Abstract
Host defense against multicellular, extracellular pathogens such as nematode parasites is believed to be mediated largely, if not exclusively, by T lymphocytes. During our investigations into the course of Brugia malayi and Brugia pahangi infections in immunodeficient mouse models, we found that mice lacking B lymphocytes were permissive for Brugian infections, whereas immunocompetent mice were uniformly resistant. Mice bearing the Btkxid mutation were as permissive as those lacking all B cells, suggesting that the B1 subset may be responsible for host protection. Reconstitution of immunodeficient recombination activating gene (Rag)-1−/− mice with B1 B cells conferred resistance, even in the absence of conventional B2 lymphocytes and most T cells. These results suggest that B1 B cells are necessary to mediate host resistance to Brugian infection. Our data are consistent with a model wherein early resistance to B. malayi is mediated by humoral immune response, with a significant attrition of the incoming infectious larval load. Sterile clearance of the remaining parasite burden appears to require cell-mediated immunity. These data raise the possibility that the identification of molecule(s) recognized by humoral immune mechanisms might help generate prophylactic vaccines.
Keywords: B cells, lymphatic filariasis, Brugia malayi, Brugia pahangi, host protection
Introduction
Filarial parasites cause lymphatic filariasis, a disease that is estimated to currently affect 120 million people worldwide 1. The parasites are transmitted to mammals by mosquitoes as infective third stage larvae (L3), which subsequently develop into L4 and adult worms in the mammalian hosts. Adult male and female parasites reside in the lymphatics and produce numerous microfilariae that find their way into the systemic circulation, from where they can be picked up by a feeding mosquito. Development in the arthropod vector is obligatory for these organisms.
Over the last 20 years, considerable progress has been made in understanding the host–parasite relationship during filarial infection using existing animal models, such as Mongolian jird 2, cat 3, or mouse 4, as well as through cross-sectional correlative studies of the affected population from the filariasis endemic areas 5. Despite the significant advances in elucidating the role of the immune system during the infection and the presence of two efficient chemotherapeutic agents, lymphatic filariasis continues to be a major public health problem and the mechanism(s) of protective immunity remain unresolved. In this report, we report our analysis of the role of B lymphocytes in host protection against Brugia infection in mice.
Materials and Methods
Mice.
C57BL/6J (hereafter B6), C57BL/6J–Rag-1null (hereafter Rag-1null), C57BL/6J-Hfh11nu (hereafter NUDE), CBA/CaHN-Btkxid/J (hereafter CBA/N), and CBA/CaJ mice were obtained from the colony of L.D. Shultz at The Jackson Laboratory. C57BL/6J-Prkdcscid(hereafter SCID) and C57BL/6J-Igh6null (hereafter μMT) mice were obtained from National Institutes for Aging, Bethesda, MD. All mice were housed in microisolator cages to reduce the incidence of intercurrent infections. To control for the confounding variables of age and sex, all mice used in the study were males between 4 and 8 wk of age.
Brugia Infection.
Brugia malayi and Brugia pahangi third stage (L3) larvae were obtained from the University of Georgia (Athens, GA) through a contract with the National Institutes of Health (U.S.–Japan Collaborative Program in Filariasis) or from TRS, Inc. (Athens, GA). The L3 larvae were injected intraperitoneally at a dose of 50 L3/mouse. The intraperitoneal route of infection was chosen because it enables us to accurately quantify worm burdens, as the worms remain within the peritoneal cavity and seldom migrate elsewhere. In addition, the larvae proceed through their developmental stages with the same kinetics after intraperitoneal injection as with the more natural subcutaneous route of infection 6.
Mice were necropsied at varying times after infection, and the peritoneal cavities were washed with RPMI 1640 (GIBCO BRL) to recover viable L4 or adult worms. In addition, the mice with their peritoneal cavities open were soaked in Tris-buffered saline to allow the remaining worms to crawl out. Worm counts were performed under a dissecting microscope. Worm burdens were expressed as a percentage of the original infective dose (50 L3).
Immune Reconstitution of Mice.
