Abstract
Although immunoglobulin (Ig)MhiIgDlo/−CD21hi marginal zone B cells represent a significant proportion of naive peripheral splenic B lymphocytes, few of the genes that regulate their development have been identified. This subset of peripheral B cells fails to emerge in mice that lack nuclear factor (NF)-κBp50. Less drastic reductions in marginal zone B cell numbers are also seen in the spleens of recombination activating gene (Rag)-2−/− mice reconstituted with NF-κBp65−/− fetal liver cells and in c-Rel−/− mice. In contrast, steady-state levels of IgDhi splenic follicular B cells are not significantly reduced in the absence of NF-κBp50, NF-κBp65, or c-Rel. Reconstitution of B cells in Rag-2−/− mice with a mixture of p50−/−/p65−/− fetal liver cells and Rag-2−/− bone marrow cells revealed that the generation of marginal zone B cells requires the expression of NF-κB in developing B cells, as opposed to supporting cells.
Keywords: transcription factors, lymphocyte development, recombination activating gene-2−/− mice, peripheral B cells, Bruton's tyrosine kinase
Introduction
A distinct population of splenic IgMhiIgDloCD21hiCD1hi peripheral B lymphocytes known as marginal zone (MZ) B cells resides close to the marginal sinus 1 2 3 4 5 6. Other B cells, including some memory cells, also reside in MZs but can be distinguished from MZ B cells. MZ B cells are large lymphocytes, have a distinct morphology 3, and make up ∼1/10 of the naive B cells in the spleen. These cells express high levels of B7-1 and B7-2 and may differentiate into plasma cells in a matter of hours when triggered in vitro by bacterial LPS, anti-CD40, or IL-4 4. MZ B cells do not divide or differentiate in response to antigen receptor ligation, and proliferate far more avidly than follicular B cells in response to LPS. They may represent specialized lymphocytes that are particularly efficient APCs, and which can be induced to rapidly secrete antibodies directed against subsets of environmental antigens 4.
Very little is understood about the signals and pathways involved in the development of MZ B cells. MZ B cells fail to develop in the absence of CD19 5 6, but these cells develop normally in mice that lack T cells 4 5, in LPS-nonresponsive mice, and in mice maintained in germ-free conditions 5. Mice that lack the Pyk2 tyrosine kinase have recently been demonstrated to lack MZ B cells 7. The only transcriptional regulator that has been linked to MZ B cell development is the Aiolos zinc finger protein 8, but, as discussed below, this nuclear protein is not directly required for MZ B cell development. It is known that MZ B cells respond particularly well to LPS 3, and that B cells from nuclear factor (NF)-κBp50−/− mice respond poorly to LPS 9. Therefore, we wished to determine if NF-κB proteins are required for MZ B cell generation. We show here that NF-κBp50 is required for MZ B cell generation, and that NF-κBp65/RelA and c-Rel play significant but less critical roles in this process. We show that NF-κB must be expressed in lymphocytes in order for B lineage cells to develop into MZ B cells. We hypothesize that NF-κBp50–containing complexes must be activated in maturing peripheral B cells to induce these cells to acquire a MZ phenotype.
Materials and Methods
Mice.
p50−/− 9, p65+/− 10, and c-Rel−/− mice 11 12 were used in these studies. Mice were analyzed between 8 and 12 wk after birth. All animal studies were approved by institutional review boards.
Reconstitution of the Lymphoid Compartment in Rag-2−/− Mice.
Reconstitutions of NF-κB− / − fetal liver cells into recombination activating gene (Rag)-2− / − mice were performed as described in Horwitz et al. 13. Rag-2− / − mice were obtained from Taconic.
Flow Cytometric Analysis.
Flow cytometry was performed essentially as described previously 14. Single cell suspensions were made from the spleen. 106 cells were incubated with 2.4 G2 (anti-CD16/CD32 [Fcγ III/II receptor], rat IgG2b, κ, culture supernatant), before staining with the following antibodies: anti-CD21/CD35–FITC clone 7G6, anti-CD1d–FITC clone 1B1, anti-CD38–FITC clone 90, anti-TNP–biotin clone A19-3, anti-IgM–PE clone R6-60, anti-CD23–PE clone B3B4, anti-CD45R–PE/Cy7 clone RA3-6B2 (all from BD PharMingen), and anti-IgD–biotin clone 11-26 (Southern Biotechnology Associates, Inc.). Biotinylated antibodies were revealed using streptavidin-allophycocyanin.
