Abstract
NY-ESO-1 is a member of the cancer-testis family of tumor antigens that elicits strong humoral and cellular immune responses in patients with NY-ESO-1–expressing cancers. Since CD4+ T lymphocytes play a critical role in generating antigen-specific cytotoxic T lymphocyte and antibody responses, we searched for NY-ESO-1 epitopes presented by histocompatibility leukocyte antigen (HLA) class II molecules. Autologous monocyte-derived dendritic cells of cancer patients were incubated with recombinant NY-ESO-1 protein and used in enzyme-linked immunospot (ELISPOT) assays to detect NY-ESO-1–specific CD4+ T lymphocyte responses. To identify possible epitopes presented by distinct HLA class II alleles, overlapping 18-mer peptides derived from NY-ESO-1 were synthetized and tested for recognition by CD4+ T lymphocytes in autologous settings. We identified three NY-ESO-1–derived peptides presented by DRB4*0101–0103 and recognized by CD4+ T lymphocytes of two melanoma patients sharing these HLA class II alleles. Specificity of recognition was confirmed by proliferation assays. The characterization of HLA class II–restricted epitopes will be useful for the assessment of spontaneous and vaccine-induced immune responses of cancer patients against defined tumor antigens. Further, the therapeutic efficacy of active immunization using antigenic HLA class I–restricted peptides may be improved by adding HLA class II–presented epitopes.
Keywords: HLA class II–restricted NY-ESO-1 epitopes, CD4+ T cell recognition
Introduction
Cancer-testis (CT) antigen NY-ESO-1, initially cloned by the SEREX (serological analysis of recombinant tumor cDNA expression libraries) approach from an esophageal cancer 1, elicits humoral and cellular immune responses in a high proportion of patients with NY-ESO-1–expressing cancers 2 3. This is in contrast to other defined tumor differentiation antigens (TAs), which either induce CTL responses but rarely antibody responses (e.g., melanocyte differentiation antigens), or are of generally low immunogenicity, eliciting cellular or humoral immune responses in <5% of patients with antigen-expressing cancers (MAGE-1 and -3 antigens; reference 2, and Jäger, E., unpublished data).
The high frequency of detectable antibody and CTL responses against NY-ESO-1 in patients with NY-ESO-1+ tumors indicates the presence of spontaneous CD4+ T cell responses, supporting the generation and maintenance of both NY-ESO-1–specific antibody and CTLs. To further analyze the role of CD4+ T cell responses in the specific recognition of NY-ESO-1, we searched for NY-ESO-1 epitopes presented by defined HLA class II alleles and recognized by HLA class II–matched CD4+ T lymphocytes of cancer patients. Monocyte-derived autologous dendritic cells incubated with recombinant NY-ESO-1 protein or lysates from an NY-ESO-1+ tumor cell line and overlapping 18-mer NY-ESO-1 peptides were used to detect peptide-specific CD4+ T cell responses in enzyme-linked immunospot (ELISPOT) and proliferation assays. We report here the identification of three new NY-ESO-1 epitopes recognized by CD4+ T lymphocytes of two melanoma patients in the context of HLA-DRB4*0101–0103.
Materials and Methods
Patients.
Seven HLA-A2+ patients with NY-ESO-1–expressing metastatic melanoma or soft tissue sarcoma were assessed for CD4+ T cell responses against recombinant NY-ESO-1 protein and HLA-DRB4*0101–0103-binding NY-ESO-1 peptides. All patients were positive for NY-ESO-1 serum antibodies in Western blotting and ELISA as described 2. Patient characteristics are presented in Table . The expression of NY-ESO-1 in tumor tissues was assessed by reverse transcriptase PCR as described 1.