For peritoneal exudate cell (PEC) transfers, the protocol was used as described by Forster and Rajewsky 7. In brief, peritoneal lavage was performed on naive age-matched male donors with 10 ml of ice-cold RPMI 1640 supplemented with 10% FBS. Peritoneal lavage was filtered through 100-μm mesh nylon fabric (Tetko, Inc.), the cells were pooled, and 3 × 106 cells were injected into recipients intraperitoneally.
Flow Cytometry.
PECs were collected from animals individually, counted, and adjusted to 107 cells/ml in staining buffer (PBS, 0.2% BSA, 0.1% NaN3). 106 cells (100 μl) were incubated with 10–100 μl of appropriately diluted antibody. Antibodies used were: CD3-FITC, CD19-biotin, CD5-PE, Mac-1–biotin, CD4-PE, and CD8-PE from PharMingen, and IgM-FITC from Kirkegaard & Perry Laboratories. After staining, cells were washed twice with staining buffer and fixed in 0.5% buffered formalin. Cells were run on a FACScalibur™ (Becton Dickinson) using CELLQuest™ software (Becton Dickinson). Additional analyses were performed using WinMDI (Joseph Trotter, The Scripps Research Institute, Palo Alto, CA).
Results and Discussion
The Absence of B Lymphocytes Renders Mice Permissive to Brugia Infection.
The importance of T cells in host protection against filarial infection is well documented in both the human parasite B. malayi 6 and the closely related parasite of cats, B. pahangi 8 9. In contrast, the role of B cells in host protection against filariasis remains controversial. Several investigators have documented the inability to transfer protective immunity with serum 10. In addition, previously published data demonstrate normal clearance of filarial infection in B cell–deficient C57BL/6J μMT mice 11. However, the latter study was performed on mice of the segregating (C57BL/6J × 129/Ola) background. Our work over the last few years has demonstrated that the background of the mouse strain has profound effects on host–parasite interactions 12. Indeed, certain backgrounds are poorly permissive to infection even in the absence of adaptive immunity 12.
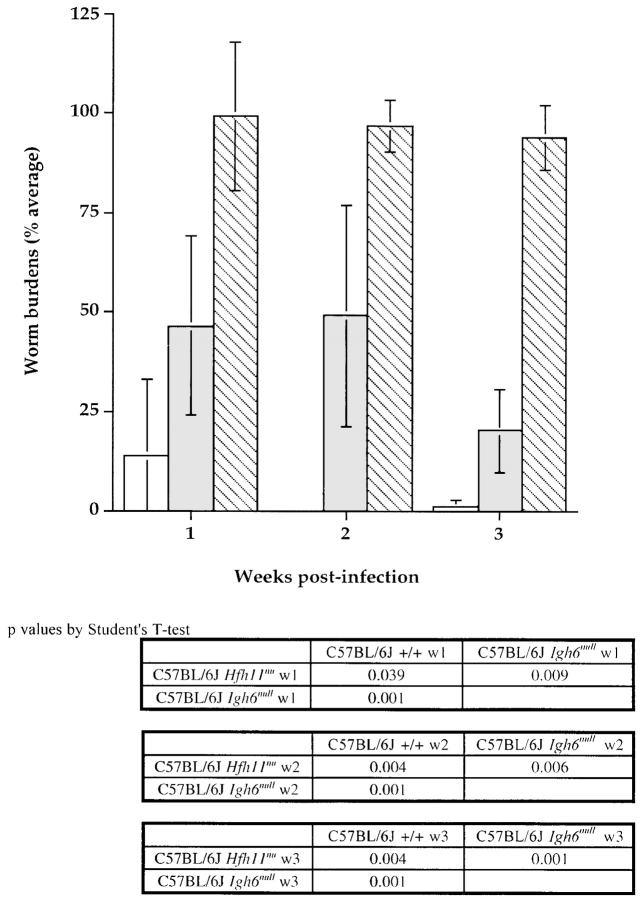
In light of these findings, we reevaluated the role of T versus B lymphocytes in filarial infection using immunodeficient mice on the B6, CBA, and BALB/c backgrounds, which are all permissive for Brugian infections. Our studies revealed that mice homozygous for the induced Igh6null mutation, which causes a profound deficiency of B lymphocytes, are permissive to a level comparable to B6 SCID mice, which lack both B and T lymphocyte subsets 13. We repeated this experiment with the closely related filarial nematode, B. pahangi. The results are shown in Fig. 1. Mice that lack B cells fail to control B. pahangi infection, just as they are unable to contain B. malayi infection. In contrast, B cell–competent but T cell–deficient B6 NUDE animals demonstrate reduced worm burdens as early as 1 wk after infection. However, it is important to stress that the reduced worm burdens in these mice persist indefinitely, and the mice become patent and productive of microfilariae. Thus, B cell–competent T cell–deficient mice reduce worm burdens early in infection through a T cell–independent mechanism, but do not accomplish sterile immunity seen in fully immunocompetent +/+ mice.
