Abstract
Salmonella typhimurium is a gram-negative bacterium that survives and replicates inside vacuolar compartments of macrophages. Infection of macrophages with S. typhimurium grown under conditions allowing expression of the type III secretion system results in apoptotic death of the infected cells. Here, we show that infection of bone marrow–derived macrophages (MΦ) with wild-type S. typhimurium 14028 results in presentation of epitopes derived from a bacteria-encoded antigen on major histocompatibility complex (MHC) class I and MHC class II molecules after internalization of apoptotic MΦ by bystander dendritic cells (DCs). In contrast, infection of MΦ with the phoP constitutive mutant strain CS022, which does not induce apoptosis in infected MΦ, does not result in presentation of a bacteria-derived antigen by bystander DCs unless the infected MΦ are induced to undergo apoptosis by treatment with lipopolysaccharide and ATP. DCs appear to be unique in their ability to present antigens derived from MΦ induced to undergo apoptosis by Salmonella, as bystander MΦ are not capable of presenting the bacteria-derived antigen despite the fact that they efficiently internalize the apoptotic cells. These data suggest that apoptosis induction by bacterial infection of MΦ may not be a quiescent death that allows the bacteria to escape recognition by the immune system, but rather may contribute to an antimicrobial immune response upon engulfment by bystander DCs.
Keywords: apoptosis, Salmonella, dendritic cells, macrophages, antigen processing
Introduction
Salmonella typhimurium is a gram-negative bacterium that causes a self-limiting gastroenteritis in humans and typhoid-like systemic disease in mice. The pathogen is acquired through the ingestion of contaminated food or water and crosses the epithelial barrier at the level of the ileum or the colon preferentially by invading M cells 1. This cell type, which functions to sample luminal antigens, overlies the lymphoid follicles 2. The bacteria subvert the normal function of M cells and are taken up by inducing membrane ruffling in these cells 1. After passing through M cells, the bacteria reach the subepithelial dome of the Peyer's patch (PP) and encounter an extensive network of resident macrophages and dendritic cells (DCs) 3 4. Rather than being destroyed by these phagocytes upon engulfment, Salmonella has evolved several mechanisms to survive in the harsh milieu of phagosomal compartments 5 and can be cytotoxic to macrophages by inducing apoptosis, as has been shown in vitro 6 7.
Apoptosis, or programmed cell death, is a process defined by a distinct set of events characterized by expression of phosphatidylserine on the cell surface, DNA fragmentation, and release of apoptotic bodies (for a review, see reference 8). Through various receptors on the cell surface of phagocytes, apoptotic cells and bodies can be rapidly recognized and engulfed, diminishing the risk of a subsequent inflammatory response 8. It has also become evident that the interaction between several genera of bacteria and phagocytes can lead to apoptosis 6 7 9 10 11. Recently, it has been shown that Shigella and Salmonella induce programmed cell death through a similar mechanism, via a bacterial invasin that directly interacts with and activates the proapoptotic enzyme caspase 1 12 13 14. In addition, DCs can acquire apoptotic material from influenza-infected monocytes and stimulate MHC class I molecule (MHC-I)–restricted CTLs 15. This suggests that apoptosis induced by microbes can lead to an immune response rather than to a “silent” nonimmunogenic death.
We have previously shown that macrophages and DCs can phagocytose and process bacteria for presentation of bacterial peptides on both MHC-I and MHC-II 16 17 18 19. In this study, we show that DCs can also acquire apoptotic material containing bacterial antigens from macrophages that have undergone apoptosis caused by S. typhimurium infection and present epitopes from a bacteria-derived antigen on both MHC-I and MHC-II. This indicates that bacterial induction of apoptosis in phagocytes may not necessarily result in a silent death, since both CD4+ and CD8+ T cells can be activated.
Materials and Methods
Mice.
BALB/c (H-2d) and C57BL/6 (H-2b) mice were bred in the animal facilities at Lund University or purchased from Charles River Laboratories. Caspase 1−/− mice (20; H-2b) were provided by Manuela Baccarini, Vienna Biocenter, Vienna, Austria.
Bacterial Strains, Plasmids, and Growth Conditions.
Bacterial strains used were S. typhimurium 14028s (American Type Culture Collection) or its rough LPS derivative 14028r 21, the phoP c mutant CS022s 22 or its rough LPS derivative CS022r, made as described 21. S. typhimurium 14028r and CS022r harbored pJLP-2H-Kan 18, which encodes the fusion protein Crl-OVA containing residues 257–277 of OVA fused near the COOH terminus of the cytoplasmic bacterial protein Crl. The OVA fragment in Crl-OVA contains the Kb-binding OVA(257–264) epitope and the I-Ab–binding OVA(265–277) epitope. S. typhimurium 14028r and CS022r containing pJLP-2H-Kan were grown in Luria broth (LB) supplemented with 50 μg/ml kanamycin while S. typhimurium bacteria not containing a plasmid were grown in LB. Bacteria were grown overnight with shaking at 37°C. Logarithmically growing bacteria were obtained by diluting an overnight culture 1:20 into fresh LB and incubating until the optical density at 600 nm (OD600) reached 1.3–1.5. Bacteria were then centrifuged at 1,700 g and resuspended in IMDM (without antibiotics; GIBCO BRL) to a density of 5 × 108 bacteria/ml. This bacterial suspension was diluted in IMDM (without antibiotics) to obtain the desired amount of bacteria to be added to assays. Heat-killed and gentamycin-treated bacteria were prepared by incubating suspensions of bacteria (5 × 108 bacteria/ml) at 65°C for 45 min or with 100 μg/ml gentamycin at 4°C for 90 min before addition to the assay.
Bone Marrow Cultures.
DCs were obtained by culturing bone marrow from C57BL/6 mice (H-2b) in IMDM supplemented with 10% culture supernatant from Ag8653 myeloma cells transfected with murine GM-CSF. DCs were separated from macrophages in the cultures by transferring nonadherent cells to new culture dishes containing IMDM plus GM-CSF and used on days 6–8 of culture as described 18. These nonadherent cells were highly enriched for DCs and were typically 60–70% pure as assessed by surface expression of MHC-II and CD11c by FACS®. In some experiments, the cells were then further enriched for CD11c-expressing cells (DCs) using anti-CD11c magnetic microbeads and a MiniMACS separation column (Miltenyi Biotec) following the manufacturer's protocol. The enriched population consisted of 80–90% CD11c+ cells as assessed using HL3 antibody (PharMingen) and FACS® analysis. Both of these DC populations have a heterogeneous expression of MHC-II, CD80, and CD86 (Svensson, M., C. Johansson, and M.J. Wick, manuscript in preparation) and are phagocytic 18 19, showing that the DCs are in an intermediate stage of maturation 23. Both the bulk culture and the CD11c-purified DC populations were used in experiments and gave identical results.
