Abstract
Clonal anergy of autoreactive B cells is a key mechanism regulating tolerance. Here, we show that anergic B cells express significant surface levels of CD5, a molecule normally found on T cells and a subset of B-1 cells. Breeding of the hen egg lysozyme (HEL) transgenic model for B cell anergy onto the CD5 null background resulted in a spontaneous loss of B cell tolerance in vivo. Evidence for this included elevated levels of anti-HEL immunoglobulin M (IgM) antibodies in the serum of CD5−/− mice transgenic for both an HEL-specific B cell receptor (BCR) and soluble lysozyme. “Anergic” B cells lacking CD5 also showed enhanced proliferative responses in vitro and elevated intracellular Ca2+ levels at rest and after IgM cross-linking. These data support the hypothesis that CD5 negatively regulates Ig receptor signaling in anergic B cells and functions to inhibit autoimmune B cell responses.
Keywords: CD5, hen egg lysozyme, B cell, anergy, signal transduction
Introduction
CD5 is a 67-kD transmembrane glycoprotein expressed constitutively on T cells and a subset of B cells, referred to as B-1a cells, that reside preferentially in the pleural and peritoneal cavities 1 2 3. CD5 levels are developmentally regulated in the T lineage, and correlate with the strength of signaling through the TCR 4 5. On normal peripheral B cells, CD5 expression can be induced after surface IgM cross-linking and B cell activation 6.
Studies with CD5 deficient mice suggest a possible role for the molecule in negative regulation of lymphocyte antigen receptor signaling 7. In CD5−/− mice, TCR transgenic (Tg) CD4+CD8+ thymocytes were hyperresponsive to selecting ligands, indicating that CD5 may regulate the threshold for positive and negative selection 8. Although normal peritoneal B-1 cells undergo apoptosis after cross-linking of surface IgM receptors 9, the same treatment of CD5-deficient B-1 cells resulted in enhanced Ca2+ mobilization and resistance to apoptosis 10. Despite these insights, the precise role for CD5 in regulating immune responses remains unclear.
The hen egg lysozyme (HEL) Tg model system has proven useful for investigating issues of tolerance in the B lineage 11 12 13 14. In mice transgenic for both an Ig receptor specific for HEL (HEL-Ig) and a membrane-bound self-antigen (mHEL), B cells arrest in development in the bone marrow and then undergo clonal deletion 15 16. In contrast, mice transgenic for HEL-Ig and a soluble form of the self-antigen HEL (sHEL) generate B cells that are functionally impaired, or anergic. Only background levels of serum anti-HEL IgM Abs are produced in these anergic animals, and the B cells display downregulated surface IgM levels and are unresponsive to further stimulation through the B cell receptor (BCR) 11 12 13 14. In the experiments described here, we show that CD5 is expressed at higher levels on HEL-Ig/sHEL anergic B cells than on naive HEL-Ig B cells, most likely as a consequence of chronic BCR stimulation. By breeding both HEL-Ig and sHEL transgenes onto the CD5-deficient background, we demonstrate that the level of CD5 expressed on anergic B cells is important for maintaining tolerance to self-antigen.
Materials and Methods
Mice.
MD4 HEL-Ig and ML5 sHEL Tg mice were originally obtained from Dr. C. Goodnow (Australian National University, Canberra, Australia), and were genotyped as described 11 17. CD5 knockout mice were provided by A. Tarakhovsky (University of Cologne, Cologne, Germany) and Randy Hardy (Fox Chase Cancer Center, Philadelphia, PA). The HEL-Ig (IgMa allotype) and sHEL transgenes were crossed onto the CD5−/− background, and the resulting CD5+/− and CD5−/− animals were on essentially identical mixed genetic backgrounds (∼25% 129/Sv and ∼75% C57Bl/6J). The CD5+/+ HEL-Ig and HEL-Ig/sHEL Tg animals used in these experiments were 100% C57Bl/6J. CD5 null offspring were identified by flow cytometry of peripheral blood using anti-CD5 mAbs (PharMingen) or by PCR genotyping of tail DNA. All mice were maintained in specific pathogen-free isolation at the University of Minnesota animal facility, and were used at 8–12 wk of age.
Flow Cytometric Analysis.
Single cell lymphoid populations were prepared as described 17, and were stained with FITC–anti-IgMa, PE–anti-CD5, and CyChrome–anti-B220 mAbs, or appropriate IgG2a control mAbs (all mAbs from PharMingen) for flow cytometric analysis. To identify HEL-binding B cells, a modified HEL sandwich assay was used. In brief, cells were incubated with 10 μg/ml HEL (Sigma Chemical Co.), washed, and then incubated with biotinylated D1.3 anti-HEL mAbs 18. After washing, the bound D1.3 anti-HEL Abs were detected with allophycocyanin (APC)-streptavidin. Three- and four-color flow cytometry was performed using a FACSCalibur™ (Becton Dickinson), and data were analyzed using Flowjo software (Treestar).