Table 1.
| Patient | Disease | NY-ESO-1 mRNA | NY-ESO-1 antibody | HLA-A alleles | HLA-DR alleles |
|---|---|---|---|---|---|
| MZ19 | Melanoma | + | + | A2 | DRB1*0101 |
| NW14 | Melanoma | + | + | A2 | DRB1*0101, 0102, 0104 |
| DRB1*0801, 0803, 0806, 0810 | |||||
| NW29 | Melanoma | Unknown | + | A2 | DRB1*1501–1505; 1601–1603; 1605 |
| DRB1*0701 | |||||
| DRB4*0101–0103 | |||||
| DRB5*0101 | |||||
| NW37 | Melanoma | + | + | A2 | DRB1*1401; 1407–1408; 0901 |
| DRB3*0201–0203 | |||||
| DRB4*0101–0103 | |||||
| NW408 | Melanoma | + | + | A2 | DRB1*0101, 0102, 0104 |
| DRB3*0201–0202, 0301 | |||||
| DRB*1101, 1104, 1106, 1108–1113 | |||||
| NW690 | Sarcoma | + | + | A2 | DRB1*1501–1505; 1601–1603; 1605 |
| DRB1*0801; 0803; 0806; 0810 | |||||
| DRB5*0101 | |||||
| NW731 | Melanoma | + | + | A2 | DRB1*0101 |
| DRB1*1501–1505 | |||||
| NW38 | Melanoma | + | + | A2 | DRB1*0101; 0701 |
| DRB4*0101–0103 | |||||
| NW-MEL-38 | Melanoma | + | NA | A2 | DRB1*0101; 0701 |
| (cell line) | DRB4*0101–0103 |
NY-ESO-1 mRNA expression in tumor tissues, status of NY-ESO-1–specific serum antibody, and HLA typing of eight tumor patients and melanoma cell line NW-MEL-38. NA, not applicable.
Peptides.
To identify potential HLA class II–binding peptides, 28 peptides derived from NY-ESO-1 consisting of 18 amino acids with overlapping sequences were synthesized using an Abimed 422 multiple peptide synthesizer. Peptides were isolated and purified by repeated ether precipitations. The purity was determined by analytical reverse phase HPLC and proved to be at least 90%. Integrity of the peptides was determined by laser desorption time-of-flight mass spectrometry on a Lasermat mass spectrometer (Finnigan MAT).
Recombinant NY-ESO-1 and SSX Proteins.
NY-ESO-1 and SSX proteins were expressed in Escherichia coli as full-length proteins with a six-histidine tag on the NH2 terminus 2. The proteins were purified from washed and solubilized inclusion bodies by nickel chelate affinity chromatography (Chelating Sepharose FF; Amersham Pharmacia Biotech) using a pH gradient. NY-ESO-1 and SSX proteins were eluted in 8 M urea, 100 mM phosphate, 10 mM Tris, pH 4.5. The purified proteins were reactive with anti–ESO-1 and anti-SSX mAbs by Western blot analysis, and purity was >80% by SDS-PAGE.
Preparation of Tumor Cell Lysate.
1.5 × 106 NW-MEL-38 tumor cells were lysed in 50 μl H2O by several cycles of rapid freeze–thawing in liquid nitrogen.
Generation of Monocyte-derived Autologous APCs.
PBMCs, depleted of CD4+ and CD8+ T lymphocytes using magnetic beads (Minimacs; Miltenyi Biotec), were seeded in 24-well plates at 4 × 106 cells/well, and allowed to adhere to plastic for 24 h. Nonadherent cells were removed, and remaining cells were used as APCs and cultured with 1,000 U/ml GM-CSF (Leukomax; Sandoz) and 1,000 U/ml IL-4 (Pharma Biotechnologie Hannover) for 5 d in X-vivo 15 medium (BioWhittaker) at 2 ml/well.
For the assessment of HLA class II–restricted presentation of NY-ESO-1 epitopes after incubation of APCs with a lysate of the NY-ESO-1–expressing melanoma cell line NW-MEL-38, APCs were treated on day 6 of in vitro culture with 1,000 U/ml IL-4, 1,000 U/ml IL-6, 10 ng/ml IL-1β, 10 ng/ml TNF-α (IL-4, IL-6, IL-1β, and TNF-α obtained from Pharma Biotechnologie Hannover), 1,000 U/ml GM-CSF, and 1 μg/ml prostaglandin (Sigma Chemical Co.). On day 7, 20 μl of NW-MEL-38 tumor cell lysate was added to the APCs and cocultured for 40 h. APCs were then washed twice and used in ELISPOT assays at 3 × 104 cells/well.
Preparation of CD4+ T Lymphocytes.
