Abstract
Natural tumor surveillance capabilities of the host were investigated in six different mouse tumor models where endogenous interleukin (IL)-12 does or does not dictate the efficiency of the innate immune response. Gene-targeted and lymphocyte subset–depleted mice were used to establish the relative importance of natural killer (NK) and NK1.1+ T (NKT) cells in protection from tumor initiation and metastasis. In the models examined, CD3− NK cells were responsible for tumor rejection and protection from metastasis in models where control of major histocompatibility complex class I–deficient tumors was independent of IL-12. A protective role for NKT cells was only observed when tumor rejection required endogenous IL-12 activity. In particular, T cell receptor Jα281 gene-targeted mice confirmed a critical function for NKT cells in protection from spontaneous tumors initiated by the chemical carcinogen, methylcholanthrene. This is the first description of an antitumor function for NKT cells in the absence of exogenously administered potent stimulators such as IL-12 or α-galactosylceramide.
Keywords: tumor immunity, perforin, natural killer T cells, interleukin 12, carcinogen
Introduction
It is generally accepted that the host immune status, particularly natural immune responses, is essential in controlling the dissemination and growth of metastatic tumors. NK cells mediate spontaneous cytotoxicity against MHC class I–deficient tumor cells and their metastases 1, and they have long been known to participate in the innate immune response against transformed cells in vivo 1 2. However, only a few studies have reported a prominent role for NK cells on the growth of primary tumors in mice without using biological response modifiers to magnify the response 2 3. Certainly cytokines, such as type I IFN and IL-12, are considered important in initiating the activation of NK cells in response to pathogens 4. More recently, NKT cells have been isolated and characterized 5 6 7 8, and their role in IL-12–mediated antitumor activity has been defined 9.
NK1.1+ T (NKT) cells are defined as specialized populations of α/β T cells that coexpress some receptors of the NK cell lineage 5. NKT cells consist mainly of CD4−CD8− double negative and CD4+ cells 6 7, most of which express a restricted TCR repertoire comprising an invariant Vα14Jα281 TCR α chain 8 associated with polyclonal Vβ8, Vβ7, and Vβ2 TCR β chains. A TCR Jα281 gene-targeted mouse produced by Cui et al. 9 confirmed the requirement of invariant Vα14Jα281 TCR for the development of most NKT cells 10. These unusual regulatory cells that bridge the innate and acquired immune systems recognize CD1d-bound glycolipid ligands such as α-galactosylceramides (α-GalCer [11, 12]) and glycosylphosphatidylinositols 13. NKT cells are present in most tissues where T cells are found, in particular the liver, bone marrow, spleen, and thymus 14 15 16. Thymus and liver contain primarily CD1d-dependent Jα281-dependent NKT cells, whereas spleen and bone marrow are enriched in CD1d-independent Jα281-independent NKT cells 17 18. NKT cells have the peculiar potential to very rapidly secrete large amounts of Th1 and Th2 cytokines 5 19 20, but also express perforin and surface molecules such as Fas ligand 21 22. After culture in the presence of IL-2, NKT cells can mediate lysis through TCR, NK1.1, or CD16, and kill classical NK-sensitive targets such as YAC-1 in vitro 23 24. The NK1.1 molecule, which activates the cytolytic function of NK cells 25 26 and NKT cells 27, could also activate IFN-γ secretion by both cell types, and IL-12 mediated a similar effect 27.
Perhaps the strongest implication for a role for NKT cells in Th1 immunity is in tumor rejection responses after exogenous IL-12 9 28 29 30 or α-GalCer 11 31 administration. The in vivo antitumor activity of α-GalCer strongly resembles the antitumor activity mediated by IL-12 9 32, and indeed α-GalCer mediates its effects by inducing IL-12 production by dendritic cells (DCs) and IL-12 receptor and IFN-γ expression by NKT cells 33. Although it is likely that endogenously produced IL-12 may also stimulate NKT cells to produce IFN-γ and activate NKT cell cytolytic function, no study to date has evaluated the natural ability (i.e., not exogenously activated by IL-12 or α-GalCer) of NKT cells to reject tumor growth or metastasis. These studies teach us something about the therapeutic benefit and mechanism of IL-12 or α-GalCer, but very little about the natural tumor surveillance capabilities of NKT cells. Here, we have evaluated several tumor rejection models in naive mice in which endogenous IL-12 does or does not regulate the efficiency of the innate immune response. For the first time, we describe a critical role for NKT cells and endogenous IL-12 in protection from spontaneous tumors initiated by the chemical carcinogen, methylcholanthrene (MCA).
Materials and Methods
Mice.
Inbred C57BL/6 (B6) mice were purchased from The Walter and Eliza Hall Institute of Medical Research. B6 TCR Jα281–deficient (B6.Jα281−/−) mice were obtained after eight backcrosses of the (129 × B6)F1 Jα281−/− mice 5 with B6 mice. B6 perforin-deficient (B6.P−/−) mice (34; from Dr. Guna Karupiah, John Curtin School of Medical Research, Canberra, Australia) and B6 IL-12p40–deficient (B6.IL-12p40−/−) mice (35; Hoffman-La Roche) were bred at the Austin Research Institute Biological Research Laboratories. Mice of 4–8 wk of age were used in all experiments in accordance with Animal Experimental Ethics Committee guidelines.
Cell Culture.
The mouse tumor cell lines, YAC-1, RM-1, and EL4-S3 (β2-microglobulin deficient; provided by Dr. James McCluskey, University of Melbourne, Melbourne, Australia) were grown in culture as described previously 36 37. Sarcomas derived from MCA-treated B6 or B6.Jα281−/− mice were maintained as described above. Flow cytometric sorting of NK, NKT, and T cells was performed using a FACStarPLUS™ (Becton Dickinson [18]), and sort purities were always 97% or higher.
51Cr-Release Assays.
The sensitivity of MCA-induced sarcomas to mouse NKT cells was determined as follows. Sorted NKT cells (as above) from the thymi or livers of B6 and B6.P−/− mice were isolated and assessed as effectors in 4- and 18-h 51Cr-release assays against labeled target cells as described 38. In some wells, NKT cells were incubated with IL-2 (50 U/ml) and IL-12 (20 pg/ml) throughout the assay. Sorted liver NK cells and thymus T cells were used as controls for NKT cells from each organ. Each experiment was performed at least twice at four different E/T ratios (20:1 shown) using duplicate samples.
