Abstract
Maturational changes at the CD4−CD8− double negative (DN) to CD4+CD8+ double positive (DP) transition are dependent on signals generated via the pre–T cell receptor (TCR) and the nonreceptor protein tyrosine kinase p56lck (Lck). How Lck activities are stimulated or relayed after pre-TCR formation remains obscure. Our structure–function mapping of Lck thymopoietic properties reveals that the noncatalytic domains of Lck are specialized to signal efficient cellular expansion at DN to DP transition. Moreover, although substitution of the Lck catalytic domain with FynT sequences minimally impacts DP development, single positive thymocytes are most efficiently produced in the presence of kinases containing both the NH2-terminal and catalytic regions of Lck. These findings demonstrate that the Lck structure is uniquely adapted to mediate signals at both major transitions in thymopoiesis.
Keywords: thymus, signal transduction, cellular differentiation, protein kinases, lymphocytes
Introduction
T cell development begins when bone marrow–derived precursors seed the thymus, where they expand and differentiate into mature T cells (for a review, see reference 1). When examined by flow cytometry, the most immature cells of the α/β lineage lack expression of both CD4 and CD8 coreceptors (CD4−CD8− double negative [DN] thymocytes). Recombination activating gene (Rag)-dependent rearrangements at the TCR-β locus are initiated in a subset of these DN cells (CD44+CD25−), and the TCR β chains subsequently produced are expressed in CD44− CD25+ intermediates in the form of a pre-TCR that consists of TCR-β, the nonpolymorphic pre-Tα chain, the CD3 complex, and associated ζ chains (for a review, see reference 2). Pre-TCR expression is required to promote transition of thymocytes from DN progenitors to CD4+ CD8+ double positive (DP) intermediates through a process marked by dramatic proliferative expansion and numerous differentiative changes (downregulation of CD25, synthesis of CD4 and CD8 coreceptors, and allelic exclusion at TCR-β). Subsequent DNA rearrangements at the TCR-α locus in DP cells permit assembly of TCR-α/β, which is required to mediate the transition from DP intermediates to mature CD4 or CD8 single positive (SP) cells. Thus, both transitions (DN to DP and DP to SP) rely on the ability of a TCR structure to trigger transmembrane signals, and on the ability of these signals to engage specific developmental programs.
Several lines of evidence suggest that the Src family nonreceptor protein tyrosine kinase (PTK) p56lck (Lck) functions as a key downstream regulator of pre-TCR signals. DP thymocyte development is severely compromised when Lck expression or activity is diminished during thymopoiesis 3 4. Similarly, transgenic TCR-β expression fails to enforce allelic exclusion at the endogenous TCR-β locus in the absence of Lck 5 6. Parallel studies demonstrated that Lck and TCR-β transgenes produce similar in vivo effects, such as inhibiting Vβ-Dβ rearrangement and rescuing DP development in Rag−/− mice 7 8. Taken together, these observations support a model in which Lck transduces pre-TCR signals that regulate differentiation, proliferative expansion, and allelic exclusion at DN to DP transition 9. These Lck-dependent signals appear to be relayed in part through kinases of the Syk/ZAP-70 family 10 11, the Src homology (SH)2 domain–containing leukocyte protein of 76 kD (SLP-76) adaptor 12 13, the guanosine triphosphatase Rho 14, and via components of the Ras/Raf/mitogen-activated protein kinase pathway 15 16 17.
Although other Src PTK family members (Src-PTKs) are expressed in thymocytes, a variety of studies have suggested that Lck is distinctive in its ability to influence early thymocyte development. FynT, the hematopoietic isoform of p59fyn, is also detectable in thymocytes and mature T cells 18 19. However, unlike Lck, loss of Fyn activity does not compromise early thymocyte development 20 21. In transgenic experiments, augmented FynT expression also failed to reproduce phenotypes associated with Lck overexpression, leaving thymocyte cellularity unchanged and subset representation intact 18 22. These findings suggested that, although highly related, FynT and Lck do not behave identically in the same cellular context. Thymic expression of a third closely related Src-PTK had no effect on the maturation or activation characteristics of transgenic thymocytes 23. Thus, Lck appears to possess specialized thymopoietic properties that cannot be readily duplicated by other Src-PTKs. The findings that endogenous FynT cannot fully compensate for loss of Lck function at DN to DP transition 24 25, and that transgenic overexpression of activated mutant FynT only partially rescues thymopoiesis in the Lck−/− strain 25, further support the view that these two related enzymes possess both unique and overlapping functional characteristics in vivo.
Src-PTKs share a common domain structure consisting of a highly conserved catalytic or SH1 domain and several noncatalytic domains, including an NH2-terminal consensus sequence for myristylation, a distinctive NH2-terminal unique region, SH3 and SH2 domains that bind proline-rich sequences and phosphotyrosine-containing peptide sequences, respectively, and a short COOH-terminal tail containing the major regulatory tyrosine residue (for a review, see reference 26). In principle, functional specialization of Src-PTKs could reflect differences in any of these Src-PTK domains, and each region has been reported to specify distinctive protein–protein interactions. Associations between Lck and the CD4/CD8 coreceptors and between Fyn and the CD3 complex are dictated by specific motifs residing in the Src-PTK unique region 27 28 29. Similarly, SH2 and SH3 regions of different Src-PTKs bind discrete sets of proteins, conferring distinct signaling characteristics to the individual enzymes 30 31 32. Moreover, recent studies suggest that some level of substrate selectivity may be imparted by unique sequences in the PTK catalytic domain 33.
The Lck CD4 or CD8 interactive motif appears to be dispensable for Lck function at DN to DP transition 4 8. In addition, although mutant forms of Lck lacking kinase activity can enhance TCR signaling in some circumstances 34, catalytic activity is an essential component of Lck function at DN to DP transition 4. Aside from the requirement for an active catalytic domain, the PTK structural features that impact Lck signal initiation or relay at DN to DP transition remain undefined. As analysis of chimeric Src-PTKs has proven to be a powerful strategy for assaying the contribution of Src-PTK domains to kinase functions in vitro, we have adopted this approach in an effort to define regions of Lck structure that are required to efficiently mediate developmental transitions in vivo. Our initial analysis has focused on examining the contribution of regulatory versus catalytic regions to Lck thymopoietic functions. This was accomplished by exchanging catalytic domains between Lck and Fyn, and by subsequently defining the ability of these altered Src-PTKs to support thymopoiesis in animals lacking endogenous Src-PTK expression. Our results suggest that Lck's distinctive ability to promote DP development with high efficiency reflects an inherent property of the Lck noncatalytic domains, whereas SP development is most efficiently mediated by enzymes bearing the Lck catalytic region. In addition, these studies demonstrate that the relative accumulation of thymocytes in CD4 or CD8 lineages is directly influenced by the structure and dose of the PTK used to mediate signals at DP to SP transition.
Materials and Methods
Construction of Chimeric Constructs.
Plasmids NT-18 35 and MM23 18 contain wild-type murine lck and fynT cDNAs, respectively. Plasmids containing cDNAs encoding LckF505 36 or FynTF528 25 37 were used to produce chimeras bearing phenylalanine substitutions at COOH-terminal regulatory tyrosine residues found in each respective kinase. To facilitate construction of the chimeras, an NcoI restriction site was introduced at the junction of the hinge and kinase domains in the murine fynT cDNA by site-directed mutagenesis. An NcoI site is present at the corresponding position of the lck cDNA. The NcoI-StuI fragment from fynT was exchanged with the analogous fragment in the lck cDNA by standard recombinant DNA technology. The integrity of the resulting chimeric constructs was confirmed by restriction mapping and sequencing.
Transfection and Transformation Assays.
Chimeric PTKs were cloned into the CMV-MNC expression vector using standard techniques 38. DNA transfection of NIH-3T3 cells was performed using a modified calcium phosphate precipitation technique 39, and transfected cells were selected in the presence of 500 μg/ml G418. To monitor focus formation, 103 or 104 NIH-3T3 fibroblasts expressing each of the constructs were admixed with 105 NIH-3T3 cells transfected with the CMV-MNC base vector, and were plated into 6-well plates in media containing 250 μg/ml G418. G418r foci were counted 10 d later. For growth in soft agar, 2 × 104 transfected cells were suspended in growth medium containing 0.33% agar (Difco), and were overlayed onto 0.55% agar gel as described previously 40. Colonies were counted after 10 and 21 d.
Transgenic Mouse Production.
Lck−/− and FynT−/− mice have been described previously 3 20, as has the p1017 vector 18 41. Chimeric PTK constructs were inserted into the BamHI site of p1017. The transgene was released from the vector by NotI digestion, purified, and injected into either C57BL/6J × DBA/2 F2 zygotes (construct Lck-fyn) or FVB zygotes (constructs Lck-fynF and Fyn-lckF). Transgene-positive animals were identified by PCR analysis 42, and lines were established by successive backcrossing of transgene-positive animals to C57BL/6. Genotyping of animals for the presence of wild-type or disrupted Lck, FynT, and Rag-1 alleles was similarly determined by PCR analysis. All procedures were performed using protocols and standards approved by the Institutional Animal Care and Use Committee of the University of Maryland Institutional Review Board.
Northern Blot Analysis.
Total RNA was isolated from mouse thymocytes using TRIzol™ Reagent (Life Technologies). 15 μg of RNA was resolved on 1% agarose formaldehyde gels, transferred to nylon membranes, hybridized, and washed according to standard procedures. Regions of cDNA corresponding to NH2-terminal or COOH-terminal coding sequences of lck or fynT were labeled to high specific activity by random priming 43, and were used as probes. Quantification was performed using a PhosphorImager® (Molecular Probes). Similar analyses failed to reveal significant transgene expression in peripheral organs of the mice.
