Abstract
Immature dendritic cells (DCs) constitutively take up large volumes of fluid by macropinocytosis and concentrate the macrosolutes in the endocytic compartment. This concentration mechanism that is the basis of their high capacity to present soluble antigens requires that DCs be capable of rapidly exchanging water across their membranes. We report that two members of the aquaporin family, AQP3 and AQP7, are expressed in immature DCs and are downregulated after maturation. Treatment of DCs with p-chloromercuribenzenesulphonate (pCMBS), a mercuric drug that blocks aquaporins, inhibited uptake and concentration of macrosolutes taken up by fluid phase endocytosis and led to dramatic cell swelling. In contrast, pCMBS did not affect receptor-mediated endocytosis via the mannose receptor. These findings indicate that aquaporins represent essential elements of a volume control mechanism that allows DCs to concentrate macrosolutes taken up via macropinocytosis.
Keywords: dendritic cells, fluid phase endocytosis, aquaporins, antigen uptake, volume control
Introduction
A high endocytic capacity is a fundamental property of immature dendritic cells (DCs) and is instrumental in allowing efficient uptake and presentation of incoming antigens. This endocytic activity is expressed at the highest level in the immature stage and is lost as DCs mature and migrate to the secondary lymphoid organs 1. Immature monocyte-derived DCs can capture soluble antigens via two distinct mechanisms. The first is represented by receptors such as the mannose receptor (MR) and FcγRII, which allow efficient uptake of mannosylated antigens or immune complexes via classical clathrin-mediated endocytosis 2 3 4. The second mechanism of antigen uptake is macropinocytosis. Whereas in other cells such as macrophages or epithelial cells macropinocytosis is induced only transiently by growth factors or phorbol ester 5 6 7, in immature DCs, macropinocytosis occurs in a constitutive fashion and at a high rate and allows continuous capture of macrosolutes present in the fluid phase 2. The macrosolutes that are taken up in the fluid phase accumulate with time in the endocytic compartment, where they are loaded on newly synthesized and recycling MHC class II molecules 8 but may also be released into the cytosol, where they become accessible to the class I antigen presentation pathway 9.
It was estimated that in one hour, a DC can take up as much fluid as its own cell volume 2. To explain how immature DCs could maintain their volume in spite of such an enormous fluid intake, we hypothesized that they should possess mechanisms to allow rapid exchange of salts and water through their membranes. These mechanisms would at the same time maintain cell volume and concentrate macrosolutes.
Cells regulate their volumes by selective transport of ions and water. The concerted action of amiloride-sensitive sodium channels and of anion antiporters results in the net transport of NaCl through the membrane 10 11 12. Water follows this salt transport passively, but its diffusion is slow and can be rate limiting. In cells that require a high capacity for water transport such as epithelial cells of the kidney and erythrocytes, this process is facilitated by specific channels known as aquaporins 13. Aquaporins can be divided into two groups depending on structural and functional properties. AQP3, AQP7, and AQP9 are structurally related to the bacterial glycerol facilitator. AQP3 and AQP7 are permeable to water as well as to small neutral solutes such as urea and glycerol, whereas AQP9 transports urea but not glycerol. The other group of aquaporins is structurally related to the bacterial water channel. With the exception of AQP4, all aquaporins are inhibited by the organomercurial sulphydryl-reactive reagent p-chloromercuribenzenesulphonate (pCMBS) that binds to critical cysteine residues of the water channel 14 15 16. This drug does not alter cell membrane conductance to the major ions of the intra- or extracellular fluids 17 18.
We report here that two aquaporins, AQP3 and AQP7, are expressed in immature DCs and are downregulated upon DC maturation. The fact that pCMBS can reversibly inhibit fluid phase but not receptor-mediated endocytosis indicates that aquaporins play an essential role in the process of antigen uptake and concentration via fluid phase macropinocytosis.
Materials and Methods
Media and Reagents.
