Abstract
Distribution of chemokine receptors CCR5 and CXCR4, which are also coreceptors for human immunodeficiency virus type 1 invasion of cells, was measured on the surfaces of CD4+ T cells and monocytes in peripheral blood samples from a group of Kenyan car washers. Patients with active schistosomiasis displayed higher cell surface densities of these receptors than did cured schistosomiasis patients.
Sub-Saharan Africa represents 10% of the world's population but more than 70% of the world's cases of AIDS (16). While many contributing factors have been proposed to explain this phenomenon (1, 2, 4, 5, 19), no clear answer has yet emerged. A prominent hypothesis is that other infections common in Africa, such as parasitic diseases, predispose persons to more readily become infected with human immunodeficiency virus type 1 (HIV-1) if they are exposed to the virus and/or that coinfections exacerbate HIV-1 replication (1, 2). However, direct evaluation of this hypothesis is difficult from both an ethical and logistical perspective. Therefore, to date, studies have utilized either in vitro or indirect in vivo study designs to understand what effect parasitic infections may have on transmission of HIV-1 or progression of AIDS.
One mechanism affecting cellular susceptibility to HIV-1 infection is the differential expression of chemokine receptors that also serve as coreceptors for viral entry into cells (10, 12, 14, 15). Higher cellular expression of the chemokine receptors CXCR4 and CCR5 confer greater susceptibility of cells to in vitro infection with HIV-1. Therefore, we wished to test whether schistosomiasis had any effect on the expression of these chemokine receptors on the surfaces of CD4+ peripheral blood T cells and monocytes of patients. To do this, we worked with a previously described cohort of male car washers in Kisumu, Kenya, where HIV-1 seroprevalence approaches 35% in sexually active adults (8, 9, 11). This study was approved by the Scientific Steering Committee of the Kenya Medical Research Institute, the Kenya National Ethical Review Committee, and the Institutional Review Board of the Centers for Disease Control and Prevention.
Inclusion criteria for this study were as follows: patient age of ≥18 years, current or previous Schistosoma mansoni infection, and willingness to be tested for HIV-1-specific antibodies. Pre- and posttest counseling were provided to patients who gave informed consent. Stool exams were performed by using the Kato Katz technique (Helm TecR P & D Pesquisa E Desenvolmento Ltd., Belo Horizonte, Brazil). Duplicate slides from each of three stool samples collected on consecutive days were examined to determine the mean number of schistosome eggs per gram for each individual. Peripheral blood samples were collected by venipuncture into heparin-coated Vacutainer tubes (Becton Dickinson, Rutherford, N.J.) and immediately transported to the laboratory for processing.
Cells were stained with Cy-Chrome anti-CD4, phycoerythrin-conjugated CD3 or CD14, and fluorescein isothiocyanate-conjugated CCR5 or CXCR4 (all from BD Pharmingen, San Diego, Calif.) for 30 min at 4°C in the dark. For controls, cells were stained with isotype- and fluorochrome-matched antibodies (BD Pharmingen). Following staining, red blood cells were lysed with fluorescence-activated cell sorting (FACS) lysing solution (Becton Dickinson) according to the manufacturer's instructions. After a washing step, cells were fixed using 4% paraformaldehyde in phosphate-buffered saline. Staining was assessed with a FACScaliber flow cytometer (Becton Dickinson) using Cell Quest software for analysis. CD4+ cells were gated, and the percent and mean channel fluorescence (MCF) of CCR5 and CXCR4 were determined for CD3+ cells (T cells) or CD14+ cells (monocytes). The flow cytometer was calibrated every day before samples were run by using four-color Calibrite beads (BD Pharmingen) to ensure that instrument settings were appropriate. In addition, for every fluorochrome-labeled antibody used, an isotype-matched control was included to control for nonspecific antibody staining.
In initial studies, we evaluated chemokine receptor expression on the surfaces of cells from HIV-1-negative individuals to avoid any independent effects HIV-1 may have had on the expression of CCR5 or CXCR4. Statistical comparisons were made by using the nonparametric Mann-Whitney test. When we compared the density (MCF) of CCR5 and CXCR4 on the surfaces of CD4+ CD3+ and CD4+ CD14+ cells, we found that patients with active schistosomiasis expressed significantly higher levels of CXCR4 on the surfaces of both T cells and monocytes compared to persons who had previously had schistosomiasis but had been treated (Table 1). In contrast, the percentages of cells positive for CXCR4 were similar for the two groups of patients (data not shown), suggesting that the density, but not distribution, of these receptors was altered by schistosomiasis. CCR5 MCF levels and percent CCR5 cells were elevated in patients with active schistosomiasis, but the values for the two groups were not significantly different (Table 1 and data not shown).
TABLE 1.