Viable lymphoid cells were identified by forward and side scatter and 30,000 events were collected. IgMhiIgDlo cells were analyzed for the expression of CD21. Flow cytometric analysis was performed on an Epics Elite ESP flow cytometer (Coulter) equipped with an ultraviolet-enhanced argon ion blue laser and a helium/neon red laser. In general, negative controls were used to set voltage and single color positive controls were used for electronic compensation. Processed samples were analyzed using Epics Elite analysis software and FloJo v3.0.5 (Tree Star Corp).
In Vivo Capture of TNP-Ficoll by MZ B Lymphocytes.
In addition to immunophenotyping approaches, MZ B cells were also revealed by a functional assay involving the in vivo capture of intravenously injected TNP-Ficoll 7. 100 μg of TNP-Ficoll (provided by Drs. Rodolphe Guinamard and Jeffrey Ravetch, The Rockefeller University, New York, NY) was injected. 30 min after injection, mice were killed and splenocytes were stained with antibodies against IgM, IgD, and TNP, or with antibodies against B220/CD45R, CD23, CD21, and TNP.
Immunofluorescence Staining of Tissue Sections.
Immunofluorescence staining was performed essentially according to the method of Oliver et al. 4, with minor modifications. Spleens were harvested and immediately frozen in OCT compound (Miles). 5-μm-thick sections were cut and stored at −80°C until use. All subsequent manipulations were done at room temperature. Sections were air dried for 15 min, fixed in ice-cold acetone for 10 min, air dried briefly, blocked with 5% normal horse serum for 20 min, and stained with MOMA-1 (rat IgG2a, tissue culture supernatant; Serotec) for 1 h. Sections were then stained with a 1:200 dilution of biotin–SP-goat anti–rat IgG (H+L) (Jackson Immunoresearch Laboratories) for 1 h and streptavidin-PE (2 μg/ml; BD PharMingen) for 30 min. The sections were then blocked with 5% normal rat serum for 10 min and stained with anti-IgM–FITC clone R6-60.2 (10 μg/ml; BD PharMingen). Sections were rinsed in PBS (five times, 3 min each) between stainings but not after the blocking steps. The sections were mounted in a polyvinyl alcohol–based aqueous medium containing N-propyl gallate (5 mg/ml) and viewed with an Axioplan 2 fluorescence microscope (ZEISS) equipped with appropriate filters. Images were acquired with a Sensys cooled CCD camera (Photometrics Ltd.) and a Power Macintosh computer, and processed using Openlab software v2.0.9 (Improvision) and Adobe Photoshop® v5.0.2.
Results and Discussion
A major defect seen in resting B cells from p50−/− mice is their inability to proliferate in response to LPS 9 15. Intriguingly, LPS induces the proliferation of MZ B cells far more readily than it does follicular B cells 4, and this difference may be ∼10–15-fold at some doses. To determine whether NF-κBp50 was required for MZ B cell development, we analyzed splenic B lymphoid cells in NF-κBp50−/− mice. Upon examination of frozen sections of splenic tissue stained with anti-IgM and MOMA-1 (which stains MZ metallophilic macrophages) we were unable to detect B cells beyond the follicular rim defined by MOMA-1, indicating that the MZ B cell population was markedly reduced (Fig. 1 a). More quantitative estimates of MZ B cell and follicular B cell numbers can be made by flow cytometry. As shown in Fig. 1 b, the proportions of B cells in the IgDhiIgMlo and IgMhiIgDhi follicular B cell populations were similar in mutant and wild-type mice. The IgMhiIgDlo population (which is largely made up of newly formed B cells and MZ B cells) in p50−/− mice was analyzed in terms of CD21 expression, and a marked reduction was noted in CD21hi MZ B cells (Fig. 1 c). Similar marked reductions in MZ B cell numbers in p50−/− mice were noted using two other markers for this population, CD1d and CD38 (Fig. 1 d).
Figure 1.
