Abstract
In addition to their well characterized role in allergic inflammation, recent data confirm that mast cells play a more extensive role in a variety of immune responses. However, their contribution to autoimmune and neurologic disease processes has not been investigated. Experimental allergic encephalomyelitis (EAE) and its human disease counterpart, multiple sclerosis, are considered to be CD4+ T cell–mediated autoimmune diseases affecting the central nervous system. Several lines of indirect evidence suggest that mast cells could also play a role in the pathogenesis of both the human and murine disease. Using a myelin oligodendrocyte glycoprotein (MOG)-induced model of acute EAE, we show that mast cell–deficient W/Wv mice exhibit significantly reduced disease incidence, delayed disease onset, and decreased mean clinical scores when compared with their wild-type congenic littermates. No differences were observed in MOG-specific T and B cell responses between the two groups, indicating that a global T or B cell defect is not present in W/Wv animals. Reconstitution of the mast cell population in W/Wv mice restores induction of early and severe disease to wild-type levels, suggesting that mast cells are critical for the full manifestation of disease. These data provide a new mechanism for immune destruction in EAE and indicate that mast cells play a broader role in neurologic inflammation.
Keywords: autoimmunity, demyelinating diseases, experimental allergic encephalomyelitis, inflammation, myelin-associated glycoprotein
Introduction
Experimental allergic encephalomyelitis (EAE), the prototypical rodent model of human multiple sclerosis (MS), is an autoimmune disease characterized by inflammation in the central nervous system (CNS) 1 2 3. Like the human disease, EAE is associated with an early breach of the blood–brain barrier, focal perivascular mononuclear cell infiltrates, and demyelination leading to paralysis of the extremities 1. The adoptive transfer of myelin-specific CD4+ T cells to naive animals passively confers EAE, demonstrating that this cell type is critical in the disease process. However, it is unclear whether these T cells directly damage the myelin sheath or if they activate other cells for this function. The underlying cause of increased vascular permeability that facilitates the entry of T cells into the CNS is also unknown. In this study, we asked if mast cells could influence the T cell response and subsequent EAE disease course. Mast cells, best known for their role in allergic inflammation, are distributed in a variety of anatomical sites, including the CNS, where they are often found adjacent to blood vessels and nerves 4 5 6 7. In addition, mast cells are an important source of several mediators, including proteases and vasoactive amines such as histamine. Mast cells also produce cytokines that have been implicated in either EAE disease pathology or protection from disease, such as TNF-α and IL-4, respectively 8 9 10 11 12.
The idea that mast cells contribute to the pathogenesis of MS is not a new concept. Over 100 years ago, mast cells were observed in the CNS plaques of MS patients 13. Subsequent studies reported a correlation between the number and/or distribution of mast cells and MS or EAE pathology 14 15 16. Sites of inflammatory demyelination are also sites of mast cell accumulation in the brain and spinal cord, and the percentage of degranulated mast cells in the CNS correlates with the clinical onset of disease symptoms in acute EAE 17. Furthermore, levels of tryptase, a mast cell–specific proteolytic enzyme, are elevated in the cerebrospinal fluid in the human disease 18. Mast cell–derived proteases are capable of degrading myelin 19 20 21, and myelin can directly stimulate mast cell degranulation in vitro 20. Finally, treatment with mast cell–stabilizing drugs or with pharmacologic antagonists of mast cell mediators such as serotonin and histamine was shown to reduce disease severity in human MS and in EAE 22 23 24. Despite this wealth of correlative data, a direct role for mast cells in the pathogenesis of neurologic disorders such as MS has not been definitively established.
Materials and Methods
Animals.
WBB6/F1-KitW/KitWv (W/Wv) female mice (8–12 wk old) and their female congenic littermates, WBB6/F1-Kit+/Kit+ (F1 +/+), were obtained from The Jackson Laboratory. Both of these groups result from the cross of WB/ReJ-KitW/+ × C57BL/6-KitWv/+ mice. Animal care was provided according to protocols approved by the Institutional Animal Care and Use Committee of Emory University.
EAE Disease Induction and Clinical Scoring.
EAE induction was performed according to the protocol of Mendel et al. 25. In brief, 300 μg of myelin oligodendrocyte glycoprotein (MOG)35–55 peptide MEVGWYRSPFSRVVHLYRNGK (Microchemical Facility, Emory University) was dissolved in 100 μl of PBS and emulsified in an equal volume of CFA (Difco Labs., Inc.) containing 5 mg/ml of Mycobacterium tuberculosis H37 RA (Difco Labs., Inc.). The emulsion (200 μl) was injected subcutaneously into the flank on days 0 and 7. Pertussis toxin, 500 ng in 500 μl of PBS (List Biological Labs.), was administered intravenously into each tail vein on days 0 and 2. Mice were scored daily according to the following clinical scoring system: 0, no clinical disease; 1, tail flaccidity; 2, hind limb weakness; 3, hind limb paralysis; 4, forelimb paralysis or loss of ability to right from supine; 5, death.
Bone Marrow–derived Mast Cell Differentiation and Reconstitution.
Bone marrow was harvested from both femurs of 6–8-wk-old wild-type F1 +/+ female mice and cultured in complete RPMI media (15% heat-inactivated FBS, 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mM glutamine, 1 mM sodium pyruvate, and 50 μM 2-β-ME) containing 25% WEHI-3B supernatant as an IL-3 source 26. In contrast to some previously described methods for culturing bone marrow–derived mast cells (BMMCs; reference 27 28 29), recombinant murine stem cell factor (12.5 ng/ml; R & D Systems, Inc.) was also added to the culture during the first 2 wk as described 30 31. This addition consistently increased the viability of the cultured cells. BMMCs were used after a minimum of 4 wk in culture at >96% purity, as determined by flow cytometric analysis. At time of reconstitution, BMMCs (5 × 106 in 300 μl) were injected intravenously into groups of five to seven W/Wv mice. Mice were housed for 10 wk before being subjected to EAE disease induction along with age-matched W/Wv and F1 +/+ controls.
Preparation of Tissue for Histologic Examination.
After animals were killed, brains, spinal columns, and other organs were removed and preserved in 10% neutral buffered formalin. Tissues were embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin or Giemsa.