Chemokine receptor expression of patients with active schistosomiasis compared to cured schistosomiasis patients
| Cell and chemokine receptor | MCF data for chemokine receptora in:
|
P value | |
|---|---|---|---|
| Patients with schistosome eggs (n = 26) | Patients with no schistosome eggs (n = 16) | ||
| CD4+ CD3+ cells | |||
| CCR5 | 49.3 (24.3; 82.5) | 27.2 (2.99; 58.9) | 0.068 |
| CXCR4 | 169.1 (147.3; 179.2) | 128.4 (87.5; 160.2) | 0.033 |
| CD4+ CD14+ cells | |||
| CCR5 | 2.5 (0.0; 13.1) | 0.0 (0.0; 7.1) | 0.423 |
| CXCR4 | 89.1 (64.6; 121.5) | 57.3 (41.3; 74.1) | 0.013 |
Data shown are group medians (25th quartile; 75th quartile) of MCF data for chemokine receptors minus the MCF of the isotype controls for each individual patient. The MCF group medians (quartiles) for the isotype controls were as follows: on the surface of CD4+ CD3+ cells, 18.5 (13.8; 26.3) for individuals with schistosome eggs and 20.9 (16.3; 24.8) for individuals with no schistosome eggs; on the surface of CD4+ CD14+ cells, 46.9 (28.6; 59.5) for individuals with schistosome eggs and 32.8 (18.1; 44.7) for individuals with no schistosome eggs. The isotype control MCF values were not significantly different for either cell type.
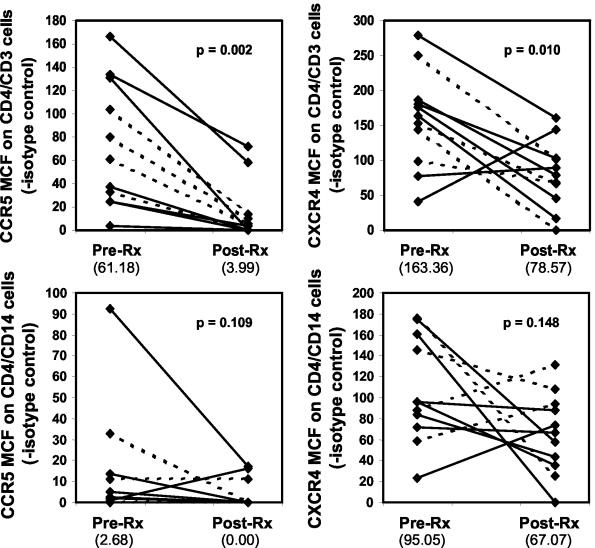
To extend these studies, we also evaluated the chemokine receptor profiles before and after treatment of individual patients. In this portion of the study, we included both HIV-1-positive and -negative individuals to determine whether the beneficial effect of treating schistosomiasis led to a reduction in chemokine receptor density irrespective of HIV-1 infection status. The median time between sampling when patients were actively infected and after they had no schistosome eggs was 11 months (range, 4 to 14 months). Figure 1 shows the chemokine receptor staining profiles of CD4+ T cells from a representative patient during active schistosomiasis and after the patient was cured. Posttreatment levels of both CCR5 and CXCR4 on the surfaces of CD4+ CD3+ cells were significantly lower than pretreatment levels (Fig. 2). Cells from HIV-1-negative and HIV-1-positive patients showed a decrease in the MCF of chemokine receptors after treatment for schistosomiasis. There was no clear association between the length of time after treatment and decrease in chemokine receptor expression, although the patient who had been clear of schistosomiasis for only 4 months demonstrated the smallest decrease in CCR5 MCF and was the only patient to show a marked increase in CXCR4 MCF. Median MCF values for CCR5 and CXCR4 expression on monocytes also decreased after treatment, but the differences were not statistically significant (Fig. 2).
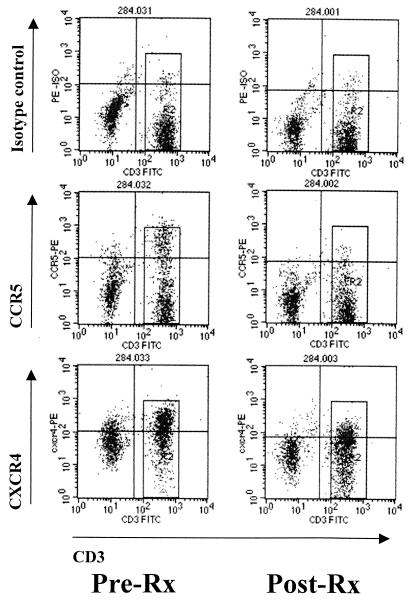
FIG. 1.
