Abstract
PD-1 is an immunoglobulin superfamily member bearing an immunoreceptor tyrosine-based inhibitory motif, and disruption of the PD-1 gene results in the development of lupus-like autoimmune diseases. In this study, we examined effects of the PD-1 deficiency on the thymocyte differentiation at the clonal level using T cell receptor (TCR)-β (Vβ8) and TCR-α/β (H-Y and 2C) transgenic mice. In these TCR transgenic lines, PD-1 expression in the thymus was variably augmented, but as in the normal mice, confined largely to the CD4−CD8− thymocytes. The transgenic mice crossed with PD-1−/− mice in the neutral genetic backgrounds exhibited selective increase in the CD4+CD8+ (DP) population with little effect on other thymocytes subsets. Similarly, the absence of PD-1 facilitated expansion of DP thymocytes in recombination activating gene (RAG)-2−/− mice by anti-CD3ε antibody injection. On the other hand, H-Y or 2C transgenic PD-1−/− mice with the positively selecting background showed significantly reduced efficiency for the generation of CD8+ single positive cells bearing the transgenic TCR-α/β in spite of the increased DP population. These results collectively indicate that PD-1 negatively regulates the β selection and modulates the positive selection, and suggest that PD-1 deficiency may lead to the significant alteration of mature T cell repertoire.
Keywords: immunoreceptor tyrosine-based inhibitory motif, knock-out mice, positive selection, T cell receptor transgenic mice, RAG-2–deficient mice
Introduction
Progenitor T cells must go through distinct check points as they differentiate in the thymus before becoming mature T cells 1. The most immature CD4−CD8− (DN) thymocytes 2 can differentiate into CD4+CD8+ (DP) cells only after the expression of TCR β chains, a process termed β selection 3 4. The DP thymocytes, which subsequently rearrange the TCR α chain genes and express TCR-α/β, are subjected to the positive and negative selection by the specificity of TCR 5 6 7. Only positively selected thymocytes can mature into either CD4+ or CD8+ single positive (SP) T cells. In the β selection of pre-T cells, the signaling from TCR-β/pre–TCR-α (pTα)–CD3 complex (8; pre-TCR) is shown to play a crucial role 9 10 11. Based on the analysis of pTα-deficient mice 10, it is indicated that pre-T cells drastically expand during β selection, which is essential for the subsequent generation of clonal diversification. However, it remains to be verified whether the β selection process somehow affects the final TCR repertoire of mature T cells.
PD-1 is a 55-kD type I transmembrane protein of the immunoglobulin superfamily bearing an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic region 12 13. In the thymus, PD-1 is selectively expressed in a very minor population of DN thymocytes 14, while it is also induced on peripheral T and B cells after activation 12. Expression of PD-1 in DN thymocytes is significantly augmented in both normal and recombination activating gene (RAG)-2–deficient mice by the injection of anti-CD3 mAb, implying that PD-1 might be involved in the β selection process 14. Recently, PD-1–deficient mice were found to spontaneously develop autoimmune diseases characterized by lupus-like glomerulonephritis with immune complex deposition and destructive arthritis as they age 16, and it has been suggested that the PD-1 deficiency results in the breakdown of peripheral tolerance against self-reactive T cells 16. The possibility that the PD-1 deficiency affects the repertoire of mature T cells also remains open 15. In the present study, we report that the absence of PD-1 significantly facilitates the transition of thymocytes from DN to DP stage in the TCR transgenic lines as well as in RAG-2−/− mice injected with anti-CD3 mAb, suggesting that PD-1 controls the threshold of β selection. On the other hand, efficiency of positive selection for the transgenic T cells in the relevant genetic background was significantly reduced in the absence of PD-1. Mechanisms for the opposing effects of PD-1 deficiency on β and positive selections as well as their possible involvement in the development of lupus-like diseases are discussed.
Materials and Methods
Mice.