The medium used throughout was RPMI 1640 supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 1% pyruvate, 50 μg/ml kanamycin (GIBCO BRL), 5 × 10−5 M 2-ME (Merck), and 10% FCS (Hyclone Labs., Inc.). Human recombinant IL-4 was produced by PCR cloning and expression in the myeloma-based expression system 19. GM-CSF (Leucomax) was purchased from Novartis. TGF-β was purchased from R & D Systems, Inc. Lucifer yellow CH potassium salt (LY) and lysine-fixable FITC–dextran (DX; M r = 40,000) was purchased from Molecular Probes, Inc. as lyophilized powder, reconstituted in RPMI buffered with 25 mM Hepes, and spun in a microfuge before use to remove aggregates. Mannan from Saccharomyces cerevisiae, pCMBS, LPS, β-ME, and 5-(N,N-dimethyl) amiloride (DMA) was purchased from Sigma Chemical Co. Purified rabbit anti–rat aquaporin 3 antiserum was purchased from Alpha Diagnostic International.
Generation of Immature and Mature DCs.
DCs were generated from human PBMCs as described 3 with minor modifications. In brief, monocytes were purified by positive selection using anti-CD14–conjugated magnetic microbeads (Miltenyi Biotec). To obtain immature DCs, the CD14+ cells (∼99% pure) were cultured at 3 × 105 per milliliter in RPMI–10% FCS supplemented with 50 ng/ml GM-CSF and 1,000 U/ml human recombinant IL-4 for 7 d. DC maturation was induced by addition of 1 μg/ml of LPS (from Salmonella abortus equi) for 40 h. Macrophages were generated from monocytes cultured for 10 d in RPMI–20% human serum and 10 ng/ml M-CSF (R & D Systems, Inc.). TGF-β (R & D Systems, Inc.) was used at 10 ng/ml.
Quantitation of Endocytosis in Single Cells by FACS® Analysis.
Cells were incubated in RPMI–10% FCS buffered with 25 mM Hepes at 37°C. FITC–DX and LY were added at a final concentration of 1 mg/ml. After different incubation times, the cells were washed four times with cold PBS containing 1% FCS and 0.01% NaN3 and were analyzed on a FACScan™ (Becton Dickinson) using propidium iodide to exclude dead cells. The background (cells pulsed at 0°C) was subtracted. In some experiments, the cells were incubated for 5 min with different concentrations of pCMBS and/or DMA before adding the marker.
Reverse Transcriptase–PCR.
Total RNA was extracted using RNAzol B (Tel-Test, Inc.). Single-stranded cDNA was synthesized from 2 μg of total RNA using AMV reverse transcriptase (RT; Promega Corp.). Primers used for the PCR were: AQP1, 5′-CATGGCCAGCGAGTTCAAG and 5′-CTGTGAGGTCACTGCTGCG, amplifying a 719-bp product; AQP3, 5′-ATGGGTCGACAGAAGGAGCT and 5′-TCAGATCTGCTCCTTGTGCT, amplifying an 879-bp product; AQP7, 5′-GTC-TCCTGGTCCGTGATAG and 5′-CTTCATACGCCACAGAATCC, amplifying an 834-bp product; AQP9, 5′-ATGCAGCCTGAGGGAGCAG and 5′-CAGAGCTGAGCATGCCACT, amplifying a 905-bp product; and β-actin, 5′-ACA-CTGTGCCCATCTACGAGGGG and 5′-ATGATGGAGTTG-AAGGTAGTTTCGTGGAT, amplifying a 340-bp product. The PCR was performed in a DNA thermal cycler (Perkin-Elmer Corp.). We performed 30 cycles for AQP3 and AQP7 and 25 cycles for AQP9 using the following conditions: 10 s at 94°C, 50 s at 63°C, and 50 s at 72°C. β-Actin mRNA amplification was performed on the cDNA as a positive control for reaction efficiency and was done for 25 cycles.
Immunoblotting.