Tumor Surveillance In Vivo.
NK and NKT cell function was examined in various different tumor models as described 36 37. In model 1, groups of five mice were injected intraperitoneally with increasing numbers of EL4-S3 or RM-1 tumor cells, and were observed daily for tumor growth for 100 d by monitoring the development of ascites in mice. Some groups of B6 mice were depleted of NK cells or T cells in vivo by treatment with 100 μg anti-NK1.1 (PK136) or anti-Thy1 (T24/31.7) mAb, respectively, on days −2 and 0 (day of intraperitoneal tumor inoculation) and weekly thereafter. Depletion of NK and NKT cells by anti-NK1.1 using this protocol was effective as determined by flow cytometric analysis of spleen, thymus, and liver. Analysis of NK1.1 versus TCR-β, IL-2Rβ versus TCR-β, and mouse Ig versus TCR-β up to 7 d after anti-NK1.1 mAb administration indicated that NK and NKT cells were depleted. In model 2, groups of five mice were injected intravenously with up to 5 × 104 EL4-S3 tumor cells. 14 d later, their livers were removed and fixed in Bouin's solution, and surface lung metastases were counted with the aid of a dissecting microscope. Some groups of B6 mice were depleted of NK cells or T cells in vivo, as in model 1. In model 3, groups of five mice were injected subcutaneously with RM-1 tumor cells (2 × 106), and tumors were established for 9 d. At this time, subcutaneous tumors were surgically resected, and RM-1 cells were injected via the dorso-lateral tail vein. Mice were killed 14 d later, the lungs were removed and fixed in Bouin's solution, and surface lung metastases were counted with the aid of a dissecting microscope. Control experiments were performed by inoculating mice with RM-1 cells intravenously and counting lung metastases 14 d later. Some groups of B6 mice were depleted of NK cells or T cells in vivo, as in model 1. The data for models 2 and 3 were recorded as the mean (n = 5) number of metastases ± SEM. Significance was determined by an unpaired t test to determine a two-tail P value. In model 4, groups of 16–35 mice were inoculated subcutaneously in the right hind leg with 0.1 ml corn oil containing 1, 5, 25, or 100 μg MCA, and mice were monitored weekly for the development of fibrosarcoma. Some groups of B6 mice were depleted of NK cells or T cells in vivo by treatment with 100 μg anti-NK1.1 or anti-Thy1 mAb, respectively, on days −2 and 0 (day of MCA inoculation) and weekly thereafter. Some MCA-induced fibrosarcomas initiated in B6 and B6.Jα281−/− mice were established in vitro, and were transplanted subcutaneously back into B6 and B6.Jα281−/− mice.
Results and Discussion
NK and NKT Cell Numbers in Gene-targeted Mice.
Multiparameter flow cytometry was used to examine the status of NK and NKT cells in the various mouse strains used in this study. NKT cells were equivalently present in the thymi of B6, B6.IL-12p40−/−, and B6.P−/− strains, whereas B6.Jα281−/− mice were markedly deficient in NKT cells in the thymus (references 9, 17, 18; data not shown). As recently reported 17 18, NKT cells were also reduced in spleen and liver of B6.Jα281−/− mice, but were not completely absent.
IL-12–independent MHC Class I–deficient Tumor Control by NK Cells.
Recently, we demonstrated that MHC class I–negative RMA-S tumor growth was controlled in the peritoneum by CD3−NK1.1+ NK cells in a perforin-dependent manner 36. In a similar fashion, EL4-S3 and RM-1 tumor cells inoculated intraperitoneally were cleared less effectively in B6.P−/− mice and B6 mice treated with anti-NK1.1 mAb compared with wild-type B6 mice (Fig. 1A and Fig. B). Rejection was IL-12 independent and did not require T cells, in particular Vα14Jα281+ NKT cells, as evidenced by normal responses in B6.IL-12p40−/− and B6.Jα281−/− mice. Similar requirements have been demonstrated using other MHC class I–deficient tumors, including RMA-S (data not shown). All three cell lines were class I deficient, and expressed low levels (RMA-S) or no (EL4-S3, RM-1) CD1d (data not shown).
Figure 1.
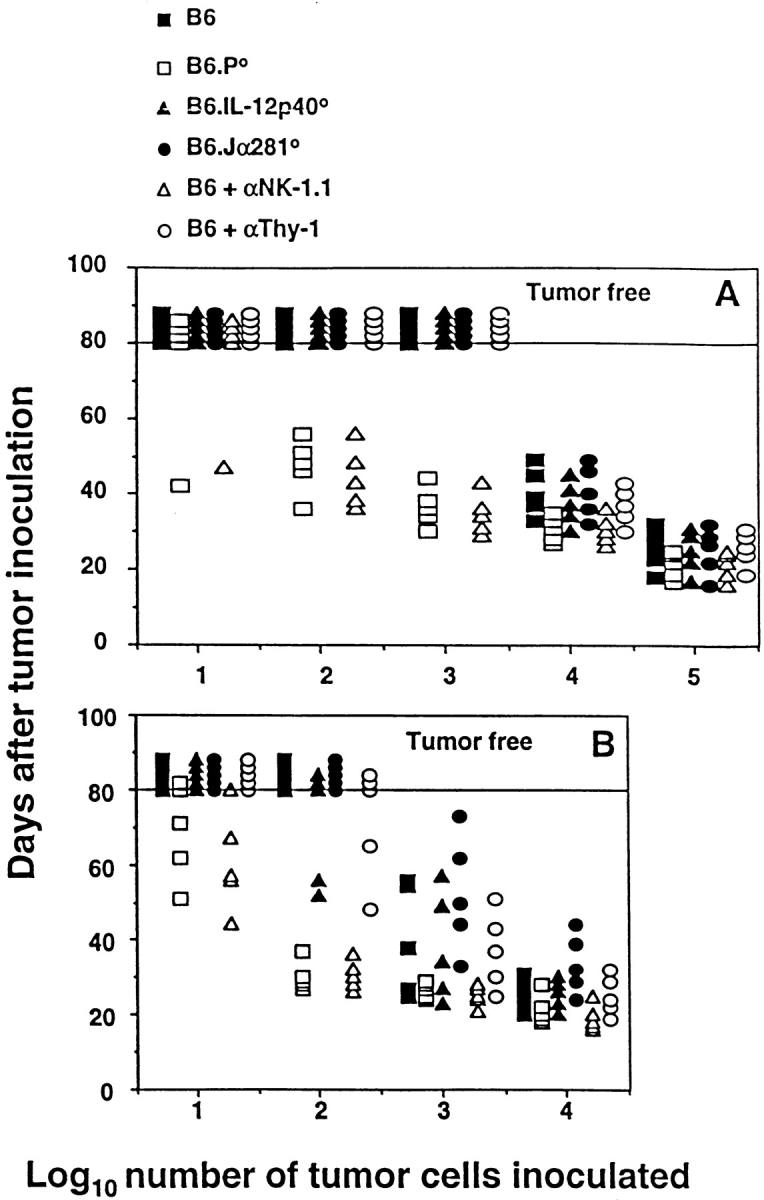
Elimination of intraperitoneally administered MHC class I–negative syngeneic tumors in vivo is mediated by NK cells and is IL-12 independent. B6, B6.P−/− (B6.P0), B6.IL-12p40−/− (B6.IL-12p400), or B6.Jα281−/− (B6.Jα2810) mice (five per group) were injected intraperitoneally with tumor cells (10–105) in 0.2 ml PBS, as indicated. Some groups of B6 mice were depleted of NK cells or T cells in vivo by treatment with mAb, 100 μg anti-NK1.1, or anti-Thy1, respectively, on days −2 and 0 (day of intraperitoneal tumor inoculation), and weekly thereafter. Mice were observed daily for tumor growth for 80 d by monitoring the development of ascites in mice. Individual mice are represented by each symbol. (A) EL4-S3 and (B) RM-1.