C57BL/6 (B6) mice were purchased from Japan SLC. PD-1–deficient mice 15 were backcrossed into B6 mice for at least eight generations. Vβ8 17, H-Y 18 and 2C transgenic 19, and RAG-2−/− mice 20 were maintained in specific pathogen-free conditions. For genotyping of mice, restricted DNAs of TCR transgenic (Vβ8, H-Y, and 2C; EcoRI) and RAG-2−/− alleles (EcoRI plus EcoRV) were probed with the 300-bp PstI-SacI fragment of Vβ8.2 and the 950-bp PstI-EcoRV fragment of RAG-2 cDNA, respectively. The transgenes and the targeted alleles were confirmed by the appearance of additional bands at ∼2.4 and 1.2 kb, respectively.
Antibodies.
mAbs for PD-1 12, H-Y TCR-α (T3.70 21), 2C TCR-α/β clonotype (1B2 22), and CD3ε (145-2C11) were all purified from ascites and biotinylated if necessary. The following mAbs were purchased from PharMingen: anti–mouse CD8α-FITC, phycoerythrin-R (PE)-conjugated anti-CD4 (RM4-5), biotinylated anti–mouse CD24 (M1/69), FITC- and biotinylated anti–mouse CD69 (H1.2F3), biotinylated anti–mouse CD8β (53-5.8), and biotinylated anti–mouse TCR-δ (GL3). Streptavidin-conjugated RED670 was obtained from GIBCO BRL.
Flow Cytometry.
Flow cytometric analysis was performed as described previously 15. Analysis was performed using a FACSCalibur™ (Becton Dickinson) and CELLQuest™ software (Becton Dickinson).
Results and Discussion
We have comparatively analyzed the expression of PD-1 in the thymus of normal and various TCR transgenic mice. As shown in Fig. 1 a, PD-1 was expressed in only ∼1% of total thymocytes of normal mice, which was confined to the DN population as reported previously 14. PD-1+ cells in the DN population showed two peaks, PD-1high and PD-1low, which represented γ/δ T cells and α/β T cells, respectively 14. In the thymus of Vβ8 transgenic mice, there was a slight yet significant increase in the PD-1+ cells (1.2 versus 3.8%), which mostly reflected the increase in the PD-1low cells in the DN population (40 versus 65%). In both mice, the CD4+CD8+ population totally lacked the expression of PD-1. We then examined the mice transgenic for both TCR α and β chain genes. In the thymus of H-Y mice, ∼20% of total thymocytes and nearly 90% of the DN population were found to express PD-1 with high intensities in both neutral (H-2d) and positively selecting (H-2b) backgrounds. The thymus of 2C mice in the positively selecting background (H-2b) exhibited as much as 63% PD-1+ cells, and again nearly 90% of the DN population strongly expressed PD-1. Unlike normal and Vβ8 transgenic mice, significant proportions of DP thymocytes in H-Y and 2C mice also exhibited PD-1, ∼15 and 50%, respectively (Fig. 1 a).
Figure 1.
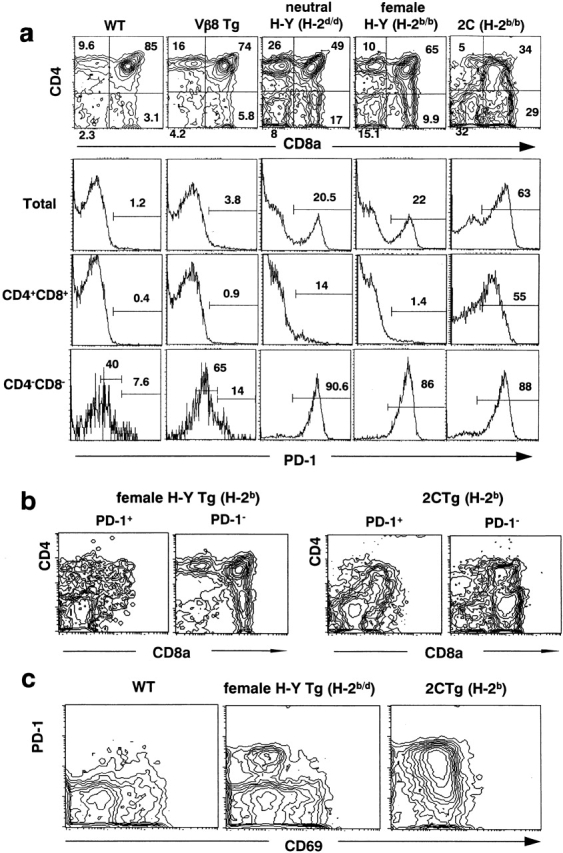
Augmented expression of PD-1 in CD4−CD8− compartment in TCR transgenic lines. (a) Thymocytes from wild-type (WT), Vβ8, H-Y (H-2d), female H-Y (H-2b), and 2C (H-2b) mice were examined for PD-1 expression. Top, CD4/CD8 contour plots. Bottom, histograms for PD-1 expression in total, CD4+CD8+, and CD4− CD8− thymocytes. (b) Thymocytes from H-Y (H-2b) and 2C (H-2b) mice were examined for the expression of PD-1. The contours of CD4/CD8 profiles gated in PD-1+ (left) or PD-1− (right) fractions are shown. (c) CD69 and PD-1 expressions are shown in total thymocytes from wild-type, female H-Y (H-2b/d), and 2C (H-2b) mice. The numbers indicate percentages in gated regions. Tg, transgenic.