Flow Cytometry.
BMMCs (106 cells in 100 μl) were blocked with antibodies to the Fcγ receptors CD16 and CD32 (PharMingen). Cells were incubated with murine IgE (PharMingen) and then surface stained with directly conjugated mAbs to murine IgE (rat anti–mouse–FITC; PharMingen) and c-kit (c-kit–PE; PharMingen). Flow cytometric analyses for BMMC purity were carried out with the appropriate isotype controls. Cells double positive for c-kit and FcεRI were considered mast cells.
Determination of Anti-MOG Antibody Levels.
Antibody level analyses were performed by specific ELISA to detect anti-MOG activity. MOG (0.25 μg/well in 0.1 M NaHC03, pH 9.6) was adsorbed onto flat-bottomed microtiter plates overnight at 4°C. After a blocking step of PBS/0.3% Tween 20/5% nonfat dry milk, plates were incubated with 1:100 dilutions of mouse sera in PBS/0.3% Tween 20. Anti-MOG antibodies bound to the MOG-coated plate were detected using peroxidase-conjugated, affinity-purified IgG fractions of isotype-specific goat anti–mouse IgG, IgG1, IgG2a, IgG2b, or IgG3 (PharMingen) diluted 1:1,000 in PBS/0.3% Tween 20. Assays were developed with 3,3′,5,5′-tetramethylbenzidine peroxidase substrate (KPL), stopped with H3PO4 (1:20 dilution), and read at a wavelength of 450 nm on a microplate reader.
Statistical Analyses.
Statistical analyses were performed using GraphPad Prism (Software for Science). Group mean clinical scores were analyzed by paired t test for comparison of two groups. Repeated measures of analysis of variance (ANOVA), followed by the Bonferroni post-test, were used for comparison of the mean clinical scores of the three groups in the reconstitution experiments. Comparison of group incidence (number of animals with disease/n) was analyzed by Fisher's exact test. Survival curves (animals positive for disease) were plotted according to the method of Kaplan-Meier, and significance was calculated by the log-rank test. Mean high scores were compared by student's t test or ANOVA with Bonferroni post-test for comparison of two or three groups, respectively.
Results
W/Wv Mice Show a Delay in Time of Disease Onset and a Reduction in Disease Severity.
To directly evaluate the in vivo role of mast cells in acute EAE, mast cell–deficient WBB6/F1-KitW/KitWv (W/Wv) mice and their congenic wild-type WBB6/F1-Kit+/Kit+ (F1 +/+), littermates (H-2bxj) were immunized with the encephalitogenic MOG35–55 peptide. MOG can induce typical EAE disease in C57BL/6 mice and other H-2b strains 25. MOG, which comprises only ∼0.05% of myelin proteins, elicits a major antibody response that has been correlated with disease severity and demyelination in both human disease and animal models of MS 32 33 34. In three independent experiments, W/Wv mice developed significantly less severe disease than wild-type mice, as indicated by lower daily mean clinical scores (P < 0.0001; Fig. 1 A). In addition, mast cell–deficient animals also demonstrated a delayed onset and lower incidence of disease when compared with their wild-type counterparts (P < 0.0003; Fig. 1 B). Sham-immunized wild-type (n = 3) and mast cell–deficient animals (n = 4) that received pertussis toxin and adjuvant alone showed no clinical signs of disease (data not shown). The cumulative analyses of disease parameters are presented in Table .
Figure 1.
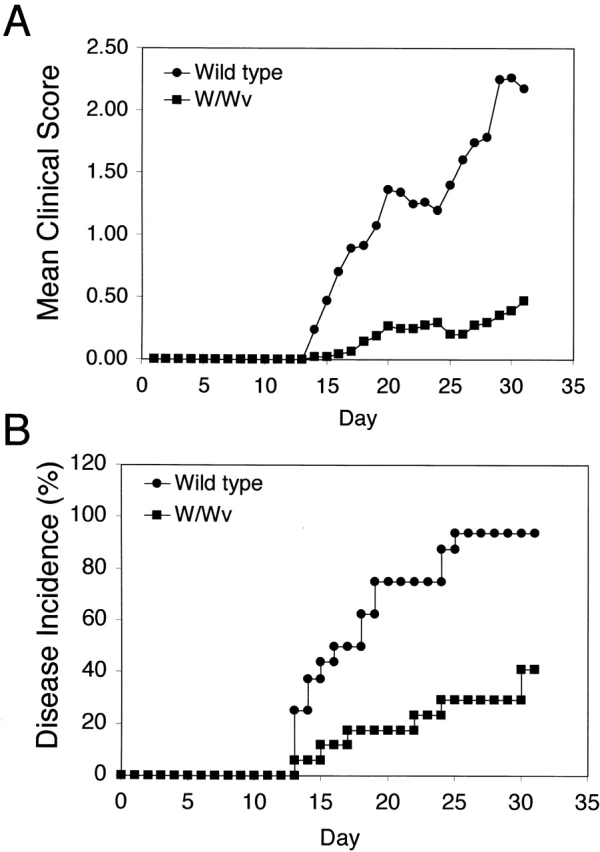
W/Wv mice show a delay in disease onset and a reduction in disease severity. (A) Clinical scores were assigned daily to wild-type (n = 16) and W/Wv (n = 17) mice, and the mean of each group was reported (P < 0.0001 as determined by paired t test). (B) Graph represents the percent of total animals that demonstrated disease by day 30 after immunization (P < 0.0003). Curve was plotted according to the method of Kaplan-Meier, and significance was calculated by the log-rank test. Results in A and B represent cumulative data from three independent experiments.
Table 1.
Cumulative Analysis of EAE Disease Parameters
| Group | Incidence | Mean day of onset | Mean high score |
|---|---|---|---|
| P < 0.003 | P < 0.03 | P < 0.008 | |
| Wild-type F1 +/+ | 15/16 | 18.0 ± 2.65 | 2.42 ± 0.28 |
| W/Wv | 7/17 | 24.3 ± 2.96 | 0.61 ± 0.22 |
Inflammatory Infiltrates Are Present in the CNS of Diseased Animals.