Bone marrow–derived macrophages (MΦ) were prepared by culturing bone marrow from BALB/c (H-2d) or caspase 1−/− mice (H-2b) in IMDM plus GM-CSF as above. After the nonadherent cells were removed onto new culture dishes, the strongly adherent cell population was washed with HBSS. These adherent cells, MΦ, were removed from the plastic with cold 10 mM EDTA (pH 8.0), washed twice with HBSS, and used in experiments. Alternatively, MΦ were generated by expanding bone marrow from BALB/c or caspase 1−/− mice in IMDM supplemented with 30% supernatant from L929 cells. The strongly adherent population, MΦ, was removed from the plastic and washed as described for MΦ derived from IMDM plus GM-CSF cultures. MΦ cultured from bone marrow using either IMDM plus GM-CSF or IMDM plus L cell supernatant gave identical results in apoptotic antigen presentation assays (data not shown).
Induction and Detection of Apoptosis.
MΦ from BALB/c mice were resuspended in IMDM containing 5% FCS (without antibiotics), and 106 cells were seeded per well in 24-well cell culture–treated or low adherence plates (Corning, Inc.). Bacteria were diluted in IMDM (without antibiotics) and added to MΦ at a bacteria to MΦ ratio of 5:1, 15:1, or 50:1 as indicated in individual experiments. Bacteria were pelleted onto the cells by centrifugation at 270 g for 4 min at room temperature, and the plates were incubated for 30–120 min at 37°C with 5% CO2. The cultures were then washed twice with HBSS to remove remaining bacteria and either incubated for an additional 2 h in the presence of 100 μg/ml gentamycin (a total of 4 h) or the cells were directly assessed for apoptosis by Annexin V staining at the indicated time points. Necrotic cells were excluded by staining with propidium iodide (PI) at 1 μg/ml (Sigma Chemical Co.). Incubations with Annexin V–FITC (Trevigene, Inc.) were for 20 min on ice. Flow cytometry analysis was performed on a Becton Dickinson FACSort™ flow cytometer (Becton Dickinson), and data were collected on 3 × 104 PI− cells. Apoptosis was further confirmed by detection of high molecular weight DNA fragments as described 24. In samples where apoptosis was chemically induced, MΦ were treated with 1 μg/ml of LPS purified from Escherichia coli (Sigma Chemical Co.) for 4 h at 37°C with 5% CO2. This was followed by incubation with 5 mM ATP for 45 min 20.
Presentation of Antigens Derived from Apoptotic Material.
After infection of MΦ with bacteria and washing with HBSS as described above, fresh IMDM supplemented with 5% FCS and 100 μg/ml gentamycin were added and the infected MΦ were incubated for an additional 2 h. After an additional wash with HBSS, the MΦ were resuspended in 500 μl of IMDM supplemented with 10% FCS and 50 μg/ml gentamycin. Then, 106 DCs or MΦ, either live or prefixed in 1% paraformaldehyde and subsequently washed thoroughly, were added to the apoptotic MΦ in 250 μl of IMDM. This resulted in a 1:1 DC or MΦ to apoptotic MΦ ratio. Finally, 5 × 105 CD8OVA 17 or OT4H.2D5 25 T hybridoma cells, which secrete IL-2 upon specific recognition of the OVA(257–264)/Kb or OVA(265–277)/I-Ab complex, respectively, were added in 250 μl of IMDM. After a 24-h incubation at 37°C, IL-2 secreted by the CD8OVA or OT4H.2D5 T hybridoma cells was quantitated using IL-2–dependent CTLL cells. In experiments where MΦ from caspase 1−/− mice (H-2b) were infected with bacteria, the assay was performed in the same way except that the T hybridoma cells were not added together with the DCs but after incubation of DCs with (apoptotic) MΦ as follows. After 20 h of DC–MΦ coculture, cells from three wells were collected and resuspended in cold PBS supplemented with 0.5% FCS and 2 mM EDTA. The suspension was then enriched for CD11c-expressing cells (DCs) using anti-CD11c magnetic microbeads (Miltenyi Biotec) following the manufacturer's protocol. The enriched population consisted of 80–90% CD11c+ cells as assessed using HL3 antibody (PharMingen) and FACS® analysis. 105 purified CD11c+ cells were then added to duplicate wells in a 96-well plate (Corning, Inc.) together with 105 T hybridoma cells. Cells were coincubated for 24 h at 37°C with 5% CO2, and IL-2 produced by the T hybridoma cells was quantified as above. Experiments where the inhibitor cytochalasin D (CCD, 5 μg/ml; Sigma Chemical Co.) was used were also performed such that the DCs were coincubated with MΦ for 20 h in the presence of CCD followed by enrichment of CD11c+ DCs by magnetic sorting. The enriched DCs were subsequently added to T hybridoma cells in fresh wells. This was done to retain viability of the DCs and to assure that equal amounts of viable DCs were added to the T hybridoma cells.
Direct Presentation of Bacterial Antigens by Infected DCs.
Experiments to assess direct presentation of bacterial antigens by DCs were conducted in 24-well or 96-well plates as described previously 18, except that DCs were not fixed after coincubation with the bacteria. Instead, bacteria were coincubated with DCs at the indicated bacteria to DC ratio for 2 h and the cells were washed three times with HBSS. Then, fresh IMDM containing 5% FCS and 50 μg/ml gentamycin was added. This was followed by addition of T hybridoma cells and quantitation of IL-2 production as described for the other antigen presentation assays.
Phagocytosis of Apoptotic Cells.
MΦ were dyed red using PKH26-GL (Sigma Biosciences), and 106 dyed cells were coincubated with 14028r or CS022r for 90 min and washed as described above. After incubation for an additional 2 h in IMDM supplemented with 50 μg/ml gentamycin, 2.5 × 105 MACS-purified DCs or MΦ dyed green using PKH67-GL (Sigma Biosciences) were added to the red MΦ. After 2 h of coincubation, flow cytometry was performed and the percentage of double positive cells among the green cells was determined. In samples where CCD was present, the green cells were first pretreated for 60 min with CCD at 10 μg/ml and the concentration of CCD was 5 μg/ml during the coincubation with the red MΦ.
Results
DCs Acquire Antigens from MΦ Induced to Undergo Apoptosis by Salmonella Infection.