Figure 1.
Comparison of B. pahangi infection in C57BL/6J Igh6null, Hfh11nu, and +/+ mice. Cohorts of age-matched B6 +/+, Hfh11nu, and Igh6null male mice received intraperitoneal injection of ∼50 B. pahangi L3. Batches of five mice of each group were necropsied at 1, 2, and 3 wk (w) after infection. Worm burdens were quantitated and expressed as a percentage of the infective dose (50). The bars represent average worm recoveries from five mice ±SD. White bars, B6 +/+; gray bars, B6 Hfh11nu; hatched bars, B6 Igh6null mice.
The Loss of B1 B Cell Function Correlates with Increased Filarial Worm Burdens in Mice.
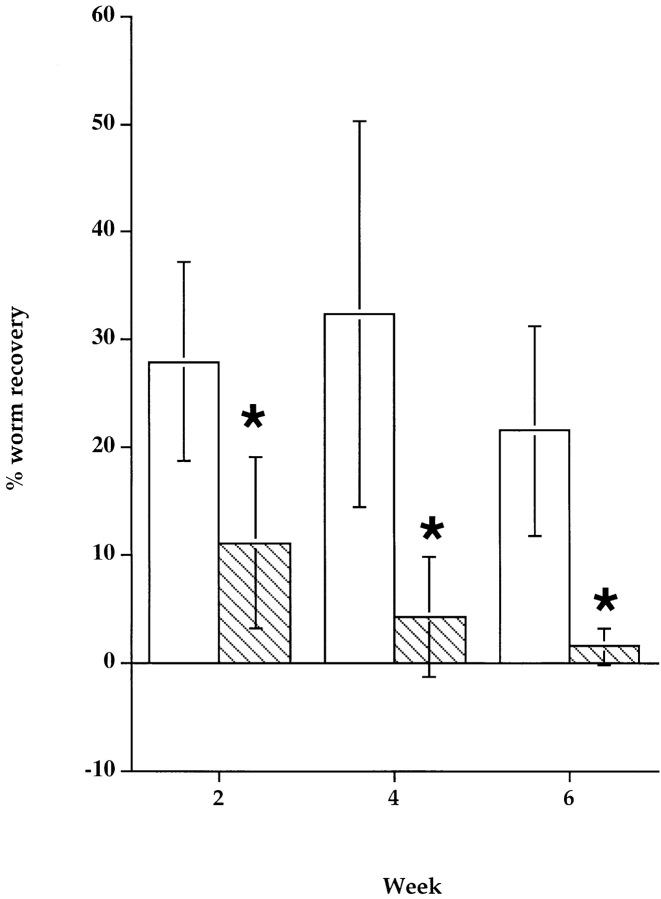
To determine whether B1 B lymphocytes are responsible for the permissiveness of B-less mice to Brugian infection, we compared the growth of B. malayi in sex- and age-matched cohorts of wild-type and Btkxid mice. The Btkxidmutation results in an X-linked immunodeficiency (xid), due to a defective Bruton's tyrosine kinase (Btk). Human patients with this genetic defect exhibit a severe agammaglobulinemia, but the murine homologue has a milder deficit. Mice bearing the Btkxid mutation lack peritoneal B1 B lymphocytes, but show only a 30–50% reduction in other B lymphocyte populations 14. Our experiments with mice bearing the Btkxid mutation, conducted on six different occasions, using both B. malayi and B. pahangi and different mouse backgrounds matched for the Btkxid and Btk +/+ genotypes reveal a striking pattern of divergence between these two strains. Whereas immunocompetent mice of all three backgrounds eliminated worms by the third week of infection, mice with the Btkxid phenotype bear significant worm burdens as late as 6 wk after infection (Fig. 2).