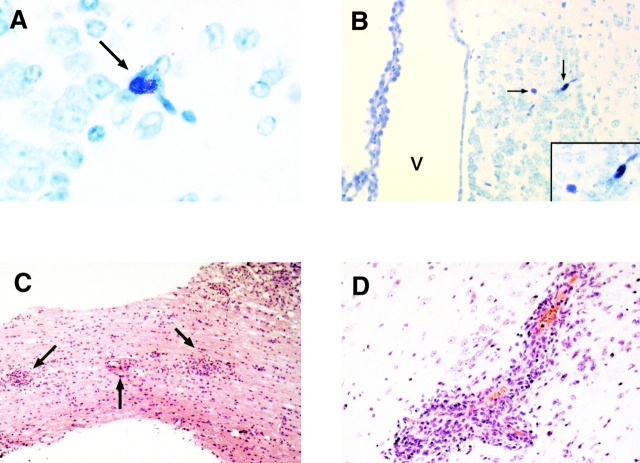
In addition to the clinical changes observed, animals were also examined for histologic evidence of disease induction. Initially, we confirmed the presence of CNS mast cells in naive animals used in this model system. Using metachromatic staining, mast cells were identified in CNS samples of wild-type mice only, particularly in perivascular regions of the hippocampus, leptomeninges, habenula, and thalamus (Fig. 2A and Fig. B). Tissue samples from immunized wild-type and W/Wv mice were also examined for the presence of inflammatory lesions. Typical mononuclear infiltrates with perivascular cuffing were noted in the brains and spinal cords of diseased mice in both groups (Fig. 2C and Fig. D). No apparent differences between the two groups were observed in the composition or distribution of inflammatory infiltrates. Mast cells were not identified in the inflammatory lesions of wild-type mice, consistent with the previous findings of Ibrahim et al. 35 in which mast cells were found in lesions from chronic but not acute disease.
Figure 2.
Histologic analyses of CNS tissues in WBB6/F1 +/+ mice. After sacrifice of the animals, brains, spinal columns, and other organs were removed and preserved in 10% neutral buffered formalin. Paraffin-embedded tissue sections were stained with Giemsa (A and B) or hematoxylin and eosin (C and D). (A) Mast cell (arrow) located within the thalamic border region of the habenula; ×40. (B) Two mast cells (arrows) located in the habenula. The third ventricle is also noted (V); ×20. Inset, the same two mast cells ×40. (C) Multiple inflammatory infiltrates (arrows) found in spinal cord section of a diseased animal; ×10. (D) Focal inflammatory infiltrate found in the brain parenchyma of a diseased animal; ×40.
Reconstitution of W/Wv Mice with BMMCs Restores EAE Disease Onset and Severity to Wild-Type Levels.
If mast cell deficiency alone accounts for the differences in EAE disease parameters observed in W/Wv mice, reconstitution of the mast cell population in these animals should restore disease incidence and severity to the level of wild-type animals. The development of functional mast cells is dependent on the interaction of stem cell factor (SCF) with its receptor, c-kit, expressed on bone marrow–derived pluripotent stem cells. The mast cell deficiency of W/Wv mice is due to mutations in c-kit that compromise its signaling function 36 37. The mast cell population can be reconstituted in these animals by intravenous injection of c-kit+ bone marrow cells or in vitro–differentiated, bone marrow–derived mast cell precursors (BMMCs) from wild-type animals 4 27 29 38 39 40 41 42 43. Mast cell numbers in the skin, respiratory tract, and gastrointestinal tissues of the W/Wv mice after transplantation with either bone marrow cells or BMMCs are comparable to those of wild-type animals by 10–12 wk after transplantation. Importantly, the phenotypic characteristics of these cells resemble the local, native populations of mast cells in normal mice 29 38 39.
We performed mast cell reconstitution in 8–10-wk-old W/Wv recipients by intravenous injection of BMMCs (>96% purity, as determined by flow cytometry; Fig. 3) to repair the mast cell deficit. To assess the establishment of mast cells in these mice, animals were killed 14–16 wk after reconstitution, and major organs were examined for the presence and distribution of mast cells. Mast cells were observed in the gut, CNS, and bone marrow as well as other organs in distribution patterns consistent with those seen in wild-type mice (Fig. 4). As expected, no mast cells were detected in tissues obtained from W/Wv mice.
Figure 3.
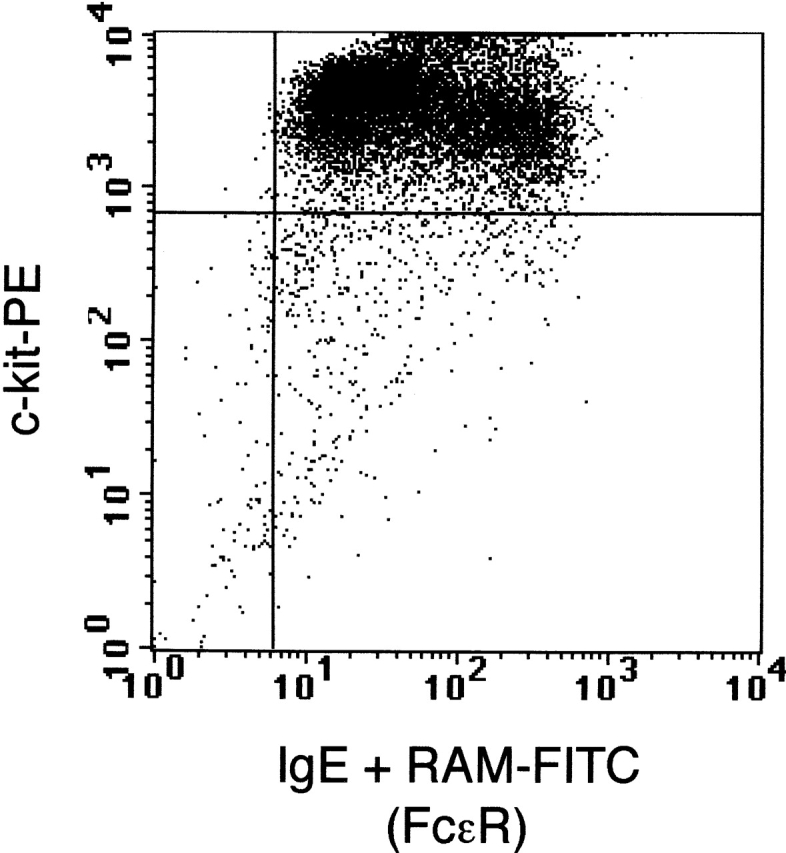
Flow cytometric analysis of the in vitro–differentiated BMMC population. Cells double positive for c-kit and FcεRI were considered mast cells. Greater than 96% of the population was positive for both mast cell markers, c-kit (c-kit–PE) and FcεRI (IgE + rat anti–mouse–FITC).