Serum anti-HEL IgM ELISAs.
Sera from HEL-Ig and HEL-Ig/sHEL mice of the various CD5 genotypes were collected by eye or tail bleed, and anti-HEL IgMa levels were measured by specific ELISA 12 17. Sera were diluted 500- or 2,500-fold for measurement in HEL-Ig mice, and 100- or 500-fold for HEL-Ig/sHEL animals. Relative serum anti-HEL IgMa levels were calculated as the product of the mean OD 490 of triplicate samples and the dilution.
In Vitro Assays.
B cells were enriched from single cell spleen preparations using anti–Thy-1 magnetic beads (Dynal) to deplete T cells. The resulting B cells from HEL-Ig or HEL-Ig/sHEL mice were cultured in triplicate wells (105 cells/200 μl medium) in complete RPMI 10%/FCS medium in the absence or presence of rIL-4 (10 ng/ml) and/or anti-μ polyclonal F(ab′)2 Abs (10 μg/ml) for 3 d. [3H]Thymidine incorporation was measured for the last 16 h of culture. For calcium mobilization studies, splenocytes were purified by red blood cell lysis, loaded with indo-1 (5 μM; Molecular Probes) at 37°C 19, and stained with PE-labeled anti-B220 (PharMingen) at 4°C. After washing, cells were warmed to 37°C for 3 min before analysis. Intracellular calcium ([Ca2+]i) levels of splenocytes were then acquired for 30 s to assure a stable baseline, and the cells were stimulated by the addition of 10 μg/ml anti-μ Abs. [Ca2+]i levels were measured as the ratio of indo-1 fluorescence at 395 versus 530 nm of gated B220+ cells.
Results and Discussion
CD5 Expression on Anergic B Cells.
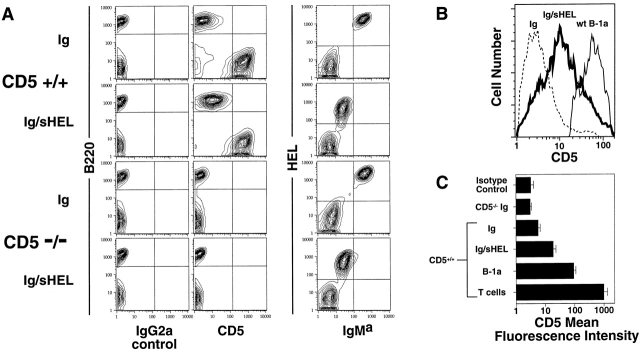
We recently reported that the antiapoptotic gene bcl-xL, when overexpressed as a transgene, allowed HEL-Ig B cells to escape central deletion in mHEL mice 17. The escaped B cells were profoundly anergic, and surprisingly were found to express significant levels of cell surface CD5 (our unpublished data). Thus, we tested whether B cells in HEL-Ig/sHEL double Tg mice, the prototypic model for B cell anergy, also expressed CD5. By flow cytometry, anergic B cells (HEL-Ig/sHEL, B220+; HEL-binding, IgMa-low) expressed significantly higher levels of CD5 than HEL-Ig B cells (Fig. 1 A). In 10 pairs of mice tested, there was a mean 3.6 ± 0.4–fold increase in CD5 levels in HEL-Ig/sHEL B cells compared with HEL-Ig B cells (Fig. 1 C, P < 0.001 by Student's paired t test). The level of CD5 expressed on anergic HEL-Ig/sHEL B cells was intermediate between the low levels found on HEL-Ig B cells and the higher levels on control peritoneal B-1a cells (Fig. 1B and Fig. C). By comparison, CD5 expression on splenic T cells (Fig. 1 A; T cells are CD5hi, B220−) was ∼10-fold higher than on peritoneal B-1a and 50-fold higher than on anergic B cells. The mean fluorescence intensities (MFIs) of CD5 on each of the relevant cell types are summarized in Fig. 1 C.
Figure 1.
CD5 expression on anergic B cells. (A) Splenocytes from CD5+/+ and CD5−/− HEL-Ig (Ig) or HEL-Ig/sHEL (Ig/sHEL) mice were stained with anti-IgMa, anti-CD5, anti-B220, and/or control IgG2a mAbs, and analyzed by flow cytometry. Results show lymphoid cells gated on the basis of forward by side scatter. All samples were analyzed in parallel using identical cytometer settings. Quadrant settings were placed to allow comparison of the relative levels of CD5, HEL-binding, and IgMa. (B) Overlaid histograms of CD5 expression on HEL-Ig (Ig, dotted line), HEL-Ig/sHEL (Ig/sHEL, bold line), and C57Bl/6J control peritoneal B-1a cells (wt B-1a, light line). HEL-Ig and HEL-Ig/sHEL B cells were gated on B220+IgMa+ cells, and B-1a cells were gated on B220loIgMhi cells. (C) CD5 MFIs (±SD) for the indicated cell populations are shown (n = 10, except for peritoneal B-1a cells where n = 4).