Comparison of chemokine receptor staining profiles for a patient during active schistosomiasis and after the patient was cured. MCF values for CCR5 and CXCR4 were calculated for the population of CD4 cells that was also positive for CD3, as represented by the rectangular box in the right half of each profile. Abbreviations: Pre-Rx and Post-Rx, before and after treatment for schistosomiasis, respectively; FITC, fluorescein isothiocyanate; PE, phycoerythrin; ISO, isotype.
FIG. 2.
Chemokine receptor expression of patients before and after treatment for schistosomiasis. MCF data for chemokine receptors minus the MCF of the isotype control for individuals before (Pre-Rx) and after (Post-Rx) treatment with praziquantel are shown. Data from HIV-1-negative individuals (solid lines) and HIV-1-positive patients (broken lines) are shown. The numbers in parentheses below the graph are the median values for the group. Statistical analyses were performed by using the nonparametric Wilcoxon matched-pair signed-rank test. The MCF group medians (quartiles shown in parentheses [25th quartile; 75th quartile]) for the isotope controls were as follows: on the surface of CD4+ CD3+ cells, 20.8 (18.3; 23.2) for individuals with schistosome eggs and 20.9 (17.1; 23.9) for individuals with no schistosome eggs; on the surface of CD4+ CD14+ cells, 46.6 (33.0; 66.2) for individuals with schistosome eggs and 57.4 (34.0; 72.2) for individuals with no schistosome eggs. The isotype control MCF values were not significantly different for either cell type.
Increased density of CXCR4 and CCR5 on the surfaces of cells from patients with schistosomiasis is consistent with observations that their expression is upregulated by the Th2-associated cytokines interleukin-4 (3, 17) and interleukin-10 (14, 18), respectively, which are commonly produced in response to helminth infections such as schistosomiasis. Because a higher number of chemokine receptors on the surfaces of CD4+ cells is associated with increased infection by HIV-1 (10, 12, 14, 15), these results imply that cells from schistosomiasis patients may be more susceptible to infection with HIV-1 and that chemotherapy for schistosomiasis may reduce the susceptibility of the patient to the virus if the patient is exposed to the virus. The reduction of HIV-1 coreceptor densities on the surfaces of CD4+ T cells from HIV-1-positive schistosomiasis patients also suggests that praziquantel treatment may provide a beneficial effect in terms of the progression of a coinfected patient to AIDS. Analogous observations of increased chemokine receptor density have previously been made in patients with generalized helminthiasis (7). These patients also had increased in vitro susceptibility to HIV-1 (13). Similarly, treatment of filariasis patients reduced the susceptibility of their cells to infection with HIV-1 in vitro (6). While it is clearly not possible to definitively conclude from these results that helminth infections confer a greater risk for contracting HIV or AIDS, these data do support the hypothesis that the public health advantages of antihelminthic treatment for persons at risk of HIV or AIDS may go beyond the simple benefit of curing their parasitic infections (1, 2).
Acknowledgments
This manuscript is published with the permission of the director of the Kenya Medical Research Institute and supported in conjunction with the VAMC Atlanta and the Atlanta Research and Education Foundation.
We thank Daniel G. Colley for helpful discussions, Patrick J. Lammie for critical reading of the manuscript, and Jose Stout for flow cytometer access. We also thank Julius Andove, Kennedy Matunda, and Nathan Mulonga for expert field assistance.