Innate Control of Experimental Metastases.
Innate protection from metastases was then examined in two different models in which NK1.1+ cells control tumor colonization 29 37. In the first, we have demonstrated that NK1.1+ cells protect B6 mice from experimental EL4-S3 liver metastases in an IL-12–independent fashion (Fig. 2 A). Neither Thy1+ nor specifically Vα14Jα281+ NKT cells appeared to control the number of hepatic colonies growing in EL4-S3–inoculated mice. A significantly greater number of lesions was observed in B6.P−/− mice (P < 0.0001), indicating that NK1.1+ effectors primarily use perforin to reduce EL4-S3 tumor colonization. Clearly, the endogenous response to EL4-S3 was NK cell mediated, and thus these data contrast with previous studies that have suggested that the anti-EL4 activity of exogenous IL-12 was mediated by NKT cells 29.
Figure 2.
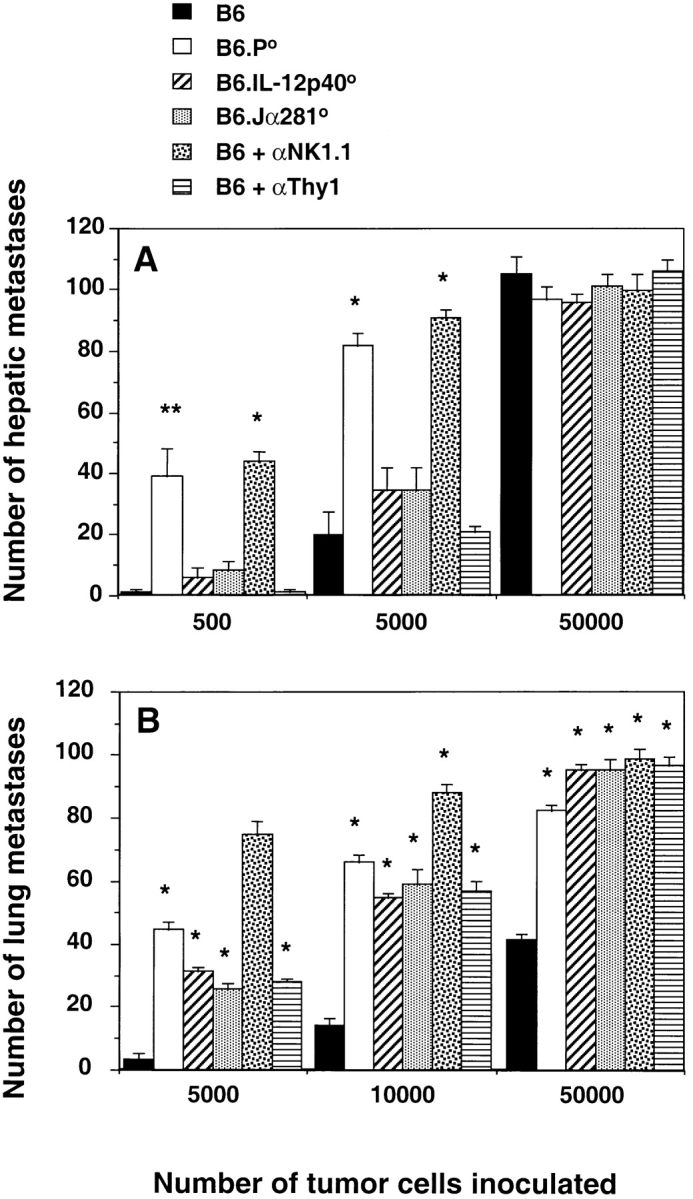
Innate control by NK and NKT cells in protection from tumor metastasis. (A) Groups of five B6, B6.P−/− (B6.P0), B6.IL-12p40−/− (B6.IL-12p400), or B6.Jα281−/− (B6.Jα2810) mice were injected intravenously with 500, 5,000, or up to 50,000 EL4-S3 tumor cells. 14 d later, their livers were removed and fixed in Bouin's solution, and surface lung metastases were counted with the aid of a dissecting microscope. Some groups of B6 mice were depleted of NK cells or T cells in vivo, as in the legend to Fig. 1. (B) Groups of five B6, B6.P−/− (B6.P0), B6.IL-12p40−/− (B6.IL-12p400), or B6.Jα281−/− (B6.Jα2810) mice were injected subcutaneously with RM-1 tumor cells (2 × 106), and tumors were established for 9 d. At this time, subcutaneous tumors were surgically resected, and RM-1 cells were injected via the dorso-lateral tail vein. Mice were killed 14 d later, the lungs were removed and fixed in Bouin's solution, and surface lung metastases were counted with the aid of a dissecting microscope. Control experiments were performed by inoculating mice with RM-1 cells intravenously and counting lung metastases 14 d later. Some groups of B6 mice were depleted of NK cells or T cells in vivo, as in the legend to Fig. 1. The data for A and B were recorded as the mean (n = 5) number of metastases ± SEM. Significant differences from B6 group were determined by an unpaired t test to determine a two-tail P value (*P < 0.0001; **P < 0.005).