Figure 4.
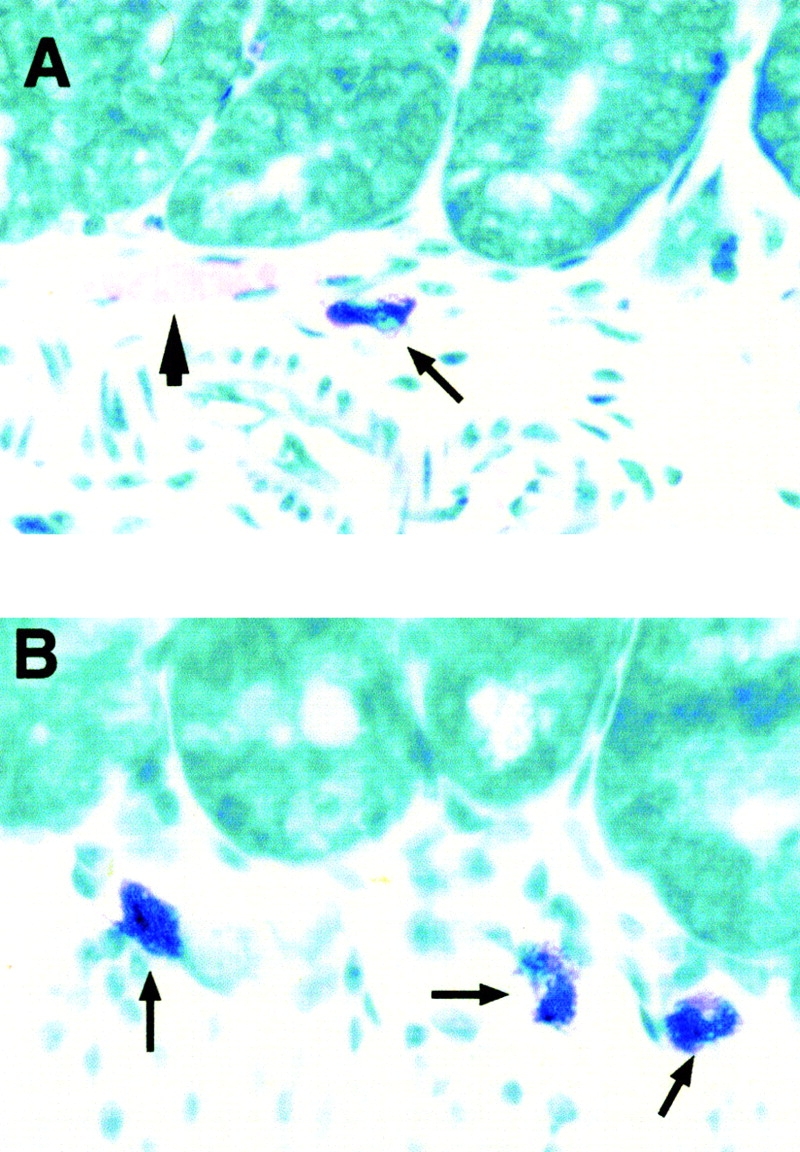
BMMC transplantation reconstitutes mast cell populations in organs of W/Wv mice. BMMCs were injected intravenously into groups of five to seven W/Wv mice. Mice were housed for 10 wk before being subjected to EAE disease induction along with age-matched W/Wv and wild-type controls. After a 30-d disease course, animals were killed, and Giemsa-stained sections were obtained from paraffin-embedded organ samples. (A) Mast cell (arrow) present in the gut of a wild-type F1 +/+ mouse. Arrowhead denotes blood vessel; ×40. (B) Mast cells (arrows) present in the gut of a BMMC-reconstituted W/Wv mouse; ×40.
The selectivity of the mast cell reconstitution was confirmed by hematocrit (Hct) determination 27 39 40 41. W/Wv mice are anemic (Hct 38.0 ± 3.0%) compared with wild-type F1 +/+ mice (Hct 51.5 ± 0.71%). Reconstituted W/Wv mice remain anemic (Hct 33.6 ± 3.1%) after BMMC transplantation, demonstrating that all hematologic deficits are not restored by this procedure.
10 wk after reconstitution, BMMC recipients as well as age-matched wild-type and W/Wv mice were subjected to the EAE disease induction protocol. As shown in Fig. 5 A, reestablishment of the mast cell population in W/Wv mice completely restored the ability of these animals to develop severe disease. When compared with wild-type mice, the mast cell–reconstituted animals showed a similar time of onset, daily mean clinical score, and disease incidence (Fig. 5). Inflammatory infiltrates in the brain and spinal cord were also similar (data not shown). In all disease parameters examined, significant differences existed between mast cell–deficient mice and those with intact mast cell compartments (Fig. 5 and Table ).
Figure 5.