Editor: J. M. Mansfield
REFERENCES
- 1.Bentwich, Z., A. Kalinkovich, and Z. Weisman. 1995. Immune activation is a dominant factor in the pathogenesis of AIDS in Africa. Immunol. Today 16:187-191. [DOI] [PubMed] [Google Scholar]
- 2.Bentwich, Z., G. Maartens, D. Torten, A. A. Lal, and R. B. Lal. 2000. Concurrent infections and HIV pathogenesis. AIDS 14:2071-2081. [DOI] [PubMed] [Google Scholar]
- 3.Bleul, C. C., L. Wu, J. A. Moxie, T. A. Springer, and C. R. Mackay. 1997. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. USA 94:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer, D. D., S. Brody, E. Drucker, D. Gisselquist, S. F. Minkin, J. J. Potterat, R. B. Rothenberg, and F. Vachon. 2003. Mounting anomalies in the epidemiology of HIV in Africa: cry the beloved paradigm. Int. J. STD AIDS 14:144-147. [DOI] [PubMed] [Google Scholar]
- 5.Buvé, A., K. Bishikwabo-Nsarhaza, and G. Mutangadura. 2002. The spread and effect of HIV-1 infection in sub-Saharan Africa. Lancet 359:2011-2017. [DOI] [PubMed] [Google Scholar]
- 6.Gopinath, R., M. Ostrowski, S. J. Justement, A. S. Fauci, and T. B. Nutman. 2000. Filarial infections increase susceptibility to human immunodeficiency virus infection in peripheral blood mononuclear cells in vitro. J. Infect. Dis. 182:1804-1808. [DOI] [PubMed] [Google Scholar]
- 7.Kalinkovich, A., Z. Weisman, Q. Leng, G. Borrow, M. Stein, Z. Greenberg, S. Zlotnikov, S. Eitan, and Z. Bentwich. 1999. Increased CCR5 expression with decreased β-chemokine secretion in Ethiopians: relevance to AIDS in Africa. J. Hum. Virol. 2:283-289. [PubMed] [Google Scholar]
- 8.Karanja, D. M. S., D. G. Colley, B. L. Nahlen, J. H. Ouma, and W. E. Secor. 1997. Studies on schistosomiasis in western Kenya. I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus co-infections. Am. J. Trop. Med. Hyg. 56:515-521. [DOI] [PubMed] [Google Scholar]
- 9.Karanja, D. M. S., A. W. Hightower, D. G. Colley, P. N. M. Mwinzi, K. Galil, J. Andove, and W. E. Secor. 2002. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and the effect of HIV-1 coinfection on susceptibility to schistosomiasis: a longitudinal study. Lancet 360:592-596. [DOI] [PubMed] [Google Scholar]
- 10.Moonis, M., B. Lee, R. T. Bailer, Q. Luo, and L. J. Montaner. 2001. CCR5 and CXCR4 expression correlated with X4 and R5 HIV-1 infection yet not sustained replication in Th1 and Th2 cells. AIDS 15:1941-1949. [DOI] [PubMed] [Google Scholar]
- 11.Mwinzi, P. N. M., D. M. S. Karanja, D. G. Colley, A. S. S. Orago, and W. E. Secor. 2001. Cellular immune responses of schistosomiasis patients are altered by human immunodeficiency virus-1 co-infection. J. Infect. Dis. 184:488-496. [DOI] [PubMed] [Google Scholar]
- 12.Nokta, M. A., X.-D. Li, J. Nichols, M. Mallen, A. Pou, D. Asmuth, and R. B. Pollard. 2001. Chemokine/CD4 receptor density ratios correlate with HIV replication in lymph nodes and peripheral blood of HIV-infected individuals. AIDS 15:161-169. [DOI] [PubMed] [Google Scholar]
- 13.Shapira-Nahor, O., A. Kalinkovich, Z. Weisman, Z. Greenberg, J. Nahmias, M. Shapiro, A. Panet, and Z. Bentwich. 1998. Increased susceptibility to HIV-1 infection of peripheral blood mononuclear cells from chronically immune-activated individuals. AIDS 12:1731-1733. [PubMed] [Google Scholar]
- 14.Sozzani, S., S. Ghezzi, G. Iannolo, W. Luini, A. Borsatti, N. Polentarutti, A. Sica, M. Locati, C. Mackay, T. N. C. Wells, P. Biwas, E. Vicenzi, G. Poli, and A. Mantovani. 1998. Interleukin 10 increases CCR5 expression and HIV infection in monocytes. J. Exp. Med. 187:439-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tokunaga, K., M. L. Greenberg, M. A. Morse, R. I. Cumming, H. K. Lyerly, and B. R. Cullen. 2001. Molecular basis for cell tropism of CXCR4-dependent human immunodeficiency virus type 1 isolates. J. Virol. 75:6776-6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United Nations. 2002. Report on the global HIV/AIDS epidemic 2002. [Online.] http://www.unaids.org/html/pub/Global-Reports/Barcelona/BRTableCountryEstimatesEnd2001_en_pdf.html. Joint United Nations Programme on HIV/AIDS (UNAIDS), United Nations, Geneva, Switzerland.
- 17.Valentin, A., W. Lu, M. Rosati, R. Schneider, J. Albert, A. Carlson, and G. N. Pavlakis. 1998. Dual effect of interleukin 4 on HIV-1 expression: implications for viral phenotypic switch and disease progression. Proc. Natl. Acad. Sci. USA 95:8886-8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, J., K. Crawford, M. Yuan, H. Wang, P. R. Gorry, and D. Gabuzda. 2002. Regulation of CC chemokine receptor 5 and CD4 expression and human immunodeficiency virus type 1 replication in human macrophage and microglia by T helper type 2 cytokines. J. Exp. Med. 185:885-897. [DOI] [PubMed] [Google Scholar]
- 19.Weiss, H. A., M. A. Quigley, and R. J. Harris. 2000. Male circumcision and risk of HIV infection in sub-Saharan Africa: a systematic review and meta-analysis. AIDS 14:2361-2370. [DOI] [PubMed] [Google Scholar]