Abstract
Gram-negative enteric bacilli, such as Escherichia coli, are common causes of nosocomial pneumonia. The interaction between pulmonary neutrophils and the infecting pathogen is a critical step in determining the outcome. Previous studies from our laboratory, for which a rat model of pneumonia was used, established that pulmonary neutrophil recruitment was modulated by the E. coli virulence factors capsule and O-specific antigen. To begin to understand the mechanism by which this recruitment occurs, we conducted in vitro and ex vivo chemotaxis assays, for which we used a clinically relevant E. coli isolate (CP9) and isogenic derivatives that were deficient in only the O antigen (CP921) or capsule (CP9.137) as chemoattractants with or without the high-affinity N-formylmethionyl-leucyl-phenylalanine receptor antagonist N-tert-butoxycarbonyl-methionine-leucine-phenylalanine (N-t-BOC). Given that only live E. coli was used for the initial in vitro chemotaxis assays, it was predicted that only N-t-BOC-sensitive chemotaxis would occur. However, both N-t-BOC-sensitive and -insensitive chemotaxis was observed. N-t-BOC-insensitive chemotaxis was mediated in part by interleukin 8, which was produced by neutrophils that had migrated toward E. coli. N-t-BOC-insensitive chemotaxis was only observed when live E. coli bacteria, not cell-free E. coli culture supernatants, were used as chemoattractants, suggesting that a direct E. coli-neutrophil interaction was necessary. The presence of both capsule and O antigen diminished total, N-t-BOC-sensitive, and N-t-BOC-insensitive neutrophil chemotaxis in vitro. The presence of capsule significantly decreased total, N-t-BOC-sensitive, and N-t-BOC-insensitive neutrophil chemotaxis ex vivo when cell-free bronchoalveolar lavage fluid from infected rats was used as the source of chemotactic factors. These effects of E. coli capsule and O antigen on neutrophil chemotaxis are novel, and they expand our understanding of the mechanisms by which these virulence traits contribute to the pathogenesis of gram-negative pneumonia and other extraintestinal infections.
Gram-negative enteric bacilli, such as Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Enterobacter spp., are the most common cause of hospital-acquired pneumonia, having been implicated previously in 55 to 85% of cases (2-5, 7, 13). Although significant institutional variation occurs, E. coli is generally the third or fourth most commonly isolated enteric gram-negative bacillus from cases of nosocomial pneumonia, accounting for 5 to 8% of episodes in both U.S.- and European-based studies (4, 8, 15). Given that pneumonia cases caused by gram-negative bacilli in the United States are associated with 36,000 to 80,000 deaths annually and that the estimated cost is greater than 1 billion dollars, an improved understanding of the pathophysiology of this infection is desirable (3, 13, 29).
A long-term goal of our laboratory is to identify strategies to decrease the morbidity and mortality caused by gram-negative pneumonia. To begin to accomplish this, we have focused on host-pathogen interactions. Hosts and their cognate pathogens have been coadapting throughout evolutionary history. During this host-pathogen “chess match,” microbial pathogens have acquired a variety of virulence factors that are required for pathogenesis, whereas the host has evolved a coordinated response to infectious insults that is designed to minimize injury and to clear invading pathogens. The interaction between neutrophils and microbial pathogens in the lungs is a critical step in determining the outcome (14, 26).
Extracellular pathogens have evolved microbial virulence factors that not only protect against the bactericidal activity of neutrophils but also have the capability to modulate neutrophil pulmonary response, effector function, and turnover. Mechanisms described to date that affect neutrophil chemotaxis include proteolytic cleavage of chemoattractants (10) and neutrophil chemoattractant receptors (11), inhibition of selectin-mediated neutrophil adherence to endothelial cells (16), inhibition of G protein-linked signal transduction (9), and perhaps desensitization or competitive blockade of receptors for chemoattractants (6, 27).
Previously reported data from our laboratory have established that the capsule and the O-antigen moiety of lipopolysaccharide (LPS) modulate the recruitment of neutrophils into the pulmonary compartment after bacterial challenge (19). In the presence of capsule, an increased influx of neutrophils occurs, whereas O antigen attenuates neutrophil recruitment into the lungs (19). The mechanism by which these bacterial surface polysaccharides modulate pulmonary neutrophil response may be direct, such as by affecting the amount of shed bacteria-derived N-formylmethionyl-leucyl-phenylalanine (FMLP) or by altering the interaction of FMLP with the neutrophil's high-affinity (formyl peptide receptor [FPR]) or perhaps low-affinity (FPRL1) receptor for FMLP (12). Bacterial polysaccharides may also indirectly modulate the pulmonary neutrophil response, such as by altering the host's generation of chemoattractants (e.g., chemokines). Also possible is that the bacterial polysaccharides act via both of these mechanisms. An understanding of the mechanism(s) by which pulmonary pathogens modulate pulmonary neutrophil recruitment may contribute to the development of novel therapeutic approaches, including immune modulators, which may result in improved outcome in cases of gram-negative pneumonia.
In this study, in vitro chemotaxis assays and a rat model of gram-negative pneumonia were used for testing the following hypotheses: (i) capsule and O antigen directly modulate neutrophil chemotaxis mediated by FPR and (ii) chemotaxis mediated by the FPR is biologically important and contributes to the pulmonary neutrophil response in vivo.
MATERIALS AND METHODS
Bacterial strains.
A human bacteremic isolate of E. coli (CP9, O4/K54/H5) and isogenic derivatives thereof were used as model pathogens for these studies (20, 23). CP9 is a well-characterized extraintestinal pathogenic E. coli strain (21). It is a human blood isolate and is virulent in a variety of in vivo infection models (17, 22). This strain possesses a group 3 capsule (K54), O4-specific antigen, α-hemolysin, cytotoxic necrotizing factor, the IroN siderophore receptor, P pilus (class I PapG adhesin), Prs pilus (class III PapG adhesin), type 1 pilus; the strain is also serum resistant. Transposon mutagenesis (TnphoA for capsule genes and TnphoA′1 for O-antigen genes) was used to generate proven isogenic derivatives of CP9 that are deficient in the O4 O antigen alone (CP921) or in the K54 capsule alone (CP9.137) (20, 23). A combination of T4 transduction of the transposon insertions from the originally identified mutants back into their wild-type parent (CP9) and Southern blot analysis of the original mutants with the transductant derivatives was used to establish that the capsule and/or O-antigen phenotypes were due to a single transposon insertion (20, 23). Sequence analysis established the precise location of the transposon insertions in the expected capsule and O-antigen gene clusters (23, 24). As a result of their locations within these gene clusters, polar effects of the transposon insertions affected only the genes that are involved in the biosynthesis, transport, and assembly of capsule or O antigen (23, 24, 28). The phenotype of CP9.137 and CP921 has been shown to be stable, and revertants have never been identified (17, 23). Nonetheless, random isolates from in vitro and in vivo experiments underwent analyses of capsule and LPS, which confirmed their phenotypic stability.
Preparation of E. coli and E. coli supernatants for use in chemotaxis assay.
E. coli cultures were grown overnight in M9 minimal medium containing 0.5% glucose and that was treated with Chelex to remove any trace Fe present (18). This minimal medium simulates the growth environment in vivo, an environment in which iron and other nutrients are limiting. Pilot studies established that the chemoattractant properties of log- and stationary-phase bacteria were similar; therefore, bacteria from overnight cultures were used for the chemotaxis assays. When bacterial supernatant was used for chemotaxis assays, it was generated fresh for each experiment by diluting an overnight bacterial culture with Chelex-treated M9 minimal medium so that 350 μl contained approximately 107 CFU of E. coli. Next, bacterial cells were removed by centrifugation (twice at 8,000 × g) followed by passage through a 45-μm-pore-size syringe filter (Acrodisc; Gelman Sciences, Ann Arbor, Mich.). Supernatant used for chemotaxis assays was confirmed by culture to be free of bacteria.
Determination of bacterial titers.