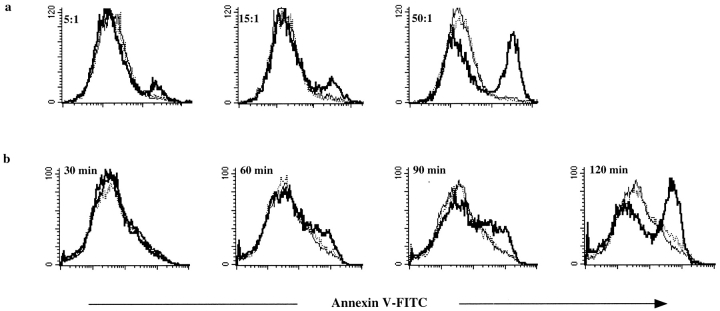
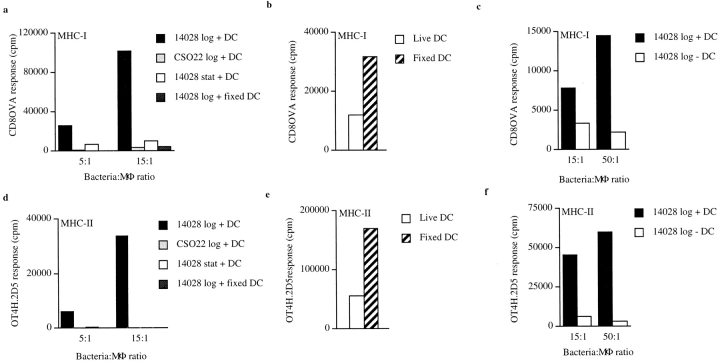
Infection of MΦ and DCs with S. typhimurium using conditions that do not alter the viability of the infected cells, such as with bacteria in the stationary phase of growth, results in processing of the bacteria for peptide presentation on MHC-I and MHC-II 16 17 18 19 21. It has also been shown that infection of MΦ with S. typhimurium grown under conditions resulting in an invasive phenotype and expression of the type III secretion apparatus 6 26 27, such as during the logarithmic growth phase, results in apoptotic death of infected MΦ 6 7. Thus, we sought to determine whether S. typhimurium–induced apoptosis of infected MΦ was a quiescent death that did not result in presentation of bacterial antigens to T cells, or whether bystander APCs could acquire bacterial antigens from MΦ induced to undergo apoptosis by S. typhimurium infection. MΦ were infected with logarithmically growing wild-type S. typhimurium 14028r, which induces apoptosis in the infected cells, or with logarithmically growing phoP constitutive (phoP c) strain CS022r, which does not induce apoptosis upon infection of MΦ as assessed by Annexin V staining (Fig. 1) or DNA fragmentation (data not shown). Both bacterial strains expressed the model antigen Crl-OVA, which is expressed in the cytosol of the bacteria 16 and encodes the MHC-I–binding OVA(257–264) as well as the MHC-II–binding OVA(265–277) epitope. Conditions that resulted in Annexin V+ PI− MΦ after infection with 14028r but not with CS022r (Fig. 1) resulted in OVA(257–264) presentation on the MHC-I molecule Kb as well as OVA(265–277) presentation on the MHC-II molecule I-Ab by bystander DCs (Fig. 2, a and d). This presentation was observed when MΦ were infected with logarithmically growing 14028r but not with logarithmically growing CS022r, bacteria which can and cannot induce apoptosis in infected MΦ, respectively (Fig. 1). In addition, no presentation of the OVA peptides on either MHC-I or MHC-II by bystander DCs was observed when MΦ were infected with 14028r in the stationary phase of growth (grown on agar plates for 20 h; Fig. 2, a and d). Stationary phase wild-type S. typhimurium does not induce apoptosis in infected MΦ (Fig. 1 b; references 6, 7). The presentation observed when MΦ were infected with logarithmically growing 14028r was not due to release of peptides into the culture that bound preformed surface MHC molecules: addition of prefixed DCs instead of viable DCs as the bystander APCs did not result in stimulation of the OVA(257–264)/Kb– or the OVA(265–277)/I-Ab–specific T cell hybridomas (Fig. 2, a and d). This was the case in spite of the enhanced ability of prefixed DCs to bind exogenously added OVA(257–264) or OVA(265–280) peptide compared with viable DCs (Fig. 2b and Fig. e). The observed presentation was not due to presentation of the bacteria-derived OVA peptides by infected MΦ. This was ensured by using MΦ from a haplotype of mouse (BALB/c; H-2d) different from that recognized by the H-2b–specific CD8OVA and OT4H.2D5 T cell hybridomas 17 25. Finally, omitting addition of bystander DCs (H-2b) to the infected MΦ (H-2d) resulted in a similar background level of stimulation of the T cell hybridomas as when DCs (H-2b) or MΦ (H-2b) were grown in medium only (Fig. 2c and Fig. f, and see Fig. 5, a–c).
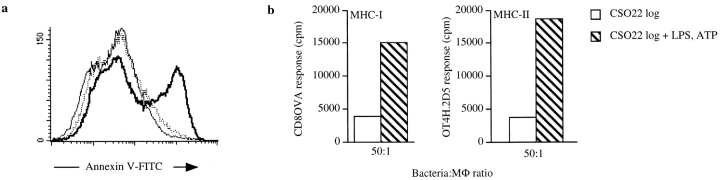
Figure 1.
Logarithmically growing S. typhimurium 14028 induces apoptosis in MΦ, whereas the phoP c mutant CS022 or stationary phase 14028 does not. (a) MΦ were coincubated with increasing bacteria to MΦ ratios (5:1, 15:1, or 50:1) for 2 h. Residual bacteria were washed away, and the cells were incubated for an additional 2 h in medium with gentamycin (100 μg/ml). The cells were stained with Annexin V–FITC and PI and were analyzed in a flow cytometer. Histograms show 3 × 104 gated PI− cells plotted against cell number of MΦ coincubated with 14028s (thick line), CS022s (dotted line), or medium (thin line). (b) MΦ were coincubated with either logarithmically growing 14028r (thick line) or stationary phase (plate-grown) 14028r (dotted line) at a bacteria to MΦ ratio of 15:1, or were incubated in medium alone (thin line). At the times indicated in the histograms, infected cells were stained and analyzed as described in a. The induction of apoptosis in the MΦ was confirmed by FACS® analysis or detection of high molecular weight DNA fragments in at least three independent experiments.
Figure 2.
Viable bystander DCs acquire antigenic material from MΦ induced to undergo apoptosis by infection with wild-type S. typhimurium. (a and c) OVA peptide presentation on MHC-I (Kb) and (d and f) MHC-II (I-Ab) by bystander DCs quantitated using the OVA(257–264)/Kb– or the OVA(265–277)/I-Ab–specific T cell hybridoma CD8OVA or OT4H.2D5, respectively. MΦ (H-2d) were coincubated for 90 min with either stationary phase (stat) 14028r, logarithmically growing (log) 14028r, or logarithmically growing CS022r expressing Crl-OVA as indicated. After washing and addition of gentamycin, live (+ DC) or paraformaldehyde-fixed (+ fixed DC) (H-2b) or no DCs (− DC) and T hybridoma cells were added as indicated. (b and e) The CD8OVA (b) or OT4H.2D5 (e) response to live or fixed DCs (H-2b) loaded with 1 nM OVA(257–264) or 100 μM OVA(265–280) peptide, respectively. Counts per minute in wells where bystander DCs were omitted or where DCs or MΦ were incubated in medium only along with the appropriate T cell hybridoma were typically 250–2,500. The results are representative of three to five independent experiments.