Figure 2.
Course of B. malayi infection in CBA/N and CBA/CaJ mice. Cohorts of age-matched CBA/N and CBA/CaJ male mice received intraperitoneal injection of ∼50 B. malayi L3. Batches of five mice of each group were necropsied at 2, 4, and 6 wk after infection. Worm burdens were quantitated and expressed as a percentage of the infective dose (50). The bars represent average worm recoveries from five mice ±SD. White bars, CBA/N; gray bars, CBA/J mice. *Significantly different worm burdens compared with matched controls (P < 0.05).
B1 Cell Reconstitution Protects Btkxid Mice against Brugia Infection.
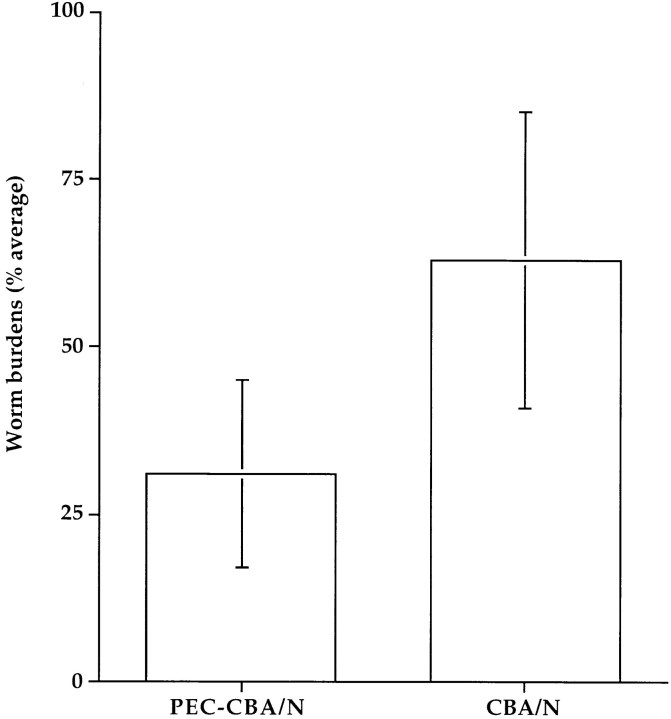
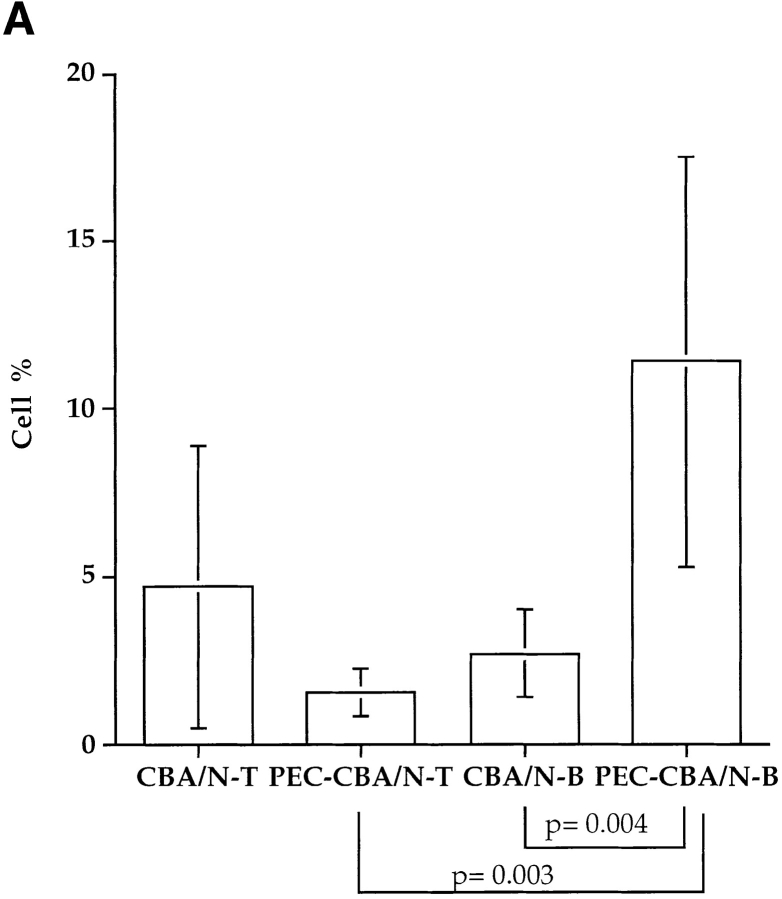
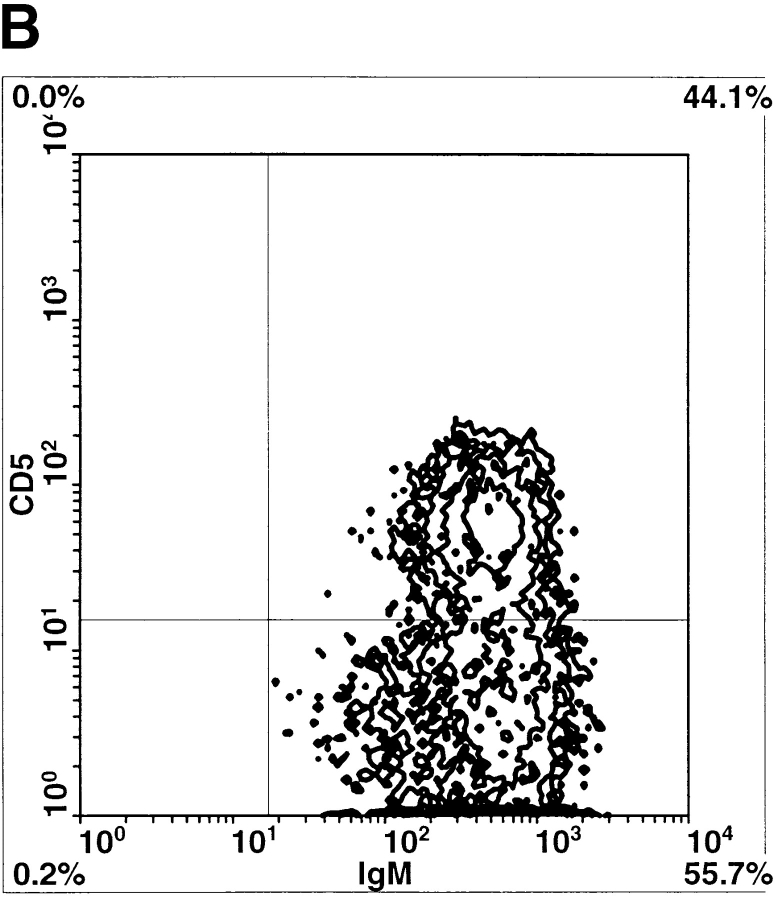
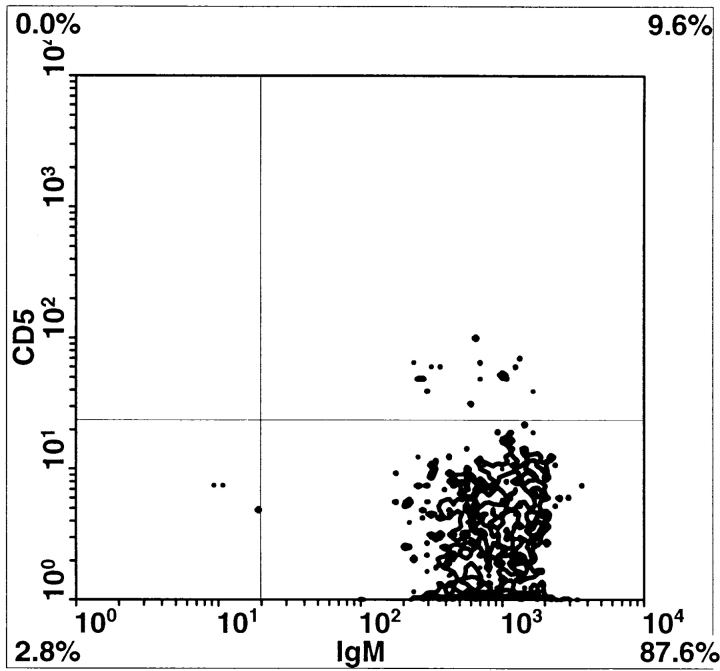
Since Btkxid mice have deficits in B1 B lymphocytes, B2 B lymphocytes, and mast cells, we sought to further document that the lack of B1 lymphocytes is responsible for the permissiveness of Btkxid mice 14. Therefore, we reconstituted CBA/N mice with PECs from sex-matched uninfected CBA/CaJ mice. PECs are known to be enriched for B1 B cells, and the protocol for their isolation and transfer is well established 7. 6 d after reconstitution, reconstituted CBA/N mice (n = 10) and nonreconstituted controls (n = 9) received 50 B. pahangi L3 intraperitoneally. Both cohorts of mice were necropsied 2 wk after infection, and worm burdens were determined. The results, shown in Fig. 