The titers of bacteria used for the pulmonary challenge inocula and in the in vitro chemotaxis assays were determined by performing serial 10-fold dilutions as described previously (19). The total pulmonary bacterial titer at the time of harvest was calculated by determining the bacterial titer in both bronchoalveolar lavage (BAL) fluid and lung homogenate, taking into account the various dilutions performed.
Pulmonary infection model.
Animal studies were reviewed and approved by the Veterans Administration Western New York Healthcare System Institutional Animal Care and Use Committee. An established rat model for studying pulmonary damage was used as reported previously (19). In brief, Long-Evans rats (weight, 300 to 400 g) were anesthetized with 3% halothane in 100% oxygen until unconscious; anesthesia levels were then maintained at 1.5% halothane. The trachea was surgically exposed for direct instillation of the bacterial inoculum. Challenge inocula of (approximately 1.0 × 107 CFU) of CP9 (wild type), CP921 (O-specific antigen deficient), and CP9.137 (capsule deficient) were prepared in normal saline and introduced intratracheally (1.2 ml/kg) via a 1-ml syringe and 26-gauge needle. Control animals were challenged with normal saline alone.
BAL and lung harvest for use in chemotaxis assay.
One hour after challenge, animals were reanesthetized. A midline incision was made from the abdomen up through the neck. After transecting the vena cava, the pulmonary vasculature was flushed by slowly injecting 20 ml of warm (37°C) Hanks' balanced salt solution into the right ventricle. A 16-gauge steel cannula was inserted into the trachea and secured with a suture. BAL was performed by instilling a total of 50 ml of normal saline into the lungs by force of gravity, and the recovered BAL fluid was placed on ice. A 500-μl aliquot of BAL fluid was removed for bacterial titer analysis. The remaining BAL fluid was centrifuged at 6,750 × g for 10 min at 4°C, the cell-free supernatant was removed, and aliquots were dispensed and stored at −80°C. The BAL fluid used for chemotaxis assays was confirmed by culture to be free of bacteria. Pilot studies established that fresh and frozen BAL fluid possessed similar chemoattractant properties; therefore, previously frozen processed BAL fluid was used. Postlavage, the lungs were resected, suspended in normal saline to a total weight of 10 g (assumed to be equivalent to 10 ml), and homogenized on ice three times for 3 s by using a Polytron PT-2000 homogenizer (Brinkman Instruments, Westbury, N.Y.). The bacterial titer of this lung homogenate was determined.
Chemotaxis assay.
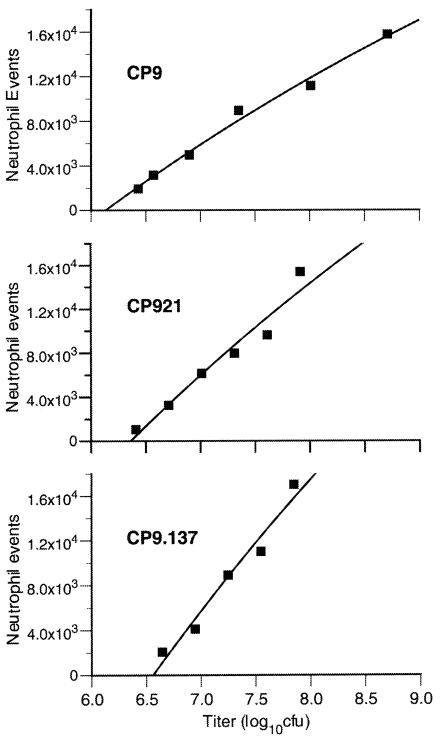
Human blood was collected from healthy individuals into endotoxin-free tubes containing heparin. Neutrophils were purified using Polymorphprep (AXIS-SHIELD PoC AS, Oslo, Norway), and chemotaxis was assessed with a 24-well plate fitted with nylon membrane well inserts (3.0-μm pore size; Transwell-Clear, Costar; Corning Inc., Corning, N.Y.). A total of 5 × 105 neutrophils in 100 μl of 20% autologous plasma-phosphate-buffered saline (pH 7.4) were placed in the top well, and 350 μl of chemotaxis incubation buffer (Migratest; ORPEGEN Pharma, Heidelberg, Germany) containing either approximately 107 CFU of live bacteria (in vitro), supernatant from bacterial culture (in vitro), or 350 μl of BAL fluid (ex vivo) was placed in the lower well. After incubation for 30 min at 37°C, 105 sulforhodamine-impregnated polystyrene beads (diameter, 6 μm) (Polysciences, Inc., Warrington, Pa.) and a cell-permeant, far red-emitting DNA fluorescent staining solution (Migratest; ORPEGEN Pharma) were added to the bottom-well cell suspension. Neutrophil chemotaxis was determined by assessing the number of neutrophils in the bottom well suspension with a FACSCalibur flow cytometer (Becton Dickinson Biosciences Immunocytometry Systems, San Jose, Calif.). Fluorescence emissions of >670 nm (i.e., from sulforhodamine beads and neutrophils containing stained DNA), resulting from 488-nm argon ion laser excitation, were measured by the FL3 detector. Data acquisition for each sample was terminated after 2,000 bead events had been detected. This assured that an equal volume of cell suspension was analyzed for each sample, since each sample contained the same bead concentration. A gate in the FL3 versus forward-scatter plot discriminated neutrophils and beads from debris, and a subsequent back-gating of the side-scatter versus forward-scatter plot discriminated the neutrophils from the beads. Pilot experiments performed with whole, live bacteria established that measurable neutrophil chemotactic events were not significantly greater after incubation times of 60 and 120 min. Titration experiments established that the chemotaxis assay for CP9 (wild type), CP921 (O-antigen deficient), and CP9.137 (capsule deficient) was linear within the range of approximately 3 × 106 to 5 × 108 CFU with E. coli in the lower well used as the chemoattractant (Fig. 1). To confirm that chemotaxis, not chemokinesis, was responsible for neutrophil migration, experiments were performed with 107 CFU of either CP9, CP921, CP9.137, or chemotaxis incubation buffer in the top well. Measured numbers of neutrophil events were 1,496, 658, 1,389, and 359 for CP9, CP921, CP9.137, and incubation buffer, respectively, compared to 20,468 events that occurred in response to FMLP (50 nM) in the bottom well. This demonstrated that a maximum of 7% of the neutrophil migration was due to chemokinesis. To account for neutrophil variability from experiment to experiment, chemoattractant stimuli (e.g., bacterial strains, supernatant, and BAL) were evaluated in parallel. To exclude monocyte contamination (or differential activation) as a potential confounding variable, interleukin 1β (IL-1β) and tumor necrosis factor alpha (TNF-α) were measured in the upper and lower wells but were not detected. Incubation buffer alone or FMLP (50 nM) added to the bottom well served as negative and positive controls, respectively. Total neutrophil chemotaxis was expressed as the ratio of neutrophils that migrated to the lower well (corrected for the negative control) to the log of the measured bacterial CFU placed in the lower well (chemotaxis index). When culture supernatant was used as the chemoattractant, neutrophil chemotaxis was expressed as the ratio of neutrophils that migrated to the lower well to the log of the E. coli count (log10 CFU) that produced the supernatant placed in the lower well. When ex vivo BAL fluid was used as the chemoattractant, neutrophil chemotaxis was expressed as the ratio of neutrophils that migrated to the lower well to the log of the given BAL-specific total lung E. coli titer at the time of harvest at 1 h (lung homogenate plus BAL [log10 CFU]). N-tert-butoxycarbonyl-methionine-leucine-phenylalanine (N-t-BOC)-insensitive chemotaxis was expressed as the ratio of neutrophils that migrated to the lower well (corrected for the negative control) to the log of the measured bacterial CFU placed in the lower well (chemotaxis index) in the presence of N-t-BOC, an antagonist of the high-affinity FPR (ICN Biomedicals, Aurora, Ohio) (direct measurement). The N-t-BOC-sensitive chemotaxis index was deduced by subtracting the N-t-BOC-insensitive chemotaxis index from the total chemotaxis index (measured in the absence of N-t-BOC).