Breeding of HEL-Ig and sHEL Transgenes onto the CD5−/− Background.
To determine the functional significance of CD5 expression on anergic B cells, the HEL-Ig and sHEL transgenes were backcrossed onto the CD5 null background. As expected, B and T cell populations in the resulting CD5−/− mice showed no detectable expression of CD5 (Fig. 1 A, bottom). The levels of HEL-binding IgMa were similar on CD5+/+ compared with CD5−/− HEL-Ig or HEL-Ig/sHEL B cells in the bone marrow, spleen, and lymph nodes (Fig. 1, and data not shown). We also observed no CD5-dependent differences in expression of the maturity markers CD21, CD22, CD23, or CD24 on HEL-Ig or HEL-Ig/sHEL B cells (data not shown).
Interestingly, the level of CD5 expressed on naive CD5+/+ HEL-Ig B cells was slightly higher than the isotype control mAb staining and was also above that observed on CD5−/− HEL-Ig B cells (Fig. 1A and Fig. C). In the original report describing the phenotype of CD5−/− mice, Tarakhovsky and colleagues also noted this low level CD5 staining on naive splenic B cells 7. They suggested that this staining might represent passive acquisition of soluble CD5 shed by CD5+ T cells, since this low level of CD5 on B cells was not observed in T cell–deficient nu/nu mice. Regardless of the source of the “background” CD5 expression on naive B cells, the data shown in Fig. 1 demonstrate that HEL-Ig/sHEL B cells have increased surface levels of CD5 compared with antigen naive HEL-Ig B cells. This difference is unlikely to be accounted for by passive acquisition from T cells, as the T cells in both cases are wild-type for CD5.
Anergic CD5−/− Mice Have Fewer B Cells in Spleen and Blood.
Table shows the size of B cell populations in the various mice generated. CD5 genotype had no effect on B cell populations in HEL-Ig mice, and the numbers of bone marrow B220+ B cells were similar in CD5+/+ and CD5−/− HEL-Ig/sHEL mice. In CD5+/+ mice, B cell numbers in the spleen of HEL-Ig/sHEL mice were decreased by ∼50% compared with HEL-Ig mice, as reported previously 14 20. In addition, there were significantly fewer splenic IgMa+ B cells in CD5−/− HEL-Ig/sHEL mice compared with CD5+/+ double Tg controls. A diminished percentage of B cells in the blood of CD5−/− HEL-Ig/sHEL mice was also noted. This phenotype was similar to the reduced peripheral B cell pool reported in HEL-Ig/sHEL mice with deficiencies in negative regulators of BCR signaling (lyn, CD22, and Src homology 2 domain–containing protein tyrosine phosphatase [SHP-1]; references 20, 21), or overexpressing the BCR costimulatory molecule CD19 22.
Table 1.
B Cell Populations in CD5+/+ and CD5−/− HEL-Ig and HEL-Ig/sHEL Mice
| Cell numbers (×10−6) | ||||
|---|---|---|---|---|
| Cells | CD5+/+ HEL-Ig | CD5−/− HEL-Ig | CD5+/+ HEL-Ig/sHEL | CD5−/− HEL-Ig/sHEL |
| Bone marrow (n = 3) | ||||
| Total cellularity | 46 ± 1 | 46 ± 16 | 49 ± 6 | 36 ± 5 |
| B220+IgMa− | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.0 |
| B220+IgMa+ | 3.3 ± 0.2 | 4.3 ± 1.9 | 3.3 ± 0.9 | 2.8 ± 0.5 |
| Spleen (n = 6) | ||||
| Total cellularity | 58 ± 7 | 56 ± 6 | 42 ± 4 | 39 ± 9 |
| B220+IgMa+ | 26 ± 6 | 23 ± 5 | 11 ± 3 | 5 ± 1 |
| Blood (n = 6) | 14 ± 3 | 15 ± 3 | 12 ± 1 | 3 ± 1 |
Single cell suspensions of bone marrow (both femurs and tibias) and spleen were enumerated, and B220/IgMa subpopulations were identified using flow cytometry. Data represent mean ± SEM for each group of mice analyzed in parallel at 8–12 wk of age. Student's paired t test was used for statistical comparisons.
Spontaneous Secretion of Autoantibodies in Anergic CD5−/− Mice.