Figure 5.
Both MΦ and DCs engulf apoptotic material by a process that requires cytoskeletal rearrangement despite the fact that OVA(257–264) presentation on MHC-I is restricted to DCs. OVA(257–264) presentation on MHC-I by bystander DCs (H-2b) (a) or MΦ (H-2b) (b) after coincubation with MΦ (H-2d) that previously phagocytosed logarithmically growing 14028r Crl-OVA or CS022r Crl-OVA. After 90 min of coincubation with bacteria, cells were washed, gentamycin was added, and OVA(257–264) presentation by added bystander cells was quantitated using CD8OVA T hybridoma cells. The response of MΦ (H-2d) and bystander cells incubated in medium only is also shown. (c) MΦ (H-2d) were coincubated with logarithmically growing 14028r Crl-OVA, CS022r Crl-OVA, or were incubated in medium only for 90 min. After washing and addition of gentamycin, DCs were added in the absence or presence of CCD (+ CCD) for 20 h. The DCs were then MACS-purified using anti-CD11c magnetic beads, and presentation of OVA(265–277) on MHC-II by the DCs was quantitated by addition of OT4H.2D5 T hybridoma cells to the MACS-purified DCs. The response of MΦ (H-2d) and bystander DCs incubated in medium only is also shown. (d) MΦ dyed red were either incubated in medium only or were coincubated with logarithmically growing 14028r or CS022r as indicated above the dot plots. These red MΦ were then coincubated with DCs (top) or MΦ (bottom) dyed green. This coincubation was done in the absence or presence (+ CCD) of CCD as indicated. Flow cytometry analysis was then performed on 3 × 104 cells. The percentage of double positive cells among green cells is indicated in each dot plot.
OVA Peptide Presentation Is Due to Uptake of Apoptotic Material and Not to Direct Presentation of Residual Bacteria.
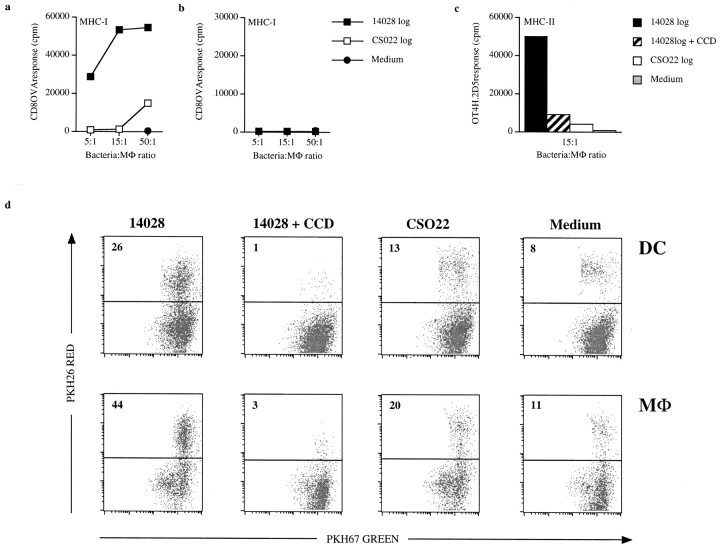
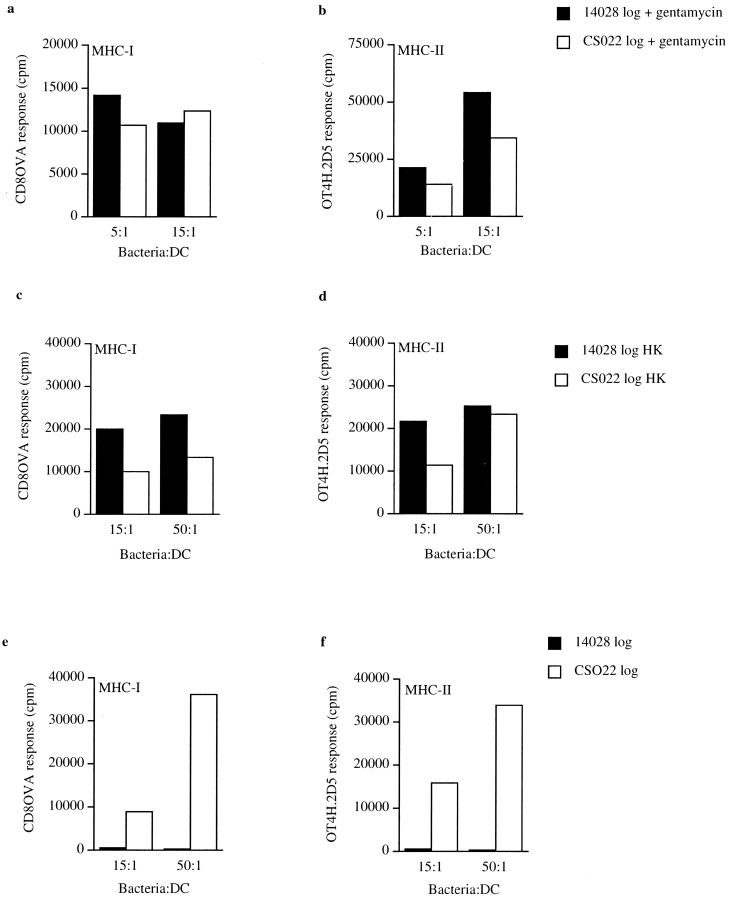
Several lines of data support that the MHC-I and MHC-II presentation shown in Fig. 2, a and d, was due to uptake of apoptotic material and not to phagocytic processing of remaining bacteria by the added DCs. First, addition of stationary phase 14028r, which does not induce apoptosis in the infected MΦ (Fig. 1 b; references 6 and 7), did not result in MHC-I or MHC-II presentation in the apoptotic antigen presentation assay (Fig. 2, a and d). This is the case despite the fact that stationary phase S. typhimurium expressing Crl-OVA is efficiently presented when phagocytosed by DCs 18 19. In addition, similar amounts of the bacteria were internalized by the MΦ. This was confirmed by coincubating MΦ with logarithmically growing or stationary phase 14028s or logarithmically growing CS022s expressing green fluorescent protein followed by flow cytometry analysis (data not shown) as described elsewhere 19. Second, we reasoned that if the presentation observed in the apoptotic presentation assay was due to remaining bacteria that were phagocytosed by the added DCs, it must be due to gentamycin-killed bacteria remaining in the wells, as no bacterial growth was observed during the 24-h incubation time. To directly test this possibility, bacterial suspensions of 14028r and CS022r were treated with gentamycin, equal numbers of these bacteria were added to viable DCs, and presentation of the OVA peptides was quantitated. This resulted in equal levels of bacterial processing by DCs for MHC-I presentation of OVA(257–264) (Fig. 3 a) and MHC-II presentation of OVA(265–277) (Fig. 3 b). Likewise, similar levels of presentation of the OVA peptides on MHC-I and MHC-II derived from the bacteria expressing Crl-OVA was observed if the bacteria were heat-killed before addition to DCs (Fig. 3c and Fig. d). The similar level of MHC-I and MHC-II presentation of OVA peptides derived from gentamycin-treated or heat-killed 14028r and CS022r expressing Crl-OVA makes it unlikely that the presentation observed in Fig. 2, a and d, was due to phagocytic processing of bacteria remaining in the wells. Furthermore, viable bacterial counts from samples removed from wells of apoptotic presentation experiments where MΦ were infected with either 14028r or CS022r consistently showed that similar quantities of 14028r and CS022r were recovered (data not shown). Such data were obtained from >10 individual experiments that gave apoptotic presentation results similar to that shown in Fig. 2, a and d, despite the fact that the absolute number of bacteria