FIG. 1.
Linearity of the in vitro neutrophil chemotaxis assay in response to live E. coli CP9 (wild type), CP921 (O-antigen deficient), and CP9.137 (capsule deficient). A total of 5 × 105 purified human neutrophils was placed in the upper well of a chemotaxis chamber, with various titers of CP9, CP921, and CP9.137 placed in the lower chamber. Neutrophils that migrated into the lower chamber (following incubation at 37°C for 30 min) in response to the E. coli were quantitated by flow cytometry. Results demonstrate that this chemotaxis assay was linear from 3 × 106 to 1 × 108 CFU of E. coli (R2 = 0.97, 0.95, and 0.96 for CP9, CP921, and CP9.137, respectively).
Cytometric bead array quantification of cytokines and chemokines.
Soluble concentrations of IL-1β, TNF-α, and IL-8 were measured with a multiplex microsphere assay (Luminex Corporation, Austin, Tex.). Antibody pairs (unconjugated capture antibody and biotinylated reporter antibody) and recombinant cytokines (standards) used in the assay were obtained from R&D Systems (Minneapolis, Minn.). A 100-μl aliquot of fluid from the upper or lower chemotaxis wells was added to 5 μl of multiplexed colored beads (each color corresponding to a specific cytokine), which contained about 500 capture antibody-coated beads for each cytokine in a well of a 96-well, 0.45-μm-pore-size filter plate (Millipore, Bedford, Mass.). Use of the filter plate allowed for efficient washing and rapid throughput of the assay. After incubating for 30 min at room temperature, the beads were washed with a total of 3 ml per well of PBS plus 0.02% Tween 20 plus 0.1% bovine serum albumin plus 0.02% NaN3. The beads were then incubated, as described above, with a cocktail of the appropriate biotinylated reporter antibodies and washed again as described above. Finally, phycoerythrin-conjugated avidin was added and incubated as described above, and the beads were washed. Data were acquired by using a Luminex 100 cytometer (Luminex Corporation) that discriminates the different colored beads and measures the mean fluorescence intensity of phycoerythrin in each bead region (which corresponds to a specific cytokine or chemokine). Concentrations were determined by using the power function generated from the trend line (R2 = 0.98) of the linear portion of a standard curve for each cytokine or chemokine.
Statistical analysis.
Normally distributed data were analyzed with either a two-tailed paired or unpaired t test. All comparisons were made between CP9 (wild type) and its isogenic derivatives that were deficient in capsule (CP9.137) or O antigen (CP921). Data are presented as the means ± the standard errors of the means (SEM), and P values of <0.05 were considered statistically significant.
RESULTS
The in vitro neutrophil chemotaxis assay measures N-t-BOC-sensitive and N-t-BOC-insensitive chemotaxis.
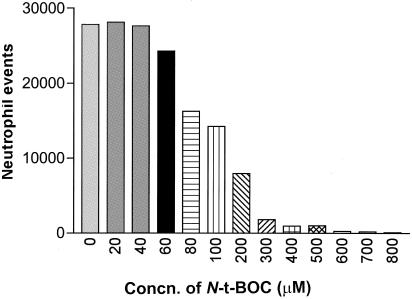
We had previously established that the virulence factors, capsule and O antigen, from an extraintestinal pathogenic E. coli strain (CP9) modulated pulmonary neutrophil influx (19). To begin to decipher the mechanism by which this occurred, an in vitro chemotaxis assay, for which live E. coli were used, was carried out to determine if capsule or O antigen modified the direct bacterial effect on chemotaxis. Given the fact that the only known neutrophil chemoattractant derived from E. coli is FMLP, it was postulated that the addition of N-t-BOC (ICN Biomedicals), an antagonist of the high-affinity FPR, to the chemotactic assay would inhibit chemoattractant activity. First, however, a titration of N-t-BOC with neutrophils in the presence of FMLP stimulant (50 nM) had to be done to determine the amount of N-t-BOC required to inhibit >95% of neutrophil chemotaxis. As can be seen in Fig. 2, concentrations of N-t-BOC ranging from 400 to 500 μM were required under the conditions of the chemotactic assay being used.
FIG. 2.
Inhibition of FMLP-mediated chemotaxis by N-t-BOC. A total of 5 × 105 purified human neutrophils was placed in the upper well of a chemotaxis chamber with various concentrations of N-t-BOC (0 to 800 μM) and an antagonist of the high-affinity FPR. FMLP (50 nM) was placed in the lower chamber. Greater than 95% inhibition of FMLP-mediated chemotaxis occurred with the use of concentrations of N-t-BOC that were greater than 400 μM.
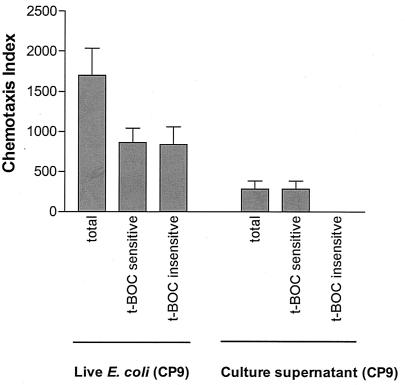
Unexpectedly, when wild-type live E. coli (CP9) was employed in a similar assay in the presence and absence of 500 μM N-t-BOC, the decrease in the amount of neutrophil chemotaxis observed was only 51% (n = 14) (Fig. 3). It was clear from this experiment that there was an additional chemotactic factor or factors responsible for the neutrophil chemotactic response to live E. coli, and it was hypothesized that an assay involving supernatants derived from the culture of E. coli (CP9) might prove useful in identifying any such factor(s). As a first step in this process, the same assay in which live wild-type E. coli (CP9) bacteria were employed was used to determine if culture supernatant from live wild-type E. coli, instead of the live E. coli bacteria themselves, would have the same effect. The results from these experiments are shown in Fig. 3. Clearly, supernatant from the culture of live wild-type E. coli was less chemotactic, but in contrast to what was observed with live E. coli, all of the chemotactic activity was sensitive to N-t-BOC. These results demonstrate that N-t-BOC-insensitive chemotaxis requires the presence of whole live bacteria. This finding suggests that a bacterium-neutrophil interaction is required for N-t-BOC-insensitive chemotaxis in this in vitro assay. In contrast, N-t-BOC-sensitive neutrophil chemotaxis was observed both when whole, live E. coli (CP9) bacteria and cell-free CP9 culture supernatant were used as the chemoattractants (Fig. 3). This suggests that, at least in part, shed E. coli components induce N-t-BOC-sensitive neutrophil chemotaxis.
FIG. 3.
In vitro chemotaxis of human neutrophils in response to live E. coli CP9 (wild type) and cell-free culture supernatant from CP9. The total deduced N-t-BOC-sensitive and the N-t-BOC-insensitive chemotaxis indices are depicted (see Materials and Methods for details on these indices). When the positive control (FMLP, 50 nM) with or without N-t-BOC was used as the chemoattractant, neutrophil chemotaxis was expressed as numbers of neutrophil events. The neutrophil response to the positive control (FMLP, 50 nM) was 30,920 ± 2,733 (mean ± SEM), of which 95% ± 1.5% was inhibited by N-t-BOC (500 μM) (number of samples, 14; data not shown on graph). Neutrophil chemotaxis toward the wild-type E. coli strain was both N-t-BOC sensitive and insensitive. In contrast, only N-t-BOC-sensitive chemotaxis was observed toward cell-free culture supernatant from the wild-type E. coli strain.
In vitro N-t-BOC-insensitive neutrophil chemotaxis in response to live wild-type E. coli (CP9) is mediated in part by IL-8.