In a second model, we have demonstrated previously that NK1.1+ cells using perforin protect B6 mice from RM-1 metastasis 37. These studies also indicated that tumor protection was compromised in B6 recombination activating gene 1 (RAG-1)–deficient mice. Here, we have extended these observations to demonstrate that NKT cells are also partially responsible for RM-1 tumor protection. Interestingly, the number of RM-1 colonies was very similar in B6.Jα281−/− mice, B6.IL-12p40−/− mice, and B6 mice depleted of T cells with anti-Thy1 mAb (Fig. 2 B). It remains to be determined what role NKT cells play in NK cell–mediated protection from metastasis in this model, but clearly endogenous IL-12 activity was required.
MCA-induced Fibrosarcoma Is Controlled by Vα14 NKT Cells in an IL-12–dependent Manner.
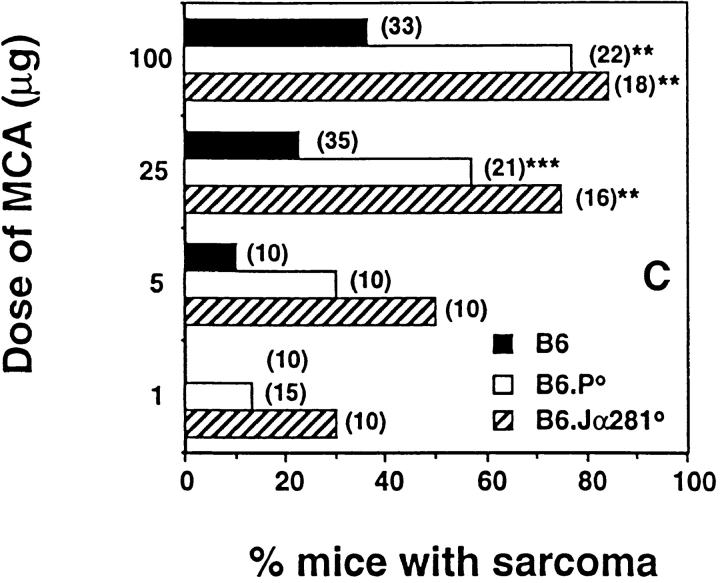
Induction of fibrosarcoma by MCA has been demonstrated previously to be controlled by effector cells in a perforin-dependent and IFN-dependent manner 38 39. To date, however, no extensive analysis has been undertaken to identify the subset of effector cells responsible for this immune surveillance. Here, groups of B6 gene-targeted mice were injected subcutaneously with different doses of MCA, and fibrosarcoma development was monitored for a period of 180 d (Fig. 3A Fig. c). At the doses examined, B6.Jα281−/− mice developed tumors more frequently and earlier than B6 control mice. A high (>70%) percentage of B6.Jα281−/− mice treated with 100 and 25 μg of MCA developed tumors, whereas only small proportions developed tumors in the corresponding wild-type mice receiving MCA. At an MCA dosage of 1 μg, 30% of B6.Jα281−/− mice developed fibrosarcoma, whereas B6 mice remained tumor free (Fig. 3 C). Although IL-12 administration has been demonstrated to protect mice from MCA-induced fibrosarcoma 40, our data in IL-12p40−/− mice are the first to describe a role for endogenous IL-12 in this model (Fig. 3 A). Further evidence supporting a role for Vα14 NKT cells in the surveillance of MCA-induced tumors was obtained in B6 mice that were chronically depleted of NK1.1+ or Thy1+ cells (Fig. 3 A).
Figure 3.
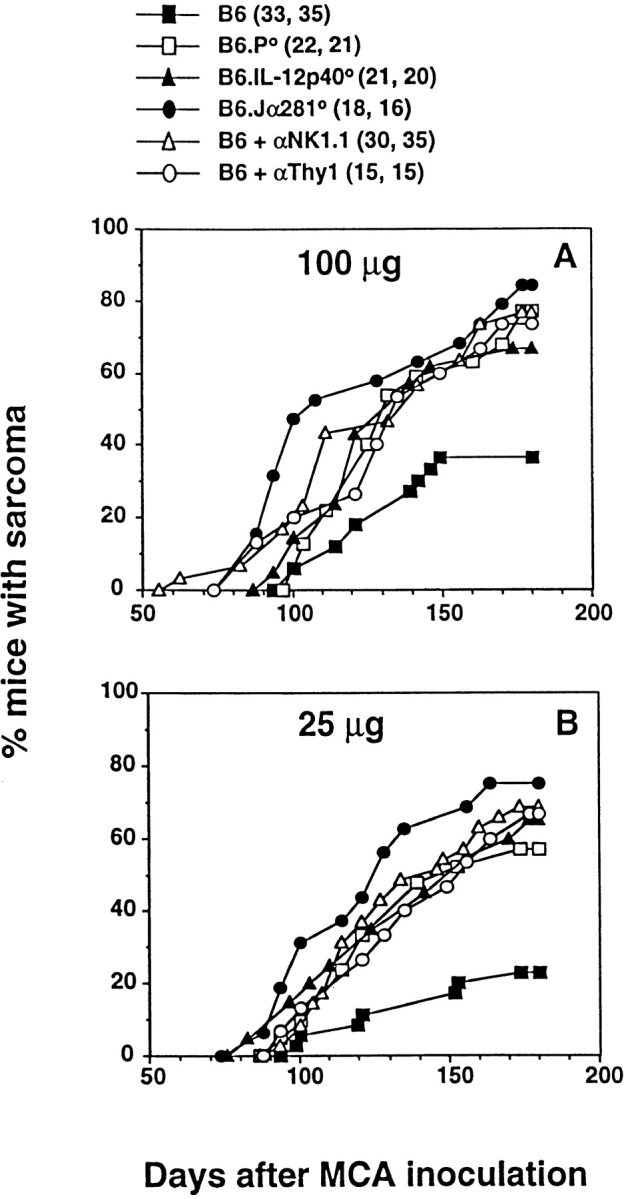
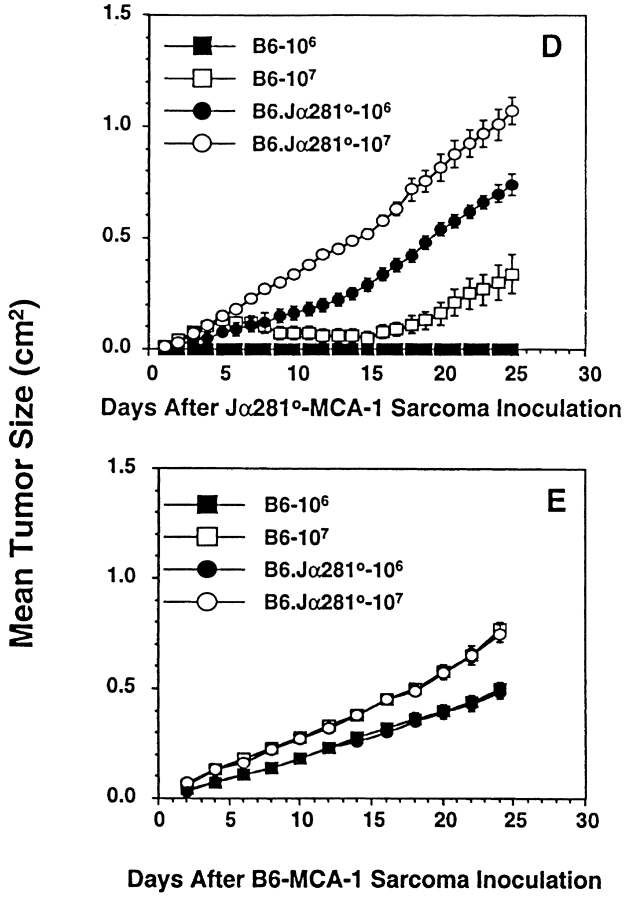
NKT cells protect mice from MCA-induced sarcoma. Groups of B6, B6.P−/− (B6.P0), B6.IL-12p40−/− (B6.IL-12p400), or B6.Jα281−/− (B6.Jα2810) mice (number of mice in parentheses) were injected subcutaneously in the hind flank with (A) 100 μg or (B) 25 μg MCA diluted in 0.1 ml corn oil. Mice were observed weekly for tumor development over the course of 50–180 d. Tumors >5 mm in diameter and demonstrating progressive growth over 3 wk were counted as positive. Some groups of B6 mice were depleted of NK cells or T cells in vivo, as in the legend to Fig. 