Abstract
The B cell receptor (BCR) regulates B cell development and function through immunoglobulin (Ig)α and Igβ, a pair of membrane-bound Ig superfamily proteins, each of which contains a single cytoplasmic immunoreceptor tyrosine activation motif (ITAM). To determine the function of Igβ, we produced mice that carry a deletion of the cytoplasmic domain of Igβ (IgβΔC mice) and compared them to mice that carry a similar mutation in Igα (MB1ΔC, herein referred to as IgαΔC mice). IgβΔC mice differ from IgαΔC mice in that they show little impairment in early B cell development and they produce immature B cells that respond normally to BCR cross-linking as determined by Ca2+ flux. However, IgβΔC B cells are arrested at the immature stage of B cell development in the bone marrow and die by apoptosis. We conclude that the cytoplasmic domain Igβ is required for B cell development beyond the immature B cell stage and that Igα and Igβ have distinct biologic activities in vivo.
Keywords: B cell receptor, immunoglobulin α, immunoglobulin β, immunoreceptor tyrosine activation motif, apoptosis
Introduction
Signals from the B cell receptor (BCR) regulate many of the essential physiologic activities in the B cell pathway. These include several different transitions in B cell development, allelic exclusion, central and peripheral tolerance, as well as B cell survival and response to antigen 1. All of these functions appear to be induced by signals emanating from the Ig-associated heterodimer of Igα and Igβ 2 3 4. Signals initiated by ligand binding to membrane (m)IgM are communicated to the Igα–Igβ transducer through a noncovalent interaction that involves polar residues in the plane of the cell membrane 5 6 7. Mutations that disrupt these polar residues interfere with signal transduction and early B cell development 5 6 7 8.
The discovery that the BCR signal transducer is a heterodimer led to the proposal that the Igα and Igβ subunits might have distinct biological functions. Biochemical studies showing that the cytoplasmic tails of Igα and Igβ bind to different sets of cellular kinases 9 and transfection experiments showing differences in the signaling activities of Igα and Igβ cytoplasmic domains support this idea 6 7 10 11 12 13 14. However, experiments performed in mice have failed to show any differences in the biologic activities of Igα and Igβ. Similarly, there are no known qualitative differences in the activities of any of the immunoreceptor tyrosine activation motifs (ITAMs) in the CD3 chains of the TCR 15 16 17 18 19 20 21.
Three approaches have been used to determine the function of Igα and Igβ in vivo: transgenic expression of chimeric proteins 8 22 23, Igβ gene deletion 24, and Igα cytoplasmic tail mutation 25. Transgenic experiments showed that the cytoplasmic domain of either Igα or Igβ was sufficient to activate allelic exclusion and pre-B cell development and led to the conclusion that Igα and Igβ are redundant in early B cell development 8 22 23. Deletion of Igβ resulted in B cells that failed to assemble a BCR and were arrested at the pre-BI cell stage, suggesting that BCR assembly is essential for B cell development 24. Deletion of 40 of the 61 cytoplasmic amino acids of Igα, including both ITAM tyrosines (IgαΔC 25), produced B cells that assembled a mutant BCR composed of mIgμ and an Igα–Igβ heterodimer with a truncated Igα tail. In agreement with the transgenic experiments, the single Igβ cytoplasmic domain in the IgαΔC BCR was enough to induce pre-B cell development and allelic exclusion 8 25. However, the number of pre-B cells in IgαΔC mice was reduced by 50%, immature B cells were reduced by 80%, and the number of mature B cells in spleen was only 1% of control. Thus, a BCR with only an Igβ cytoplasmic domain was unable to support later stages of B cell development. Furthermore, increased tyrosine phosphorylation in IgαΔC B cells and increased calcium flux in response to receptor cross-linking suggested a unique negative regulatory role for the Igα cytoplasmic domain 26 27.
To compare the biologic function of Igα and Igβ directly, we produced mice that carry a targeted deletion of the cytoplasmic domain of Igβ.
Materials and Methods
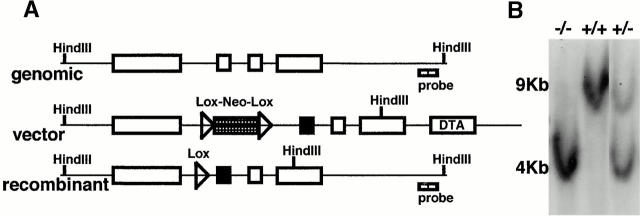
Mice.