Flow Cytometry.
Single cell suspensions were obtained from lymphoid organs as described 22. Thymocytes were stained for surface expression of CD4, CD8, and CD3 molecules as described previously 16, using PE-conjugated anti-CD4 (CT-CD4), FITC-conjugated anti-CD8 (CT-CD8α), and biotinylated anti-CD3ε (500.A2; all from Caltag Laboratories). Binding of biotinylated antibodies was detected using streptavidin-TriColor conjugate (Caltag Laboratories). Each analysis was performed on 2 × 104 events acquired in list mode using a FACSort™ flow cytometer, and was analyzed using CELLQuest™ software (Becton Dickinson).
Immunoblot Analysis.
Thymocytes were lysed in NP-40 lysis buffer (50 mM Tris, pH 7.4, 1% NP-40, 150 mM NaCl, 1 mM EGTA, 1 mM PMSF, 1 μg/ml aprotinin and leupeptin, 1 mM NaF, and 1 mM Na3VO4). Samples were gently rocked on ice for 30 min, and were centrifuged at 12,000 rpm for 10 min at 4°C. Total protein concentrations in the supernatants were determined using a modified Lowry assay (Bio-Rad). Cell lysates were separated by SDS-PAGE, and were transferred to nitrocellulose membranes. An mAb specific for the NH2-terminal region of p56lck(Santa Cruz Biotechnology), rabbit antisera specific for the COOH-terminal region of human p56lck (Upstate Biotechnology) or mouse p56lck 35, and specific Ab for Fyn (Santa Cruz Biotechnology) were used to document expression of the chimeric kinases. The blots were developed using enhanced chemiluminescence (Amersham Pharmacia Biotech). Densitometric analyses of immunoblots verified that when expressed at similar mRNA concentrations, wild-type Lck, Lck-fyn, and Fyn-lckF proteins accumulate to comparable levels in Lck+/−, Lck−/− [A10247], and Lck−/−[116] strains, respectively. Dose-matched levels of Lck-fynF transcripts yielded a somewhat lower steady-state kinase accumulation (0.17 Lck-FynF:Lck+/−) when measured in Lck−/−[490] mice.
Results
Lck/Fyn Chimeras Have Differing Transforming Efficiencies in NIH-3T3 Fibroblasts.
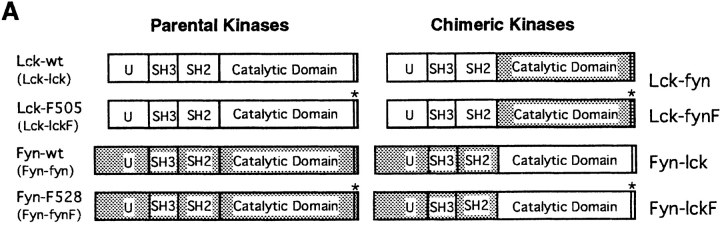
To monitor the contribution of Src-PTK domains to Lck thymopoietic function, chimeric kinases were produced by exchanging catalytic domains between Lck and the related Src-PTK FynT (Fig. 1 A). The Lck-fyn construct carries a replacement of amino acids 234–510 of Lck with corresponding residues 257–534 of FynT, generating a chimera consisting of the Lck NH2-terminal regions fused to the Fyn catalytic domain. The reciprocal construct Fyn-lck substitutes residues 255–534 of FynT with amino acids 232–510 of Lck, encoding a chimera in which the NH2-terminal regions of Fyn have been fused to the Lck catalytic domain. In addition, as the efficiency of enzyme autorepression may be altered in chimeras containing SH2 and COOH-terminal regulatory domains recovered from heterologous sources 44, we also produced genetically derepressed versions of these recombinant PTKs, in which the regulatory COOH-terminal tyrosine residue was substituted with phenylalanine (Lck-fynF and Fyn-lckF).
Figure 1.
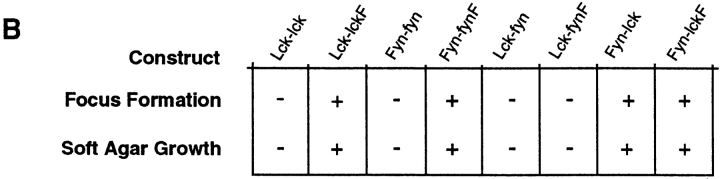
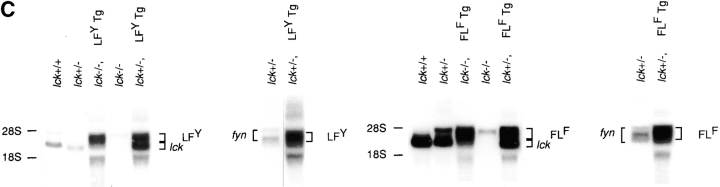
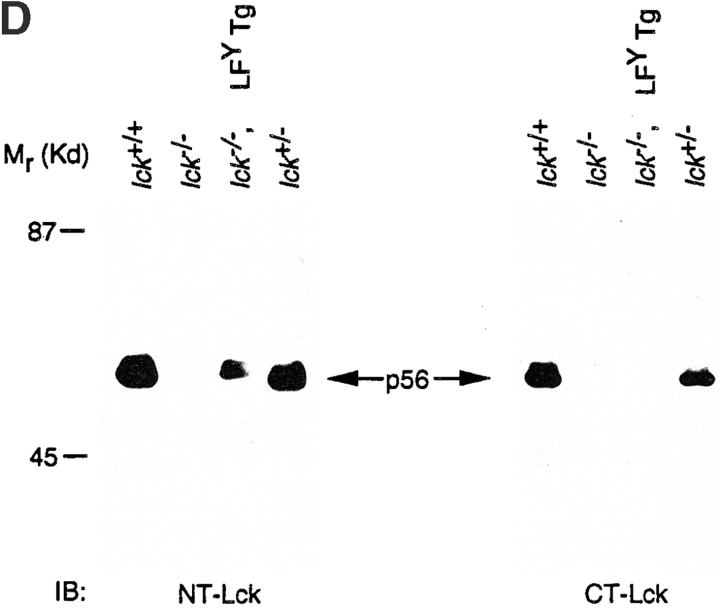
(A) Schematic representation of parental and Lck/Fyn chimeric structures. wt, wild-type; U, unique. (B) Transforming efficiency of Src-PTKs in NIH-3T3 fibroblasts as measured in focus formation and soft agar growth assays. (C) Expression of transgene-encoded mRNAs in thymocytes from littermates representing indicated genotypes. Northern blot analysis using probes specific for the NH2-terminal regions of Lck or the COOH-terminal regions of FynT (left), or probes specific for the COOH-terminal regions of Lck or the NH2-terminal region of FynT (right). LFY, Lck-fyn chimera; FLF, Fyn-lckF chimera; Tg, transgene. (D) Immunoblot (IB) analysis of chimera expression using antibodies specific for NH2-terminal (NT) or COOH-terminal (CT) regions of p56lck.
Fidelity of the chimeric constructs was examined after cloning of the recombinant Src-PTKs into expression vectors and transfection of the constructs into the NIH-3T3 fibroblast cell line. Stable fibroblast clones expressing each kinase were obtained after drug selection, and immunoblotting with antibodies specific for NH2-terminal or COOH-terminal regions of p56lck and p59fynTconfirmed that all chimeras were expressed in correct immunoreactive forms in these cells (data not shown). Src-PTK function was evaluated in this context by measuring the transforming efficiency of individual PTK constructs in focus formation and soft agar growth assays. Consistent with previous reports 36 45, expression of wild-type forms of Lck and FynT did not promote transformation of NIH-3T3 cells, whereas parental enzymes bearing Tyr to Phe substitutions at COOH-terminal regulatory tyrosine residues behaved as dominant oncogenes in the fibroblasts (Lck-lckF and Fyn-fynF; Fig. 1 B). Both Fyn-lck and Fyn-lckF were as potent as the mutant parental enzymes in transforming NIH-3T3 cells, suggesting that both chimeras behave as activated kinases in fibroblasts. In contrast, neither Lck-fyn nor Lck-fynF was transforming as measured by these criteria. The inability of Lck-fyn PTKs to transform NIH-3T3 cells does not reflect a grossly aberrant conformation or loss of kinase activity in these chimeras, as both Lck-fyn and Lck-fynF operate efficiently in the lymphocyte background (see below). Rather, this phenotype in fibroblasts likely reflects a negative impact of Lck NH2-terminal regions on Fyn catalytic function in these cells, a phenomenon similarly noted on analysis of chimeras bearing Lck regulatory domains coupled to the Src catalytic region 32.
Generation of Lck Mutant Mice Expressing Chimeric PTKs.
The ability of the chimeric PTKs to influence thymocyte development was examined first in transgenic mice expressing these enzymes under the control of the lck proximal promoter. Lck gene expression is regulated by two promoter elements, proximal and distal 46 47 48, and sequences including the proximal promoter element have been used to direct expression of a variety of heterologous genes in thymocytes of transgenic mice (vector p1017 [18, 41]). The chimeric PTK constructs were inserted into the p1017 vector and then injected into zygotes, and transgenic lines expressing Lck-fyn, Lck-fynF, and Fyn-lckF were obtained. Transgenic mice expressing the chimeras did not exhibit major changes in thymus size and cellularity, and transgene expression did not significantly alter thymocyte subset representation with respect to CD3, CD4, and CD8 expression (Table ; data not shown).
