedine tetrahydrochloride solution (Sigma Chemical Co.). AP activity was detected using naphthol AS-MX phosphate (Sigma Chemical Co.) and chromogen Fast Blue BB salt (Sigma Chemical Co.) in 50 mM Tris-buffered saline (pH 9.2) containing 0.8 mg/ml levamisole to block endogenous AP activity (Sigma Chemical Co.). To detect cells that had incorporated BrdU, sections were treated with 0.1 M HCl (Sigma Chemical Co.) at 60°C for 20 min to make the BrdU-containing DNA accessible to the anti-BrdU Ab (Dako). Bound Ab was detected with biotin-conjugated goat anti–mouse Ig followed by StreptABComplex/AP. Enzyme activity was demonstrated using naphthol AS-MX phosphate with Fast Red TR salt (Sigma Chemical Co.). Slides were mounted in glycerol jelly.
Cells undergoing apoptosis were detected by terminal deoxynucleotidyltransferase (TdT)-mediated dUTP nick end labeling (TUNEL) staining. Frozen sections were fixed in 3% paraformaldehyde for 3 min at room temperature, then washed twice in PBS. TdT buffer (80 μl; GIBCO BRL) was added for 15 min, and then 60 μl of warm TdT mix (5% TdT buffer, 0.2 nM digoxigenin-11-dUTP; Boehringer Mannheim), 5 U TdT (Boehringer Mannheim), and 2 mM dATP (Promega) were added. The slides were incubated for 40 min at 37°C in a humidified chamber. Slides were washed twice in 0.5 mM EDTA, 2% BSA (pH 8.0). Antidigoxigenin Fab fragments conjugated to AP (Boehringer Mannheim) were added at 1:50 in blocking solution (5× SSC, 5% nonfat milk powder, 0.15% Triton X-100) and incubated for 30 min at room temperature, then washed and developed as described above. Double stainings with rat anti–mouse IgM were carried out as described above.
Quantification of Immunohistology.
The germinal centers were stained with PNA, and the extrafollicular plasmablasts were identified by the high content of cytoplasmic Ig and expression of syndecan-1. The NP specificity or idiotype staining was checked in adjacent tissue sections. The relative areas in square millimeters were determined using a point counting technique 25. An approximation of the total number of positively identified cells per cubic millimeter was calculated as the cube of the square root of the number of cells per spleen area. The values obtained were corrected for spleen mass to give relative volumes.
NP-specific Ab ELISA.
Serum NP-specific IgM, IgG1, IgG2a, IgG2b, and IgG3 Ab titers were assessed by ELISA using 4-hydroxy-3-iodo-5-nitrophenyl (NIP)-BSA–coated and BSA-blocked plates. Bound Ab was detected using AP-conjugated rat Abs against the appropriate mouse Ig isotype (Sigma Chemical Co.). The amount of enzyme bound to each well was quantitated using a phosphatase substrate system (Kirkegaard & Perry Laboratories). Standard calibration curves were plotted from the positive control sera, and the test serum titers were calculated as an OD50 of these standard controls.
Cell Culture.
B cells were purified from spleen by magnetic depletion of CD43+ cells using the VarioMacs system (Miltenyi Biotec). This resulted in B cell purity of >97%. Cells were cultured in RPMI, 10% FCS. 5 × 105 cells were added to each well of a sterile flat-bottomed 96-well plate, stimulated with NP-Ficoll with or without anti-CD40 mAb (3.23), and cultured at 37°C in 5% CO2.
Results
Germinal Centers Form in QM Mice in Response to Immunization with NP-Ficoll.
When QM mice are immunized intraperitoneally with 30 μg of the polysaccharide antigen NP-Ficoll, they form germinal centers in the spleen (Fig. 1, a–d). These germinal centers are large and can be found in every follicle, but contain very few T cells (Fig. 1c and Fig. d). The phenotype of B cells in NP-Ficoll–induced germinal centers is indistinguishable from germinal centers induced by conventional protein antigen 12 26. They stain with PNA, downregulate IgD and B220, and express Bcl-6. The large size and paucity of T cells contrast with background germinal centers seen in QM mice. The background germinal centers are not NP specific; they are small, and contain normal numbers of T cells (Fig. 1, a and b).
Figure 1.
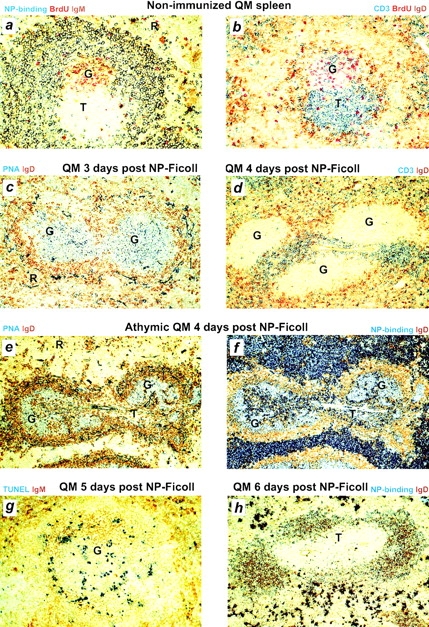
The formation and demise of germinal centers in QM mouse splenic follicles in response to NP-Ficoll. (a and b) Sections of spleen from nonimmunized QM mice. NP-specific cells (blue) in the follicular mantle and marginal zones surround a small germinal center, which is not NP-specific (a) and contains normal numbers of CD3+ T cells (blue, b). Red-stained nuclei of cells in the germinal center have taken up BrdU, given to mice 2 h before the spleen was taken. (c and d) Sections of spleen from QM mice after intraperitoneal immunization with NP-Ficoll. Large T cell–independent germinal centers are PNA+ (blue, c) and contain few CD3+ T cells (blue, d). (e and f) Sections of spleen from QM nude mouse chimeras 4 d after intraperitoneal immunization with NP-Ficoll. Germinal centers are PNA+ (blue, e) and contain NP-binding B cells (blue, f). Heavy blue staining in the red pulp and around the central arteriole are NP-specific plasmablasts. (g) TUNEL staining (blue) of a germinal center in spleen from a QM mouse 5 d after intraperitoneal immunization with NP-Ficoll. (h) Section of spleen from a QM mouse in which no germinal centers can be seen 6 d after intraperitoneal immunization with NP-Ficoll. G, germinal center; T, T zone; R, red pulp.