However, the CD4/CD8 expression of thymocytes in the TCR-α/β transgenic mice was quite diffuse, particularly in 2C mice. To have a better picture of the distribution of PD-1+ cells, the CD4/CD8 profiles of PD-1+ and PD-1− populations were analyzed separately. As shown in Fig. 1 b, PD-1+ cells were present in the CD4low and/or CD8low in addition to DN populations in both H-Y (H-2b) and 2C (H-2b) mice. The vast majority of CD4highCD8high DP cells as well as CD4highCD8− and CD4−CD8high SP populations lacked PD-1 expression. In the female H-Y (H-2b/d) transgenic as well as control mice, CD69+ thymocytes barely expressed PD-1 and vice versa (Fig. 1 c), implying that thymocytes during the positive selection process lacked PD-1 expression. In 2C (H-2b) mice, the majority of thymocytes expressed CD69 weakly, including both PD-1+ and PD-1− cells (Fig. 1 c). In the TCR transgenic mice, β selection largely proceeds based on the expression of transgenic TCR β chain, either as TCR-β homodimer 23 24 in Vβ8 transgenic mice or as TCR-α/β complex in H-Y and 2C transgenic mice. Although the studies on pTα−/− mice suggest that the three types of pre-TCR complex mentioned above achieve β selection in somewhat distinct fashions 25 26, signaling downstream of the CD3 complex is expected to be similar 11. Our results here have reinforced that PD-1 expression is induced during the β selection process of DN thymocytes, which is variably enhanced in the TCR transgenic mice. It remains to be further analyzed whether PD-1 is also expressed during the later selection processes such as positive and negative selection, particularly in 2C mice (see below).
To explore the functions of PD-1, we crossed Vβ8 and H-Y (H-2d/d) transgenic mice with PD-1−/− mice and analyzed their thymocytes. As summarized in Table , the total cell number of thymocytes in Vβ8 × PD-1−/− transgenic mice was significantly increased compared with that in Vβ8 × PD-1+/+ transgenic mice (1.49 ± 0.17 vs. 0.99 ± 0.3 × 10−8, P < 0.005). The increase was ascribed mostly to DP thymocytes (109.82 ± 12.65 vs. 73.51 ± 2.27 × 10−6, P < 0.01), whereas the number of DN thymocytes remained unaffected. Essentially similar results were obtained in the H-Y (H-2d) mice, DP thymocytes being increased selectively in the absence of PD-1 (80.04 ± 30.83 vs. 125.11 ± 27.92 × 10−6, P < 0.005). The results strongly suggest that PD-1 negatively regulates the transition of DN thymocytes to the DP stage in the TCR transgenic mice. The numbers of CD4+ and CD8+ SP thymocytes were increased proportionally to DP thymocytes in both Vβ8 × PD-1−/− and H-Y (H-2d/d) × PD-1−/− mice, suggesting that the overall efficiency for the positive selection based on the TCRs of transgenic β chains and endogenous α chains was largely unaffected in the absence of PD-1.