Neutrophils have been reported to produce IL-8 (25, 30). Therefore, we hypothesized that the observed N-t-BOC-insensitive neutrophil chemotaxis in response to E. coli (CP9 present in the bottom well) was due, at least in part, to IL-8 production by neutrophils that had already migrated into the bottom well. To test this, IL-8, TNF-α, and IL-1β were measured in the top and bottom wells of the chemotaxis chamber. The results are summarized in Table 1. IL-1β was not detected, and TNF-α levels were negligible. The fact that monocytes are potent producers of TNF-α strongly suggests that monocytes were not contaminating the purified neutrophils and therefore were not confounding the observed results. In contrast, while IL-8 was present in the top well, considerably higher IL-8 levels (4.5- to 11-fold) were measured in the bottom well (Table 1). These data support the contention that neutrophils that had moved into the bottom well containing E. coli were producing IL-8, but not TNF-α, and IL-1β, which in turn served as a chemoattractant for N-t-BOC-insensitive chemotaxis. Whether the measurable levels of IL-8 in the top well were due to diffusion from the bottom well or to the low level of production from neutrophils that had not yet moved into the bottom well is unclear.
TABLE 1.
Measurement of TNF-α and IL-8 levels in an in vitro chemotaxis assay
| Chemoattractanta | Level ofb:
|
|||
|---|---|---|---|---|
| TNF-α
|
IL-8
|
|||
| Top well | Bottom well | Top well | Bottom well | |
| CP9 (wild type) | 0.8 ± 0.8 | 2.9 ± 0.3 | 343 ± 78.9 | 1,559 ± 84.7 |
| CP921 (O4-antigen deficient) | 0.0 ± 0.0 | 1.8 ± 0.9 | 159 ± 19.5 | 1,182 ± 50.9 |
| CP9.137 (capsule deficient) | 0.6 ± 0.6 | 2.9 ± 0.2 | 232 ± 61.4 | 2,543 ± 175 |
Live bacteria (107 CFU) from the indicated E. coli strains were used.
Values are given in picograms per milliliter.
To confirm that the IL-8 present in the lower wells of the chemotaxis chamber was responsible, at least in part, for the N-t-BOC-insensitive chemotaxis (Fig. 3), neutrophil chemotaxis in response to CP9 (wild type) was measured in the presence and absence of IL-8 antibodies (25 μg/ml in the lower well) or of the FPR antagonist N-t-BOC (500 μM). Antibody titration experiments were performed to confirm that this concentration of antibody maximally inhibited IL-8 (26 mM)-induced neutrophil chemotaxis (data not shown). In seven independent experiments, the presence of anti-IL-8 antibodies inhibited 23% ± 11% (mean ± SEM) of N-t-BOC-insensitive chemotactic events in response to live CP9 (wild type). In these experiments, IL-8 antibodies inhibited a mean of 92% ± 2% of total chemotactic events in response to purified IL-8. Chemoattractant strains CP921 and CP9.137 had inhibition levels of N-t-BOC-insensitive chemotaxis by anti-IL-8 antibodies of 29% ± 18% and 71% ± 26%, respectively. Taken together with detection of IL-8 in the lower wells, as described above, these results establish that IL-8 is responsible in part for N-t-BOC-insensitive neutrophil chemotaxis in response to live E. coli.
Effects of E. coli's capsule and O antigen on neutrophil chemotaxis in vitro.
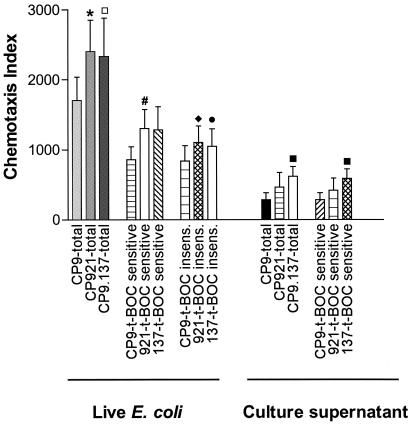
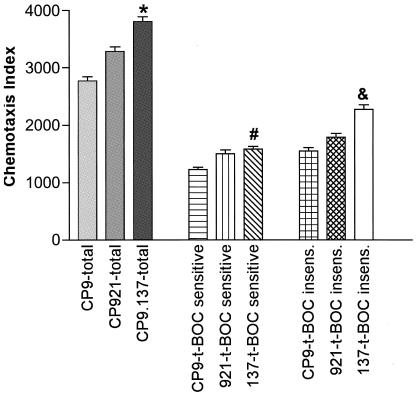
In vivo data from a rat model of gram-negative pneumonia demonstrated that the capsule (K54) and O antigen (O4) from an extraintestinal pathogenic isolate of E. coli (CP9) modulated the influx of neutrophils into the pulmonary compartment. The described in vitro chemotaxis assay was performed, with live E. coli organisms used as chemoattractants, to determine whether O antigen and/or capsule affected the chemoattractant properties of E. coli. Neutrophil chemotaxis was measured for live CP9 (wild type) (n = 14), CP921 (O-antigen deficient) (n = 14), and CP9.137 (capsule deficient) (n = 14) (Fig. 4). Total chemotaxis was significantly decreased toward the wild-type E. coli strain that possessed an O antigen and capsule (CP9; 1,700 ± 336 [mean ± SEM]) compared to the isogenic O-antigen-deficient derivative (CP921; 2,404 ± 451 [mean ± SEM]; P = 0.002, paired t test) and the total chemotaxis trended toward being significantly decreased compared to the isogenic capsule-deficient derivative (CP9.137; 2,330 ± 552 [mean ± SEM]; P = 0.10). These data demonstrated that in the presence of bacterial O antigen and capsule, the overall effect was that neutrophils were less likely to migrate toward wild-type E. coli in vitro. However, given that we had demonstrated that in our in vitro chemotaxis assay, live E. coli induced both N-t-BOC-sensitive and N-t-BOC-insensitive neutrophil chemotaxis, it was necessary to establish whether O antigen and capsule attenuated one or both of these mechanisms for neutrophil chemotaxis.
FIG. 4.
In vitro chemotaxis of human neutrophils in response to the live E. coli strains CP9 (wild type), CP921 (O-antigen deficient), and CP9.137 (capsule deficient) and to cell-free culture supernatant from CP9, CP921, and CP9.137. The total deduced N-t-BOC-sensitive and the N-t-BOC-insensitive chemotaxis indices are depicted (see Materials and Methods for details on these indices). The neutrophil response to the positive control (FMLP, 50 nM) was 30,920 ± 2,733 (mean ± SEM), of which 95% ± 1.5% was inhibited by N-t-BOC (500 μM) (n = 14; data not shown in graph). Total chemotaxis was significantly decreased toward the wild-type E. coli strain that possessed an O antigen and capsule (CP9) compared to the isogenic O-antigen-deficient derivatives (CP921) (*, P = 0.002 compared to CP9, paired t test; n = 14 for each strain), and total chemotaxis trended toward being significantly decreased compared to the isogenic capsule-deficient derivative (CP9.137) (□, P = 0.10). Neutrophil chemotaxis toward live E. coli strains was both N-t-BOC sensitive and insensitive. N-t-BOC-sensitive chemotaxis was significantly decreased toward the live, wild-type E. coli strain (CP9) that possessed an O antigen compared to an isogenic O-antigen-deficient derivative (CP921) (#, P = 0.03 compared to CP9, paired t test; n = 14 for each strain). N-t-BOC-insensitive chemotaxis was significantly decreased toward the live, wild-type E. coli strain (CP9) that possessed an O antigen compared to an isogenic O-antigen-deficient derivative (CP921) (⧫, P = 0.008 compared to CP9, paired t test; n = 14 for each strain), and N-t-BOC-insensitive chemotaxis trended toward being significantly decreased compared to the isogenic capsule-deficient derivative (CP9.137) (•, P = 0.052). Neutrophil chemotaxis toward cell-free culture supernatants was primarily N-t-BOC sensitive (100, 97.4, and 96% for CP9, CP921, and CP9.137, respectively); therefore, N-t-BOC-insensitive chemotaxis toward cell-free culture supernatants is not shown. Total and N-t-BOC-sensitive chemotaxis was significantly decreased toward cell-free supernatant from the wild-type stain (CP9) that possessed a capsule compared to an isogenic capsule-deficient derivative (CP9.137) (▪, P = 0.003, paired t test; n = 5 for each supernatant).