Table 1.
Reconstitution of Lck−/− Thymopoiesis with Src-PTK Transgenes
| Transgenic line (Chimera) | Transgene mRNA | Genotype | n | No. of thymocytes |
|---|---|---|---|---|
| mean % ± SD | ||||
| A10247 | 0.18 | Lck-fyn | 11 | 89 ± 29 |
| (Lck-fyn) | lck −/− Lck-fyn | 5 | 1.9 ± 4 | |
| A10247 (2×) | 0.36 | Lck-fyn | 1 | 95 |
| (Lck-fyn) | lck −/− Lck-fyn | 2 | 11.5 ± 5 | |
| A10244 | 0.87 | Lck-fyn | 8 | 98 ± 38 |
| (Lck-fyn) | lck −/− Lck-fyn | 3 | 67 ± 24 | |
| A10262 | 4.45 | Lck-fyn | 5 | 67 ± 23 |
| (Lck-fyn) | lck −/− Lck-fyn | 5 | 56 ± 25 | |
| 490 | 1.02 | Lck-fynF | 9 | 131 ± 33 |
| (Lck-fynF) | lck −/− Lck-fynF | 3 | 98 ± 25 | |
| 116 | Fyn-lckF | 7 | 90 ± 32 | |
| (Fyn-lckF) | 0.96 | lck −/− Fyn-lckF | 4 | 2.1 ± 0.4 |
| 668 | Fyn-lckF | 5 | 120 ± 13 | |
| (Fyn-lckF) | 2.20 | lck −/− Fyn-lckF | 3 | 4.3 ± 1.0 |
| 687 | 3.50 | Fyn-lckF | 3 | 82 ± 24 |
| (Fyn-lckF) | lck −/− Fyn-lckF | 3 | 4.7 ± 1.8 | |
| 685 | 5.32 | Fyn-lckF | 7 | 132 ± 33 |
| (Fyn-lckF) | lck −/− Fyn-lckF | 8 | 12.9 ± 1.9 |
Chimera function was examined subsequently by documenting the ability of individual PTKs to reconstitute thymopoiesis in Lck−/− mice 3. Levels of chimera mRNA expression were defined relative to endogenous Lck through simultaneous measurement of endogenous and transgene-derived PTK expression in thymocytes of transgene-positive Lck+/− progeny (Fig. 1 C; summarized in Table ). Consistent with previous reports 3, no Lck protein, either full-length or truncated, was detected in Lck−/− mice when immunoblots were probed using antisera specific for either the NH2-terminal or COOH-terminal region of Lck (Fig. 1 D). However, a large transcript could be detected by probes specific for either the NH2-terminal or COOH-terminal region of the lck mRNA in these mice (Fig. 1 C), presumably reflecting lck transcripts containing the neoR cassette in exon 12. Expression of the chimeric PTK transgenes was documented by the appearance of transcripts slightly larger than the wild-type lck mRNA (Fig. 1 C), and by detection of chimeric proteins reactive with antiserum specific for either NH2-terminal (Lck-fyn and Lck-fynF) or COOH-terminal regions of Lck (Fyn-lckF) in Lck−/− mice (Fig. 1 D; data not shown).
Lck-fyn Restores DP Development in Lck Mutant Mice.
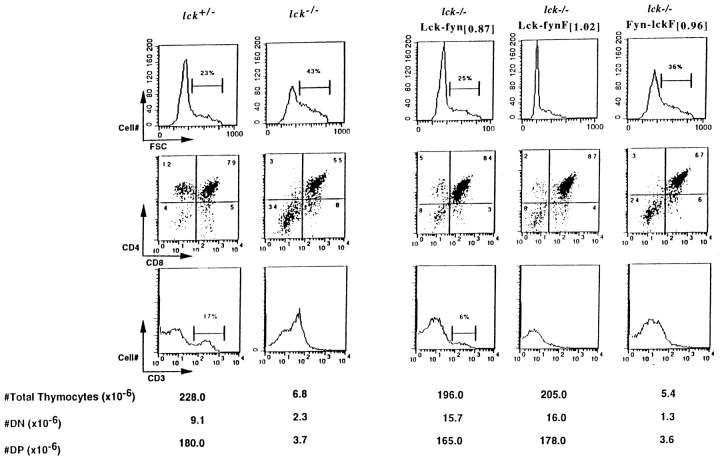
As reported previously, thymocyte numbers are drastically reduced in Lck mutant mice, with the remaining cells distributed between DN (70%) and DP (30–80%) compartments (Fig. 2, and Table ; references 3, 8). One allele of wild-type Lck is sufficient to restore normal thymocyte numbers and subset representation in these mice (Lck+/−; Fig. 2, and reference 3), suggesting that one allele represents a maximal Lck dose necessary to efficiently rescue Lck−/− thymopoiesis. Accordingly, we have assayed chimera function at transgene doses that approximate PTK expression from a single Lck allele in an effort to enhance the sensitivity of our in vivo analysis. Significantly, many aspects of Lck−/− thymopoiesis could be restored by expression of the Lck-fyn PTK at this physiological dose (Lck-fyn[0.87]; Fig. 2). An increase in DP cell numbers and a proportional shift of thymocytes between DN and DP compartments occurred when Lck-fyn[0.87] was expressed in the Lck−/− strain (Fig. 2, and Table ). In addition, transgene-dependent changes in Lck−/− thymocyte size were observed coincident with the appearance of DP and SP subsets in Lck-fyn+ Lck−/− mice (Fig. 2). Thus, the Lck-fyn PTK efficiently relays pre-TCR signals that are necessary to mediate two key facets of the DN to DP transition: cellular expansion, and acquisition of CD4 and CD8 expression.
Figure 2.
Reconstitution of Lck−/− thymopoiesis using Lck-fyn and Fyn-lck chimeras. Thymocytes were recovered from animals of indicated genotypes, and were counted and analyzed by flow cytometry. Shown are representative flow cytometric profiles of thymocyte forward scatter (FSC, top), CD4 and CD8 expression (middle), and CD3 density (bottom). Numbers of thymocytes recovered in each analysis are indicated.
Surface CD3 expression is increased in Lck−/− thymocytes (Fig. 2; reference 3), a phenomenon that reflects the impact of Lck signals on CD3 surface density 8 49. Like wild-type Lck, the Lck-fyn PTK modulates surface CD3 expression in Lck−/− thymocytes (Fig. 2). Thus, although endogenous Fyn cannot appropriately regulate CD3 density in the absence of Lck, CD3 expression is normalized when the Fyn catalytic region is expressed in the context of Lck NH2-terminal domains (the Lck-fyn chimera). This finding indicates that Fyn's inability to modulate CD3 density cannot be ascribed to a major deficiency of the Fyn catalytic region.
Lck-fyn and Lck-fynF Fail to Fully Restore SP Development in Lck Mutant Mice.
Although DP production and normal TCR/CD3 expression were restored by Lck-fyn[0.87], fewer SP thymocytes accumulate in this strain (17% in Lck+/− versus 6% in Lck-fyn[0.87]; Fig. 2). The suboptimal rescue of SP cells observed at this chimera dose cannot be attributed either to the suballelic level of PTK expression achieved in the cross, or to modulation of transgene promoter activity at positive selection 50, as no improvement in SP production was observed in Lck−/− animals expressing Lck-fyn at four to five times higher levels (strain A10262 Lck-fyn[4.45]; Table , and data not shown). Thus, the inability of Lck-fyn to mediate full reconstitution of SP thymocytes appears to reflect a deficiency in the Lck-fyn structure that preferentially impacts SP production. To examine Lck-fyn PTK efficiency in greater detail, a constitutively active form of the Lck-fyn kinase was produced (Lck-fynF), and its function was monitored in the Lck−/− background.
Expression of this activated mutant form of Lck-fyn (construct Lck-fynF) also restored wild-type DP numbers in Lck−/− mice (Fig. 2). Although DN to DP transition was effectively mediated by Lck-fynF, this PTK structure once again failed to fully restore SP development. Taken together, these findings suggest that substitution of the Lck catalytic and COOH-terminal regulatory regions with FynT sequences has minimal impact on the efficiency of pre-TCR signaling as measured by development and expansion of DP thymocytes. The fact that SP production is not similarly restored using this PTK further suggests that DN to DP and DP to SP transitions have differential requirements for Lck structures or catalytic activities.
Fyn-lckF Mediates DN to DP Transition, but Fails to Reconstitute DP Thymocyte Numbers in Lck Mutant Mice.
As substitution of the Lck catalytic region with FynT domains appeared to have a negligible impact on pre-TCR signaling, we next examined the contribution of Lck noncatalytic domains to this process. Accordingly, a chimera consisting of the Fyn NH2-terminal regions fused to the Lck catalytic domain was produced (Fyn-lckF), and the ability of this PTK to restore thymopoiesis in the Lck mutant background was monitored. Despite its potent transforming effects in NIH-3T3 cells (Fig. 1 B), Fyn-lckF failed to restore thymopoiesis in Lck−/− mice when expressed at levels approximating a single allele of Lck (Fig. 2, Fyn-lckF [0.96]). Thus, in contrast to the effects of Lck-fyn and Lck-fynF at this gene dose, DP numbers were not increased in Lck−/− mice expressing Fyn-lckF [0.96]. These differential activities of the chimeras cannot be ascribed to major differences in protein stability, as in agreement with their mRNA levels, both Lck-fyn[0.87] and Fyn-lckF [0.96] yield a degree of PTK protein accumulation that is comparable to one allele of Lck (see Materials and Methods; data not shown). Nonetheless, the DP compartment was not restored in Fyn-lckF [0.96]:Lck−/− mice. However, a slight but appreciable enhancement in DN to DP transition and a transgene-dependent modulation of thymocyte CD3 density were observed in this reconstituted strain (Fig. 2). These findings suggested the possibility that Fyn-lckF may be able to subserve some Lck functions at DN to DP transition, albeit with low efficiency.