CD4+ T lymphocytes were separated from PBMCs using magnetic beads (Minimacs; Miltenyi Biotec) according to the manufacturer's instructions and frozen until use. T cell fractions were confirmed to contain 98% CD4+ T lymphocytes by FACS® analysis.
Generation of Peptide-specific CD4+ T Cell Lines.
106 autologous irradiated APCs pulsed with DRB4*0101-0103–binding NY-ESO-1 peptides at 10 μg/ml were seeded in 24-well plates, and CD4+ T lymphocytes were added at 2.5 × 105 cells/well. Culture medium consisted of RPMI 1640 medium supplemented with 10% human serum, l-asparagine (50 mg/l), l-arginine (242 mg/l), l-glutamine (300 mg/l), IL-2 (2.5 ng/ml), and IL-4 (5 U/ml). CD4+ T cells were restimulated weekly with autologous APCs pulsed with the same peptides.
ELISPOT Assay.
APCs were harvested and pooled on day 5, and reseeded in 96-well flat-bottomed nitrocellulose plates (MAHAS4510; Millipore) at 3.5 × 104 cells/well. Before the addition of cells, plates had been coated overnight at 4°C with 5 μg/ml anti–IFN-γ antibody (Hoelzel Diagnostik). APCs were pulsed with NY-ESO-1 peptides at 4 μg/well, or recombinant NY-ESO-1 or SSX protein at 2 μg/well for control. APCs pulsed with NW-MEL-38 tumor cell lysate were used at 3 × 104 cells/well. Freshly isolated CD4+ T lymphocytes (105 cells/well) or peptide-specific CD4+ T cell lines (104 cells/well) were added in RPMI 1640 medium, supplemented with 10% human serum, l-asparagine (50 mg/l), l-arginine (242 mg/l), l-glutamine (300 mg/l) in a final volume of 100 μl. After incubation for 48 h (16 h for testing the tumor cell lysate) at 37°C in a water-saturated atmosphere, the plates were washed extensively (six times with a solution of 0.05% Tween 20/PBS) and supplemented with the biotinylated anti–IFN-γ detection antibody (Hoelzel Diagnostik) at 0.5 μg/ml. After incubation for 2 h at 37°C, plates were washed and developed with ABC-AP Vectastain (Vector Labs) for 1 h. After addition of substrate (BCIP/NPT) and 10 min of incubation, plates were prepared for microscopic counting of the blue spots. The number of blue spots per well was determined as the frequency of CD4+ T lymphocytes specifically recognizing NY-ESO-1–derived, HLA class II–restricted peptides, or the processed recombinant NY-ESO-1 or SSX protein. Controls were the reagents alone, effector cells alone (spontaneous IFN-γ release), and APCs alone. The difference between the numbers counted in peptide- or protein-stimulated versus nonstimulated wells was calculated as the effective number of peptide-specific T lymphocytes above background. In HLA class II blocking experiments, DR-specific antibody L243 was added at 2 μg/well before incubation of APCs with peptides, and antibody W6/32 was used at 2 μg/well as a control.
Proliferation Assay.
Autologous or allogeneic HLA-DRB4*0101–0103-matched irradiated APCs derived from melanoma patients NW29 and NW37 pulsed with peptides were plated in 96-well round-bottomed plates at 105 cells/well in RPMI 1640 medium supplemented with 10% human serum, l-asparagine (50 mg/l), l-arginine (242 mg/l), l-glutamine (300 mg/l), IL-2 (2.5 ng/ml), and IL-4 (5 U/ml), and CD4+ selected T lymphocytes were added at 5 × 104 cells/well. On day 13, after one restimulation with peptide-pulsed APCs on day 7, cultures were incubated with [3H]TdR (Amersham Pharmacia Biotech) at 0.5 μCi/well for 16 h. Cells were then collected, and thymidine incorporation was measured in a liquid scintillation counter.
Results
NY-ESO-1–specific CD4+ T Cell Responses.
