Abstract
Signals generated through CD28–B7 and CD40 ligand (CD40L)–CD40 interactions have been shown to be crucial for the induction of long-term allograft survivability. We have recently demonstrated that humanized anti-CD40L (hu5C8) prevents rejection of mismatched renal allografts in primates. To investigate potential mechanisms of CD40L–induced allograft acceptance, we coimmobilized hu5C8 with suboptimal amounts of anti-CD3 to stimulate CD4+ T cells. We now report that anti-CD3/CD40L costimulation results in CD28-independent activation and subsequent deletion of resting T cells. Coligation of CD3 and CD40L increased expression of CD69, CD25, and CD54 on CD4+ T cells. We also found that costimulation with anti-CD3/CD40L resulted in enhanced production of interleukin (IL)-10, interferon γ, and tumor necrosis factor α but not IL-2 or IL-6. Interestingly, after several days, anti-CD3/CD40L–mediated activation was followed by apoptosis in a significant population of cells. Consistent with that observation, anti-CD3/CD40L did not enhance the antiapoptotic proteins Bcl-2 and Bcl-xL. Further, the addition of CD28 at 24 h failed to rescue those cells induced to die after costimulation with anti-CD3/CD40L. Together, these data suggest that the graft-sparing effect of hu5C8 in vivo may result in part from early and direct effects on CD4+ T cells, including a vigorous induction of immunomodulatory cytokines and/or apoptosis of allograft-specific T cells.
Keywords: costimulatory molecules, T lymphocytes, transplantation, cytokines, apoptosis
Introduction
Binding of CD40 with its counterreceptor, CD40 ligand (CD40L), acts on APCs and T cells in a bidirectional fashion, mediating both humoral and cellular immune responses. In B cells, cross-linking CD40 drives differentiation, proliferation, and isotype switching while preventing B cell apoptosis 1. In T cells, CD40L expression is rapidly but transiently induced after CD3 stimulation 2 3 4. Much evidence suggests that CD40L is an important regulator of T cell responses. In CD40L-deficient mice, it has been demonstrated that antigen-specific T cell responses were impaired and that therefore the expression of CD40L on T cells was required for the in vivo priming of CD4+ cells 5 6. Enhancement of T cell responses by CD40L results partially from upregulation of CD80 and CD86 on the APCs 7 8. CD40L has been shown to induce proliferation and cytokine production in small resting human T cells stimulated with soluble anti-CD3 and CD40-transfected mouse P815 cells 9 10 and to directly provide signals to CD40L+ Jurkat T cells, which results in neutral sphingomyelinase, c-Jun NH2-terminal kinase (JNK), and p38 mitogen-activated protein (MAP) kinase induction 11 12.
It is becoming clear that peripheral tolerance to allografts is dependent on the interplay of several costimulatory molecules that work cooperatively to regulate effector functions 13 14. Acceptance of alloantigens can be induced in adult animals by injection of anti-CD40L antibodies or CD40-deficient B cells 15. Recent reports have shown that antibodies to CD40L in combination with CTL-associated antigen 4–Ig (CTLA4-Ig) or donor-specific T cells could induce prolonged rejection-free survival to islet, heart, and skin allografts in mice 16 17 18. Anti-CD40L treatment has also been shown to preserve kidney function in mice with established nephritis 19. We have effectively used a strategy to block costimulatory signals to prevent rejection of primate renal allografts using CTLA4-Ig and the anti-CD40L mAb hu5C8. Whereas costimulation blockade with CTLA4-Ig alone was unsuccessful, anti-CD40L treatment resulted in sustained graft survival 20. More recently, we have shown that anti-CD40L (hu5C8) alone prevented acute rejection and increased kidney allograft survival in nonhuman primates. We also demonstrated that additional immunosuppressants abrogated the affects mediated by hu5C8 21.
Here, we test the functional effects of the anti-CD40L mAb hu5C8 on purified human CD4+ T cells when coimmobilized with anti-CD3. We compared these findings with results generated in cultures of CD4+ T cells stimulated with anti-CD3 and CD32+CD40+ transfectants. Our data demonstrate that CD40L can costimulate CD4+ T cells in the absence of CD28, causing transient thymidine incorporation and activation. CD40L-mediated costimulation was characterized by an increase in T cell activation and adhesion antigens and enhanced production of IFN-γ, TNF-α, and IL-10. However, in anti-CD3/CD40L–stimulated cultures, apoptosis occurred within 3 d in many cells. The paradox of first allowing and then failing to sustain T cell proliferation may suggest a critical role for CD40L in driving short-term T cell effector responses that do not lead to the development of memory.
Materials and Methods
Cells and Antibodies.
Freshly isolated PBLs were separated by Percoll (Amersham Pharmacia Biotech) gradient centrifugation from leukopacks obtained by apheresis from healthy donors. CD4+ T cells were purified by negative selection. Hu5C8, an anti-CD40L mAb, was a gift from Dr. Linda Burkly (Biogen, Inc., Cambridge, MA). Anti-CD3 huOKT3 (human IgG1; Ortho Biotech) was a gift of Dr. Jeffrey Bluestone (University of Chicago, Chicago, IL); anti-CD28 9.3 (mouse IgG2a), anti–monomorphic HLA class I mAb W6/32 (mouse IgG2a; American Type Culture Collection), anti–class II mAb 2.06 (mouse IgG1; American Type Culture Collection), and anti-glycophorin A 10FTMC (IgG1; American Type Culture Collection) served as binding and nonbinding controls. Humanized anti-CD80 (1F1, IgG1) and anti-CD86 (3D1, IgG2a) were gifts from Dr. Gary Gray (Genetics Institute, Cambridge, MA). The following antibodies were used to immunophenotype T cells: anti-CD4 (clone SK3, IgG1; Becton Dickinson), anti-CD69–PE (clone L78, IgG1; Becton Dickinson), anti-CD25–PE (clone 2A3, IgG1; Becton Dickinson), and anti-CD54 (IgG2a; Immunotech).
Bead Preparation.
Anti-CD3 and control or anti-CD40L mAbs were covalently attached to 0.5-μm polyurethane-coated tosyl-activated Dynabeads® (Dynal) according to the manufacturer's instructions. The bead to cell ratio used in cultures was 3:1. Beads were prepared with a constant amount of anti-CD3 antibody representing 12.5% of bound protein, and with control, anti-CD28, or anti-CD40L mAb to make up the remaining 87.5%.
CD40+ Transfectants.
A full-length cDNA for human (h)CD40 was generated from Raji RNA using primers flanked by KpnI and NotI sites. This cDNA was cloned into pcDNA 3.1 (Invitrogen), digested with ScaI, and electroporated into CD32+ L cells (a gift of G. Delespesse, University of Montreal, Montreal, Canada) using standard procedures to create CD32+CD40+ L cells. Stable transfectants were generated by staining CD32-Fcγ–treated cells with an mAb against CD40 (Becton Dickinson) and then performing multiple sterile sorts using an EPICS® ELITE ESP cell sorter (Coulter). CD86-transfected CD32+ L cells were a gift of G. Delespesse.
T Cell Function Assay.
CD4+ T cells were plated in 96-well flat-bottomed microtiter plates at a density of 106 cells/ml in a total volume of 200 μl RPMI (GIBCO BRL). Proliferation was measured after a pulse with 1 μCi of [3H]thymidine. Cytokine concentrations in cell-free supernatants were assayed by ELISA (R&D Systems). Apoptosis was analyzed using a modified terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL)-based procedure 22.