3, demonstrate a 50% reduction in worm burdens in PEC-reconstituted CBA/N mice compared with controls. At the time of necropsy, PECs from both reconstituted and nonmanipulated animals were recovered and stained for the presence of B1 or B2 lymphocytes, as well as T cells. FACS® analyses were performed on each mouse individually; however, since the animals within a cohort showed remarkable homogeneity, data are shown in Fig. 4 B from only one animal. Whereas there was little difference in the percentages of T cells between PEC-reconstituted and nonreconstituted CBA/N mice, the former show a significant increase in B lymphocyte percentage (Fig. 4 A). In addition, a population of CD5+ cells, missing in the control group, is seen in the CD19+IgM+ lymphocyte subset of PEC-reconstituted CBA/N mice. Thus, it would appear that host resistance to Brugia infection requires B1 B lymphocytes.
Figure 3.
Comparison of B. pahangi infection in CBA/N mice vs. PEC-reconstituted littermates. PECs were collected from uninfected CBA/CaJ adult male mice by peritoneal lavage, pooled, and 3 × 106 cells were injected intraperitoneally per recipient into CBA/N male mice (n = 10). 6 d later, reconstituted mice and nonreconstituted age-matched CBA/N controls (n = 9) received 50 B. pahangi L3 intraperitoneally. Both groups were necropsied at 2 wk after infection, and worm burdens were determined as described above. PEC-CBA/N, CBA/N mice reconstituted with PEC from CBA/CaJ mice. PEC-CBA/N mice had statistically significantly lower worm burdens than controls, P = 0.002.