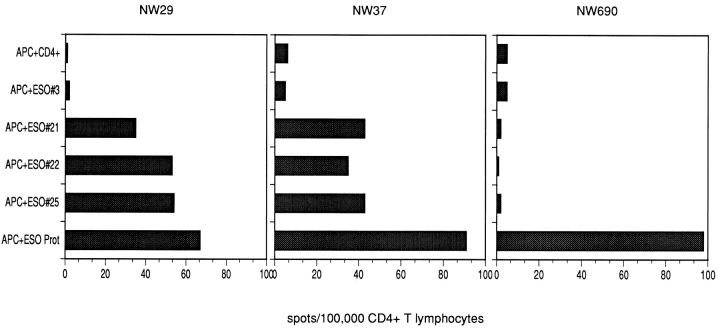
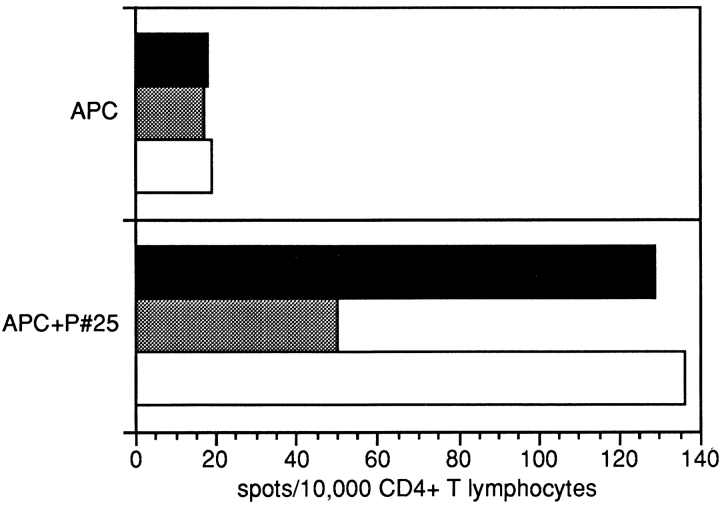
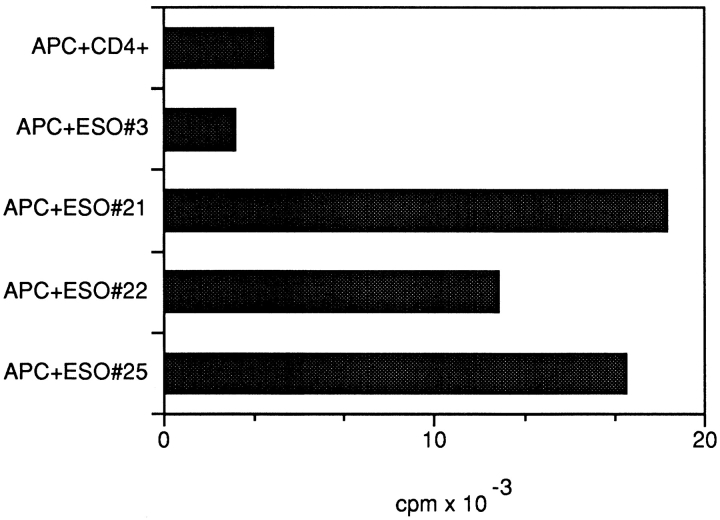
CD4+ T cell responses against autologous APCs pulsed with the recombinant NY-ESO-1 protein were observed in all patients with NY-ESO-1–expressing tumors. 28 overlapping 18-mer NY-ESO-1 peptides were tested for recognition by CD4+ T lymphocytes in the same assays. Two patients, NW29 and NW37, showed a similar pattern of recognition: with three NY-ESO-1–derived peptides (peptide 21, PLPVPGV- LLKEFTVSGNI, p115–132; peptide 22, VLLKEFTVSGNI- LTIRLT, p121–138; peptide 25, AADHRQLQLSISSCL- QQL, p139–156), significant numbers of IFN-γ spots were induced in at least three independent experiments (Fig. 1). Induction of peptide-specific IFN-γ spots was efficiently blocked with anti-DR antibody L243, suggesting that peptide recognition was DR-restricted (Fig. 2), whereas antibody W6/32 had no detectable blocking effect. ELISPOT results were confirmed in proliferation assays, which show specific proliferation against the same peptides (representative results are presented in Fig. 3). Since only two of seven patients displayed the same pattern of peptide recognition, it is suggested that the peptides recognized are presented by HLA class II alleles shared by both patients, which are DRB4*0101–0103 (Table ). Results presented here have been confirmed in at least two independent experiments.
