Abstract
C57BL/6 mice genetically deficient in interleukin 15 (IL-15−/− mice) were generated by gene targeting. IL-15−/− mice displayed marked reductions in numbers of thymic and peripheral natural killer (NK) T cells, memory phenotype CD8+ T cells, and distinct subpopulations of intestinal intraepithelial lymphocytes (IELs). The reduction but not absence of these populations in IL-15−/− mice likely reflects an important role for IL-15 for expansion and/or survival of these cells. IL-15−/− mice lacked NK cells, as assessed by both immunophenotyping and functional criteria, indicating an obligate role for IL-15 in the development and functional maturation of NK cells. Specific defects associated with IL-15 deficiency were reversed by in vivo administration of exogenous IL-15. Despite their immunological defects, IL-15−/− mice remained healthy when maintained under specific pathogen-free conditions. However, IL-15−/− mice are likely to have compromised host defense responses to various pathogens, as they were unable to mount a protective response to challenge with vaccinia virus. These data reveal critical roles for IL-15 in the development of specific lymphoid lineages. Moreover, the ability to rescue lymphoid defects in IL-15−/− mice by IL-15 administration represents a powerful means by which to further elucidate the biological roles of this cytokine.
Keywords: interleukin 15, knockout mice, natural killer cells, CD8+ T lymphocytes, immunologic memory
Introduction
The cytokine IL-15 was discovered as a result of its “IL-2–like” stimulatory activity on T cells 1 2. The similar in vitro activities of IL-2 and IL-15 stem from shared use of common receptor signaling subunits. IL-15 and IL-2 both interact with receptor complexes that contain the common gamma (γc) and IL-2Rβ chains. The γc subunit is also used for signaling by IL-4, IL-7, and IL-9 3, whereas the IL-2Rβ subunit is used for signaling only by IL-2 and IL-15 4 5. The ability of a cell to respond to IL-2 or IL-15 can also be controlled by the presence or absence of specific receptor subunits for IL-2 or IL-15. These distinct (nonshared) receptors, known as IL-2Rα and IL-15Rα, respectively, do not appear to be involved in signaling but are required for high-affinity binding of these cytokines. Thus, despite the fact that IL-2 and IL-15 share receptor subunits used for signaling, differential expression of the IL-2Rα or IL-15Rα receptor subunits allows the two cytokines to exert qualitatively and/or quantitatively differential effects on several cell types. In addition, IL-2 and IL-15 are produced by different cells and in different tissues. Thus, the in vivo responses to IL-2 and IL-15 are likely to be temporally and physically compartmentalized (for a review, see reference 6).
Studies of mice carrying induced mutations within the IL-2/IL-15 system provide definitive evidence that IL-2 and IL-15 have unique roles in vivo 7 8 9 10 11 12. Mice lacking IL-2, IL-2Rα, or IL-2Rβ develop an autoimmune disorder. However, IL-2Rβ2/− mice have additional defects that are not observed in mice lacking IL-2 or IL-2Rα. These additional defects include an absence of functional NK cells and dramatic decreases in numbers of NK T cells and intestinal intraepithelial lymphocytes (IELs) that are believed to develop extrathymically. The differences in the phenotypes of mice lacking IL-2, IL-2Rα, or IL-2Rβ led to the speculation that IL-2 plays a primary role in maintaining homeostasis of thymus-derived T cells, whereas IL-15 promotes extrathymic development and survival of T cells and NK cells (for a review, see reference 13). More recent studies in normal mice indicate that IL-15 is a selective growth factor for memory CD8+, but not CD4+, T cells 14. Other studies implicate IL-15 as an anabolic factor for muscle cells 15, a differentiation factor for osteoclast progenitors 16, a growth factor for mast cells and intestinal epithelial cells 17 18, and a general inhibitor of apoptosis in T cells, B cells, and fibroblasts 19.
To further explore the role of IL-15 in vivo, we generated C57BL/6 mice that are genetically deficient in IL-15. Gross histological analysis of IL-15−/− mice revealed no evidence of increased cellular apoptosis or generalized defects in mast cells, muscle, or bone. However, the phenotypes of mice lacking either IL-15 (described here) or IL-15Rα 20 confirm a critical role for IL-15 in regulating the development and/or expansion of NK cells, NK T cells, and distinct IEL populations. In addition, both of these strains of mice reveal a role for IL-15 in the maintenance of the memory phenotype CD8+ T cell population. IL-15−/− mice should prove useful for studying the roles of IL-15 in infectious disease, antitumor immunity, autoimmune responses, and inflammation.
Materials and Methods
Mice.
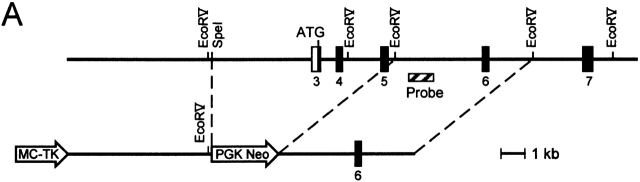
C57BL/6 mice genetically deficient in IL-15 were generated by homologous recombination in embryonic stem (ES) cells. The structure of the murine IL-15 genomic locus has been described 21. A gene targeting vector was constructed in which a 7.5-kb SpeI-EcoRV fragment containing IL-15 exons 3–5, encoding amino acids 1–65 of the primary translation product, was replaced with a PGK-neo cassette. A thymidine kinase cassette (MC-TK) was inserted into the 5′ end of the vector. C57BL/6-derived ES cells were electroporated with the IL-15 targeting vector and selected in G418 and ganciclovir as described 22. ES cell clones carrying a targeted IL-15 gene were identified by a combination of PCR and genomic Southern blot analyses and were injected into BALB/c blastocysts. Resulting male chimeras were bred to C57BL/6 females. The offspring were analyzed for germline transmission of the mutant IL-15 allele by PCR and genomic Southern blot analyses. Mice heterozygous for the IL-15 mutation (IL-15+/−) were intercrossed to generate IL-15–deficient (IL-15−/−) mice.
The IL-15−/− mice used throughout these studies were bred at Immunex and maintained on a C57BL/6 genetic background. All mice were housed under specific pathogen-free conditions and were used between 9 and 20 wk of age. Age- and sex-matched littermates (IL-15+/+ or IL-15+/−) or C57BL/6 mice (Taconic Farms, Inc.) were used as controls as indicated. Initial studies revealed no apparent difference between IL-15+/+ and IL-15+/− mice in cellularity of their secondary lymphoid tissues, phenotype of spleen or LN cells, or splenic NK cell responses.
Blood and Serum Analyses.
Peripheral blood was harvested by cardiac puncture, and blood smears were prepared. Complete blood counts and serum chemistry analyses were conducted by Phoenix Laboratory for Veterinarians (Everett, WA) using a CellDyne 3500 blood analyzer and a Hitachi chemistry analyzer. Basal levels of serum IgA, IgG, and IgM were determined by isotype-specific ELISA as described 23.
Histological Analyses.
Mice were killed by exposure to CO2 and weighed. A midline incision was made through the skin but not into the abdominal wall, and 8 ml of sterile HBSS was injected into the peritoneal cavity. The abdomen was gently massaged, the fluid was extracted, and the cells were counted. To evaluate mast cells, quadruplicate 100 μl aliquots of fluid were diluted to 106 cells/ml and centrifuged onto slides at 500 rpm for 5 min using a Shandon Cytospin® II. All tissues were examined grossly, and 37 tissues representing all major tissues of all organ systems (skin and special senses, nervous, respiratory, digestive, urogenital, endocrine, lymphoid, hematopoietic, and musculoskeletal systems) were collected for histopathology. Tissues were fixed in 10% neutral buffered formalin, processed and embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. Body weight and selected absolute organ weights were determined, and relative organ to body weight ratios were calculated for spleen, thymus, and LNs.
Immunohistochemistry.
