Since its discovery in 1994, IL-15 has been studied in the context of its structural cousin, IL-2 1. Early biochemical and in vitro lymphocyte studies revealed many similarities between IL-15 and IL-2: (a) IL-2 and IL-15 constitute the only two members of a family of cytokines containing a four α helix bundle; (b) the heterotrimeric receptors for IL-2 and IL-15 share both the IL-2/15Rβ and IL-2R common γ (IL-2Rγ or γc) chains; (c) the shared IL-2/15Rβ and γc chains are responsible for downstream cytoplasmic signals; and (d) the IL-2Rα and IL-15Rα chains, which confer specificity to IL-2R and IL-15R for their respective ligands, are closely related proteins 2. However, the highly restricted expression of IL-2 and IL-2Rα to activated T cells suggests that IL-2R signals perform unique functions in adaptive T cells, whereas the pleiotropic expression of IL-15 and IL-15Rα hints at a much broader role for IL-15R in multiple cell types. Indeed, distinct functions for IL-2 and IL-15 have been suggested from in vivo studies. IFN regulatory factor (IRF-1)–deficient mice lack NK cells due to an inability of their bone marrow stroma to elaborate IL-15, demonstrating a unique role for IL-15 in supporting NK cell development 3. Comparative studies of IL-2Rα–deficient and IL-2/15Rβ–deficient mice (where only the latter should lack IL-15R signals) suggest that IL-15 might also be important for the differentiation of NK T cells and certain subsets of intraepithelial lymphocytes (IELs) 4 5. Finally, exogenous IL-15, but not IL-2, selectively induces the proliferation of memory phenotype CD8+ T cells in normal mice 6. Thus, emerging from the shadow of IL-2, IL-15 may serve a wide range of immune functions.
The unique roles for IL-15 in vivo have become clearer with the initial reports of IL-15–deficient (IL-15−/−) mice by Kennedy et al. in this issue 7 and IL-15Rα−/− mice 8. While IL-2−/− and IL-2Rα−/− mice spontaneously accumulate activated T and B cells and die prematurely from autoimmune disease, IL-15−/− and IL-15Rα−/− mice are generally healthy, lymphopenic, and specifically lack NK cells, NK T cells, intestinally derived subsets of IELs, and activated CD8+ T cells 7 8 9 10. The loss of these cells demonstrates that IL-15 signals via IL-15Rα are critical for lymphoid development and/or maintenance, particularly for innate immune cells. By showing that 7 d of IL-15 treatment salvages these cells, Kennedy et al. suggest that the peripheral maintenance of these cells may be a prominent aspect of IL-15 function 7. This observation is consistent with prior work indicating that IL-15 supports peripheral memory phenotype CD8+ T cells 6 8. It also suggests that CD8+ T cells may partially resemble innate immune cells. Furthermore, IL-15 also induces the activation and cytotoxicity of mature NK cells 11. In addition, IL-15 clearly regulates innate immune cell differentiation. Bone marrow stroma elaborates IL-15 to support NK cell differentiation 3 12, and IL-15 skews the differentiation of thymic progenitors towards NK T cells (13; Fig. 1). Thus, IL-15 is critical for mediating the development, homeostasis, and activation of innate immune cells.
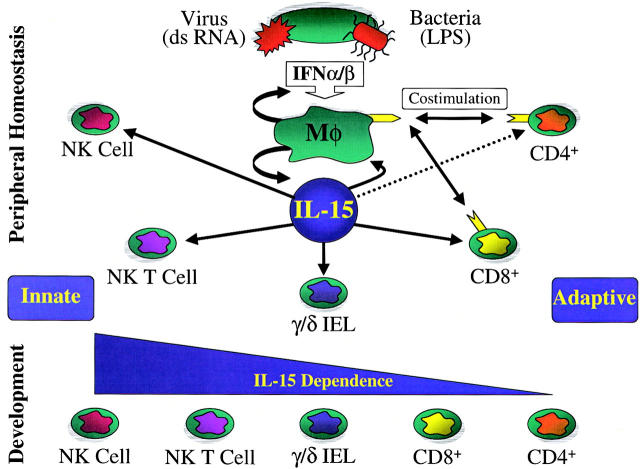
Figure 1.
IL-15 preferentially supports innate immune cell development and homeostasis. Upon bacterial or viral infection, conserved motifs (such as double-stranded [ds] RNA or LPS) result in the release of type I IFNs (IFN-α/β) from infected host cells. Type I IFNs activate macrophages (Mφ), stimulating them to produce IL-15 as well as inducing the upregulation of costimulatory molecules. IL-15 selectively activates NK cells, NK T cells, intestinal γ/δ T cells (γ/δ IEL), and CD8+ T cells. From a developmental standpoint, innate immune cells also appear to rely more on IL-15R signals.