Cells (5 × 106) were lysed at 4°C in lysis buffer (100 mM Tris/HCL, pH 7.4, 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin). Lysates were solved by 10% SDS-PAGE under reducing conditions, and proteins were transferred to nitrocellulose membranes (Hybond-C super; Amersham). After blocking with PBS/0.1% Tween/5% nonfat dry milk, the membrane was incubated with rabbit anti–rat AQP3 antiserum followed by horseradish peroxidase–conjugated goat anti–rabbit IgG (Southern Biotechnology Associates). Proteins were visualized by chemiluminescence using ECL detection reagents (Amersham).
Results
AQP3 and AQP7 Are Expressed in Immature DCs and Downregulated upon Maturation.
Preliminary experiments using RT-PCR revealed that AQP3, AQP7, and AQP9 mRNAs were present in immature DCs, whereas AQP1 and AQP2 were absent. As shown in Fig. 1 A, the expression of AQP3 and AQP7 is developmentally regulated in monocyte-derived DCs. These aquaporins were absent in monocytes, which represent the precursors of immature DCs, but were induced when monocytes differentiated into immature DCs in cultures supplemented with GM-CSF and IL-4 and were downregulated when DCs matured in response to LPS. AQP3 and AQP7 were not expressed in macrophages but were expressed in monocytes cultured with GM-CSF, IL-4, and TGF-β, a cytokine combination that promotes generation of Langerhans cells 20. AQP3 and AQP7 were also expressed in monocytes cultured with GM-CSF and TGF-β, indicating that IL-4 is not required for their induction. In contrast, AQP9 mRNA was detected in all CD14-derived cells and was not influenced by the maturation stage.
Figure 1.
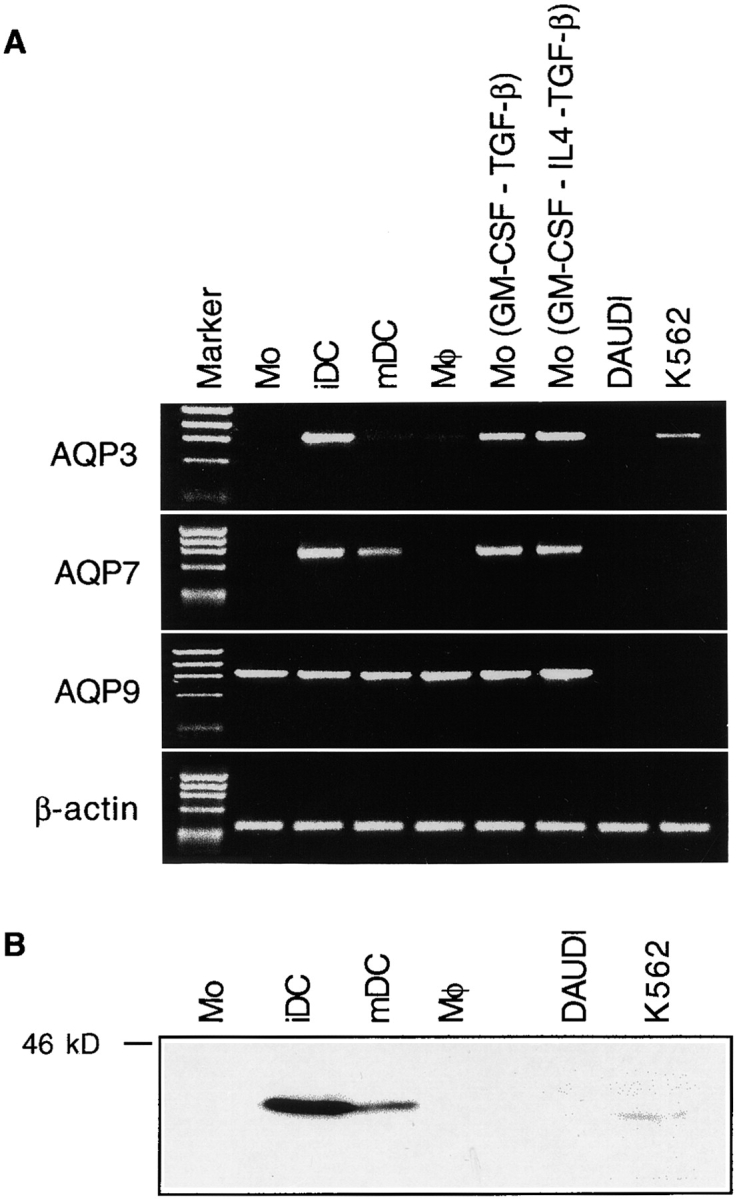
AQP3 and AQP7 are selectively expressed in immature DCs and downregulated after maturation. (A) RT-PCR results showing AQP3, AQP7, AQP9, and β-actin expression in monocytes (Mo), immature DCs (iDC), LPS-matured DCs (mDC), macrophages (Mφ), monocytes cultured with GM-CSF and TGF-β (GM-CSF-TGF-β), GM-CSF, IL-4, and TGF-β (GM-CSF-IL4-TGF-β), DAUDI, and K-562. One representative experiment out of six performed with cDNA from different donors. (B) Western blot with rabbit polyclonal anti-AQP3 antiserum (300 μg protein per lane). One representative experiment out of three performed.