Figure 1.
ELISPOT assays showing CD4+ T cell recognition of APCs pulsed with NY-ESO-1. Numbers of IFN-γ spots/100,000 CD4+ selected T lymphocytes are shown. CD4+ T cells were tested against autologous APCs, either alone or after pulsing with NY-ESO-1–derived peptides or recombinant NY-ESO-1 protein. Peptides tested were different NY-ESO-1–derived 18-mer peptides. Patients NW29 and NW37, who share the HLA-DRB4*0101–0103 alleles, show the same pattern of peptide recognition, whereas the HLA-DRB4* 0101–0103− patient NW690 shows recognition of NY-ESO-1 protein only, suggesting that peptides 21, 22, and 25 are presented by HLA-DRB4* 0101–0103.
Figure 2.
ELISPOT assays showing the blocking effect of HLA-DR–specific antibody L243 on CD4+ T cell recognition of NW29 APCs pulsed with the DRB4*0101–0103-binding NY-ESO-1 peptide 25. Experiments were performed as in the legend to Fig. 1, with L243 added at 2 μg/well to one set of experiments. HLA class I–binding antibody W6/32 was used at 2 μg/well as a control. Results showed a decrease of positive spots by >50% in the presence of L243 (gray bars), whereas W6/32 (white bars) did not affect the specific recognition of peptide 25.
Figure 3.
Standard [3H]TdR proliferation assay showing specific proliferative response of peptide-stimulated NW29 CD4+ T cells to autologous APCs alone or pulsed with the respective DRB4*0101–0103-restricted NY-ESO-1 peptides (21, 22, and 25).
Peptide-specific CD4+ T Cell Lines.
Of patients NW29 and NW37, peptide-specific CD4+ T cell lines were generated by repetitive stimulation with peptides 22 and 25. Fig. 4 shows the specific recognition of the stimulating peptide, the recombinant NY-ESO-1 protein, but not of the recombinant SSX protein used as a control.
Figure 4.
ELISPOT assay with autologous APCs pulsed with NY-ESO-1–derived peptide 22, and the recombinant NY-ESO-1 and SSX proteins. The CD4+ T cell line NW37 CD4/22 was used as effectors. Bars indicate the number of spots/104 NW37 CD4/22 cells. Values represent the results of three independent experiments.
Recognition of Naturally Processed NY-ESO-1 Epitopes by the Peptide-specific NW29 CD4/25 and NW37 CD4/22 T Cell Lines.
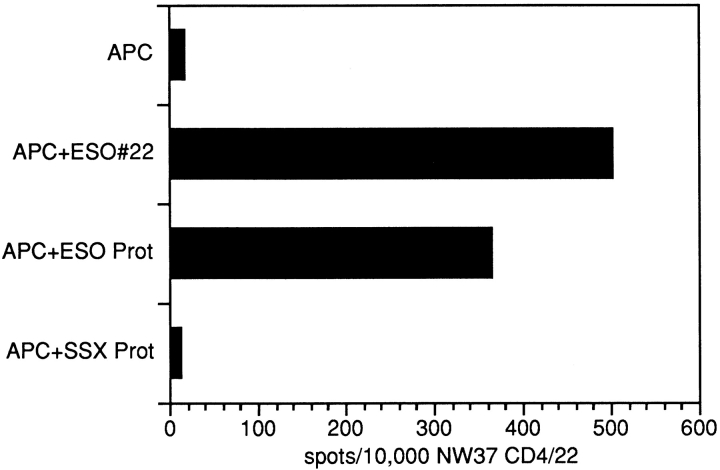
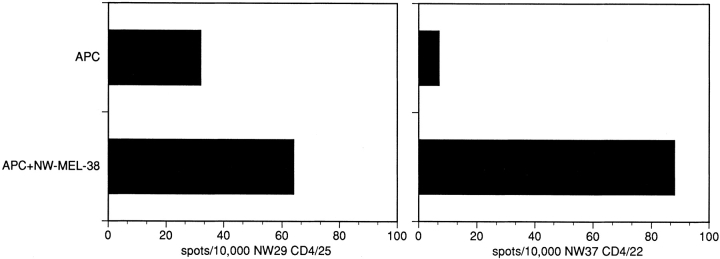
The CD4+ T cell lines NW29 CD4/25 and NW37 CD4/22 were generated by repetitive stimulation with the DRB4*0101–0103-restricted synthetic NY-ESO-1–derived peptides 25 and 22. To show that these CD4+ T cell lines also recognize naturally processed NY-ESO-1 epitopes in the context of HLA-DRB4*0101–0103, ELISPOT assays were performed with autologous APCs pulsed with a lysate of the NY-ESO-1–expressing tumor cell line, NW-MEL-38. The significant recognition of NW-MEL-38–pulsed APCs by both CD4+ T cell lines suggests that the NY-ESO-1–derived peptides 22 and 25 represent naturally processed epitopes (Fig. 5).