Figure 4.
(A) Relative amounts of peritoneal T and B lymphocytes in CBA/N mice vs. PEC-reconstituted littermates 2 wk after B. pahangi infection. PECs were collected from both cohorts of mice by peritoneal lavage, stained with anti–mouse CD19-biotin (for B lymphocytes) and anti–mouse CD3-FITC (for T lymphocytes) antibodies, and analyzed by flow cytometry. Lymphocytes recovered from each individual mouse were analyzed separately. Bars represent the average percentages of T or B cells in total PEC populations ±SD. (B) Comparison of peritoneal B lymphocytes in PEC-reconstituted vs. nonmanipulated CBA/N mice. Peritoneal lymphocytes were collected from B. pahangi–infected mice at 2 wk after infection and stained with IgM-FITC, CD5-PE, and CD19-biotin. B lymphocytes were gated as CD19+ cells and further divided on the basis of surface IgM and CD5 expression. In both panels, CD19+ gated peritoneal lymphocytes are analyzed for expression of CD5 (y-axis) and IgM (x-axis). Left, representative CBA/N mice reconstituted with PECs from uninfected CBA/CaJ mouse; right, representative unmanipulated CBA/N mouse. Note that the unmanipulated mouse has no CD5+ cells; the mouse with PECs from CBA/CaJ has abundant CD5+ cells.
Reconstitution of B6 Rag-1null Mice with PECs from Naive B6 Donors Confers Resistance to Brugia Infection.