In vitro N-t-BOC-sensitive neutrophil chemotaxis is decreased toward live wild-type E. coli (CP9) compared to an isogenic O-antigen-deficient derivative (CP921).
It was hypothesized that capsule and O antigen decreased host recognition of FMLP by diminishing the shedding or the biologic availability of bacterial formylated peptides. To test this hypothesis, in vitro chemotaxis assays were performed with live CP9 (wild type) (n = 14), CP921 (O-antigen deficient) (n = 14), and CP9.137 (capsule deficient) (n = 14) strains used as chemoattractants, in the presence and absence of the FPR antagonist N-t-BOC (Fig. 4). N-t-BOC-sensitive neutrophil chemotaxis (i.e., neutrophil events inhibited in the presence of the FPR antagonist N-t-BOC) was significantly increased by 51% (P = 0.03, paired t test) when an isogenic O-antigen-deficient derivative was used as a chemoattractant (CP921; 1,300 ± 270 [mean ± SEM]) compared to its wild-type parent (CP9; 861 ± 182 [mean ± SEM]). N-t-BOC-sensitive neutrophil chemotaxis was increased by 49%, but was not statistically significant (P = 0.17), when an isogenic capsule-deficient derivative was used as a chemoattractant (CP9.137; 1,280 ± 334 [mean ± SEM]) compared to its wild-type parent (CP9; 861 ± 182 [mean ± SEM]). These data demonstrate that E. coli's O antigen diminishes N-t-BOC-sensitive neutrophil chemotaxis in vitro, which is primarily mediated through the FPR.
In vitro N-t-BOC-sensitive neutrophil chemotaxis is decreased toward cell-free culture supernatant from wild-type E. coli (CP9) compared to an isogenic capsule-deficient derivative (CP9.137).
To gain insight as to the mechanism by which E. coli's O antigen and/or capsule decreased N-t-BOC sensitive-neutrophil chemotaxis, in vitro chemotaxis was performed, with cell-free culture supernatants of CP9 (wild type), CP921 (O-antigen deficient), and CP9.137 (capsule deficient) as chemoattractants in the presence and absence of N-t-BOC (Fig. 4). In five independent experiments, N-t-BOC-sensitive neutrophil chemotaxis was significantly increased by 108% (P = 0.003, paired t test) when an isogenic capsule-deficient derivative was used as a chemoattractant (CP9.137; 591.2 ± 133.4 [mean ± SEM]) compared to its wild-type parent (CP9; 284.8 ± 98.3 [mean ± SEM]). N-t-BOC-sensitive neutrophil chemotaxis was increased by 48%, but was not statistically significant (P = 0.38), when an isogenic O-antigen-deficient derivative was used as a chemoattractant (CP921; 422.2 ± 174 [mean ± SEM]) compared the chemotaxis of its wild-type parent (CP9; 284.8 ± 98.3 [mean ± SEM]). These results support the supposition that N-t-BOC-sensitive neutrophil chemotaxis is mediated, at least in part, by shed E. coli components and demonstrate that E. coli's capsule diminishes N-t-BOC-sensitive neutrophil chemotaxis in vitro when cell-free culture supernatants were used as chemoattractants.
Cell-free culture supernatant from wild-type E. coli (CP9) does not inhibit N-t-BOC-sensitive chemotaxis induced by culture supernatants from isogenic O-antigen-deficient (CP921) or capsule-deficient (CP9.137) derivatives.
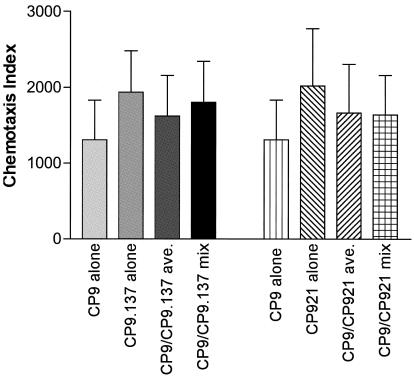
Possible mechanisms by which neutrophil chemotaxis was decreased in the presence of capsule and O antigen included (i) cell-associated capsule or O antigen decreased the shedding of FMLP, (ii) free (shed) capsule or O antigen biologically inactivated free FMLP, or (iii) free capsule or O antigen down-regulated FPR or its signal transduction pathway. To begin to resolve these possibilities, in vitro chemotaxis assays in the presence and absence of N-t-BOC were performed with cell-free culture supernatants of CP9 (wild type), CP921 (O-antigen deficient), and CP9.137 (capsule deficient) used as chemoattractants alone (relative chemoattractant activity, 1.0) as well as mixtures of cell-free culture supernatants from CP9-CP921 and CP9-CP9.137 (relative chemoattractant activity, 0.5 from each strain for a total activity of 1.0) (Fig. 5). It was predicted that if free capsule or O antigen biologically inactivated FMLP or down-regulated FPR or its signal transduction pathway, then the chemotaxis index measured when supernatant mixtures (CP9-CP921 or CP9-CP9.137) were tested would be less than the average index of CP9 plus CP921 and CP9 plus CP9.137 when supernatant from CP9, CP9.137, or CP921 was tested alone. However, in two independent experiments in which similar results were obtained, these findings were not observed (Fig. 5). Therefore, these results do not support a model for which shed capsule and/or O antigen inhibit the biologic activity of FMLP or down-regulate FRR or its signal transduction pathway. These results indirectly support a model for which decreased shedding of FMLP is the most likely mechanism by which E. coli's capsule and O antigen decreases N-t-BOC-sensitive neutrophil chemotaxis.
FIG. 5.
Total in vitro chemotaxis of human neutrophils in response to cell-free culture supernatant from CP9 (wild type), CP921 (O-antigen deficient), and CP9.137 (capsule deficient) alone and in response to the mixture of supernatants from CP9 plus CP921 and CP9 plus CP9.137. Neutrophil chemotaxis was similar (not less) in response to the mixtures of supernatants from CP9 plus CP921 and CP9 plus CP9.137 compared to when the chemotactic activity due to supernatant from CP9 and CP921 alone and CP9 and CP9.137 alone was averaged (ave.) (n = 2).
In vitro N-t-BOC-insensitive neutrophil chemotaxis is decreased toward live wild-type E. coli (CP9) compared to isogenic O-antigen- and capsule-deficient derivatives (CP921 and CP9.137, respectively).
To determine if O antigen and/or capsule modulated N-t-BOC-insensitive chemotaxis in vitro, chemotaxis assays were performed, with live CP9 (wild type), CP921 (O-antigen deficient), and CP9.137 (capsule deficient) used as chemoattractants, in the presence and absence of the FPR antagonist N-t-BOC (Fig. 4). The neutrophil chemotaxis index for N-t-BOC-insensitive events (i.e., neutrophil events not inhibited in the presence of the FPR antagonist N-t-BOC) was significantly increased by 32% when isogenic O antigen derivative (CP921; 1,104 ± 235 [mean ± SEM]; P = 0.008, paired t test) was used as a chemoattractant compared to its wild-type parent (CP9; 838 ± 221 [mean ± SEM]) (n = 14). N-t-BOC-insensitive neutrophil chemotaxis was increased by 25%, but was not statistically significant (P = 0.052), when an isogenic capsule-deficient derivative was used as a chemoattractant (CP9.137; 1,050 ± 250 [mean ± SEM]) compared to its wild-type parent (CP9; 838 ± 221 [mean ± SEM]). These data demonstrate that E. coli's O antigen diminishes and that there is a trend toward the presence of capsule diminishing N-t-BOC-insensitive neutrophil chemotaxis, which is mediated through non-FPR chemotaxis receptor(s).