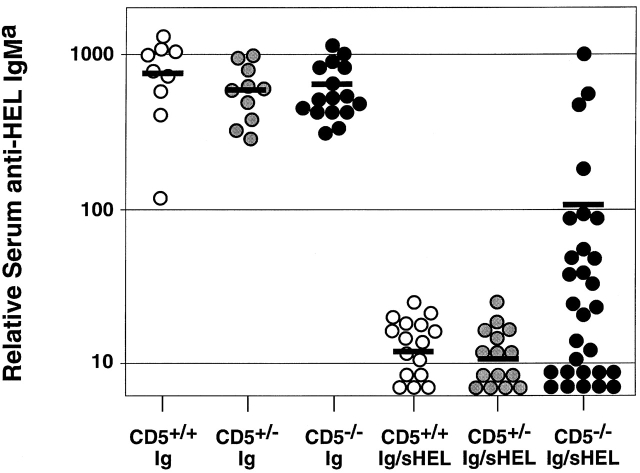
Despite the lower numbers of IgMa+ B cells in HEL-Ig/sHEL mice on the CD5 null background, many of these animals exhibited elevated levels of anti-HEL IgMa Abs in serum (Fig. 2). Although specific Ab levels in CD5+/+ and CD5+/− HEL-Ig/sHEL mice were consistently near or below the limit of detection, 13 of 29 CD5−/− HEL-Ig/sHEL mice tested demonstrated anti-HEL IgMa titers above the highest level measured in CD5+/+ or CD5+/− anergic mice. Remarkably, the titers in 4 of 29 mice were within the range observed for HEL-Ig mice. Thus, many of the “anergic” mice lacking CD5 showed a spontaneous in vivo loss of B cell tolerance.
Figure 2.
Elevated serum anti-HEL IgMa Ab levels in CD5−/− HEL-Ig/sHEL mice. Sera from CD5+/+, CD5+/−, or CD5−/− HEL-Ig and HEL-Ig/sHEL mice were collected, and anti-HEL IgMa levels were measured by specific ELISA. All samples were analyzed in parallel. Each data point represents the mean value for a single mouse, while horizontal bars indicate the mean value for each group of animals. Differences in mean anti-HEL IgMa levels between CD5−/− and either CD5+/− or CD5+/+ HEL-Ig/sHEL mice were significant (P < 0.02, Student's paired t test).
Serial bleeds of CD5−/− HEL-Ig/sHEL mice indicated that levels of serum anti-HEL Abs remained stable over a period of at least 4 mo. In preliminary experiments, we immunized CD5−/− HEL-Ig/sHEL mice that displayed low basal levels of HEL-specific IgM with HEL in adjuvant to test whether tolerance could be broken in these animals. In approximately half the mice, immunization led to a >18-fold increase in anti-HEL titers at 14 d, compared with no more than 2-fold increases in control CD5+/+ anergic mice (our unpublished data). The reason for the heterogeneity observed in Ab responses (both basal and induced) in CD5−/− HEL-Ig/sHEL animals is currently unknown. One possibility is that this reflects subtle differences in background genes, since these animals are on a mixed 129/B6 background, and the 129/Sv genetic background has recently been shown to be permissive for autoantibody production in other knockout models 23 24. Alternatively, this heterogeneity might reflect differing exposure of the mice to infectious agents, or incomplete penetrance of the CD5 genetic effect.
CD5−/− HEL-Ig/sHEL B Cells Are Hyperproliferative in Response to BCR Cross-linking.
A hallmark of B cell anergy is reduced in vitro proliferation in response to antigen or BCR cross-linking 12 14. Therefore, we isolated splenic B cells from CD5+/+ and CD5−/− HEL-Ig and HEL-Ig/sHEL mice and stimulated them in vitro with anti-μ Abs in the presence of IL-4, followed by measurement of DNA synthesis. In the representative experiment shown (Fig. 3 A), purified B cells from CD5−/− HEL-Ig mice showed approximately a twofold greater proliferative response than CD5+/+ HEL-Ig animals after anti-μ or anti-μ plus IL-4 treatment. This result differs from previous published data 10, where the proliferation of stimulated polyclonal CD5+/+ and CD5−/− B-2 cells was reported to be similar. These differences are likely attributable to the fact that we are studying the responses of Ig transgenic B cells, where the activation state and response to stimulation may be more uniform, and/or to technical differences in the preparation of the B cells. Importantly, CD5−/− HEL-Ig/sHEL B cells demonstrated an eightfold increase in proliferation compared with CD5+/+ anergic cells in response to anti-μ plus IL-4. In other experiments, this fold increase ranged from 4 to 14. Thus, CD5−/− “anergic” B cells, and even CD5−/− HEL-Ig cells, were hyperproliferative after BCR cross-linking, suggesting that CD5 functions to downregulate BCR signaling in these cells.
Figure 3.