1. In C, sarcoma development in B6, B6.P−/− (B6.P0), and B6.Jα281−/− (B6.Jα2810) mice was compared at several doses of MCA with data recorded at 180 d as a percentage of the mice in each group (in parentheses). Significant differences from the B6 group were determined by a Fisher's exact test (**P < 0.005; ***P < 0.02). Sarcomas derived from (D) B6.Jα281−/− mice (Jα2810-MCA-1) and (E) B6 mice (B6-MCA-1) were transplanted at 106 or 107 cells subcutaneously into groups of five B6.Jα281−/− or B6 mice. Tumor growth was measured daily with a caliper square as the product of two diameters. Results were recorded as the mean tumor size (in cm2) ± SEM, and are representative of two similar sarcomas transplanted into B6 and B6.Jα281−/− mice.
Depletion of CD8+ cells did not increase the proportion of mice developing fibrosarcoma (data not shown), in concert with a similar study with CD8-deficient mice 38. These data would argue against an important role for CD8+ T cells or CD8+ lymphoid DCs that have been demonstrated to contribute to some NK1.1+ cell–mediated tumor rejection responses driven by Flt3 ligand 41. Nevertheless, Flt3 ligand treatment has been demonstrated previously to cause complete regression of tumors in mice challenged with a syngeneic MCA-induced fibrosarcoma 42, and thus endogenous responses to MCA-induced spontaneous tumors may also involve intermediary DC populations. In agreement with van den Broek et al. 38, B6.P−/− mice were more prone to MCA-induced sarcoma, but always less so than B6.Jα281−/− mice, suggesting that more than one effector mechanism might be employed by NK1.1+ cells in tumor surveillance. Given the demonstrated surveillance defect in IFN-γR–deficient mice 39, we are currently evaluating the level of protection in IFN-γ–deficient mice and mice deficient for both perforin and IFN-γ.
In concert with previous studies 38 43, MCA-induced sarcomas arising in B6 and B6.Jα281−/− mice were generally both MHC class I negative and CD1d− (data not shown). Interestingly, when tumors were transplanted subcutaneously from B6.Jα281−/− mice, they grew far more effectively in B6.Jα281−/− mice than in control B6 mice. Indeed, doses as high as 106 cells were effectively controlled by B6 mice (Fig. 3 D) and (B6 × 129)F1 mice (as a control for any potential minor 129 alloantigen expressed by the tumor; data not shown). By contrast, sarcomas arising in B6 mice grew equally well in B6.Jα281−/− and B6 mice after transfer (Fig. 3 E). This observation is analogous to other studies that have indicated that tumor initiation in immunocompetent mice compared with immunocompromised mice (e.g., B6.Jα281−/− mice) may select for the growth of less immunogenic tumors 43.
NKT Cells Can Directly Lyse Spontaneous Sarcomas Arising in MCA-treated Mice.