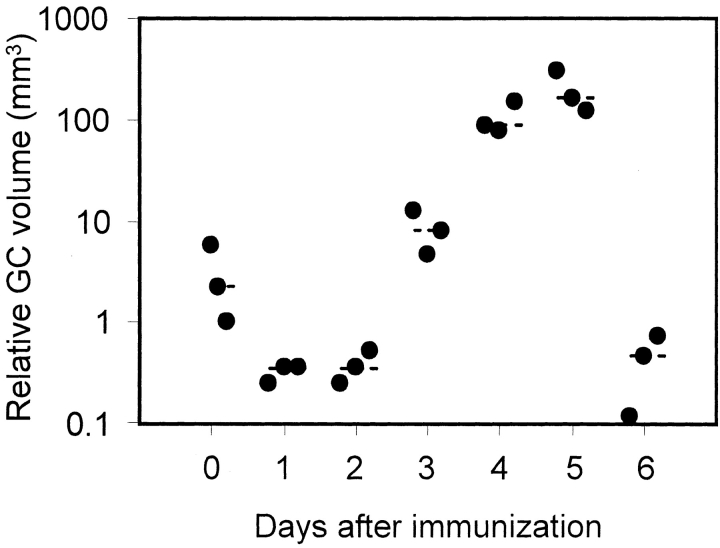
The kinetics of NP-Ficoll–induced germinal center formation in QM mice were analyzed by flow cytometry and immunohistology. By 2 d after immunization, PNAhigh NP-specific B cells are detectable by flow cytometry (Fig. 2). At this time, PNAhigh B cells already represent >20% of B220+ B cells. NP-specific germinal centers are clearly detectable immunohistologically by 72 h, when large clusters of NP-binding PNAhigh blasts fill the follicles (Fig. 1 c). Growth of the germinal centers continues through day 4, when they approach their maximum size (Fig. 3). By 96 h, pulse labeling for 2 h with BrdU shows demarcation of dark zones, with many BrdU+ centroblasts, and light zones containing the follicular dendritic cell network and few BrdU+ cells. The kinetics and peak sizes of NP-Ficoll–induced germinal centers in QM mice up to this stage are very similar to those found in responses to haptenated protein when primed T cells are made available at the time of challenge through prior immunization with carrier 7 8.
Figure 2.
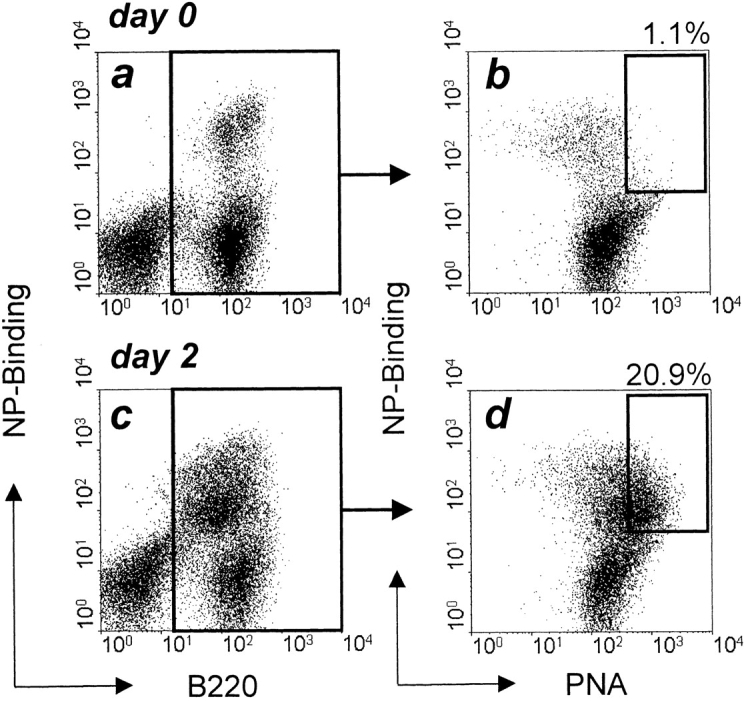
Three-color flow cytometric analysis of splenic B cells from QM mice before (a and b) and 2 d after (c and d) intraperitoneal immunization with 30 μg NP-Ficoll. Lymphocytes were gated on forward and side scatter, and then PNA expression and NP binding (b and d) were analyzed on B220+ cells (a and c). The percentages of NP-binding B cells that are also PNAhigh are shown.
Figure 3.