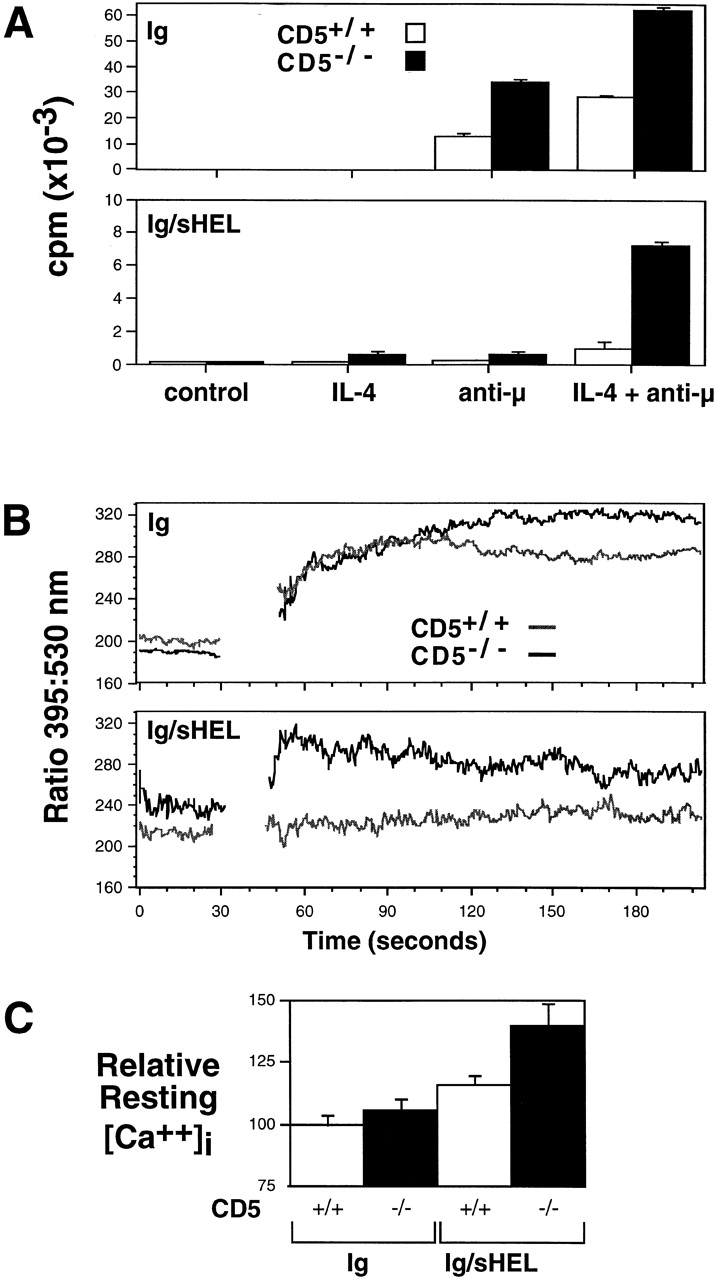
(A) Proliferative responses of splenic B cells in vitro. B cells from CD5+/+ or CD5−/− HEL-Ig (top) or HEL-Ig/sHEL (bottom) mice were cultured in triplicate in the absence or presence of rIL-4 (10 ng/ml) and/or anti-μ polyclonal F(ab′)2 Abs (10 μg/ml) for 3 d. [3H]Thymidine incorporation was measured for the last 16 h of culture. Data represent mean ± SD of the triplicate cultures, and are representative of six experiments. (B and C) Elevated resting and stimulated [Ca2+]i levels in CD5−/− HEL-Ig/sHEL B cells. Indo-1–loaded splenocytes from CD5+/+ and CD5−/− HEL-Ig or HEL-Ig/sHEL mice were stained with anti-B220 mAbs, and [Ca2+]i of B220+ B cells was measured at rest and after stimulation with 10 μg/ml anti-μ Abs (break in tracing). The data shown are representative of five independent experiments. C summarizes data from six experiments (mean ± SEM), showing relative resting [Ca++]i of gated B cells, with the indo-1 ratio for HEL-Ig B cells set at 100%.
Calcium Responses in CD5−/− B Cells.
To begin to address the molecular nature of the dysregulated responses observed in CD5−/− HEL-Ig/sHEL B cells, we measured [Ca2+]i levels by flow cytometry using indo-1 19, both in resting and anti-μ–stimulated B cells. Previous work has shown that B cells from anergic HEL-Ig/sHEL mice have a higher resting [Ca2+]i than HEL-Ig mice 25 26, and this was confirmed here (Fig. 3B and Fig. C). Interestingly, the mean basal [Ca2+]i of CD5−/− HEL-Ig/sHEL B cells was 36% higher than CD5+/+ HEL-Ig B cells (P < 0.004) and 22% higher than CD5+/+ HEL-Ig/sHEL B cells (P < 0.006) (Fig. 3 C).