Figure 5.
ELISPOT assay with autologous APCs derived from patients NW29 and NW37 pulsed with NW-MEL-38 lysate and tested for specific recognition with the CD4+ T cell lines NW29 CD4/25 and NW37 CD4/22. Bars indicate the number of spots/104 NW37 CD4/22 cells.
Discussion
The CT antigen NY-ESO-1 appears to be highly immunogenic and is therefore regarded as a promising target for immunotherapeutic interventions in patients with NY-ESO-1+ cancers. NY-ESO-1 is expressed in different types of malignant tumors, e.g., melanoma, and breast, lung, head and neck, gastrointestinal tract, bladder, and prostate cancer 1. In contrast to other CTL-defined TAs, spontaneous humoral and cellular NY-ESO-1–specific immune responses can be observed in ∼50% of patients with advanced NY-ESO-1+ tumors 2 3, suggesting efficient activation of specific CD4+ and CD8+ T lymphocytes during cancer development. Much attention has been paid to CD8+ CTLs, regarded as the effectors that directly mediate lysis of tumor cells upon specific interaction with peptide–HLA class I complexes expressed on the tumor cell surface. Early clinical trials with HLA class I–restricted peptides derived from different TAs have shown tumor regressions in single patients, which could be correlated in some of the studies with a measurable increase of peptide-specific CTL reactivity in vivo 4 5 6. We recently succeeded in identifying HLA class I–restricted NY-ESO-1 peptide epitopes recognized by CTLs of cancer patients 3. These HLA class I–binding NY-ESO-1 epitopes are now used in clinical trials to evaluate their efficacy in the induction of peptide-specific CTL responses in vivo. However, active immunization may become more efficient if antigen-specific CD4+ T cell responses are stimulated simultaneously by antigen-specific HLA class II peptides, as suggested by several murine tumor models 7. Further, the role of CD4+ T cells in antitumor immunity can be explored in more detail using antigen-specific HLA class II peptide epitopes for monitoring antigen-specific immune responses in cancer patients.
We describe here the identification of HLA class II–restricted NY-ESO-1 peptides using the ELISPOT technique, a method that is sensitive enough to reflect the status of antigen-specific T cell immunity without prestimulation of effector cells in vitro for extended periods of time 8. To evaluate antigen-specific CD4+ T cell responses, we employed APCs derived from PBMCs, which are capable of efficiently processing extracellular proteins into the endogenous HLA class II presentation pathway. Our results show that CD4+ T cell responses against NY-ESO-1 protein processed by autologous APCs were observed in all seven patients examined. Of a panel of 18-mer NY-ESO-1 peptides, three were recognized by CD4+ T cells of two patients who shared the HLA-DRB4*0101–0103 alleles. Peptide-specific CD4+ T cell reactivity assessed in ELISPOT experiments was confirmed by standard proliferation assays. To further confirm the specificity of recognition, we established CD4+ T cell lines from two patients sharing the HLA-DRB4*0101–0103 alleles by repetitive stimulation with NY-ESO-1–derived peptides (peptides 22 and 25). These peptide-specific CD4+ T cell lines showed specific recognition of the stimulating peptides and the recombinant NY-ESO-1 protein, but not of the recombinant control protein SSX. The peptide-specific CD4+ T cell lines were further used to confirm the specificity against naturally processed NY-ESO-1–derived epitopes. A lysate of the NY-ESO-1–expressing melanoma cell line NW-MEL-38 was loaded onto autologous APCs, and specific recognition by both CD4+ T cell lines was shown. This observation suggests (a) that the NY-ESO-1–derived peptides identified here represent the naturally processed NY-ESO-1 epitopes, and (b) that the PBMC-derived APCs used in the ELISPOT experiments efficiently process and present antigenic peptides in the context of HLA class II.