The finding that IL-15−/− and IL-15Rα−/− mice are lymphopenic suggests that IL-15 may also support adaptive CD4+ and CD8+ T cells 7 8. Whether IL-15 regulates adaptive lymphocyte differentiation remains to be elucidated, but the reduced thymic cellularity of IL-15Rα−/− mice suggests that this may be the case 8. Nevertheless, it is clear that IL-15 provides tonic proliferative and/or survival signals to support mature lymphocytes 6 7 8. As IL-15 is elaborated by nonlymphoid (stromal and epithelial) cells, physiological delivery of IL-15 to adaptive T lymphocytes may predominantly occur within nonlymphoid stroma. For example, both muscle and epithelial cells express and respond to IL-15, so tissue-bound lymphocytes may share these tissue pools of IL-15 and use them for homeostatic maintenance 14 15. In this context, the intestinal mucosa—which harbors large numbers of innate immune cells responding to luminal microbes, and whose epithelial and stromal cells elaborate IL-15—is an excellent microenvironment for such interactions. Future studies of mucosal and other tissue-bound lymphocytes are thus likely to reveal novel insights into how IL-15 supports lymphocyte homeostasis.
The propensity of antigen-experienced, or memory, T lymphocytes to circulate through nonlymphoid tissues suggests that IL-15 function may extend to the maintenance of immunological memory. Because memory T cells may express elevated levels of IL-15Rα and IL-2/15Rβ as a result of antigenic exposure, they may proliferate and survive in response to tissue-derived IL-15. As noted above, such stimulation could occur within nonlymphoid stroma, in the relative absence of cognate antigens, active inflammation, or lymphocyte-derived cytokines. Moreover, IL-15 is expressed by activated endothelial cells and may regulate the capacity of T lymphocytes to migrate across endothelial barriers to target tissues 16. Thus, several approaches may be required to fully understand the scope of IL-15's potential memory function. It will also be important to determine which cellular sources of IL-15 are responsible for supporting memory cells. How IL-15 may be involved in the maintenance of immunological memory is clearly an important and testable issue that is greatly facilitated by the availability of IL-15−/− and IL-15Rα−/− mice.
An essential role for IL-15 in immune responses is highlighted by the inability of IL-15−/− mice to survive vaccinia virus infection 7. While NK cell antiviral responses are likely to be compromised in these mice, the selective loss of memory phenotype CD8+ T cells also raises the possibility that CD8+ T cell antiviral responses may also be defective. Since CD8+ T cells resemble classical innate immune cells in their homeostatic reliance on IL-15, and since IL-15 activates NK cells, it is possible that IL-15 also stimulates CD8+ T cell cytolytic competence. Additional studies are now needed to define which IL-15 function(s) is essential for successful antiviral responses. Other immune responses may be less reliant on IL-15, and understanding the role(s) of IL-15 in different systems should lead to a better understanding of the mechanisms by which different immunogens induce distinct innate (and subsequently, adaptive) immune responses (17; Fig. 1). Moreover, manipulating the expression or activity of IL-15 and IL-15Rα (perhaps in conjunction with other costimulatory molecules) will likely be an important area for modulating immune responses.
The fate of activated T lymphocytes to undergo programmed cell death or persist as memory cells is central to the regulation of immune responses. In this context, it is essential to note that activated cells express both IL-2Rα and IL-15Rα. IL-2 predisposes activated T cells to undergo programmed cell death, whereas IL-15 appears to support proliferation (and possibly survival), so these cells must distinguish between IL-2R and IL-15R signals. The importance of this distinction is emphasized by the divergent phenotypes of the mutant mice. This difference forces us to return to the IL-2R and IL-15R signaling complexes and ask how the distinction is made. Ligand availability is likely to be a major factor; activated T cells are the only cells expressing IL-2, whereas multiple cell types express IL-15. Differential induction of IL-2Rα and IL-15Rα is another potential factor. Also, IL-15 binds to IL-15Rα alone with far greater affinity than IL-2 binds to IL-2Rα alone, so cells expressing both receptors may preferentially bind to IL-15 under conditions where both cytokines are present 18. Finally, the possibility remains that IL-15Rα engagement induces IL-2/15Rβ and γc signaling events that are quantitatively distinct from those initiated by IL-2Rα binding 19. The strength of these signals may then translate into biologically distinct outcomes for the T cell (e.g., a stronger activation or proliferation stimulus may predispose to programmed cell death).