Spleen, inguinal and mesenteric LNs, and thymus were quick-frozen in 2-methyl butane cooled with dry ice. A panel of anti–mouse immunophenotypic markers for T cells, B cells, macrophages, follicular dendritic cells, and germinal centers were applied to 5-μm cryosections cut onto charged slides. The reagents and protocols used are described in detail elsewhere 24.
Cell Preparation.
Single cell suspensions of liver, thymus, spleen, and LN were prepared by gently pressing the tissues through fine nylon or wire mesh screens. Erythrocytes were depleted from spleen cell suspensions by hypotonic lysis. Intrahepatic mononuclear cells were separated from hepatocytes by density centrifugation over Lympholyte M (Cedarlane) as described 12. IELs were obtained from the small intestine and isolated on discontinuous Percoll (Pharmacia Fine Chemicals) gradients as described 25.
Antibodies and Flow Cytometry.
Expression of cell surface markers was analyzed by standard two or three-color flow cytometry on FACScalibur™ and FACScan™ II instruments (Becton Dickinson) and with CELLQuest™ software (Becton Dickinson). FITC-, PE-, and biotin-conjugated mAbs and isotype-matched controls were purchased from PharMingen. Secondary reagents to detect binding of biotinylated mAb included streptavidin-allophycocyanin (Molecular Probes), streptavidin-RED670 (GIBCO BRL), and streptavidin-CyChrome (PharMingen).
In Vivo Treatment with Polyinosilic-Polycytidylic Acid or IL-15.
Mice were injected intraperitoneally with 0.2 ml pyrogen-free PBS or PBS containing either polyinosilic-polycytidylic acid (polyI:C) or human IL-15. PolyI:C-treated mice received a single injection of 100 μg polyI:C (Sigma Chemical Co.). IL-15–treated mice received 10 μg human IL-15 once daily for 7 d. Chinese hamster ovary (CHO)-derived human rIL-15 was produced and purified at Immunex. Endotoxin levels were <3.7 ng/mg of protein. The IL-15 for injection was prepared daily from a concentrated stock solution. Spleens and/or LNs were harvested 24 h after the last injection of PBS, polyI:C, or IL-15.
Analysis of NK Cell Function.
A chromium release assay was used to assess the cytolytic function of splenocytes from naive or treated mice. RBC-free splenocytes from individual mice were used as effector cells and incubated overnight in 96-well flat-bottomed plates in the presence of 5 × 103 51Cr-labeled YAC-1 or P815 target cell lines. There were four replicates per sample. Cell-free supernatants were harvested using a Skatron harvester. Spontaneous release (from targets incubated without effector cells) and maximum release (from targets incubated in the presence of 1% NP-40) were also determined. The percentage of specific lysis was calculated as: (experimental release − spontaneous release/maximum release − spontaneous release) × 100.
Viral Infections.
The neurotropic wild-type WR strain of vaccinia virus 26 was prepared and titered on CV-1 cells using standard techniques 27. Virus stocks were dispersed by sonication and diluted in endotoxin-free saline. 8-wk-old female mice were infected intravenously with 106 PFU of virus. Mice were observed daily for signs of morbidity and mortality.
Results and Discussion
Generation and Gross Characterization of IL-15−/− Mice.
Mice genetically deficient in IL-15 were generated by homologous recombination in ES cells using the construct depicted in Fig. 1 A. Correct targeting of the IL-15 locus was confirmed by genomic Southern blot analysis (Fig. 1 B). Although reagents for detecting murine IL-15 protein are not available, the IL-15 mutation is expected to represent a null mutation, as it deletes the initiation codon, the leader sequence, and the first 17 amino acids of the mature protein. IL-15−/− mice were generated from IL-15+/− intercrosses at the expected Mendelian frequency, did not display any gross phenotypic anomalies, and had normal life spans in a specific pathogen-free facility (data not shown).
Figure 1.
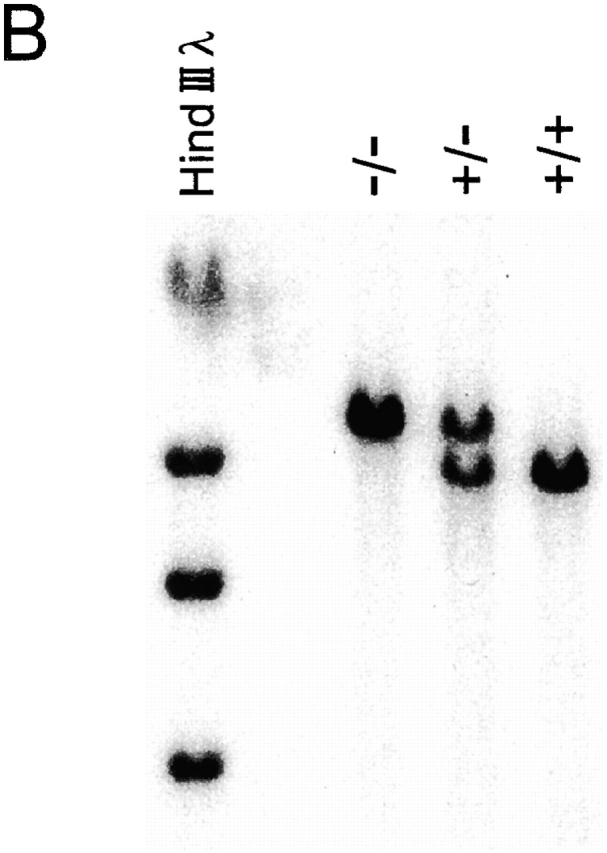
Generation of IL-15−/− mice. (A) A gene targeting vector was constructed in which a 7.5-kb SpeI-EcoRV fragment containing IL-15 exons 3–5, encoding amino acids 1–65 of the primary translation product, was replaced with a PGK-neo cassette. A thymidine kinase cassette (MC-TK) was inserted into the 5′ end of the vector. C57BL/6-derived ES cells were electroporated with the IL-15 targeting vector and selected in G418 and ganciclovir. (B) ES cell clones carrying a targeted IL-15 gene were identified by a combination of PCR and genomic Southern blot analyses and injected into BALB/c blastocysts. Resulting male chimeras were bred to C57BL/6 females, and offspring were analyzed for germline transmission of the mutant IL-15 allele by PCR and genomic Southern blot analyses.
Serological and Histological Characterization of IL-15−/− Mice.
All hematological and clinical chemistry values (n = 12/group), absolute and relative body and organ weights (n = 18/group), abdominal cavity cytology, tissue morphology, and immunophenotyping (n = 3/group) were within normal ranges for this strain of mouse in our laboratory (data not shown). None of the IL-15−/− mice exhibited any evidence of autoimmune disease, inflammatory bowel disease, or any alterations in resident tissue leukocytes including mast cells. Basal levels of serum IgA, IgG, and IgM (n = 14/group) were determined in mice up to 19 wk of age and were within normal ranges in both IL-15−/− and their littermate (IL-15+/+ and IL-15+/−) controls (data not shown).
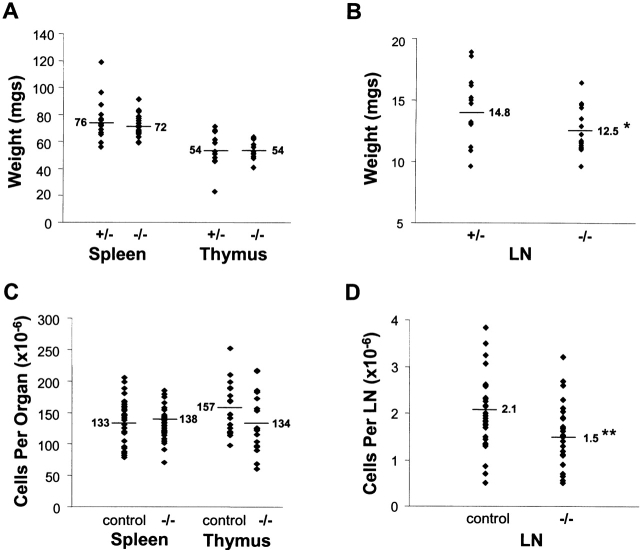
Thymic, Splenic, and LN Weight and Cellularity.