NF-κBp50 is required for MZ B cell generation. (a) Immunofluorescence performed on splenic cryosections with anti-IgM–FITC and MOMA-1–PE revealed a marked reduction in MZ B cells in the p50−/− mouse (right). Multiple sections revealed similar results. WT, wild-type. (b) Splenic IgMhiIgDhi and IgDhiIgMlo follicular B cell populations appear grossly normal in the p50−/− mouse. (c) IgMhiIgDloCD21hi MZ B cells are markedly reduced in p50−/− mice. Three wild-type and three p50−/− mice were analyzed in the above studies. The results shown are representative. (d) IgMhiIgDloCD1dhi and IgMhiIgDloCD38hi MZ B cells are markedly reduced in p50−/− mice. Three wild-type and three p50−/− mice were analyzed in the above studies. The results shown are representative.

We used a functional assay for the detection of MZ B cells based on the ability of these cells to capture T cell–independent type II antigens. It has been shown that TNP-Ficoll injected intravenously decorates MZ B lymphocytes but not follicular B cells 7. The in vivo capture of a T cell–independent type II antigen by MZ B cells does not occur efficiently in mice that lack CD21 or C3 7, suggesting that complement-coated T cell–independent type II antigens bind to MZ B cells in vivo. As seen in Fig. 2, TNP-Ficoll can be seen in association with B220+CD21hiCD23lo MZ B cells isolated from wild-type spleens. Very few cells that capture TNP-Ficoll were seen in the B220+CD21hiCD23lo population from p50−/− spleens. Newly formed B cells (B220+CD23loCD21lo) and follicular B cells (B220+CD23hiCD21int) from p50−/− spleens also failed to capture TNP-Ficoll, confirming that the development of MZ B cells is significantly impaired in the absence of p50. Experiments performed in parallel with antibodies to IgM, IgD, and TNP confirmed that p50−/− spleens lack detectable numbers of B cells that associate with intravenously injected TNP-Ficoll (data not shown).
Figure 2.

A T cell–independent type II antigen is captured in vivo by wild-type (WT) MZ B cells but not by any of the B cell populations in p50−/− spleens. Splenocytes were isolated from wild-type and p50−/− mice that were or were not immunized with TNP-Ficoll. In immunized mice, cells that could be stained with anti-TNP antibodies were readily visualized within the CD45R+CD21hiCD23lo MZ B cell gate. Significant numbers of TNP-staining cells were not seen in any of the splenic B cell pools in p50−/− mice.
Because p50 forms complexes in B cells with both p65/RelA and c-Rel, we examined MZ B cells in Rag-2−/− mice reconstituted with fetal liver cells lacking p65, and in c-Rel−/− mice. In the absence of p65, mice die during embryogenesis. To examine the role of this NF-κB protein in lymphocyte development, Rag-2−/− mice were irradiated and reconstituted with mixtures of p65−/− fetal liver cells and Rag-2−/− bone marrow cells. As seen in Fig. 3 a, although splenic follicular B cells are well reconstituted in the absence of p65, MZ B cells are nevertheless reduced to ∼1/3 their normal numbers. A similar observation can be made in the absence of c-Rel (Fig. 3 b). We conclude that although the requirement for p65 or for c-Rel is not as stringent as the requirement for p50, complexes containing p65 or c-Rel contribute to MZ B cell development.
Figure 3.
NF-κBp65 and c-Rel contribute to MZ B cell development. (a) IgMhiIgDloCD21hi MZ B cells are markedly reduced in Rag-2−/− mice reconstituted with p65−/− fetal liver and Rag-2−/− bone marrow cells (bottom panels). Splenic IgDhiIgMlo follicular B cell numbers appear grossly normal (top panels). Two p65−/− chimeras were analyzed in these studies. The results shown are representative. WT, wild-type. (b) IgMhiIgDloCD21hi MZ B cells are markedly reduced in c-Rel−/− mice (bottom panels), but splenic IgDhiIgMlo follicular B cell numbers appear grossly normal (top panels). Two control and two c-Rel−/− mice were analyzed. The results shown are representative.