Kinetics of germinal center formation in spleens from QM mice after intraperitoneal immunization with 30 μg NP-Ficoll. Spleens were taken at the indicated times after immunization. Each dot represents the total volume in 1 mm3 of NP-specific germinal centers in the spleen from one mouse.
NP-Ficoll–induced Germinal Center B Cells Undergo Apoptosis at the Stage When Centrocytes Start to Be Selected.
The similarity with normal germinal centers ends on day 5, when the NP-Ficoll–induced germinal centers in QM mice abort synchronously. Within 24 h (by day 6), no trace of germinal centers remains (Fig. 1 h, and Fig. 3). This dissolution is associated with progressive loss of proliferating cells and massive apoptosis among the remaining centrocyte population. To confirm the morphological evidence of apoptosis, spleen sections were subjected to TUNEL staining throughout the time course of the germinal center reaction. Although very few nuclei stained positive on day 4, a large number of germinal center cells was stained on day 5 (Fig. 1 g). Possible interpretations of this sequence of events are set out in the Discussion.
Germinal Centers Induced by NP-Ficoll in QM Mice are Thymus Independent.
Ab responses to NP-Ficoll are independent of T cell help, as they occur normally in nude mice and in mice that are T cell deficient because of disruption of the β and δ TCR genes. Several observations suggest that the germinal centers induced in QM mice by NP-Ficoll are also T cell independent. First, the germinal centers are only formed in primary T cell–dependent responses to protein-based antigens after T cell priming has occurred. The lag of 2–3 d associated with priming is not seen in the germinal centers induced by NP-Ficoll in QM mice, where the kinetics match those seen when T cell help is immediately available 7 8. Second, very few or no T cells are present in the NP-specific germinal centers in QM mice (Fig. 1 d). Finally, the NP-specific Ab response in QM mice immunized with NP-Ficoll is predominantly IgM and IgG3 (Table ), the isotype pattern associated with lack of T cell help in response to thymus-independent type 2 antigens in normal 27 and T cell–deficient mice (Table ).
Table 1.
Median Serum Class and Subclass Anti-NP Ab Titers Produced in Response to NP-Ficoll
| C57BL/6 | QM | |||||
|---|---|---|---|---|---|---|
| Days after immunization | 0 | 5 | 7 | 0 | 5 | 7 |
| Log2 anti-NP titer | ||||||
| IgM | <0 | 8 | 9 | 4 | 19 | 16 |
| IgG1 | <0 | 2 | <0 | 9 | ||
| IgG2a | <0 | <0 | 4 | 0 | ||
| IgG2b | <0 | 0 | <0 | <0 | ||
| IgG3 | <0 | 8 | <0 | 14 | ||
Six C57BL/6 mice and four QM mice were studied at each time point after intraperitoneal immunization with 30 μg NP-Ficoll.
The thymus independence of germinal center formation by QM mouse B cells responding to NP-Ficoll was tested in nude mouse chimeras. Adult nude mice were subject to 400-cGy total body irradiation, followed by transfer of QM fetal liver as a source of hemopoietic cells. The resulting chimeras had donor-derived B cells, but no thymus-processed T cells. 6 wk after transfer, the QM nude chimeras were immunized intraperitoneally with 30 μg NP-Ficoll. Large NP-specific germinal centers formed in splenic follicles (Fig. 1e and Fig. f).
NP-Ficoll–induced Germinal Centers Are Independent of CD40 Ligation or Signaling Triggered by CD80 or CD86.
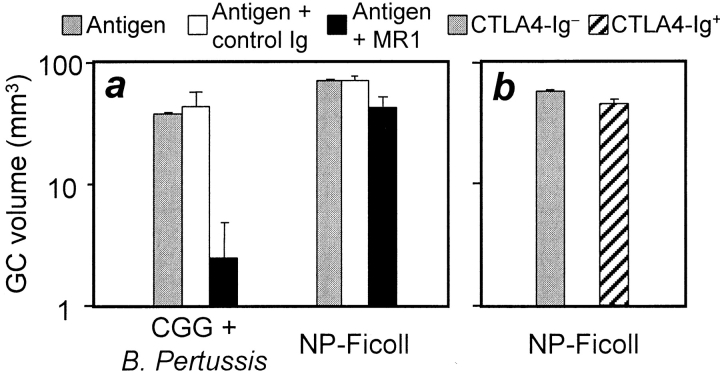
CD40 signaling is critical for the formation of germinal centers in normal mice immunized with protein-based antigens 13 14 28 29. In addition, mice unable to signal through CD80 or CD86 engagement of CD28 or CTLA-4 fail to form germinal centers in response to protein antigens 24 30. The experiments described above indicate that NP-Ficoll–induced germinal centers in QM mice are T cell independent, but it was important to exclude a role for CD40 ligand expressed by B cells or another type of cell in the absence of T cells. This was tested in (QM × C57BL/6)F1 mice, which also form germinal centers in response to NP-Ficoll, and form germinal centers normally after immunization with alum-precipitated chicken γ-globulin. The effect of blocking Ab against CD40 ligand on germinal centers to these two antigens was compared: 250 μg blocking Ab MR1 was given to mice 12 h before and 24 and 72 h after immunization with either antigen. Consistent with previous reports, the formation of germinal centers after protein immunization was reduced to <5% of that in mice given control Ab (Fig. 3 a). The blocking Ab had only a small effect on germinal centers formed in response to NP-Ficoll (Fig. 4 a); the small but significant effect might reflect an enhancing role for non–T cell CD40 ligand in germinal center formation. The effect of CD40 signaling on NP-specific QM mouse B cells exposed to NP-Ficoll was then assessed in vitro. Dilutions of NP-Ficoll were added to purified QM splenic B cells with and without added mAb against CD40. On culture, high level PNA binding developed in B cells exposed to NP-Ficoll alone; the addition of CD40 enhanced this effect (Fig. 5).