PCR-based Liquid Hybridization Assay.
Steady state cytokine mRNA levels were assayed by semiquantitative reverse transcriptase (RT)-PCR–based liquid hybridization using the following primers and probes as described previously 23. RT-generated PCR products were diluted as indicated in the figure legends. To ensure that the PCR reactions were performed in the linear range of the assay, a twofold dilution of the RT product was amplified at 95°C for 0.5 min, 55°C for 0.5 min, and 72°C for 1.5 min for 25 cycles with the primers listed below. Liquid hybridization of the PCR products was conducted as reported previously. In brief, 20,000 counts of the probes listed below were hybridized in 30-μl reactions. After hybridization, samples were loaded into 10% acrylamide gels, run at 140 V for 75 min, and then exposed to a PhosphorImager® screen (Molecular Probes). Assays were validated by determining whether twofold differences in signal strength could be detected. Primers were as follows: for IL-2 (sense) 5′-CAA CTC CTG TCT TGC ATT GC-3′, (antisense) 5′-TTC TGT GGC CTT CTT GGG-3′, (probe) 5′-ACA AGA ATC CCA AAC TCA CCA GG-3′; for IL-6 (sense) 5′-AAG ATT CCA AAG ATG TAG CC-3′, (antisense) 5′-CCT CAA ACT CCA AAA GAC CA-3′, (probe) 5′-GAG AAA GGA GAC ATG TAA C-3′; for IL-10 (sense) 5′-TTG CCT GGT CCT CCT GAC TG-3′, (antisense) 5′-GAT GTC TGG GTC TTG GTT CT-3′, (probe) 5′-ATG AAG GAT CAG CTG GAC AA-3′; for IFN-γ (sense) 5′-TGC AGG TCA TTC AGA TGT AG-3′, (antisense) 5′-TCT CGT TTC TTT TTG TTG CT-3′, (probe) 5′-GGA GAC CAT CAA GGA AGA CA-3′; for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (sense) 5′-ATG GGG AAG GTG AAG GTC GGA GTC AAC GGA-3′, (antisense) 5′-AGG GGG CAG AGA TGA TGA CCC TTT TGG CTC-3′, (probe) 5′-TCG CTC CTG GAA GAT GGT GAT GGG ATT TCC-3′.
Immunoblotting.
Bcl-x and Bcl-2 (PharMingen) proteins were immunoprecipitated and Western blotted as described previously 22. Bcl-x antibodies were a gift from Craig Thompson (University of Chicago, Chicago, IL).
Results and Discussion
Anti-CD3/CD40L Stimulation Provides a Costimulatory Signal to Resting Human CD4+ T Cells.
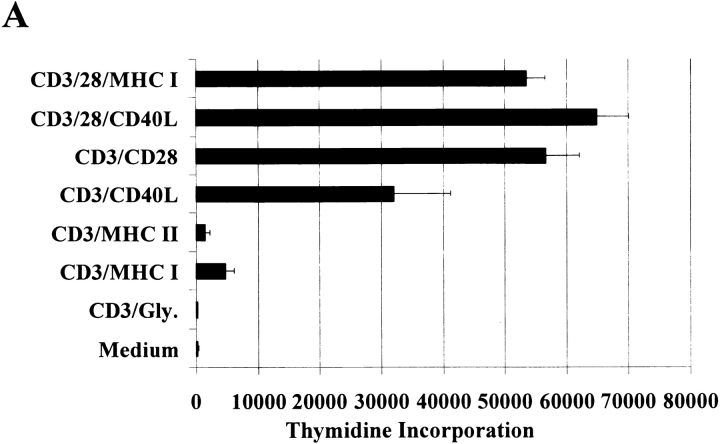
CD40L is rapidly induced on the surface of T cells after CD3 ligation 3 4. Given our published findings 20 21 demonstrating the dramatic ability of anti-CD40L to prolong primate kidney allograft survival, we have examined the costimulatory effects of the hu5C8 antibody on purified CD4+ T cell function by binding suboptimal amounts of anti-CD3 antibodies in combination with anti-CD40L on 5-μm microspheres. As shown in Fig. 1 A, we compared thymidine incorporation in highly enriched (>95%) CD4+ T cells stimulated with anti-CD3/CD40L to responses in cultures stimulated by equivalent amounts of anti-CD3 plus antibodies against either MHC class I, MHC class II, or CD28. In addition, some experiments included beads prepared with anti-CD3 plus antiglycophorin as a nonbinding control antibody. We saw insignificant thymidine incorporation in CD4+ T cell cultures stimulated by anti-CD3/glycophorin– or by anti-CD3/MHC–coated beads. As anticipated, stimulation with anti-CD3/CD28–coated beads led to robust proliferative responses. Surprisingly, anti-CD3/CD40L–coated beads also induced significant CD4+ T cell thymidine incorporation. Both humanized and nonhumanized 5C8 (anti-CD40L) performed similarly in this assay. Our result is in contrast to earlier work that demonstrated no anti-CD3/CD40L proliferative responses in purified CD4+ T cells but a dramatic increase in anti-CD3/CD28 proliferative responses after the addition of anti-CD40L 24. In further contrast, we found that adding anti-CD28 to anti-CD3/CD40L–coated beads led to only a modest increase in thymidine incorporation, especially when compared with the effect of adding anti-CD28 to anti-CD3/MHC class I–coated beads. These differences could be attributed to our use of antibodies presented to T cells on three-dimensional beads, as opposed to plate-bound antibodies. Alternatively, it is also possible that there are special agonistic properties of the hu5C8 mAb that are not found with other anti-CD40L reagents.
Figure 1.
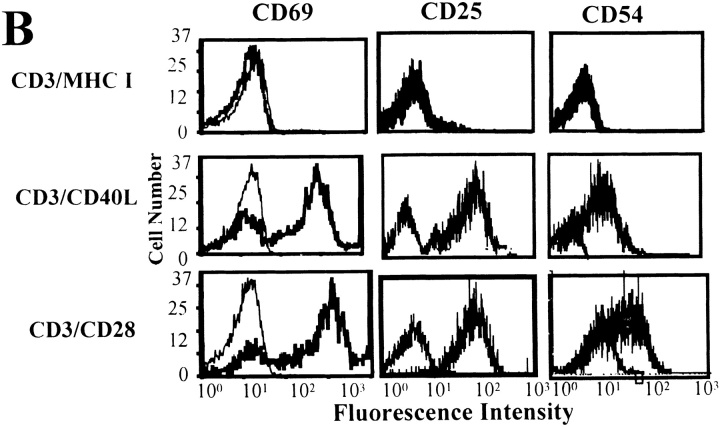
Anti-CD3/CD40L stimulation provides a costimulatory signal for purified human CD4+ T cells. (A) Purified CD4+ T cells were cultured with beads coated with anti-CD3 antibody in combination with anti-CD40L, anti-CD28, or control MHC class I, MHC class II, or glycophorin (Gly.) antibodies at a 3:1 cell to bead ratio for 54 h. Cells were also cultured with anti-CD3/CD28–coated beads in combination with either MHC class I or CD40L mAbs. Cultures were pulsed and harvested after an additional 18 h. Data represent the mean ± SEM from three separate assays. (B) Expression of activation and adhesion molecules in purified CD4+ T cells after 24-h stimulation with either anti-CD3/MHC class I (control), anti-CD3/CD40L, or anti-CD3/CD28. Representative fluorescence histograms of isotype control reagents (thin lines) and either CD69, CD25, or intracellular adhesion molecule (ICAM) (thick lines) are shown. Histograms were first gated on live CD4-FITC–stained cells.