Within the course of these studies, we noted no consistent or marked difference in the size or the cellularity of the spleens, thymi, or peripheral LNs between small groups of age- and sex-matched IL-15−/− and littermate control (either IL-15+/+ or IL-15+/−) mice. To compare the groups in a quantitative fashion, the lymphoid organs were removed and weighed from a cohort of age-matched male mice (n = 17–18/group). There were no significant differences in the average absolute weights or organ to body weight ratios of the spleens or thymi between IL-15−/− and their heterozygote littermate controls (Fig. 2 A, and data not shown). However, the average absolute weight and organ to body weight ratio of the peripheral LNs from IL-15−/− mice were significantly lower than those of the controls (Fig. 2 B, and data not shown). To compare cellularity of these organs, data were pooled from numerous experiments. Although groups of mice were age and sex matched within each experiment, the pooled data samples were derived from both male and female mice between 9 and 20 wk of age (Fig. 2C and Fig. D). The pooled data revealed a significant decrease in the average cellularity of the peripheral LNs, but not the spleen or thymi, between IL-15−/− mice and the controls.
Figure 2.
Lymphoid organ weights and cellularity. Average values are indicated by the horizontal lines. Significant differences in organ weights or cellularity between groups are indicated with asterisks. (A) Weights of individual spleens or thymi from 10–12.5-wk-old male IL-15+/− or IL-15−/− mice (n = 17–18/group). (B) Total weight of six peripheral LNs (two each of proper axillary, accessory axillary, and inguinal) from individual 10–12.5-wk-old male IL-15+/− or IL-15−/− mice (n = 17–18/group). *P < 0.05 (Student's t test). (C and D) Cellularity data were collected in multiple experiments, each of which used age- and sex-matched mice. The values shown are from both male and female mice between 9 and 20 wk of age. Control mice included IL-15+/− littermates (used in the majority of cases), IL-15+/+ littermates, and C57BL/6 mice. The average number of cells per LN was calculated from the combined cellularity of the six LNs described above. **P < 0.005 (mixed models analysis of variance).
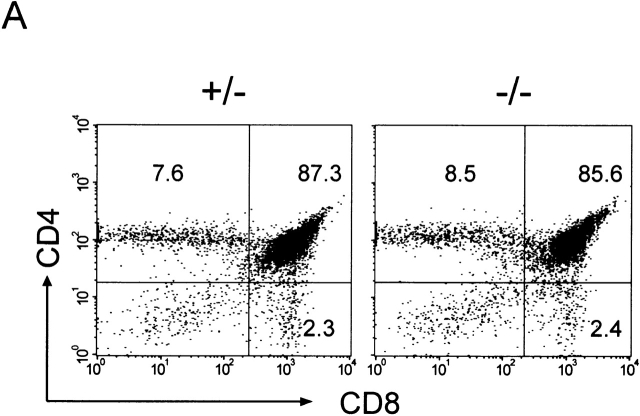
IL-15−/− Mice Have Normal Numbers of Conventional Thymic T Cells but Reduced Numbers of Thymic and Peripheral NK T Cells.
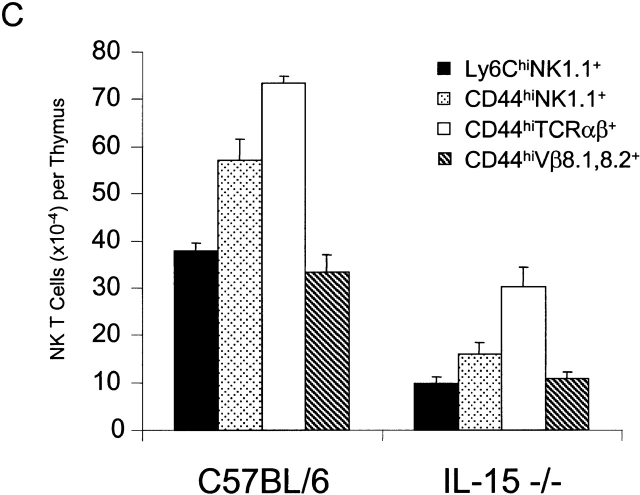
The relative proportions and absolute numbers of double positive CD4+CD8+ immature thymocytes and single positive CD4+ or CD8+ mature thymocytes were similar in control and IL-15−/− mice (Fig. 3 A, and data not shown). Thus, conventional thymic T cell development appeared normal in IL-15−/− mice. In contrast, the relative proportion and absolute numbers of thymic NK T cells were significantly reduced in IL-15−/− mice compared with controls (Fig. 3b and Fig. c). Decreases in the relative proportions and absolute numbers of NK T cells were also observed in the spleen and liver of IL-15−/− mice (Fig. 3 B, and data not shown).
Figure 3.
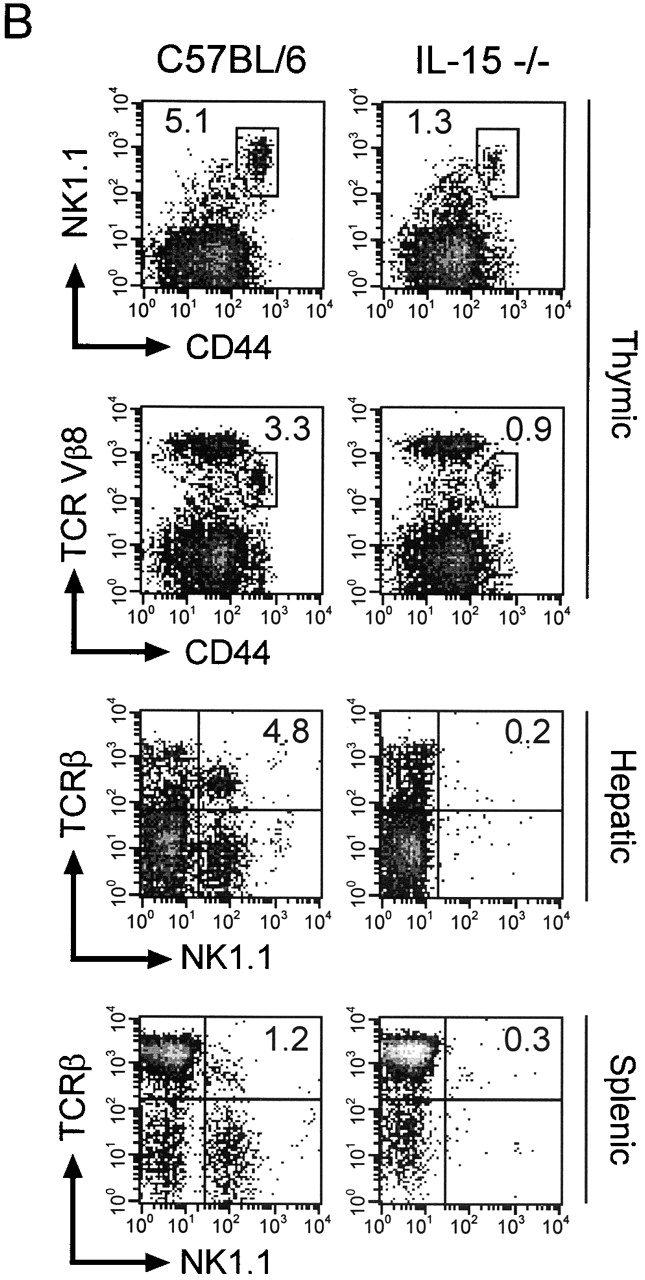
IL-15−/− mice have normal numbers of conventional T cells but reduced numbers of thymic NK T cells. (A) Thymocytes from individual male IL-15+/− (left) or IL-15−/− (right) littermates were analyzed for expression of CD4 and CD8 (n = 2/group; 12–13 wk of age). The numbers shown represent the percentages of cells within each quadrant. The thymic cellularity was similar in both groups. The results shown are representative of three experiments. (B) NK T cell populations of thymocytes (top), intrahepatic mononuclear cells (middle), and splenocytes (bottom) from individual C57BL/6 or IL-15−/− mice. Live gating of the HSAlowCD8low thymocyte population was used to acquire data on CD44hiNK1.1+ or CD44hiTCR Vβ8+ thymocytes. Similarly, live gating of B220null splenocytes was used to acquire data on NK1.1+TCR-β+ splenic NK T cells. The numbers shown represent the percentage of cells with an NK T cell phenotype. (C) Absolute numbers of thymic NK T populations in control and IL-15−/− mice. The numbers shown represent the mean absolute number ± SEM of thymic NK T cell populations from C57BL/6 or IL-15−/− mice (n = 3/group). NK T cell numbers were calculated from the percentages of HSAlow CD8low thymocytes that were Ly6Chi NK1.1+ or CD44hi and NK1.1+, TCR-α/β+, or Vβ8.1,8.2+. The results shown are representative of two experiments.
