Abstract
Peptides derived from the sequence of a single-chain, recombinant, antiidiotypic antibody (IdAb; KT-scFv) acting as a functional internal image of a microbicidal, wide-spectrum yeast killer toxin (KT) were synthesized and studied for their antimicrobial activity by using the KT-susceptible Candida albicans as model organism. A decapeptide containing the first three amino acids (SAS) of the light chain CDR1 was selected and optimized by alanine replacement of a single residue. This peptide exerted a strong candidacidal activity in vitro, with a 50% inhibitory concentration of 0.056 μM, and was therefore designated killer peptide (KP). Its activity was neutralized by laminarin, a β1-3 glucan molecule, but not by pustulan, a β1-6 glucan molecule. KP also competed with the binding of a KT-like monoclonal IdAb to germinating cells of the fungus. In a rat model of vaginal candidiasis, local, postchallenge administration of KP was efficacious in rapidly abating infections caused by fluconazole-susceptible or -resistant C. albicans strains. In systemic infection of BALB/c or SCID mice preinfected intravenously with a lethal fungal load, KP caused a highly significant prolongation of the median survival time, with >80% of the animals still surviving after >60 days, whereas >90% of control mice died within 3 to 5 days. KP is therefore the first engineered peptide derived from a recombinant IdAb retaining KT microbicidal activity, probably through the interaction with the β-glucan KT receptor on target microbial cells.
A killer toxin (KT) produced by the yeast Pichia anomala is a glycoprotein able to kill other microorganisms presenting specific cell wall receptors (KTR) and competing in natural habitats for the same ecological niche (19, 31). Although a therapeutically attractive tool for its wide spectrum of microbicidal activity in vitro, inclusive of such diverse pathogens as Candida albicans, Pneumocystis carinii, and Mycobacterium tuberculosis, KT is by itself of no practical use because of its instability in the physiological milieu of mammals as well as its antigenicity and toxicity (2, 8, 22, 25). We postulated the possibility of exploiting KT antimicrobial activity without KT's undesired effects by mimicry through the Id network (4, 14). Thus, a KT-neutralizing monoclonal antibody (MAb) (mAbKT4) was used to raise antiidiotypic antibodies (IdAb) representing the internal image of the KT active domain (KT-IdAb) and endowed with effective binding to KTR, KT-like microbicidal activity in vitro, and pronounced protective effects in vivo (23, 26-28). The KTR has recently been identified as a β-glucan (12, 13), thus indirectly supporting the concept that KT-IdAb's could indeed recognize this cell wall polysaccharide and then exert their cytocidal action on a large variety of glucan-possessing pathogens (4).
To obtain standardizable reagents in sufficient amounts for therapeutic assays, KT-IdAb were produced in the monoclonal (KT-MAb) and recombinant single chain fragment variable (KT-scFv) format by hybridoma and phage display molecular technologies from animals vaccinated with mAbKT4 (18, 24). All these novel KT-mimicking antibodies displayed in vitro and in vivo microbicidal activity against KTR-possessing microbial pathogens (6, 18, 30).
In an attempt to identify biologically active fragments for possible therapeutic use, we have sequenced KT-scFv and synthesized a number of peptides, with special focus on those pertaining to CDR domains which are expected to express antigen specificity (15). Here we report on the synthesis and optimization, through alanine scanning, of a decapeptide derived from the sequence of the variable region and containing part of the CDR1 segment of the KT-scFv light chain. By the use of C. albicans as a model of a variety of KT- and KT-IdAb-susceptible microorganisms, we demonstrate the potent in vitro and in vivo activities of this novel microbicidal peptide. We also provide some circumstantial evidence that killer peptide (KP), like KT and KT-IdAb, interacts with β-glucan constituents of the cell wall. Overall, this is to our knowledge the first engineered peptide fragment derived from a microbicidal recombinant IdAb capable of exerting a remarkable antimicrobial activity in vitro and in vivo.
MATERIALS AND METHODS
Sequencing of KT-scFv.
The sequencing of KT-scFv was carried out according to the conventional Sanger method (29) of dideoxy-mediated chain termination using a Sequenase kit (U.S. Biochemicals, Cleveland, Ohio) with primers 5′-CAACGTGAAAAAATTATTATTCGC-FOR and 5′-GTAAATGAATTTTCTGTATGAGG-BACK.
Synthesis of decapeptides from the KT-scFv sequence.
Studies have been carried out on the correlation between amino acid sequences and functions by synthesizing a series of decapeptides belonging to the KT-scFv sequence, with special regard to CDR regions and CDR peptides as well. A synthetic decapeptide (P6) containing the first three amino acids of the CDR-L1 region (SAS; sequence positions 24 to 26) and exerting remarkable candidacidal activity in vitro on a molar basis (see below) was selected for large-scale synthesis as soluble product. Solid-phase synthesis was carried out in a MultiSynTech Syro automatic peptide synthesizer (Witten, Germany) employing Fmoc chemistry with HOBt activation and Rink amide MBHA resin as solid support. Peptides were cleaved from the resins and deprotected by treatment with trifluoroacetic acid containing ethanedithiol, water, triisobutylsilane, and anisole (93:2.5:2:1.5:1). After precipitation by ethylic ether, the crude peptides were purified by using a Vydac C18 column (25 cm by 1 cm) and characterized by amino acid analysis and mass spectrometry.