Both CD54 and CD44H are rapidly induced by CD40L ligation 25 26. After CD40L ligation, the role of these molecules is not one of costimulation but of adhesion, presumably to amplify and sustain signals through the TCR. To further test the effects of anti-CD3/CD40L beads on CD4+ T cell activation, we measured the expression of several T cell activation and adhesion molecules (Fig. 1 B). 24 h after anti-CD3/CD40L–mediated activation, the early activation markers CD69, CD25, and CD54 were all expressed at levels comparable to those seen in anti-CD3/CD28–stimulated cells. The intensity of anti-CD3/CD40L–induced responses at 24 h was surprising; therefore, we determined the kinetics of CD25, CD54, and CD69 expression (Table ). Cells were stimulated optimally with anti-CD3/CD28–coated, with anti-CD3/CD40L–coated beads, or with control beads that either bound (anti–MHC I) or did not bind (glycophorin) T cells. Interesting, our results showed that CD3/CD40L coligation had the capacity to induce an early activation response in T cells, resulting in significant increases in the expression of these markers. However, the duration of anti-CD3/CD40L responses decreased markedly by 48 h. These results are comparable to the anti-CD3/CD40L–mediated thymidine incorporation we had previously measured (Fig. 1) and suggest that coligation of CD3 and CD40L on T cells alone does not lead to sustained activation.
Table 1.
Immunophenotyping Kinetics on Stimulated CD4+ T Cells
| Stimulation | Time | % CD25 | % CD54 | % CD69 |
|---|---|---|---|---|
| h | ±SEM | ±SEM | ±SEM | |
| 0 | 6.6 | 1.9 | 15.2 | |
| CD3/glycophorin | 12 | 4.0 ± 0.4 | 0.7 ± 0.1 | 20.8 ± 5.3 |
| 24 | 6.7 ± 0.4 | 0.9 ± 0.1 | 23.9 ± 6.1 | |
| 48 | 7.1 ± 0.2 | 0.8 ± 0.4 | 25.4 ± 6.7 | |
| 72 | 4.8 ± 0.2 | 1.3 ± 0.5 | 23.3 ± 5.5 | |
| 96 | 3.9 ± 0.5 | 0.9 ± 0.2 | 17.7 ± 5.6 | |
| CD3/MHC I | 12 | 2.8 ± 0.1 | 1.2 ± 0.1 | 12.6 ± 5.3 |
| 24 | 3.7 ± 0.4 | 2.2 ± 0.4 | 12.3 ± 4.0 | |
| 48 | 7.6 ± 0.8 | 1.6 ± 0.3 | 14.5 ± 2.3 | |
| 72 | 6.8 ± 1.0 | 1.6 ± 0.4 | 13.4 ± 6.2 | |
| 96 | 4.4 ± 1.2 | 1.2 ± 0.2 | 12.9 ± 5.2 | |
| CD3/CD40L | 12 | 14.9 ± 3.5 | 3.7 ± 2.0 | 57.3 ± 5.0 |
| 24 | 48.3 ± 7.1 | 21.5 ± 4.0 | 68.4 ± 7.1 | |
| 48 | 31.9 ± 4.3 | 11.0 ± 2.3 | 56.0 ± 3.8 | |
| 72 | 24.4 ± 4.5 | 7.1 ± 1.2 | 39.2 ± 7.5 | |
| 96 | 17.2 ± 3.2 | 4.1 ± 0.8 | 29.8 ± 1.3 | |
| CD3/CD28 | 12 | 69.1 ± 1.0 | 14.4 ± 0.5 | 78.1 ± 2.6 |
| 24 | 83.3 ± 2.5 | 31.1 ± 4.2 | 84.8 ± 1.5 | |
| 48 | 92.0 ± 0.7 | 45.3 ± 5.4 | 94.5 ± 0.5 | |
| 72 | 95.6 ± 0.2 | 62.0 ± 5.0 | 91.4 ± 1.0 | |
| 96 | 97.3 ± 1.2 | 44.5 ± 3.5 | 86.2 ± 1.3 |
Cells were stained as described in Materials and Methods and labeled with the indicated mAbs. Percent frequencies are shown for 5 × 104 lymphocytes first gated as CD4-PE+. SEM shown from three to four separate donors.
CD28-independent Effects after Anti-CD3/CD40L Costimulation.
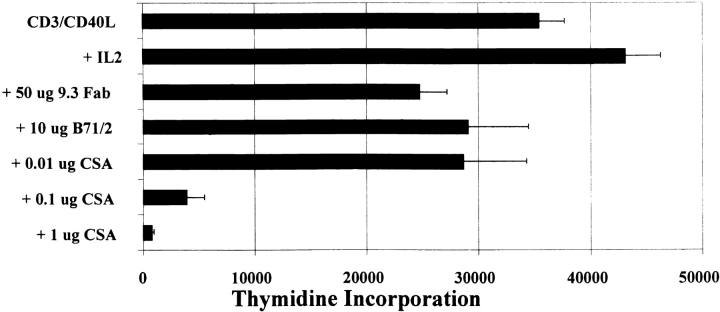
CD28 is necessary for sustained antigen-dependent T cell proliferation 27. Anti-CD28 Fab fragments and antibodies to CD80 and CD86 block T cell proliferation induced by plant lectins or anti-CD3 28. To test whether the CD40L receptor–induced costimulatory effects were CD28 dependent, we treated anti-CD3/CD40L–stimulated cultures with agents that prevent CD28-mediated responses. As shown in Fig. 2, CD40L–induced T cell activation is largely CD28 and CD80/CD86 independent, as judged by the relative inability of these reagents to block T cell thymidine incorporation. In separate reagent control experiments, we found that the CD28 Fab fragments and CD80/CD86 antibodies had retained full inhibitory activity (data not shown). Costimulation through CD28 drives cyclosporin A (CSA)-independent T cell proliferation, cytotoxic activity, and the increased production of cytokines including IL-2, IFN-γ, and TNF-α through both de novo synthesis and mRNA stabilization 29. CSA, which inhibits calcineurin activation through the TCR 30, has been shown to block induction of T cell apoptosis 31. In Fig. 2, we found that, unlike CD28 32, CD40L-induced thymidine incorporation was sensitive to CSA treatment in a dose-dependent fashion. Subsequently, we determined that CD40L expression decreased on human CD4+ T cells as CSA concentrations increased (data not shown). This observation is consistent with previous studies 33 and suggests that concurrent treatment of transplant recipients with CSA and hu5C8 could reduce the effectiveness of events mediated through CD40L. Finally, our results demonstrate that the addition of exogenous IL-2 further augmented the thymidine incorporation induced by anti-CD3/CD40L–coated beads (Fig. 2). This is also consistent with the notion that anti-CD3/CD40L– and anti-CD3/CD28–stimulated T cell activation are distinct, as the addition of exogenous IL-2 did not further stimulate anti-CD3/CD28–stimulated T cells (data not shown).