Table 1.
The Frequency of Thymocyte Subpopulations in Vβ8 or H-2d/d H-Y Transgenic Mice in the Nonselection Environment with or without PD-1 Deficiency
| Genotype | No. of mice examined | Total cell no. | CD4+CD8+ cell no. ×10−6 | CD8+ cell no. ×10−6 | CD4+ cell no. ×10−6 | CD4−CD8− cell no. ×10−6 |
|---|---|---|---|---|---|---|
| ×10−8 | (%) | (%) | (%) | (%) | ||
| Vβ8 | 3 | 0.99 ± 0.3 | 73.51 ± 2.27 | 5.76 ± 0.59 | 16.29 ± 0.82 | 4.10 ± 0.64 |
| (74.25 ± 0.17) | (5.83 ± 0.66) | (16.45 ± 0.36) | (4.14 ± 0.63) | |||
| Vβ8 × PD-1+/− | 3 | 1.04 ± 0.19 | 74.04 ± 16.31 | 6.37 ± 0.52 | 19.34 ± 3.00 | 4.25 ± 0.59 |
| (70.94 ± 3.20) | (6.24 ± 1.04) | (18.70 ± 1.89) | (4.12 ± 0.39) | |||
| Vβ8 × PD-1−/− | 4 | 1.49 ± 0.17 | 109.82 ± 12.65 | 7.70 ± 0.92 | 25.90 ± 2.33 | 5.84 ± 1.30 |
| (73.56 ± 0.52) | (5.16 ± 0.13) | (17.41 ± 1.02) | (3.88 ± 0.47) | |||
| P values between PD-1+/− and PD-1−/− background | 0.0048 | 0.0055 | 0.009 | <0.001 | 0.06 | |
| H-Y (H-2d/d) | 9 | 1.27 ± 0.39 | 80.04 ± 30.83 | 6.02 ± 2.10 | 23.27 ± 5.72 | 17.86 ± 4.43 |
| (61.58 ± 7.17) | (5.08 ± 1.84) | (18.89 ± 3.65) | (14.58 ± 3.24) | |||
| H-Y (H-2d/d) × PD-1+/− | 7 | 1.43 ± 0.42 | 92.61 ± 31.09 | 5.77 ± 2.10 | 26.18 ± 7.16 | 18.45 ± 4.49 |
| (64.20 ± 4.32) | (4.18 ± 1.63) | (18.53 ± 2.39) | (13.08 ± 1.79) | |||
| H-Y (H-2d/d) × PD-1−/− | 7 | 1.81 ± 0.36 | 125.11 ± 27.92 | 7.29 ± 1.99 | 31.53 ± 6.22 | 17.69 ± 4.72 |
| (68.74 ± 3.67) | (4.11 ± 1.37) | (17.48 ± 1.72) | (9.81 ± 2.58) | |||
| P values between PD-1+/− and PD-1−/− background | 0.006 | 0.0042 | 0.135 | 0.009 | 0.47 |
Numbers in parentheses indicate percentages of subsets based on the expression of CD4 and CD8. All numbers are shown as mean ± SD.