recovered varied between individual experiments. In addition, OVA-specific ELISA was performed on lysates prepared from bacteria used in apoptotic antigen presentation assays that gave results similar to those shown in Fig. 2, a and d, to quantitate the level of Crl-OVA expressed in logarithmically growing 14028r and CS022r. These data consistently showed similar levels of Crl-OVA expression in the two bacterial strains and never exceeded a twofold difference in relative expression, sometimes slightly greater in 14028r and sometimes greater in CS022r. Despite this, we never observed significant presentation of the bacteria-encoded antigens by DCs in our apoptotic bystander assay when MΦ were infected with CS022r. Finally, no presentation of either OVA(257–264) on Kb or OVA(265–277) on I-Ab was detected when logarithmic 14028r Crl-OVA was coincubated with the DCs (Fig. 3e and Fig. f), as logarithmically growing 14028r is cytotoxic to these cells (our unpublished results). In contrast, logarithmically growing CS022r expressing Crl-OVA, which does not induce apoptosis in infected cells (Fig. 1 a), was processed for OVA peptide presentation on both MHC-I and MHC-II by DCs (Fig. 3e and Fig. f). Together, these data support the notion that the presentation observed in Fig. 2, a and d, is due to uptake of material from MΦ that were induced to undergo apoptosis by infection with wild-type S. typhimurium by bystander DCs rather than to direct presentation of residual bacteria in the wells by bystander DCs.
Figure 3.
DCs process residual 14028 and CS022 expressing Crl-OVA for OVA peptide presentation on MHC-I and MHC-II equally well. Logarithmically growing (OD600 = 1.3–1.5) 14028r Crl-OVA or CS022r Crl-OVA bacteria were treated with 100 μg/ml of gentamycin (a and b), heat killed (HK) at 65°C (c and d), or were left untreated (e and f) before coincubation with DCs for 90 min. After several washes, OVA(257–264)/Kb (a, c, and e) and OVA(265–277)/I-Ab (b, d, and f) were quantitated using CD8OVA and OT4H.2D5 T hybridoma cells, respectively. The results are representative of three independent experiments.
Chemical Induction of Apoptosis in MΦ Infected with CSO22r Results in Presentation of Bacterial Antigens by Bystander DCs.
To determine if inducing MΦ to undergo apoptosis upon phagocytosis of S. typhimurium was sufficient to result in presentation of apoptotic material by bystander DCs, MΦ infected with CS022r expressing Crl-OVA were induced to undergo apoptosis by addition of LPS and ATP 20. This treatment induced apoptosis as assessed by Annexin V staining (Fig. 4 a). Presentation of OVA(257–264) on MHC-I as well as OVA(265–277) on MHC-II by bystander DCs was significantly enhanced when MΦ (MHC-mismatched) coincubated with logarithmically growing CS022r were induced to undergo apoptosis by treatment with LPS and ATP (Fig. 4 b).
Figure 4.
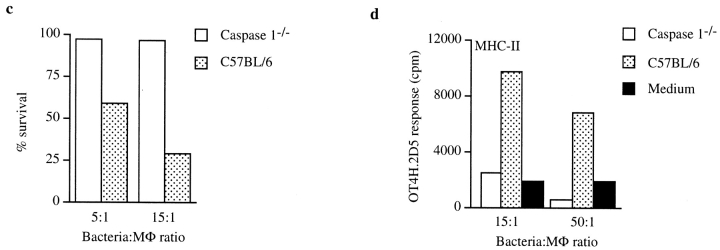
Chemical induction of apoptosis in MΦ infected with CS022 results in MHC-I and MHC-II presentation of bacteria-derived antigen by bystander DCs. (a) Histogram of flow cytometry analysis of Annexin V–FITC binding to 3 × 104 PI− MΦ that have phagocytosed logarithmically growing (log) CS022r Crl-OVA (dotted line) or CS022r Crl-OVA and were subsequently treated with LPS and ATP (thick line) or that were incubated in medium alone (thin line). Flow cytometry analysis was carried out 20 h after the apoptosis induction. (b) OVA(257–264)/Kb and OVA(265–277)/I-Ab presentation on MHC-I and MHC-II by bystander DCs (H-2b) after coincubation with MΦ (H-2d) previously coincubated with logarithmically growing CS022r Crl-OVA and left untreated or treated with LPS plus ATP as indicated. OVA(257–264)/Kb and OVA(265–277)/I-Ab presentation was quantitated using CD8OVA and OT4H.2D5 T hybridoma cells, respectively. (c) Viability of MΦ from wild-type and caspase 1−/− mice after coincubation with logarithmically growing 14028r Crl-OVA for 90 min measured as exclusion of PI staining. (d) MΦ derived from C57BL/6 or caspase 1−/− mice were coincubated with logarithmically growing 14028r Crl-OVA or medium only for 90 min. After washing and addition of gentamycin, DCs were added for 24 h. The DCs were then MACS-purified using anti-CD11c magnetic beads, and presentation of OVA(265–277) on MHC-II by the DCs was quantitated by addition of OT4H.2D5 T hybridoma cells to the MACS-purified DCs.
To further investigate the role of apoptosis induction in generating material for presentation by bystander DCs, MΦ from caspase 1−/− mice 20, which were previously shown to be resistant to the cytotoxic effects of Salmonella infection 14, were used in the apoptotic antigen presentation assay. Although a 2-h coincubation of caspase 1−/− MΦ with 14028r Crl-OVA resulted in no detectable cell death, significant death occurred upon coincubation of 14028r with C57BL/6 MΦ (Fig. 4 c). Apoptotic antigen presentation assays were performed by adding DCs to MΦ from either caspase 1−/− or C57BL/6 mice that had been coincubated with 14028r expressing Crl-OVA. In contrast to the experiments presented above, which use MHC-mismatched MΦ for bacterial infections, MΦ from caspase 1−/− mice are of the correct haplotype (H-2b) to be recognized by the OT4H.2D5 T cell hybridoma. To avoid detecting direct presentation by caspase 1−/− or C57BL/6 MΦ, the infected MΦ were first coincubated with the bystander DCs (also H-2b) for 20 h. After this incubation, CD11c+ cells, i.e., the added bystander DCs, were positively selected from the cultures using anti-CD11c–coated magnetic beads. These purified DCs were then added to wells containing OT4H.2D5 T hybridoma cells, and presentation of OVA(265–277) on I-Ab was subsequently quantitated in the absence of the caspase 1−/− or C57BL/6 MΦ. These experiments showed that DCs coincubated with MΦ from wild-type but not caspase 1−/− mice could sensitize the MHC-II–restricted T cell hybridoma (Fig. 4 d).
DCs but Not MΦ Present Antigens from Apoptotic Material on MHC-I Despite the Fact that Both Cell Types Internalize Apoptotic MΦ.
To investigate if MΦ also had the capacity to acquire antigens from bacteria-induced apoptotic MΦ, experiments were performed where MΦ or DCs were added as the bystander APCs. Thus, MΦ (MHC-mismatched, H-2d) were coincubated with 14028r or CS022r expressing Crl-OVA, and either MΦ or DCs from C57BL/6 mice (H-2b) were added as the bystander cells. Quantification of OVA(257–264)/Kb presentation showed that DCs (Fig. 5 a) but not MΦ (Fig. 5 b) could present the OVA epitope on MHC-I from bacteria-encoded Crl-OVA. No presentation by bystander MΦ was observed even at a higher bacteria to MΦ infection ratio (50:1). However, the MΦ could present bacterial antigens on MHC-I when stationary phase 14028r or CS022r expressing Crl-OVA was added directly to the cells (data not shown).
We next investigated if the lack of presentation of antigens from apoptotic material on MHC-I by bystander MΦ was due to an inability of MΦ to engulf apoptotic material. To test this, MΦ were dyed red and were coincubated with logarithmically growing 14028r or CS022r expressing Crl-OVA. These red MΦ were then coincubated with MΦ or DCs that were dyed green. Flow cytometry was subsequently used to analyze the number of double positive cells among the green cells. These data showed that MΦ and DCs efficiently internalized apoptotic material from MΦ that were coincubated with 14028r. In contrast, significantly less uptake of apoptotic material was apparent when the red MΦ were coincubated with CS022r or when they were incubated in medium alone before coincubation with green bystander cells (Fig. 5 d). Furthermore, the uptake of apoptotic material was inhibited when CCD was present during the coculture (Fig. 5 d). This demonstrates that cytoskeletal rearrangements are required for the uptake of apoptotic material. In addition, CCD also abolished presentation of OVA(265–277) on MHC-II by bystander DCs when it was present during the coincubation of the bystander DCs and the MΦ that had previously phagocytosed 14028r expressing Crl-OVA (Fig. 5 c). Finally, the uptake of apoptotic material by bystander DCs after coincubation with bacterially induced apoptotic MΦ was further confirmed by electron microscopy, showing apoptotic bodies within DCs (data not shown).
Discussion
An increasing number of bacterial species have been shown to induce apoptosis in phagocytes, including Yersinia pseudotuberculosis, Shigella flexneri, and S. typhimurium 6 7 9 10. Although these studies have examined the mechanism underlying the observed apoptosis, none of them have investigated if this process generates antigenic material. Thus, this is the first demonstration that bacteria-induced apoptosis of infected cells results in presentation of a bacteria-encoded antigen on both MHC-I and MHC-II by bystander DCs. Apoptosis induction in infected cells is critical for presentation of bacteria-derived material by bystander APCs. This was demonstrated here using bacterial strains that can or cannot induce apoptosis in infected MΦ, and by chemically inducing apoptosis in MΦ infected with bacteria that cannot themselves induce apoptosis. Interestingly, it has been suggested that Salmonella also can induce cells to undergo a necrotic type of cell death 28. However, conditions inducing such death in infected cells, for example using a very high bacteria to macrophage ratio, resulted in drastic reduction of the presentation of bacteria-encoded antigens on MHC-I by bystander DCs, whereas the presentation on MHC-II was not as dramatically affected (our unpublished observations). These observations support the important role of apoptosis induction for presentation of antigenic material by bystander DCs and suggest a different role for MHC-I versus MHC-II presentation of apoptotic material by bystander DCs.
We also showed that the observed presentation of epitopes to T cells after bacterial induction of apoptosis in infected MΦ occurred when live DCs but not MΦ were present as bystander cells, and furthermore that this presentation involved active phagocytosis of antigenic material. The mechanism underlying the ability of DCs but not MΦ to present antigens derived from apoptotic material, despite the fact that both cell types actively internalize material from neighboring apoptotic cells, remains to be clarified. In preliminary experiments where bystander MΦ were added to bystander DCs coincubated with bacteria-induced apoptotic MΦ, presentation of the bacteria-encoded antigens on MHC-I and MHC-II was diminished. Whether the observed reduction in presentation by bystander DCs in the presence of simultaneously added bystander MΦ was due to a soluble factor secreted by the macrophages or a result of competition for the antigenic material between the DCs and the MΦ remains to be determined. Interestingly, a similar ability of bystander DCs but not MΦ to present antigenic material from monocytes induced to undergo apoptosis by influenza virus infection to CD8+ cytotoxic T cells, as well as reduced presentation by bystander DCs in the presence of added bystander MΦ, has also been observed 15. Such differences in the ability of these different APCs to present antigens from apoptotic material may be due to different functions they may have in initiating an immune response. For example, DCs are the most efficient stimulators of naive T cells due to their extensive antigen capture capacity before they have been exposed to antigenic stimuli and their enhanced antigen presentation capacity after encountering antigenic stimuli or after exposure to immunomodulatory factors such as LPS or TNF-α (for a review, see reference 29). MΦ, on the other hand, are not as efficient stimulators of naive T cells as DCs. This is due, at least in part, to low or absent expression of MHC-II and costimulatory molecules, unless the cells are activated by, for example, IFN-γ or LPS 30 31. MΦ also have extensive antigen degradative capacity, which is important in their role in innate protection against microbes. This latter property of MΦ may result in extensive degradation of phagocytosed apoptotic material and concomitant lack of antigenic peptide presentation for T cell recognition.
S. typhimurium infection naturally occurs by the oral route. After reaching the intestine, the bacteria penetrate into deeper tissue through M cells by inducing membrane ruffling 1. The bacteria then pass through the epithelium of the PP and enter the subepithelial dome. This consists of a network of MΦ and DCs intermingled with CD4+ T cells and B cells from the underlying follicle 4. The potential interaction of S. typhimurium with MΦ and DCs in the follicle dome after oral infection, combined with the bacteria's ability to survive inside phagocytic cells 32 and its ability to induce apoptosis in infected MΦ (Fig. 1; references 6, 7), raises the question of the significance of bacteria-induced apoptosis of infected MΦ and its influence on the immune response to oral Salmonella infection. Indeed, it has been shown that apoptosis occurs in vivo during infection not only with S. typhimurium, but also with Y. pseudotuberculosis and S. flexneri 33 34. Although apoptotic CD18+ cells have been identified in the liver of mice infected with S. typhimurium 35, apoptotic MΦ were present in the lymphoid follicles of the PP in a rabbit ligated ileal loop model of S. flexneri infection 34. Furthermore, Zychlinsky et al. 34 showed that apoptotic MΦ were present when virulent (apoptosis-inducing) S. flexneri was used, whereas no increase in the frequency of apoptotic cells above background levels was apparent using avirulent bacteria not capable of inducing apoptosis. Not only were apoptotic MΦ present after infection with virulent S. flexneri, but also large lymphoid follicle cells containing multiple apoptotic nuclei were demonstrated 34. This suggests that these cells had phagocytosed apoptotic cells, presumably apoptotic MΦ. Orogastric inoculation with Y. pseudotuberculosis revealed Mac1+ cells in spleen and mesenteric lymph nodes that were terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling assay–positive (TUNEL+) upon infection with wild-type bacteria 33. In contrast, no increase in Mac1+ TUNEL+ cells relative to uninfected mice was apparent when the mice were infected with a yopJ mutant 33. Furthermore, a yopJ mutant strain was more readily cleared from infected mice 33. These data suggest that apoptosis induction may contribute to spread of the bacteria and severity of the infection. However, the yopJ locus is also required for Yersinia-mediated inhibition of IL-8 and TNF-α expression 36, which could affect clearance of the bacteria from the host. Thus, although three genera of bacteria that infect hosts by penetrating the intestinal epithelium and accessing the lymphoid follicles of the PPs via M cells 37 induce apoptosis in vivo, the relative contribution of apoptosis by itself or apoptosis induction in combination with bacteria-mediated effects on cytokine production has not clearly been established. However, the ability of bacterial antigens to be presented to T cells from bacteria-induced apoptotic material may be yet another factor to consider when evaluating the influence of bacteria-induced apoptosis on the host immune response.
Immature DCs have an extensive capacity to internalize antigens 38, including apoptotic material 15 39 40 41, for presentation on MHC-I and MHC–II. DCs in the subepithelial dome are endocytic and can process protein in vivo for presentation to naive T cells 42. It could be envisioned that when bacteria reach MΦ underlying the epithelial layer, the cells undergo apoptosis after phagocytosing the bacteria; such a scenario has been suggested for Shigella infection 34. In this way, MΦ neutralize the internalized bacteria and generate apoptotic material containing bacterial antigens in a “package” that does not induce apoptosis in a subsequently phagocytosing cell. In this study, we have shown that apoptotic material generated by Salmonella infection can be efficiently phagocytosed by DCs and MΦ. In the case of uptake by DCs, these cells can then present bacterial antigens on MHC-I and MHC-II. LPS, TNF-α, IL-1β 43 44, and uptake of apoptotic material 40 have all been shown to induce DC maturation. This in turn triggers enhanced surface expression of costimulatory molecules and DC migration (for a review, see reference 29). In the case of Salmonella, DCs containing apoptotic material may migrate to lymphoid organs and activate naive T cells.
Indeed, apoptotic bodies can affect the immune system. For example, apoptotic bodies can have an immunosuppressive affect by reducing secretion of the proinflammatory cytokines TNF-α, IL-1β, and IL-12 by LPS-stimulated monocytes 45 46. Apoptotic cells have also been shown to induce autoantibody production when injected into syngeneic mice 47. The recent report by Ronchetti et al. 48 also demonstrated that injection of apoptotic RMA cells primes a functional tumor-specific immune response in vivo. Furthermore, DCs but not MΦ pulsed with apoptotic material prime a specific antitumor CTL response despite the fact that MΦ engulf apoptotic cells in vivo 48. MΦ and DCs have been suggested to use different integrin receptors, αvβ3 and αvβ5, respectively, to phagocytose apoptotic material 39. This may result in differences in the intracellular trafficking of the phagocytosed apoptotic material and concomitant differences in ability to present the antigens to T cells, as has been shown for MΦ here and elsewhere 15 39. The cytokine production profile of MΦ and DCs after uptake of apoptotic material has also been shown to differ. For example, MΦ synthesize TGF-β 45 and downregulate production of the proinflammatory cytokines TNF-α, IL-1β, and IL-12 46, whereas DCs increase secretion of IL-10, TNF-α, and IL-1β 40. The differences in cytokine profiles produced by the different APCs as well as the differential use of receptors to phagocytose apoptotic material may suggest that DCs and MΦ have different functions in the clearance of apoptotic material. Interestingly, S. flexneri apoptosis of infected MΦ also results in release of IL-1β in its biologically active form 49. Although IL-1β production by MΦ induced to undergo apoptosis by Salmonella infection has not yet been assessed, it is interesting to note that a similar mechanism of apoptosis induction, direct binding of a homologous effector protein to caspase 1 12 13 14, is responsible for Salmonella- and Shigella-induced apoptosis of infected MΦ. Given the ability of apoptotic material to influence the immune response and the vast number of different bacteria that induce apoptosis in infected cells, the ability to combat microbial infections and develop effective recombinant bacterial vaccines must include understanding the relationship between microbe-induced apoptosis and antimicrobial immunity.
Acknowledgments
The authors acknowledge Dr. Anders Håkansson (Lund University) for assistance with analysis of high and low molecular weight fragments in apoptotic cells, Dr. Manuela Baccarini and Veronika Jesenberger (Vienna Biocenter) for providing caspase 1−/− mice, and Dr. Judith A. Kapp (Emory University School of Medicine, Atlanta, GA) for providing the OT4H.2D5 T hybridoma cells.
This work was supported by the Swedish Natural Sciences Research Council (project 10610-306), Lund University Medical Faculty, The Österlund Foundation, Kock's Foundation, Kungliga Fysiografiska Foundation, The Crafoord Foundation, Åke Wiberg's Foundation, and the Swedish Society for Medical Research.
Footnotes
Abbreviations used in this paper: CCD, cytochalasin D; DC, dendritic cell; LB, Luria broth; MΦ, bone marrow–derived macrophage(s); MHC-I and MHC-II, MHC class I and II molecules, respectively; PI, propidium iodide; PP, Peyer's patch.
References
- Jones B.D., Ghori N., Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutra M.R., Frey A., Kraehenbuhl J.-P. Epithelial M cellsgateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- Jones B.D., Falkow S. Salmonellosishost immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- Neutra M.R., Pringault E., Kraehenbuhl J.-P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immunol. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- Foster J.W., Spector M.P. How Salmonella survive against the odds. Annu. Rev. Microbiol. 1995;49:145–174. doi: 10.1146/annurev.mi.49.100195.001045. [DOI] [PubMed] [Google Scholar]
- Chen L.M., Kaniga K., Galán J.E. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- Monack D.M., Raupach B., Hromockyj A.E., Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.J., Duke R.C., Fadok V.A., Sellins K.S. Apoptosis and programmed cell death in immunity. Annu. Rev. Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A., Prevost M.C., Sansonetti P.J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- Monack D.M., Mecsas J., Ghori N., Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A., Gunther D., Dux F., Naumann M., Meyer T.F., Rudel T. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:339–352. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Smith M.R., Thirumalai K., Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- Hilbi H., Moss J.E., Hersh D., Chen Y., Arondel J., Banerjee S., Flavell R.A., Yuan J., Sansonetti P.J., Zychlinsky A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 1998;273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- Hersh D., Monack D.M., Smith M.R., Ghori N., Falkow S., Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.D., Wick M.J., Russell D.G., Normark S.J., Harding C.V. Recombinant Escherichia coli express a defined, cytoplasmic epitope that is efficiently processed in macrophage phagolysosomes for class II MHC presentation to T lymphocytes. J. Immunol. 1992;149:2576–2584. [PubMed] [Google Scholar]
- Pfeifer J.D., Wick M.J., Roberts R.L., Findlay K., Normark S.J., Harding C.V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Svensson M., Stockinger B., Wick M.J. Bone marrow-derived dendritic cells can process bacteria for MHC-I and MHC-II presentation to T cells. J. Immunol. 1997;158:4229–4236. [PubMed] [Google Scholar]
- Svensson M., Wick M.J. Classical MHC class I peptide presentation of a bacterial fusion protein by bone marrow-derived dendritic cells. Eur. J. Immunol. 1999;29:180–188. doi: 10.1002/(SICI)1521-4141(199901)29:01<180::AID-IMMU180>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Wick M.J., Harding C.V., Normark S.J., Pfeifer J.D. Parameters that influence the efficiency of processing antigenic epitopes expressed in Salmonella typhimurium . Infect. Immun. 1994;62:4542–4548. doi: 10.1128/iai.62.10.4542-4548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.I., Mekalanos J.J. Constitutive expression of the PhoP regulon attenuates Salmonella virulence and survival within macrophages. J. Bacteriol. 1990;172:2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre P., Turley S.J., Gatti E., Hull M., Meltzer J., Mirza A., Inaba K., Steinman R.M., Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- Håkansson A., Andreasson J., Zhivotovsky B., Karpman D., Orrenius S., Svanborg C. Multimeric α-lactalbumin from human milk induces apoptosis through a direct effect on cell nuclei. Exp. Cell Res. 1999;246:451–460. doi: 10.1006/excr.1998.4265. [DOI] [PubMed] [Google Scholar]
- Li Y., Ke Y., Gottlieb P.D., Kapp J.A. Delivery of exogenous antigen into the major histocompatibility complex class I and class II pathways by electroporation. J. Leukoc. Biol. 1994;56:616–624. doi: 10.1002/jlb.56.5.616. [DOI] [PubMed] [Google Scholar]
- Lee C.A., Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc. Natl. Acad. Sci. USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo C.M., Galán J.E. The invasion-associated type-III protein secretion system in Salmonella—a review. Gene. 1997;192:51–59. doi: 10.1016/s0378-1119(96)00825-6. [DOI] [PubMed] [Google Scholar]
- Lindgren S.W., Heffron F. To sting or be stungbacteria-induced apoptosis. Trends Microbiol. 1997;5:263–264. doi: 10.1016/S0966-842X(97)88832-4. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Beller D.I. Functional significance of the regulation of macrophage Ia expression. Eur. J. Immunol. 1984;14:138–143. doi: 10.1002/eji.1830140207. [DOI] [PubMed] [Google Scholar]
- Hathcock K.S., Laszlo G., Pucillo C., Linsley P., Hodes R.J. Comparative analysis of B7-1 and B7-2 costimulatory ligandsexpression and function. J. Exp. Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P.I., Swanson R.V., Haidaris C.G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack D.M., Mecsas J., Bouley D., Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky A., Thirumalai K., Arondel J., Cantey J.R., Aliprantis A.O., Sansonetti P.J. In vivo apoptosis in Shigella flexneri infections. Infect. Immun. 1996;64:5357–5365. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Dahlfors A., Buchan A.M.J., Finlay B.B. Murine salmonellosis studied by confocal microscopySalmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schesser K., Spiik A.-K., Dukuzumuremyi J.-M., Neurath M.F., Pettersson S., Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expressionYopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- Jepsen M.A., Clark M.A. Studying M cells and their role in infection. Trends Microbiol. 1998;6:359–365. doi: 10.1016/s0966-842x(98)01337-7. [DOI] [PubMed] [Google Scholar]
- Austyn J.M. New insights into the mobilization and phagocytic activity of dendritic cells. J. Exp. Med. 1996;183:1287–1292. doi: 10.1084/jem.183.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M.L., Pearce S.F., Francisco L.M., Sauter B., Roy P., Silverstein R.L., Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovere P., Vallinoto C., Bondanza A., Crosti M.C., Rescigno M., Ricciardi-Castagnoli P., Rugarli C., Manfredi A.A. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J. Immunol. 1998;161:4467–4471. [PubMed] [Google Scholar]
- Inaba K., Turley S., Yamaide F., Iyoda T., Mahnke K., Inaba M., Pack M., Subklewe M., Sauter B., Sheff D. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products by dendritic cells. J. Exp. Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsall B.L., Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer's patch. J. Exp. Med. 1996;183:237–247. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Cella M., Danieli C., Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartmentdownregulation by cytokines and bacterial products. J. Exp. Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzler C., Rovere P., Rescigno M., Granucci F., Penna G., Adorini L., Zimmermann V.S., Davoust J., Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor–dependent long-term cultures. J. Exp. Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V.A., Bratton D.L., Konowal A., Freed P.W., Westcott J.Y., Henson P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll R.E., Herrmann M., Roth E.A., Stach C., Kalden J.R., Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- Mevorach D., Zhou J.L., Song X., Elkon K.B. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J. Exp. Med. 1998;188:387–392. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchetti A., Rovere P., Iezzi G., Galati G., Heltai S., Protti M.P., Garancini M.P., Manfredi A.A., Rugarli C., Bellone M. Immunogenicity of apoptotic cells in vivorole of antigen load, antigen-presenting cells, and cytokines. J. Immunol. 1999;163:130–136. [PubMed] [Google Scholar]
- Zychlinsky A., Fitting C., Cavaillon J.-M., Sansonetti P.J. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri . J. Clin. Invest. 1994;94:1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]