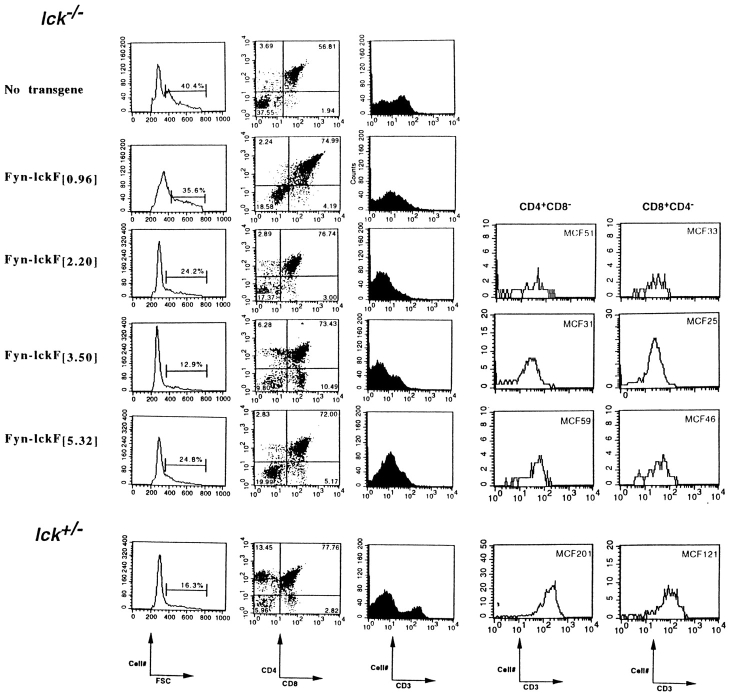
To examine Fyn-lckF thymopoietic efficiency in greater detail, the dose of Fyn-lckF introduced into the Lck−/− background was raised incrementally, and thymocyte development was monitored in these reconstituted strains (Fig. 3, and Table ). Fyn-lckF failed to reconstitute thymus cellularity at any dose tested, including expression levels that exceeded by five times the amount of wild-type Lck, Lck-fyn, or Lck-fynF required to restore DP numbers (Fyn-lckF [5.32] versus Lck-fyn[0.87] and Lck-fynF [1.02]; Table ). However, a transgene dose–dependent restoration of CD3 density and DN to DP differentiation occurred in Fyn-LckF:Lck−/− strains lacking full reconstitution of the DP compartment. For example, CD3 density was modulated in Lck−/− CD4+CD8+ thymocytes in the presence of Fyn-lckF[0.96], and became normalized at Fyn-lckF [2.2], whereas reconstitution of wild-type proportions of DN and DP cells appeared to require higher levels of transgene expression (Fig. 3, Fyn-lckF [3.5]). These findings demonstrate that the structure and dose of the Src-PTK expressed differentially impacts CD3 density, DN to DP differentiation, and proliferative expansion of developing thymocytes.
Figure 3.
Dose-dependent effects of Fyn-lckF chimera expression in Lck−/− mice. Thymocytes were recovered from animals of indicated genotypes, and were analyzed by flow cytometry. Shown are representative flow cytometric profiles depicting thymocyte forward scatter (FSC), CD4 and CD8 expression, and CD3 density as determined using three-color immunofluorescence. Numbers of CD3+CD4+ or CD8+ thymocytes observed in equivalent flow cytometric analyses of Lck−/− animals expressing Fyn-lckF at [2.2], [3.5], and [5.32] are shown, with CD3 density expressed as mean channel fluorescence (MCF). CD4+ and CD8+ thymocytes did not accumulate at levels that were sufficient to permit a similar analysis of CD3 density in animals expressing Fyn-lckF[0.96].
Fyn-lckF Mediates DP to SP Transition in Lck Mutant Mice.
A transgene-dependent recovery of SP production was observed when Fyn-lckF expression was raised from [0.96] to [3.50]; representation of SP thymocytes in Lck−/− increases from <1% at Fyn-lckF [0.96] to 5–7% at Fyn-lckF [2.2], and reaches wild-type proportions at Fyn-lckF [3.5] (Fig. 3; data not shown). Our preliminary analysis of animals expressing Fyn-lckF at [4.4] (Fyn-lckF [2.2] homozygotes) is also consistent with this apparent dose-dependent relationship between Fyn-lckF expression and SP generation, as Fyn-lckF [4.4] and Fyn-lckF [3.5] yield similar proportions of SPs in the Lck−/− background (data not shown). The fact that a higher level of Fyn-lckF expression was required to effect DP to SP transition than was necessary to influence DN to DP transition suggests that, although both differentiation programs possess overlapping characteristics, DP to SP transition may be a more stringent process, requiring higher levels of kinase expression or activity. Interestingly, accumulation of the CD8 SP subset is enhanced relative to the CD4 subset when Fyn-lckF is substituted for Lck during DP to SP transition (Fig. 3). CD4/CD8 ratios of <1.0 are routinely observed in CD3+ thymocytes produced via Fyn-lckF [2.2], Fyn-lckF [3.5], and Fyn-lckF [5.32] activity. Thus, the natural bias in thymopoiesis toward generation of CD4 SPs appears to be a phenotype superimposed on thymic selection by PTKs bearing Lck NH2-terminal domains. When SP selection operates in the absence of wild-type Lck, as for example in the case of Lck−/−:Fyn-lckF [3.5], this bias is minimized to yield a CD4/CD8 ratio of 0.79 ± 0.28 (mean ± SD, n = 4; Fig. 3; data not shown).
CD3 density is upregulated during repertoire selection as cells transition from DP to SP stages of development 51. However, SP subsets selected by Fyn-lckF in the absence of Lck failed to express wild-type levels of surface CD3 (Fig. 3). This finding indicates that signals required to upregulate CD3 density at DP to SP transition may be distinguished from signals necessary to mediate coreceptor modulation by their differential dependence on PTK activity. In support of this view, a recent study revealed a correlation between mitogen-activated protein kinase kinase (MEK) activity and the extent of CD3 upregulation in SP thymocytes 52, suggesting that inefficient transduction of signals to MEK may underlie the inability of Fyn-lckF to fully upregulate CD3 density at DP to SP transition.
Src-PTK Collaboration in Lck−/− Thymocyte Differentiation.
As the majority of kinase structure in Fyn-lckF is contributed by Fyn sequences, we considered the possibility that Fyn-lckF function in Lck−/− thymocytes could be influenced by the presence of endogenous Fyn. This influence could take the form of enhancing chimera function through collaborative effects, or of inhibiting chimera function through dominant negative interference. To test this possibility, dose-dependent effects of Fyn-lckF transgene expression were monitored in Lck−/−Fyn−/− mice, and results were compared with analyses performed in mice expressing one or both wild-type Fyn alleles (Fig. 4). As described previously, DPs were virtually absent in Lck−/−Fyn−/− mice (Fig. 4 A; references 24, 25). Notably, we observed a reproducible, dose-dependent effect of Fyn expression in the Lck−/− background (from 39% DPs in Lck−/−Fyn+/− to 65% DPs in Lck−/−Fyn+/+). This dose-dependent effect of Fyn alleles in Lck−/− was not apparent in prior analyses of Lck−/−Fyn−/− recombinant strains. We believe this discrepancy may reflect the influence of background gene effects contributed by the founder knockout strains, as the severity and reproducibility of both the Lck−/−Fyn+/+ and Lck−/−Fyn+/− developmental arrests increase as the Lck−/− and FynT−/− parental strains are continually backcrossed to C57BL/6. Accordingly, we have limited our analyses to reconstitutions in which the transgenes and disrupted alleles are contributed by parental strains that have been backcrossed to C57BL/6 for at least four or five generations.
Figure 4.