NK T cells, which express NK cell markers as well as markers expressed on activated T cells, display a restricted TCR repertoire characterized by invariant usage of Vα14 and preferential usage of Vβ8, V8β2, and Vβ7 (for a review, see reference 28). NK T cells are present in normal numbers in IL-2−/− mice 11 12. In contrast, NK T cells are present, but dramatically reduced in number, in mice lacking either IL-2Rβ, IL-15Rα 11 20, or IL-15 (Fig. 3b and Fig. c). The lower number of NK T cells in the IL-15−/− thymus was not likely due to a defect in selection, as the NK T cells that were present exhibited the skewed Vβ repertoire expected of such cells (Fig. 3 C). Additionally, IL-15−/− thymocytes exhibited normal expression and function of CD1d (data not shown), which is the antigen-presenting molecule that positively selects NK T cells 29 30 31. This observation provides additional evidence that the basic elements required for NK T cell selection remain intact in IL-15–deficient mice. The observation that the NK T cells present in IL-15−/− mice appear to have undergone a normal selection process suggests that IL-15 is not obligatory for NK T cell differentiation but is important for expansion/survival or functional maturation of committed NK T cells.
IL-15 Deficiency Affects Selected IEL Subpopulations.
IEL subsets were examined to determine whether IL-15 is required for development or expansion of specific subsets of IELs. In normal mice, the IEL compartment consists of heterogenous populations of TCR-α/β+ and TCR-γ/δ+ T cells. The development or maturation of certain IEL subsets requires an intact thymus or factors that are produced by thymic-dependent T cell populations. Most “thymic-dependent” populations of IELs express Thy1 and are TCR-α/β+CD8αβ+ or TCR-α/β+CD4+. In contrast, most of the IELs that are thought to mature extrathymically (possibly within the gut epithelium itself) are Thy1− and bear TCR-α/β or TCR-γ/δ in conjunction with CD8αα 32 33.
IELs were isolated from individual C57BL/6 or IL-15−/− mice, and cell yields were determined. Once isolated, the IEL populations from individual mice were pooled and analyzed by three-color flow cytometry for expression of various cell surface markers. The absolute number of CD3+ IELs isolated from IL-15−/− mice was estimated to be approximately twofold lower than that of control mice. Within the CD3+ populations, the ratio of α/β to γ/δ IELs was ∼1:1 in control and 2:1 in IL-15−/− mice (data not shown). Thy1− IELs, which made up ∼50% of IEL populations in control mice (Fig. 4A and Fig. C), accounted for <5% of the total IELs in IL-15−/− mice (Fig. 4B and Fig. D). Within gated populations of TCR-α/β+ IELs, there were increases in the relative proportions of CD4+CD8− and CD4+CD8+ IELs in IL-15−/− mice compared with the controls (Fig. 4E and Fig. F). However, as the yield of CD3+ IELs from IL-15−/− mice was approximately twofold lower than that of control mice, the changes in the relative proportions of these IEL populations likely arise solely from a decrease in the absolute number of TCR-α/β+ CD4−CD8+ IELs in IL-15−/− mice compared with controls. The decrease in the TCR-α/β+CD4−CD8+ IEL population in IL-15−/− mice resulted from a selective decrease in the subset of IELs that express the CD8αα homodimer (Fig. 4G and Fig. H). Thus, IL-15−/− mice have a dramatic reduction in the specific IEL populations that are thought to arise or mature independently of the thymus.
Figure 4.
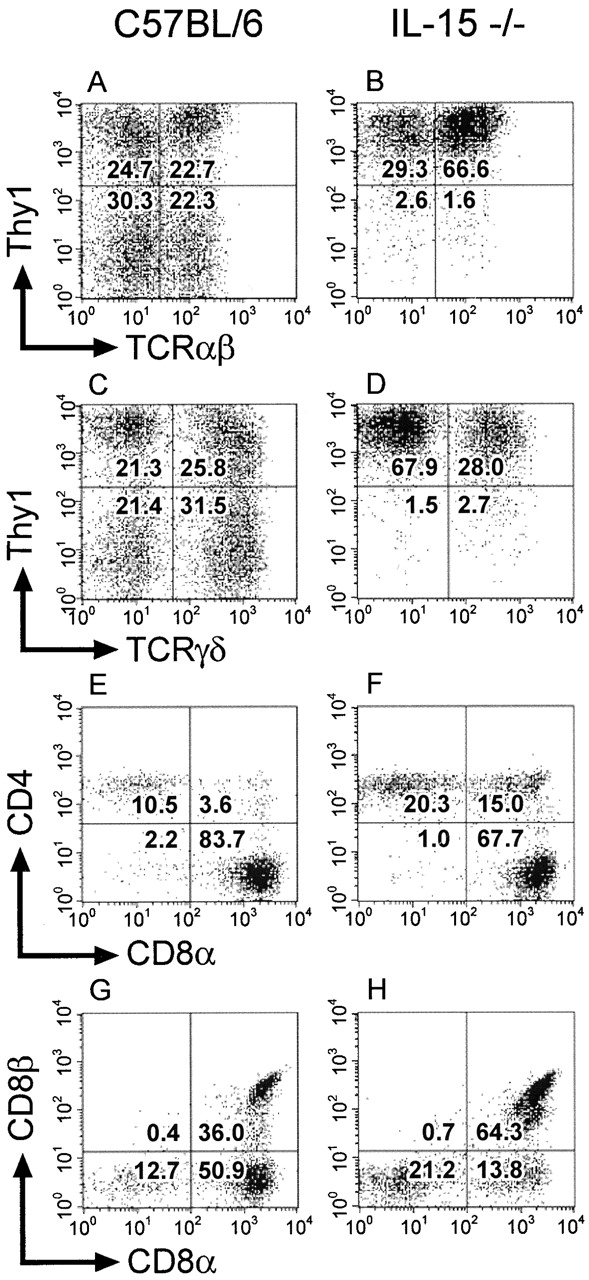
IL-15−/− mice have a deficiency in selective populations of IELs. IELs were isolated from individual 8-wk-old female C57BL/6 or IL-15−/− mice (n = 3/group). The isolated IELs were pooled after the individual cell yields were determined for each mouse. The pooled samples were incubated with the indicated mAb and analyzed using three-color flow cytometry. Viable lymphocytes were gated on the basis of forward and side scatter. A total of 200,000 events was collected for each sample. (A–D) IELs were incubated with mAb specific for CD3, Thy1.2, and TCR-α/β or TCR-γ/δ. Gated populations of CD3+ IELs were analyzed for expression of Thy1.2 and TCR-α/β (A and B) or Thy1.2 and TCR-γ/δ (C and D). The numbers shown represent the percentage of CD3+ IELs within each quadrant. (E–H) IELs were incubated with mAb specific for TCR-α/β, CD8α, and CD4 or CD8β. Gated populations of TCR-α/β+ IELs were analyzed for expression of CD4 and CD8α (E and F) or CD8β and CD8α (G and H). The numbers shown represent the percentage of TCR-α/β+ IELs within each quadrant.