To test the possibility that MCA-induced sarcomas were sensitive to direct lysis by NKT cells, we determined the basal and activated cytotoxicity of NKT cells against MCA-1 (a sarcoma derived from B6.Jα281−/− mice) and other tumor target cell lines (Fig. 4). Sorted NKT cells from the liver of B6 mice displayed significant lysis of MCA-1 in a 4-h assay, particularly with IL-2/IL-12 stimulation (Fig. 4 A). The lytic activity of sorted resting liver NK cells was higher than resting NKT cells against all the tumor target cells, with RM-1 tumor cells comparatively insensitive to both NK and NKT cells. Thymus NKT cells demonstrated lower levels of lysis of MCA-1 than liver NKT cells, but they too demonstrated enhanced lysis with IL-2/IL-12 stimulation. Although resting NKT cells did not display significantly greater lysis of target cells in 18-h assays, both thymus and liver NKT cells stimulated in IL-2/IL-12 over the assay period were considerably more lytic than in the 4-h assay (Fig. 4 B). By contrast, NKT cells sorted from the thymi of B6.P−/− mice were unable to lyse MCA-1, even after IL-2/IL-12 stimulation for 18 h, suggesting that NKT cell lysis of MCA-1 was strictly perforin dependent (Fig. 4 B). Control thymus T cells did not display lytic activity in the absence or presence of IL-2/IL-12 against YAC-1 or MCA-1.
Figure 4.
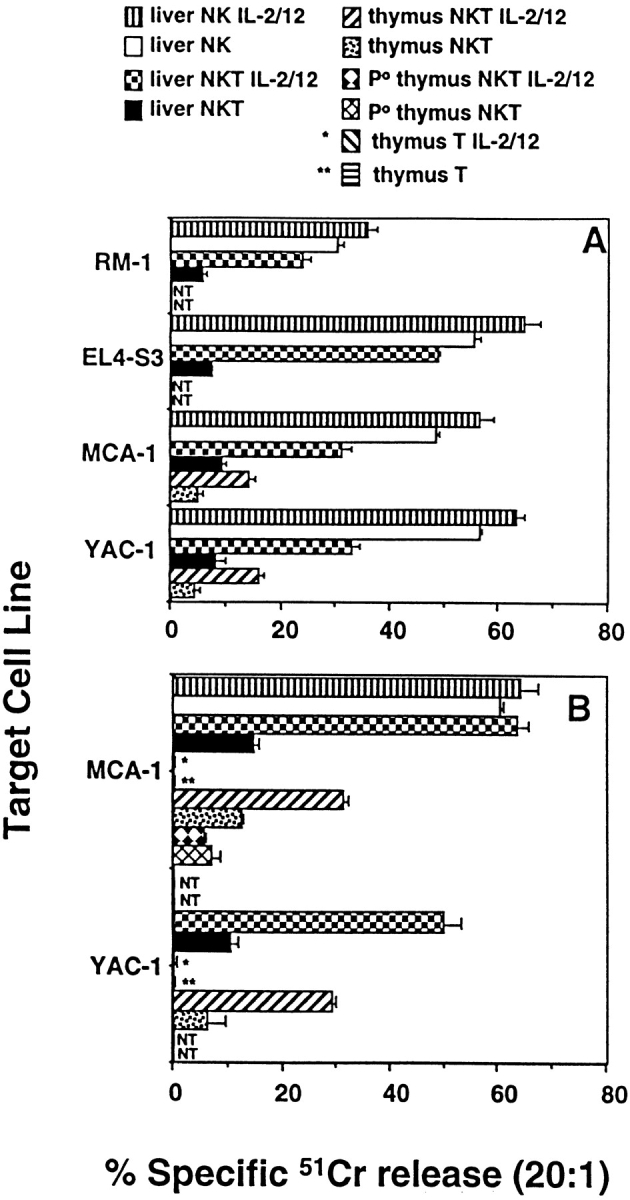
NKT cells directly lyse MCA-induced sarcoma. Thymus and liver NKT cells (NK1.1+TCR-β+) were sorted from B6 and B6.P−/− mice, and were examined in (A) 4-h and (B) 18-h 51Cr-release assays for direct lysis of a panel of labeled target cells as indicated. Some effector cells were cultured in IL-2 (50 U/ml) and IL-12 (20 pg/ml) throughout the course of the assay (+ IL-2/12). NKT effectors were tested at four different E/T ratios (20, 5, 1, 0.5), with an E/T ratio of 20:1 shown. In A, sorted liver NKT cells are compared with sorted liver NK cells. In B, sorted thymus NKT cells are with sorted thymus T cells (CD4+CD8− HSA−). The data is recorded as the mean ± SEM, the spontaneous release of 51Cr was always <15% (subtracted from all tests), and each test was performed using duplicate samples. NT, not tested.
Concluding Remarks.
The deficiency of a TCR Vα14 Jα281–expressing NKT cell population in TCR Jα281 gene-targeted mice confirmed a critical function for NKT cells in protection from spontaneous tumors initiated by the chemical carcinogen, MCA. This is the first description of an antitumor function for NKT cells in the absence of exogenously administered IL-12 or α-GalCer. It remains unclear whether NK cells are also contributing directly to protection from MCA-induced sarcoma, as depletion of NK1.1+ cells serves to eliminate both NK and NKT cells. The observations that the frequency of sarcomas is higher in TCR Jα281 gene-targeted mice than in anti-NK1.1 mAb–treated mice, and that anti-NK1.1 mAb treatment did not further increase the frequency of sarcomas in TCR Jα281 gene-targeted mice (data not shown) suggested that NK cells may not necessarily play any additional role. However, the mAb depletion of NK1.1+ cells over 6 mo is not optimal, and a mouse deficient in NK cells but not NKT or T cells would offer an ideal model mouse in which to address this issue further.
The question of whether the antitumor activity of exogenous IL-12 is mediated by NKT cells rather than NK cells also remains a topic of great debate. Data using bg/bg beige 44 and Jα281−/− 9 mice supports this hypothesis. However, by contrast, other experiments (even using the same tumors) suggest that only NK cells mediate IL-12 antitumor activities 45. We believe our data support the concept that in some models both NK and NKT cells can have important activities that protect against tumor metastasis or development. However, in some tumor-rejection settings, NK cell–mediated tumor rejection clearly does not require NKT cell activity. Although NK cells were the primary effector cells in the intraperitoneal RM-1 tumor model, in the intravenous RM-1 lung tumor model, NKT cells appeared to also contribute to tumor protection. These data support previous observations that NK cell–mediated clearance of tumors from the peritoneum is quite a distinct process, dependent strictly on perforin, and requiring TNF for NK cell recruitment 36. In the lung, RM-1 rejection requires NKT cells and IFN-γ (data not shown), but not TNF, and thus it is possible that the cytokine network initiating the NK cell response may be distinct at different tumor sites. Importantly, a recent study by Carnaud et al. 46 has suggested that with the appropriate activation, NKT cells can rapidly produce IFN-γ that activates NK cells.