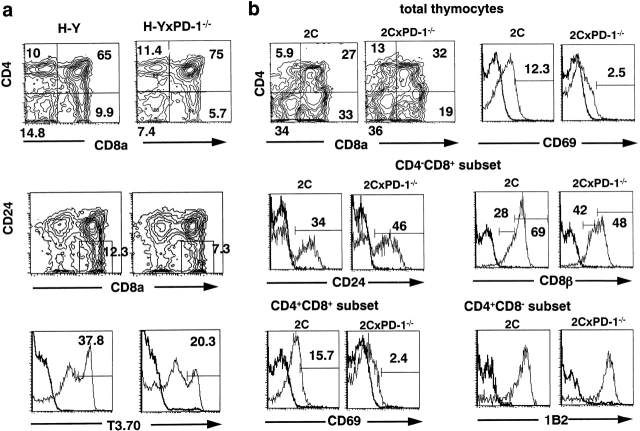
Next, to define the effect of PD-1 deficiency on the positive selection for the transgenic TCR-α/β, we crossed PD-1−/− mice with the female H-Y (H-2b) and 2C (H-2b) mice, in which the CD8+ cells expressing the transgenic TCR-α/β are positively selected. The number of DP thymocytes in female H-Y (H-2b) mice was significantly increased in the absence of PD-1, as before (Table ). In the female H-Y (H-2b) × PD-1+/− mice, the number of CD8+ SP cells was also increased proportionally to both DP and CD4+ SP cells (∼1.5-fold; Table ). In contrast, the number of CD8+ SP cells in the female H-Y (H-2b) × PD-1−/− mice remained the same as in the control female H-Y (H-2b) mice (17.6 ± 7.63 vs. 16 ± 3.89 × 106) in spite of the fact that the numbers of DP and CD4+ SP cells were increased in parallel ∼1.5-fold (Table ). FACS® analysis indicated that the relative proportions of both T3.70 (transgenic TCR-α)high and CD24−CD8+ cells were indeed decreased in the absence of PD-1 (Fig. 2 a), indicating that the efficiency of positive selection for the clonotypic T3.70+CD8+ SP cells was significantly reduced. On the other hand, the proportion of CD4+ SP thymocytes, which are positively selected on the basis of diversified TCRs of the transgenic TCR-β and endogenous TCR-α, was unaffected (Fig. 2 a).
Table 2.
The Frequency of Thymocyte Subpopulations in Female H-2b/b H-Y and H-2b/b2C Transgenic Mice with or without PD-1 Deficiency
| Genotype | No. of mice examined | Total cell no. | CD4+CD8+ cell no. ×10−6 | CD8+ cell no. ×10−6 | CD4+ cell no. ×10−6 | CD4−CD8− cell no. ×10−6 |
|---|---|---|---|---|---|---|
| ×10−8 | (%) | (%) | (%) | (%) | ||
| H-Y (H-2b/b) | 10 | 1.57 ± 0.44 | 98.07 ± 32.15 | 16 ± 3.89 | 17.74 ± 4.87 | 25.01 ± 7.17 |
| (61.84 ± 4.08) | (10.79 ± 3.70) | (11.51 ± 2.85) | (16.1 ± 2.85) | |||
| H-Y (H-2b/b) × PD-1+/− | 10 | 2.45 ± 0.62 | 168 ± 44 | 23.2 ± 10.8 | 27.47 ± 7.3 | 25.52 ± 11.0 |
| (69.22 ± 6.13) | (9.67 ± 4.40) 0.03 | (11.30 ± 1.78) | (10.12 ± 3.02) | |||
| H-Y (H-2b/b) × PD-1−/− | 15 | 2.41 ± 0.72 | 172 ± 52 | 17.6 ± 7.63 | 27.0 ± 10.0 | 23.44 ± 9.14 |
| (71.62 ± 5.37) | (7.40 ± 2.21) | (11.23 ± 1.87) | (9.75 ± 2.47) | |||
| P values between PD-1+/+ and PD-1−/− background | 0.0001 | 0.0001 | 0.08 | 0.002 | 0.63 | |
| 2C (H-2b/b) | 5 | 0.21 ± 0.09 | 5.72 ± 2.74 | 6.00 ± 2.78 | 1.46 ± 0.55 | 8.15 ± 2.82 |
| (26.1 ± 4.7) | (28.0 ± 4.1) | (6.9 ± 0.8) | (39.0 ± 6.8) | |||
| 2C (H-2b/b) × PD-1−/− | 5 | 0.19 ± 0.05 | 6.28 ± 1.74 | 3.33 ± 1.08 | 2.64 ± 0.84 | 6.91 ± 2.06 |
| (34.0 ± 3.6) | (17.3 ± 1.7) | (13.8 ± 1.4) | (36.0 ± 3.7) | |||
| P values between PD-1+/+ and PD-1−/− background | 0.36 | 0.35 | 0.05 | 0.017 | 0.23 |
Numbers in parentheses indicate percentages of subsets based on the expression of CD4 and CD8. All numbers are shown as mean ± SD.