Abnormal IEL profiles have also been observed in IL-2Rβ2/− and IL-15Rα2/− mice 10 20. It has been suggested that the abnormal IEL profile in IL-2Rβ2/− mice might be due to defects in activation-induced cell death that promote the preferential outgrowth and accumulation of thymic-dependent IEL populations within the gut epithelia, rather than due to a decrease in expansion or survival of thymic-independent IEL populations 34. However, the decreases in the relative proportions of Thy1− IELs and TCR-α/β+CD8αα+ IEL populations in IL-15−/− mice (Fig. 4) were very similar to those observed in IL-2Rβ2/− mice 10. In addition, the absolute number of IELs isolated from IL-15Rα2/− mice is reduced approximately twofold and the relative proportions of CD8αα+ and TCR-γ/δ+ IELs are reduced three- to fourfold compared with controls 20. The selective reduction of these particular subsets of IELs in IL-15−/− and IL-15Rα2/− mice, neither of which appear to have defects in activation-induced cell death, points to a critical role for IL-15 in the development or maintenance of IEL populations that mature extrathymically.
IL-15−/− Mice Have a Reversible NK Cell Defect.
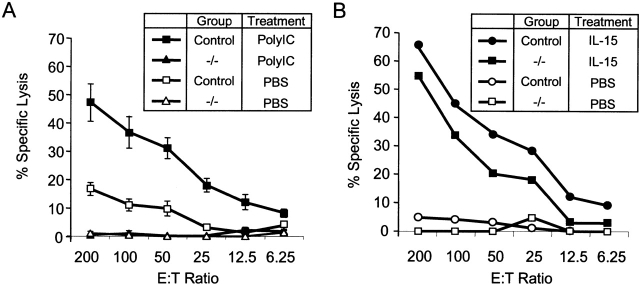
Flow cytometric analysis of splenocytes from naive IL-15−/− mice revealed that they had a severe reduction or absence of cells with an NK (NK1.1+CD3−) phenotype (data not shown). To determine whether IL-15−/− mice lacked functional splenic NK cells, their splenocytes were tested for cytolytic activity against NK-sensitive targets. Splenocytes from PBS-treated control mice exhibited low to weak NK activity, whereas splenocytes from polyI:C-treated control mice exhibited strong NK activity (Fig. 5 A). In contrast, splenocytes from IL-15−/− mice exhibited no demonstrable spontaneous or polyI:C-inducible NK activity.
Figure 5.
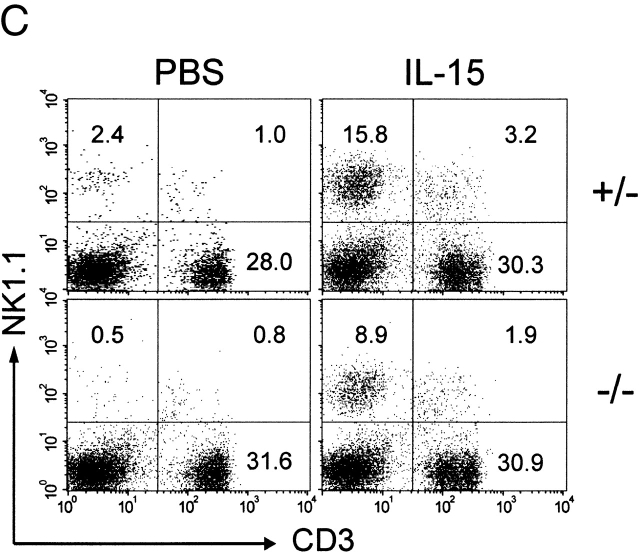
IL-15−/− mice have a reversible NK cell defect. (A) In vitro lysis of 51Cr-labeled YAC-1 targets by splenocytes from IL-15−/− (triangles) or littermate control (IL-15+/+ or IL-15+/−; squares) mice treated 24 h previously with PBS (open symbols) or 100 μg polyI:C (filled symbols). Data shown are the mean ± SEM for a total of five mice per group, analyzed in two experiments. The NK activity of splenocytes from IL-15+/− mice was indistinguishable from that of IL-15+/+ mice. None of the splenocytes induced significant lysis of NK-resistant P815 targets (data not shown). (B) In vitro lysis of YAC-1 targets by splenocytes from control (circles) or IL-15−/− (squares) mice treated with PBS (open symbols) or IL-15 (filled symbols). Groups of two IL-15−/− or control (IL-15+/+ or IL-15+/−) mice were injected intraperitoneally with PBS or 10 μg human IL-15 once daily for 7 d. Spleens were removed 24 h after the last injection. The responses of the individual mice in each group were similar, and the data are shown as the average response of two mice per group. Significant lysis of P815 targets was observed only with splenocytes from IL-15–treated control mice and only at the highest E/T ratio (30% lysis at E/T of 200:1; data not shown). The results shown are representative of two experiments. (C) Splenocytes from mice treated as described in B were analyzed for cell surface expression of NK1.1 and CD3. The numbers shown represent the percentage of cells within each quadrant. The results are representative of four experiments. Data not shown: splenic cellularity was similar in PBS-treated control and IL-15−/− mice. IL-15 treatment increased the splenic cellularity ∼1.5–2-fold in both groups of mice.
Treatment of normal mice with IL-15 induces strong splenic NK cell responses and increases the relative proportion and absolute number of splenic NK cells 35 36. To determine whether the functional NK cell defect in IL-15−/− mice was reversible, adult mice were treated with human IL-15 for 7 d. Splenocytes were harvested 24 h later and assessed for cytolytic activity and cell surface phenotype. Control or IL-15−/− mice injected with PBS exhibited low or undetectable NK activity (Fig. 5 B). In contrast, both control and IL-15−/− mice developed strong splenic NK activity after in vivo treatment with IL-15.
Both control and IL-15−/− mice responded to IL-15 treatment with similar increases (1.5–2-fold) in splenic cellularity and similar phenotypic changes in splenic populations (Fig. 5 C, and data not shown). Both groups of IL-15–treated mice had dramatic increases (>12-fold) in their absolute numbers of splenic NK cells. In addition, the absolute numbers of splenic NK T and CD8+ T cells increased by approximately three- to fivefold in both groups. In contrast, the treatment had no effect on the absolute numbers of splenic B or CD4+ T cells (data not shown).
In vitro studies provide strong evidence that IL-15 is required for the later stages of NK cell maturation and acquisition of cytolytic activity 37 38. However, further studies are needed to determine whether IL-15 reverses the functional NK cell defect in IL-15−/− mice by expanding and activating a severely reduced population of mature NK cells and/or by driving the proliferation and maturation of NK cell precursors.
IL-15−/− Mice Have a Reversible Defect in Memory Phenotype CD8+ T Cells.
Splenocytes and peripheral LN cells were analyzed for cell surface expression of lymphoid and myeloid cell surface markers (CD3ε, CD4, CD8, CD11b, CD19, CD45, Gr1, Ter119, and NK1.1). No dramatic differences were noted in the relative proportions of B cells, macrophages, granulocytes, or CD4+ T cells between IL-15−/− and control mice (Table , and data not shown). In contrast, the relative proportions of splenic and LN CD8+ T cells were reduced approximately twofold in IL-15−/− mice compared with their littermate controls (Table ).
Table 1.
IL-15−/− Mice Have Reduced Levels of Peripheral CD8+ T Cells
| Percent B cells | Percent CD4+ T cells | Percent CD8+ T cells | CD4/CD8 ratio | |
|---|---|---|---|---|
| Spleen | ||||
| Control | 39.0 ± 2.4 | 19.8 ± 0.4 | 10.4 ± 0.5 | 2.1 ± 0.1 |
| IL-15−/− | 43.2 ± 2.8 | 22.0 ± 0.6 | 5.8 ± 0.2 | 3.9 ± 0.2 |
| LN | ||||
| Control | 24.4 ± 1.0 | 33.2 ± 0.7 | 26.5 ± 0.9 | 1.3 ± 0.1 |
| IL-15−/− | 30.9 ± 0.8 | 40.4 ± 0.8 | 14.4 ± 0.4 | 2.8 ± 0.1 |
Splenocytes and LN cells from IL-15−/− and controls (IL-15+/+ and IL-15+/−) were analyzed by flow cytometry. Mice were between 9 and 15 wk of age and were sex- and age-matched in each experiment. The subsets were defined as follows: CD19+CD45R+ (B cells); CD3+CD4+ or CD4+CD8− (CD4+ T cells), and CD3+CD8+ or CD4−CD8+ (CD8+ T cells). Data represent the mean ± SEM percentage of cell populations from 11–24 mice, determined in 4–6 experiments.