Given that signals from neighboring cells may induce the differentiation of precursor B cells into MZ-type cells, we wished to establish whether NF-κBp50 is required in lymphocytes themselves, as opposed to being required in supporting cells such as macrophages or other nonlymphoid cells. For instance, defects in MZ formation and the reduced numbers of peripheral B cells seen in the absence of NF-κBp52 are believed to reflect a requirement for this protein in accessory cells 16. In the combined absence of both p50 and p65, lymphopoiesis is blocked but can be rescued by wild-type hematopoietic cells, which suggests that this defect is not cell autonomous 13. To address whether NF-κB is required in lymphocytes or in supporting cells for MZ B cell generation, Rag-2−/− mice were lethally irradiated and reconstituted with mixtures containing equal numbers of NF-κB–deficient fetal liver cells and Rag-2–deficient bone marrow cells as described 13 17. As seen in the top panel of Fig. 4, splenic B cells were reconstituted in Rag-2−/− recipients that received fetal liver cells from mice lacking both p50 and p65 in conjunction with Rag-2−/− bone marrow. Similar reconstitution experiments were performed using p50−/− fetal liver cells (data not shown). Mature follicular IgMhiIgDhi and IgDhiIgMlo fractions were well reconstituted in the absence of both p50 and p65. Examination of the IgMhiIgDlo/− fraction revealed the near total lack of CD21hi MZ B cells in the absence of p50 and p65 (Fig. 4, bottom panel). These results clearly established that NF-κB is required within lymphoid cells for MZ B cells to be generated.
Figure 4.
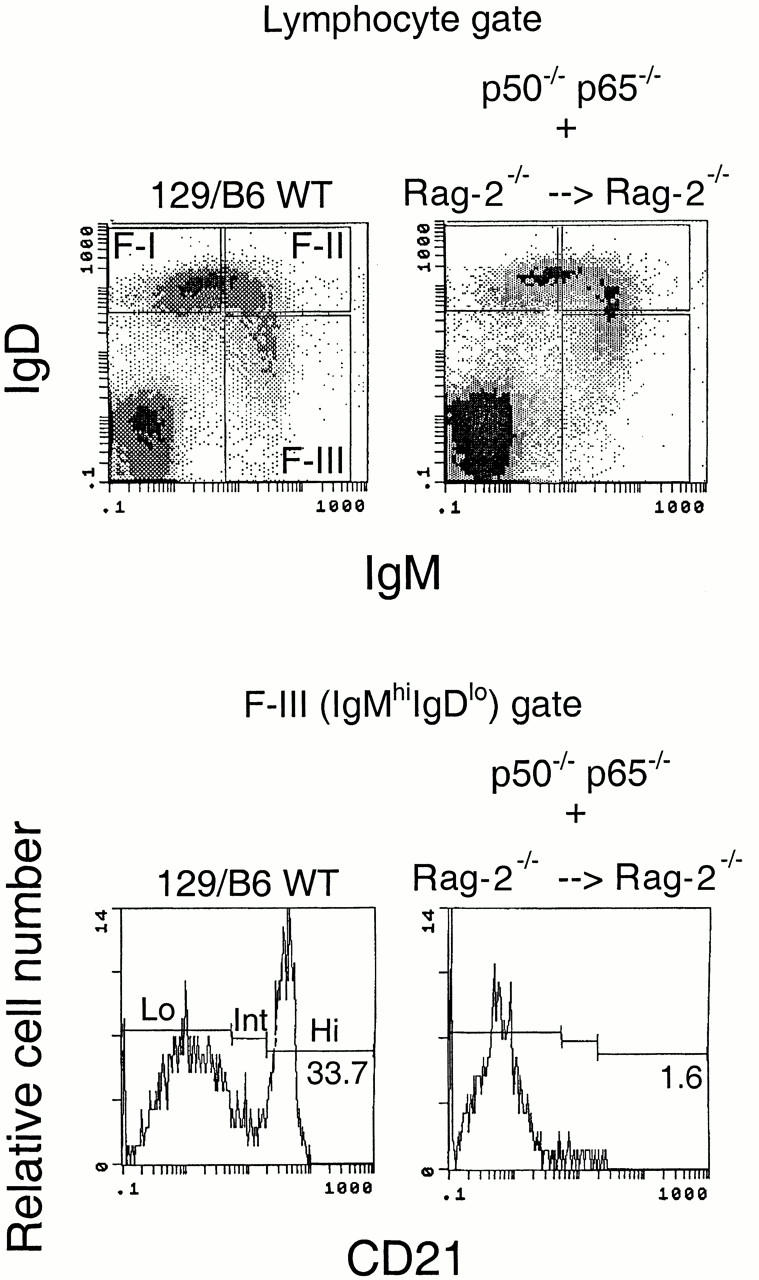
NF-κBp50 must be expressed in developing lymphocytes for MZ B cell generation. Rag-2−/− mice were reconstituted with fetal liver from p50−/−/p65−/− mice, and bone marrow from Rag-2−/− mice. Reconstitution results in clearly identifiable IgMhiIgDlo, IgMhiIgDhi, and IgDhi IgMlo subpopulations (top panels). In the IgMhiIgDlo fraction, CD21hi cells were markedly reduced in the p50−/−/p65−/− chimeras (bottom panels). Six separate p50−/−/p65−/− chimeric mice were analyzed in these studies. The results shown are representative. WT, wild-type.