Anti-μ stimulation resulted in rapid and sustained Ca2+ mobilization in CD5+/+ and CD5−/− HEL-Ig B cells (Fig. 3 B, top; differences in the curves after the 100-s time point were not always observed). Neither CD5+/+ nor CD5−/− HEL-Ig/sHEL B cells responded to soluble HEL protein by mobilizing Ca2+ (data not shown). Similarly, anti-μ stimulation, which provides higher levels of Ig cross-linking, failed to induce a significant Ca2+ flux in CD5+/+ HEL-Ig/sHEL B cells (Fig. 3 B, bottom). However, anti-μ induced a rapid Ca2+ mobilization in CD5−/− HEL-Ig/sHEL B cells that peaked at levels equivalent to HEL-Ig B cells, and then drifted slowly towards baseline.
These results indicate that the levels of CD5 expressed on anergic B cells are functionally important for maintaining B cell tolerance. Approximately half of the HEL-Ig/sHEL mice lacking CD5 exhibited spontaneously elevated levels of serum anti-HEL IgMa, and purified B cells from CD5−/− anergic mice were hyperresponsive in vitro. This observed break in B cell tolerance is most likely due to an increased sensitivity of the anti-HEL BCR to cross-linking in the absence of CD5. Although the signaling pathways downstream of CD5 are not well defined, it was recently reported that SHP-1, a phosphatase that negatively regulates Ig signaling 20 21 27 28 29, associates with the CD5 cytoplasmic domain 30 31. In the absence of CD5, B cells with chronically engaged BCRs may have insufficient SHP-1 (or other negative regulators) available at the membrane to downmodulate BCR signaling, leading to the observed phenotype.
The data presented do not exclude the possibility that defects in T cell tolerance may be contributing to the observed phenotype in CD5−/− HEL-Ig/sHEL mice. However, several lines of evidence suggest that the phenotype is more likely to reflect an intrinsic defect in CD5−/− HEL-Ig/sHEL B cells. First, in the data shown in Fig. 3, CD5−/− HEL-Ig/sHEL B cells fluxed calcium in response to BCR cross-linking while CD5+/+ anergic B cells did not, and T cell–depleted splenic HEL-Ig/sHEL B cells from CD5−/− mice were hyperproliferative in response to BCR cross-linking compared with CD5+/+ anergic B cells. BCR cross-linking in both these settings is T cell independent, suggesting an intrinsic defect in B cells. Also arguing against a significant role for T cells in this system are data from previous studies showing exquisite sensitivity of HEL-reactive T cells to negative selection in mice expressing even very low levels of soluble HEL 32 33. Finally, while CD5 likely does have a role in fine tuning selection events in the thymus 5 8, Tarakhovsky and colleagues reported that negative selection of H-Y–specific TCR transgenic T cells was not affected in male CD5−/− H-Y Tg mice 8. To more definitively address the issue of T cell tolerance in this model, we are currently breeding the CD5 knockout alleles and the HEL-Ig and sHEL transgenes onto the T cell–deficient recombination activating gene (Rag) knockout background.
Although CD5 can be induced on mature peripheral B cells after activation 3, constitutive expression of CD5 in the B lineage (above that found on naive B cells; see Fig. 1) has previously been observed only on the B-1a population. B-1a cells are found in the serous cavities of mice and humans, including the peritoneum, and they comprise a significant percentage of peripheral B cells in humans 34 35. B-1 cells are a major source of natural autoantibody production, and require antigen exposure for their maturation into Ab-secreting cells 36. Although B-1 cells are not anergic as classically defined in the HEL system, there are some interesting parallels. Both populations have received antigen stimulation 12 36, both cell types respond suboptimally to IgM cross-linking 9 14, and both show evidence for excessive receptor editing (17 37; and Tze, L.E., and T.W. Behrens, unpublished data). In addition, B-1 cells generally use germline-encoded heavy and light chain V genes with little evidence for somatic mutation and are reported to be excluded from germinal centers 34 35. Likewise, anergic cells are excluded from follicles 38, and thus are unable to effectively participate in germinal center responses. These similarities raise the possibility that B cell anergy, or an anergy-like state, might be an intermediate step in the development of the B-1 compartment.
The hypothesis that low-to-intermediate levels of CD5 identify B cells with a history of antigen exposure has potentially interesting implications for human disease. The numbers of peripheral CD5+ B cells are increased in several autoimmune diseases, including rheumatoid arthritis, primary Sjogren's syndrome, autoimmune thyroid disease, and multiple sclerosis 39. The accumulation of CD5+ B cells in autoimmunity may, at least in part, be secondary to antigen-driven activation and subsequent anergy induction of autoreactive B cells. Mechanisms that then contribute to a loss of tolerance in CD5+ anergic B cells, including polymorphisms in molecules that negatively regulate BCR signaling, might be important in the pathogenesis of autoimmune disorders. Finally, >95% of human chronic lymphocytic leukemias and many small cell non-Hodgkin's lymphomas are derived from a CD5+ B cell precursor 40. Repeated encounters of CD5+ B cells with self-antigen and resultant chronic signaling through the BCR might contribute to the genetic mutations responsible for their malignant transformation.