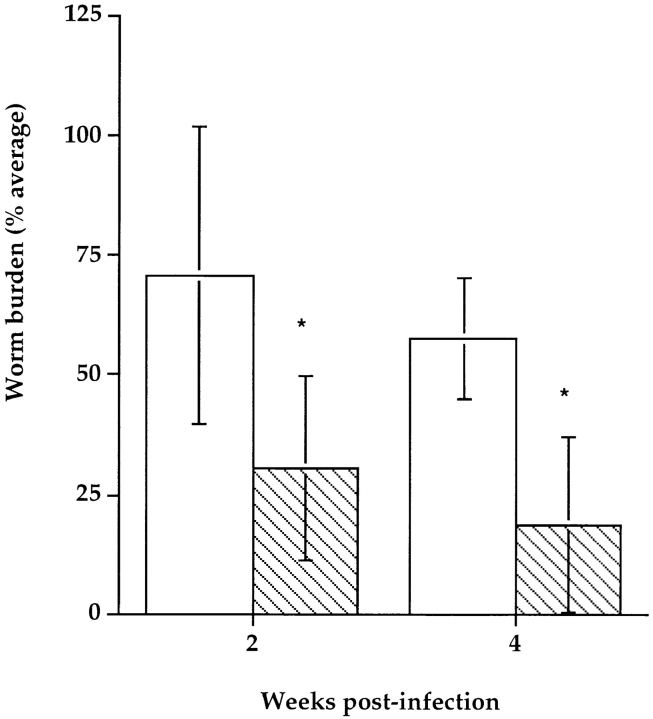
We next wanted to determine whether B1 B lymphocytes are sufficient for host protection. To address this question, we reconstituted B6 Rag-1null male mice with peritoneal lymphocytes from naive immunocompetent B6 male donors. Each recipient animal received 3 × 106 peritoneal lymphocytes. 3 wk later, cohorts of reconstituted and wild-type B6 Rag-1null mice were injected intraperitoneally with 50 B. pahangi. Batches of five mice from each group were necropsied at 2 and 4 wk after infection. Fig. 5 demonstrates that at both time points reconstituted mice had significantly lower worm burdens than nonreconstituted controls. Analysis of peritoneal lymphocytes revealed that although reconstituted mice harbored a few CD3+ cells, the majority of lymphocytes in the peritoneal cavity were B cells (CD19+). Moreover, all of the B lymphocytes manifested the B1 phenotype (IgMhi, Mac-1+, CD5+/−).
Figure 5.
Comparison of B. pahangi infection in PEC-reconstituted Rag-1null mice vs. nonmanipulated littermates. PECs were collected from adult B6 +/+ males as described above and injected into age-matched B6 Rag-1null recipients intraperitoneally. 3 wk later, cohorts of reconstituted mice and nonreconstituted B6 Rag-1null controls were injected intraperitoneally with 50 B. pahangi L3. Batches of five mice from each group were necropsied at 2 and 4 wk after infection, and quantitative worm recovery was performed. Each bar represents average worm burdens from five animals ±SD. White bars, naive B6 Rag-1null mice; hatched bars, PEC-reconstituted B6 Rag-1null mice. At both time points, comparisons of the two groups reveal that the worm burdens are statistically significantly different (*P < 0.05).
How might B1 lymphocytes provide host protection against this large, multicellular, extracellular organism that is surrounded by a thick, impervious cuticle? They might act as early antigen-presenting cells to initiate T cell responses. In addition, B1 lymphocytes are known to produce IL-10 15, which might polarize the immune response towards Th2 dominance, believed to be important in host defense against nematode parasitism. Finally, B1 lymphocytes may combat filarial parasites via antibody production. Although all three are possible and not mutually exclusive, we favor the last. B1 cells are known to produce antibodies to a variety of nonpeptide antigens, many of which seem to be conserved among pathogenic organisms. Phosphorylcholine (PC), a small amphipathic molecule that is present abundantly on excretory/secretory (E/S) molecules of filarial parasites, is one such immunogen. We propose that the presence of PC on E/S products is not accidental and that it is required for either communication between worms themselves or for host–parasite interaction that is necessary for successful development of the larvae. One such interaction may involve host NK cells. We have recently shown that some product and/or function of NK cells is critically required for B. malayi to mature in the mammalian host 12. E/S products of Brugia origin may act directly or indirectly on NK cells, causing them to produce a morphogen required for parasite development. Host anti-PC antibodies can successfully sequester these E/S products and prevent the obligate host–parasite interaction. The possibility of eliminating the infection by blocking the “parasite” function of filarial nematodes opens up new opportunities towards vaccine development that have not yet been explored in infectious disease research. Although a 50% protection might seem trivial, it is important to point out that in the natural state, target individuals are challenged with 1–3 L3 per day, rather than 50 as in our experimental model. B1 B cell immunity might be more quantitatively effective under those circumstances.