Figure 2.
Specific effects after anti-CD3/CD40L costimulation. (A) Purified cells were cultured for 72 h with anti-CD3/CD40L–coated beads in combination with titrated amounts of CSA, anti-CD28 (9.3) Fab fragments, anti–B7-1 and anti–B7-2 antibodies, or rIL-2 (300 U/ml). Proliferation was measured as indicated in the legend to Fig. 1.
Measurement of Cytokine mRNA and Protein Levels in Anti-CD3/CD40L–stimulated CD4+ T Cells.
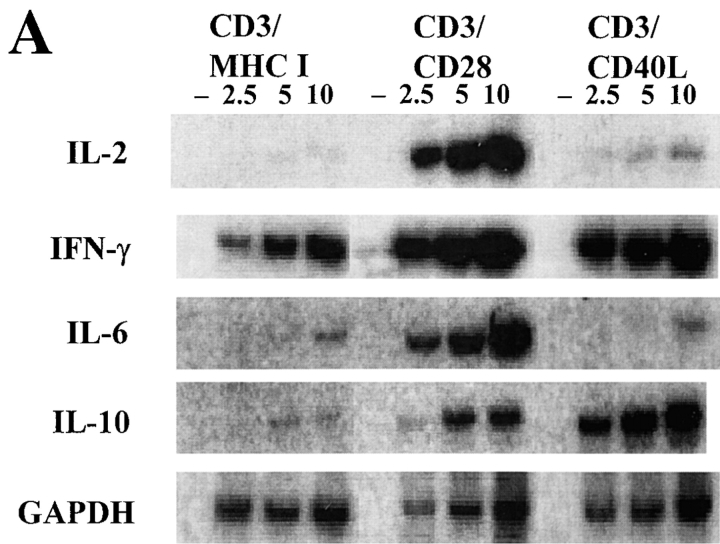
Experiments in CD40L-deficient and CD40-deficient mice have shown that CD40–CD40L interactions play an important role in regulating cytokine responses 34 35. Ligation of CD40L on T cells is crucial for the T cell contact–dependent signaling to induce nitric oxide and cytokine production 36, indicating that CD40L may be involved in inhibiting T cell activation responses. While most available information indicates that CD40L regulates cytokine secretion indirectly through its effects on APCs, the ability of CD40L to induce CD4+ T cell activation suggested that CD40L might have direct effects on T cell cytokine secretion. To test this possibility, we assayed cytokine levels in cells stimulated with anti-CD3/CD40L–coated beads compared with anti-CD3/CD28–coated beads or with beads coated with anti-CD3/MHC class I mAbs (Fig. 3). Using first a semiquantitative RT-PCR–based assay, we measured IL-2, IL-6, IL-10, and IFN-γ mRNA transcripts in cells activated for 24 h (Fig. 3 A). We found high levels of IL-10 and IFN-γ transcripts induced in CD4+ T cell cultures stimulated with anti-CD3/CD40L–coated beads and low or undetectable levels of IL-2 and IL-6 transcripts. In contrast, in cells simulated with anti-CD3/CD28–coated beads, we identified high amounts of IL-2, IFN-γ, IL-6, and IL-10 transcripts. When cultures were examined earlier (after 4–8 h of culture), high levels of TNF-α transcripts were seen in both CD3/CD40L- and CD3/CD28-stimulated cultures while we continued to observe that IL-2 transcripts were restricted to the CD3/CD28-stimulated cultures (data not shown).
Figure 3.
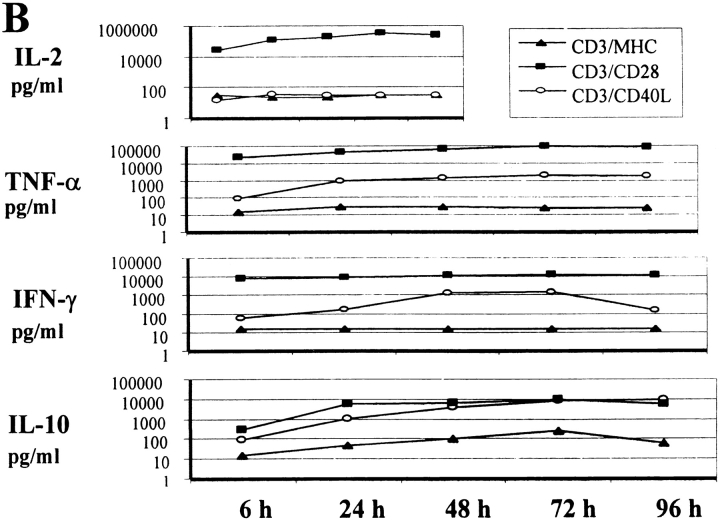
Measurement of cytokine mRNA and protein levels in stimulated CD4+ T cells. (A) Total RNA was extracted from cells cultured as indicated and a semiquantitative RT-PCR–based assay using liquid hybridization was performed on 2.5, 5, or 10 μl of RT products. Samples that lacked RT (–) were included for each condition. Results represent at least three separate assays. (B) Kinetics of cytokine secretion (in pg/ml) after stimulation. Cell-free supernatants from cells cultured as indicated were assayed by ELISA for IL-2, TNF-α, IFN-γ, and IL-10.
We also examined the accumulation of cytokines in the supernatants of CD4+ cells by ELISA (Fig. 3 B). These results confirmed the large (>100-fold) difference in IL-2 secretion between anti-CD3/CD28– and anti-CD3/CD40L–stimulated cultures. In marked contrast, the anti-CD3/CD40L–stimulated cultures had high and sustained levels of IL-10 that were equivalent to those found in CD3/CD28-stimulated cultures. The increase in IL-10 was specific to anti-CD40L, as cultures stimulated with the control CD3/MHC class I beads did not accumulate IL-10. Levels of TNF-α and IFN-γ were also elevated in the supernatants from the CD3/CD40L-stimulated cultures, demonstrating that both Th1- and Th2-type cytokines had been secreted.
CD32+CD40+ Transfectants Induce CD4+ T Cell Proliferation and Production of Immunomodulatory Cytokines, but Not IL-2.
To determine whether the cytokine profiles we found in anti-CD3/CD40L bead–stimulated cells were unique to this model system and/or to the anti-CD40L mAb 5C8, we generated CD40+ transfectants by electroporation of human CD40 into CD32+ mouse L cells (Table ). The CD32 receptor transfected previously in these cells bound soluble anti-CD3 (huOKT3). We found that upon addition of anti-CD3, CD40+ L cells were potent stimulators of purified populations of CD4+ T cells, leading to activation responses similar to those results generated in our studies with anti-CD3/CD154–coated beads. In these experiments, we measured cell proliferation, and IL-2, IL-10, IFN-γ, and TNF-α production. Responses were compared against CD86-transfected CD32+ L cells and CD32+ L cells that bound anti-CD3. As evident in Table , we saw significant thymidine incorporation in anti-CD3–treated CD40+ L cells that was comparable to levels seen in CD80 and CD86 transfectants and almost double the levels in L cells treated with anti-CD3 alone. Of interest, we noted that while anti-CD3–treated CD40+ L cells did not produce IL-2, significant amounts of IL-10 and IFN-γ were made. These results compliment what we have documented above using anti-CD3/CD40L–coated microspheres and suggest that stimulation through CD40–CD40L without concurrent ligation through CD28 has a direct effect on CD4+ T cells that results in the upregulation of the immunosuppressive cytokines IL-10, TNF-α, and IFN-γ, but not IL-2.