Specific CD4+ T cell responses against different human tumor antigens have been described 9 10 11, some of which directly mediate lysis of tumor cells 12. However, the major role of CD4+ T cell immunity is believed to be in the regulation and coordination of different immune effector functions 13 14. Although the lytic capacity of adoptively transferred CD8+ CTLs is not further improved by coadministration of CD4+ T cells 15 16, it is well documented that the priming of a CTL response is significantly enhanced by CD4+ T cells specific for the same antigen 17. The helper effect of CD4+ T cells is dependent on the CD40 receptor–ligand interaction between APCs and antigen-specific CD4+ T cells 18 19. Signaling through CD40 ligation results in the release of proinflammatory cytokines, upregulation of adhesion and costimulatory molecules, and the production of IL-12 20 21. Therefore, immunization with peptides derived from TAs may lead to improved immune responses if HLA class II–binding epitopes stimulating CD4+ T cells are injected simultaneously with HLA class I–restricted epitopes derived from the same antigen.
The central role of CD4+ T cells for the induction and regulation of antigen-specific CTL responses is well established. Less is known about the significance of CD4+ T cells for the initiation of specific antibody responses. Since NY-ESO-1 elicits both cellular and humoral immune responses that can be detected simultaneously in cancer patients, it may be assumed that the function of CD4+ T cells is modulated towards a Th1 or Th2 type of immune response by different cytokine profiles in the tumor environment 22. In addition, the frequency and activity of macrophages, dendritic cells, and B lymphocytes functioning as potential APCs in tumor tissues may affect the type of Th response 23. We have found that the development of NY-ESO-1 antibody titers correlates with the clinical persistence of NY-ESO-1–expressing tumors 24. With the definition of HLA class II–binding peptides in NY-ESO-1, it is now feasible to investigate the specific role of CD4+ T cells induced by HLA class II–binding peptides, and its correlation to cellular and/or humoral immune responses to NY-ESO-1–expressing tumors in cancer patients.
Acknowledgments
The assistance of Claudia Wahle and Anja Melzer is greatly appreciated.
This work was supported by Krebsforschung Rhein-Main.
Footnotes
Abbreviations used in this paper: CT antigen, cancer-testis antigen; ELISPOT, enzyme-linked immunospot; TA, tumor antigen.
References
- Chen Y.-T., Scanlan M. J., Sahin U., Türeci Ö., Gure A.O., Tsang S., Williamson B., Stockert E., Pfreundschuh M., Old L.J. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc. Natl. Acad. Sci. USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E., Jäger E., Chen Y.-T., Scanlan M.J., Gout I., Karbach J., Arand M., Knuth A., Old L.J. A survey of the humoral response of cancer patients to a panel of human tumor antigens. J. Exp. Med. 1998;187:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger E., Chen Y.-T., Drijfhout J.W., Karbach J., Ringhoffer M., Jäger D., Arand M., Wada H., Noguchi Y., Stockert E. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1definition of human histocompatibility leukocyte antigen (HLA)-A2–binding peptide epitopes. J. Exp. Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger E., Bernhard H., Romero P., Ringhoffer M., Arand M., Karbach J., Ilsemann C., Hagedorn M., Knuth A. Generation of cytotoxic T cell responses with synthetic melanoma associated peptides in vivoimplications for tumor vaccines with melanoma associated antigens. Int. J. Cancer. 1996;66:162–169. doi: 10.1002/(SICI)1097-0215(19960410)66:2<162::AID-IJC4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Marchand M., van Baren N., Weynants P., Brichard V., Dreno B., Tessier M.-H., Rankin E., Parmiani G., Arienti F., Humblet Y. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int. J. Cancer. 1998;80:219–230. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A. Development of cancer immunotherapies based on identification of the genes encoding cancer regression antigens. J. Natl. Cancer Inst. 1996;88:1635–1644. doi: 10.1093/jnci/88.22.1635. [DOI] [PubMed] [Google Scholar]
- Ossendorp F., Menged E., Camps M., Filius R., Melief C.J.M. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II–negative tumors. J. Exp. Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Wölfel T., Heike M., Meyer zum Büschenfelde K.-H., Knuth A. Frequency analysis of tumor-reactive cytotoxic T lymphocytes in peripheral blood of a melanoma patient vaccinated with autologous tumor cells. Cancer Immunol. Immunother. 1994;39:93–99. doi: 10.1007/BF01525314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper R., Christian R.E., Gonzales M.I., Nishimura M.I., Gupta G., Settlage R.E., Shabanowitz J., Rosenberg S.A., Hunt D.F., Topalian S.L. Biochemical identification of a mutated human melanoma antigen recognized by CD4+ T cells. J. Exp. Med. 1999;189:757–765. doi: 10.1084/jem.189.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S.L., Rivoltini L., Mancini M., Markus N.R., Robbins P.F., Kawakami Y., Rosenberg S.A. Human CD4+ T cells specifically recognize a shared melanoma-associated antigen encoded by the tyrosinase gene. Proc. Natl. Acad. Sci. USA. 1994;91:9461–9465. doi: 10.1073/pnas.91.20.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S.L., Gonzales M.I., Parkhurst M., Li Y.F., Southwood S., Sette A., Rosenberg S.A., Robbins P.F. Melanoma-specific CD4+ T cells recognize nonmutated HLA-DR–restricted tyrosinase epitopes. J. Exp. Med. 1996;183:1965–1971. doi: 10.1084/jem.183.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manici S., Sturniolo T., Imro M.A., Hammer J., Sinigaglia F., Noppen C., Spagnioli G., Mazzi B., Bellone M., Dellabona P., Protti M.P. Melanoma cells present a MAGE-3 epitope to CD4+ cytotoxic T cells in association with histocompatibility leukocyte antigen DR11. J. Exp. Med. 1999;189:871–876. doi: 10.1084/jem.189.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D.M., Topalian S.L. The role of CD4+ T cell responses in antitumor immunity. Curr. Opin. Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- Greenberg P.D., Kern D.E., Cheever M.A. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+, 2− T cells. Tumor eradication does not require participation of cytotoxic T cells. J. Exp. Med. 1985;161:1122–1134. doi: 10.1084/jem.161.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast W.M., Bronkhorst A.M., de Waal L.P., Melief C.J.M. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. J. Exp. Med. 1986;164:723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief C.J.M. Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv. Cancer Res. 1992;58:143–175. doi: 10.1016/s0065-230x(08)60294-8. [DOI] [PubMed] [Google Scholar]
- Bennett S.R.M., Carbone F.R., Karamalis F., Miller J.F.A.P., Heath W.R. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett S.R.M., Carbone F.R., Karamalis F., Flavell R.A., Miller J.F.A.P., Heath W.R. Help for cytotoxic T cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Schoenberger S.P., Toes R.E.M., van der Voort E.I.H., Offringa R., Melief C.J.M. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Koch F., Stanzl U., Jennewein P., Janke K., Heufler C., Kämpgen E., Romani N., Schuler G. High level IL-12 production by murine dendritic cellsupregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Scheidegger D., Palmer-Lehmann K., Lane P., Lanzavecchia A., Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of IL-12 and enhances T cell stimulatory capacityT–T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hseih C.S., Macatonia S.E., Tripp C.S., Wolf S.F., O'Garra A., Murphy K.M. Development of Th1 CD4+ T-cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Qin Z., Richter G., Schüler T., Ibe S., Cao X., Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat. Med. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- Jäger E., Stockert E., Zidianakis Z., Chen Y.-T., Karbach J., Jäger D., Arand M., Ritter G., Old L.J., Knuth A. Humoral immune responses of cancer patients against “Cancer-Testis” antigen NY-ESO-1correlation with clinical events. Int. J. Cancer. 1999;84:506–510. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]