The presence of AQP3 protein was studied by Western blot with a specific antiserum (Fig. 1 B). This analysis confirmed that the highest levels of AQP3 protein were present in immature DCs and showed that this level decreased upon maturation. Monocytes and macrophages did not express AQP3 protein at detectable levels. Altogether, these results indicate that AQP3 and AQP7 expression is developmentally regulated in DCs and decreases after maturation as does macropinocytosis 2.
pCMBS Inhibits Uptake and Concentration of Macrosolutes Taken up by Fluid Phase But Not by Receptor-mediated Endocytosis.
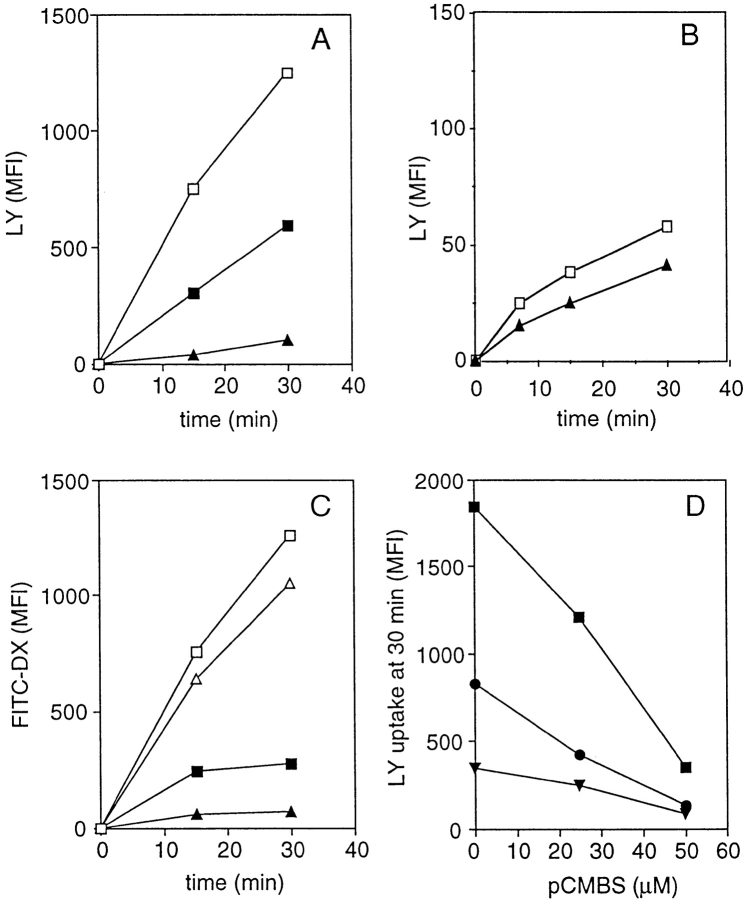
We have previously shown that in immature DCs, LY is taken up exclusively by fluid phase endocytosis, whereas FITC–DX is taken up via both fluid phase and MR-mediated endocytosis 2. pCMBS is a mercuric drug that inhibits AQP channel function by binding to two cysteine residues, an effect that leads to a block of the aqueous pore and which can be reversed by reducing reagents such as β-ME 14 15 16. We tested whether in immature DCs pCMBS could differentially affect the uptake and accumulation of macrosolutes taken up via fluid phase versus receptor-mediated endocytosis.