Table 2.
Cytokine Expression at 72 h in Supernatants from Stimulated CD4+ T Cells
| Stimulation | IL-2 | IL-10 | IFN-γ | TNF-α | Thymidine incorporation |
|---|---|---|---|---|---|
| CD40+ L cells | <31.2 | 0 | 10 | <15.2 | 2,597 |
| L cells + anti-CD3 | 42 | 163 | 333 | <15.2 | 19,201 |
| CD40+ L cells + anti-CD3 | 63 | 494 | 8,936 | 1,471 | 31,308 |
| B7-2+ L cells + anti-CD3 | 5,418 | 191 | 7,388 | 7,286 | 21,022 |
Supernatants were collected from stimulated cells after 72 h culture, and IL-2, IL-10, IFN-γ, and TNF-α production were measured by ELISA as described in Materials and Methods. Results (in pg/ml) are a representation from two to three separate assays using different donors.
In both anti-CD3/CD40L–ligated and anti-CD3 CD40+–transfected CD4+ T cells, we noted significant IL-10 production. IL-10 is a potent immunosuppressive cytokine that acts to prevent autoimmune disease onset (insulin-dependent diabetes mellitus) in nonobese diabetic (NOD) mice 37 38. Others have shown that chronic activation of both human and murine CD4+ T cells in the presence of IL-10 gives rise to CD4+ T cells with low proliferative capacity that produce high levels of IL-10 and low levels of IL-2. These antigen-specific T cells suppress the proliferation of CD4+ T cells in response to antigen 39. We speculate that CD3/CD40L-activated CD4+ T cells may result in the unique cytokine production profile described, and that these cytokines may contribute to the overall immunosuppressive effects of CD40L mAb observed in vivo.
The production of IL-2 by activated T cells is an autocrine signal that leads to T cell proliferation and the development of effector functions, and thus is important in the understanding of costimulator regulators such as CD40L in T cell responses. Interestingly, unlike earlier reports 9 10, which used the same transfected mouse cell line, we found that in both our bead and CD40-transfected cell models costimulation through anti-CD3/CD40L did not lead to an increase in IL-2. In fact, levels of IL-2 induced by anti-CD3/CD40L stimulation were similar to levels seen in anti-CD3/MHC class I–stimulated cells and dramatically lower than levels seen in anti-CD3/CD28 bead cultures or B7-2–transfected L cells (Fig. 3 B, and Table ). These results clearly demonstrate that although CD25 is upregulated on the surface of anti-CD3/CD40L–stimulated human CD4+ T cells, little or no IL-2 is actually produced after anti-CD3/CD40L ligation. We cannot rule out the possibility that small amounts of IL-2 induced by CD40L costimulation may have been rapidly reincorporated by the cultured cells. Our findings are supported by other studies. Johnson-Leger et al. demonstrated that CD3-primed CD40L+ T cells were incompetent helper cells because they secreted insufficient IL-2 40. They proposed that T cells must first encounter antigen in conjunction with CD28–B7 interactions before CD40L is stabilized on the surface and IL-2 is produced 41. Additionally, Gray and colleagues used CD40L knockout mice to show that CD40L delivers signals to T cells that enhanced the maturation of IFN-γ and played a role in the development of Th2 cells to optimize IL-4 secretion. However, they found that CD40L ligation had no effect on IL-2 production 42. In many regards, our results are similar to those seen by Cayabyab et al. 9 and Peng et al. 10. For instance, they (as we) measured appreciable levels of IL-10 and IFN-γ after culture of T cells with anti-CD3 and CD40+ transfectants. Within the transfected mouse cell system, however, the impact of additional costimulatory pathways cannot be ruled out. For instance, recently an additional and novel homologue of CD80/CD86 was discovered that is induced by TNF-α 43. Further, unlike previous work, we used either purified CD4+ T cells that were stimulated by anti-CD3/CD40L on three-dimensional beads or anti-CD3–treated CD32+CD40+ L cells, both of which allowed close proximity of the coligated signals. Overall, these data strongly suggest that although signals through CD3 and CD40L are sufficient to induce short-term proliferation, they are inadequate to drive IL-2 production and cell cycle progression.
Effects of Anti-CD3/CD40L Costimulation on Apoptosis and Expression of Cell Survival Proteins.
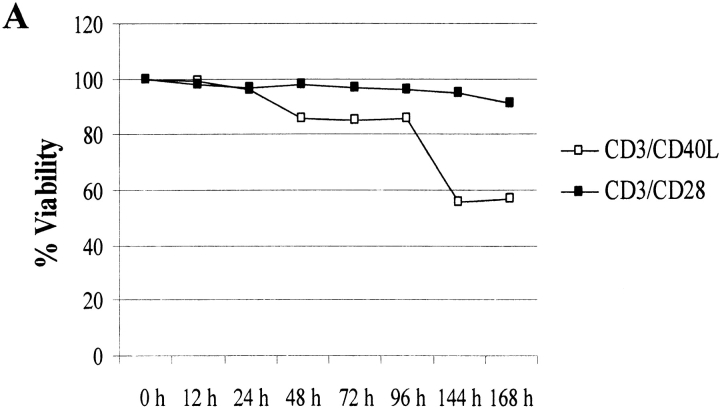
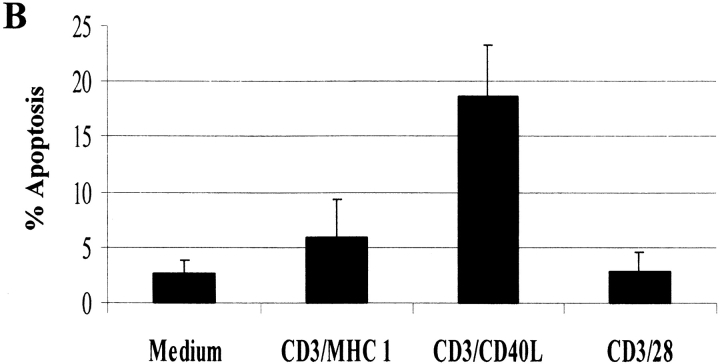
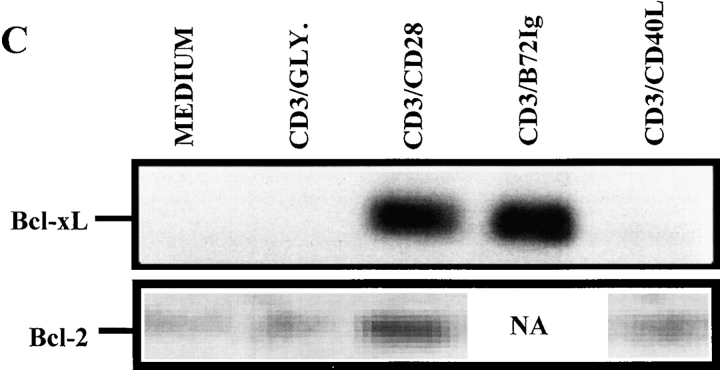
Programmed cell death is an important mediator of homeostasis within the immune system. To test whether engagement of CD40L might enhance the probability of apoptosis, we first measured viability kinetics using trypan blue exclusion. Consistent with our hypothesis, cell viability plummeted to <60% by 144 h (Fig. 4 A) after anti-CD3/CD40L stimulation. In comparison, anti-CD3/CD28–stimulated cultures maintained >95% viability. To test whether CD4+ T cells were dying via apoptosis, we analyzed DNA fragmentation using the TUNEL assay (Fig. 4 B). Compared with anti-CD3/MHC class I–treated (<6%) or anti-CD3/CD28–treated cultures (<3%), high levels of apoptosis were evident at 72 h in anti-CD3/CD40L–coligated cultures (18%). To test whether apoptosis was mediated by Fas or in an autocrine fashion by TNF-α secretion, we treated anti-CD3/CD40L–stimulated cultures with neutralizing antibodies to FasL or TNF-α (data not shown). Treatment with these neutralizing antibodies did not appreciatively decrease levels of anti-CD3/CD40L–induced apoptosis, suggesting an independent mechanism of death. As CD28-mediated costimulation is known to stimulate production of the cell survival protein Bcl-xL within T cells 44, we assayed anti-CD3/CD40L–stimulated T cells to see if a failure to induce Bcl-xL might correlate with the apoptosis we had observed. Consistent with the increased apoptosis observed in anti-CD3/CD40L–stimulated T cells, using immunoprecipitation and Western blotting we found a complete lack of Bcl-xL (Fig. 4 C). In contrast, CD4+ T cells exhibited high levels of Bcl-xL after stimulation with anti-CD3/CD28 or with anti-CD3/B7-2–Ig. We saw only minor differences in Bcl-2 expression when CD28 and CD40L-costimulated T cells were assayed (Fig. 4 C). It is likely that the failure to enhance Bcl-xL in an environment where IL-2 was limited could result in significant apoptosis in these cultures.
Figure 4.
Effects of anti-CD3/CD40L costimulation on apoptosis and expression of cell survival proteins. (A) Viability kinetics in anti-CD3/CD40L– and anti-CD3/CD28–stimulated cultures. Trypan blue uptake was assessed in cell suspensions. (B) Apoptosis in cultured cells was measured using the TUNEL method to measure dUTP incorporation into single-strand DNA breaks. CD4+ T cells irradiated with 1,000-Gy γ rays 24 h previously served as a positive control for the assay. Values represent the mean ± SEM from five individual experiments. (C) Expression of the cell survival gene Bcl-xL and Bcl-2. Protein lysates from 107 cells stimulated for 72 h were subjected to sequential immunoprecipitation with a Bcl-xL–specific mAb and immunoblotted with a Bcl-xL rabbit polyclonal antibody as described.
CD28 Fails to Rescue CD4+ T Cells from Apoptosis after Anti-CD3/CD40L Coligation.
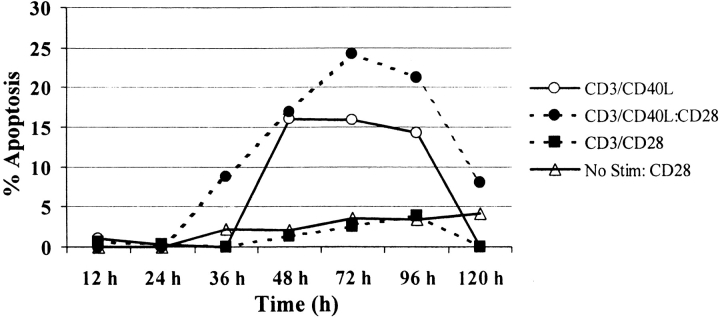
While T cell costimulatory molecules can work synergistically, fundamental differences exist among these molecules in their capacity to regulate T cell responses 45. To test whether CD28 ligation could reverse CD40L-induced apoptosis, we analyzed the kinetics of apoptosis among CD4+ T cells after the addition of beads containing anti-CD3/CD28 in cultures that were previously either unstimulated or stimulated with anti-CD3/CD40L (Fig. 5). Apoptosis was measured 24 h after restimulation of cultured cells. Interestingly, we found that even short culture (12 h) with anti-CD3/CD40L induced considerable apoptosis upon restimulation with anti-CD3/CD28 24 h later. Conversely, relatively little apoptosis was seen in unstimulated cultures restimulated with anti-CD3/CD28. In all cases, anti-CD3/CD28 failed to decrease apoptosis in cells previously stimulated with anti-CD3/CD40L.
Figure 5.
CD28 fails to rescue CD4+ T cells from apoptosis after anti-CD3/CD40L coligation. Purified CD4+ T cells were initially cultured at 106 cells/ml with or without (open triangles) anti-CD3/CD28–coated (filled squares) or anti-CD3/CD40L–coated (circles) microspheres at a cell to bead ratio of 3:1. At the times indicated, cultures were split. In half of the samples the beads were removed, and the cells were then restimulated with anti-CD3/CD28 beads. Apoptosis was measured by TUNEL analysis (as in the legend to Fig. 3) 24 h after restimulation.
Our results point to an early and important role for CD40L in the modulation of T cell responses. Others have shown that CD40L expression is transient, and that the receptor is rapidly internalized after binding CD40 46. Recently, it has been shown that CD40L is specifically regulated at the level of mRNA stability and that this regulation is not influenced by anti-CD3/CD28 costimulation 47 48. Our in vivo findings show that the effects of hu5C8 are not solely mediated through the upregulation of CD80/CD86, as, in a kidney allograft transplantation model, blocking anti-B7 mAbs did not yield similar results as those evident after treatment with hu5C8. Additionally, anti-CD80/CD86 mAbs act synergistically with hu5C8. This implies that hu5C8 imparts effects on transplanted grafts apart from B7 and therefore CD28-induced events (our unpublished data).
In this study, we have demonstrated that ligation of CD40L using the anti-CD40L mAb hu5C8 leads to specific CD28-independent short-term CD4+ T cell activation. However, anti-CD3/CD40L–mediated activation is aborted due to the enhanced production of immunomodulatory cytokines. Ultimately, this resulted in CD4+ T cell apoptosis consequent to the failure to induce IL-2, Bcl-2, or Bcl-xL expression. We believe that these in vitro results could point to a mechanism by which hu5C8 is working in vivo within our primate kidney allograft transplantation model. Others have recently highlighted the importance of apoptosis in the induction of long-term graft survivability 49 50. Li et al. demonstrated that treatments that enhance the induction of apoptosis, such as CD28–B7 and CD40–CD40L blockade or costimulation blockade in conjunction with rapamycin treatment, promote peripheral allograft tolerance 50. Comparable to our results, anti-CD3/CD40L responses were blocked upon the addition of CSA. Wells et al. 49 provided additional support for a crucial role of apoptosis in transplantation tolerance. In studies that used Bcl-xL–transgenic and IL-2–deficient mice, they found that models that contained defective passive or active T cell apoptotic pathways were resistant to the induction of transplantation tolerance. Interestingly, the transplantation tolerance induced in the models presented in these studies and their previous work did not appear to depend on Fas-induced apoptosis 51. Similarly, in our work, a neutralizing mAb against Fas did not diminish the level of apoptosis that we measured after anti-CD3/CD40L ligation (data not shown). The mechanism by which costimulation blockage induces apoptosis of alloresponsive T cells is as yet unknown. It will be important to determine how current costimulatory regimens influence this process.
Our results imply that CD40L may directly signal downstream pathways in T cells. This is supported by data that indicate that the cytoplasmic domain of human and mouse CD40L is 82% identical 52 and contains structural elements that are functionally important 46. Aglycosylated hu5C8, which is unable to cross-link CD40L on the surface of T cells, does not prevent acute allograft rejection. Thus, anti-CD40L may exert its effect, at least in part, by direct T cell ligation (our unpublished data). CD40–CD40L interactions influence the gradual amplification of an immune response through the step-wise upregulation of adhesion and activation molecules. The ability of CD40L to regulate a response may depend on the availability of addition coreceptors and/or their cognate ligands as well as the activation status of the T cell. Teleologically, in the absence of additional costimulation, regulation of short-term responses through CD40L ligation could control damage from a prolonged immune response. An emerging question in the field of transplantation biology is how treatment regimens that act through the CD28–B7 and CD40L–CD40 pathways exert long-term effects after cessation of treatment. Defining the diverse roles of CD40–CD40L interactions and gaining a better understanding of the molecular pathways elicited after CD40L ligation will allow clinicians to identify additional therapeutic targets.
CD40L-mediated T Cell Regulation
Acknowledgments
The authors are grateful for the administrative assistance provided by Amy Mosquera and Randal A. Carr and the technical assistance of Mark Shoemaker. We thank Dr. Bruce Levine and Julio Cotte for coordinating and assisting in blood draws at the National Naval Medical Center Blood Bank.
Views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. government. Work was funded under Naval Medical Research Center Work Unit M0095.003.1921.
Footnotes
Abbreviations used in this paper: CSA, cyclosporin A; CTLA4-Ig, CTL-associated antigen 4–Ig; hu5C8, humanized anti-CD40L mAb clone 5C8; RT, reverse transcriptase; TUNEL, terminal deoxynucleotidyl transferase–mediated dUTP nick end-labeling.
References
- Ochs H.D., Hollenbaugh D., Aruffo A. The role of CD40L (gp39)/CD40 in T/B cell interaction and primary immunodeficiency. Semin. Immunol. 1994;6:337–341. doi: 10.1006/smim.1994.1042. [DOI] [PubMed] [Google Scholar]
- Roy M., Waldschmidt T., Aruffo A., Ledbetter J.A., Noelle R.J. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J. Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- Castle B.E., Kishimoto K., Stearns C., Brown M.L., Kehry M.R. Regulation of expression of the ligand for CD40 on T helper lymphocytes. J. Immunol. 1993;151:1777–1788. [PubMed] [Google Scholar]
- Lederman S., Yellin M.J., Krichevsky A., Belko J., Lee J.J., Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help) J. Exp. Med. 1992;175:1091–1101. doi: 10.1084/jem.175.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal I.S., Xu J., Flavell R.A. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- van Essen D., Kikutani H., Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature. 1995;378:620–623. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]
- Kennedy M.K., Mohler K.M., Shanebeck K.D., Baum P.R., Picha K.S., Otten-Evans C.A., Janeway C.A., Jr., Grabstein K.H. Induction of B cell costimulatory function by recombinant murine CD40 ligand. Eur. J. Immunol. 1994;24:116–123. doi: 10.1002/eji.1830240118. [DOI] [PubMed] [Google Scholar]
- Caux C., Massacrier C., Vanbervliet B., Dubois B., van Kooten C., Durand I., Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayabyab M., Phillips J.H., Lanier L.L. CD40 preferentially costimulates activation of CD4+ T lymphocytes. J. Immunol. 1994;152:1523–1531. [PubMed] [Google Scholar]
- Peng X., Kasran A., Warmerdam P.A., de Boer M., Ceuppens J.L. Accessory signaling by CD40 for T cell activationinduction of Th1 and Th2 cytokines and synergy with interleukin-12 for interferon-gamma production. Eur. J. Immunol. 1996;26:1621–1627. doi: 10.1002/eji.1830260732. [DOI] [PubMed] [Google Scholar]
- Brenner B., Koppenhoefer U., Grassme H., Kun J., Lang F., Gulbins E. Evidence for a novel function of the CD40 ligand as a signalling molecule in T-lymphocytes. FEBS Lett. 1997;417:301–306. doi: 10.1016/s0014-5793(97)01306-9. [DOI] [PubMed] [Google Scholar]
- Koppenhoefer U., Brenner B., Lang F., Gulbins E. The CD40-ligand stimulates T-lymphocytes via the neutral sphingomyelinasea novel function of the CD40-ligand as signalling molecule. FEBS Lett. 1997;414:444–448. doi: 10.1016/s0014-5793(97)01035-1. [DOI] [PubMed] [Google Scholar]
- Zheng X.X., Markees T.G., Hancock W.W., Li Y., Greiner D.L., Li X.C., Mordes J.P., Sayegh M.H., Rossini A.A., Strom T.B. CTLA4 signals are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J. Immunol. 1999;162:4983–4990. [PubMed] [Google Scholar]
- Saito K., Sakurai J., Ohata J., Kohsaka T., Hashimoto H., Okumura K., Abe R., Azuma M. Involvement of CD40 ligand-CD40 and CTLA4-B7 pathways in murine acute graft-versus-host disease induced by allogeneic T cells lacking CD28. J. Immunol. 1998;160:4225–4231. [PubMed] [Google Scholar]
- Larsen C.P., Pearson T.C. The CD40 pathway in allograft rejection, acceptance, and tolerance. Curr. Opin. Immunol. 1997;9:641–647. doi: 10.1016/s0952-7915(97)80043-x. [DOI] [PubMed] [Google Scholar]
- Parker D.C., Greiner D.L., Phillips N.E., Appel M.C., Steele A.W., Durie F.H., Noelle R.J., Mordes J.P., Rossini A.A. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc. Natl. Acad. Sci. USA. 1995;92:9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson R.N., Chang A.C., Blum M.G., Blair K.S., Scott M.A., Atkinson J.B., Collins B.J., Zhang J.P., Thomas D.W., Burkly L.C., Miller G.G. Prolongation of primate cardiac allograft survival by treatment with anti-CD40 ligand (CD154) antibody. Transplantation. 1999;68:1800–1805. doi: 10.1097/00007890-199912150-00026. [DOI] [PubMed] [Google Scholar]
- Larsen C.P., Elwood E.T., Alexander D.Z., Ritchie S.C., Hendrix R., Tucker-Burden C., Cho H.R., Aruffo A., Hollenbaugh D., Linsley P.S. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- Kalled S.L., Cutler A.H., Datta S.K., Thomas D.W. Anti-CD40 ligand antibody treatment of SNF1 mice with established nephritispreservation of kidney function. J. Immunol. 1998;160:2158–2165. [PubMed] [Google Scholar]
- Kirk A.D., Harlan D.M., Armstrong N.N., Davis T.A., Dong Y., Gray G.S., Hong X., Thomas D., Fechner J.H., Jr., Knechtle S.J. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc. Natl. Acad. Sci. USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk A.D., Burkly L.C., Batty D., Baumgartner R.E., Berning J.D., Fechner J.H., Jr., Germond R.L., Kampen R.L., Patterson N.B., Swanson S.J. Humanized anti-CD154 monoclonal antibody treatment prevents renal allograft rejection in non-human primates. Nat. Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- Blair P.J., Boise L.H., Perfetto S.P., Levine B.L., McCrary G., Wagner K.F., St. Louis D.C., Thompson C.B., Siegel J.N., June C.H. Impaired induction of the apoptosis-protective protein Bcl-XL in asymptomatic HIV-infected individuals. J. Clin. Immunol. 1997;17:234–246. doi: 10.1023/a:1027310612323. [DOI] [PubMed] [Google Scholar]
- Wong M.T., Dolan M.J., Kozlow E., Doe R., Melcher G.P., Burke D.S., Boswell R.N., Vahey M. Patterns of virus burden and T cell phenotype are established early and are correlated with the rate of disease progression in human immunodeficiency virus type 1-infected persons. J. Infect. Dis. 1996;173:877–887. doi: 10.1093/infdis/173.4.877. [DOI] [PubMed] [Google Scholar]
- Blotta M.H., Marshall J.D., DeKruyff R.H., Umetsu D.T. Cross-linking of the CD40 ligand on human CD4+ T lymphocytes generates a costimulatory signal that up-regulates IL-4 synthesis. J. Immunol. 1996;156:3133–3140. [PubMed] [Google Scholar]
- Shinde S., Wu Y., Guo Y., Niu Q., Xu J., Grewal I.S., Flavell R., Liu Y. CD40L is important for induction of, but not response to, costimulatory activity. ICAM-1 as the second costimulatory molecule rapidly up-regulated by CD40L. J. Immunol. 1996;157:2764–2768. [PubMed] [Google Scholar]
- Gurunathan S., Irvine K.R., Wu C.Y., Cohen J.I., Thomas E., Prussin C., Restifo N.P., Seder R.A. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J. Immunol. 1998;161:4563–4571. [PMC free article] [PubMed] [Google Scholar]
- Chambers C.A., Allison J.P. Co-stimulation in T cell responses. Curr. Opin. Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- June C.H., Bluestone J.A., Nadler L.M., Thompson C.B. The B7 and CD28 receptor families. Immunol. Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Thompson C.B., Lindsten T., Ledbetter J.A., Kunkel S.L., Young H.A., Emerson S.G., Leiden J.M., June C.H. CD28 activation pathway regulates the production of multiple T- cell-derived lymphokines/cytokines. Proc. Natl. Acad. Sci. USA. 1989;86:1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S.L., Crabtree G.R. The mechanism of action of cyclosporin A and FK506. Immunol. Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Shi Y.F., Sahai B.M., Green D.R. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989;339:625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- June C.H., Ledbetter J.A., Gillespie M.M., Lindsten T., Thompson C.B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol. Cell. Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuleihan R., Ramesh N., Horner A., Ahern D., Belshaw P.J., Alberg D.G., Stamenkovic I., Harmon W., Geha R.S. Cyclosporin A inhibits CD40 ligand expression in T lymphocytes. J. Clin. Invest. 1994;93:1315–1320. doi: 10.1172/JCI117089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong L., Xu J.C., Grewal I.S., Kima P., Sun J., Longley B.J., Ruddle N.H., McMahon-Pratt D., Flavell R.A. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- Kamanaka M., Yu P., Yasui T., Yoshida K., Kawabe T., Horii T., Kishimoto T., Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- Shu U., Kiniwa M., Wu C.Y., Maliszewski C., Vezzio N., Hakimi J., Gately M., Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur. J. Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- Moritani M., Yoshimoto K., Ii S., Kondo M., Iwahana H., Yamaoka T., Sano T., Nakano N., Kikutani H., Itakura M. Prevention of adoptively transferred diabetes in nonobese diabetic mice with IL-10–transduced islet-specific Th1 lymphocytes. A gene therapy model for autoimmune diabetes. J. Clin. Invest. 1996;98:1851–1859. doi: 10.1172/JCI118986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.X., Steele A.W., Hancock W.W., Stevens A.C., Nickerson P.W., Roy-Chaudhury P., Tian Y., Strom T.B. A noncytolytic IL-10/Fc fusion protein prevents diabetes, blocks autoimmunity, and promotes suppressor phenomena in NOD mice. J. Immunol. 1997;158:4507–4513. [PubMed] [Google Scholar]
- Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Johnson-Leger C., Christenson J.R., Holman M., Klaus G.G. Evidence for a critical role for IL-2 in CD40-mediated activation of naive B cells by primary CD4 T cells. J. Immunol. 1998;161:4618–4626. [PubMed] [Google Scholar]
- Johnson-Leger C., Christenson J., Klaus G.G. CD28 co-stimulation stabilizes the expression of the CD40 ligand on T cells. Int. Immunol. 1998;10:1083–1091. doi: 10.1093/intimm/10.8.1083. [DOI] [PubMed] [Google Scholar]
- Poudrier J., van Essen D., Morales-Alcelay S., Leanderson T., Bergthorsdottir S., Gray D. CD40 ligand signals optimize T helper cell cytokine productionrole in Th2 development and induction of germinal centers. Eur. J. Immunol. 1998;28:3371–3383. doi: 10.1002/(SICI)1521-4141(199810)28:10<3371::AID-IMMU3371>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Swallow M.M., Wallin J.J., Sha W.C. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFα. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- Boise L.H., Minn A.J., Accavitti M.A., June C.H., Lindsten T., Thompson C.B. CD28 costimulation can promote T cell survival by inducing the expression of Bcl-XL . Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Yashiro Y., Tai X.G., Toyo-oka K., Park C.S., Abe R., Hamaoka T., Kobayashi M., Neben S., Fujiwara H. A fundamental difference in the capacity to induce proliferation of naive T cells between CD28 and other co-stimulatory molecules. Eur. J. Immunol. 1998;28:926–935. doi: 10.1002/(SICI)1521-4141(199803)28:03<926::AID-IMMU926>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Yellin M.J., Sippel K., Inghirami G., Covey L.R., Lee J.J., Sinning J., Clark E.A., Chess L., Lederman S. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J. Immunol. 1994;152:598–608. [PubMed] [Google Scholar]
- Rigby W.F., Waugh M.G., Hamilton B.J. Characterization of RNA binding proteins associated with CD40 ligand (CD154) mRNA turnover in human T lymphocytes. J. Immunol. 1999;163:4199–4206. [PubMed] [Google Scholar]
- Ford G.S., Barnhart B., Shone S., Covey L.R. Regulation of CD154 (CD40 ligand) mRNA stability during T cell activation. J. Immunol. 1999;162:4037–4044. [PubMed] [Google Scholar]
- Wells A.D., Li X.C., Li Y., Walsh M.C., Zheng X.X., Wu Z., Nunez G., Tang A., Sayegh M., Hancock W.W. Requirement for T-cell apoptosis in the induction of peripheral transplantation. Nat. Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- Li Y., Li X.C., Zheng X.X., Wells A.D., Turka L.A., Strom T.B. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft. Nat. Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- Li X.C., Li Y., Dodge I., Wells A.D., Zheng X.X., Turka L.A., Strom T.B. Induction of allograft tolerance in the absence of Fas-mediated apoptosis. J. Immunol. 1999;163:2500–2507. [PubMed] [Google Scholar]
- Farrah T., Smith C.A. Emerging cytokine family. Nature. 1992;358:26. doi: 10.1038/358026b0. [DOI] [PubMed] [Google Scholar]