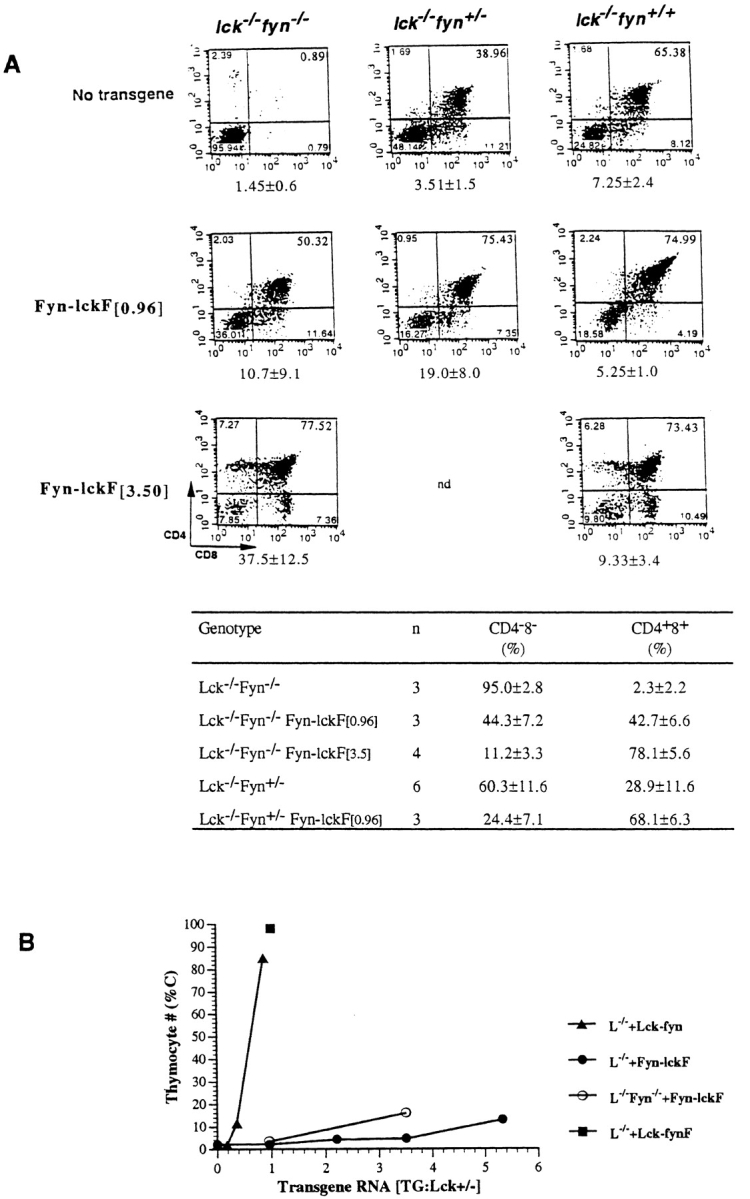
Endogenous Fyn enhances Fyn-lckF function in Lck−/− mice. (A, top) FynT enhances Fyn-lckF–mediated thymocyte differentiation. Thymocytes were recovered from animals of indicated genotypes and were analyzed by flow cytometry. Shown are representative flow cytometric profiles depicting CD4 and CD8 expression determined in three-color immunofluorescence analyses as described in the legends to Fig. 2 and Fig. 3. Numbers of thymocytes recovered from animals in each experimental group are shown beneath the corresponding dual parameter histograms (mean × 10−6 ± SD). (A, bottom) A cumulative analysis of DN and DP proportions in mouse strains as determined by flow cytometry (mean percentage ± SD; n = number of animals of indicated genotypes analyzed). Statistical analyses by two-sample t tests verify that expression of Fyn-lckF is associated with a significant increase in DP thymocytes in both Lck−/−Fyn−/− ([0.96], P = 0.005; [3.5], P = <0.001) and Lck−/−Fyn+/− mice ([0.96], P = <0.001), and that there is a statistically significant impact of Fyn alleles on DP generation in the presence of Fyn-lckF activity (P = 0.009). (B) FynT modulates Fyn-lckF–mediated proliferative expansion. Thymocyte cellularity (C) in reconstituted strains plotted as a function of chimera RNA expression levels. Symbols are mean percentages (adapted from Table ).
The ability of Fyn-lckF [0.96] to promote DN to DP transition is easily appreciated in the Lck−/−Fyn−/− background (Fig. 4 A). Moreover, endogenous Fyn enhanced Fyn-lckF's ability to promote DN to DP transition in what appeared to be an additive fashion. Although both Fyn-lckF [0.96] and one allele of wild-type Fyn enhanced transition into the DP compartment in Lck−/−, generating 42.7 and 28.9% DPs, respectively (Fig. 4 A), their combination yielded 68.1% DPs (Lck−/−Fyn+/− Fyn-lckF [0.96]). This increment in Fyn-lckF DN to DP differentiative function was not appreciably changed by introduction of the second Fyn allele (Lck−/− Fyn+/+), and further evidence of enhanced Fyn-lckF function, such as generation of SPs, was not observed.
SPs emerged in the Lck−/−Fyn−/− background only as the level of Fyn-lckF was increased, with wild-type proportions once again observed at Fyn-lckF [3.5]. The additive effects of endogenous Fyn and Fyn-lckF [3.5] appear to influence the extent of CD8 SP bias associated with Fyn-lckF activity (Fig. 3). Although CD4 and CD8 SPs were produced in equivalent proportions in Fyn-lckF [3.5] mice lacking both Lck and Fyn (Fyn-lckF [3.5]:Lck−/−Fyn−/−), a relative enrichment in CD8 SPs was observed in Fyn-lckF [3.5]:Lck−/−Fyn+/+ mice (Fig. 4 A). As observed previously in the Lck−/−Fyn+/+ background, signals generated by Fyn-lckF in Lck−/−Fyn−/− mice were sufficient to mediate coreceptor modulation, but failed to fully upregulate CD3 in either SP subset (Fig. 4; data not shown). Because CD69 density is upregulated in these cells (data not shown), we speculate that the phenotype of Fyn-lckF[3.5]:Lck−/−Fyn−/− SP thymocytes reflects the outcome of a DP to SP transition event in which signals were initiated via the Ras pathway (CD69hi 53), but failed to sustain full activation of MEK (CD3lo 52).
Although Fyn-lckF could mediate both DN to DP and DP to SP transitions in Lck−/−Fyn−/− mice, this kinase once again failed to reconstitute proliferative expansion in this background (Fig. 4A and Fig. B). Moreover, parallel analyses of Fyn-lckF–mediated thymocyte expansion in the Lck−/− and Lck−/−Fyn−/− backgrounds suggest that the modest proliferative signals associated with Fyn-lckF [0.96] and Fyn-lckF [3.5] were reduced in the presence of endogenous Fyn (by two to three times; Fig. 4A and Fig. B). We conclude that endogenous Fyn influences Fyn-lckF function in two ways: (a) by facilitating Fyn-lckF–mediated DN to DP transition signals, and (b) by modulating Fyn-lckF–mediated proliferative expansion signals.
Pre-TCR Regulation of Chimeric PTK Function.
Transgenic expression of activated mutant Lck (LckF) promotes DP development in the absence of pre-TCR formation, such as in mice lacking TCR-β or pre–TCR-α expression (strain LGF 2954 [8, 54]). These findings have supported the general view that Lck activation is a key event induced by the pre-TCR at DN to DP transition. As high levels of PTK activity can bypass the requirement for pre-TCR formation at DN to DP transition, we asked whether the pre-TCR was required to mediate DP development in the presence of the chimeric PTKs. The contribution of the pre-TCR to Lck-fynF– or Fyn-lckF–dependent DP production was measured by comparing the ability of the chimeras to promote DN to DP transition in the presence or absence of pre-TCR assembly in PTK−/− or Rag−/− strains, respectively (Fig. 5).
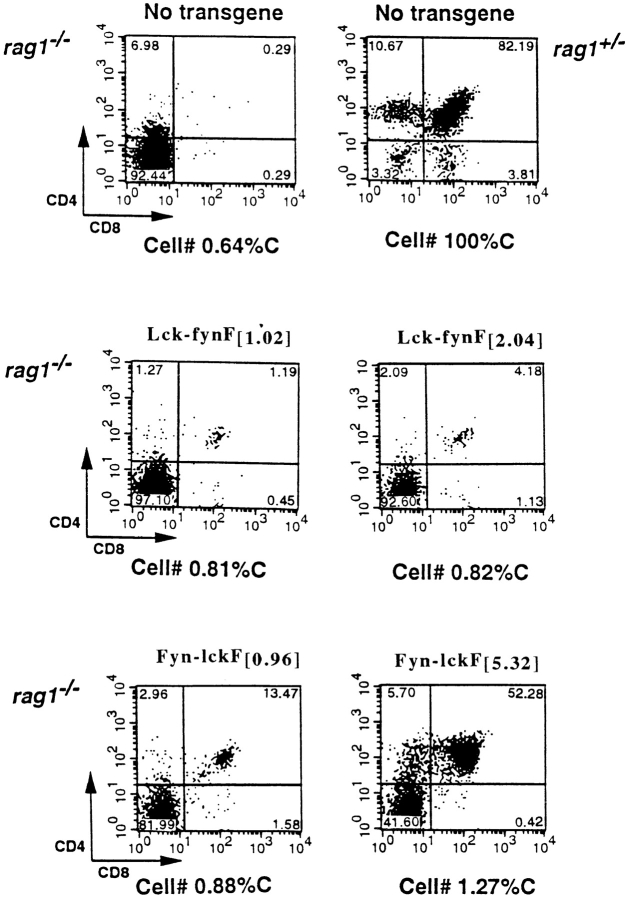
Figure 5.
DP production in the absence of pre-TCR assembly. Thymocytes from Rag+/−, Rag−/−, and Rag−/− mice expressing chimeras at indicated concentrations were recovered, counted, and analyzed by flow cytometry. Shown are representative dual parameter flow cytometric profiles depicting CD4 and CD8 expression in each strain. Cellularity (C) is expressed as a percentage of thymocyte numbers recovered from nontransgenic Rag+/− littermates. In each case, the data are representative of at least three independently derived transgene-positive Rag−/− progeny.