NF-κB and IκB proteins are critical for several events during B cell development, although it is clear that in this context NF-κB has both cell-autonomous and non–cell-autonomous functions (for a review, see reference 18). NF-κBp50 and c-Rel are required for lymphocyte survival and proliferation, and p50, p65, and c-Rel also participate in isotype switching in activated B lymphocytes 10 11 12 13 15 17 18 19 20 21. The proliferative responses of B lymphocytes are significantly impaired in mice that lack c-Rel 11 12, and these B cells fail to induce the Bcl-2 family member A1 after antigen receptor ligation 21.
In Xid mice, Bruton's tyrosine kinase (Btk)−/− mice, and CD45−/− mice (see reference 22 for further references on these mutants), absolute numbers of IgDhiIgMlo B cells are markedly diminished, and this is apparent in flow cytometric profiles. These cells represent the most mature subpopulation of naive follicular B cells, and their loss in the absence of Btk reflects ongoing apoptosis 14. In addition to the apoptotic loss of IgDhiIgMlo/− follicular B cells, Xid and Btk−/− mice also have defects in antigen receptor–mediated proliferation, but MZ B cells are readily identified in these mice 5 23. The defect in antigen receptor–induced proliferation in the absence of Btk has been tied to the failure to activate NF-κB 24 25. This defect may be distinct from the apoptotic loss of IgDhiIgMlo/− B cells noted in the absence of Btk, as steady-state levels of these follicular B cells are not significantly diminished in the absence of p50 (9 11 12; Fig. 1 Fig. 2 Fig. 3, and Fig. 5 a). Although p50 may contribute to the maintenance of follicular B cells 20, this contribution cannot account for the far more obvious steady-state loss of follicular B cells seen in the absence of Btk. Although the absolute numbers of follicular B cells are not reduced in the absence of p50 or c-Rel (follicular B cell numbers are actually enhanced in the absence of c-Rel; Fig. 5 a), these proteins as well as p65 are required for MZ B cell generation. p50 appears to be stringently required for the generation of MZ B cells, whereas p65 and c-Rel appear to make important but lesser contributions to the development of this B cell subpopulation. Quantitative comparisons of the absolute numbers of newly formed, follicular and MZ B cells in wild-type and mutant mice are depicted in Fig. 5, a and b. It is possible that p50/p65 and p50/c-Rel complexes contribute in roughly equal measure to the transcriptional events required for the acquisition of the MZ B cell phenotype.
Figure 5.
Absolute numbers of splenic B cells in wild-type (WT) and mutant mice. (a) Absolute numbers of newly formed, follicular, and MZ B cells in wild-type, p50−/−, c-Rel−/−, and Rag-2−/− mice reconstituted with mixtures including wild-type, p65−/−, and p50−/−/p65−/− fetal liver cells, respectively, in combination with Rag-2−/− bone marrow cells. (b) Absolute numbers of MZ B cells in the above mice depicted using an expanded scale.
We have demonstrated that NF-κB is required in lymphocytes during the development of MZ B cells. It is likely that a cell surface receptor on B lymphocytes must signal to activate NF-κBp50–containing complexes in order for mature B cells to acquire the MZ phenotype. NF-κBp50 is essential for the survival/differentiation of MZ B cells but is apparently not critical for the development of naive follicular B cells. Whether NF-κB is involved in the differentiation of MZ B cell precursors or is required for the survival of MZ B cells is unclear.
MZ B cells fail to develop in the absence of CD19 5 6, suggesting that the B cell receptor (BCR) may be required for MZ B cell generation. However, although BCR signaling is enhanced in the absence of Aiolos, Aiolos−/− mice lack MZ B cells 8, suggesting that a direct relationship might not exist between the strength of BCR signaling and MZ B cell generation. We have recently shown that Aiolos−/−/Xid double mutants have normal numbers of MZ B cells (Cariappa, A., K. Georgopoulos, and S. Pillai, unpublished results), indicating that Aiolos is not directly required for MZ B cell development. Although NF-κBp50–containing complexes are known to be induced when splenic B cells are triggered to proliferate with anti-IgM 26, MZ B cells do not proliferate in response to antigen receptor ligation 4, and it is unclear whether the BCR is required to induce NF-κB during MZ B cell generation. Candidate signaling molecules on B cells that may be involved in MZ B cell development are the TNF receptor family members, transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) and B cell maturation antigen (BCMA) 27, which are triggered by B lymphocyte stimulator (BlyS)/B cell activator belonging to the TNF family (BAFF) 28 29. BlyS/BAFF is made in membrane-bound and cleaved/secreted forms by macrophages, dendritic cells, and T cells. Transgenic overexpression of BAFF led to an increase in MZ B cell generation 30. It is conceivable that NF-κBp50 may be induced downstream of TACI and BCMA during the generation of MZ B cells.
Acknowledgments
We thank Ranjan Sen for comments on the manuscript; John Daley, Suzan Kallanian, Yong-Guang Yang, Dennis Sgroi, Rebbecca Levangie, Steve Matheson, Mike Boxem, and Chuenlei Parng for help and advice; and Rodolphe Guinamard for the kind gift of TNP-Ficoll.
This work was supported by grants from the National Institutes of Health (AI33507 and CA69618).
Footnotes
Abbreviations used in this paper: BCR, B cell receptor; Btk, Bruton's tyrosine kinase; MZ, marginal zone; NF, nuclear factor; RAG, recombination activating gene.
References
- MacLennan I.C.M., Gray D. Antigen driven selection of virgin and memory B cells. Immunol. Rev. 1986;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Kraal G. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- Oliver A.M., Martin F., Gartland G.L., Carter R.H., Kearney J.F. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur. J. Immunol. 1997;27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- Oliver A.M., Martin F., Kearney J.F. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J. Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- Makowska A., Faizunnessa N.N., Anderson P., Midtvedt T., Cardell S. CD1high B cellsa population of mixed origin. Eur. J. Immunol. 1999;29:3285–3295. doi: 10.1002/(SICI)1521-4141(199910)29:10<3285::AID-IMMU3285>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Martin F., Kearney J.F. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk . Immunity. 2000;12:39–49. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- Guinamard R., Okigaki M., Schlessinger J., Ravetch J.V. Absence of marginal zone B cells in Pyk-2 deficient mice defines their role in the humoral response. Nat. Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- Wang J.H., Avitahl N., Cariappa A., Friedrich C., Ikeda T., Renold A., Andrikopoulos K., Liang L., Pillai S., Morgan B.A., Georgopoulos K. Aiolos regulates B cell activation and maturation to effector state. Immunity. 1998;9:543–554. doi: 10.1016/s1074-7613(00)80637-8. [DOI] [PubMed] [Google Scholar]
- Sha W.C., Liou H.C., Toumanen E.I., Baltimore D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- Beg A.A., Sha W.C., Bronson R.T., Ghosh S., Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Kontgen F., Grumont R.J., Strasser A., Metcalf D., Li R., Tarlinton D., Gerondakis S. Mice lacking the c-Rel protooncogene exhibit defects in lymphocyte proliferation, humoral immunity and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- Tumang J.R., Owyang A., Andjelic S., Jin Z., Hardy R.R., Liou M.L., Liou H.C. c-Rel is essential for B lymphocyte survival and cell cycle progression. Eur. J. Immunol. 1998;28:4299–4312. doi: 10.1002/(SICI)1521-4141(199812)28:12<4299::AID-IMMU4299>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Horwitz B.H., Scott M.L., Cherry S.R., Bronson R.T., Baltimore D. Failure of lymphopoiesis after adoptive transfer of NFκB deficient fetal liver cells. Immunity. 1997;6:765–772. doi: 10.1016/s1074-7613(00)80451-3. [DOI] [PubMed] [Google Scholar]
- Cariappa A., Kim T.J., Pillai S. Accelerated emigration of B lymphocytes in the Xid mouse. J. Immunol. 1999;162:4417–4423. [PubMed] [Google Scholar]
- Snapper C.M., Zelazowski P., Rosas F.R., Kehry M.R., Tian M., Baltimore D., Sha W.C. B cells from p50/NF-κB knockout mice have selective defects in proliferation, differentiation, germline CH transcription, and Ig class switching. J. Immunol. 1996;156:183–191. [PubMed] [Google Scholar]
- Franzoso G., Carlson L., Poljak L., Shores E.W., Epstein S., Leonardi A., Grinberg A., Tran T., Scharton-Kesten T., Anver M. Mice deficient in NFκB/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J. Exp. Med. 1998;187:147–159. doi: 10.1084/jem.187.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B.H., Zelazowski P., Shen Y., Wolcott K.M., Scott M.L., Baltimore D., Snapper C.M. The p65 subunit of NFκB is redundant with p50 during B cell proliferative responses, and is required for germline CH transcription and class switching to IgG3. J. Immunol. 1999;162:1941–1946. [PubMed] [Google Scholar]
- Sha W.C. Regulation of immune responses by NF-κB/Rel transcription factors. J. Exp. Med. 1998;187:143–146. doi: 10.1084/jem.187.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T.S., Takahashi T., Taguchi O., Takachika A., Obata Y. NF-κB RelA deficient lymphocytesnormal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. J. Exp. Med. 1997;185:953–961. doi: 10.1084/jem.185.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumont R.J., Rourke I.J., O'Reilly L.A., Strasser A., Miyake K., Sha W., Gerondakis S. B lymphocytes differentially use the Rel and NF-κB1 transcription factors to regulate cell cycle progression and apoptosis in quiescent and mitogen-activated cells. J. Exp. Med. 1998;187:663–674. doi: 10.1084/jem.187.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumont R.J., Rourke I.J., Gerondakis S. Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation induced apoptosis. Genes Dev. 1999;13:400–411. doi: 10.1101/gad.13.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S. The chosen few? Positive selection and the generation of naive B lymphocytes. Immunity. 1999;10:493–502. doi: 10.1016/s1074-7613(00)80049-7. [DOI] [PubMed] [Google Scholar]
- Loder F., Mutschler B., Ray R.J., Paige C.J., Sideras P., Torres R., Lamers M.C., Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor–derived signals. J. Exp. Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai U.D., Zhang K., Teutsch M., Sen R., Wortis H.J. Bruton's tyrosine kinase links the B cell receptor to nuclear factor κB activation. J. Exp. Med. 2000;191:1735–1744. doi: 10.1084/jem.191.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro J.B., Rahman S.M., Ballard D.W., Khan W.N. Bruton's tyrosine kinase is required for activation of IκB kinase and nuclear factor κB in response to B cell receptor engagement. J. Exp. Med. 2000;191:1745–1754. doi: 10.1084/jem.191.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D.A., Sen R., Rice N., Rothstein T.L. Receptor-specific induction of NFκB components in primary B cells. Int. Immunol. 1998;10:285–293. doi: 10.1093/intimm/10.3.285. [DOI] [PubMed] [Google Scholar]
- Gross J.A., Johnston J., Mudri S., Enselman R., Dillon S.R., Madden K., Xu W., Parrish-Novak J., Foster D., Lofton-Day C. TACI and BCMA are receptors for a TNF homologue implicated in B cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- Moore P.A., Belvedere O., Orr A., Pieri K., LaFleur D.W., Feng P., Soppet D., Charters M., Gentz R., Parmelee D. BLySmember of the tumor necrosis family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- Schneider P., Mackay F., Steiner V., Hofmann K., Bodmer J.L., Holler N., Ambrose C., Lawton P., Bixler S., Acha-Orbea H. BAFF, a new ligand of the tumor necrosis factor family stimulates B cell growth. J. Exp. Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F., Woodcock S.A., Lawton P., Ambrose C., Baetscher M., Schneider P., Tschopp J., Browning J.L. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]