IgβΔC mice were created by gene targeting in 129/Sv embryonic stem cells 24. To shorten the cytoplasmic tail of Igβ by 45 amino acids and delete the ITAM, the stop codon TGA was introduced by PCR at amino acid 184 4. A unique HindIII site was placed into the targeting vector between the exons as indicated (see Fig. 1 A). A lox-P–flanked neomycin resistance gene was inserted between two XbaI sites, and sequence coding for diphtheria toxin (DTA 28) was added to the 3′ end of the targeted locus at the XhoI site (see Fig. 1 A). Homologous recombination was confirmed by Southern blotting after digestion with HindIII (see Fig. 1 B). The rate of homologous recombination was 1:80. The genomic fragment used as a probe for Southern blotting was amplified by PCR using the specific primers GGATTCGAATGGTGAATGTTGG and AGGCTCTAGCTCAGTGAAGGGAG. PCR conditions were: 94°C for 5 min, and 30 cycles of 94°C for 30 s, 48°C for 45 s, and 72°C for 1 min, followed by extension at 72°C for 7 min. To delete the neomycin gene, mice carrying the targeted Igβ gene were bred to C57BL/6 Cre transgenic mice 29. Deletion of the neomycin gene was confirmed by PCR using neomycin-specific primers ATGATTGAACAAGATGGATTGCAC and TCGTCCAGATCATCCTGATCGAC. PCR conditions were: 94°C for 3 min, and 30 cycles of 94°C for 1 min, 58°C for 45 s, and 72°C for 1 min, followed by extension at 72°C for 7 min. Heterozygous IgβΔC mice were backcrossed to C57BL/6 mice for three generations before intercrossing to produce homozygous IgβΔC mice. All mice were bred and maintained under specific pathogen–free conditions.
Figure 1.
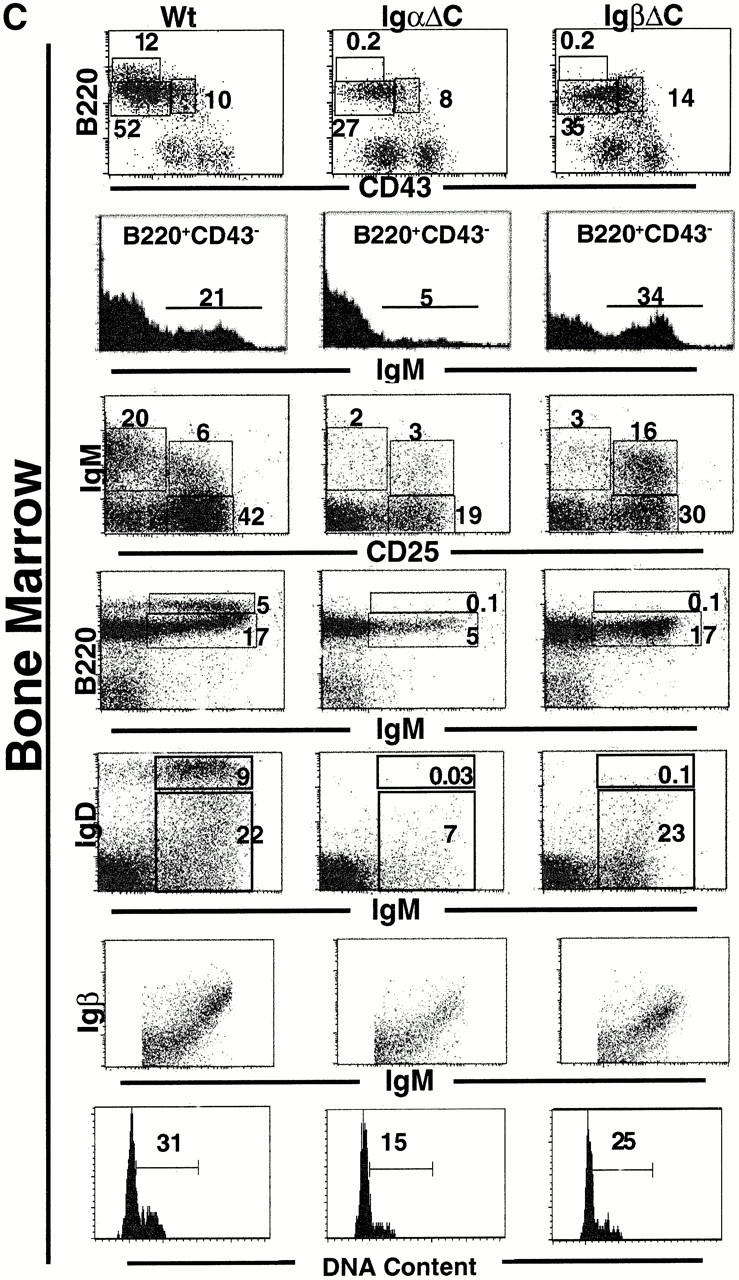
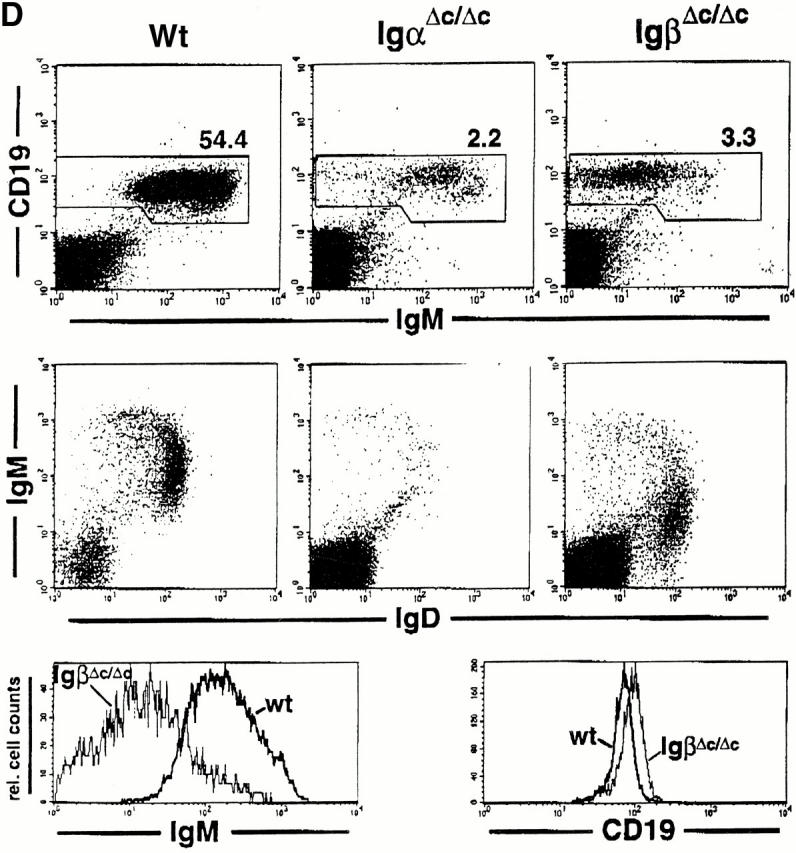
IgβΔC gene targeting. (A) Scheme for IgβΔC gene targeting showing the genomic Igβ locus (top), targeting vector (middle), and recombinant locus after deletion of the neo gene (bottom). Exons are represented by open boxes, loxP sites by open triangles, and the exon containing the mutant stop codon at position 184 by a filled box. The probe used to identify homologous recombinants is shown. (B) Southern blot analysis of wild-type, homozygous, and heterozygous mice. HindIII digestion of genomic DNA yields restriction fragments of 4 or 9 kb corresponding to targeted or wild-type alleles, respectively. (C) Analysis of bone marrow B cells in wild-type (Wt), IgαΔC, and IgβΔC mice. In B220 vs. CD43 and B220 vs. IgM plots, numbers indicate the percentages of lymphocytes determined by forward versus side scatter parameters. In IgD vs. IgM and IgM vs. CD25 plots, numbers indicate the percentages of B220+ cells in the boxed region. In