As noted above, treatment of IL-15−/− and control mice with exogenous IL-15 increased the relative proportion and absolute numbers of splenic CD8+ T cells. This observation is in accord with a previous study in which IL-15 was shown to be a selective T cell growth factor for memory phenotype CD8+ T cells in vitro and in vivo 14. In that study, IL-15 induced strong in vitro proliferation of purified memory (CD44hi) phenotype CD8+ T cells but failed to stimulate proliferation of naive phenotype CD8+ T cells or naive or memory phenotype CD4+ T cells. In addition, injection of mice with IL-15 induced selective turnover of the memory phenotype CD8+ T cell population. Thus, it was of interest to determine whether the reduction in peripheral CD8+ T cells in IL-15−/− mice preferentially affected the memory phenotype (CD44hi) CD8+ T cell population.
Three-color flow cytometry was used to analyze spleen and LN cells for the expression of CD8, CD4, and CD44. In IL-15−/− mice, there was a dramatic reduction in the relative proportion of CD44hiCD8+ cells in both the spleen (Fig. 6 A) and LN (data not shown) compared with the controls. The CD44hi population accounted for 19 ± 2% of CD8+ splenocytes and 14.2 ± 2% of CD8+ LN cells in control mice but only 6.5 ± 0.5% of CD8+ splenocytes and 2.8 ± 0.2% of CD8+ LN cells in IL-15−/− mice. In contrast, the relative proportion of CD44hi cells within the CD4+ population was similar in controls and IL-15−/− mice in both spleen (22.2 ± 2 vs. 22 ± 1%, respectively; Fig. 6 B) and LN (10.6 ± 0.4 vs. 11 ± 1%).
Figure 6.
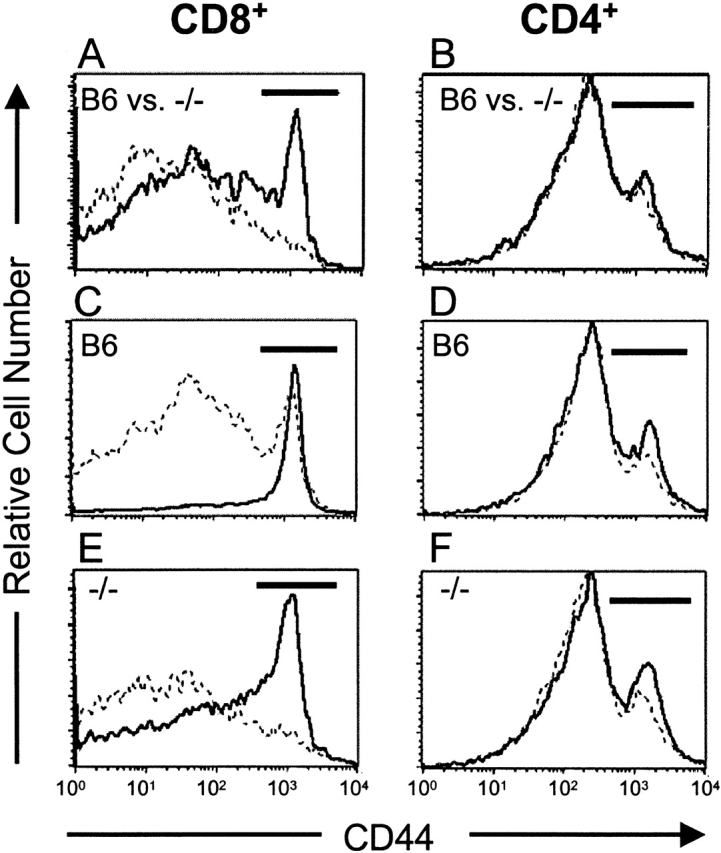
IL-15−/− mice have a reversible defect in CD8+ memory phenotype (CD44hi) T cells. Splenocytes and LN cells were incubated with mAb specific for CD4, CD8, and CD44. Gated populations of CD8+ or CD4+ cells were analyzed for expression of CD44. The CD44hi populations are indicated by the horizontal bars on each histogram. (A and B) CD44 expression was analyzed on CD8+ and CD4+ splenocytes and LN cells from individual 15-wk-old naive C57BL/6 (B6) or IL-15−/− female mice (n = 4/group). Similar patterns were observed in both LNs (not shown) and spleen. (A) CD44 expression on CD8+ splenocytes from control (solid line) or IL-15−/− (dashed line) mice. The mean percentage ± SEM of CD44hi cells within the CD8+ population was 19 ± 2% for control mice and 6.5 ± 0.5% for IL-15−/− mice. (B) CD44 expression on CD4+splenocytes from control (solid line; 22.2 ± 2% CD44hi) or IL-15−/− (dashed line; 22 ± 1% CD44hi) mice. (C–F) 9-wk-old C57BL/6 or IL-15−/− female mice (n = 3/group) were injected intraperitoneally with PBS (dashed line) or 10 μg human IL-15 (solid line) once daily for 7 d. Spleens and LNs were removed 24 h after the last injection. Single cell suspensions from individual mice were analyzed for CD4, CD8, and CD44 expression as described above. Similar changes were observed in both LNs (not shown) and spleen (see Table for summary of data). (C and E) CD44 expression on CD8+ splenocytes from PBS- or IL-15–treated control (C) or IL-15−/− (E) mice. (D and F) CD44 expression on CD4+ splenocytes from PBS- or IL-15–treated control (D) or IL-15−/− (F) mice.
The normal numbers of thymic CD8+ T cells but reduced numbers of memory phenotype CD8+ T cells in IL-15−/− mice suggest that IL-15 is not required for development of CD8+ T cells but is important for expansion and/or maintenance of memory phenotype CD8+ T cells in the periphery. In this regard, short term treatment with exogenous IL-15 induced dramatic increases in the relative proportions and absolute numbers of memory phenotype CD8+ splenocytes and LN cells in both control and IL-15−/− mice (Fig. 6C and Fig. E, and Table ). In contrast, IL-15 treatment had minimal effects on the relative proportions or absolute numbers of memory phenotype CD4+ splenocytes and LN cells (Fig. 6D and Fig. F, and Table ). The ability of IL-15 to reverse the memory phenotype CD8+ T cell defect in otherwise naive IL-15−/− mice provides additional evidence to support a role for IL-15 in expansion and/or maintenance rather than generation of these cells. Future studies that track the fate of wild-type memory phenotype CD8+ T cells after transfer into IL-15−/− hosts will likely clarify the relationship between IL-15 and the memory phenotype CD8+ T cell population.
Table 2.
In Vivo Treatment with IL-15 Expands Memory Phenotype CD44hiCD8+ T Cells in Normal and IL-15−/− Mice
| Percent CD44hi cells within CD8 gate | Percent CD44hi cells within CD4 gate | |||
|---|---|---|---|---|
| PBS | IL-15 | PBS | IL-15 | |
| Splenocytes | ||||
| C57BL/6 | 19.7 ± 1.3 | 75.9 ± 2.1 | 21.3 ± 0.3 | 28.8 ± 1.2 |
| IL-15−/− | 9.5 ± 0.5 | 45.6 ± 4.8 | 20.2 ± 1.1 | 27.1 ± 1.7 |
| LN cells | ||||
| C57BL/6 | 12.4 ± 0.4 | 55.5 ± 1.4 | 11.5 ± 1.2 | 13.4 ± 0.4 |
| IL-15−/− | 3.4 ± 0.1 | 31.2 ± 2.8 | 9.3 ± 0.6 | 14.1 ± 0.8 |
9-wk-old C57BL/6 or IL-15−/− female mice (n = 3/group) were injected intraperitoneally with PBS or 10 μg human IL-15 once daily for 7 d. Spleens and LNs were removed 24 h after the last injection. Single cell suspensions of spleen and LN cells from individual mice were incubated with mAb specific for CD4, CD8, and CD44. Gated populations of CD8+ or CD4+ cells were analyzed for expression of CD44. Results are expressed as the mean ± SEM percentage of CD44hi cells within the gated CD8+ or CD4+ populations. Data not shown: IL-15 treatment had minimal effects on the absolute number of CD4+ cells but increased the absolute number of CD8+ cells in spleen and LNs by two- to threefold in C57BL/6 mice and three- to fivefold in IL-15−/− mice.