Figure 2.
Effects of PD-1 deficiency on T cell development in the TCR-α/β transgenic mice in positively selecting background. (a) Thymocytes from female H-Y (H-2b) mice with or without the PD-1 mutation were analyzed for expression of CD4, CD8a, CD24, and transgenic TCR-α (T3.70). (b) Thymocytes from 2C (H-2b) mice with or without the PD-1 mutation were analyzed for expression of CD4, CD8a, transgenic TCR-α/β (1B2), CD24, CD69, and CD8β for total thymocytes (top), CD4−CD8+ (middle), and CD4+CD8+ and CD4+CD8− (bottom) subsets. Numbers indicate percentages of subsets in gated regions.
In 2C (H-2b) mice, the number of total as well as DP thymocytes was far less and the relative proportion of CD8+ SP thymocytes was much higher than in female H-Y (H-2b) mice (Table , and Fig. 1), probably reflecting higher affinity and/or avidity of 2C TCR-α/β for the selecting ligands 27 28. In the 2C (H-2b) × PD-1−/− mice, a significant reduction of CD8+ SP thymocytes was observed in terms of both absolute cell number and relative proportion, whereas the DP population was increased slightly compared with 2C (H-2b) × PD-1+/+ mice (Table , and Fig. 2 b). Furthermore, within the CD4−CD8+ population, the percentages of those with the mature phenotypes (CD24− and CD8βhigh) were significantly reduced in 2C (H-2b) × PD-1−/− (50%) than in 2C (H-2b) × PD-1+/+ mice (70%) (Fig. 2 b), suggesting that the reduction of positively selected CD8+ SP thymocytes in the former mice was apparently underestimated by the presence of transient CD4low/−CD8+ cells. Consistently, expression of CD69, a marker associated with positive selection, was also significantly reduced in both total and DP thymocytes in the absence of PD-1 (Fig. 2 b). Although there was an apparent increase in the CD4+ SP thymocytes (Table , and Fig. 2 b), the vast majority of CD4+ SP thymocytes in both groups highly expressed the clonotypic epitope (1B2) of transgenic TCR-α/β (Fig. 2 b). Therefore, it probably reflects the increase in the transitional thymocytes rather than positively selected mature CD4+ SP cells, which is consistent with the accelerated β selection in the absence of PD-1. These results have collectively suggested that the efficiency of positive selection for the cells expressing transgenic TCR-α/β is significantly reduced in the absence of PD-1.
We have reported previously that the injection of anti-CD3ε mAb into RAG-2−/− mice induces significant expansion of DP cells expressing PD-1 14. Since the procedure is considered to mimic the β selection process 29, we have generated RAG-2−/− × PD-1−/− mice and injected varying amounts of anti-CD3ε mAb. 50 μg of the anti-CD3ε mAb induced more expansion of the thymocytes in RAG-2−/− × PD-1−/− than in RAG-2−/− × PD-1+/+ mice (47.1 ± 21.8 vs. 27.3 ± 14.5 × 10−6; n = 9, P < 0.05), whereas no significant difference was observed any more by 100 μg or more (54.5 ± 26.1 vs. 53.1 ± 12.3 × 10−6; n = 6, P > 0.05). Our preliminary biochemical studies indicate that the cross-linking of PD-1 indeed inhibits the cell activation signals such as Ca2+ influx mediated by B cell receptor (BCR) cross-linking in a B cell line transfected with PD-1 cDNA (our unpublished data). Therefore, it is suggested that PD-1 functions as a negatively regulating receptor for antigen stimulation.