the IgM histograms, numbers indicate the percentage of B220+CD43− that are IgM positive (immature B cells). IgD vs. IgM, IgM vs. CD25, and Igβ vs. IgM dot plots show bone marrow cells that were pregated on B220+ cells. DNA content is shown through Hoechst 33342 staining of large B220+CD25+ pre-B cells, and the percentage of cells in S and G2/M phase is indicated. (D) Analysis of spleen B cells in wild-type (Wt), IgαΔC, and IgβΔC mice. Spleen plots for IgαΔC and IgβΔC mice show a fivefold greater number of events than the wild-type. Antibodies used for staining are indicated.
IgαΔC mice 25 were crossed with IgβΔC mice to create IgαΔC/IgβΔC mixed heterozygous and homozygous mice 25. IgβΔC mice were also bred to C57BL/6 IgHEL Ig transgenic mice 30.
Flow Cytometry.
Single cell suspensions from bone marrow, spleen, and peritoneal cavity were stained with FITC, PE, allophycocyanin, and biotin-conjugated monoclonal antibodies visualized with streptavidin red 613 (GIBCO BRL). Monoclonal antibodies were anti-CD43, anti-IgM, anti-B220, anti-CD25, anti-IgD, anti-CD19, anti-IgMa, anti-IgMb (BD PharMingen), biotin anti-Igβ (a gift from H. Karasuyama, The Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan), and anti-493 (a gift from A. Rolink, Basel Institute for Medical Science, Basel, Switzerland). For intracellular Igμ staining, cells were first surface stained with anti-B220–allophycocyanin, anti-CD25–PE, and/or anti-IgM–PE, and anti-CD43–biotin, permeabilized with Intraprep permeabilization kit (Immunotech), and incubated with Fab′ fragments of FITC-goat anti–mouse IgM. Data were collected on a FACSCalibur™ and analyzed using CELLQuest™ software (Becton Dickinson).
Cell Cycle Analysis.
Bone marrow cells were incubated at 37°C for 40 min with Hoechst 33342 (Molecular Probes) diluted 1:1,000, then stained for cell surface markers with anti-B220, anti-IgM, anti-CD43, and anti-CD25. Data were collected on a FACS Vantage™ and analyzed with CELLQuest™ software (Becton Dickinson).
Ca2+ Flux.
Bone marrow cells were adjusted to 5 × 106/ml in PBS plus 1% FCS plus 1 mM CaCl2 plus 1 mM MgCl2 (loading buffer), and incubated with 1.5 μM Indo-1-AM (Molecular Probes) for 30 min at 37°C. Cells were stained with PE–anti-B220 and Fab′ FITC-goat anti–mouse IgM (Jackson ImmunoResearch Laboratories). Calcium flux was measured by fluorescence emission ratios of Indo-1-AM on a dual laser FACS Vantage™ (Becton Dickinson) at 395/510 nm on B220lowIgM+ cells. Data were acquired for 60 s before BCR cross-linking with F(ab′)2 goat anti–mouse IgM (Southern Biotechnology Associates, Inc.) at 10 or 20 μg/ml.