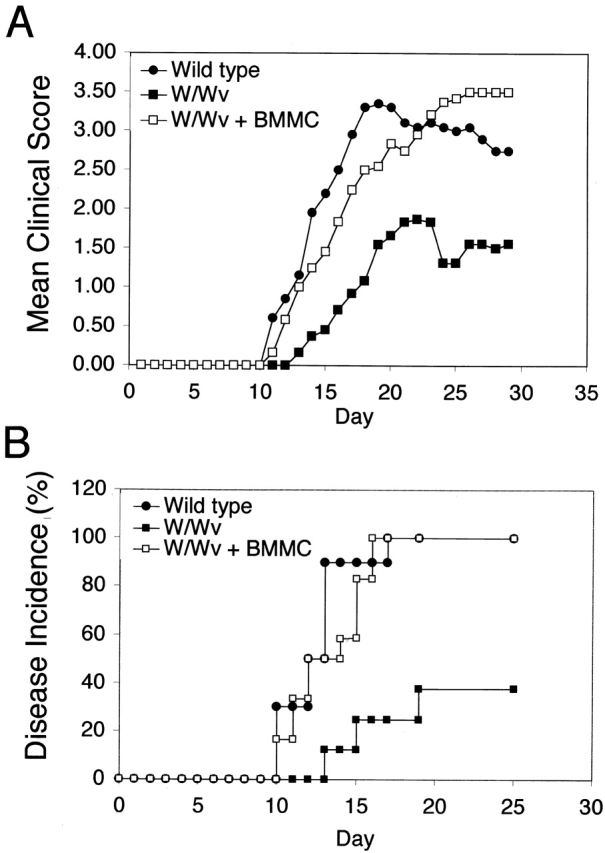
Reconstitution of W/Wv mice with BMMCs restores EAE disease onset and severity to wild-type levels. (A) Clinical scores were assigned daily to wild-type (n = 10), W/Wv (n = 8), and W/Wv + BMMCs (n = 12) mice, and the mean of each group was reported (P < 0.0001; post-test results comparing W/Wv to wild-type or W/Wv to reconstituted group: P < 0.001). Repeated measures of ANOVA followed by the Bonferroni post-test was used for comparison of the mean clinical scores of the three groups in the reconstitution experiments. (B) Graph represents the percent of total animals who demonstrated disease by day 30 after immunization (P < 0.004). Curve was plotted according to the method of Kaplan-Meier, and significance was calculated by the log-rank test. Results from A and B represent cumulative data from two independent experiments.
Table 2.
Cumulative Analysis of EAE Disease Parameters in BMMC Reconstitution Experiments
| Group | Incidence | Mean day of onset | Mean high score |
|---|---|---|---|
| P < 0.03 | P < 0.008 | P < 0.002 | |
| Wild-type F1 +/+ | 10/10 | 12.4 ± 0.64¶ | 3.45 ± 0.31¶ |
| W/Wv | 4/8 | 18.0 ± 2.64 | 1.63 ± 0.65 |
| W/Wv + BMMC | 12/12 | 13.1 ± 0.67 | 3.75 ± 0.17¶ |
In the reconstitution experiments, it was noted that wild-type and W/Wv animals demonstrated higher mean clinical scores than those observed in younger animals of respective genotypes (Fig. 1 A). In addition, some individual W/Wv mice had clinical scores as high as those of the wild-type animals. The explanation for these observations is unclear, but they may be due to age-related differences in host sensitivity to pertussis toxin or peptide dose. These possibilities are presently being examined.
Immunized W/Wv Mice Mount Anti-MOG–specific T and B Cell Responses Similar to Wild-Type F1 +/+ Mice.
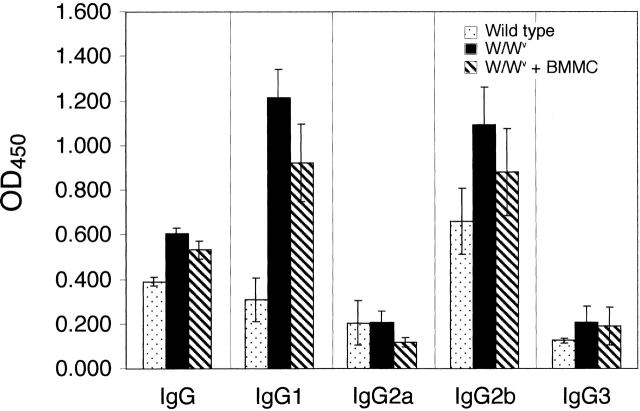
While it is formally possible that W/Wv mice have T cell deficits that could account for the differences in disease parameters demonstrated between wild-type and mast cell–deficient animals, we believe this is unlikely. Thymocytes are c-kit+, and the defect in c-kit carried by W/Wv mice could potentially hinder T cell development in these animals; however, previous characterizations of W/Wv mice revealed no such T cell deficits 44 45. It has also been demonstrated that IL-7, which has many activities that overlap with SCF, can direct the development of normal T cells in c-kit–deficient mice 44. In addition, we evaluated MOG-specific proliferative responses, cytokine profiles, and antibody production in both groups. Splenocytes from MOG-immunized wild-type and W/Wv mice mounted equivalent proliferative responses and IFN-γ cytokine production in response to in vitro stimulation with MOG peptide (data not shown). No IL-4 was detected in these assays. Wild-type and W/Wv mice, as well as BMMC-reconstituted W/Wv animals, produced similar levels of MOG-specific IgG (Fig. 6). MOG-specific IgG1 and IgG2b subtypes were also detected in all three groups. Interestingly, the MOG-specific IgG1 levels of W/Wv and BMMC-reconstituted W/Wv mice were significantly higher (P < 0.05, ANOVA) than those of wild-type mice. The biological significance of this observation is unclear. However, it may indicate that c-kit signaling pathways play an as yet unidentified role in B cell isotype switching. Alternatively, the kinetics of IgG1 antibody production may be altered in these mutant animals. Despite these differences in IgG1 levels, it is unlikely that this has a major effect on the development of EAE, because wild-type and BMMC-reconstituted mice exhibit similar disease courses. Also of note, total serum IgE was high in immunized animals within all groups, yet MOG-specific IgE was undetectable (data not shown). These results indicate that there are no global T or B cell deficits in W/Wv mice. Taken together with the demonstration that mast cell reconstitution with a virtually pure BMMC population restores disease susceptibility, these data support the hypothesis that it is the absence of mast cells in the W/Wv animals that predisposes them to delayed onset and less severe disease.
Figure 6.
Detection of MOG-specific IgG and IgG subclasses. Upon sacrifice, serum was obtained from wild-type (n = 16), W/Wv (n = 16), and BMMC-reconstituted W/Wv (n = 10) mice and analyzed for MOG-specific isotype and IgG subclass levels by ELISA. Results represent cumulative data from four experiments.