In summary, the report by Kennedy et al. strongly reinforces the concept that IL-15 performs pleiotropic developmental and homeostatic immune functions, particularly for innate immune cells 7. Curiously, despite the biochemical similarities of IL-2R and IL-15R signals, none of the in vivo biological functions of IL-15 appear to overlap with IL-2's critical role in downregulating activated T cells. The initial findings in IL-15−/− and IL-15Rα−/− mice prompt many new questions about how IL-15 mediates these functions. For example, do all IL-15 signals use IL-15Rα, and how are IL-2R and IL-15R signals biochemically distinguished? To what extent does IL-15 support lymphocyte proliferation versus survival? Additional important future directions include understanding IL-15's diverse roles in: NK cell differentiation, activation, and proliferation; NK T cell function; CD8+ T cell activation and homeostasis; mucosal immunity; innate and adaptive immune responses; and immunological memory. It will also be important to determine whether IL-15 mediates more subtle functions in CD4+ T cells. As these investigations are completed, it may become apparent that IL-15 will encompass far broader biological functions than its scientifically older (but perhaps evolutionarily younger) cousin, IL-2.
Acknowledgments
This work is supported by National Institutes of Health grants RO1 DK52751 and AI45860, Digestive Disease Research Center grant DK42086, and the Gastrointestinal Research Foundation.
References
- Grabstein K.H., Eisenman J., Shanebeck K., Rauch C., Srinivasan S., Fung V., Beers C., Richardson J., Schoenborn M.A., Ahdieh M. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- DiSanto J.P. Cytokinesshared receptors, distinct functions. Curr. Biol. 1997;7:R424–R426. doi: 10.1016/s0960-9822(06)00208-9. [DOI] [PubMed] [Google Scholar]
- Ogasawara K., Hida S., Azimi N., Tagaya Y., Sato T., Yokochi-Fukuda T., Waldmann T.A., Taniguchi T., Taki S. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature. 1998;391:700–703. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- Ohteki T., Ho S., Suzuki H., Mak T.W., Ohashi P.S. Role for IL-15/IL-15 receptor beta-chain in natural killer 1.1+ T cell receptor-alpha beta+ cell development. J. Immunol. 1997;159:5931–5935. [PubMed] [Google Scholar]
- Suzuki H., Duncan G.S., Takimoto H., Mak T.W. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor beta chain. J. Exp. Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Sun S., Hwang I., Tough D.F., Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- Kennedy M.K., Glaccum M., Brown S.N., Butz E.A., Viney J.L., Embers M., Matsuki N., Charrier K., Sedger L., Willis C.R., Brasel K., Morrissey P.J., Stocking K., Schuh J.C.L., Joyce S., Peschon J.J. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15–deficient mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce J.P., Boone D.L., Chai S., Swain R.E., Dassopoulos T., Trettin S., Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A.C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Willerford D.M., Chen J., Ferry J.A., Davidson L., Ma A., Alt F.W. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- Carson W.E., Giri J.G., Lindemann M.J., Linett M.L., Ahdieh M., Paxton R., Anderson D., Eisenmann J., Grabstein K., Caligiuri M.A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrozek E., Anderson P., Caligiuri M.A. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–2640. [PubMed] [Google Scholar]
- Leclercq G., Debacker V., de Smedt M., Plum J. Differential effects of interleukin-15 and interleukin-2 on differentiation of bipotential T/natural killer progenitor cells. J. Exp. Med. 1996;184:325–336. doi: 10.1084/jem.184.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn L.S., Haugk K.L., Grabstein K.H. Interleukin-15a novel anabolic cytokine for skeletal muscle. Endocrinology. 1995;136:3669–3672. doi: 10.1210/endo.136.8.7628408. [DOI] [PubMed] [Google Scholar]
- Reinecker H.C., MacDermott R.P., Mirau S., Dignass A., Podolsky D.K. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology. 1996;111:1706–1713. doi: 10.1016/s0016-5085(96)70036-7. [DOI] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Brezinschek R.I., Mohamadzadeh M., Vita R., Lipsky P.E. Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells in vitro and in the SCID mouse–human rheumatoid arthritis model in vivo. J. Clin. Invest. 1998;101:1261–1272. doi: 10.1172/JCI1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron C.A., Nguyen K.B., Pien G.C., Cousens L.P., Salazar-Mather T.P. Natural killer cells in antiviral defensefunction and regulation by innate cytokines. Annu. Rev. Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Giri J.G., Kumaki S., Ahdieh M., Friend D.J., Loomis A., Shanebeck K., DuBose R., Cosman D., Park L.S., Anderson D.M. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Parijs L., Refaeli Y., Lord J.D., Nelson B.H., Abbas A.K., Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]