Optimization of a killer decapeptide.
The decapeptide P6 was analyzed by alanine scanning in order to identify the functional contribution of each residue. The derivative showing the highest candidacidal activity in vitro (hereafter designated KP) was reduced by COOH-terminal deletions of up to three residues (KP derivatives) to establish the ability to retain the candidacidal activity.
In vitro evaluation of killer activity of synthetic peptides.
The candidacidal activity in vitro was determined essentially as previously reported (18, 24). In brief, approximately 150 viable KT-susceptible germinating C. albicans UP10 cells, suspended in 10 μl of H2O, were added to 90 μl of H2O containing synthetic decapeptides or peptides representing CDRs to obtain a final concentration of 100 μg/ml. The same concentration of an irrelevant peptide (TSTTSLELD) was used as a control. P6 and the products obtained by alanine scanning were further tested at the concentrations of 100, 25, and 6.25 μg/ml in comparison with a scramble decapeptide properly synthesized as P6 altered sequence as a control. After incubation for 6 h at 37°C with the respective reagents, the fungal cells were dispensed and streaked on the surface of Sabouraud dextrose agar plates which were then incubated at 30°C, and CFU were enumerated after 48 h. Each experiment was performed in triplicate. KP was also tested in vitro in comparison with its own scramble decapeptide (SP) at scalar dilutions (100 to 0.8 μg/ml) in order to establish the minimal fungicidal concentration corresponding to 100% killing of C. albicans cells. The above-mentioned CFU assay was also carried out in the presence of scalar concentrations (800 to 12.5 μg/ml) of pustulan (β1,6-glucan; Calbiochem, La Jolla, Calif.; approximate molecular weight [MW] of 20,000) and laminarin (β1,3-glucan; Sigma Chemical Co., St. Louis, Mo; approximate MW of 5,800; see also reference 21). Both polysaccharides were coincubated with KP or SP (both 25 μg/ml) for 6 h at 37°C. KP and equivalent molar concentrations of its derivatives were also tested at scalar dilutions to establish the peptide concentration (in moles per liter) corresponding to the 50% inhibitory concentration (IC50). Assays were performed in triplicate, and the IC50 of each peptide was calculated by nonlinear regression analysis of curves obtained by plotting the number of CFU versus log peptide concentration using GraphPad Prism 3.02 software.
In vivo evaluation of KP therapeutic activity. (i) Vaginal infection model.
A previously established experimental model of vaginal infection by C. albicans in oophorectomized, estrogen-treated rats was used (9). Estrogen-conditioned rats (five animals/group) were inoculated intravaginally with 107 cells of fluconazole-sensitive (SA-40) or fluconazole-resistant (AIDS 68) C. albicans strains and intravaginally administered different doses of KP at 1, 24, and 48 h postchallenge. Both yeast strains were originally isolated from a human vaginal infection and maintained in stock collections in the Department of Bacteriology and Medical Mycology of the Istituto Superiore di Sanità, Rome, Italy. The vaginal C. albicans burden was quantitated by CFU enumeration from the vaginal fluid taken each day by using a special calibrated loop. Vaginal smears were also stained by the periodic acid-Schiff-van Gieson method. The outcome of treatment was assessed in terms of microscopic reduction of the hyphal growth in the vagina as well as by acceleration of fungal CFU clearance over a period of 28 days. Negative controls (untreated rats and rats treated with SP) were included. As a positive control, rats received 50 or 100 μg of fluconazole (Pfizer)/ml in phosphate-buffered saline (0.1 ml) at 1, 24, and 48 h after the yeast challenge. The person dealing with animal treatments and vaginal smears was blinded with regard to the nature of the material being injected.
(ii) Systemic infection model.
Groups of eight BALB/c or severe combined immunodeficient (SCID) female mice, both obtained from Charles River (Calco, Italy), weighing 18 to 21 g, were challenged with five 50% lethal doses (LD50's; corresponding to 107 cells of C. albicans strain SA-40) by the intravenous route. Fifty micrograms of KP was therefore administered intraperitoneally for 3 days starting on day 0, i.e., 1 h after the fungal challenge, and at 24 and 48 h thereafter. Untreated animals or animals treated with SP (same dosage and treatment schedule as those treated with KP) were the controls. The person who injected the animals was blinded with regard to the nature of the material being injected. The animals were then followed for mortality and internal organ invasion for 60 days.
RESULTS
Sequence of KT-scFv and peptide selection for candidacidal activity in vitro.
The nucleotide and the deduced amino acid sequence of KT-scFv, representing the functional internal image of KT (18), were determined (Fig. 1). The CDRs of both light and heavy chains were synthesized and tested for candidacidal activity in vitro. In addition, the whole protein was dissected into decapeptides, each displaced by two amino acids, and all decapeptides containing parts of CDRs (15) were also synthesized and tested.
FIG. 1.