B Cell Cultures.
Bone marrow B cells from mutant or wild-type mice were enriched by positive selection using MACS mouse CD19 microbeads (Miltenyi Biotech) and stained with Fab′ anti-IgM, and monoclonal anti-CD25, anti-CD43, and anti-B220. B cells were then sorted into B220+CD43−IgMlow immature B cells and cocultured at 106/ml with irradiated S17 stromal cells in RPMI 1640 supplemented with 10% FCS and 10 ng/ml IL-7 (BD PharMingen 31). B cell viability was assessed on days 0 and 1 by flow cytometry using PE–annexin V (BD PharMingen) and propidium iodide staining.
Immunization.
6–8-wk-old IgβΔC and C57BL/6 mice were immunized intraperitoneally with either 50 μg alum-precipitated 4-hydroxy-3-nitrophenylacetyl coupled to chicken gamma globulin (NP-CGG) or 50 μg NP-Ficoll in PBS. Blood was collected from the tail vein of each mouse before immunization and at days 7, 14, 21, and 28 after immunization. NP-specific IgM and IgG levels were measured by ELISA using plates coated with NP16BSA (5 μg/ml) and developed with anti-IgM coupled to horseradish peroxidase or anti-IgG coupled to horseradish peroxidase (Southern Biotechnology Associates, Inc. 32). Immunoabsorbance was read at 415 nm and titers were calculated relative to control sera from unimmunized mice. Four mice were used in each group.
Results
B Cell Development in IgβΔC Mice.
Gene targeting was used to introduce a stop codon at position 184 in Igβ (Fig. 1 A). The mutant gene directs the expression of a truncated Igβ protein that resembles mIgμ in having only three charged cytoplasmic anchor amino acids (DKD).
B cell development in IgβΔC mice was analyzed by multiparameter flow cytometry. When compared with wild-type controls, IgαΔC and IgβΔC mice showed an increase in the number of IgM−B220+CD43+CD25− pro-B cells (25; Table and Fig. 1 C). Both strains also showed smaller numbers of IgM−B220+CD43−CD25+ pre-BII cells (fraction C′/D) than wild-type, although the 25% decrease found in IgβΔC mice was less substantial than the 50% decrease found in IgαΔC mice (25; Table and Fig. 1 C).
Table 1.
B Cell Development and Cell Cycle Analysis
| Percentage of B220+ bone marrow cells | Percentage of B220+ cells in S/G2/M | |||||
|---|---|---|---|---|---|---|
| Pre-BI | Pre-BII | Immature | Recirculating | Pre-BII (small) | Pre-BII (large) | |
| C57BL/6 | 27 ± 2.2 | 37 ± 5.3 | 23 ± 12.6 | 7.0 ± 3.6 | 0.4 ± 0.3 | 33 ± 6.0 |
| IgβΔC | 55 ± 4.0 | 27 ± 2.8 | 28 ± 11.9 | 0.1 ± 0.1 | 0.7 ± 0.8 | 20 ± 6.5 |
Data represent the mean from four mice ± SD.
After H chain expression, pre-BI cells become large dividing pre-BII cells (fraction C′ 33 34 35 36). To determine whether the single Igα cytoplasmic domain in the IgβΔC BCR is sufficient to trigger normal pre-BII cell division, we measured the DNA content of these cells. We found that the cell cycle distribution of large pre-BII cells in IgβΔC mice was similar to that of control mice (Fig. 1 C and Table ). Thus, the single cytoplasmic tail of Igα in the IgβΔC BCR is sufficient to trigger pre-BII cell (fraction C′) proliferation.
After mIgμ triggered proliferative expansion, B cells rearrange Ig L chain genes, express surface IgM, lose CD25 expression, and then express IgD 37. Few B cells in IgαΔC mice progress to the B220+CD43−IgM+IgD− “immature” B cell stage (fraction E) (25; Fig. 1 C, second row; B220+CD43− gated IgM histograms; IgM versus CD25, B220 versus IgM, and IgM versus IgD dot plots). In contrast, IgβΔC B cells proceed to this immature B cell stage in normal numbers, although the level of IgM expressed on the cell surface is lower than control (Fig. 1 C). Immature IgβΔC B cells fail to progress further to the CD25−IgM+IgD+ transitional B cell stage (Fig. 1 C; IgM versus CD25, B220 versus IgM, and IgM versus IgD). Failure to progress to the CD25−IgM+IgD+ transitional B cell stage is reflected in the near absence of recirculating B cells in the bone marrow and mature B cells in spleen (Fig. 1c and Fig. d). To determine whether this failure to mature is due to low levels of surface Igα–Igβ expression, we stained developing B cells with anti-Igβ monoclonal antibody 38. We found that for any given level of surface IgM expression, the level of cell surface Igβ on B220+IgM+ immature B cells was similar in IgβΔC B cells and controls (Fig. 1 C). Thus, IgαΔC mice suffer a continuous loss of B cell precursors beginning at the pre-B cell stage, whereas B cell development is terminated abruptly at the immature B cell stage in IgβΔC mice (25; Fig. 1 C and Table ).