Importantly, although Lck-fynF efficiently restored DP production in Lck−/− mice (Fig. 2), this kinase failed to reconstitute the DP compartment in the Rag−/− background at either dose tested (Lck-fynF [1.02] and Lck-fynF [2.04]; Fig. 5). We conclude that Lck-fynF promotes DN to DP transition in Lck−/− mice through processes that require pre-TCR–dependent regulation of Lck-fynF activity. In contrast, a dose-dependent increase in DP production was apparent in Rag−/− mice expressing the Fyn-lckF transgene. As shown above, Fyn-lckF [0.96] dramatically increased DN to DP transition in Lck−/−Fyn−/− mice (from 0.89 to 50.32% DP; Fig. 4 A). Rag−/− mice expressing Fyn-lckF at this concentration exhibited a modest, but nonetheless appreciable transgene-dependent increase in DP production (from 0.29 to 13.47% DP in transgene-negative and transgene-positive, respectively), indicating that Fyn-lckF can mediate DN to DP transition in the absence of pre-TCR assembly, but with low efficiency. As pre-TCR expression facilitates Fyn-lckF [0.96] function at DN to DP transition, we conclude that the Fyn-lckF chimera retains sequences that are necessary to functionally couple the pre-TCR to a subset of intracellular transduction pathways.
Importantly, phenotypes associated with overexpression of LckF emerge when Fyn-lckF expression is raised five times to the levels achieved in Fyn-lckF [5.32]. These LckF-associated phenotypes include a relative accumulation of DN thymocytes (Fig. 3) and production of DP thymocytes in Rag−/− mice (to 52% in Rag−/− Fyn-lckF [5.32]; Fig. 5; references 8, 22). Similar phenotypes are observed in animals expressing Fyn-lckF at levels >5.32, such as mice homozygous for the Fyn-lckF [3.5] transgene (Fyn-lckF [7.0]; data not shown). Thus, titration of Fyn-lckF into Lck−/− at doses that exceed a range of Lck expression of between one and four allele equivalents (>[0.96], [2.20], [3.5], and [4.4]) yields a phenotype that is reminiscent of LckF overexpression 22. Nonetheless, unlike LckF, the Fyn-lckF enzyme structure fails to signal efficient cellular expansion in either Lck−/− or Rag−/− mice at these high doses (Fig. 4 B and Fig. 5). Taken together, these findings suggest that although Fyn-lckF lacks structures required to relay signals to the cell cycle, the enzyme may retain sequences that are necessary to link the PTK to signaling phenomena previously associated with Lck hyperactivity, such as pathways regulating rearrangement at the TCR-β locus 7 22.
Discussion
Several lines of evidence indicate that expression of the TCR β chain is necessary for efficient progression from CD4−CD8− DN to CD4+CD8+ DP stages of α/β thymocyte development. These β chain–associated maturational changes are dependent on formation of a pre-TCR complex consisting of TCR-β, pre-Tα, and CD3 components, and on expression of Lck (for a review, see reference 9). In this report, we define the relative contributions of catalytic versus noncatalytic domains to Lck functions in thymopoiesis by monitoring the activity of recombinant chimeric PTKs in PTK-deficient mouse strains. Our observations support the view that among the Src-PTKs, Lck is specialized in its ability to promote DN to DP development with high efficiency, and that this functional distinction is encoded within the Lck noncatalytic regions. In addition, the ability of the pre-TCR to regulate proliferative expansion is most profoundly dependent on the participation of a Src-PTK bearing Lck NH2-terminal domains.
The ability of Src-PTKs to transform fibroblasts does not correlate with their functional efficiency in the lymphocyte background. Although only activated forms of Lck and FynT are capable of transforming NIH-3T3 cells (COOH-terminal Y→F), both wild-type and activated forms of Lck induce thymic tumorigenesis when overexpressed in transgenic thymocytes 55. Neither wild-type nor constitutively active FynT readily promotes lymphocyte transformation 18 25. Accordingly, the transforming ability of the chimeric kinases in fibroblasts is inversely related to their ability to promote maturation of Lck-deficient thymocytes. As a corollary to the diminished effectiveness of Lck in thymocytes that results when Fyn noncatalytic regions are affixed to the Lck catalytic domain, the potent transforming ability of FynT is dramatically decreased in fibroblasts when noncatalytic regions of Lck are substituted into the FynT structure. These apparent inhibitory effects of the Lck regulatory domains on FynT transforming activity indicate that specialized functions are imparted to Fyn by its noncatalytic regions that cannot be substituted by Lck domains within the fibroblast cellular context. This view is consistent with the fact that FynB is normally expressed in fibroblasts, whereas Lck is not. Similar inhibitory effects of the Lck SH3 domain have been observed in fibroblasts expressing Lck-Src chimeras 32.
The presence of small numbers of DP thymocytes in Lck mutant mice prompted speculation that kinases such as Itk/Tsk 56 57 or Zap-70 58 may compensate for loss of Lck during thymopoiesis 8. Consistent with previous reports 24 25, our data identifies the Src-PTK FynT as the compensatory element required for generation of Lck−/− DP cells. The fact that endogenous FynT cannot fully compensate for Lck loss at DN to DP transition could reflect an inadequate level of FynT expression, a functional deficiency in FynT, or a combination of these characteristics. Indeed, transgenic expression of activated mutant FynT in Lck−/− increases DP production 25, suggesting that apparent differences in Fyn and Lck potency might simply reflect their differing expression levels in DN thymocytes. However, striking differences in Fyn and Lck potency are apparent when dose-dependent effects of the two activated mutant PTKs are compared 22 25 55, suggesting that intrinsic differences in these two enzyme structures likely impact their differential thymopoietic activities.
Fyn exists as two isoforms that differ in their alternative usage of Fyn exon 7a (FynB) or 7b (FynT). These isoforms differ exclusively within a sequence of ∼50 amino acids overlapping the end of the SH2 and beginning of the catalytic domains 37 59. The hematopoietic isoform FynT is the only PTK in which the last glycine of the canonical GXGXXG motif (glycine 280) found in the catalytic region is replaced by alanine 60. By creation and analysis of FynT-FynB chimeras, it was determined that it is the distinctive FynT catalytic domain and not its divergent SH2 motif that confers improved FynT biological function during antigen stimulation 59. Based on these findings, it was suggested that the enhanced catalytic function of FynT may be directed toward a limited subset of TCR-regulated substrates.
Our data demonstrate that when expressed in the context of the Lck regulatory domains, the distinctive FynT catalytic region directs DN to DP transition with an efficiency and fidelity that is indistinguishable from the Lck catalytic region. These results indicate that differences in Lck and Fyn thymopoietic potency that impact DN to DP transition are not inherent properties of the individual catalytic domains. Nonetheless, it is clear that the Fyn catalytic region does not completely duplicate Lck catalytic domain function, as enzymes containing this region fail to efficiently promote development of SP thymocytes. The fact that DP to SP transition cannot be effectively restored by PTK structures that efficiently mediate DN to DP transition indicates that these two developmental processes have distinctive PTK requirements. These differential requirements could be imposed by the need to transduce signals downstream of unique receptor structures at each transition (the pre-TCR versus the TCR-α/β), by the participation of additional PTK-coupled receptors (such as CD4/CD8), or by the need to interact with unique substrates, effectors, or regulators of kinase activity at each developmental stage.
As their catalytic domains appear to operate interchangeably at DN to DP transition, it is apparent that functional distinctions between Lck and FynT that impact this stage of development reflect individual or cooperative characteristics of the PTK noncatalytic regions. In support of this view, transgene dose–response curves demonstrate that PTKs containing FynT NH2-terminal domains are 5–20 times less efficient in reconstituting Lck−/− thymopoiesis than enzymes containing the Lck NH2-terminal domains. This difference cannot be attributed to interference from endogenous Fyn, and in fact endogenous Fyn assists in promoting Fyn-lckF–dependent DN to DP transition. The possibility that apparent differences in Lck and Fyn NH2-terminal domain functions may reflect both quantitative and qualitatitive distinctions is supported by the finding that activated FynT can restore some aspects of Lck−/− thymopoiesis, including pre-TCR–dependent differentiation and proliferation 25. As the level of FynTF expression was not quantitated relative to endogenous Lck in the experiments, and only a single dose of FynTF was used, it is impossible to deduce whether FynTF is more or less potent than Fyn-lckF in mediating pre-TCR signals. Nonetheless, it is apparent that the thymopoietic efficiency of activated mutant Fyn is substantially inferior compared with wild-type and activated Lck 22 and Lck-fyn (this report). Moreover, our data convincingly demonstrate that, when operating in the context of the same catalytic domain, the Fyn and Lck NH2-terminal regions exhibit striking functional differences that impact the efficiency of pre-TCR signaling.
Pre-TCR assembly appears to regulate the majority of DP development associated with Fyn-lckF [0.96] kinase activity. This finding suggests that triggering of PTK activation by the pre-TCR may not be dramatically affected by substitution of the Lck NH2-terminal regions with FynT domains. However, proliferative expansion does not occur when the Fyn-lckF kinase is used by the pre-TCR, indicating that kinases containing the FynT NH2-terminal regions fail to efficiently engage those downstream signaling pathways that are necessary to initiate this expansion event. Importantly, although proliferative signals are dramatically compromised when PTKs containing FynT NH2-terminal domains provide pre-TCR signals, this enzyme structure is relatively efficient in directing DN to DP transition. As signals generated by this PTK are both regulated by pre-TCR assembly and sufficient to signal upregulation of CD4 and CD8 expression, we conclude that this enzyme structure can couple to the pre-TCR and relay signals to downstream signaling pathways. The fact that the PTK signals transduced are sufficient to stimulate developmental transition but fail to signal proliferative expansion suggests that these two outcomes of pre-TCR signaling require either differing thresholds of kinase activity or distinct kinase functions. As increasing the concentration of this PTK increases differentiative activity but only modestly enhances proliferative expansion, it seems unlikely that the deficiency lies in a suboptimal level of kinase expression or activity. Rather, based on these results we speculate that FynT NH2-terminal domains may fail to efficiently couple to downstream elements necessary to promote proliferative expansion after pre-TCR triggering. Among these elements may be one or both of the secondary PTKs required for this transition (ZAP-70 or Syk [10, 11]) and/or adaptor molecules required to direct signals to specific downstream signaling pathways 12 13 15 17.