As shown in Fig. 2 A, pCMBS inhibited the uptake of LY by immature DCs up to 90%, whereas in monocytes, which show a 20-fold lower LY uptake, the inhibition was only 30% (Fig. 2 B). In contrast to the dramatic inhibition of LY uptake, the uptake of FITC–DX by immature DCs was only slightly affected by pCMBS (∼10–15%). This small decrease reflects the small contribution of macropinocytosis to FITC–DX uptake that occurs mainly via MR. Indeed, when MR was blocked by high concentrations of mannan, the residual uptake of FITC–DX (which was then entirely mediated by fluid phase endocytosis) could be blocked by pCMBS up to ∼80% (Fig. 2 C). These results indicate that pCMBS inhibits uptake and concentration of macrosolutes taken up via fluid phase endocytosis, whereas it does not interfere with receptor-mediated endocytosis.
Figure 2.
pCMBS blocks fluid phase but not receptor-mediated endocytosis in immature DCs. (A) Time-dependent accumulation of LY in immature DCs in the absence (□) or presence of 50 (▪) or 100 μM (▴) of pCMBS. (B) Time-dependent accumulation of LY in monocytes in the absence (□) or presence of 100 μM of pCMBS (▴). (C) Accumulation of FITC–DX in immature DCs in the absence (□, ▪) or presence (▵, ▴) of 100 μM of pCMBS. The experiment was done in the absence (□, ▵) or presence (▪, ▴) of 2 mg/ml of mannan to block MR. (D) Additive inhibitory effect of pCMBS and DMA on LY accumulation in immature DCs. Immature DCs were incubated with LY for 30 min in the presence of different concentrations of pCMBS alone (▪) or with added DMA at 125 μM (•) or 500 μM (▾).
Macropinocytosis can also be inhibited by amiloride and amiloride analogues, which inhibit Na+/H+ exchange 2 7. Treatment with DMA inhibited LY uptake as with pCMBS, and the two drugs showed additive effects when used at suboptimal concentrations (Fig. 2 D). These results suggest that uptake and concentrations of fluid phase markers requires both aquaporins and amiloride-sensitive Na+/H+ channels.
Loss of Volume Control in DCs Treated with pCMBS.
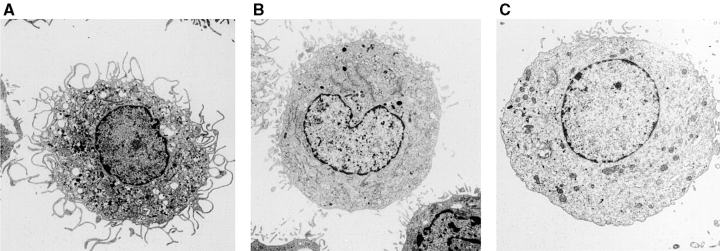
The inhibition of fluid phase uptake after treatment with pCMBS was accompanied by a prominent swelling of DCs. pCMBS-treated DCs showed increased size, loss of veils, and plumpness (Fig. 3). The drug-induced swelling was accelerated in the presence of an oncotic load. If pCMBS was added in the presence of medium alone, swelling occurred slowly within 2 h. In contrast, in the presence of 1 mg/ml FITC–DX (or DX alone), swelling occurred rapidly within 5 min (Fig. 4 A). By measuring the diameter of cells in suspension, the increase in cell volume was estimated to be on average 3.7-fold (i.e., from 1,000 to 3,760 μm3). This effect cannot be explained by the osmotic gradient induced by FITC–DX but is rather due to the colloid oncotic pressure of the DX macromolecules (M r = 40,000). Importantly, the effects of pCMBS on fluid phase endocytosis and cell volume could be reversed by addition of β-ME, which is known to reverse the effect of the drug, indicating that the effect observed was not due to toxicity (Fig. 4B and Fig. C). In contrast, incubation with DMA caused a rapid and marked shrinkage of the cell that was independent of an oncotic load and led with time to cell death (data not shown).