The ScFv H6 gene nucleotide and translated amino acid sequences. P6 amino acids are shown in italics. For other details, see the text.
At the end of this preliminary screening, the decapeptide P6 (sequence EKVTMTCSAS; residues 17 to 26, with amino acids 24 to 26, SAS, being the first three residues of the CDR1 of the VL chain) was selected for further studies because of its higher candidacidal activity in vitro (IC50 = 1.07 × 10−5 mol/liter) than that of the other peptides, in particular the heavy and light chain CDRs, which had an IC50 always higher than 10−4 mol/liter. After P6 sequence optimization through alanine scanning, a killer decapeptide (KP), with A replacing E in the above sequence, was generated which showed the highest enhancement of candidacidal activity (IC50 = 5.6 × 10−8 mol/liter) in the in vitro assay among all alanine-substituted decapeptides (whose IC50 values ranged between 1.1 × 10−5 and 7.9 × 10−6 mol/liter). On the basis of KP sequence, a scramble peptide named SP (MSTAVSKCAT), was also synthesized which showed no candidacidal activity at all and was thereafter used as negative control.
The IC50 values of KP derivatives shortened by progressive COOH-terminal amino acid deletion were also calculated. There was a decay of candidacidal activity of almost 3 orders of magnitude with the deletion of the COOH-terminus serine and of >4 orders of magnitude with the deletion of the last four COOH-terminal residues. This shows that the amino acids belonging to CDR1 were indeed critical for KP activity.
Inhibition of KP activity by glucan.
Since (i) KT and its mimicking KT-IdAb interact with cell surface β-glucan (4, 12, 13), (ii) KP contained part of the sequence of a CDR domain potentially involved in the binding, and (iii) as suggested above, this sequence was critical for KP activity, we examined whether KP activity was affected by soluble glucan molecules. In addition, we tested for any competition by KP on an IdAb (KT-MAb) binding to C. albicans germ tubes, the growth forms of the fungus mostly expressing KT- and KT-IdAb receptor components.
We observed that the candidacidal activity of KP was strongly and dose dependently inhibited by laminarin, a soluble β1-3 glucan, but not by pustulan, a soluble β1-6 glucan (Table 1). In addition KP, but not SP, completely inhibited the binding of the KT-MAb to germinating cells of C. albicans, as seen by immunofluorescence assay (data not shown; see also reference 24). Altogether, these data suggest that the candidacidal decapeptide did effectively compete for the site of binding of KT-IdAb on the fungal cell wall, presumably constituted by, or containing, β1-3 glucan as receptor component.
TABLE 1.
Neutralization of KP candidacidal activity in vitro by laminarin
| Addition and concn (μg/ml) | CFUa in the presence of:
|
|
|---|---|---|
| KP | SP | |
| None | 0 | 2,800 ± 120 |
| Laminarinb | ||
| 12.5 | 0 | 2,500 ± 90 |
| 25 | 1 ± 1 | 2,800 ± 260 |
| 50 | 110 ± 10 | 2,700 ± 100 |
| 100 | 2,800 ± 60 | 2,700 ± 280 |
| Pustulanb | ||
| 12.5 | 0 | 2,300 ± 150 |
| 25 | 0 | 2,500 ± 50 |
| 50 | 0 | 2,700 ± 300 |
| 100 | 0 | 2,700 ± 120 |
| 200 | 0 | 3,700 ± 60 |
| 400 | 10 ± 6 | 3,800 ± 220 |
| 800 | 20 ± 6 | 4,000 ± 140 |
CFU are the means ± standard deviations of triplicate counts. KP and SP were used at a single concentration of 25 μg/ml.
Laminarin and pustulan are commercial preparations of β1-3 and β1-6 glucans, respectively, with approximate molecular weights of 20,000 and 5,800, respectively, as per information obtained from the manufacturers (see also references 16 and 21).
In vivo therapeutic activity of KP. The results of the above experiments established that (i) KP has a strong candidacidal activity in vitro and (ii) this activity, as that previously shown with KT-MAb and KT-scFv, is probably mediated through an interaction with a β-glucan KT receptor. Since killer activity of KT-IdAb in vitro was reflected in an effective in vivo activity (18, 23, 24), we performed a series of experiments aimed at detecting any potential KP activity against experimental candidiasis. For this purpose, well-established mucosal and systemic infection models in rodents were used.
(i) Vaginal infection in oophorectomized, estrogen-treated rats.
In a preliminary, dose-finding experiment, rats were treated intravaginally with 10, 25, 50, and 100 μg of KP, 1, 24, and 48 h after the fungus challenge, and monitored for C. albicans clearance from the vagina as described in Materials and Methods. A dose-response therapeutic effect, but with 50 and 100 μg of the compound being similarly efficacious, was observed. Thus, in all subsequent experiments, 50 μg of KP was used in the established three-dose administration schedule.