Interestingly, the proportion of thymocytes adopting the CD4 or CD8 SP cell fate can be influenced by the dose and structure of Src-PTKs expressed at DP to SP transition. Thus, the propensity toward CD4 lineage development that is characteristic of murine thymopoiesis is minimized when PTKs lacking Lck NH2-terminal domains are used to mediate signals at DP to SP transition (Fyn-lck). This finding may reflect the fact that PTKs bearing Lck NH2-terminal domains are required to facilitate the high level of signaling necessary to promote adoption of the CD4 SP cell fate, a view consistent with existing threshold models 17. However, the finding that provision of additional Fyn activity in the context of Fyn-lck appears to enhance CD8 SP as opposed to CD4 SP development would seem inconsistent with the concept that PTK activity by itself is the determining factor driving the CD4 bias. A slightly modified model incorporating our data would postulate that the Lck NH2-terminal domains possess specialized features that enable efficient transduction of qualitatively distinct signals at this transition. In this scenario, the Lck NH2 termini would be more efficient than Fyn NH2 termini in engaging those adaptors or mediators of downstream signaling pathways that promote preferential CD4 SP development, such as elements in the Ras/Raf/MEK/extracellular signal–regulatory kinase pathway 16 17 52 61. Importantly, the shift we observe in SP development toward the CD8 lineage when Fyn expression is increased in Fyn-lckF:Lck−/− mice, a manipulation that would be expected to enhance signals downstream of the TCR 18, further suggests that it is the balance of signals generated via Fyn and Lck during selection that may direct progenitor commitment preferentially toward CD8 versus CD4 SP lineages, respectively. This notion would be consistent with recent findings indicating that the extent of Lck participation during selection influences lineage commitment, and with current models linking restricted signaling via the TCR to development of the CD8 lineage 62. These unique combinations of signaling events that reflect the relative participation of Lck and Fyn during selection could then serve as preferential targets of amplification or modification by other signaling pathways, including Notch 63. In summary, our data support the view that specific features of the Lck structure make this member of the Src family uniquely suited to relay TCR-dependent signals at both DN to DP and DP to SP checkpoints. To gain a deeper understanding of Lck action at these transitions, it will be necessary to identify the specific PTK structures involved in conferring Lck potency, and the downstream signaling pathways influenced by these structures.
Acknowledgments
We thank Dr. Roger Perlmutter for helpful discussions and for furnishing the FynT−/− strain, Katherine Forbush for assistance in generating the Lck-fyn transgenics, Dr. Tak Mak for graciously supplying the Lck−/− strain, and Drs. Steve Anderson, Steve Levin, and Donna Farber for their critical review of the manuscript.
This work was supported by National Institutes of Health grant GM48484 to K.M. Abraham.
Footnotes
K. Lin's present address is Department of Biochemistry and Biophysics, University of California at San Francisco, San Francisco, CA 94143-0554. J.A. Hewitt's present address is National Institute of Allergy and Infectious Diseases, National Institutes of Health–Frederick Cancer Research and Development Center, Frederick, MD 21702.
Abbreviations used in this paper: DN, double negative; DP, double positive; Lck, p56lck; LckF, activated mutant Lck; MEK, mitogen-activated protein kinase kinase; PTK, protein tyrosine kinase; Rag, recombination activating gene; Src-PTK, Src family PTK; SH, Src homology; SP, single positive.
References
- Robey E., Fowlkes B.J. Selective events in T cell development. Annu. Rev. Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Fehling H.J. Structure and function of the pre-T cell receptor. Annu. Rev. Immunol. 1997;15:433–452. doi: 10.1146/annurev.immunol.15.1.433. [DOI] [PubMed] [Google Scholar]
- Molina T.J., Kishihara K., Siderovski D.P., van Ewijk W., Narendran A., Timms E., Wakeham A., Paige C.J., Hartmann K.U., Veillette A. Profound block in thymocyte development in mice lacking p56lck . Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- Levin S.D., Anderson S.J., Forbush K.A., Perlmutter R.M. A dominant-negative transgene defines a role for p56lck in thymopoiesis. EMBO (Eur. Mol. Biol. Organ.) J. 1993;4:1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S.J., Levin S.D., Perlmutter R.M. The protein tyrosine kinase p56lck controls allelic exclusion of T-cell receptor β chain genes. Nature. 1993;365:552–554. doi: 10.1038/365552a0. [DOI] [PubMed] [Google Scholar]
- Wallace V.A., Kawai K., Levelt C.N., Kishihara K., Molina T., Timms E., Pircher H., Penninger J., Ohashi P.S., Eichmann K., Mak T. T lymphocyte development in p56lck deficient miceallelic exclusion of the TcR beta locus is incomplete but thymocyte development is not restored by TcR beta or TcR alpha beta transgenes. Eur. J. Immunol. 1995;25:1312–1318. doi: 10.1002/eji.1830250527. [DOI] [PubMed] [Google Scholar]
- Anderson S.J., Abraham K.M., Nakayama T., Singer A., Perlmutter R.M. Inhibition of T-cell receptor β-chain rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck . EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Anderson S.J., Perlmutter R.M., Mak T.W., Tonegawa S. An activated lck transgene promotes thymocyte development in RAG-1 mutant mice. Immunity. 1994;1:261–267. doi: 10.1016/1074-7613(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Anderson S.J., Perlmutter R.M. A signaling pathway governing early thymocyte maturation. Immunol. Today. 1995;16:99–105. doi: 10.1016/0167-5699(95)80096-4. [DOI] [PubMed] [Google Scholar]
- Cheng A.M., Negish I., Anderson S.J., Chan A.C., Bolen J., Loh D.Y., Pawson T. The Syk and ZAP-70 SH2-containing tyrosine kinases are implicated in pre-T cell receptor signaling. Proc. Natl. Acad. Sci. USA. 1997;94:9797–9801. doi: 10.1073/pnas.94.18.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers N.S., Killeen N., Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J. Exp. Med. 1996;183:1053–1062. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J.L., Yang B., Ross-Barta S.E., Eliason S.L., Hrstka R.F., Williamson R.A., Koretzky G.A. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- Pivniouk V., Tsitsikov E., Swinton P., Rathbun G., Alt F.W., Geha R.S. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- Henning S.W., Cantrell D.A. p56lck signals for regulating thymocyte development can be distinguished by their dependency on Rho function. J. Exp. Med. 1998;188:931–939. doi: 10.1084/jem.188.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton T., Gilmour K.C., Owen M.J. The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell. 1996;86:243–251. doi: 10.1016/s0092-8674(00)80096-3. [DOI] [PubMed] [Google Scholar]
- Lin K., Abraham K.M. Targets of p56lck activity in immature thymoblastsstimulation of the Ras/Raf/MAPK pathway. Int. Immunol. 1997;19:291–306. doi: 10.1093/intimm/9.2.291. [DOI] [PubMed] [Google Scholar]
- Sharp L.L., Schwarz D.A., Bott C.M., Marshall C.J., Hedrick S.M. The influence of the MAPK pathway on T cell lineage commitment. Immunity. 1997;7:609–618. doi: 10.1016/s1074-7613(00)80382-9. [DOI] [PubMed] [Google Scholar]
- Cooke M.P., Abraham K.M., Forbush K.A., Perlmutter R.M. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn) Cell. 1991;65:281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- Olszowy M.W., Leuchtmann P.L., Veillette A., Shaw A.S. Comparison of p56lck and p59fyn protein expression in thymocyte subsets, peripheral T cells, NK cells, and lymphoid cell lines. J. Immunol. 1995;155:4236–4240. [PubMed] [Google Scholar]
- Appleby M.W., Gross J.A., Cooke M.P., Levin S.D., Zian X., Perlmutter R.M. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992;70:751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- Stein P.L., Lee H.M., Rich S., Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- Abraham K.M., Levin S.D., Marth J.D., Forbush K.A., Perlmutter R.M. Delayed thymocyte development induced by augmented expression of p56lck . J. Exp. Med. 1991;173:1421–1432. doi: 10.1084/jem.173.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin S.D., Abraham K.M., Anderson S.J., Forbush K.A., Perlmutter R.M. The protein tyrosine kinase p56lck regulates thymocyte development independently of its interaction with CD4 and CD8 coreceptors. J. Exp. Med. 1993;178:245–255. doi: 10.1084/jem.178.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers N.S., Lowin-Kropf B., Finlay D., Connolly K., Weiss A. αβ T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- Groves T., Smiley P., Cooke M.P., Forbush K., Perlmutter R.M., Guidos C.J. Fyn can partially substitute for Lck in T lymphocyte development. Immunity. 1996;5:417–428. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- Bolen J.B., Rowley R.B., Spana C., Tsygankov A.Y. The Src family of tyrosine protein kinases in hemopoietic signal transduction. FASEB J. 1992;6:3403–3409. doi: 10.1096/fasebj.6.15.1281458. [DOI] [PubMed] [Google Scholar]