Figure 3.
Morphological changes induced by pCMBS treatment of immature DCs. Representative electron micrographs of immature DCs exposed for 15 min to 1 mg/ml of FITC–DX in the absence (A) or presence of 100 μM pCMBS (B and C).
Figure 4.
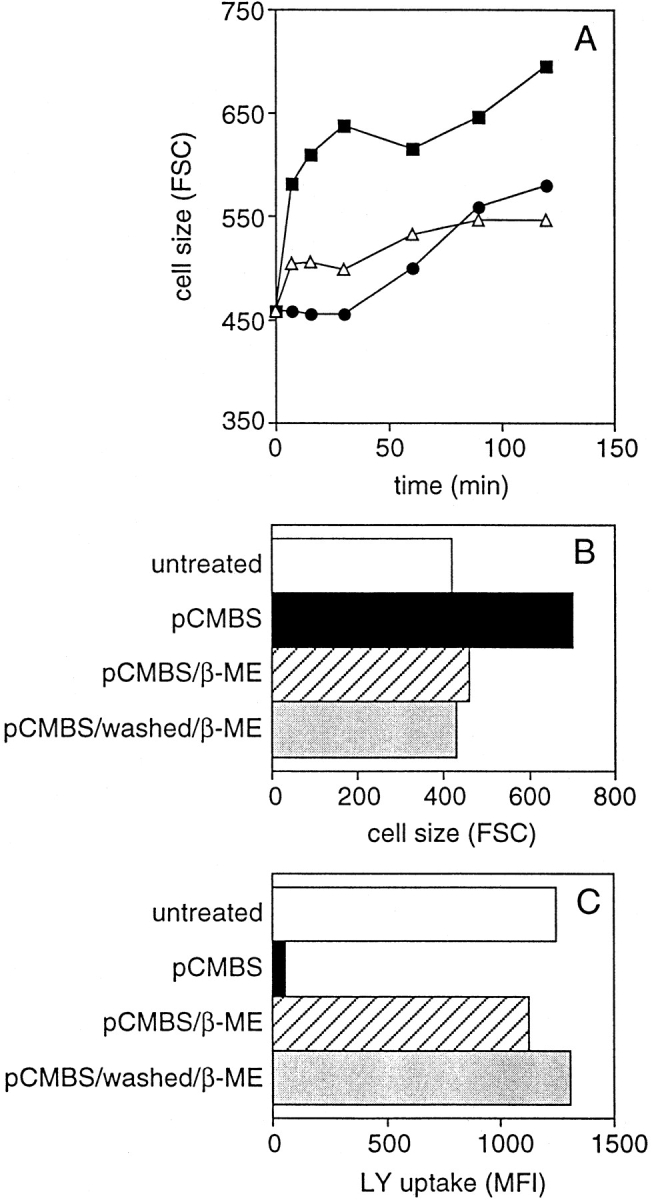
Reversibility of the pCMBS effect after its removal or neutralization. (A) Kinetics of swelling of immature DCs incubated with 1 mg/ml of DX (▵), with 100 μM pCMBS (•), or with both DX and pCMBS (▪). (B and C) Immature DCs were incubated with 1 mg/ml of DX in the absence (untreated) or presence of pCMBS. After 30 min, pCMBS was either left in culture (pCMBS) or neutralized by adding β-ME (pCMBS/β-ME) or removed by washing and neutralized (pCMBS/washed/β-ME). After a further 15 min, cell size (B) and uptake of LY (C) were measured.
Discussion
We have shown that aquaporins are essential components of a volume regulatory system that acts in immature DCs to balance the high volume of fluid taken up by constitutive macropinocytosis. In DCs, this system is instrumental to allow sustained capture and concentration of antigens present in the fluid phase 2.