Figure 4.
The effect of CD40, CD80, and CD86 blockade on germinal center formation. (a) Volume of germinal centers in spleens from (QM × C57BL/6)F1 mice 4 d after intraperitoneal immunization with 30 μg NP-Ficoll (right) or 10 d after intraperitoneal immunization with 50 μg alum-precipitated chicken γ-globulin (CGG), plus 1 × 109 killed B. pertussis bacteria (left). Mice represented by the black bars were given 250 μg of MR1 (reference 13), a hamster anti–mouse CD40 ligand mAb, on days −0.5, 1, and 3 after immunization; white bars show values for control mice receiving normal hamster Ig. The values shown by the gray bars are responses of mice receiving no Ig. (b) Volume of germinal centers in spleens from (QM × CTLA-4–Hγ1 transgenic)F1 mice 4 d after intraperitoneal immunization with 30 μg NP-Ficoll. Values for mice carrying the CTLA-4–Hγ1 transgene are shown by the gray bar, and those for congenic littermates without the transgene are shown by the hatched bar.
Figure 5.
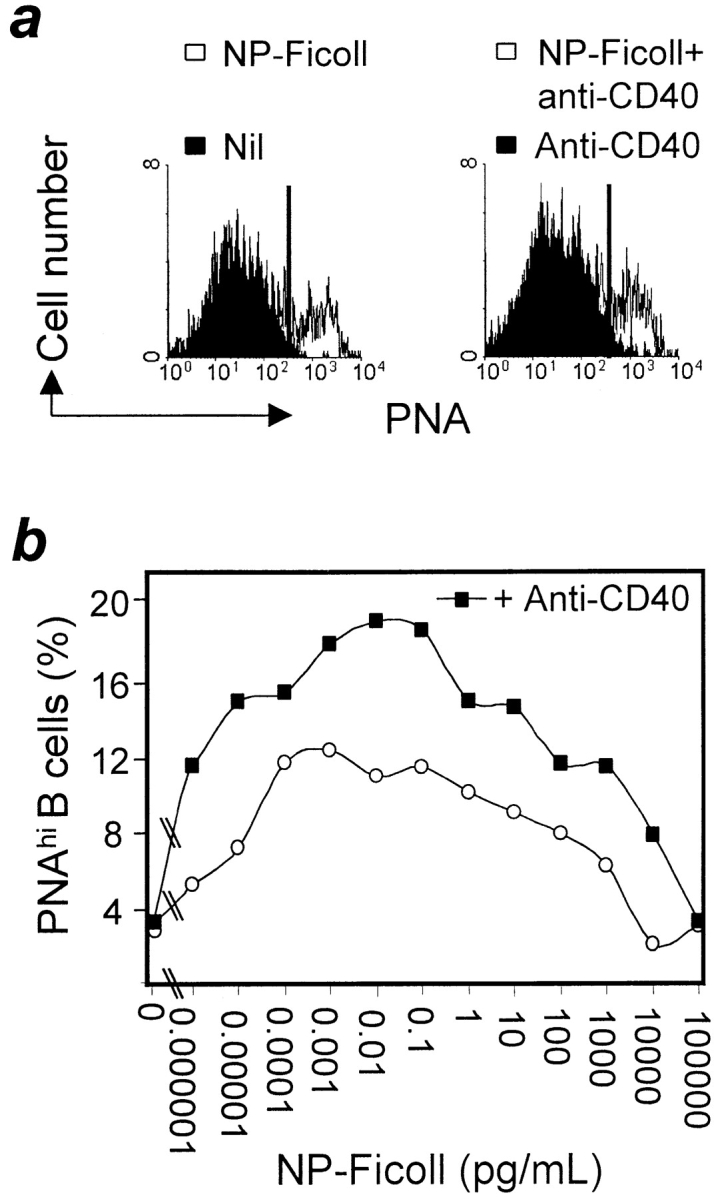
Induction of PNA on QM B cells after stimulation in vitro. Purified QM B cells were cultured with graded doses of NP-Ficoll with or without anti-CD40 Ab (0.1 μg/ml 3.23). Cultures were harvested on day 2, and the percentage of PNAhigh cells was determined by flow cytometry. (a) Representative histograms of PNA binding generated by flow cytometric analysis of gated B220+ cells that were stimulated with NP-Ficoll (left) or NP-Ficoll and anti-CD40 (right). In each case, black overlays show that there was minimal induction of PNA-binding B cells in cultures without NP-Ficoll. (b) Summary of induction of PNA-binding cells by NP-Ficoll (○), or NP-Ficoll plus anti-CD40 (▪).
Further studies were made to test whether CD28 signaling is required for the formation of germinal centers in QM mice immunized with NP-Ficoll. Mice constitutively expressing the CTLA-4–Hγ1 transgene were crossed to QM mice. The response to NP-Ficoll by their progeny expressing the transgene was compared with that in their transgene-negative littermates. The CTLA-4–Hγ1 fusion protein is an effective competitive inhibitor of CD86 or CD80 engagement of CD28; mice carrying this transgene do not form germinal centers in response to protein-based antigens in otherwise normal mice 24 30. The presence of the CTLA-4–Hγ1 fusion protein did not prevent (QM × CTLA-4–Hγ1)F1 hybrids from forming germinal centers in response to NP-Ficoll (Fig. 4 b).