When comparing sarcoma induction by MCA in B6.P−/− and B6.Jα281−/− mice (Fig. 3 C), it is apparent that additional effector mechanisms may contribute to the protective response. In particular, the response of NKT cells to cytokines (such as IL-12) or production of cytokines (such as IFN-γ) may also provide tumor protection by mechanisms other than perforin-mediated cytotoxicity. Indeed, ongoing experiments in IFN-γ–deficient mice and mice doubly deficient for IFN-γ and perforin support an important role for IFN-γ in addition to perforin (Smyth, M.J., unpublished data). We have demonstrated here that after activation with IL-12, NKT cells can directly lyse MCA-induced tumors with negligible levels of MHC class I or CD1d. This is not to say that NKT cells protect mice from sarcoma development by direct lysis, as NK cells also lyse MCA-1 tumor cells, but the data illustrate that direct tumor cell lysis cannot be ruled out as a potential mechanism of control. It remains to be determined whether TCR–CD1d interactions are involved in direct lysis of MCA tumors by activated NKT cells, and the role of CD1d in surveillance against MCA-induced sarcoma needs to be examined in CD1d-deficient mice. Several recent studies suggest that DCs expressing CD1d may act as important APCs in innate antitumor responses by NK and/or NKT cells 33 41. Further work will be required to dissect the dynamics between NK and NKT cells and APCs in vivo in this tumor model.
Our work provides the first evidence that TCR Vα14 Jα281 NKT cells may be critical in natural immune responses to spontaneous tumors. The demonstrated critical role of Vα14 NKT cells in controlling what are considered weakly immunogenic sarcomas raises the question of whether NKT cells may also play an important role in protection from more immunogenic tumors, such as those induced by oncogenic viruses or UV light. Future work evaluating the role of NKT cells in other spontaneous tumor models should provide greater insight into the immune networks that constitute tumor surveillance.
Acknowledgments
We thank the staff of the Austin Research Institute Biological Research Laboratories for their maintenance and care of the mice in this project.
M.J. Smyth is currently supported by a National Health and Medical Research Council (NHMRC) of Australia Principal Research Fellowship. D.I. Godfrey was supported by the NHMRC and by an AdCorp-Diabetes Australia Postdoctoral Fellowship. This work was supported by an NHMRC Project Grant.
Footnotes
Abbreviations used in this paper: α-GalCer, α-galactosylceramide(s); B6, C57BL/6; B6.Jα281−/−, TCR Jα281–deficient B6; B6.P−/−, perforin-deficient B6; B6.IL-12p40−/−; IL-12p40–deficient B6; DC, dendritic cell; MCA, methylcholanthrene; NKT cell, NK1.1+ T cell.
References
- Trinchieri G. Biology of natural killer cells. Adv. Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge J.E., Meyers K.M., Prieur D.J., Starkey J.R. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980;284:622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Karre K., Ljunggren H.G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Salazar-Mather T.P., Ishikawa R., Biron C.A. NK cell trafficking and cytokine expression in splenic compartments after IFN induction and viral infection. J. Immunol. 1996;157:3054–3064. [PubMed] [Google Scholar]
- Bendelac A., Rivera M.N., Park S.H., Roark J.H. Mouse CD1-specific NK1 T cellsdevelopment, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- Sugie T., Kubota H., Sato M., Nakamura E., Imamura M., Minato N. NK1+ CD4−CD8− αβ T cells in the peritoneal cavityspecific T cell receptor-mediated cytotoxicity and selective IFN-γ production against B cell leukemia and myeloma cells. J. Immunol. 1996;157:3925–3935. [PubMed] [Google Scholar]
- MacDonald H.R. NK1.1+ T cell receptor–α/β+ cellsnew clues to their origin, specificity, and function. J. Exp. Med. 1995;182:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz O., Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I–specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Shin T., Kawano T., Sato H., Kondo E., Toura I., Kaneko Y., Koseki H., Kanno M., Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Taniguchi M., Koseki H., Tokuhisa T., Masuda K., Sato H., Kondo E., Kawano T., Cui J., Perkes A., Koyasu S., Makino Y. Essential requirement of an invariant Vα14 T cell antigen receptor expression in the development of natural killer T cells. Proc. Natl. Acad. Sci. USA. 1996;93:11025–11028. doi: 10.1073/pnas.93.20.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Burdin N., Brossay L., Koezuka Y., Smiley S.T., Grusby M.J., Gui M., Taniguchi M., Hayakawa K., Kronenberg M. Selective ability of mouse CD1 to present glycolipidsα-galactosylceramide specifically stimulates Vα14+ NK T lymphocytes. J. Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- Schofield L., McConville M.J., Hansen D., Campbell A.S., Fraser-Reid B., Grusby M.J., Tachado S.D. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Killeen N., Littman D.R., Schwartz R.H. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- Ohteki T., MacDonald H.R. Major histocompatibility complex class I–related molecules control the development of CD4+8− and CD4−8− subsets of natural killer 1.1+ T cell receptor–α/β+ cells in the liver of mice. J. Exp. Med. 1994;180:699–704. doi: 10.1084/jem.180.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Bendelac A., Watson C., Hu-Li J., Paul W.E. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- Eberl G., Lees R., Smiley S.T., Taniguchi M., Grusby M.J., MacDonald H.R. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J. Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]
- Hammond K.J., Pelikan S.B., Crowe N.Y., Hooi H.L., Randle-Barrett E., Smyth M.J., Baxter A.G., van Driel I.R., Scollay R., Godfrey D.I. NKT cells are a phenotypically and functionally diverse population. Eur. J. Immunol. 1999;29:3768–3781. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Godfrey D.I., Fischer M., Suda T. Cytokine production by mature and immature CD4−CD8− T cells. αβ-T cell receptor+ CD4−CD8− T cells produce IL-4. J. Immunol. 1992;149:1211–1215. [PubMed] [Google Scholar]