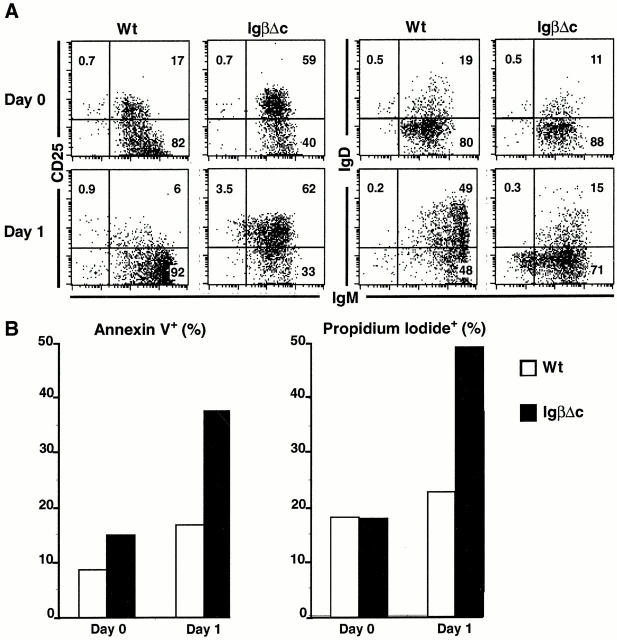
To determine whether arrest at the CD25+IgM+IgD− immature B cell stage is associated with increased cell death, we established in vitro bone marrow cultures 31. Immature B cells were purified by cell sorting using a Fab′ anti-IgM to avoid receptor cross-linking. Cell death was measured by propidium iodide exclusion and annexin V staining (Fig. 2). Annexin V staining varies between different stages of B cell development and is therefore unreliable when comparing B cells in different stages 39. However, annexin is a reliable marker for apoptosis when comparing cells at similar stages in development 39. Freshly isolated immature IgβΔC and control B cells were equally viable as measured by exclusion of propidium iodide. In culture, the control immature B cells developed into CD25−IgMhiIgD+ transitional B cells, whereas the IgβΔC B cells did not progress beyond the CD25+IgMloIgD− immature B cell stage. Instead, IgβΔC B cells became increasingly annexin V and propidium iodide positive (Fig. 2). Thus, IgβΔC B cells that reach the CD25+IgM+IgD− immature B cell stage fail to progress and die by apoptosis.
Figure 2.
B cell development and death in bone marrow cultures. Bone marrow cells from IgβΔC and control mice were selected using CD19 MACS beads, sorted for B220+CD43−IgMlow immature B cells, and then cocultured with S17 stromal cells. (A) Dot plots show staining with anti-CD25 or anti-IgD and anti-IgM at the initiation of culture (left) and after 24 h (right). Numbers indicate percentages of B220+ cells in each quadrant. Wt, wild-type. (B) Bar graphs show the percentage of B220+ cells that were annexin V or propidium iodide. Results are the average of duplicate cultures performed on two independent mice. The variation between samples and mice was <5%.
Allelic Exclusion.
In addition to supporting pre-B cell development, cell surface expression of the BCR induces Ig H chain allelic exclusion 40 41. To determine whether the single Igα cytoplasmic domain in IgβΔC mice is sufficient for allelic exclusion, we bred IgβΔC mice to IgHEL transgenic mice which carry an allotype marked Igμa H chain 30. IgHEL transgenic IgβΔC mice resemble nontransgenic IgβΔC mice in that their B cells fail to progress beyond the immature stage of B cell development, they express lower levels of surface IgM than controls, and there are few detectable B cells in the spleen and the peritoneal cavity (Fig. 3). Nevertheless, allelic exclusion is established normally in IgHEL transgenic IgβΔC B cells. 96% of the immature B cells in the bone marrow of both IgHEL transgenic IgβΔC mice and control mice expressed the IgHEL Igμa H chain, whereas only 3–4% coexpressed the endogenous Igμb H chains (Fig. 3). We conclude that the single Igα cytoplasmic domain in IgβΔC BCRs is sufficient to maintain H chain allelic exclusion and that transgenic antibody expression is not sufficient to induce further B cell differentiation in IgβΔC mice.
Figure 3.