Given the prior observations with CP9 (Fig. 3), we hypothesized that the observed N-t-BOC-insensitive neutrophil chemotaxis modulated by O antigen and capsule required live E. coli cells. In five independent experiments, N-t-BOC-insensitive chemotaxis accounted for only 2.6 and 4.0% of the neutrophil migration induced by cell-free culture supernatant from CP921 and CP9.137, respectively. In contrast, in 14 independent experiments, N-t-BOC-insensitive chemotaxis was significantly greater, accounting for 46 and 45% of the neutrophil migration induced by whole, live CP921 (P = 0.017, unpaired t test) and CP9.137 (P = 0.028), respectively. These results demonstrate that the modulation of N-t-BOC-insensitive chemotaxis by O antigen and perhaps by capsule occurs in the presence of whole bacteria, not supernatant; the results also suggest that a bacterium-neutrophil interaction is required.
Given the prior observations with CP9 (Table 1), we also hypothesized that the observed N-t-BOC-insensitive neutrophil chemotaxis modulated by O antigen and capsule was due, at least in part, to IL-8 production from neutrophils that had already migrated into the bottom well. As seen in Table 1, IL-8 was also present in the lower well when CP921 (O-antigen deficient) and CP9.137 (capsule deficient) were used as chemoattractants. Likewise, anti-IL-8 antibodies inhibited 29% ± 18% and 71% ± 26% of N-t-BOC-insensitive neutrophil chemotaxis in response to live CP921 and CP9.137, respectively. These results further confirm that IL-8 is responsible in part for N-t-BOC-insensitive neutrophil chemotaxis in response to E. coli. Although the necessary studies are beyond the focus of this report, these results also suggest the possibility that E. coli's O antigen and capsule decrease N-t-BOC-insensitive neutrophil chemotaxis by decreasing the chemokine production and/or the chemoattractant effects of chemokines that are produced from a neutrophil-E. coli interaction.
Effects of E. coli capsule and O antigen on neutrophil chemotaxis in the rat model of gram-negative pneumonia.
In vitro studies established that O antigen and capsule modulated neutrophil chemotaxis in vitro (Fig. 4). To determine the effects of capsule and O antigen on total, N-t-BOC-sensitive, and N-t-BOC-insensitive chemotaxis in vivo, rats were infected via intratracheal challenge with approximately 107 CFU of CP9 (wild type), CP921 (O-antigen deficient), or CP9.137 (capsule deficient). One hour after bacterial challenge, BAL was performed, and cell-free BAL fluid was used ex vivo as the chemoattractant in the neutrophil chemotaxis assay (Fig. 6).
FIG. 6.
Ex vivo chemotaxis of human neutrophils in response to cell-free BAL fluid from rats infected with approximately 107 CFU of the E. coli strains CP9 (wild type), CP921 (O-antigen deficient) or CP9.137 (capsule deficient). One hour after bacterial challenge, BAL was performed and cell-free BAL fluid was used as the chemoattractant. The total deduced N-t-BOC-sensitive and the N-t-BOC-insensitive chemotaxis indices are depicted (see Materials and Methods for details on these indices). Total, N-t-BOC-sensitive, and N-t-BOC-insensitive chemotaxis was significantly decreased toward the wild-type E. coli strain that possessed a capsule compared to an isogenic capsule-deficient derivative at 1 h post-bacterial challenge. *, for total chemotaxis, P was ≤0.001 compared to CP9; #, for N-t-BOC-sensitive chemotaxis, P was 0.048 compared to CP9; &, for N-t-BOC-insensitive chemotaxis, P was <0.004 (P values are per paired t test; n = 18).
Total chemotaxis.
The total neutrophil chemotaxis index was significantly increased by 37% when an isogenic capsule-deficient derivative (CP9.137; 3,804 ± 373 [mean ± SEM]; P = 0.001, paired t test) was used as a chemoattractant compared its wild-type parent (CP9; 2,770 ± 321 [mean ± SEM]) (n = 18). There was a trend toward a significant increase (19%) in chemotaxis when an isogenic O-antigen-deficient derivative (CP921; 3,283 ± 347 [mean ± SEM]; P = 0.079, paired t test) was used as a chemoattractant compared its wild-type parent (CP9; 2,770 ± 321 [mean ± SEM]). These data demonstrate that, in the presence of bacterial capsule and perhaps O antigen, the overall effect was that neutrophils were less likely to migrate toward wild-type E. coli in vivo.
N-t-BOC-sensitive chemotaxis.
N-t-BOC-sensitive chemotaxis accounted for 44, 46, and 42% of the total chemotactic activity for CP9, CP921, and CP9.137, respectively. These data demonstrate that N-t-BOC-sensitive chemotaxis contributes to neutrophil migration in vivo. Further, N-t-BOC-sensitive neutrophil chemotaxis was significantly increased by 29% when an isogenic capsule-deficient derivative was used as a chemoattractant (CP9.137; 1,581 ± 193 [mean ± SEM]; P = 0.048) compared to its wild-type parent (CP9; 1,224 ± 182 [mean ± SEM]) (n = 18). N-t-BOC-sensitive neutrophil chemotaxis was increased by 23%, but was not statistically significant (P = 0.38), when an isogenic O-antigen-deficient derivative was used as a chemoattractant (CP921; 1,499 ± 277 [mean ± SEM]) compared to its wild-type parent (CP9; 1,224 ± 182 [mean ± SEM]) (Fig. 6). These data were consistent with in vitro results and demonstrate that E. coli's capsule decreases N-t-BOC-sensitive chemotaxis in vivo.
N-t-BOC-insensitive chemotaxis.
N-t-BOC-insensitive chemotaxis accounted for 56, 54, and 58% of the total chemotactic activity for CP9, CP921, and CP9.137, respectively. These data demonstrate that N-t-BOC-insensitive chemotaxis also contributes to neutrophil migration in vivo. Further, N-t-BOC-insensitive neutrophil chemotaxis was significantly increased by 47% when an isogenic capsule-deficient derivative was used as a chemoattractant (CP9.137; 2,271 ± 337 [mean ± SEM]) compared to its wild-type parent (CP9; 1,546 ± 259 [mean ± SEM]) (P = 0.004, paired t test) (n = 18). N-t-BOC-insensitive neutrophil chemotaxis was increased by 13%, but was not statistically significant (P = 0.38), when an isogenic O-antigen-deficient derivative was used as a chemoattractant (CP921; 1,785 ± 291 [mean ± SEM]) compared to its wild-type parent (CP9; 1,546 ± 259 [mean ± SEM]) (Fig. 6). These data were also consistent with in vitro results and demonstrate that E. coli's capsule decreases N-t-BOC-insensitive chemotaxis in vivo.
DISCUSSION
Previous studies from our laboratory, for which we used a rat model of gram-negative pneumonia, established that pulmonary neutrophil recruitment was modulated by the E. coli virulence factors capsule and O-specific antigen (19). To begin to understand the mechanism by which this occurs, in vitro and ex vivo chemotaxis assays were performed.
An important aspect of the experimental design of these studies was the use of a clinically relevant extraintestinal pathogenic strain of E. coli (CP9) (not a commensal or intestinal pathogenic strain of E. coli) that possesses the full repertoire of virulence factors needed to function as a successful extraintestinal pathogen and the use of isogenic derivatives deficient only in O antigen (CP921) or capsule (CP9.137) (20, 23). Further, live organisms were used for in vitro assays and in a relevant in vivo rat model of gram-negative pneumonia (19). The use of live E. coli guaranteed the appropriate level of expression for virulence factors, despite unpredictable and changing environmental signals in vivo. Moreover, the manner in which microbial virulence factors such as capsule and O antigen are presented to the host may differ when a purified product is used rather than a whole organism. For example, in vivo, the host interacts both with cell-bound LPS and with shed complete LPS, not purified lipid A. Additionally, direct bacterium-host cell interactions may modify host response, which would not be detected with the use of purified bacterial components. Finally, although in vitro systems are unable to reproduce the complexities of the in vivo milieu, we also realize that mechanistic studies are difficult when only in vivo systems are used. Therefore, an integrated approach to in vitro and ex vivo evaluations was utilized to maximize the chances of deciphering the complex host-pathogen interactions that affect neutrophil chemotaxis.