These results have strongly suggested that PD-1 negatively regulates the β selection process probably by affecting the threshold of pre-TCR/CD3 complex–mediated signaling. It has been further indicated that the efficiency of positive selection for SP thymocytes expressing transgenic TCR-α/β, but not for those expressing diversified endogenous TCR α chains, is significantly reduced in the absence of PD-1. Two explanations may be considered for this phenomenon. First, reduced efficiency of positive selection in the absence of PD-1 can be an indirect consequence of the accelerated β selection. Since PD-1 deficiency is suggested to lower the threshold for pre–TCR-β/CD3 signaling, the DP thymocytes developed in the absence of PD-1 would include those that were selected by a weaker activation signal that otherwise could not allow them to expand. Such DP cells that expanded by suboptimal β selection signaling may not be qualified for positive selection as long as the transgenic TCR-α/βs are fixed, resulting in the reduced efficiency of the subsequent positive selection. However, these unqualified thymocytes may be rescued by the expression of endogenous TCR α chain to form new TCR-α/βs with compensatory higher avidity for the selecting ligands, which may explain the unchanged efficiency of positive selection for such thymocytes in the absence of PD-1. Alternatively, PD-1 might directly affect the positive selection process, independently of the effect on the β selection. Unlike in normal and Vβ8 transgenic mice, the H-Y and 2C mice expressed PD-1 in a portion of DP thymocytes, the CD4lowCD8low population, which may undergo positive selection 30. It may then be possible that the deficiency of PD-1 causes the hyperactivation of this population upon interaction with selecting ligands for TCR, resulting in negative rather than positive selection. We consider the latter less likely because the efficiency of the positive selection for CD4+ SP thymocytes was unaffected in H-Y × PD-1−/− mice in both neutral and positive selecting backgrounds. If PD-1 directly regulates the positive selection process, the PD-1 deficiency should affect both CD4+ SP and CD8+ SP thymocytes similarly. In addition, at least in the H-Y model, PD-1 is not significantly expressed in the CD69+ population (Fig. 1 c).
Whichever is the case, PD-1 deficiency is strongly suggested to result in the significant alteration of the final T cell repertoire either directly or indirectly. We have reported that B6 mice deficient for PD-1 develop autoimmune diseases, including lupus-like glomerulonephritis and destructive arthritis, as they age, which are greatly accelerated by the additional lpr/lpr mutation 16. In addition to the dysregulation of peripheral tolerance 16, it is tempting to speculate that the altered T cell repertoire formation in the absence of PD-1 may also increase the likelihood of the emergence of mature autoreactive T cells and thereby predispose the mice to the systemic autoimmune diseases.
Acknowledgments
We thank Ms. Y. Tabuchi and M. Yamamoto for their technical assistance, and Ms. K. Saito and K. Hirano for their preparation of the manuscript. We also thank Drs. D. Loh, F. Alt, H. von Boehmer, Y. Takahama, S. Habu, and T. Nakayama for providing TCR transgenic and RAG-2−/− mice.
This work was supported by grants from the Ministry of Education, Science, Sports, and Culture of Japan, from a Searle Scientific Research Fellowship, and from Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
References
- Kisielow P., von Boehmer H. Development and selection of T cellsfacts and puzzles. Adv. Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- Adkins B., Mueller C., Okada C.Y., Reichert R.A., Weissman I.L., Spangrude G.J. Early events in T-cell maturation. Annu. Rev. Immunol. 1987;5:325–365. doi: 10.1146/annurev.iy.05.040187.001545. [DOI] [PubMed] [Google Scholar]
- Godfrey D.I., Zlotnik A. Control points in early T-cell development. Immunol. Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Aifantis I., Azogui O., Feinberg J., Saint Ruf C., Zober C., Garcia C., Buer J. Crucial function of the pre-T-cell receptor (TCR) in TCR beta selection, TCR beta allelic exclusion and alpha beta versus gamma delta lineage commitment. Immunol. Rev. 1998;165:111–119. doi: 10.1111/j.1600-065x.1998.tb01234.x. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu. Rev. Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Nossal G.J. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Saint Ruf C., Ungewiss K., Groettrup M., Bruno L., Fehling H.J., von Boehmer H. Analysis and expression of a cloned pre-T cell receptor gene. Science. 