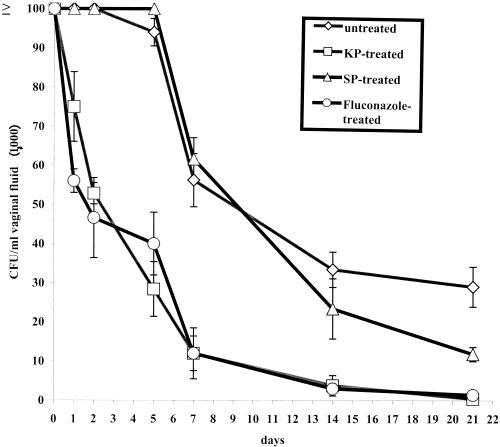
Figure 2 shows the results of a typical experiment (out of three performed with comparable results) where the animals were intravaginally infected with C. albicans cells and then each treated with 50 μg of KP. SP and fluconazole (an efficacious, widely used anticandidal drug) served as negative and positive controls, respectively. KP was seen to accelerate the early rate of clearance (1 to 5 days) of the fungus from rat vagina as significantly as fluconazole. KP also provided a substantial resolution of the infection (less than 103 CFU/ml of vaginal fluid) within 3 weeks of challenge, when the untreated controls still had from 2 × 104 to 4 × 104 Candida CFU/ml of vaginal fluid. No acceleration of the fungal clearance and no effect on resolution of infection was provided by SP administration. Overall, the therapeutic benefit of KP was substantially comparable with that of fluconazole (Fig. 2). Noteworthy, KP proved to have a therapeutic effect also in rat vaginal infection caused by a C. albicans strain (AIDS 68) which was resistant to treatment with up to 100 μg of fluconazole (Table 2).
FIG. 2.
Clearance of vaginal candidiasis in rats intravaginally administered KP, SP, or fluconazole or untreated. All rats (five per group) were challenged intravaginally with 107 C. albicans cells (strain SA-40) in 0.1 ml of physiological saline on day 0 and then sampled for initial intravaginal CFU. The therapeutics (KP, SP, and fluconazole; 50 μg each) were administered 1, 24, and 48 h after the challenge. Starting on day 1, there was always a statistically significant difference (P < 0.05) in the vaginal CFU counts between untreated or SP-treated and KP- or fluconazole-treated rats. No statistically significant difference was at any time point found between SP-treated and untreated animals (except at the last day of measurement), and no statistically significant difference was similarly found at any time point between KP- and fluconazole-treated rats. Statistical significance was assessed by using Student's t test (two-tailed).
TABLE 2.
Protection conferred by KP upon rats intravaginally infected with fluconazole-susceptible strain SA-40 and fluconazole-resistant strain AIDS 68 of C. albicansa
| Exptl group |
C. albicans vaginal CFU (103) (± SD) on dayb
|
|||
|---|---|---|---|---|
| 0 | 7 | 14 | 28 | |
| C. albicans only, strain SA-40 | >100 | 98 ± 12 | 42 ± 7 | 38 ± 6 |
| C. albicans only, strain AIDS 68 | >100 | 70 ± 10 | 55 ± 8 | 18 ± 3 |
| C. albicans SA-40 + fluconazole | >100 | 13 ± 4 | 2 ± 1 | <1 |
| C. albicans SA-40 + KP | >100 | 12 ± 7 | 1 ± 1 | <1 |
| C. albicans AIDS 68 + fluconazole | >100 | 64 ± 6 | 48 ± 3 | 12 ± 4 |
| C. albicans AIDS 68 + KP | >100 | 40 ± 5 | 18 ± 2 | <1 |
All rats (five per group) were given 107 cells in 0.1 ml of physiological solution on day 0 and were sampled for initial intravaginal CFU. The therapeutics (KP, 50 μg, and fluconazole, 50 μg in group 3 and 100 μg in group 5) were administered 1, 24, and 48 h after the infectious challenge.
On days 7, 14, and 28, all the differences in the CFU vaginal counts between group 1 and groups 3 and 4 and between group 2 and group 6 (but not group 5) were statistically significant (P < 0.05). The differences in vaginal CFU counts between groups 5 and 6 were statistically significant (P < 0.05) on the same days. There were no statistically significant differences in the vaginal CFU counts, at any day, between groups 3 and 4. The statistical significance was assessed by two-tailed Student's t test. Data are from one of two experiments performed with similar results. C. albicans SA-40 and C. albicans AIDS 68 are in vitro fluconazole susceptible and fluconazole resistant, respectively.
(ii) Lethal systemic infection in normal and SCID mice.