Acknowledgments
We would like to thank the U.S.–Japan Filariasis Program for the B. malayi L3 larvae; Dr. James Kenny, the National Institutes for Aging, Baltimore, MD, for some of the mice used in this study; and members of the Rajan laboratory for critical reading of the manuscript and helpful comments.
This work was made possible by National Institutes of Health grants AI39705 and AI42362 to T.V. Rajan and grants AI30389 and CA34196 to L.D. Shultz.
References
- Ottesen E.A., Duke B.O., Karam M., Behbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull. World Health Organ. 1997;75:491–503. [PMC free article] [PubMed] [Google Scholar]
- Ash L., Riley J. Development of subperiodic Brugia malayi in the jird, Meriones unguiculatus, with notes on infections in other rodents. J. Parasitol. 1970;56:969–973. [PubMed] [Google Scholar]
- Denham D.A., Fletcher C. The cat infected with Brugia pahangi as a model of human filariasis. Ciba Found. Symp. 1987;127:225–235. doi: 10.1002/9780470513446.ch15. [DOI] [PubMed] [Google Scholar]
- Lawrence R.A. Lymphatic filariasiswhat mice can tell us. Parasitol. Today. 1996;12:267–271. doi: 10.1016/0169-4758(96)10025-9. [DOI] [PubMed] [Google Scholar]
- Nanduri J., Kazura J.W. Clinical and laboratory aspects of filariasis. Clin. Microbiol. Rev. 1989;2:39–50. doi: 10.1128/cmr.2.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson F.K., Greiner D.L., Shultz L.D., Rajan T.V. The immunodeficient scid mouse as a model for human lymphatic filariasis. J. Exp. Med. 1991;173:659–663. doi: 10.1084/jem.173.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster I., Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur. J. Immunol. 1987;17:521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- Vincent A.L., Sodeman W.A., Winters A. Development of Brugia pahangi in normal and nude mice. J. Parasitol. 1980;66:448. [PubMed] [Google Scholar]
- Suswillo R.R., Owen D.G., Denham D.A. Infections of Brugia pahangi in conventional and nude (athymic) mice. Acta Trop. 1980;37:327–335. [PubMed] [Google Scholar]
- Vickery A.C., Vincent A.L., Sodeman W.A. Effect of immune reconstitution on resistance to Brugia pahangi in congenitally athymic nude mice. J. Parasitol. 1983;69:478–485. [PubMed] [Google Scholar]
- Rajan T.V., Shultz L.D, Yates J., Greiner D.L. B lymphocytes are not required for murine resistance to the human filarial parasite, Brugia malayi . J. Parasitol. 1995;81:490–493. [PubMed] [Google Scholar]
- Babu S., Porte P., Klei T.R., Shultz L.D, Rajan T.V. Host NK cells are required for the growth of the human filarial parasite Brugia malayi in mice. J. Immunol. 1998;161:1428–1432. [PubMed] [Google Scholar]
- Babu S., Shultz L.D., Klei T.R., Rajan T.V. Immunity in experimental murine filariasisroles of T and B cells revisited. Infect. Immun. 1999;67:3166–3167. doi: 10.1128/iai.67.6.3166-3167.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite A.B., Li Z., Witte O.N. Btk function in B cell development and response. Semin. Immunol. 1998;10:309–316. doi: 10.1006/smim.1998.0123. [DOI] [PubMed] [Google Scholar]
- O'Garra A., Chang R., Go N., Hastings R., Haughton G., Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur. J. Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]