- Arase H., Arase N., Nakagawa K., Good R.A., Onoe K. NK1.1+ CD4+ CD8− thymocytes with specific lymphokine secretion. Eur. J. Immunol. 1993;23:307–310. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- Dao T., Mehal W.Z., Crispe I.N. IL-18 augments perforin-dependent cytotoxicity of liver NK-T cells. J. Immunol. 1998;161:2217–2222. [PubMed] [Google Scholar]
- Arase H., Arase N., Kobayashi Y., Nishimura Y., Yonehara S., Onoe K. Cytotoxicity of fresh NK1.1+ T cell receptor α/β+ thymocytes against a CD4+CD8+ thymocyte population associated with intact Fas antigen expression on the target. J. Exp. Med. 1994;180:423–432. doi: 10.1084/jem.180.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S. CD3+CD16+NK1.1+B220+ large granular lymphocytes arise from both alpha-beta TCR+CD4−CD8− and gamma-delta TCR+CD4−CD8− cells. J. Exp. Med. 1994;179:1957–1972. doi: 10.1084/jem.179.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S., D'Adamio L., Arulanandam A.R., Abraham S., Clayton L.K., Reinherz E.L. T cell receptor complexes containing FcεRI gamma homodimers in lieu of CD3 ζ and CD3 ζ componentsa novel isoform expressed on large granular lymphocytes. J. Exp. Med. 1992;175:203–209. doi: 10.1084/jem.175.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J.C., Niemi E.C., Goldfien R.D., Hiserodt J.C., Seaman W.E. NKR-P1, an activating molecule on rat natural killer cells, stimulates phosphoinositide turnover and a rise in intracellular calcium. J. Immunol. 1991;147:3244–3250. [PubMed] [Google Scholar]
- Karlhofer F.M., Yokoyama W.M. Stimulation of murine natural killer (NK) cells by a monoclonal antibody specific for the NK1.1 antigen. IL-2-activated NK cells possess additional specific stimulation pathways. J. Immunol. 1991;146:3662–3673. [PubMed] [Google Scholar]
- Arase H., Arase N., Saito T. Interferon gamma production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto W., Takeda K., Anzai R., Ogasawara K., Sakihara H., Sugiura K., Seki S., Kumagai K. Cytotoxic NK1.1 Ag+ αβ T cells with intermediate TCR induced in the liver of mice by IL-12. J. Immunol. 1995;154:4333–4340. [PubMed] [Google Scholar]
- Kawamura T., Takeda K., Mendiratta S.K., Kawamura H., Van Kaer L., Yagita H., Abo T., Okumura K. Critical role of NK1+ T cells in IL-12-induced immune responses in vivo. J. Immunol. 1998;160:16–19. [PubMed] [Google Scholar]
- Takahashi M., Ogasawara K., Takeda K., Hashimoto W., Sakihara H., Kumagai K., Anzai R., Satoh M., Seki S. LPS induces NK1.1+ αβ T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J. Immunol. 1996;156:2436–2442. [PubMed] [Google Scholar]
- Kobayashi E., Motoki K., Uchida T., Fukushima H., Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol. Res. 1995;7:529–534. [PubMed] [Google Scholar]
- Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Sato H., Kondo E., Harada M., Koseki H., Nakayama T. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc. Natl. Acad. Sci. USA. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H., Iwakabe K., Yahata T., Nishimura S.-I., Ohta A., Ohmi Y., Sato M., Takeda K., Okumura K., Van Kaer L. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 1999;189:1121–1127. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagi D., Ledermann B., Burki K., Seiler P., Odermatt B., Olsen K.J., Podack E.R., Zinkernagel R.M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Magram J., Connaughton S.E., Warrier W.W., Carvajal D.M., Wu C.Y., Ferrante J., Stewart C., Sarmiento U., Faherty D.A., Gately M.K. IL-12 deficient mice are defective in IFN-γ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- Smyth M.J., Kelly J.M., Baxter A.G., Korner H., Sedgwick J.D. An essential role for tumor necrosis factor in natural killer cell–mediated tumor rejection in the peritoneum. J. Exp. Med. 1998;188:1611–1619. doi: 10.1084/jem.188.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth M.J., Thia K.Y., Cretney E., Kelly J.M., Snook M.B., Forbes C.A., Scalzo A.A. Perforin is the major contributor to NK cell control of tumor metastasis. J. Immunol. 1999;162:6658–6662. [PubMed] [Google Scholar]
- van den Broek M.E., Kagi D., Ossendorp F., Toes R., Vamvakas S., Lutz W.K., Melief C.J., Zinkernagel R.M., Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D.H., Shankaran V., Dighe A.S., Stockert E., Aguet M., Old L.J., Schreiber R.B. Demonstration of an interferon γ-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi Y., Jungbluth A., Richards E.C., Old L.J. Effect of interleukin 12 on tumor induction by 3-methylcholanthrene. Proc. Natl. Acad. Sci. USA. 1996;93:11798–11801. doi: 10.1073/pnas.93.21.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez N.C., Lozier A., Flament C., Ricciardi-Castagnoli P., Bellet D., Suter M., Perricaudet M., Tursz T., Maraskovsky E., Zitvogel L. Dendritic cells directly trigger NK cell functionscross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- Lynch D.H. Induction of dendritic cells (DC) by Flt3 ligand (FL) promotes the generation of tumor-specific immune responses in vivo. Crit. Rev. Immunol. 1998;18:99–107. doi: 10.1615/critrevimmunol.v18.i1-2.110. [DOI] [PubMed] [Google Scholar]
- Svane I.M., Engel A.M., Nielsen M.B., Ljunggren H.G., Rygaard J., Werdelin O. Chemically induced sarcomas from nude mice are more immunogenic than similar sarcomas from congenic normal mice. Eur. J. Immunol. 1996;26:1844–1850. doi: 10.1002/eji.1830260827. [DOI] [PubMed] [Google Scholar]
- Brunda M.J., Luistro L., Warrier R.R., Wright R.B., Hubbard B.R., Murphy M., Wolf S.F., Gately M.K. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J. Exp. Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Takeda K., Shimozato O., Hayakawa Y., Atsuta M., Kobayashi K., Ito M., Yagita H., Okumura K. Perforin-dependent NK cell cytotoxicity is sufficient for anti-metastatic effect of IL-12. Eur. J. Immunol. 1999;29:1390–1396. doi: 10.1002/(SICI)1521-4141(199904)29:04<1390::AID-IMMU1390>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Carnaud C., Lee D., Donnars O., Park S.-H., Beavis A., Koezuka Y., Bendelac A. Cutting edgeCross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]