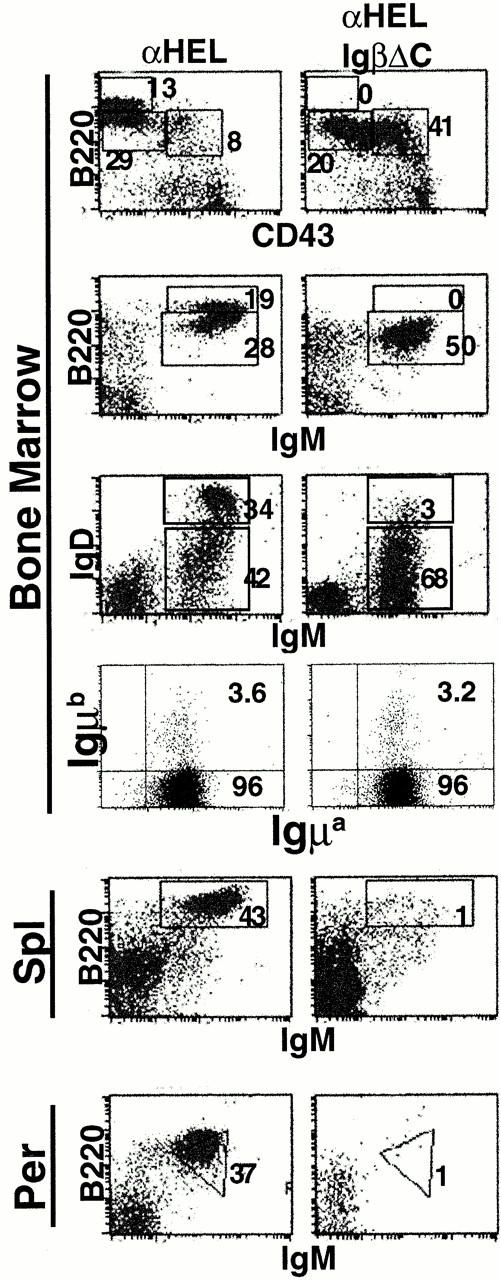
B cell development and allelic exclusion in IgβΔC IgHEL transgenic mice. In the B220 vs. CD43 and B220 vs. IgM plots, numbers indicate percentages of lymphocytes as determined by forward vs. side scatter parameters. In the IgD vs. IgM plots, numbers indicate percentages of B220+ cells in the boxed region. For H chain allelic exclusion, dot plots show B cells gated on B220+IgM+ cells stained with IgMa and IgMb, and the numbers indicate the percentage of B220+IgM+ cells in each quadrant. B220 vs. IgM staining in the spleen (Spl) and peritoneal cavity (Per) is shown.
Peripheral B Cells and Antibody Responses.
Splenic B cell numbers were reduced to ∼2% in IgβΔC mice in comparison with wild-type controls (0.64 ± 0.66 × 106 B cells, n = 5 versus 27.51 ± 5.86 × 106 B cells, n = 8). A similar block in the development and maintenance of mature B lymphocytes was present in IgαΔC mice (splenic B cell numbers are reduced to ∼1% [0.21 ± 0.14 × 106, n = 5; reference 25). The maturation status of splenic B lymphocytes was examined in IgαΔC and IgβΔC mice. More than 80% of B lymphocytes did not stain for the immature B cell marker 493 42 and displayed a mature phenotype (data not shown). We also examined splenic B cells for surface expression of CD23 and MHC class II and found no effect of the cytoplasmic truncations (data not shown). However, peripheral B lymphocytes in IgαΔC and IgβΔC mice expressed higher levels of CD19 (Fig. 1 D). Splenic B cells in IgαΔC mice expressed normal levels of cell surface IgM. In contrast, the splenic B cells found in IgβΔC mice resembled their bone marrow precursors and continued to express 10 times lower levels of surface IgM and 0.5 times lower levels of IgD than controls (Fig. 1 D).
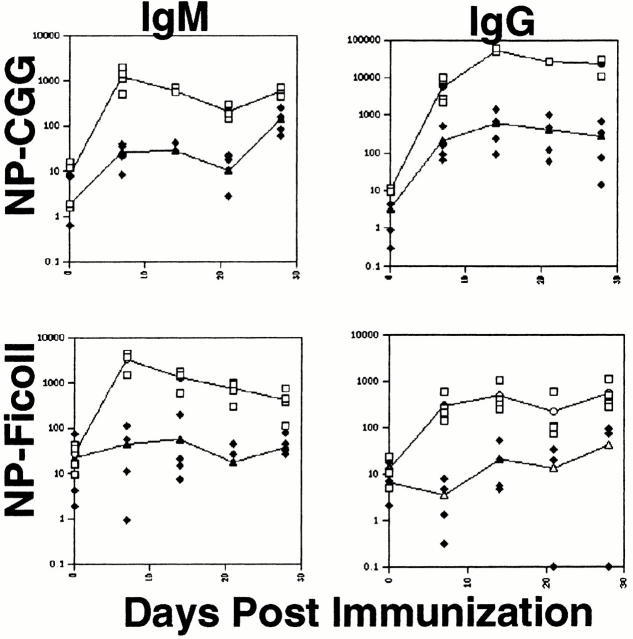
The scarce peripheral B cells in IgαΔC mice produce specific antibody responses to T cell–dependent but not to T cell–independent antigens 25. To determine whether IgβΔC B cells can respond to antigens, we immunized mice with T cell–dependent (NP-CGG) and T cell–independent (NP-Ficoll) antigens and measured specific antibody responses by ELISA. IgβΔC B cells mount a hapten specific immune response to NP-CGG with class switching to IgG, but do not appear to respond to NP-Ficoll. Consistent with the small number of peripheral B cells in the IgβΔC mice, anti-NP antibody titers were two orders of magnitude lower than controls (Fig. 4). We conclude that like IgαΔC B cells, IgβΔC B cells respond to T cell–dependent but not T cell–independent antigens.