Discussion
The data reported in this study provide direct evidence that mast cells influence both the initiation and the severity of EAE in vivo, yet many questions regarding mast cell activation and effector mechanisms remain to be answered. Although cross-linkage of the high-affinity IgE receptor (FcεRI) on mast cells is a well characterized pathway of mast cell activation, there are several alternative pathways that could be operational in this disease. Ig-dependent mechanisms may include involvement of anti-MOG antibodies, which have been implicated in both human and rodent forms of the disease 34 46. Levels of IgG2b in particular are correlated with disease severity in MOG-induced EAE in NOD mice 47. Our finding that both IgG1 and IgG2b are produced in MOG-induced EAE, coupled with the fact that mast cells express FcγRIIB/III (receptors that specifically interact with these Ig subtypes; reference 48), is consistent with the possibility that these antibodies have a role in FcγR-mediated mast cell activation.
Mast cells can also be directly activated via Ig-independent pathways by neuropeptides, such as substance P, certain complement components, and estradiol, an observation that may explain the increased susceptibility of females to MS 49 50. It was recently shown that activated T lymphocytes can induce degranulation and cytokine production by human mast cells after cell–cell contact 51 52. These data indicate that direct interaction with autoreactive T cells may be sufficient for mast cell activation.
The site of mast cell activation and influence in this model of EAE is also unknown. We did not detect mast cells in the CNS lesions from wild-type or mast cell–reconstituted W/Wv mice. This may be due to the difficulty of detecting degranulated mast cells using classic histologic stains. Because of the potent activity of mast cell mediators, very few mast cells may be required to exert profound local effects. Alternatively, mast cells may act at sites distant from the site of CNS destruction. Activated mast cells can migrate to local lymph nodes 53, indicating their potential to influence naive T cell activation and differentiation. Once mast cell activation occurs, the release of numerous mast cell mediators could act at several levels to influence disease induction and/or progression. For example, alteration of the blood–brain barrier through release of vasoactive amines may facilitate entry of autoreactive T cells into the CNS 54 55 56. Proinflammatory cytokines such as TNF-α could regulate endothelial expression of adhesion molecules, kill myelin-producing cells, and degrade myelin components 57 58. TNF-α has also been shown to promote local presentation of autoantigen in the diabetic model of NOD mice 59. Mast cell proteases may directly damage the myelin sheath and adjacent nerves 19 21 22. Finally, regulatory cytokines such as IL-4 and IL-10 could influence the development of an autoimmune T cell response or modulate an ongoing response both in the periphery and within the CNS 60 61.
Until recently, the contribution of mast cells to nonspecific and specific inflammatory processes was virtually ignored outside the realm of allergy research. It is becoming increasingly clear that mast cells can provide protection in bacterial infections 27 42. Through their ability to regulate a myriad of both adaptive and innate immune responses, mast cells may play a major role in many immune-mediated diseases as well. The demonstration that mast cells are significant effector cells in EAE alters the way we have classically thought about this disease in humans. These data pave the way for completely new avenues of immunotherapy that could complement treatment regimens based solely on altering the autoreactive T cell response.
Acknowledgments
We thank J.A. Kapp, S.W. Caughman, B.D. Evavold, R.D. Lopez, and A.E. Lukacher for helpful discussions, J. Holden for assistance with histological analyses, and A.W. Hightower for assistance with statistical analyses.
This work was supported in part by the National Multiple Sclerosis Society. M.A. Brown was supported by a scholarship from the Leukemia Society of America.
Footnotes
Abbreviations used in this paper: ANOVA, analysis of variance; BMMCs, bone marrow–derived mast cells; CNS, central nervous system; EAE, experimental allergic encephalomyelitis; Hct, hematocrit; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis.
References
- Steinman L. Multiple sclerosisa coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- French-Constant C. Pathogenesis of multiple sclerosis. Lancet. 1994;343:271–275. doi: 10.1016/s0140-6736(94)91118-5. [DOI] [PubMed] [Google Scholar]
- Kermode A.G. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Brain. 1990;113:1477–1489. doi: 10.1093/brain/113.5.1477. [DOI] [PubMed] [Google Scholar]
- Johnson D., Yasui D., Seeldrayers P. An analysis of mast cell frequency in the rodent nervous systemnumbers vary between different strains and can be reconstituted in mast cell-deficient mice. J. Neuropathol. Exp. Neurol. 1991;50:227–234. doi: 10.1097/00005072-199105000-00005. [DOI] [PubMed] [Google Scholar]
- Orr E.L. Presence and distribution of nervous system-associated mast cells that may modulate experimental autoimmune encephalomyelitis. Ann. NY Acad. Sci. 1988;540:723–726. doi: 10.1111/j.1749-6632.1988.tb27226.x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt R.C., Hough L.B., Glick S.D., Padawer J. Mast cells in the rat thalamusnuclear localization, sex differences, and left-right asymmetry. Brain Res. 1984;323:209–217. doi: 10.1016/0006-8993(84)90291-9. [DOI] [PubMed] [Google Scholar]
- Ibrahim M.Z.M. Mast cells in the mammalian central nervous system. Part 1. Morphology, distribution and histochemistry. J. Neurol. Sci. 1974;21:431–478. doi: 10.1016/0022-510x(74)90044-6. [DOI] [PubMed] [Google Scholar]
- Gordon J.R., Galli S.J. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Begolka W.S., Vanderlugt C.L., Rahbe S.M., Miller S.D. Differential expression of inflammatory cytokines parallels progression of CNS pathology in two clinically distinct models of MS. J. Immunol. 1998;161:4437–4446. [PubMed] [Google Scholar]