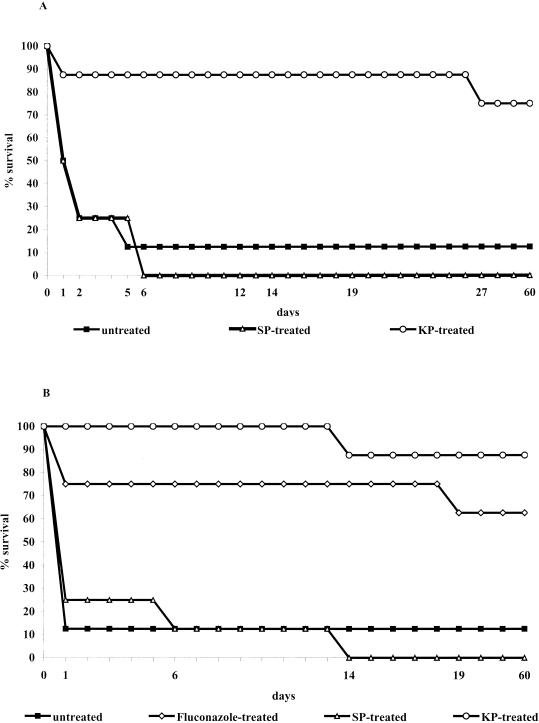
Three independent experiments were also performed to assess the capacity of KP to exert therapeutic activity in a rapidly lethal model of systemic infection by C. albicans. In one of the three experiments, mice with SCID were used instead of their immunocompetent BALB/c counterparts to verify whether the curative effect of the peptide required the participation of host adaptive immunity. In all experiments, untreated or SP-treated mice were used as negative controls, while fluconazole was used (in one experiment) as a positive control. In all experiments, KP exerted a similar beneficial therapeutic effect in terms of mortality delay and animal cure (60 days). As a typical example, Fig. 3 shows the Kaplan-Meier survival curve of normal BALB/c and SCID mice robustly challenged with five LD50's of C. albicans cells and treated with 50 μg of KP, SP, or fluconazole, or untreated. In both BALB/c and SCID mice, KP was seen to increase the median survival time from 1 day in the untreated control to >60 days. In addition, only two and one of the eight KP-treated BALB/c and SCID animals, respectively, died compared to the eight out of eight dead mice treated with SP (in both normal and SCID) or seven out of eight dead BALB/c and SCID untreated mice. The KP curative effect was comparable to that of fluconazole (Fig. 3B). In all cases, the death of the animals was attributable to the C. albicans challenge as shown by the fungus burden in the kidneys.
FIG. 3.
BALB/c (A) or SCID (B) mice (eight per group) were infected intravenously on day 0 with 107 C. albicans cells (strain SA-40; predetermined to correspond to five LD50's) and then intraperitoneally injected with 50 μg of KP, SP, or fluconazole in saline at 1, 24, and 48 h after the infectious challenge. Untreated mice received saline only. Mortality was evaluated for 60 days, and dead mice were subjected to necropsy to assess the presence of the fungus in one target organ (kidney). The differences in the median survival time (in days) or in the ratio of dead versus total animals on day 60 were assessed by using the Mann-Whitney U test or by using Fisher's exact test, respectively. For both endpoint parameters, there was a highly significant (P < 0.01) statistical difference between untreated (or SP-treated) and KP-treated (or fluconazole-treated) mice. No statistical difference was found between KP- and fluconazole-treated SCID mice or between untreated and SP-treated mice.
DISCUSSION
Infections by microbial pathogens remain a major threat to public health, a threat that is now more widely appreciated because of the increasing antimicrobial drug resistance, the paucity of novel antibiotics, and the widening of the spectrum of opportunistic infections in immunocompromised subjects and the elderly. Thus, the need of adding novel, in particular immunotherapeutic, weapons to our antimicrobial armamentarium is largely shared.
In this line, we have originally exploited the KT phenomenon and the Id network to produce KT-IdAb, in the polyclonal, monoclonal, and recombinant format, capable of mimicking in vitro and in vivo the potent wide-spectrum, microbicidal activity of the KT from P. anomala (19). These antibodies were effective in vitro against a panel of relevant opportunistic and frank pathogens such as C. albicans, P. carinii, and M. tuberculosis, including multidrug-resistant strains and, more recently, penicillin-, methicillin-, and vancomycin-resistant cocci (7). They were also effective in several experimental models of infection (5, 18, 23). In particular, KT-scFv exerted a therapeutic effect against C. albicans rat vaginal infection when intravaginally administered as such or vectored by the human commensal Streptococcus gordonii colonizing the vaginal mucosa (3, 18).
Nonetheless, the intrinsic limitations of the reported approach in terms of potential for treatment of human infections are easily recognized. A practical problem related to therapeutic treatment with KT-scFv is the requirement for continuous administration of a potentially expensive and labile product. The mucosal delivery of the candidacidal antibody fragment permanently expressed by a colonizing human commensal bacterium would appear to be safe and cost-effective, but the means for large-scale production and standardization and the regulatory pathway for the acceptance of new transgenic commensals would need to be defined. In addition, this last approach could not be considered for the treatment of systemic infections.
In recognition of these constraints, and because of our interest in dissecting the biological activity of KT-scFv, we addressed the biological activity of peptides synthesized and optimized from the KT-scFv sequence, with special regard to the CDR domains which express the antibody-specific binding to the susceptible cells. For this, we were also encouraged by the recent identification of KTR as a β-glucan component of the cell wall (12). Importantly, glucan and glucan-like molecules are largely present in the microbial world, where they exert critical structural and/or virulence properties. Thus, targeting these components with antibodies or antibody derivatives could provide novel and general therapeutic approaches.
To prove the above concept, we have selected as a model organism C. albicans, a KT-, KT-MAb-, and KT-scFv-susceptible opportunistic pathogen of ever-increasing medical importance. This fungus is one of the most frequently involved microbial pathogens in the etiology of mucosal infections, being the principal agent of vulvo-vaginal (acute, chronic, and recurrent) infection and oral pathology in human immunodeficiency virus-infected subjects (17). In addition, it causes serious, highly lethal systemic infections in immunocompromised or otherwise debilitated hosts against which the current therapeutic choices are limited, also due to increasing antimycotic resistance problems (1, 10). Nonetheless, other KT-sensitive microbial pathogens are, in principle, to be considered in this approach since they have also been demonstrated to be susceptible to KT-MAb and KT-scFv (5-7).
Through KT-scFv sequence determination, a synthetic decapeptide (P6) inclusive of three CDR1 residues of the VL chain was selected for large-scale synthesis as soluble product because of its strong candidacidal activity in vitro in comparison with other decapeptides pertaining to CDRs as well as CDR-constituting peptides.
P6 was analyzed by alanine scanning in an attempt to potentiate its activity, thus obtaining a potent in vitro killer decapeptide denominated KP. Importantly, the candidacidal activity of KP required the presence of the three CDR1 amino acids SAS and was clearly inhibited, in a dose-dependent fashion, by laminarin, a β1-3 glucan preparation. This latter effect was highly specific, since it was not reproduced by pustulan, a β1-6 glucan preparation, thus suggesting that the peptide preferentially interacts with a receptorial component containing β1-3 glucan. This would also suggest that germinating C. albicans cells, which in contrast to nongerminating yeast cells are highly susceptible to KP, express β1-3 glucan on their surface. Indeed, β1-6 glucan is rather expressed by nongerminating yeast cells (32). Germination of yeast cells is mandatory for tissue invasion by C. albicans, and β-glucan synthesis is absolutely required for this event to occur. In fact inhibitors of β1-3 glucan synthase are candidacidal (11). Whichever the precise β-glucan configuration recognized by KP, our data suggest that, similarly to KT and KT-IdAb, the decapeptide also interacts with β-glucans of KTR, as strengthened by the observation that KP, like KT, was able to inhibit the binding of a KT-MAb to KTR of C. albicans cells in a direct immunofluorescence assay (data not shown; see also references 24 and 26). Taken together, our findings constitute circumstantial evidence that the candidacidal activity of KP is, by still-unknown mechanisms, mediated in part or as a whole by KP interaction with β-glucan.
The true reason why P6 and consequently KP, rather than the whole light chain CDR1 or other CDRs, proved to be a particularly strong KP is not known. The expectation that the anticandidal activity, if present, would have been expressed at the highest level by one or more CDRs was not supported by the experimental data. For instance, the VH CDR3 (which was the most active, on a molar basis, among all CDRs) was slightly less active than P6, and its activity was substantially unaffected by alanine replacement which, in contrast, increased P6 activity by more than 2 logs. Even though KP cannot be considered a structural mimotope of KT, it acted as a functional mimotope and was adopted for therapeutic purposes with reference to its enhanced activity in vitro. The functional mimicry, based on a critical interaction of KP with β-glucan, i.e., the KTR, deserves further investigations aimed at unravelling the mechanism of action of KP on sensitive microbial cells. Central to these ongoing investigations are those studies concerning the nature of the molecular interaction between KP and its glucan receptor, which is expected to be a rather widespread molecule in consideration of the fact that KP, exactly as its source antibody molecules KT-MAb and Sc-Fv (5, 7) are, is active in vitro against several fungi and some bacteria, for instance, M. tuberculosis (unpublished data).
The consolidated experimental model of rat vaginal candidiasis (9) as well as a routinely adopted and well-established systemic mouse model of Candida infection (20) were used to test the therapeutic activity of KP in vivo. The results proved that KP was capable of significantly accelerating the clearance from the vagina a high fungus burden, similarly to a therapeutic course of fluconazole, a highly active, universally used anticandidal drug. KP greatly accelerated the fungus elimination from rat vagina in the first 2 to 3 days of infection, a critical period when the infecting yeast-form cells of the fungus undergo massive germination to hyphal elements and secrete critical virulence enzymes, such as the aspartyl proteinases (9). Importantly, KP did also prove to eradicate the infection caused by a fluconazole-resistant strain of C. albicans, a finding of special interest in view of the increased clinical concern about fluconazole resistance in this and allied fungi (1, 10).
A potent therapeutic effect of KP in the mouse model of systemic candidiasis was also observed. Although various natural or artificial peptides have been used to this aim, we are not aware of any of them being capable of curing a systemic experimental infection resulting in death in 2 to 3 days. Noteworthy, this curative effect was similarly exerted in normal immunocompetent mice as well as in SCID mice, demonstrating that the therapeutic benefit did not require the participation of host adaptive immunity. By its presumptive killing activity in vivo, the peptide could reduce the burden of the fungus to levels affordable by the host phagocytes, being the professional phagocytes normally active, if not more active, against the fungus in SCID mice. Neutrophils could be particularly involved, since they have a recognized critical role in the fight against systemic Candida infection (10). However, it is also possible that the KP activity does not require any help by the host because of its rapid and direct cytocidal effects, as also suggested by the results of treatment with KT-MAb in an aspergillosis model in neutropenic mice (5).
Finally, a major interest of our approach is the potential antimicrobial spectrum of KP, which may include most microorganisms possessing β-glucan in their cell wall, inclusive of yeasts, most filamentous fungi, and some bacteria (5, 7, 16, 21). The observation that an engineered antibody fragment exerts an antimicrobial activity in vitro and in vivo comparable to that displayed by the original KT-scFv could open a new scenario in the biochemistry of antimicrobial peptides for the prevention and therapy of the numerous superficial and systemic diseases caused by the numerous KT-susceptible microorganisms.
Acknowledgments
This work was supported in part by grants from the National AIDS Project under contracts 50E and 50D.26.
We express our gratitude to Andrea Bernini and Simona Vaccari, who helped in sequence analysis, as well as to A. Botzios and A. M. Marella for their help in the preparation of the manuscript.
Editor: T. R. Kozel
REFERENCES
- 1.Alexander, B. D., and J. R. Perfect. 1997. Antifungal resistance trends toward the year 2000. Implications for therapy and new approaches. Drugs 54:657-678. [DOI] [PubMed] [Google Scholar]
- 2.Aliouat, E. M., J. C. Cailliez, N. Sèguy, E. Dei-Cas, L. Polonelli, M. Gerloni, S. Conti, and D. Camus. 1993. Inhibitory effect of a yeast killer toxin to the in vitro Pneumocystis carinii attachment. Serodiagn. Immunother. Infect. Dis. 5:102-106. [Google Scholar]
- 3.Beninati, C. M., R. Oggioni, M. Boccanera, M. R. Spinosa, T. Maggi, S. Conti, W. Magliani, F. De Bernardis, G. Teti, A. Cassone, G. Pozzi, and L. Polonelli. 2000. Therapy of mucosal candidiasis by expression of an antiidiotype in human commensal bacteria. Nat. Biotechnol. 18:1060-1064. [DOI] [PubMed] [Google Scholar]
- 4.Cassone, A., S. Conti, F. De Bernardis, and L. Polonelli. 1997. Antibodies, killer toxins and antifungal immunoprotection: a lesson from nature? Immunol. Today 18:164-169. [DOI] [PubMed] [Google Scholar]
- 5.Cenci, E., A. Mencacci, A. Spreca, C. Montagnoli, A. Bacci, K. Perruccio, A. Velardi, W. Magliani, S. Conti, L. Polonelli, and L. Romani. 2002. Killer antiidiotypes protect from early invasive aspergillosis in a murine model of allogeneic T-cell-depleted bone marrow transplantation. Infect. Immun. 70:2375-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conti, S., F. Fanti, W. Magliani, M. Gerloni, D. Bertoletti, S. Salati, A. Cassone, and L. Polonelli. 1998. Mycobactericidal activity of human natural, monoclonal, and recombinant yeast killer toxin-like antibodies. J. Infect. Dis. 177:807-811. [DOI] [PubMed] [Google Scholar]
- 7.Conti, S., W. Magliani, S. Arseni, E. Dieci, R. Frazzi, A. Salati, P. E. Varaldo, and L. Polonelli. 2000. In vitro activity of monoclonal and recombinant yeast killer toxin-like antibodies against antibiotic-resistant gram-positive cocci. Mol. Med. 7:613-619. [PMC free article] [PubMed] [Google Scholar]
- 8.Conti, S., C. Cantelli, M. Gerloni, P. Fisicaro, W. Magliani, D. Bertoletti, P. Mozzoni, D. Sullivan, D. Coleman, and L. Polonelli. 1996. Killer factor interference in mixed opportunistic yeast cultures. Mycopathologia 135:1-8. [DOI] [PubMed] [Google Scholar]
- 9.De Bernardis, F., R. Lorenzini, and A. Cassone. 1999. Rat model of Candida vaginal infection, p. 735-740. In O. Zak and M. A. Sande (ed.), Handbook of animal models of infection. Academic Press, New York, N.Y.
- 10.Edwards, J. E. 1991. Invasive Candida infections: evolution of a fungal pathogen. N. Engl. J. Med. 324:1060-1062. [DOI] [PubMed] [Google Scholar]
- 11.Green, L. J., P. Marder, L. L. Mann, L. C. Chio, and W. L. Current. 1999. LY 303366 exhibits rapid and potent fungicidal activity in flow cytometric assays of yeast viability. Antimicrob. Agents Chemother. 43:830-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyard, C., E. Dehecq, J. P. Tissier, L. Polonelli, E. Dei-Cas, J. C. Cailliez, and F. D. Menozzi. 2002. Involvement of beta-glucan in the wide spectrum antimicrobial activity of Williopsis saturnus var. mrakii MUCL 41968 killer toxin. Mol. Med. 8:686-694. [PMC free article] [PubMed] [Google Scholar]
- 13.Guyards, C., N. Sèguy, J. C. Cailliez, H. Drobecq, L. Polonelli, E. Dei-Cas, A. Mercenier, and F. D. Menozzi. 2002. Characterization of a Williopsis saturnus var. mrakii high molecular weight secreted killer toxin with broad-spectrum antimicrobial activity. J. Antimicrob. Chemother. 49:961-971. [DOI] [PubMed] [Google Scholar]
- 14.Jerne, N. K. 1974. Towards a network theory of the immune system. Ann. Immunol. (Inst. Pasteur) 125C:373-389. [PubMed]
- 15.Kabat, E. A. 1988. Antibody complementarity and antibody structure. J. Immunol. 141(Suppl. 7):S25-S36. [PubMed] [Google Scholar]
- 16.Klarzynski, O., B. Plesse, J. M. Joubert, J. C. Yvin, M. Kopp, B. Kloareg, and B. Fritig. 2000. Linear β-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 124:1027-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein, R. S., C. A. Harris, C. Butkus Small, B. Moll, M. Lesser, and G. H. Friendland. 1984. Oral candidiasis in high risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N. Engl. J. Med. 311:354-358. [DOI] [PubMed] [Google Scholar]
- 18.Magliani, W., S. Conti, F. De Bernardis, M. Gerloni, D. Bertoletti, P. Mozzoni, A. Cassone, and L. Polonelli. 1997. Therapeutic potential of antiidiotypic single chain antibodies with yeast killer toxin activity. Nat. Biotechnol. 15:155-158. [DOI] [PubMed] [Google Scholar]
- 19.Magliani, W., S. Conti, M. Gerloni, D. Bertolotti, and L. Polonelli. 1997. Yeast killer systems. Clin. Microbiol. Rev. 10:369-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mencacci, A., A. Torosantucci, R. Spaccapelo, L. Romani, F. Bistoni, and A. Cassone. 1994. A mannoprotein constituent of Candida albicans that elicits different levels of delayed-type hypersensitivity, cytokine production, and anticandidal protection in mice. Infect. Immun. 62:5353-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milton, D. K., K. U. Alwis, L. Fisette, and M. Muilenberg. 2001. Enzyme-linked immunosorbent assay specific for (1→6) branched, (1→3)-β-d-glucan detection in environmental samples. Appl. Environ. Microbiol. 67:5420-5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettoello-Mantovani, M., A. Noverino, L. Polonelli, G. Morace, S. Conti, L. di Martino, G. De Ritis, M. Iafusco, and M. Guendalini. 1995. Hansenula anomala killer toxin induces secretion, and severe acute injury in the rat intestine. Gastroenterology 109:1900-1906. [DOI] [PubMed] [Google Scholar]
- 23.Polonelli, L., F. De Bernardis, S. Conti, M. Boccanera, M. Gerloni, G. Morace, W. Magliani, C. Chezzi, and A. Cassone. 1994. Idiotypic intravaginal vaccination to protect against candidal vaginitis by secretory, yeast killer toxin-like antiidiotypic antibodies. J. Immunol. 152:3175-3182. [PubMed] [Google Scholar]
- 24.Polonelli, L., N. Sèguy, S. Conti, M. Gerloni, D. Bertoletti, C. Cantelli, W. Magliani, and J. C. Cailliez. 1997. Monoclonal yeast killer toxin-like candidacidal antiidiotypic antibodies. Clin. Diagn. Lab. Immunol. 4:142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polonelli, L., and G. Morace. 1986. Reevaluation of the yeast killer phenomenon. J. Clin. Microbiol. 24:866-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polonelli, L., F. Fanti, S. Conti, L. Campani, M. Gerloni, M. Castagnola, G. Morace, and C. Chezzi. 1990. Detection by immunofluorescent anti-idiotypic antibodies of yeast killer toxin cell wall receptors of Candida albicans. J. Immunol. Methods 132:205-209. [DOI] [PubMed] [Google Scholar]
- 27.Polonelli, L., R. Lorenzini, F. De Bernardis, M. Gerloni, S. Conti, G. Morace, W. Magliani, and C. Chezzi. 1993. Idiotypic vaccination: immunoprotection mediated by antiidiotypic antibodies with antibiotic activity. Scand. J. Immunol. 37:105-110. [DOI] [PubMed] [Google Scholar]
- 28.Polonelli, L., and G. Morace. 1987. Production and characterization of yeast killer toxin monoclonal antibodies. J. Clin. Microbiol. 25:460-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Séguy, N., L. Polonelli, E. Dei-Cas, and J. C. Cailliez. 1997. Monoclonal killer toxin-like antiidiotypic antibodies to control rat pneumocystosis. J. Eukaryot. Microbiol. 44:37S. [DOI] [PubMed]
- 31.Tipper, D. J., and K. A. Bostian. 1984. Double-stranded ribonucleic acid killer systems in yeasts. Microbiol. Rev. 48:125-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torosantucci, A., P. Chiani, and A. Cassone. 2000. Differential chemokine response of human monocytes to yeast and hyphal forms of Candida albicans and its relation to the β 1-6 glucan of the fungal cell wall. J. Leukoc. Biol. 68:923-932. [PubMed] [Google Scholar]