IL-15−/− Mice Have Enhanced Susceptibility to Vaccinia Virus Infection.
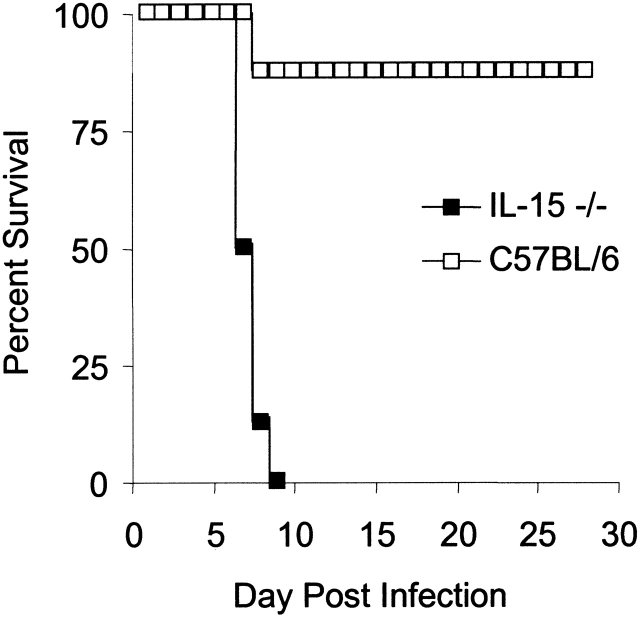
IL-15−/− mice remained healthy when maintained under specific-pathogen free conditions. However, given their severe defects in NK cell function and their selective reduction in peripheral CD8+ T cells, it was of interest to determine whether these mice would be compromised in their ability to respond to infectious agents. Vaccinia virus was chosen for initial challenge studies, as both NK cell and CD8+ T cell responses are crucial for mounting protective responses to this virus 39 40 41. IL-15−/− and C57BL/6 female mice (n = 8/group) were infected with the virulent neurotropic strain of vaccinia virus and assessed daily for morbidity and mortality. By day 4 after infection, the IL-15−/− mice showed severe signs of virus-induced morbidity (ruffled fur, hunched appearance, reduced activity, and overt signs of cachectic fever), whereas the control mice showed less severe clinical symptoms (data not shown). The IL-15−/− mice were unable to resolve the infection and died by day 9 after infection (Fig. 7). In contrast, only one of the control mice died during this time period. The remaining seven control mice cleared the infection and showed no signs of morbidity by day 28 after infection. Thus, IL-15 is essential for the host response to vaccinia virus.
Figure 7.
Susceptibility of IL-15−/− mice to infection with vaccinia virus. Groups of eight female C57BL/6 or IL-15−/− mice were inoculated intravenously with 106 PFU of vaccinia virus. Mice were monitored daily for morbidity (data not shown) and mortality. Data shown are representative of two experiments.
Phenotypic Differences between IL-15−/− and IL-15Rα 2/− Mice.
Recently, Lodolce et al. reported on the generation and characterization of IL-15Rα2/− mice 20. The phenotypes of IL-15Rα2/− and IL-15−/− mice appear similar in terms of their NK, NK T, IEL, and peripheral CD8+ T cell defects. The observation that the defects in IL-15−/− mice are not more severe than those observed in IL-15Rα2/− mice suggests that the high-affinity IL-15R is required for normal physiological responses to IL-15.
Interestingly, the phenotypes that are observed in IL-15−/− and IL-15Rα2/− mice are not identical. IL-15−/− mice, like IL-2Rβ2/− mice 8 42, appear to have normal CD4+ and CD8+ thymic T cell development (Fig. 3). In contrast, IL-15Rα2/− mice may have impaired development of CD8+ T cells, as young mice exhibit an increased ratio of single positive CD4+ to CD8+ thymocytes and a slight thymic hypocellularity (25% decrease relative to controls). In addition, IL-15Rα2/− mice are markedly lymphopenic (30–80% decreased cellularity compared with controls), whereas IL-15−/− mice exhibited only a slight degree of lymphopenia (15% decrease in average weight and 29% decrease in average cellularity of LNs relative to controls; Fig. 2B and Fig. D). Elucidation of the mechanism(s) behind these phenotypic differences will require a systematic comparison of IL-15−/− and IL-15Rα2/− mice that are of matched genetic backgrounds and housed under identical conditions.
IL-15−/− mice will likely prove useful for defining the role of IL-15 (and/or IL-15–dependent cell populations) in host defense mechanisms against various pathogens, tumors, and allografts. In particular, the ability to rescue the NK and memory phenotype CD8+ T cell defects in IL-15−/− mice provides an ideal means by which to further dissect the in vivo relationship between IL-15 and these cell populations. When used in conjunction with appropriate animal models of disease, these mice should also prove useful for determining whether dysregulated expression of IL-15 contributes to the pathogenesis of acute or chronic autoimmune and inflammatory diseases.
Acknowledgments
We thank Randy Hall, Kathy Rohrbach, Kim Harrington, Eileen Roux, Brenda Balogh, Steve Braddy, and Daniel Hirschstein for technical assistance, Dana Schack for animal husbandry, Drs. Kendall Mohler and Hilary McKenna for critical review of the manuscript, Dr. Michael Butine for assistance with statistical analyses, Gary Carlton for graphics, and Anne Aumell for assistance with the manuscript.
Funding for S. Joyce was provided by National Institutes of Health grant AI42284 and by the Juvenile Diabetes Foundation International, NY.
Footnotes
Abbreviations used in this paper: ES, embryonic stem; IEL, intestinal intraepithelial lymphocyte; polyI:C, polyinosilic-polycytidylic acid.