- Selmaj K., Raine C.S., Cannella B., Brosnan C.F. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J. Clin. Invest. 1991;87:949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renno T., Krakowski M., Piccirillo C., Lin J.Y., Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J. Immunol. 1995;154:944–953. [PubMed] [Google Scholar]
- Khoury S.J., Hancock W.W., Weiner H.L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor β, interleukin 4, and prostaglandin E expression in the brain. J. Exp. Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman J. Ueber das Vorkommen der sogneannten “Mastzellen” bei pathologischen Veraenderungen des Gehirns. Arch. Pathol. Anat. Physiol. Virchows. 1890;122:378–381. [Google Scholar]
- Toms R., Weiner H.L., Johnson D. Identification of IgE-positive cells and mast cells in frozen sections of multiple sclerosis brains. J. Neuroimmunol. 1990;30:169–177. doi: 10.1016/0165-5728(90)90101-r. [DOI] [PubMed] [Google Scholar]
- Olsson Y. Mast cells in plaques of multiple sclerosis. Acta Neurol. Scand. 1974;50:611–618. doi: 10.1111/j.1600-0404.1974.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Lafaille J.J., Van de Keere F., Hsu A.L., Baron J.L., Haas W., Raine C.S., Tonegawa S. Myelin basic protein–specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J. Exp. Med. 1997;186:307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner T., Soffer D., Shalit M., Levi-Schaffer F. Mast cells in experimental allergic encephalomyelitischaracterization, distribution in the CNS and in vitro activation by myelin basic protein and neuropeptides. J. Neurol. Sci. 1994;122:210–213. doi: 10.1016/0022-510x(94)90300-x. [DOI] [PubMed] [Google Scholar]
- Rozniecki J.J., Hauser S.L., Strein M., Lincoln R., Theoharides T.C. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann. Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- Watson S.L., Westlan K., Pollard J.D. An electrophysiological and histological study of trypsin induced demyelination. J. Neurol. Sci. 1994;126:116–125. doi: 10.1016/0022-510x(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Johnson D., Seeldrayers P.A., Weiner H.L. The role of mast cells in demyelination. 1. Myelin proteins are degraded by mast cell proteases and myelin basic protein and P2 can stimulate mast cell degranulation. Brain Res. 1988;44:195–198. doi: 10.1016/0006-8993(88)90929-8. [DOI] [PubMed] [Google Scholar]
- Dietsch G.N., Hinrichs D.J. Mast cell proteases liberate stable encephalitogenic fragments from intact myelin. Cell. Immunol. 1991;135:541–548. doi: 10.1016/0008-8749(91)90297-o. [DOI] [PubMed] [Google Scholar]
- Dietsch G.N., Hinrichs D.J. The role of mast cells in the elicitation of experimental allergic encephalomyelitis. J. Immunol. 1989;142:1476–1481. [PubMed] [Google Scholar]
- Babington R., Wedeking P. The influence of cinanserin and selected pharmacologic agents on experimental allergic encephalomyelitis (EAE) J. Pharmacol. Exp. Ther. 1971;177:455–460. [PubMed] [Google Scholar]
- Waxman F., Taguiam J., Whitacre C. Modification of the clinical and histopathologic expression of experimental allergic encephalomyelitis by the vasoactive amine antagonist cyproheptadine. Cell. Immunol. 1984;85:82–93. doi: 10.1016/0008-8749(84)90280-6. [DOI] [PubMed] [Google Scholar]
- Mendel I., Kerlero de Rosbo N., Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b micefine specificity and T cell receptor Vβ expression of encephalitogenic T cells. Eur. J. Immunol. 1995;26:1951–1959. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- Yung Y., Eger R., Tertian G., Moore M.A.S. Long-term in vitro culture of murine mast cells. II. Purification of a mast cell growth factor and its dissociation from TCGF. J. Immunol. 1981;127:794–799. [PubMed] [Google Scholar]
- Echtenacher B., Mannel D.N., Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- Galli S.J., Tsai M., Gordon J.R., Geissler E.N., Wershil B.K. Analyzing mast cell development and function using mice carrying mutations at W/c-kit or Sl/MGF (SCF) loci. Ann. NY Acad. Sci. 1992;664:69–88. doi: 10.1111/j.1749-6632.1992.tb39750.x. [DOI] [PubMed] [Google Scholar]
- Sonoda S., Sonoda T., Nakano T., Kanayama Y., Kanakura Y., Asai H., Yonezawa T., Kitamura Y. Development of mucosal mast cells after injection of a single connective tissue-type mast cell in the stomach mucosa of genetically mast cell-deficient W/Wv mice. J. Immunol. 1986;137:1319–1322. [PubMed] [Google Scholar]
- Rottem M., Hull G., Metcalfe D.D. Demonstration of differential effects of cytokines on mast cells derived from murine bone marrow and peripheral blood mononuclear cells. Exp. Hematol. 1994;22:1147–1155. [PubMed] [Google Scholar]
- Smith T.J., Ducharme L.A., Weis J.H. Preferential expression of interleukin-12 or interleukin-4 by murine bone marrow mast cells derived in mast cell growth factor or interleukin-3. Eur. J. Immunol. 1994;24:822–826. doi: 10.1002/eji.1830240408. [DOI] [PubMed] [Google Scholar]
- Bernard C.C.A., Johns T.G., Slavin A., Ichikawa M., Ewing C., Liu J., Bettadapura J. Myelin oligodendrocyte glycoproteina novel candidate autoantigen in multiple sclerosis. J. Mol. Med. 1997;75:77–88. doi: 10.1007/s001090050092. [DOI] [PubMed] [Google Scholar]
- Lebar R., Lubetzki C., Vincent C., Robineaus R., Voisin G.A. The M2 autoantigen of central nervous system myelin, a glycoprotein present in oligodendrocyte membrane. Clin. Exp. Immunol. 1976;66:423–443. [PMC free article] [PubMed] [Google Scholar]
- Genain C.P., Cannella B., Hauser S.L., Raine C.S. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat. Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- Ibrahim M.Z.M., Reder A.T., Lawand R., Takash W., Sallouh-Khatib S. The mast cells of the multiple sclerosis brain. J. Neuroimmunol. 1996;70:131–138. doi: 10.1016/s0165-5728(96)00102-6. [DOI] [PubMed] [Google Scholar]