Minimum Requirements for T Cell–independent Germinal Centers: Antigen-specific Precursor Frequency and Antigen Dose.
The experiments described above establish that NP-Ficoll–induced germinal centers in QM mice are independent of T cell help and CD40 ligation. The question remains: why do B cells from QM mice form germinal centers, whereas those in congenic nontransgenic littermates do not? There are two important characteristics of QM mice, not found in conventional mice, that could provide an explanation. First, QM mice have an abnormally high number of antigen-specific cells. Second, the transgene-encoded antigen receptors have a uniform high affinity for NP. These two factors could increase the strength of signals delivered to the B cells, either via the high-avidity interaction between antigen and BCR, or because increased numbers of antigen-reactive cells could result in autocrine potentiation of responses. To investigate these possibilities, we assessed the minimum frequency of NP-specific QM B cells and the minimum dose of antigen required to induce (a) an extrafollicular plasmablast response and (b) a germinal center reaction.
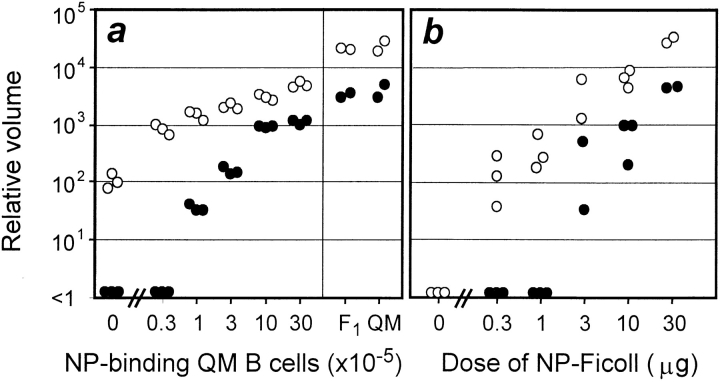
Serial dilutions of QM spleen cells were transferred into nonirradiated congenic recipients, which were then immunized with NP-Ficoll (Fig. 6 a). The numbers of NP-specific B cells in the inocula were determined by flow cytometry before the cells were transferred. NP-specific germinal centers and extrafollicular responses, identified by their expression of donor-specific H chain idiotype, formed in mice that had received 1 × 105 or more NP-specific QM B cells. Recipients of 3 × 104 B cells produced an extrafollicular response, but no germinal centers. In the next experiment, intact (QM × C57BL/6)F1 mice were immunized with graded doses of NP-Ficoll. Mice immunized with 0.3 and 1 μg of NP-Ficoll made an extrafollicular response, but did not form NP-specific germinal centers. Immunization with doses of 3 μg NP-Ficoll or more induced both germinal centers and extrafollicular responses (Fig. 6 b).
Figure 6.
Antigen dose and B cell precursor frequency required to form germinal centers. (a) Volume of NP-specific germinal centers (•) and extrafollicular foci (○) in spleens obtained from nonirradiated congenic recipients of the indicated number of QM-derived B cells 4 d after intraperitoneal immunization with 30 μg NP-Ficoll. NP-specific germinal center volumes in spleens obtained from intact QM and (QM × C57BL/6)F1 mice 4 d after intraperitoneal immunization with 30 μg NP-Ficoll are also shown. (b) Volume of NP-specific germinal centers (•) and extrafollicular responses (○) in spleens obtained from (QM × C57BL/6)F1 mice 4 d after intraperitoneal immunization with the indicated quantities of NP-Ficoll.
Discussion
The studies reported indicate that there is no absolute requirement for T cell–derived signals for the induction of germinal centers and acquisition of the germinal center B cell phenotype. Further, these signals are necessary for neither the exponential phase of B cell growth nor the differentiation of germinal centers into dark and light zones. The spontaneous involution of the germinal centers on day 5 might reflect the absence of T cell signals, which are required for physiological germinal center maturation, at the time of initial B cell activation. Alternatively, the loss of germinal centers on day 5 might reflect failed centrocyte selection.
Previous studies have shown involution of established germinal centers by CD40 blockade, and this provided the most compelling evidence that T cells are required for positive selection of germinal center B cells 14. The possible role of B cell–expressed CD40 ligand in maintaining germinal centers could not be excluded as an explanation for this result. The results of the present study are consistent with the view that T cell–derived signals are required to sustain germinal centers; if B cell CD40 ligand has a role in germinal center maintenance, it must be in addition to CD40 ligation delivered by T cells.
How are T cells involved in maintaining germinal centers? B cells in germinal centers proliferate as centroblasts in the dark zone, which is largely devoid of T cells; they then come out of cell cycle as centrocytes and enter the light zone 31. In the light zone, centrocytes can bind antigen held on follicular dendritic cells and present this to the abundant T cells, which are found at the outer edge of this compartment 32. Kepler and Perelson developed a mathematical model predicting that successive rounds of hypermutation and selection are necessary to account for the kinetics of affinity maturation 33. In practice, this is likely to result from hypermutation being active in centroblasts, which only undergo a finite number of divisions before maturing into centrocytes. If this is the case, the pool of centroblasts will be rapidly exhausted unless it is renewed from a proportion of the centrocytes that are positively selected in the light zone. In the NP-Ficoll–induced germinal centers of QM mice, the antigen is not processed and presented to T cells; consequently, selection is not possible and centroblasts are not renewed. This gives us a tentative indication of the number of cell divisions centroblasts go through before differentiating to centrocytes, as the period between the appearance of the dark zone and germinal center dissolution is between 24 and 36 h. Allowing for the time centrocytes take to die, the lifespan of centroblasts is likely to be 18–24 h, sufficient time for three to four cell cycles 34.