References
- Giri J.G., Anderson D.M., Kumaki S., Park L.S., Grabstein K.H., Cosman D. IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J. Leukoc. Biol. 1995;57:763–766. doi: 10.1002/jlb.57.5.763. [DOI] [PubMed] [Google Scholar]
- Burton J.D., Bamford R.N., Peters C., Grant A.J., Kurys G., Goldman C.K., Brennan J., Roessler E., Waldmann T.A. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W.J., Shores E.W., Love P.E. Role of the common cytokine receptor γ chain in cytokine signaling and lymphoid development. Immunol. Rev. 1995;148:97–114. doi: 10.1111/j.1600-065x.1995.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Giri J.G., Ahdieh M., Eisenman J., Shanebeck K., Grabstein K., Kumaki S., Namen A., Park L.S., Cosman D., Anderson D. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson W.E., Giri J.G., Lindemann M.J., Linett M.L., Ahdieh M., Paxton R., Anderson D., Eisenman J., Grabstein K., Caligiuri M.A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M.K., Park L.S., Paxton R.J. Interleukin-15. In: Thomson A., editor. The Cytokine Handbook. Academic Press; San Diego: 1998. pp. 443–464. [Google Scholar]
- Kündig T.M., Schorle H., Bachmann M.F., Hengartner H., Zinkernagel R.M., Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kündig T.M., Furlonger C., Wakeman A., Timms E., Matsuyama T., Schmits R., Simard J.J.L., Ohashi P.S., Griesser H. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- Willerford D.M., Chen J., Ferry J.A., Davidson L., Ma A., Alt F.W. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Duncan G.S., Takimoto H., Mak T.W. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor β chain. J. Exp. Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohteki T., Ho S., Suzuki H., Mak T.W., Ohashi P.S. Role for IL-15/IL-15 receptor β-chain in natural killer 1.1+ T cell receptor-αβ+ cell development. J. Immunol. 1997;159:5931–5935. [PubMed] [Google Scholar]
- Boesteanu A., De Silva A.D., Nakajima H., Leonard W.J., Peschon J.J., Joyce S. Distinct roles for signals relayed through the common cytokine receptor γ chain and interleukin 7 receptor α chain in natural T cell development. J. Exp. Med. 1997;186:331–336. doi: 10.1084/jem.186.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo J.P. Cytokinesshared receptors, distinct functions. Curr. Biol. 1997;7:R424–R426. doi: 10.1016/s0960-9822(06)00208-9. [DOI] [PubMed] [Google Scholar]
- Zhang X., Sun S., Hwang I., Tough D.F., Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- Quinn L.S., Haugk K.L., Grabstein K.H. Interleukin-15a novel anabolic cytokine for skeletal muscle. Endocrinology. 1995;136:3669–3672. doi: 10.1210/endo.136.8.7628408. [DOI] [PubMed] [Google Scholar]
- Ogata Y., Kukita A., Kukita T., Komine M., Miyahara A., Miyazaki S., Kohashi O. A novel role of IL-15 in the development of osteoclastsinability to replace its activity with IL-2. J. Immunol. 1999;162:2754–2760. [PubMed] [Google Scholar]
- Tagaya Y., Burton J.D., Miyamoto Y., Waldmann T.A. Identification of a novel receptor/signal transduction pathway for IL-15/T in mast cells. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:4928–4939. [PMC free article] [PubMed] [Google Scholar]
- Reinecker H.-C., MacDermott R.P., Mirau S., Dignass A., Podolsky D.K. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology. 1996;111:1706–1713. doi: 10.1016/s0016-5085(96)70036-7. [DOI] [PubMed] [Google Scholar]
- Bulfone-Paus S., Ungureanu D., Pohl T., Lindner G., Paus R., Rückert R., Krause H., Kunzendorf U. Interleukin-15 protects from lethal apoptosis in vivo . Nat. Med. 1997;3:1124–1128. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- Lodolce J.P., Boone D.L., Chai S., Swain R.E., Dassopoulos T., Trettin S., Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Anderson D.M., Johnson L., Glaccum M.B., Copeland N.G., Gilbert D.J., Jenkins N.A., Valentine V., Kirstein M.N., Shapiro D.N., Morris S.W. Chromosomal assignment and genomic structure of Il15 . Genomics. 1995;25:701–706. doi: 10.1016/0888-7543(95)80013-c. [DOI] [PubMed] [Google Scholar]
- Peschon J.J., Torrance D.S., Stocking K.L., Glaccum M.B., Otten C., Willis C.R., Charrier K., Morrissey P.J., Ware C.B., Mohler K.M. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- Renshaw B.R., Fanslow W.C., III, Armitage R.J., Campbell K.A., Liggitt D., Wright B., Davison B.L., Maliszewski C.R. Humoral immune responses in CD40 ligand–deficient mice. J. Exp. Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall W.C., Glaccum M., Charrier K., Rohrbach K., Brasel K., De Smedt T., Daro E., Smith J., Tometsko M.E., Maliszewski C.R. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey P.J., Charrier K., Horovitz D.A., Fletcher F.A., Watson J.D. Analysis of the intra-epithelial lymphocyte compartment in SCID mice that received co-isogeneic CD4+ T cells. Evidence that mature post-thymic CD4+ T cells can be induced to express CD8α in vivo. J. Immunol. 1995;154:2678–2686. [PubMed] [Google Scholar]
- Wokatsch R. Vaccinia virus. In: Majer M.A., Plotkin S.A., editors. Strains of Human Viruses. S. Karger; Basel: 1972. pp. 241–257. [Google Scholar]
- Earl, P.L., N. Cooper, and B. Moss. 1991. Preparation of cell cultures and vaccinia virus stocks. In Current Protocols in Molecular Biology. F.M. Ausubel, R. Brent, R.E. Kingston, D.D. Moore, J.A. Seidman, J.A. Smith, and K. Struhl, editors. John Wiley & Sons, Inc., New York. 16.16.11–16.16.17.
- MacDonald H.R. Development and function of natural killer 1+ T cells. Biochem. Soc. Trans. 1997;25:696–699. doi: 10.1042/bst0250696. [DOI] [PubMed] [Google Scholar]
- Chen Y.H., Chiu N.M., Mandal M., Wang N., Wang C.R. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- Mendiratta S.K., Martin W.D., Hong S., Boesteanu A., Joyce S., Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- Smiley S.T., Kaplan M.H., Grusby M.J. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- Viney J.L., MacDonald T.T., Kilshaw P.J. T-cell receptor expression in intestinal intra-epithelial lymphocyte subpopulations in normal and athymic mice. Immunology. 1989;66:583–587. [PMC free article] [PubMed] [Google Scholar]
- Rocha B., Vassalli P., Guy-Grand D. Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J. Exp. Med. 1994;180:681–686. doi: 10.1084/jem.180.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo J.P., Colucci F., Guy-Grand D. Natural killer and T cells of innate and adaptive immunitylymphoid compartments with different requirements for common γ chain-dependent cytokines. Immunol. Rev. 1998;165:29–38. doi: 10.1111/j.1600-065x.1998.tb01227.x. [DOI] [PubMed] [Google Scholar]
- Evans R., Fuller J.A., Christianson G., Krupke D.M., Troutt A.B. IL-15 mediates anti-tumor effects after cyclophosphamide injection of tumor-bearing mice and enhances adoptive immunotherapythe potential role of NK cell subpopulations. Cell. Immunol. 1997;179:66–73. doi: 10.1006/cimm.1997.1132. [DOI] [PubMed] [Google Scholar]
- Munger W., DeJoy S.Q., Jeyaseelan R., Sr., Torley L.W., Grabstein K.H., Eisenman J., Paxton R., Cox T., Wick M.M., Kerwar S.S. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factorcomparison with interleukin-2. Cell. Immunol. 1995;165:289–293. doi: 10.1006/cimm.1995.1216. [DOI] [PubMed] [Google Scholar]
- Puzanov I.J., Williams N.S., Schatzle J., Sivakumar P.V., Bennett M., Kumar V. Ontogeny of NK cells and the bone marrow microenvironmentwhere does IL15 fit in? Res. Immunol. 1997;148:195–201. doi: 10.1016/s0923-2494(97)84225-3. [DOI] [PubMed] [Google Scholar]
- Williams N.S., Klem J., Puzanov I.J., Sivakumar P.V., Schatzle J.D., Bennett M., Kumar V. Natural killer cell differentiationinsights from knockout and transgenic mouse models and in vitro systems. Immunol. Rev. 1998;165:47–61. doi: 10.1111/j.1600-065x.1998.tb01229.x. [DOI] [PubMed] [Google Scholar]
- Karupiah G., Woodhams C.E., Blanden R.V., Ramshaw I.A. Immunobiology of infection with recombinant vaccinia virus encoding murine IL-2. Mechanisms of rapid viral clearance in immunocompetent mice. J. Immunol. 1991;147:4327–4332. [PubMed] [Google Scholar]
- Buller R.M., Palumbo G.J. Poxvirus pathogenesis. Microbiol. Rev. 1991;55:80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby J., Ramshaw I. The antiviral activity of immune CD8+ T cells is dependent on interferon-gamma. Lymphokine Cytokine Res. 1991;10:353–358. [PubMed] [Google Scholar]
- Suzuki H., Hayakawa A., Bouchard D., Nakashima I., Mak T.W. Normal thymic selection, superantigen-induced deletion and Fas-mediated apoptosis of T cells in IL-2 receptor β chain-deficient mice. Int. Immunol. 1997;9:1367–1374. doi: 10.1093/intimm/9.9.1367. [DOI] [PubMed] [Google Scholar]