- Nocka K., Majumder S., Chabot B., Ray P., Cervone M., Bernstein A., Besmer P. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice—evidence for an impaired c-kit kinase in mutant mice. Genes Develop. 1989;3:816–826. doi: 10.1101/gad.3.6.816. [DOI] [PubMed] [Google Scholar]
- Nocka K., Tan J.C., Chiu E., Chu T.Y., Ray P., Traktman P., Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locusW37, Wv, W41 and W. EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S.J. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. J. Exp. Med. 1985;162:1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu K., Nakano T., Kanakura Y., Asai H., Katz H.R., Austen K.F., Stevens R.L., Galli S.J., Kitamura Y. Phenotypic changes of bone marrow–derived mast cells after intraperitoneal transfer into W/Wv mice that are genetically deficient in mast cells. J. Exp. Med. 1987;165:615–627. doi: 10.1084/jem.165.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M., Echtenacher B., Hultner L., Kollias G., Mannel D.N., Langley K.E., Galli S.J. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J. Exp. Med. 1998;188:2343–2348. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R., Ikeda T., Ross E., Abraham S.N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- Galli S.J., Kitamura Y. Genetically mast-cell-deficient W/Wv and Sl/Sld micetheir value for the analysis of the roles of mast cells in biologic responses in vivo. Am. J. Pathol. 1987;127:191–198. [PMC free article] [PubMed] [Google Scholar]
- Rodewald H.R., Ogawa M., Haller C., Waskow C., DiSanto J.P. Pro-thymocyte expansion by c-kit and the common cytokine receptor gamma chain is essential for repertoire formation. Immunity. 1997;6:265–272. doi: 10.1016/s1074-7613(00)80329-5. [DOI] [PubMed] [Google Scholar]
- Yu C.Z., Hisha H., Li Y., Lian Z., Nishino T., Toki J., Adachi Y., Inaba M., Fan T.R.X., Jin T. Stimulatory effects of hepatocyte growth factor on hemopoiesis of SCF/c-kit system-deficient mice. Stem Cells. 1998;16:66–77. doi: 10.1002/stem.160066. [DOI] [PubMed] [Google Scholar]
- Ichikawa M., Johns T.G., Adelmann M., Bernard C.C.A. Antibody response in Lewis rats injected with myelin oligodendrocyte glycoprotein derived peptides. Int. Immunol. 1996;8:1667–1674. doi: 10.1093/intimm/8.11.1667. [DOI] [PubMed] [Google Scholar]
- Ichikawa M., Koh C.-S., Inaba Y., Seki C., Inoue A., Itoh M., Ishihara Y., Bernard C.C.A., Komiyama A. IgG subclass switching is associated with the severity of experimental autoimmune encephalomyelitis induced with myelin oligodendrocyte glycoprotein peptide in NOD mice. Cell. Immunol. 1999;191:97–104. doi: 10.1006/cimm.1998.1414. [DOI] [PubMed] [Google Scholar]
- Ravetch J.V., Kinet J.-P. Fc receptors. Annu. Rev. Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Ebertz J.M., Hirschman C.A., Kettlekamp N.S., Uno H., Hanifin J.M. Substance P induced histamine release in human cutaneous mast cells. J. Invest. Dermatol. 1987;88:682–685. doi: 10.1111/1523-1747.ep12470339. [DOI] [PubMed] [Google Scholar]
- Theoharides T.C., Dimitriadou V., Letourneau R., Rozneicki J.J., Vliagoftis H., Boucher W. Synergistic action of estradiol and myelin basic protein on mast cell secretion and brain myelin changes resembling early stages of demyelination. Neuroscience. 1993;57:861–871. doi: 10.1016/0306-4522(93)90030-j. [DOI] [PubMed] [Google Scholar]
- Inamura N., Mekori Y.A., Bhattacharyya S.P., Bianchine P.J., Metcalfe D.D. Induction and enhancement of FceRI-dependent mast cell degranulation following coculture with activated T cellsdependency on ICAM-1- and leukocyte function-associated antigen (LFA)-1-mediated heterotypic aggregation. J. Immunol. 1998;160:4026–4033. [PubMed] [Google Scholar]
- Bhattacharyya S.P., Drucker I., Reshef T., Kirshenbaum A.S., Metcalfe D.D., Mekori Y.A. Activated T lymphocytes induce degranulation and cytokine production by human mast cells following cell-to-cell contact. J. Leukoc. Biol. 1998;63:337–341. doi: 10.1002/jlb.63.3.337. [DOI] [PubMed] [Google Scholar]
- Wang H.-W., Tedia N., Lloyd A.R., Wakefield D., McNeil H.P. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J. Clin. Invest. 1998;102:1617–1626. doi: 10.1172/JCI3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr E.L., Stanley N.C. Brain and spinal cord levels of histamine in Lewis rats with acute experimental autoimmune encephalomyelitis. J. Neurochem. 1989;53:111–118. doi: 10.1111/j.1471-4159.1989.tb07301.x. [DOI] [PubMed] [Google Scholar]
- Bebo B.F., Jr., Lee C.H., Orr E.L., Linthicum D.S. Mast cell-derived histamine and tumor necrosis factordifferences between SJL/J and BALB/c inbred strains of mice. Immunol. Cell Biol. 1996;74:225–230. doi: 10.1038/icb.1996.41. [DOI] [PubMed] [Google Scholar]
- Rosenblum W.I. A possible role for mast cells in controlling the diameter of arterioles on the surface of the brain. Brain Res. 1973;42:75–82. doi: 10.1016/0006-8993(73)90402-2. [DOI] [PubMed] [Google Scholar]
- Cannella B., Cross A.H., Raine C.S. Upregulation and coexpression of adhesion molecules correlated with relapsing autoimmune demyelination in the central nervous system. J. Exp. Med. 1990;172:1521–1524. doi: 10.1084/jem.172.5.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K., Raine C.S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann. Neurol. 1988;1988:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Green E.A., Eynon E.E., Flavell R.A. Local expression of TNF-alpha in neonatal NOD mice promotes diabetes by enhancing presentation of islet antigens. Immunity. 1998;9:733–743. doi: 10.1016/s1074-7613(00)80670-6. [DOI] [PubMed] [Google Scholar]
- Rott O., Fleischer B., Cash E. Interleukin-10 prevents experimental autoimmune encephalomyelitis in rats. Eur. J. Immunol. 1994;24:1434–1440. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- Inobe J.I., Chen Y., Weiner H.L. In vivo administration of IL-4 induces TGF-β producing cells and protects animals from experimental autoimmune encephalomyelitis. Ann. NY Acad. Sci. 1996;778:390–392. doi: 10.1111/j.1749-6632.1996.tb21153.x. [DOI] [PubMed] [Google Scholar]