Studies of cocultures of human centrocytes with autologous germinal center T cells show that T cells can prevent centrocytes from entering apoptosis, and induce differentiation of a proportion of the cells to centroblasts, whereas others acquire a memory B cell phenotype 35. In addition to lack of centroblast renewal, failed selection of centrocytes will also prevent memory B cells and plasma cells from emerging from the germinal centers. This provides a fail-safe mechanism that prevents the emergence of cells rendered autoreactive by hypermutation from germinal centers formed in the absence of T cell help.
An alternative explanation for the demise of germinal center cells induced in QM mice by NP-Ficoll is that they are negatively selected by signals delivered through the BCR. Precedents for this mechanism are provided by the studies that indicate that massive death is induced in established germinal centers by giving large amounts of the antigen used to induce the germinal center 36 37 38. This phenomenon appears to differ in three ways from the sudden death that occurs in germinal center B cells during the QM mouse response to NP-Ficoll. First, large amounts of antigen (4 mg [38] or 5 mg [36, 37]) were required to induce the dissolution of established germinal centers. By contrast, germinal centers were induced in QM mice by a single injection of 3 μg NP-Ficoll (Fig. 6). Some NP-Ficoll–containing immune complex is identified immunohistologically on follicular dendritic cells. As this antigen induces a particularly strong cross-linking signal, it might be argued that this is sufficient to induce apoptosis of germinal center cells. The second difference militates against this possibility, since high-dose antigen induces apoptosis of germinal center cells within 3 h 38. This timing is hardly consistent with that of cell death in the germinal centers in the NP-Ficoll response, which starts only at the end of the day 4 after immunization. Third, the effect of high-dose soluble antigen on germinal center cells is dramatic, but is not complete, and there is some subsequent recovery 38; however, the loss of germinal center B cells in QM mice is complete and permanent.
Transfer of QM B cells into intact recipients reveals a precursor frequency threshold for the T cell–independent germinal center formation induced by NP-Ficoll. Germinal centers were observed when the frequency of NP-specific B cells reached 1:1,000 B cells. In conventional mice that have undergone repeated immunization with NP–chicken γ-globulin, the number of high-affinity NP-specific memory B cells may well be >1:1,000. Nevertheless, when these mice are immunized with NP-Ficoll, they only form extrafollicular Ab responses 39. This suggests that memory cells may be resistant to being induced to form germinal centers, and supports the circumstantial evidence that virgin B cells have a greater predilection than memory B cells for giving rise to germinal centers (for a review, see reference 40).
The threshold number of antigen-specific cells required to form germinal centers might reflect cross-talk between B cells associated with the presence of increased numbers of responding B cells, either at the time of B cell activation or within nascent germinal centers. This could be through interaction between cell surface molecules or secreted cytokines or both. The studies with CD40 ligand neutralization reported here make a critical role for CD40 ligation by B cell interaction with B cells unlikely. Another possibility is interaction between the CD11a, CD18 heterodimer, and CD54 41. There are several potential B cell autocrine effects that could involve cytokines; these include the production of IL-10 and IL-6 42 43.
We have also demonstrated an antigen threshold; doses of NP-Ficoll <3 μg/ml stimulated extrafollicular responses, but not germinal center formation. Since there are thresholds for antigen-specific precursor frequency and antigen dose, NP-Ficoll–induced germinal center formation in QM mice may also reflect an exceptionally strong signal delivered through BCRs because of B cell–B cell cross-linking via NP-Ficoll. Precedents for an altered outcome resulting from B cells binding membrane-bound versus soluble antigen are provided by studies of immature B cells in the marrow. When these bind antigen expressed by another cell 44 45 or are agglutinated by anti-IgM 46 47, they die in situ. If, on the other hand, they bind soluble antigen, they leave the marrow for secondary lymphoid tissues where they can elicit and respond to T cell help (48).
As well as identifying the minimal requirements for germinal center formation, these experiments reveal that the threshold of signaling for B cells to mount an extrafollicular response in the absence of T cells is substantially lower than that required for germinal center formation. An explanation has yet to be found for why some B cells differentiate into Ab-forming cells in extrafollicular foci, and others form germinal centers.
This model for germinal center formation provides the opportunity to assess whether T cells are critical in activating somatic mutation in germinal center cells, or whether this is triggered by the same signals that induce B cell growth in follicles. Currently, studies are underway comparing the appearance of Ig V region mutations in T cell–dependent and –independent germinal centers. The use of carrier priming to induce synchronous early T cell–dependent NP-specific germinal center formation allows direct comparison with germinal centers formed in QM mice.
Acknowledgments
The authors are particularly grateful to Peter Lane for his helpful discussions and for providing the CTLA-4–Hγ1 transgenic mice. We are grateful to Shirley Peach and Dale Taylor for assistance with the genetic typing and care of mice.
This work is supported by the British Medical Research Council with a program grant to I.C.M. MacLennan and a clinical research fellowship to C. García de Vinuesa. M. Wabl is supported by National Institutes of Health grant R01 AI41570 and by a Howard Hughes grant for transgenic mice.