1994;266:1208–1212. doi: 10.1126/science.7973703. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A.R., Rudnicki M.A., Iacomini J., Itohara S., Lafaille J.J., Wang L., Ichikawa Y., Jaenisch R., Hooper M.L. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Fehling H.J., Krotkova A., Saint Ruf C., von Boehmer H. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- Malissen M., Gillet A., Ardouin L., Bouvier G., Trucy J., Ferrier P., Vivier E., Malissen B. Altered T cell development in mice with a targeted mutation of the CD3-epsilon gene. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:4641–4653. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agata Y., Kawasaki A., Nishimura H., Ishida Y., Tsubata T., Yagita H., Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H., Agata Y., Kawasaki A., Sato M., Imamura S., Minato N., Yagita H., Nakano T., Honjo T. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4−CD8−) thymocytes. Int. Immunol. 1996;8:773–780. doi: 10.1093/intimm/8.5.773. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Nakano T., Minato N., Honjo T. Immunological studies on PD-1 deficient miceimplication of PD-1 as a negative regulator for B cell responses. Int. Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Nose M., Hiai H., Minato N., Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- Uematsu Y., Ryser S., Dembic Z., Borgulya P., Krimpenfort P., Berns A., von Boehmer H., Steinmetz M. In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell. 1988;52:831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Bluthmann H., Staerz U.D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Sha W.C., Nelson C.A., Newberry R.D., Kranz D.M., Russell J.H., Loh D.Y. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K.P., Oltz E.M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A.M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Teh H.S., Kisielow P., Scott B., Kishi H., Uematsu Y., Bluthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988;335:229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- Kranz D.M., Sherman D.H., Sitkovsky M.V., Pasternack M.S., Eisen H.N. Immunoprecipitation of cell surface structures of cloned cytotoxic T lymphocytes by clone-specific antisera. Proc. Natl. Acad. Sci. USA. 1984;81:573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Bonneville M., Ishida I., Ryser S., Lincoln G., Smith R.T., Kishi H., Scott B., Kisielow P., Tonegawa S. Early expression of a T-cell receptor beta-chain transgene suppresses rearrangement of the V gamma 4 gene segment. Proc. Natl. Acad. Sci. USA. 1988;85:9729–9732. doi: 10.1073/pnas.85.24.9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi H., Borgulya P., Scott B., Karjalainen K., Traunecker A., Kaufman J., von Boehmer H. Surface expression of the beta T cell receptor (TCR) chain in the absence of other TCR or CD3 proteins on immature T cells. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:93–100. doi: 10.1002/j.1460-2075.1991.tb07924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotkova A., von Boehmer H., Fehling H.J. Allelic exclusion in pTα-deficient miceno evidence for cell surface expression of two T cell receptor (TCR)-β chains, but less efficient inhibition of endogenous Vβ→(D)Jβ rearrangements in the presence of a functional TCR-β transgene. J. Exp. Med. 1997;186:767–775. doi: 10.1084/jem.186.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer J., Aifantis I., DiSanto J.P., Fehling H.J., von Boehmer H. Role of different T cell receptors in the development of pre-T cells. J. Exp. Med. 1997;185:1541–1547. doi: 10.1084/jem.185.9.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey E.A., Ramsdell F., Kioussis D., Sha W., Loh D., Axel R., Fowlkes B.J. The level of CD8 expression can determine the outcome of thymic selection. Cell. 1992;69:1089–1096. doi: 10.1016/0092-8674(92)90631-l. [DOI] [PubMed] [Google Scholar]
- Crooks M.E., Littman D.R. Disruption of T lymphocyte positive and negative selection in mice lacking the CD8 beta chain. Immunity. 1994;1:277–285. doi: 10.1016/1074-7613(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Alt F.W. CD3 epsilon-mediated signals rescue the development of CD4+CD8+ thymocytes in RAG-2−/− mice in the absence of TCR beta chain expression. Int. Immunol. 1994;6:995–1001. doi: 10.1093/intimm/6.7.995. [DOI] [PubMed] [Google Scholar]
- Lucas B., Germain R.N. Unexpectedly complex regulation of CD4/CD8 coreceptor expression supports a revised model for CD4+CD8+ thymocyte differentiation. Immunity. 1996;5:461–477. doi: 10.1016/s1074-7613(00)80502-6. [DOI] [PubMed] [Google Scholar]