The large volume of fluid that is continuously taken up via macropinocytosis must be eliminated by the DC to maintain its volume and to avoid swelling. As discussed by Swanson 5, the fluid taken up by macropinocytosis cannot be simply eliminated by recycling vesicles, as these vesicles are much smaller than the macropinosomes and therefore will recycle membrane more efficiently than fluid. Consequently, this process will lead to an increase in the membrane tension of the maturing macropinosome 21. This stress may lead to bursting of some pinosomes, thus accounting for the striking finding that soluble antigens taken up via macropinocytosis can gain access to the class I presentation pathway 9. How can macropinocytosing DCs cope with the problem of maintaining their volume? Previous work has shown that amiloride blocks macropinocytosis in both endothelial cells 7 and immature DCs 2, suggesting a role for the amiloride-sensitive epithelial sodium channel (ENaC; reference 22). These channels can be activated by various mechanisms, including an increase in membrane tension, and thus provide a plausible mechanism for increasing the transport of Na+ in macropinosomes after mechanical stress 23 24. Interestingly, we found that the ENaC regulatory γ subunit is highly expressed in immature DCs and downregulated after DC maturation (our unpublished data).
In this study, we have identified aquaporins as a second essential component in this volume-controlling system. We have shown that AQP3 and AQP7 are highly expressed in immature DCs (coincident with the high level of macropinocytosis) and are downregulated upon maturation (coincident with a downregulation of macropinocytosis). Furthermore, treatment of immature DCs with pCMBS results in the selective and reversible block of macropinocytosis but not of receptor-mediated endocytosis, demonstrating that aquaporins have a selective effect on macropinocytosis. In contrast to AQP3 and AQP7, AQP9 is constitutively expressed in leukocytes 25 and is present throughout DC development from monocytes to mature DCs. We do not have a way to address the relative importance of the three aquaporins. However, the regulated expression of AQP3 and AQP7 in immature DCs is compatible with their prominent role in allowing immature DCs to cope with the increased water turnover related to macropinocytosis.
Based on these observations, we propose the following model to explain how DCs can concentrate macrosolutes. In immature DCs, the high load of water and solutes taken in via constitutive macropinocytosis is balanced by a high activity of volume regulating channels. These include: (a) the amiloride sensitive Na+/H+ channels that increase the transport of ions and (b) the aquaporins that facilitate the flow of water following the salt gradient. This mechanism is geared to allow efficient presentation of soluble antigens and illustrates how DCs can adapt their physiology to function as professional APCs. It is tempting to speculate that aquaporins may also participate in other aspects of DC function, such as the release of small endocytosed molecules into the cytosol for class I–restricted presentation.
Acknowledgments
We thank Federica Sallusto and Jean Pieters for critical reading and comments and Dr. Fred Gudat and Mrs. Ivon Litzisdorf (Institute of Pathology, University of Basel) for electron microscopy.
The Basel Institute for Immunology was founded and is supported by Hoffmann-La Roche, Basel, Switzerland.
References
- Steinman R.M., Swanson J. The endocytic activity of dendritic cells. J. Exp. Med. 1995;182:283–288. doi: 10.1084/jem.182.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Cella M., Danieli C., Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartmentdownregulation by cytokines and bacterial products. J. Exp. Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault A., Lankar D., Lacabanne V., Rodriguez A., Thery C., Rescigno M., Saito T., Verbeek S., Bonnerot C., Ricciardi-Castagnoli P. Fcγ receptor–mediated induction of dendritic cell maturation and major histocompatibility complex class I–restricted antigen presentation after immune complex internalization. J. Exp. Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J.A. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J. Cell Sci. 1989;94:135–142. doi: 10.1242/jcs.94.1.135. [DOI] [PubMed] [Google Scholar]