Figure 4.
Antibody responses in IgβΔC mice. Plots show anti-NP IgM and IgG responses measured by ELISA on days 7, 14, 21, and 28 after immunization with NP-CGG or NP-Ficoll. The open squares represent individual wild-type controls and the filled diamonds represent individual IgβΔC mice. The y axis indicates OD415 relative to unimmunized controls. The lines represent the means.
Ca2+ Flux.
Ca2+ flux responses are enhanced in immature B cells from IgHEL transgenic, IgαΔC mice 27. This increase in the Ca2+ response could be due to a unique negative regulatory role for Igα in developing B cells or, alternatively, to a difference in Ca2+ responses induced by IgHEL transgene expression in the IgαΔC background 27. To determine whether altered responses to BCR cross-linking were IgαΔC specific, we measured Ca2+ flux in response to BCR cross-linking in immature IgβΔC bone marrow cells. B cells expressing similar levels of surface IgM were compared by electronically gating on surface IgM expression after staining with an Fab′ anti-IgM. We found no measurable differences in Ca2+ responses to anti-BCR cross-linking between immature IgβΔC B cells and control immature B cells (Fig. 5). In contrast, IgHEL transgenic IgβΔC B cells produced a higher magnitude Ca2+ response than either wild-type controls or IgHEL transgenic B cells despite lower surface IgM expression (Fig. 5). We conclude that cross-linking the BCR in immature IgβΔC B cells induces normal Ca2+ flux responses, whereas B cells in IgβΔC mice carrying the IgHEL transgene have hyperactive receptors.
Figure 5.
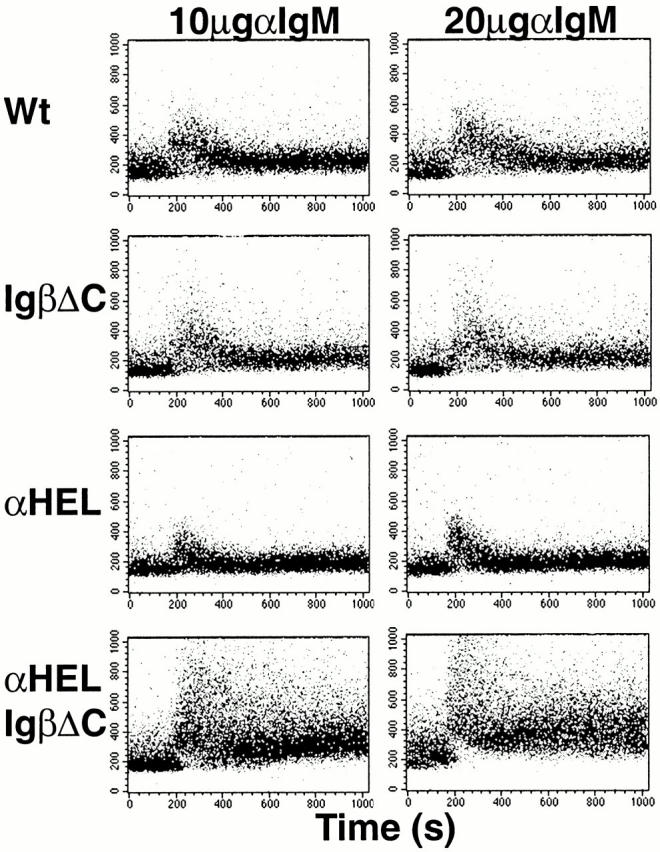
Ca2+ flux response to BCR cross-linking in immature B cells in IgβΔC and IgHEL transgenic IgβΔC mice. Dot plots represent Ca2+ flux of immature B cells measured by the fluorescence 395/510 nm ratio of Indo-1-AM emission accumulated over 512 s. Immature B cells were gated by staining with anti-B220 and Fab′ anti-IgM fragment. Baseline fluorescence was acquired for 60 s before the agonist, F(ab′)2 goat anti–mouse IgM at a final concentration of either 10 or 20 μg/ml, was added. Wt, wild-type.
B Cell Development in the Absence of Igα and Igβ Tails.
To determine whether the cytoplasmic domain of either Igα and Igβ is essential for pre-B cell development, we produced double mutant IgαΔC/IgβΔC mice by crossing IgαΔC and IgβΔC mice. IgαΔC/IgβΔC mice resembled Igβ−/− mice in that B cell development was arrested at the B220+CD43+CD25− pre-BI stage (24; Fig. 6 A). We conclude that B cell development cannot proceed beyond the pre-BI stage in the absence of the cytoplasmic domains of both Igα and Igβ.
Figure 6.