Footnotes
Abbreviations used in this paper: AP, alkaline phosphatase; BCR, B cell receptor; BrdU, 5-bromo-2′-deoxyuridine; CTLA-4, CTL antigen 4; Hγ1, human γ1; NP, (4-hydroxy-3-nitrophenyl)acetyl; PNA, peanut agglutinin; QM, quasimonoclonal; TdT, terminal deoxynucleotidyltransferase; TUNEL, TdT-mediated dUTP nick end labeling.
References
- Jacobson E.B., Caporale L.H., Thorbecke G.J. Effect of thymus cell injections on germinal center formation in lymphoid tissues of nude (thymusless) mice. Cell. Immunol. 1974;13:416–430 . doi: 10.1016/0008-8749(74)90261-5. [DOI] [PubMed] [Google Scholar]
- Kindred B. Nude mice in immunology. Prog. Allergy. 1979;26:137–238. [PubMed] [Google Scholar]
- Schuurman H.J., Bell E.B., Gartner K., Hedrich H.J., Hansen A.K., Kruijt B.C., de-Vrey P., Leyten R., Maeder S.J., Moutier R. Comparative evaluation of the immune status of congenitally athymic and euthymic rat strains bred and maintained at different institutes2. Athymic rats. J. Exp. Anim. Sci. 1992;35:33–43. [PubMed] [Google Scholar]
- Dianda L., Gulbranson-Judge A., Pao W., Hayday A.C., MacLennan I.C., Owen M.J. Germinal centre formation in mice lacking alpha beta T cells. Eur. J. Immunol. 1996;26:1603–1607. doi: 10.1002/eji.1830260729. [DOI] [PubMed] [Google Scholar]
- Wang D., Wells S.M., Stall A.M., Kabat E.A. Reaction of germinal centers in the T-cell-independent response to the bacterial polysaccharide alpha(1→6)dextran. Proc. Natl. Acad. Sci. USA. 1994;91:2502–2506. doi: 10.1073/pnas.91.7.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverremark E., Fernandez C. Role of T cells and germinal center formation in the generation of immune responses to the thymus-independent carbohydrate dextran B512. J. Immunol. 1998;161:4646–4651. [PubMed] [Google Scholar]
- Liu Y.-J., Zhang J., Lane P.J., Chan E.Y., MacLennan I.C. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- Toellner K.-M., Gulbranson-Judge A., Taylor D.R., Sze D.M., MacLennan I.C. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J. Exp. Med. 1996;183:2303–2312. doi: 10.1084/jem.183.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kassir R., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther S.A., Gulbranson-Judge A., Acha-Orbea H., MacLennan I.C. Viral superantigen drives extrafollicular and follicular B cell differentiation leading to virus-specific antibody production. J. Exp. Med. 1997;185:551–562. doi: 10.1084/jem.185.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitroprenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath–associated foci and germinal centers. J. Exp. Med. 1992;176:679–687. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M.L., Birbeck M.S., Wallis V.J., Forrester J.A., Davies A.J. Peanut lectin binding properties of germinal centers of mouse lymphoid tissue. Nature. 1980;284:364–366. doi: 10.1038/284364a0. [DOI] [PubMed] [Google Scholar]
- Foy T.M., Laman J.D., Ledbetter J.A., Aruffo A., Claassen E., Noelle R.J. gp39–CD40 interactions are essential for germinal center formation and the development of B cell memory. J. Exp. Med. 1994;180:157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Hathcock K., Zheng B., Kepler T.B., Hodes R., Kelsoe G. Cellular interactions in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- Wykes M., Poudrier J., Lindstedt R., Gray D. Regulation of cytoplasmic, surface and soluble forms of CD40 ligand in mouse B cells. Eur. J. Immunol. 1998;28:548–559. doi: 10.1002/(SICI)1521-4141(199802)28:02<548::AID-IMMU548>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Grammer A.C., Bergman M.C., Miura Y., Fugita K., Davis L.S., Lipsky P.E. The CD40 ligand expressed by human B cells costimulates B cell responses. J. Immunol. 1995;154:4996–5010. [PubMed] [Google Scholar]
- Cascalho M., Ma A., Lee S., Masat L., Wabl M. A quasi-monoclonal mouse. Science. 1996;272:1649–1652. doi: 10.1126/science.272.5268.1649. [DOI] [PubMed] [Google Scholar]
- Snapper C.M., Mond J.J. T cell-independent type 2 antigens. Annu. Rev. Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- Goodlad J.R., Macartney J.C. Germinal-center cell proliferation in response to T-independent antigensa stathmokinetic, morphometric and immunohistochemical study in vivo. Eur. J. Immunol. 1995;25:1918–1926. doi: 10.1002/eji.1830250719. [DOI] [PubMed] [Google Scholar]
- Claassen E., Kors N., Dijkstra C.D., van Rooijen N. Marginal zone of the spleen and the development and localization of specific antibody-forming cells against thymus-dependent and thymus-independent type-2 antigens. Immunology. 1986;57:399–403. [PMC free article] [PubMed] [Google Scholar]
- García de Vinuesa C., O'Leary P., Sze D.M., Toellner K.-M., MacLennan I.C. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur. J. Immunol. 1999;29:1314–1323. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Cascalho M., Wong J., Wabl M. VH gene replacement in hyperselected B cells of the quasimonoclonal mouse. J. Immunol. 1997;159:5795–5801. [PubMed] [Google Scholar]
- Lopez-Macias C., Kalinke U., Cascalho M., Wabl M., Hengartner H., Zinkernagel R.M., Lamarre A. Secondary rearrangements and hypermutation generate sufficient B cell diversity to mount protective antiviral immunoglobulin responses. J. Exp. Med. 1999;189:1791–1798. doi: 10.1084/jem.189.11.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P., Burdet C., Hubele S., Scheidegger D., Müller U., McConnell F., Kosco-Vilbois M. B cell function in mice transgenic for mCTLA4-Hγ1lack of germinal centers correlated with poor affinity maturation and class switching despite normal priming of CD4+ T cells. J. Exp. Med. 1994;179:819–830. doi: 10.1084/jem.179.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible E.R. Principle and methods for the morphometric study of the lung and other organs. Lab. Invest. 1963;12:131–135. [PubMed] [Google Scholar]
- Onikuza T., Moriyama M., Yamochi T., Kuroda T., Kazama A., Kanazawa N., Sato K., Kato T., Ota H., Mori S. BCL-6 gene product, a 92-kD nuclear phosphoprotein, is highly expressed in germinal center B cells and their neoplastic counterparts. Blood. 1995;86:28–37. [PubMed] [Google Scholar]
- Perlmutter R.M., Hansburg D., Briles D.E., Nicolotti R.A., Davie J.M. Subclass restriction of murine anti-carbohydrate antibodies. J. Immunol. 1978;121:566–572. [PubMed] [Google Scholar]
- Kawabe T., Naka T., Yoshida K., Tanaka T., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T., Kikutani H. The immune response in CD40-deficient miceimpaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Xu J., Foy T.M., Laman J.D., Elliott E.A., Dunn J.J., Waldschmidt T.J., Elsemore J., Noelle R.J., Flavell R.A. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Ferguson S.E., Han S., Kelsoe G., Thompson C.B. CD28 is required for germinal center formation. J. Immunol. 1996;156:4576–4581. [PubMed] [Google Scholar]
- Liu Y.-J., Joshua D.E., Williams G.T., Smith C.A., Gordon J., MacLennan I.C. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- Hardie D.L., Johnson G.D., Khan M., MacLennan I.C. Quantitative analysis of molecules which distinguish functional compartments within germinal centers. Eur. J. Immunol. 1993;23:997–1004. doi: 10.1002/eji.1830230502. [DOI] [PubMed] [Google Scholar]
- Kepler T.B., Perelson A.S. Cyclic re-entry of germinal center B cells and the efficiency of affinity maturation. Immunol. Today. 1993;14:412–415. doi: 10.1016/0167-5699(93)90145-B. [DOI] [PubMed] [Google Scholar]
- Zhang J., MacLennan I.C., Liu Y.-J., Lane P.J. Is rapid proliferation in B centroblasts linked to somatic mutation in memory B cell clones? Immunol. Lett. 1988;18:297–299. doi: 10.1016/0165-2478(88)90178-2. [DOI] [PubMed] [Google Scholar]
- Casamayor-Palleja M., Feuillard J., Ball J., Drew M., MacLennan I.C. Centrocytes rapidly adopt a memory B cell phenotype on co-culture with autologous germinal centre T cell-enriched preparations. Int. Immunol. 1996;8:737–744. doi: 10.1093/intimm/8.5.737. [DOI] [PubMed] [Google Scholar]
- Pulendran B., Kannourakis G., Nouri S., Smith K.G., Nossal G.J. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995;375:331–334. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- Shokat K.M., Goodnow C.C. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375:334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- Han S., Zheng B., Dal Porto J., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl IV. Affinity-dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J. Exp. Med. 1995;182:1635–1644. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze, D.M. 1998. Selection and selective use of the B cell repertoire. PhD thesis. University of Birmingham, Birmingham, United Kingdom. 184 pp.
- MacLennan I.C. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Katada Y., Tanaka T., Ochi H., Aitani M., Yokota A., Kikutani H., Suemura M., Kishimoto T. B cell-B cell interaction through intercellular adhesion molecule-1 and lymphocyte functional antigen-1 regulates immunoglobulin E synthesis by B cells stimulated with interleukin-4 and anti-CD40 antibody. Eur. J. Immunol. 1996;26:192–200. doi: 10.1002/eji.1830260130. [DOI] [PubMed] [Google Scholar]
- Voorzanger N., Touitou R., Garcia E., Delecluse H.J., Rousset F., Joab I., Favrot M.C., Blay J.Y. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin's lymphoma cells and act as cooperative growth factors. Cancer Res. 1996;56:5499–5505. [PubMed] [Google Scholar]
- Burdin N., Rousset F., Banchereau J. B-cell-derived IL-10production and function. Methods. 1997;11:98–111. doi: 10.1006/meth.1996.0393. [DOI] [PubMed] [Google Scholar]
- Nemazee D., Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C.C., Crosbie J., Adelstein S., Lavoie T.B., Smith-Gill S.J., Brink R.A., Pritchard-Briscoe H., Wotherspoon J.S., Loblay R.H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Lawton A.R., III, Cooper M.D. Modification of B lymphocyte differentiation by anti-immunoglobulins. Contemp. Top. Immunobiol. 1974;3:193–225. doi: 10.1007/978-1-4684-3045-5_8. [DOI] [PubMed] [Google Scholar]
- Cook M.C., Basten A., Fazekas de St. Groth B. Rescue of self-reactive B cells by provision of T cell help in vivo . Eur. J. Immunol. 1998;28:2549–2558. doi: 10.1002/(SICI)1521-4141(199808)28:08<2549::AID-IMMU2549>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]