In the process of developing the in vitro chemotaxis assay, it was established that the use of live E. coli as the sole chemoattractant resulted in both N-t-BOC-sensitive and N-t-BOC-insensitive human neutrophil chemotaxis (Fig. 3). This surprising finding suggested that in our in vitro system, not only was the predicted direct bacterial effect of FMLP on chemotaxis being measured but an effect on non-FPR chemotaxis receptors was also. Subsequent experiments demonstrated that this effect on non-FPR chemotaxis receptors was mediated in part by the induction of IL-8 from neutrophils (Table 1). IL-8 was measured in the lower wells, and anti-IL-8 antibodies decreased N-t-BOC-insensitive chemotaxis. Further, N-t-BOC-insensitive chemotaxis required the presence of viable E. coli. When cell-free E. coli culture supernatant was used as the chemoattractant, little to no N-t-BOC-insensitive neutrophil chemotaxis was observed. This suggests that either direct bacterial contact or bacterial components that are neither shed nor secreted stimulate neutrophils to produce IL-8 and probably other chemokines as well. No IL-1β and little to no TNF-α were detected in either the top or bottom well in the in vitro chemotaxis assay (Table 1). This strongly suggested that neutrophils, and not contaminating monocytes, are responsible for the IL-8 production. Although some controversy exists, increasing data support the concept that neutrophils are capable of producing physiologic quantities of cytokines and chemokines (1, 25, 30). Further, our data suggest that there may be fundamental differences in the ability of neutrophils to detect and respond to microbes compared to that of other cells which are capable of producing chemokines such as monocytes or macrophages. It is logical for neutrophils to maximize the chemokine gradient when in direct contact with bacteria, thus ensuring that “reinforcements” will localize to the precise location of the invading pathogens.
To begin to understand the effect of capsule and/or O antigen on neutrophil chemotaxis, in vitro chemotaxis was measured in response to wild-type E. coli (CP9) and isogenic derivatives that were deficient in O antigen (CP921) or capsule (CP9.137). In the presence of either O antigen or capsule, there was a significant reduction of human neutrophil migration toward E. coli (Fig. 4). Having established that our in vitro system was capable of measuring both N-t-BOC-sensitive and N-t-BOC-insensitive chemotaxis, we were able to evaluate the effect of E. coli capsule and O antigen on each of these chemotactic pathways.
One of the objectives of this study was to test the hypotheses that capsule and O antigen directly modulate neutrophil chemotaxis mediated by the FPR. The measurement of N-t-BOC-sensitive chemotaxis is a surrogate for this end point. In both in vitro and in vivo (Fig. 4, 6) environments, N-t-BOC-sensitive neutrophil chemotaxis was always decreased in response to the wild-type E. coli (CP9) compared to isogenic derivatives that were deficient in O antigen (CP921) or capsule (CP9.137) or to cell-free supernatants generated from these strains. These differences were statistically significant in vitro for CP921, for which live E. coli was used as the chemoattractant (P = 0.03), and for CP9.137, both in vitro when supernatant was used as the chemoattractant (P = 0.003) and, importantly, in vivo (P = 0.048). Taken together, these data demonstrate that both of these surface polysaccharides modulate neutrophil chemotaxis mediated through the FPR. The in vivo results validate the in vitro studies and support physiologic relevance. These findings are novel and expand our understanding of how O antigen and capsule contribute to the pathogenesis of infection. Logical possibilities by which O antigen and capsule diminish neutrophil chemotaxis toward the bacterium include (i) diminished shedding of FMLP, (ii) biologic inactivation of FMLP by shed capsule and/or O antigen, and (iii) down-regulation (or inactivation) of FRR or its signal transduction pathway by shed capsule or O antigen. Although the precise mechanism has not been definitively established, in vitro chemotaxis experiments in which a mix of cell-free culture supernatants from wild-type E. coli and each isogenic derivative deficient in capsule or O antigen did not identify inhibitory activity against neutrophil chemotaxis (Fig. 5). This finding indirectly supports a model of decreased shedding of FMLP as being the most likely mechanism by which E. coli's capsule and O antigen decrease N-t-BOC-sensitive neutrophil chemotaxis, a microbial equivalent of “stealth” technology.
Capsule and O antigen also diminished N-t-BOC-insensitive neutrophil chemotaxis, which is a surrogate measure of non-FPR-mediated chemotaxis. Both in vitro and in vivo (Fig. 4 and 6), N-t-BOC-insensitive neutrophil chemotaxis was always decreased in response to the wild-type E. coli (CP9) compared to isogenic derivatives that were deficient in O antigen (CP921) or capsule (CP9.137). These differences were statistically significant in vitro for CP921 (P = 0.008), and there was a trend toward significance for CP9.137 (P = 0.052), using live E. coli as the chemoattractant, and, importantly, in vivo for CP9.137 (P = 0.004). Taken together, these data demonstrate that both O antigen and capsule attenuate neutrophil chemotaxis mediated through non-FPR chemotaxis receptors. This finding is also novel, but it is logical to envision that virulence would be enhanced if the pathogen has the ability to attenuate the host's acute inflammatory response that has evolved in order to clear invading microbes. In our in vitro chemotaxis system, N-t-BOC-insensitive neutrophil chemotaxis is mediated in part by IL-8 (Table 1) and appears to require a direct E. coli-neutrophil interaction. The most likely means by which capsule and O antigen decrease N-t-BOC-insensitive chemotaxis would be by decreasing the chemokine production that results from an E. coli-neutrophil interaction. Logical mechanisms by which this could be accomplished include (i) affecting the efficiency of E. coli interacting with a neutrophil, (ii) affecting the host cell N-t-BOC-insensitive chemotactic receptor-E. coli-ligand interaction, or (iii) in the absence of capsule and O antigen, lipid A could be more available to interact with pattern recognition receptors on neutrophils. These models are currently being tested directly.
To confirm that our in vitro studies were meaningful, rats were infected with wild-type E. coli (CP9) and isogenic derivatives thereof that were deficient solely in O antigen (CP921) or capsule (CP9.137). At 1 h post-E. coli challenge, BAL fluid was obtained and cell-free BAL fluid was used as the chemoattractant in chemotaxis studies. An important observation from these studies was that N-t-BOC-sensitive chemotactic activity was present in BAL fluid after bacterial challenge, accounting for 42 to 46% of the chemoattractant activity present in BAL fluid (Fig. 6). Secondly, data generated from the rat model of gram-negative pneumonia supported the results of our in vitro studies, which demonstrated that capsule and O antigen diminish N-t-BOC-sensitive and -insensitive chemotaxis. Because N-t-BOC-sensitive chemotaxis can be due to host components, such as mitochondrial proteins, and to formylated bacterial peptides (12), the relative contribution of bacterial products to N-t-BOC-sensitive neutrophil chemotaxis in vivo is unclear. However, given that this response occurs soon after bacterial challenge and is modulated by bacterial capsule and O antigen, it is reasonable to assume that bacterial formylated peptides are contributory.
It is clear that the overall effect of capsule and O antigen on neutrophil chemotaxis is due to the sum of both direct bacterial effects (N-t-BOC sensitive) and indirect effects on the activation and induction of host chemoattractants (N-t-BOC insensitive). Data for the rat pneumonia model previously published by workers in our laboratory demonstrated that O antigen decreased pulmonary neutrophil influx in response to a pulmonary challenge with E. coli that resulted in pneumonia (19). Findings from this study are consistent with those results. In contrast, in that study (19) we had demonstrated that capsule increased pulmonary neutrophil influx. Findings from this study are not consistent with those prior results. This apparent discrepancy emphasizes that we must exercise caution, as the mechanisms by which capsule and O antigen modulate neutrophil chemotaxis are being elucidated. Other chemoattractant factors that have not yet been evaluated will undoubtedly prove to be important and perhaps dominant. Although we have begun to decipher this complex biological process that is critical to determining the outcome of infection, additional studies are needed in order to fully understand the multifactorial mechanism by which capsule and O antigen modulate pulmonary neutrophil recruitment.