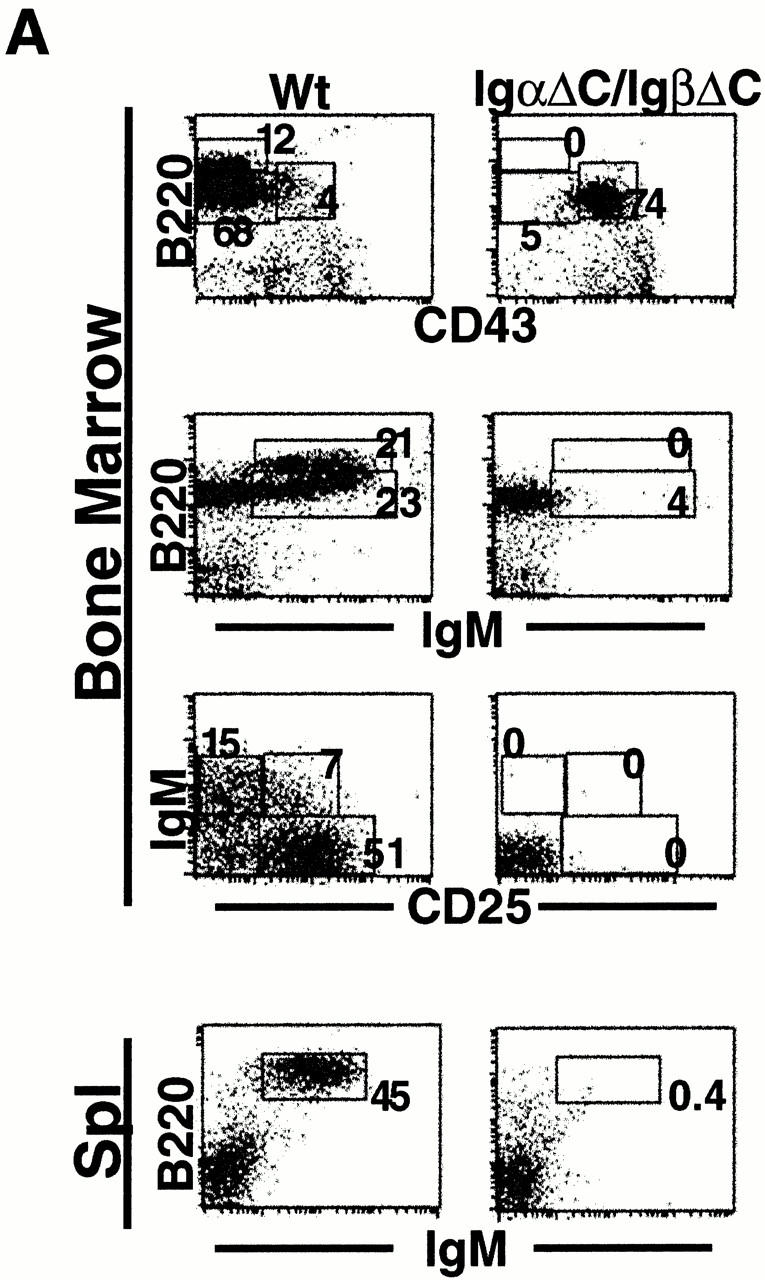
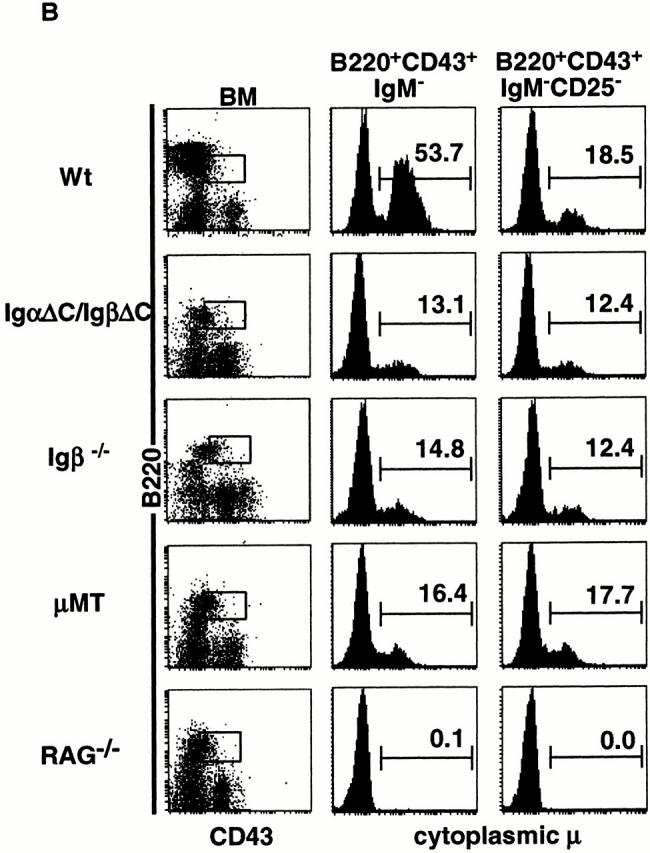
Cytoplasmic domains of Igα and Igβ are essential for B cell development. (A) