Abstract
Recently, biochemical, cell biological, and genetic studies have converged to reveal that integral membrane heparan sulfate proteoglycans (HSPGs) are critical regulators of growth and differentiation of epithelial and connective tissues. As a large number of cytokines involved in lymphoid tissue homeostasis or inflammation contain potential HS-binding domains, HSPGs presumably also play important roles in the regulation of the immune response. In this report, we explored the expression, regulation, and function of HSPGs on B lymphocytes. We demonstrate that activation of the B cell antigen receptor (BCR) and/or CD40 induces a strong transient expression of HSPGs on human tonsillar B cells. By means of these HSPGs, the activated B cells can bind hepatocyte growth factor (HGF), a cytokine that regulates integrin-mediated B cell adhesion and migration. This interaction with HGF is highly selective since the HSPGs did not bind the chemokine stromal cell–derived factor (SDF)-1α, even though the affinities of HGF and SDF-1α for heparin are similar. On the activated B cells, we observed induction of a specific HSPG isoform of CD44 (CD44-HS), but not of other HSPGs such as syndecans or glypican-1. Interestingly, the expression of CD44-HS on B cells strongly promotes HGF-induced signaling, resulting in an HS-dependent enhanced phosphorylation of Met, the receptor tyrosine kinase for HGF, as well as downstream signaling molecules including Grb2-associated binder 1 (Gab1) and Akt/protein kinase B (PKB). Our results demonstrate that the BCR and CD40 control the expression of HSPGs, specifically CD44-HS. These HSPGs act as functional coreceptors that selectively promote cytokine signaling in B cells, suggesting a dynamic role for HSPGs in antigen-specific B cell differentiation.
Keywords: proteoglycans, B lymphocytes, hepatocyte growth factor, CD44, signaling
Introduction
Proteoglycans are proteins that are covalently linked to sulfated glycosaminoglycan (GAG) chains composed of repeating disaccharide units 1. These molecules, which are widespread throughout mammalian tissues as extracellular matrix components and membrane-bound molecules, have been implicated in several important biological processes including cell adhesion and migration, angiogenesis, tissue morphogenesis, and regulation of blood coagulation 1 2 3. In these processes, proteoglycans are believed to function as scaffold structures, designed to accommodate proteins through noncovalent binding to their GAG chains. In particular, heparan sulfate proteoglycans (HSPGs) have been shown to function as versatile protein coreceptors. Their ligand-binding sites reside within discrete sulfated domains formed by complex, cell-specific, chemical modifications of the HS disaccharide repeat 4. Binding of proteins, including growth factors/cytokines, to HS chains may serve a variety of functions ranging from immobilization and concentration, to distinct modulation of biological function 5 6. This functional importance is illustrated by fibroblast growth factor (FGF)-2, whose binding to its signal-transducing receptors and consequent biological effects are critically dependent on its interaction with cell surface HSPGs 7 8. Recently, several cell biological and genetic studies have provided compelling evidence for an in vivo role of cell surface HSPGs in growth control and morphogenesis in Drosophila, mice, and humans 9 10 11 12 13 14.
Most studies concerning the expression and function of cell surface HSPGs have focussed on epithelial cells and fibroblasts, but these molecules presumably also play important roles in the immune system. A vast number of cytokines involved in lymphoid tissue homeostasis or inflammation bind to heparin, a GAG structurally related to HS. These cytokines, which include chemokines as well as interleukins and hematopoietic growth factors, e.g., IL-3, IL-8, GM-CSF, and hepatocyte growth factor (HGF) 15 16 17 18 19 20, can thus be potentially immobilized by HSPGs. HSPGs expressed on the luminal surface of endothelial cells have been shown to bind chemokines produced at sites of inflammation 21, thereby preventing their immediate dilution by the blood stream. Presentation of HSPG-bound chemokines, e.g., macrophage inflammatory protein 1β and IL-8, to leukocytes plays a crucial role in activating the leukocyte integrins that mediate stable adhesion to and transmigration across the vessel wall 22 23. However, chemokines and other heparin-binding cytokines do not exclusively act at the endothelial–blood interface. They also play key roles in the regulation of lymphocyte trafficking within lymphoid tissues and are involved in the control of lymphocyte growth, differentiation, and survival 24. This suggests that cell surface HSPGs on cells of the immune system, such as lymphocytes and antigen-presenting cells, might also be involved in the regulation of cytokine responsiveness. To explore this hypothesis, we have studied the expression, identity, regulation, and function of HSPGs on human tonsillar B cells. We show that ligation of the B cell antigen receptor (BCR) or CD40, two key receptors in the initiation of antigen-specific B cell differentiation 25 26 27 28 29, induces a strong upregulation of cell surface HSPGs, specifically of CD44-HS. These HSPGs enable B cells to selectively bind HGF, a growth factor that induces integrin-dependent adhesion and migration of B cells 30 31. Moreover, we show that CD44-HS strongly potentiates HGF-induced signaling in B cells.
Materials and Methods
Antibodies.
Mouse mAbs used were anti–pan-CD44, Hermes-3 (IgG2a 32); anti-CD44v3, 3G5 (IgG2b; R&D Systems); anti-HS, 10E4 (IgM; Seikagaku); anti-desaturated uronate from heparitinase-treated HS (anti–ΔHS-stub), 3G10 (IgG2b; Seikagaku); anti-HGF, 24612.111 (IgG1; R&D Systems); anti-Met, DO24 (IgG2a; Upstate Biotechnology); anti–CXC chemokine receptor (CXCR)4, 12G5 (IgG2a; BD PharMingen); anti–syndecan-1, 1D4 (IgG1; CLB); anti–syndecan-2, 10H4 (IgG1 33); anti–syndecan-4, 8G3 (IgG1 34); anti–glypican-1, S1 (IgG1 35); antiphosphotyrosine, PY-20 (IgG2b; Affiniti BioReagents, Inc.); and IgG1, IgG2a, IgG2b, and IgM control Abs (ICN Biomedicals). Polyclonal Abs used were rabbit anti-Met, C-12 (IgG; Santa Cruz Biotechnology, Inc.); rabbit anti–Grb2-associated binder 1 (Gab1; Upstate Biotechnology); rabbit anti–stromal cell–derived factor (SDF)-1α (PeproTech); rabbit anti-Akt (H-136; Santa Cruz Biotechnology, Inc.); phospho-specific rabbit anti-Akt (Ser 473; New England Biolabs, Inc.); RPE-conjugated goat anti–mouse (Southern Biotechnology Associates); biotin-conjugated swine anti–rabbit (Dako); horseradish peroxidase (HRP)-conjugated goat anti–rabbit (Dako); and HRP-conjugated rabbit anti–mouse (Dako). In addition, we used RPE-conjugated streptavidin (Dako).
B Cell Isolation and Culturing.
B cells were isolated from human tonsils as described previously 36. Total B cell fractions were >97% pure as determined by FACS® analysis. B cells were cultured in RPMI 1640 containing 10% FCS, 2 mM l-glutamine, 100 IU/ml penicillin, and 100 IU/ml streptomycin (all from GIBCO BRL/Life Technologies). Some media were supplemented with either 50 ng/ml PMA (Sigma-Aldrich), 0.002% Staphylococcus aureus Cowan strain I (SAC; Calbiochem-Novabiochem), 1 μg/ml Immunobeads with covalently bound rabbit anti–human Ig (Irvine Scientific), 100 U/ml IL-2 (Eurocetus), 100 U/ml IL-4 (Genzyme), 0.5 ng/ml IL-6 (CLB), or 25 ng/ml IL-10 (Genzyme).
For CD40 ligation, B cells were cultured on irradiated (7,000 rads) CD40L (CD154)-transfected or, as a control, wild-type L cells 37.
Cell Lines and Transfectants.
The Burkitt's lymphoma cell line Namalwa was purchased from American Type Culture Collection. The cells were cultured in RPMI 1640 (GIBCO BRL/Life Technologies) supplemented with 10% Fetal Clone I serum (HyClone Laboratories), 10% FCS (Integro), 2 mM l-glutamine, 100 IU/ml penicillin, and 100 IU/ml streptomycin (all GIBCO BRL/Life Technologies). The Namalwa cell lines transfected with CD44s (Nam-SM) or CD44v3-10 (Nam-V3M) were described previously 30 38.
Enzyme Treatments.
For enzymatic cleavage of HS, cells or tissue sections were treated with 10 mU/ml heparitinase (Flavobacterium heparinum, EC 4.2.2.8; ICN Biomedicals) in RPMI 1640 (GIBCO BRL/Life Technologies) at 37°C for 3 h. The cleavage of HS by heparitinase was determined by the loss of cell surface–expressed HS (mAb 10E4), and the simultaneous gain of HS-stub expression (mAb 3G10). Chondroitinase treatment was used as a specificity control.
FACS® Analysis.
FACS® analyses using a single or triple staining technique were described previously 39 40. For cytokine binding assays, cells were incubated with saturating concentrations (20 nM) of recombinant human HGF or recombinant human SDF-1α (both R&D Systems) in PBS for 1 h, before the Ab incubations. This step was followed by washing with FACS buffer. For blocking studies, cells were incubated with 125 nM recombinant human FGF-2 or recombinant human SDF-1α (both R&D Systems) in PBS at 4°C for 1 h, before the incubation with HGF.
Immunoprecipitation and Western Blot Analysis.
Immunoprecipitation and Western blotting were performed as described 39. For the immunodepletion experiments, the lysates were immunoprecipitated twice and the lysate remaining after the second immunodepletion and the immunoprecipitate obtained during the first immunoprecipitation were analyzed by Western blotting.
Results
Expression and Regulation of HSPGs on Human B Cells.
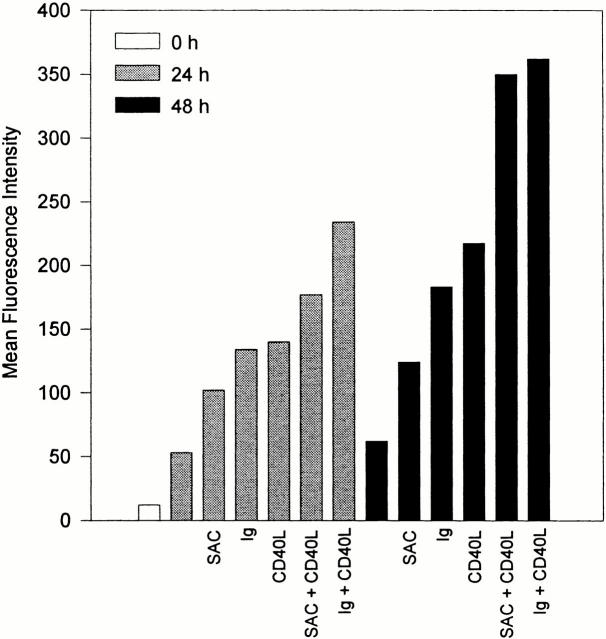
We investigated the expression of HSPGs on the cell surface of resting and activated human tonsillar B cells by means of FACS® analysis, using mAb 10E4, against an epitope on HS chains. In addition, we used mAb 3G10, recognizing the ΔHS-stubs that remain present on HSPG core proteins after heparitinase treatment. HSPGs were hardly detectable on freshly isolated tonsillar B cells. However, upon stimulation of these cells with the phorbol ester PMA, we observed a strong induction of HSPGs (data not shown). This observation prompted us to explore whether HSPGs can also be induced by physiological B cell activators. Since engagement of the BCR and CD40 plays a key role in the initiation of T cell–dependent B cell responses and in the formation of germinal centers (GCs) 25 26 27 28 29, we assessed whether activation via these receptors also leads to HSPG upregulation. Tonsillar B cells were cultured on CD40 ligand (CD40L)-transfected L cells or, as a control, on wild-type L cells, in the presence or absence of BCR stimuli (anti-Ig Abs or SAC). As is shown in Fig. 1, concurrent ligation of CD40 and the BCR induced a strong induction of HSPGs on the B cells. Single triggering of either the BCR or CD40 also led to enhanced HSPG expression, although the HSPG levels were lower than those obtained upon dual receptor ligation. In contrast, stimulation by various cytokines including IL-2, IL-4, IL-6, and IL-10 did not lead to a significant induction of HSPGs (data not shown). These data identify activation via the BCR and CD40 as major signals for the induction of HSPG expression on tonsillar B cells.
Figure 1.
Activation via the BCR and CD40 induces strong expression of HSPGs on B cells. Tonsillar B cells were cultured on CD40L-transfected L cells or wild-type L cells, in the presence or absence of BCR stimuli, i.e., anti-Ig immunobeads (Ig) or SAC, for 0, 24, or 48 h, and analyzed by FACS®. Expression of HSPGs is given as the mean fluorescence intensity of the anti–ΔHS-stub staining after heparitinase treatment minus its staining before treatment.
HSPGs on Activated B Cells Selectively Bind HGF.
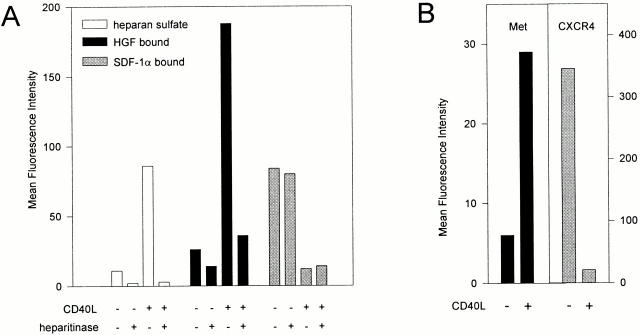
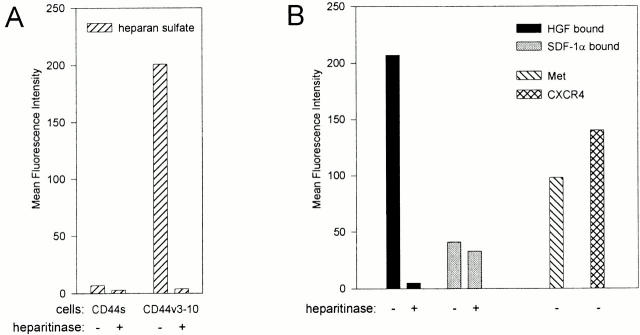
HSPGs are capable of highly selective cytokine binding and presentation 4. To explore the cytokine-binding ability and specificity of the HSPGs expressed on activated B cells, we tested their capacity to bind two distinct cytokines with established heparin-binding capacity, i.e., HGF and SDF-1α 18 41. Although they are structurally unrelated—HGF belongs to the plasminogen-related growth factor family 42 and SDF-1α is a chemokine 43—these cytokines have both been implicated in the regulation of B cell adhesion and migration 30 31 41 43. In agreement with the data presented in Fig. 1, culturing of tonsillar B cells on CD40L-transfected, but not on wild-type, L cells led to a strong induction of HS (Fig. 2 A). In parallel, these B cells acquired a vast capacity to bind HGF (Fig. 2 A). This HGF binding was largely dependent on HS, since >80% was lost after heparitinase treatment of the B cells (Fig. 2 A). The HGF that remained present on the cells after heparitinase treatment most probably was bound to its receptor tyrosine kinase Met, as Met was also induced by CD40 ligation (Fig. 2 B). In contrast to HGF, binding of SDF-1α to the B cells was completely independent of HS: the HSPGlow B cells that were cultured on wild-type L cells had a much greater SDF-1α binding capacity than the HSPGhigh B cells cultured on CD40L-transfected L cells (Fig. 2 A). Moreover, heparitinase treatment did not have any effect on SDF-1α binding. The differences in SDF-1α binding between resting and activated cells were directly related to differential expression of CXCR4, the high affinity receptor for SDF-1α: expression of this receptor strongly decreased as a result of CD40 ligation (Fig. 2 B).
Figure 2.
HSPGs on activated B cells bind HGF but not SDF-1α. (A) CD40 stimulation induced HS expression and HGF binding, but not SDF-1 binding. Tonsillar B cells were analyzed by FACS® for HS expression and for their capacity to bind HGF or SDF-1α after being cultured on CD40L-transfected L cells or wild-type L cells for 48 h. To determine the involvement of HS, the cells were analyzed after control or heparitinase treatment. Expression of HS is given as the mean fluorescence intensity after staining with anti-HS mAb 10E4 minus staining with an isotype-matched control mAb. Binding of HGF or SDF-1α is given as the mean fluorescence intensity of cells that were incubated with one of the cytokines, washed, and stained with a cytokine-specific Ab, minus the mean fluorescence intensity of identically stained control cells. (B) Effect of CD40 stimulation on Met and CXCR4 expression. Expression of the receptor for HGF, Met, and the receptor for SDF-1α, CXCR4, on unstimulated or CD40L-stimulated tonsillar B cells was analyzed by FACS®. Expression of Met or CXCR4 is given as the mean fluorescence intensity after staining with receptor-specific mAbs, minus the mean fluorescence intensity after staining with an isotype-matched control mAb.
Activated B Cells Express HSPG Forms of CD44.
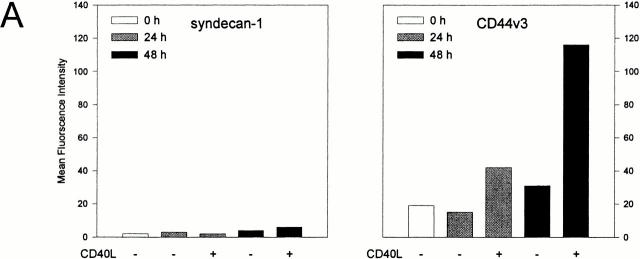
The above data show that, upon their activation, B cells acquire cell surface–expressed HS chains that are capable of selective growth factor binding. This may be the consequence of either upregulation of proteoglycan core protein(s) or upregulation or activation of the enzymes involved in HS synthesis. To address this issue and to identify the proteoglycan core proteins carrying the HS chains, we employed mAbs against a panel of defined proteoglycan core proteins, i.e., the syndecans-1, -2, and -4, glypican-1, and CD44. As is shown in Fig. 3 A, B cell activation enhanced expression of CD44 splice variants containing epitopes encoded by exon v3, which can be decorated with HS 39 44. By contrast, no basal expression, or induction of the distinct syndecans or glypican-1, was observed after B cell activation via CD40 and/or the BCR (Fig. 3 A, and data not shown).
Figure 3.
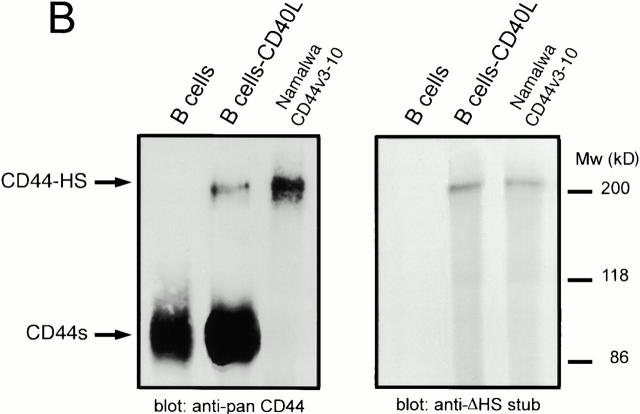
CD40 stimulation induces expression of CD44-HS on tonsillar B cells. (A) CD44-HS, but not syndecan-1, is induced by CD40 stimulation. Expression of syndecan-1 or CD44v3 by tonsillar B cells cultured on CD40L-transfected L cells or wild-type L cells for 0, 24, or 48 h was analyzed by FACS®. Expressions are given as the mean fluorescence intensity after staining with anti–syndecan-1 or anti-CD44v3, minus the staining with isotype-matched control mAbs. (B) CD44v3 isoform(s) on CD40-activated B cells are decorated with HS. CD44 was immunoprecipitated from tonsillar B cells that had been cultured on wild-type L cells or CD40L-transfected L cells for 48 h, and immunoblotted with anti–pan-CD44 (left), or with anti–ΔHS-stub (right). To allow the detection of ΔHS-stubs, the cells had been treated with heparitinase before immunoprecipitation. Namalwa B cells transfected with the HSPG CD44v3-10 (Nam-V3M) were used as a positive control.
To ensure that the CD44 isoforms expressed by activated tonsillar B cells are indeed decorated with HS chains, CD44 was immunoprecipitated from resting and activated B cells and the immunoprecipitates were analyzed on Western blot for the presence of CD44 and HS. Whereas both unstimulated and CD40L-stimulated B cells expressed the 90-kD “standard” isoform of CD44 (CD44s; Fig. 3 B), activation via CD40, in addition, induced expression of a 200-kD CD44 isoform (Fig. 3 B). Upon restaining the blot, only this 200-kD CD44 isoform was found to be modified with HS. By its size, the CD44-HS isoform on activated B cells resembles the HS-modified CD44v3-10 isoform expressed on Namalwa cells (Fig. 3 B), suggesting that they are the products of similar or identical transcripts.
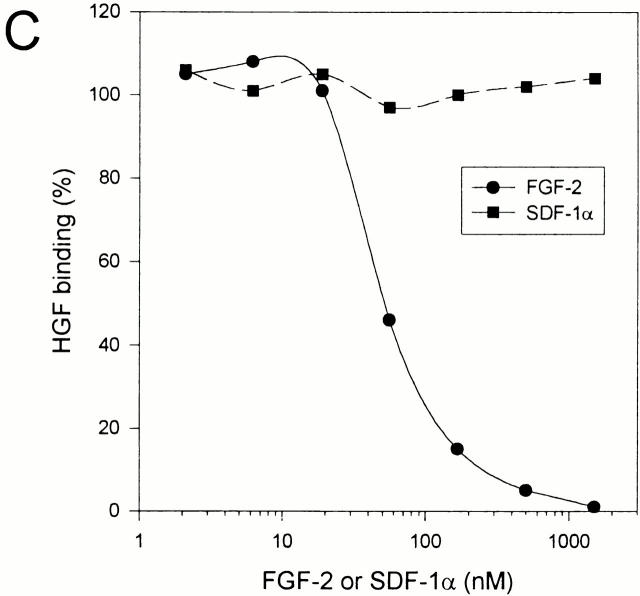
Since we demonstrated in Fig. 2 that the HSPGs induced upon B cell activation specifically bound HGF but not SDF-1α, we investigated the ability of CD44-HS to bind HGF and SDF-1α. For this purpose, we employed Namalwa B cells transfected with either the CD44-HS isoform CD44v3-10, or the isoform CD44s, which does not contain an HS-attachment site 44. Indeed, as shown in Fig. 4 A, only the Namalwa cells transfected with CD44v3-10 expressed HS. Moreover, in contrast to HGF, SDF-1α did not bind to these cells in an HS-dependent manner (Fig. 4 B). Furthermore, in contrast to the heparin-binding growth factor FGF-2, SDF-1α did not compete with HGF for binding to the cells, even at concentrations exceeding those of FGF-2 by more than a factor of 10 (Fig. 4 C). Taken together, these results indicate that ligation of CD40 on tonsillar B cells induces the expression of CD44-HS, most likely the CD44v3-10 isoform. This CD44 isoform is capable of selectively recruiting HGF to the B cell surface.
Figure 4.
HGF, but not SDF-1α, binds to CD44v3-10 (CD44-HS). (A) Namalwa B cells stably transfected with CD44v3-10 (Nam-V3M) express HS, whereas those transfected with CD44s (Nam-SM) do not. (B) Namalwa B cells stably transfected with CD44v3-10 (Nam-V3M) express Met and CXCR4 and show HS-dependent binding of HGF but not of SDF-1α. Nam-V3M cells were analyzed by FACS® for their expression of HS, Met, or CXCR4. In addition, their capacity to bind HGF or SDF-1α is shown. To determine the involvement of HS, the cells were analyzed after control or heparitinase treatment. Expression of HS, Met, or CXCR4 is given as the mean fluorescence intensity after staining with specific mAbs minus the mean fluorescence intensity after staining with an isotype-matched control mAb. Binding of HGF or SDF-1α is given as the mean fluorescence intensity of cells that were incubated with one of the cytokines, washed, and stained with a cytokine-specific Ab, minus the mean fluorescence intensity of identically stained control cells. (C) HGF binding to CD44v3-10 is cross-blocked by FGF-2 but not by SDF-1α. HGF binding to Namalwa CD44v3-10 transfectants was analyzed by FACS® as described above. Before the incubation with HGF, the cells were incubated with a range of concentrations of either FGF-2 or SDF-1α.
Cell Surface HSPGs Regulate Met Signaling in B Cells.
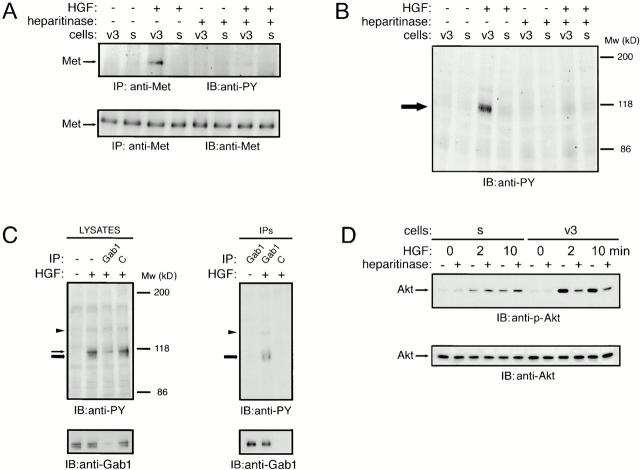
The above data suggest a role for HSPGs, specifically CD44-HS, in the regulation of HGF/Met signaling. To address this hypothesis, we employed Met+ Namalwa B cells transfected with either a CD44-HS isoform (CD44v3-10), or, as a control, with a CD44 isoform that cannot be decorated with HS (CD44s) 39. Upon HGF stimulation, a strong phosphorylation of Met was induced in the cells expressing CD44-HS, whereas phosphorylation in the cells expressing CD44s was weak (Fig. 5 A). HS moieties decorating CD44 were responsible for the strongly enhanced Met phosphorylation in the cells carrying CD44-HS, as heparitinase treatment reduced the HGF induced phosphorylation to the control level observed in the cells expressing CD44s (which lack HS) (Fig. 5 A). The strong potentiation of Met signaling by HS on the B cell surface was not present only at the level of receptor phosphorylation: as is show in Fig. 5 B, upon HGF stimulation a broad band representing (a) hyperphosphorylated protein(s) of ∼110–120 kD was detected in the lysates of B cells expressing CD44-HS, but not in those expressing CD44s. Like the Met phosphorylation, this hyperphosphorylation was HS dependent (Fig. 5 B). By performing immunodepletion experiments, we observed that the broad band was actually composed of (at least) two bands (Fig. 5 C). The lower of these bands was identified as Gab1 (Fig. 5 C), an adapter protein that can associate with the cytoplasmic docking site of Met 45. Indeed, in anti-Gab1 immunoprecipitates, we detected a phosphorylated protein of ∼145 kD which probably represents Met (Fig. 5 C). In addition to Gab1 phosphorylation, a strongly enhanced activation of Akt, also referred to as protein kinase B (PKB), was found upon HGF stimulation in cells expressing CD44-HS compared with cells expressing CD44s (Fig. 5 D). Again, enhanced Akt activation was HS dependent, as it could be abrogated by heparitinase treatment of the cells expressing CD44-HS but not CD44s (Fig. 5 D). Taken together, these data demonstrate that CD44-HS is capable of regulating HGF/Met signaling in B cells in an HS-dependent fashion.
Figure 5.
CD44-HS promotes HGF/Met signaling in B cells in an HS-dependent fashion. (A) CD44-HS promotes HGF-induced tyrosine phosphorylation of Met. Namalwa B cells, stably transfected with either CD44v3-10 (v3), which is HS decorated, or CD44s (s), were treated with heparitinase, as indicated, and subsequently stimulated with HGF. Anti-Met immunoprecipitates were immunoblotted with antiphosphotyrosine (PY-20, top), or with anti-Met (bottom). (B) CD44-HS promotes HGF-induced tyrosine phosphorylation of proteins downstream of Met. An immunoblot of total cell lysates prepared from cells treated as described in A was stained with antiphosphotyrosine. The arrow indicates (a) highly phosphorylated protein(s) at 115–120 kD. (C) HGF-induced Gab1 phosphorylation is enhanced in the presence of CD44-HS. An immunoblot of immunodepleted Namalwa CD44v3-10 cell lysates (left) or their corresponding immunoprecipitates (right) is shown. Immunoprecipitation was performed with anti-Gab1 or control mAbs (C) as indicated. The blots were stained with antiphosphotyrosine (anti-PY, top) or anti-Gab1 (bottom). The arrowhead indicates a band probably representing coimmunoprecipitated Met, while the thin and thick arrows indicate an unidentified protein of ∼115 kD and Gab1, respectively. (D) CD44-HS promotes HGF-induced activation of Akt/PKB. Control or heparitinase-treated cells were incubated with HGF for 0, 2, or 10 min as indicated. Total cell lysates from the transfectants described in A were immunoblotted with anti–phospho-Akt (top) or anti-Akt (bottom).
Discussion
HS proteoglycans are involved in regulating the growth, migration, and differentiation of epithelial cells and fibroblasts 1 3 7 8 9. During these processes, HSPGs immobilize and oligomerize cytokines and present them to their high affinity receptors 5 46 47 48. In this way, HSPGs create niches in the microenvironment and regulate cytokine responses. Since a large number of cytokines involved in lymphoid tissue homeostasis or inflammation contain potential HS-binding sites, HSPGs presumably also play important roles in the regulation of the immune response. However, the expression and function of HSPGs on the cell surface of lymphocytes, as well as within the extracellular matrix of the lymphoid tissues, have thus far remained largely unexplored. In this study, we investigated the regulation and function of HSPGs on human B cells. We demonstrate that expression of HSPGs on human B cells is dynamic and that HSPGs are capable of selective cytokine binding and regulation of cytokine-induced signaling.
We observed that freshly isolated tonsillar B cells express low levels of HSPGs but that single or concurrent ligation of the BCR and CD40 induced a strong expression of HSPGs on the B cell surface (Fig. 1). These observations for the first time show that B cell triggering by physiological stimuli has a profound effect on their HSPG expression and suggest that B cells use cell surface HSPGs as a means of controlling their cytokine-binding capacity and responsiveness. To explore this hypothesis, we analyzed the binding of the cytokines HGF and SDF-1α to HSPGs on activated B cells. These cytokines were selected because they bind heparin, a heavily sulfated HS proteoglycan, with similar affinities 41 49 50. Moreover, although HGF and SDF-1α are structurally unrelated, they have both been implicated in the regulation of B cell adhesion and migration 30 31 51 52. HGF is a 90-kD cytokine that induces complex responses in target cells, e.g., stimulation of motility, growth, and morphogenesis, by binding to the receptor tyrosine kinase Met 53 54 55 56 57 58. HGF is essential for vertebrate development, since knockout of the HGF or Met genes is lethal, causes abnormal development of the liver and placenta, and disrupts the migration of myogenic precursors to the limb buds 59 60 61. Other studies suggest important roles for HGF in tissue regeneration and in tumor growth, invasion, and metastasis 53 62 63 64. The CXC chemokine SDF-1 was originally identified as a pre-B cell growth-stimulating factor 65 and, more recently, has been implicated in a variety of processes, including hematopoiesis, cerebellar and vascular development, and cardiogenesis, by activating its receptor CXCR4 66 67 68 69. SDF-1α is a potent chemoattractant for hematopoietic progenitors, and induces migration of naive and memory, but not GC, B cells 43 52 69 70.
We observed that the inducibly expressed HSPGs on CD40-activated B cells are capable of binding large quantities of HGF, but not SDF-1α. Instead, B cell activation resulted in a strongly decreased binding of SDF-1α to the B cells (Fig. 2 A). This could be explained by the observation that CXCR4, the SDF-1α receptor, was downregulated in response to CD40 triggering, in analogy to BCR-induced downregulation of CXCR4 (71; Fig. 2 B). The finding that HS moieties on B cells do not bind SDF-1α was further corroborated by our observation that SDF-1α, unlike the heparin-binding cytokine FGF-2, does not compete with HGF for HSPG binding (Fig. 4 C). Hence, upon their activation via the BCR and CD40, B cells not only gain expression of the HGF receptor Met 30 (Fig. 2 B), but also acquire the appropriate HSPGs, i.e, HSPGs with the capacity to bind large quantities of HGF. Thus, unlike interactions with heparin, the interaction between cytokines and the natural HSPGs that are induced during B cell activation appear to be highly selective, suggesting that HSPGs contribute an additional level of specificity to B cell–cytokine interactions and may coregulate B cell differentiation. Selectivity of HSPG–protein interactions has also been observed in other biological systems, including blood coagulation and embryonic development 2 3 4 9. It is determined by the structural modifications of the HS chains, which take place within the Golgi complex, as well as by the nature of the core protein 4 18.
HSPGs consist of HS chains covalently attached to a core protein. Ligation of CD40 resulted in a strong induction of cell surface–expressed CD44 splice variants containing the domain encoded by exon v3 (Fig. 3 A). This domain contains a consensus motif for HS attachment 39 44, and we indeed confirmed that CD44 isoforms on activated B cells are decorated with HS (Fig. 3 B). The relative molecular mass of the HSPG form of CD44 on activated B cells was indistinguishable from that of a CD44v3-10 isoform expressed by Namalwa cells, suggesting that they are products of similar or identical transcripts (Fig. 3 B). Although we cannot exclude a contribution of other (unknown) core proteins, our findings identify CD44 as an important cell surface HSPG on activated B cells. As of yet, data on the expression and function of HSPGs in lymphocytes are scarce. The HSPG syndecan-1 can be expressed by human plasma cells, myeloma cells, and Reed-Sternberg cells of classical Hodgkin's disease 72 73 74, and syndecan-4 expression has been demonstrated in mouse B cells 75. Apart from a possible role in growth factor presentation, analogous to that observed for CD44-HS in this study, syndecan-1 and -4 may be important mediators of cell–cell adhesion since their transfection to B lymphoblastoid cell lines results in cell spreading and aggregation 76 77.
Incubation of HGF with heparin or HS-derived oligosaccharides has been reported to promote phosphorylation of the HGF receptor Met 15. This prompted us to explore the impact of CD44-HS expression on HGF-induced signal transduction. Interestingly, we observed that the autophosphorylation of Met, as well as the phosphorylation of the kinase Akt/PKB, and of two proteins of 110–120 kD is strongly promoted by expression of CD44-HS at the B cell surface (Fig. 5). Immunodepletion experiments indicated that the smaller of the two proteins represents Gab1, an adapter protein that can associate with Met (45; Fig. 5 C). The other hyperphosphorylated protein might represent p120-Cbl, a protein tyrosine kinase that participates in signal transduction via receptor tyrosine kinases, and that has been implicated in the regulation of integrin activation 78 79. This is of particular interest, since stimulation of B cells with HGF results in enhanced integrin-dependent adhesion (see below).
Our observations suggest a scenario in which B cells, upon their activation by antigen and T cells, become insensitive to the migration-promoting activity of SDF-1α as a result of downregulation of CXCR4. At the same time, they acquire the receptor tyrosine kinase Met 30 as well as HSPG, viz. CD44-HS, which allow them to selectively recruit HGF to the B cell surface, resulting in efficient HGF/Met signaling. We have previously shown that HGF is produced by follicular dendritic cells and enhances integrin-dependent adhesion of B cells to fibronectin and vascular cell adhesion molecule (VCAM)-1. Hence, activation of the HGF/Met pathway may strengthen B cell adhesion, specifically to follicular dendritic cells, which is mediated by α4β1/VCAM-1 80. Interestingly, apart from establishing physical contact, outside-in signaling via α4β1 presumably contributes to the B cell selection process in the GC by inhibiting apoptosis of B cells 36 40. Since both integrin engagement and Met stimulation may lead to activation of Akt/PKB, a pathway reported to suppress apoptosis 81, it will be of interest to explore the collaborative effects of integrin and Met signaling on B cell survival.
In conclusion, our data demonstrate that the BCR and CD40 control the expression of HSPGs, specifically CD44-HS, on B cells. By selectively binding HGF to the B cell surface, these HSPGs act as functional coreceptors for HGF promoting signaling through Met, which suggests a role for these HSPGs in the regulation of antigen-specific B cell differentiation.
Acknowledgments
We thank Drs G. David, C.G. Figdor, and S. Jalkanen for mAbs.
This study was supported by grants from the University of Amsterdam and the Association for International Cancer Research (AICR).
Footnotes
R. van der Voort's present address is Department of Tumor Immunology, University Medical Center St. Radboud, 6525 EX Nijmegen, The Netherlands.
Abbreviations used in this paper: BCR, B cell antigen receptor; CD44s, CD44 standard isoform; CXCR, CXC chemokine receptor; FGF, fibroblast growth factor; Gab1, Grb2-associated binder 1; GAG, glycosaminoglycan; GC, germinal center; HGF, hepatocyte growth factor; HS, heparan sulfate; HSPG, HS proteoglycan; PKB, protein kinase B; SAC, Staphylococcus aureus Cowan strain I; SDF, stromal cell-derived factor.
References
- Kjellen L., Lindahl U. Proteoglycansstructures and interactions. Annu. Rev. Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Jackson R.L., Busch S.J., Cardin A.D. Glycosaminoglycansmolecular properties, protein interactions, and role in physiological processes. Physiol. Rev. 1991;71:481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- Iozzo R.V. Matrix proteoglycansfrom molecular design to cellular function. Annu. Rev. Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Kusche-Gullberg M., Kjellen L. Regulated diversity of heparan sulfate. J. Biol. Chem. 1998;273:24979–24982. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Lax I., Lemmon M. Regulation of growth factor activation by proteoglycanswhat is the role of the low affinity receptors? Cell. 1995;83:357–360. doi: 10.1016/0092-8674(95)90112-4. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991;64:867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Rapraeger A.C., Krufka A., Olwin B.B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J.D., Leder P., Ornitz D.M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Selleck S. Genetic analysis of functions for cell surface proteoglycans. Matrix Biol. 1998;17:473–476. doi: 10.1016/s0945-053x(98)90094-4. [DOI] [PubMed] [Google Scholar]
- Reichsman F., Smith L., Cumberledge S. Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J. Cell Biol. 1996;135:819–827. doi: 10.1083/jcb.135.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilia G., Hughes-Benzie R.M., MacKenzie A., Baybayan P., Chen E.Y., Huber R., Neri G., Cao A., Forabosco A., Schlessinger D. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat. Genet. 1996;12:241–247. doi: 10.1038/ng0396-241. [DOI] [PubMed] [Google Scholar]
- Jackson S.M., Nakato H., Sugiura M., Jannuzi A., Oakes R., Kaluza V., Golden C., Selleck S.B. dally, a Drosophila glypican, controls cellular responses to the TGF-beta-related morphogen, Dpp. Development. 1997;124:4113–4120. doi: 10.1242/dev.124.20.4113. [DOI] [PubMed] [Google Scholar]
- Binari R.C., Staveley B.E., Johnson W.A., Godavarti R., Sasisekharan R., Manoukian A.S. Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development. 1997;124:2623–2632. doi: 10.1242/dev.124.13.2623. [DOI] [PubMed] [Google Scholar]
- Lin X., Buff E.M., Perrimon N., Michelson A.M. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development. 1999;126:3715–3723. doi: 10.1242/dev.126.17.3715. [DOI] [PubMed] [Google Scholar]
- Zioncheck T.F., Richardson L., Liu J., Chang L., King K.L., Bennett G.L., Fugedi P., Chamow S.M., Schwall R.H., Stack R.J. Sulfated oligosaccharides promote hepatocyte growth factor association and govern its mitogenic activity. J. Biol. Chem. 1995;270:16871–16878. doi: 10.1074/jbc.270.28.16871. [DOI] [PubMed] [Google Scholar]
- Webb L.M., Ehrengruber M.U., Clark-Lewis I., Baggiolini M., Rot A. Binding to heparan sulfate or heparin enhances neutrophil responses to interleukin 8. Proc. Natl. Acad. Sci. USA. 1993;90:7158–7162. doi: 10.1073/pnas.90.15.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R., Gallagher J., Spooncer E., Allen T.D., Bloomfield F., Dexter T.M. Heparan sulphate bound growth factorsa mechanism for stromal cell mediated haemopoiesis. Nature. 1988;332:376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- Lyon M., Deakin J.A., Mizuno K., Nakamura T., Gallagher J.T. Interaction of hepatocyte growth factor with heparan sulfate. Elucidation of the major heparan sulfate structural determinants. J. Biol. Chem. 1994;269:11216–11223. [PubMed] [Google Scholar]
- Gordon M.Y., Riley G.P., Watt S.M., Greaves M.F. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. Nature. 1987;326:403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- Ashikari S., Habuchi H., Kimata K. Characterization of heparan sulfate oligosaccharides that bind to hepatocyte growth factor. J. Biol. Chem. 1995;270:29586–29593. doi: 10.1074/jbc.270.49.29586. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Fujii K., Hubscher S., Aso M., Takazawa A., Saito K., Ota T., Eto S. Heparan sulfate proteoglycan on endothelium efficiently induces integrin-mediated T cell adhesion by immobilizing chemokines in patients with rheumatoid synovitis. Arthritis Rheum. 1998;41:1365–1377. doi: 10.1002/1529-0131(199808)41:8<1365::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Adams D.H., Hubscher S., Hirano H., Siebenlist U., Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Rot A. Endothelial cell binding of NAP-1/IL-8role in neutrophil emigration. Immunol. Today. 1992;13:291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Dewald B., Moser B. Human chemokinesan update. Annu. Rev. Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Bazan F., Blanchard D., Briere F., Galizzi J.P., van Kooten C., Liu Y.J., Rousset F., Saeland S. The CD40 antigen and its ligand. Annu. Rev. Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Lindhout E., Koopman G., Pals S.T., de Groot C. Triple check for antigen specificity of B cells during germinal centre reactions. Immunol. Today. 1997;18:573–577. doi: 10.1016/s0167-5699(97)01160-2. [DOI] [PubMed] [Google Scholar]
- Foy T.M., Laman J.D., Ledbetter J.A., Aruffo A., Claassen E., Noelle R.J. gp39–CD40 interactions are essential for germinal center formation and the development of B cell memory. J. Exp. Med. 1994;180:157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Hathcock K., Zheng B., Kepler T.B., Hodes R., Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- MacLennan I.C. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- van der Voort R., Taher T.E., Keehnen R.M., Smit L., Groenink M., Pals S.T. Paracrine regulation of germinal center B cell adhesion through the c-met–hepatocyte growth factor/scatter factor pathway. J. Exp. Med. 1997;185:2121–2131. doi: 10.1084/jem.185.12.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimar I.S., de Jong D., Muller E.J., Nakamura T., van Gorp J.M., de Gast G.C., Gerritsen W.R. Hepatocyte growth factor/scatter factor promotes adhesion of lymphoma cells to extracellular matrix molecules via alpha 4 beta 1 and alpha 5 beta 1 integrins. Blood. 1997;89:990–1000. [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R.F., de los Toyos J., Butcher E.C. Lymphocyte recognition of high endotheliumantibodies to distinct epitopes of an 85–95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J. Cell Biol. 1987;105:983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lories V., Cassiman J.J., Van den Berghe H., David G. Multiple distinct membrane heparan sulfate proteoglycans in human lung fibroblasts. J. Biol. Chem. 1989;264:7009–7016. [PubMed] [Google Scholar]
- David G., van der Schueren B., Marynen P., Cassiman J.J., van den Berghe H. Molecular cloning of amphiglycan, a novel integral membrane heparan sulfate proteoglycan expressed by epithelial and fibroblastic cells. J. Cell Biol. 1992;118:961–969. doi: 10.1083/jcb.118.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boeck H., Lories V., David G., Cassiman J.J., van den Berghe H. Identification of a 64 kDa heparan sulphate proteoglycan core protein from human lung fibroblast plasma membranes with a monoclonal antibody. Biochem. J. 1987;247:765–771. doi: 10.1042/bj2470765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman G., Keehnen R.M., Lindhout E., Newman W., Shimizu Y., van Seventer G.A., de Groot C., Pals S.T. Adhesion through the LFA-1 (CD11a/CD18)-ICAM-1 (CD54) and the VLA-4 (CD49d)-VCAM-1 (CD106) pathways prevents apoptosis of germinal center B cells. J. Immunol. 1994;152:3760–3767. [PubMed] [Google Scholar]
- Arpin C., Dechanet J., Van Kooten C., Merville P., Grouard G., Briere F., Banchereau J., Liu Y.J. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- van der Voort R., Manten-Horst E., Smit L., Ostermann E., van den Berg F., Pals S.T. Binding of cell-surface expressed CD44 to hyaluronate is dependent on splicing and cell type. Biochem. Biophys. Res. Commun. 1995;214:137–144. doi: 10.1006/bbrc.1995.2267. [DOI] [PubMed] [Google Scholar]
- van der Voort R., Taher T.E., Wielenga V.J., Spaargaren M., Prevo R., Smit L., David G., Hartmann G., Gherardi E., Pals S.T. Heparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-Met. J. Biol. Chem. 1999;274:6499–6506. doi: 10.1074/jbc.274.10.6499. [DOI] [PubMed] [Google Scholar]
- Koopman G., Keehnen R.M., Lindhout E., Zhou D.F., de Groot C., Pals S.T. Germinal center B cells rescued from apoptosis by CD40 ligation or attachment to follicular dendritic cells, but not by engagement of surface immunoglobulin or adhesion receptors, become resistant to CD95-induced apoptosis. Eur. J. Immunol. 1997;27:1–7. doi: 10.1002/eji.1830270102. [DOI] [PubMed] [Google Scholar]
- Amara A., Lorthioir O., Valenzuela A., Magerus A., Thelen M., Montes M., Virelizier J.L., Delepierre M., Baleux F., Lortat-Jacob H., Arenzana-Seisdedos F. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J. Biol. Chem. 1999;274:23916–23925. doi: 10.1074/jbc.274.34.23916. [DOI] [PubMed] [Google Scholar]
- Donate L.E., Gherardi E., Srinivasan N., Sowdhamini R., Aparicio S., Blundell T.L. Molecular evolution and domain structure of plasminogen-related growth factors (HGF/SF and HGF1/MSP) Protein Sci. 1994;3:2378–2394. doi: 10.1002/pro.5560031222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul C.C., Fuhlbrigge R.C., Casasnovas J.M., Aiuti A., Springer T.A. A highly efficacious lymphocyte chemoattractant, stromal cell–derived factor 1 (SDF-1) J. Exp. Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D.G., Bell J.I., Dickinson R., Timans J., Shields J., Whittle N. Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. J. Cell Biol. 1995;128:673–685. doi: 10.1083/jcb.128.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner K.M., Di Cesare S., Sachs M., Brinkmann V., Behrens J., Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- Aviezer D., Hecht D., Safran M., Eisinger M., David G., Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79:1005–1013. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- DiGabriele A.D., Lax I., Chen D.I., Svahn C.M., Jaye M., Schlessinger J., Hendrickson W.A. Structure of a heparin-linked biologically active dimer of fibroblast growth factor. Nature. 1998;393:812–817. doi: 10.1038/31741. [DOI] [PubMed] [Google Scholar]
- Spivak-Kroizman T., Lemmon M.A., Dikic I., Ladbury J.E., Pinchasi D., Huang J., Jaye M., Crumley G., Schlessinger J., Lax I. Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell. 1994;79:1015–1024. doi: 10.1016/0092-8674(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Hartmann G., Prospero T., Brinkmann V., Ozcelik C., Winter G., Hepple J., Batley S., Bladt F., Sachs M., Birchmeier C. Engineered mutants of HGF/SF with reduced binding to heparan sulphate proteoglycans, decreased clearance and enhanced activity in vivo. Curr. Biol. 1998;8:125–134. doi: 10.1016/s0960-9822(98)70059-4. [DOI] [PubMed] [Google Scholar]
- Rahmoune H., Rudland P.S., Gallagher J.T., Fernig D.G. Hepatocyte growth factor/scatter factor has distinct classes of binding site in heparan sulfate from mammary cells. Biochemistry. 1998;37:6003–6008. doi: 10.1021/bi972468t. [DOI] [PubMed] [Google Scholar]
- Soede R.D., Wijnands Y.M., Van Kouteren-Cobzaru I., Roos E. ZAP-70 tyrosine kinase is required for LFA-1–dependent T cell migration. J. Cell Biol. 1998;142:1371–1379. doi: 10.1083/jcb.142.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul C.C., Schultze J.L., Springer T.A. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J. Exp. Med. 1998;187:753–762. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Voort R., Taher T.E.I., Derksen P.W.B., Spaargaren M., van der Neut R., Pals S.T. The hepatocyte growth factor-Met pathway in development, tumorigenesis, and B cell differentiation. Adv. Cancer Res. 2000;79:39–90. doi: 10.1016/s0065-230x(00)79002-6. [DOI] [PubMed] [Google Scholar]
- Bottaro D.P., Rubin J.S., Faletto D.L., Chan A.M., Kmiecik T.E., Vande Woude G.F., Aaronson S.A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Montesano R., Matsumoto K., Nakamura T., Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Naldini L., Vigna E., Narsimhan R.P., Gaudino G., Zarnegar R., Michalopoulos G.K., Comoglio P.M. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991;6:501–504. [PubMed] [Google Scholar]
- Stoker M., Gherardi E., Perryman M., Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- Bladt F., Riethmacher D., Isenmann S., Aguzzi A., Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Schmidt C., Bladt F., Goedecke S., Brinkmann V., Zschiesche W., Sharpe M., Gherardi E., Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Minowa O., Mori C., Shiota K., Kuno J., Noda T., Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Weidner K.M., Behrens J., Vandekerckhove J., Birchmeier W. Scatter factormolecular characteristics and effect on the invasiveness of epithelial cells. J. Cell Biol. 1990;111:2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong S., Segal S., Anver M., Resau J.H., Vande Woude G.F. Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc. Natl. Acad. Sci. USA. 1994;91:4731–4735. doi: 10.1073/pnas.91.11.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano S., Zhen Z., Medico E., Gaudino G., Galimi F., Comoglio P.M. Transfer of motogenic and invasive response to scatter factor/hepatocyte growth factor by transfection of human MET protooncogene. Proc. Natl. Acad. Sci. USA. 1993;90:649–653. doi: 10.1073/pnas.90.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T., Kikutani H., Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc. Natl. Acad. Sci. USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T., Hirota S., Tachibana K., Takakura N., Nishikawa S., Kitamura Y., Yoshida N., Kikutani H., Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Tachibana K., Hirota S., Iizasa H., Yoshida H., Kawabata K., Kataoka Y., Kitamura Y., Matsushima K., Yoshida N., Nishikawa S. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Zou Y.R., Kottmann A.H., Kuroda M., Taniuchi I., Littman D.R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- Aiuti A., Webb I.J., Bleul C., Springer T., Gutierrez-Ramos J.C. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J. Exp. Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Apuzzo M., Rolink A., Loetscher M., Hoxie J.A., Clark-Lewis I., Melchers F., Baggiolini M., Moser B. The chemokine SDF-1, stromal cell-derived factor 1, attracts early stage B cell precursors via the chemokine receptor CXCR4. Eur. J. Immunol. 1997;27:1788–1793. doi: 10.1002/eji.1830270729. [DOI] [PubMed] [Google Scholar]
- Guinamard R., Signoret N., Masamichi I., Marsh M., Kurosaki T., Ravetch J.V. B cell antigen receptor engagement inhibits stromal cell–derived factor (SDF)-1α chemotaxis and promotes protein kinase C (PKC)-induced internalization of CXCR4. J. Exp. Med. 1999;189:1461–1466. doi: 10.1084/jem.189.9.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijdenes J., Vooijs W.C., Clement C., Post J., Morard F., Vita N., Laurent P., Sun R.X., Klein B., Dore J.M. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. Br. J. Haematol. 1996;94:318–323. doi: 10.1046/j.1365-2141.1996.d01-1811.x. [DOI] [PubMed] [Google Scholar]
- Ridley R.C., Xiao H., Hata H., Woodliff J., Epstein J., Sanderson R.D. Expression of syndecan regulates human myeloma plasma cell adhesion to type I collagen. Blood. 1993;81:767–774. [PubMed] [Google Scholar]
- Carbone A., Gloghini A., Gattei V., Degan M., Improta S., Aldinucci D., Canzonieri V., Perin T., Volpe R., Gaidano G. Reed-Sternberg cells of classical Hodgkin's disease react with the plasma cell-specific monoclonal antibody B-B4 and express human syndecan-1. Blood. 1997;89:3787–3794. [PubMed] [Google Scholar]
- Yamashita Y., Oritani K., Miyoshi E.K., Wall R., Bernfield M., Kincade P.W. Syndecan-4 is expressed by B lineage lymphocytes and can transmit a signal for formation of dendritic processes. J. Immunol. 1999;162:5940–5948. [PubMed] [Google Scholar]
- Lebakken C.S., Rapraeger A.C. Syndecan-1 mediates cell spreading in transfected human lymphoblastoid (Raji) cells. J. Cell Biol. 1996;132:1209–1221. doi: 10.1083/jcb.132.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M.J., Liebersbach B.F., Liu W., Anhalt D.J., Sanderson R.D. Heparan sulfate-mediated cell aggregation. Syndecans-1 and -4 mediate intercellular adhesion following their transfection into human B lymphoid cells. J. Biol. Chem. 1995;270:5077–5083. doi: 10.1074/jbc.270.10.5077. [DOI] [PubMed] [Google Scholar]
- Meng F., Lowell C.A. A beta 1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:4391–4403. doi: 10.1093/emboj/17.15.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit L., Borst J. The Cbl family of signal transduction molecules. Crit. Rev. Oncog. 1997;8:359–379. doi: 10.1615/critrevoncog.v8.i4.50. [DOI] [PubMed] [Google Scholar]
- Koopman G., Parmentier H.K., Schuurman H.J., Newman W., Meijer C.J., Pals S.T. Adhesion of human B cells to follicular dendritic cells involves both the lymphocyte function-associated antigen 1/intercellular adhesion molecule 1 and very late antigen 4/vascular cell adhesion molecule 1 pathways. J. Exp. Med. 1991;173:1297–1304. doi: 10.1084/jem.173.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffer P.J., Jin J., Woodgett J.R. Protein kinase B (c-Akt)a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]