- Racoosin E.L., Swanson J.A. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J. Cell Sci. 1992;102:867–880. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- West M.A., Bretscher M.S., Watts C. Distinct endocytotic pathways in epidermal growth factor–stimulated human carcinoma A431 cells. J. Cell Biol. 1989;109:2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Engering A., Pinet V., Pieters J., Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- Norbury C.C., Chambers B.J., Prescott A.R., Ljunggren H.G., Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur. J. Immunol. 1997;27:280–288. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Woodside M., Sardet C., Pouyssegur J., Rotin D. Activation of the Na+/H+ antiporter during cell volume regulation. Evidence for a phosphorylation-independent mechanism. J. Biol. Chem. 1992;267:23823–23828. [PubMed] [Google Scholar]
- Agre P., Preston G.M., Smith B.L., Jung J.S., Raina S., Moon C., Guggino W.B., Nielsen S. Aquaporin CHIPthe archetypal molecular water channel. Am. J. Physiol. 1993;265:463–476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- Verkman A.S. Water channels in cell membranes. Annu. Rev. Physiol. 1992;54:97–108. doi: 10.1146/annurev.ph.54.030192.000525. [DOI] [PubMed] [Google Scholar]
- King L.S., Agre P. Pathophysiology of the aquaporin water channels. Annu. Rev. Physiol. 1996;58:619–648. doi: 10.1146/annurev.ph.58.030196.003155. [DOI] [PubMed] [Google Scholar]
- Preston G.M., Jung J.S., Guggino W.B., Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J. Biol. Chem. 1993;268:17–20. [PubMed] [Google Scholar]
- Zhang R., van Hoek A.N., Biwersi J., Verkman A.S. A point mutation at cysteine 189 blocks the water permeability of rat kidney water channel CHIP28k. Biochemistry. 1993;32:2938–2941. doi: 10.1021/bi00063a002. [DOI] [PubMed] [Google Scholar]
- Kuwahara M., Gu Y., Ishibashi K., Marumo F., Sasaki S. Mercury-sensitive residues and pore site in AQP3 water channel. Biochemistry. 1997;36:13973–13978. doi: 10.1021/bi9711442. [DOI] [PubMed] [Google Scholar]
- Echevarria M., Frindt G., Preston G.M., Milovanovic S., Agre P., Fischbarg J., Windhager E.E. Expression of multiple water channel activities in Xenopus oocytes injected with mRNA from rat kidney. J. Gen. Physiol. 1993;101:827–841. doi: 10.1085/jgp.101.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarria M., Windhager E.E., Frindt G. Selectivity of the renal collecting duct water channel aquaporin-3. J. Biol. Chem. 1996;271:25079–25082. doi: 10.1074/jbc.271.41.25079. [DOI] [PubMed] [Google Scholar]
- Traunecker A., Oliveri F., Karjalainen K. Myeloma based expression system for production of large mammalian proteins. Trends Biotechnol. 1991;9:109–113. doi: 10.1016/0167-7799(91)90038-j. [DOI] [PubMed] [Google Scholar]
- Geissmann F., Prost C., Monnet J.P., Dy M., Brousse N., Hermine O. Transforming growth factor β1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J. Exp. Med. 1998;187:961–966. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racoosin E.L., Swanson J.A. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J. Cell Biol. 1993;121:1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firsov D., Gautschi I., Merillat A.M., Rossier B.C., Schild L. The heterotetrameric architecture of the epithelial sodium channel (ENaC) EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:344–352. doi: 10.1093/emboj/17.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard J.M., Bubien J.K., Benos D.J., Warnock D.G. Stretch modulates amiloride sensitivity and cation selectivity of sodium channels in human B lymphocytes. Am. J. Physiol. 1996;270:224–234. doi: 10.1152/ajpcell.1996.270.1.C224. [DOI] [PubMed] [Google Scholar]
- Ismailov I.I., Berdiev B.K., Shlyonsky V.G., Benos D.J. Mechanosensitivity of an epithelial Na+ channel in planar lipid bilayersrelease from Ca2+ block. Biophys. J. 1997;72:1182–1192. doi: 10.1016/S0006-3495(97)78766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi K., Kuwahara M., Gu Y., Tanaka Y., Marumo F., Sasaki S. Cloning and functional expression of a new aquaporin (AQP9) abundantly expressed in the peripheral leukocytes permeable to water and urea, but not to glycerol. Biochem. Biophys. Res. Commun. 1998;244:268–274. doi: 10.1006/bbrc.1998.8252. [DOI] [PubMed] [Google Scholar]