Acknowledgments
This work was supported by the VA Merit Review of the Department of Veterans Affairs (T.A.R.). Grants were also received from the National Institutes of Health (HL69763 to T.A.R. and HL48889 and AI46534 to P.R.K.) as well as The John R. Oishei Foundation (T.A.R.).
Editor: B. B. Finlay
REFERENCES
- 1.Al-Mohanna, F., S. Saleh, R. Parhar, and K. Collison. 2002. IL-12-dependent nuclear factor-κB activation leads to de novo synthesis and release of IL-8 and TNF-α in human neutrophils. J. Leukoc. Biol. 72:995-1002. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1984. National nosocomial infections study report. Annual summary 1984. Morb. Mortal. Wkly. Rep. 35:17SS-29SS. [Google Scholar]
- 3.Chastre, J., and J.-Y. Fagon. 2002. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 165:867-903. [DOI] [PubMed] [Google Scholar]
- 4.Cook, D., G. Guyatt, J. Marshall, D. Leasa, H. Fuller, R. Hall, S. Peters, F. Rutledge, L. Griffith, A. McLellan, G. Wood, and A. Kirby. 1998. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N. Engl. J. Med. 338:791-797. [DOI] [PubMed] [Google Scholar]
- 5.Craven, D., T. Barber, K. Steger, and M. Montecalvo. 1990. Nosocomial pneumonia in the 1990s: update of epidemiology and risk factors. Semin. Respir. Infect. 5:157-172. [PubMed] [Google Scholar]
- 6.Dong, Z., and J. Murphy. 1995. Intravascular cryptococcal culture filtrate (CneF) and its major component, glucuronoxylomannan, are potent inhibitors of leukocyte accumulation. Infect. Immun. 63:770-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emori, T., and R. Gaynes. 1993. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 6:428-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fluit, A., F. Schmitz, J. Verhoef, et al. 2001. Frequency of isolation of pathogens from bloodstream, nosocomial pneumonia, skin and soft tissue, and urinary tract infections occurring in European patients. Eur. J. Clin. Microbiol. Infect. Dis. 20:188-191. [DOI] [PubMed] [Google Scholar]
- 9.Goldman, D., F. Chang, L. Gifford, E. Goetzl, and H. Bourne. 1985. Pertussis toxin inhibition of chemotactic factor-induced calcium mobilization and function in human polymorphonuclear leukocytes. J. Exp. Med. 162:145-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill, H., J. Bohnsack, E. Morris, N. Augustine, C. Parker, P. Cleary, and J. Wu. 1988. Group B streptococci inhibit the chemotactic activity of the fifth component of complement. J. Immunol. 141:3551-3556. [PubMed] [Google Scholar]
- 11.Jagels, M., J. Travis, J. Potempa, R. Pike, and T. Hugli. 1996. Proteolytic inactivation of the leukocyte C5a receptor by proteinases derived from Porphyromonas gingivalis. Infect. Immun. 64:1984-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le, Y., P. Murphy, and J. Wang. 2002. Formyl-peptide receptors revisited. Trends Immunol. 23:541-548. [DOI] [PubMed] [Google Scholar]
- 13.Lynch, J. I. 2001. Hospital-acquired pneumonia. Chest 119:373S-384S. [DOI] [PubMed] [Google Scholar]
- 14.Malech, H., and J. Gallin. 1987. Neutrophils in human disease. N. Engl. J. Med. 317:687-694. [DOI] [PubMed] [Google Scholar]
- 15.National Nosocomial Infections Surveillance System. 1996. National nosocomial infections surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996. Am. J. Infect. Control 24:380-388. [PubMed] [Google Scholar]
- 16.Rozdzinski, E., T. Jones, W. Burnette, M. Burroughs, and E. Tuomanen. 1993. Antiinflammatory effects in experimental meningitis of prokaryotic peptides that mimic selectins. J. Infect. Dis. 168:1422-1428. [DOI] [PubMed] [Google Scholar]
- 17.Russo, T., J. Brown, S. Jodush, and J. Johnson. 1996. The O4 specific antigen moiety of lipopolysaccharide but not the K54 group 2 capsule is important for urovirulence in an extraintestinal isolate of Escherichia coli. Infect. Immun. 64:2343-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo, T., U. Carlino, A. Mong, and S. Jodush. 1999. Identification of genes in an extraintestinal isolate of Escherichia coli with increased expression after exposure to human urine. Infect. Immun. 67:5306-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo, T., B. Davidson, R. Priore, U. Carlino, J. Helinski, and P. I. Knight. 2000. Capsular polysaccharide and O-specific antigen divergently modulate pulmonary neutrophil influx in a rat Escherichia coli model of gram-negative pneumonitis. Infect. Immun. 68:2854-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo, T., J. Guenther, S. Wenderoth, and M. Frank. 1993. Generation of isogenic K54 capsule-deficient Escherichia coli strains through TnphoA-mediated gene disruption. Mol. Microbiol. 9:357-364. [DOI] [PubMed] [Google Scholar]
- 21.Russo, T., and J. Johnson. 2000. A proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 181:1753-1754. [DOI] [PubMed] [Google Scholar]
- 22.Russo, T., Y. Liang, and A. Cross. 1994. The presence of K54 capsular polysaccharide increases the pathogenicity of Escherichia coli in vivo. J. Infect. Dis. 169:112-118. [DOI] [PubMed] [Google Scholar]
- 23.Russo, T., G. Sharma, C. Brown, and A. Campagnari. 1995. The loss of the O4 antigen moiety from the lipopolysaccharide of an extraintestinal isolate of Escherichia coli has only minor effects on serum sensitivity and virulence in vivo. Infect. Immun. 63:1263-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo, T., S. Wenderoth, U. Carlino, J. Merrick, and A. Lesse. 1998. Identification, genomic organization and analysis of the group III capsular polysaccharide genes kpsD, kpsM, kpsT, and kpsE from an extraintestinal isolate of Escherichia coli (CP9, O4/K54/H5). J. Bacteriol. 180:338-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scapini, P., J. Lapinet-Vera, S. Gasperini, F. Calzetti, F. Bazzoni, and M. Cassatella. 2000. The neutrophil as a cellular source of chemokines. Immunol. Rev. 177:195-203. [DOI] [PubMed] [Google Scholar]
- 26.Shelhamer, J., G. Toews, H. Masur, A. Suffredini, P. Pizzo, T. Walsh, and D. Henderson. 1992. Respiratory disease in the immunosuppressed host. Ann. Intern. Med. 117:415-431. [DOI] [PubMed] [Google Scholar]
- 27.Veldkamp, K., H. Heezius, J. Verhoef, J. van Strijp, and K. van Kessel. 2000. Modulation of neutrophil chemokine receptors by Staphylococcus aureus supernate. Infect. Immun. 68:5908-5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, L., W. Qu, and P. Reeves. 2001. Sequence analysis of four Shigella boydii O-antigen loci: implication for Escherichia coli and Shigella relationships. Infect. Immun. 69:6923-6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wenzel, R. 1989. Hospital-acquired pneumonia: overview of the current state of art for prevention and control. Eur. J. Clin. Microbiol. 8:56-60. [DOI] [PubMed] [Google Scholar]
- 30.Witko-Sarsat, V., P. Rieu, B. Descamps-Latscha, P. Lesavre, and L. Halbwachs-Mecarelli. 2000. Neutrophils: molecules, functions and pathophysiological aspects. Lab. Investig. 80:617-653. [DOI] [PubMed] [Google Scholar]