Acknowledgments
We thank A. Tarakhovsky, R. Hardy, C. Goodnow, M. Neuberger, and R. Poljak for the gifts of mice and reagents; E. Baness for technical assistance; M. Jenkins, C. Pennell, and D. Mueller for helpful comments and advice; and H. Wortis for extensive discussions and encouragement.
This work is supported by an Arthritis Foundation Investigator Award (to K.L. Hippen), a Leukemia Society of America Scholar Award (to T.W. Behrens), and National Institutes of Health grant R01 AR43805 (to T.W. Behrens).
Footnotes
Abbreviations used in this paper: [Ca2+]i, intracellular calcium concentration; BCR, B cell receptor; HEL, hen egg lysozyme; MFI, mean fluorescence intensity; mHEL, membrane-bound self-antigen HEL; sHEL, soluble self-antigen HEL; SHP-1, Src homology 2 domain–containing protein tyrosine phosphatase 1; Tg, transgenic.
References
- Cantor H., Boyse E.A. Functional subclasses of T lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T cell subclasses in a differentiative process independent of antigen. J. Exp. Med. 1975;141:1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J.A., Rouse R.V., Micklem H.S., Herzenberg L.A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigenstwo parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J. Exp. Med. 1980;152:280–294. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Parks D.R., Herzenberg L.A. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J. Exp. Med. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes B.J., Edison L., Mathieson B.J., Chused T.M. Early T lymphocytesdifferentiation in vivo of adult intrathymic precursor cells. J. Exp. Med. 1985;162:802–822. doi: 10.1084/jem.162.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam H.S., Grinberg A., Lui K., Shen H., Shores E.W., Love P.E. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y.Z., Rabin E., Wortis H.H. Treatment of murine CD5− B cells with anti-Ig, but not LPS, induces surface CD5two B-cell activation pathways. Int. Immunol. 1991;3:467–476. doi: 10.1093/intimm/3.5.467. [DOI] [PubMed] [Google Scholar]
- Tarakhovsky A., Muller W., Rajewsky K. Lymphocyte populations and immune responses in CD5-deficient mice. Eur. J. Immunol. 1994;24:1678–1684. doi: 10.1002/eji.1830240733. [DOI] [PubMed] [Google Scholar]
- Tarakhovsky A., Kanner S.B., Hombach J., Ledbetter J.A., Muller W., Killeen N., Rajewsky K. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- Tsubata T., Murakami M., Honjo T. Antigen-receptor cross-linking induces peritoneal B-cell apoptosis in normal but not autoimmunity-prone mice. Curr. Biol. 1994;4:8–17. doi: 10.1016/s0960-9822(00)00003-8. [DOI] [PubMed] [Google Scholar]
- Bikah G., Carey J., Ciallella J.R., Tarakhovsky A., Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science. 1996;274:1906–1909. doi: 10.1126/science.274.5294.1906. [DOI] [PubMed] [Google Scholar]
- Goodnow C.C., Crosbie J., Adelstein S., Lavoie T.B., Smith-Gill S.J., Brink R.A., Pritchard-Briscoe H., Wotherspoon J.S., Loblay R.H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C.C., Crosbie J., Jorgensen H., Brink R.A., Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C.C. Balancing immunity and tolerancedeleting and tuning lymphocyte repertoires. Proc. Natl. Acad. Sci. USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke M.P., Heath A.W., Shokat K.M., Zeng Y., Finkelman F.D., Linsley P.S., Howard M., Goodnow C.C. Immunoglobulin signal transduction guides the specificity of B cell–T cell interactions and is blocked in tolerant self-reactive B cells. J. Exp. Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley S.B., Crosbie J., Brink R., Kantor A.B., Basten A., Goodnow C.C. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- Hartley S.B., Cooke M.P., Fulcher D.A., Harris A.W., Cory S., Basten A., Goodnow C.C. Elimination of self-reactive B lymphocytes proceeds in two stagesarrested development and cell death. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Fang W., Weintraub B.C., Dunlap B., Garside P., Pape K.A., Jenkins M.K., Goodnow C.C., Mueller D.L., Behrens T.W. Self-reactive B lymphocytes overexpressing Bcl-xL escape negative selection and are tolerized by clonal anergy and receptor editing. Immunity. 1998;9:35–45. doi: 10.1016/s1074-7613(00)80586-5. [DOI] [PubMed] [Google Scholar]
- Mariuzza R.A., Jankovic D.L., Boulot G., Amit A.G., Saludjian P., Le Guern A., Mazie J.C., Poljak R.J. Preliminary crystallographic study of the complex between the Fab fragment of a monoclonal anti-lysozyme antibody and its antigen. J. Mol. Biol. 1983;170:1055–1058. doi: 10.1016/s0022-2836(83)80206-x. [DOI] [PubMed] [Google Scholar]