- Shaw A.S., Amrein K.E., Hammond C., Stern D.F., Sefton B.M., Rose J.K. The Lck tyrosine protein kinase interacts with the cytoplasmic tail of the CD4 glycoprotein through its unique amino terminal domain. Cell. 1989;59:627–636. doi: 10.1016/0092-8674(89)90008-1. [DOI] [PubMed] [Google Scholar]
- Turner J.M., Brodsky M.H., Irving B.A., Levin S.D., Perlmutter R.M., Littman D.R. Interaction of the unique N-terminal region of the tyrosine kinase p56lck with the cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990;60:755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- Timson Gauen L.K., Kong A.-N., Samelson L.E., Shaw A.S. p59fyn tyrosine kinase associates with multiple T-cell receptor subunits through its unique amino-terminal domain. Mol. Cell. Biol. 1992;12:5438–5446. doi: 10.1128/mcb.12.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek S.N., Desiderio S. SH2 domains of the protein-tyrosine kinases Blk, Lyn, and Fyn(T) bind distinct sets of phosphoproteins from B lymphocytes. J. Biol. Chem. 1993;268:22557–22565. [PubMed] [Google Scholar]
- Weng Z., Thomas S.M., Rickles R.J., Taylor J.A., Brauer A.W., Seidel-Dugan C., Michael W.M., Dreyfuss G., Brugge J.S. Identification of Src, Fyn, and Lyn SH3-binding proteinsimplications for a function of SH3 domains. Mol. Cell. Biol. 1994;14:4509–4521. doi: 10.1128/mcb.14.7.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erpel T., Alonso G., Roche S., Courtneidge S.A. The Src SH3 domain is required for DNA synthesis induced by platelet-derived growth factor and epidermal growth factor. J. Biol. Chem. 1996;271:16807–16812. doi: 10.1074/jbc.271.28.16807. [DOI] [PubMed] [Google Scholar]
- Chang C.-M., Shu H.-K., Kung H.-J. Disease specificity of kinase domainsthe src-encoded catalytic domain converts erbB into a sarcoma oncogene. Proc. Natl. Acad. Sci. USA. 1995;92:3928–3932. doi: 10.1073/pnas.92.9.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Littman D.R. A kinase-independent function of Lck in potentiating antigen-specific T cell activation. Cell. 1993;74:633–643. doi: 10.1016/0092-8674(93)90511-n. [DOI] [PubMed] [Google Scholar]
- Marth J.D., Peet R., Krebs E.G., Perlmutter R.M. A lymphocyte-specific protein-tyrosine kinase is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985;43:393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Marth J.D., Cooper J.A., King C.S., Ziegler S.F., Tinker D.A., Overell R.W., Krebs E.G., Perlmutter R.M. Neoplastic transformation induced by an activated lymphocyte-specific protein kinase (p56lck) Mol. Cell. Biol. 1988;8:540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke M.P., Perlmutter R.M. Expression of a novel form of the fyn proto-oncogene in hematopoietic cells. New Biol. 1989;1:66–74. [PubMed] [Google Scholar]
- Sleckman B.P., Peterson A., Jones W.K., Foran J.A., Greenstein J.L., Seed B., Burakoff S.J. Expression and function of CD4 in a murine T-cell hybridoma. Nature. 1987;328:351–353. doi: 10.1038/328351a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L.S., Pellicer A., Cheng Y.C., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977;11:223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Kawakami T., Kawakami Y., Aaronson S.A., Robbins K.C. Acquisition of transforming properties by FYN, a normal SRC-related human gene. Proc. Natl. Acad. Sci. USA. 1988;85:3870–3874. doi: 10.1073/pnas.85.11.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin K.E., Beals C.R., Forbush K.A., Wilkie R.M., Simon M.I., Perlmutter R.M. Dissection of thymocyte signaling pathways by in vivo expression of pertussis-toxin ADP ribosyltransferase. EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:3821–3829. doi: 10.1002/j.1460-2075.1990.tb07600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham K.M., Longo N.S., Hewitt J.A. Detection of transgene integrants and homologous recombinants in mice by polymerase chain reaction. Methods Mol. Biol. 1998;92:245–250. doi: 10.1385/0-89603-497-6:245. [DOI] [PubMed] [Google Scholar]
- Feinberg A.P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Cantley L.C., Auger K.R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991;64:281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Davidson D., Fournel M., Veillette A. Oncogenic activation of p59fynT tyrosine protein kinase by mutation of its carboxyl-terminal site of tyrosine phosphorylation, tyrosine 528. J. Biol. Chem. 1994;269:10956–10963. [PubMed] [Google Scholar]
- Voronova A.F., Adler H.T., Sefton B.M. Two lck transcripts containing different 5′ untranslated regions are present in T cells. Mol. Cell. Biol. 1987;7:4407–4413. doi: 10.1128/mcb.7.12.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin A.M., Pawar S., Marth J.D., Perlmutter R.M. Structure of the murine lck gene and its rearrangement in a murine lymphoma cell line. Mol. Cell. Biol. 1988;8:3058–3064. doi: 10.1128/mcb.8.8.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin R.S., Garvin A.M., Pawar S., Lewis D.B., Abraham K.M., Forbush K.A., Ziegler S.F., Allen J.M., Perlmutter R.M. Developmental regulation of lck gene expression in T lymphocytes. J. Exp. Med. 1991;173:383–393. doi: 10.1084/jem.173.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Wiest D.L., Abraham K.M., Munitz T.I., Perlmutter R.M., Singer A. Decreased signaling competence as a result of receptor overexpressionoverexpression of CD4 reduces its ability to activate p56lck tyrosine kinase and to regulate T-cell antigen receptor expression in immature CD4+CD8+ thymocytes. Proc. Natl. Acad. Sci. USA. 1993;90:10534–10538. doi: 10.1073/pnas.90.22.10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves T., Parsons M., Miyamoto N.G., Guidos C. TCR engagement of CD4+CD8+ thymocytes in vitro induces early aspects of positive selection, but not apoptosis. J. Immunol. 1997;158:65–75. [PubMed] [Google Scholar]
- Lundberg K., Heath W., Kontgen F., Carbone F.R., Shortman K. Intermediate steps in positive selectiondifferentiation of CD4+8int TCRint thymocytes into CD4−8+ TCRhi thymocytes. J. Exp. Med. 1995;181:1643–1651. doi: 10.1084/jem.181.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommhardt U., Basson M.A., Krummrei U., Zamoyska R. Activation of the extracellular signal-related kinase/mitogen-activated protein kinase pathway discriminates CD4 versus CD8 lineage commitment in the thymus. J. Immunol. 1999;163:715–722. [PubMed] [Google Scholar]
- D'Ambrosio D., Cantrell D.A., Frati L., Santoni A., Testi R. Involvement of p21ras activation in T cell CD69 expression. Eur. J. Immunol. 1994;24:616–620. doi: 10.1002/eji.1830240319. [DOI] [PubMed] [Google Scholar]
- Fehling H.J., Iritani B.M., Krotkova A., Forbush K.A., Laplace C., Perlmutter R.M., von Boehmer H. Restoration of thymopoiesis in pTα−/− mice by anti-CD3ε antibody treatment or with transgenes encoding activated Lck or tailless pTα. Immunity. 1997;6:703–714. doi: 10.1016/s1074-7613(00)80446-x. [DOI] [PubMed] [Google Scholar]
- Abraham K.M., Levin S.D., Marth J.D., Forbush K.A., Perlmutter R.M. Thymic tumorigenesis induced by overexpression of p56lck . Proc. Natl. Acad. Sci. USA. 1991;88:3977–3981. doi: 10.1073/pnas.88.9.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano J.D., Morrow T.A., Desiderio S.V. itk, a T-cell-specific tyrosine kinase gene inducible by interleukin 2. Proc. Natl. Acad. Sci. USA. 1992;89:11194–11198. doi: 10.1073/pnas.89.23.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyeck S.D., Berg L.J. Developmental regulation of a murine T-cell-specific tyrosine kinase gene, Tsk. Proc. Natl. Acad. Sci. USA. 1993;90:669–673. doi: 10.1073/pnas.90.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A.C., Iwashima M., Turck C.W., Weiss A. ZAP-70a 70 kd protein-tyrosine kinase that associates with the TCR ζ chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Davidson D., Viallet J., Veillette A. Unique catalytic properties dictate the enhanced function of p59fynT, the hemopoietic cell-specific isoform of the fyn tyrosine protein kinase, in T cells. Mol. Cell. Biol. 1994;14:4554–4564. doi: 10.1128/mcb.14.7.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S.K., Quinn A.M. Protein kinase catalytic domain sequence databaseidentification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Denny M.F., Kaufman H.C., Chan A.C., Straus D.B. The lck SH3 domain is required for activation of the mitogen-activated protein kinase pathway but not the initiation of T-cell antigen receptor signaling. J. Biol. Chem. 1999;274:5146–5152. doi: 10.1074/jbc.274.8.5146. [DOI] [PubMed] [Google Scholar]
- Albert Basson M., Bommhardt U., Cole M.S., Tso J.Y., Zamoyska R. CD3 ligation on immature thymocytes generates antagonist-like signals appropriate for CD8 lineage commitment, independently of T cell receptor specificity. J. Exp. Med. 1998;187:1249–1260. doi: 10.1084/jem.187.8.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey E., Chang D., Itano A., Cado D., Alexander H., Lans D., Weinmaster G., Salmon P. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996;87:483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]