- Justement L.B., Krieger J., Cambier J.C. Production of multiple cytokines by the A20.1 B cell lymphoma following crosslinking of membrane immunoglobulins by immobilized anti-Ig. J. Immunol. 1989;143:881–889. [PubMed] [Google Scholar]
- Cornall R.J., Cyster J.G., Hibbs M.L., Dunn A.R., Otipoby K.L., Clark E.A., Goodnow C.C. Polygenic autoimmune traitsLyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- Cyster J.G., Goodnow C.C. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Inaoki M., Sato S., Weintraub B.C., Goodnow C.C., Tedder T.F. CD19-regulated signaling thresholds control peripheral tolerance and autoantibody production in B lymphocytes. J. Exp. Med. 1997;186:1923–1931. doi: 10.1084/jem.186.11.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto M., Dell'Agnola C., Bygrave A.E., Thompson E.M., Cook H.T., Petry F., Loos M., Pandolfi P.P., Walport M.J. Homozygous C1q deficiency causes glomerulonephritis associated with apoptotic bodies. Nat. Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Bickerstaff M.C., Botto M., Hutchinson W.L., Herbert J., Tennent G.A., Bybee A., Mitchell D.A., Cook H.T., Butler P.J., Walport M.J., Pepys M.B. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat. Med. 1999;5:694–697. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- Healy J.I., Dolmetsch R.E., Timmerman L.A., Cyster J.G., Thomas M.L., Crabtree G.R., Lewis R.S., Goodnow C.C. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- Dolmetsch R.E., Lewis R.S., Goodnow C.C., Healy J.I. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Schweitzer P.A., Rajan T.V., Yi T., Ihle J.N., Matthews R.J., Thomas M.L., Beier D.R. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- Tsui H.W., Siminovitch K.A., de Souza L., Tsui F.W. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat. Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio D., Hippen K.L., Minskoff S.A., Mellman I., Pani G., Siminovitch K.A., Cambier J.C. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by Fc gamma RIIB1. Science. 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- Pani G., Fischer K.D., Mlinaric-Racan I., Siminovitch K.A. Signaling capacity of the T cell antigen receptor is negatively regulated by the PTP1C tyrosine phosphatase. J. Exp. Med. 1996;184:839–852. doi: 10.1084/jem.184.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Villar J.J., Whitney G.S., Bowen M.A., Hewgill D.H., Aruffo A.A., Kanner S.B. CD5 negatively regulates the T-cell antigen receptor signal transduction pathwayinvolvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol. Cell. Biol. 1999;19:2903–2912. doi: 10.1128/mcb.19.4.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelstein S., Pritchard B.H., Anderson T.A., Crosbie J., Gammon G., Loblay R.H., Basten A., Goodnow C.C. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991;251:1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- Peterson D.A., DiPaolo R.J., Kanagawa O., Unanue E.R. Quantitative analysis of the T cell repertoire that escapes negative selection. Immunity. 1999;11:453–462. doi: 10.1016/s1074-7613(00)80120-x. [DOI] [PubMed] [Google Scholar]
- Herzenberg L.A., Stall A.M., Lalor P.A., Sidman C., Moore W.A., Parks D.R., Herzenberg L.A. The Ly-1 B cell lineage. Immunol. Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Hardy R.R., Carmack C.E., Li Y.S., Hayakawa K. Distinctive developmental origins and specificities of murine CD5+ B cells. Immunol. Rev. 1994;137:91–118. doi: 10.1111/j.1600-065x.1994.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Asano M., Shinton S.A., Gui M., Allman D., Stewart C.L., Silver J., Hardy R.R. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- Qin X.F., Schwers S., Yu W., Papavasiliou F., Suh H., Nussenzweig A., Rajewsky K., Nussenzweig M.C. Secondary V(D)J recombination in B-1 cells. Nature. 1999;397:355–359. doi: 10.1038/16933. [DOI] [PubMed] [Google Scholar]
- Cyster J.G., Hartley S.B., Goodnow C.C. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- Talal N., Dauphinee M., Ahmed S.A. CD5 B cells in autoimmunity. Ann. NY Acad. Sci. 1992;651:551–556. doi: 10.1111/j.1749-6632.1992.tb24661.x. [DOI] [PubMed] [Google Scholar]
- Pangalis G.A., Angelopoulou M.K., Vassilakopoulos T.P., Saikantaris M.P., Kittas C. B-chronic lymphocytic leukemia, small lymphocytic lymphoma, and lymphoplasmacytic lymphoma, including Waldenstrom's macroglobulinemiaa clinical, morphologic, and biologic spectrum of similar disorders. Semin. Hematol. 1999;36:104–114. [PubMed] [Google Scholar]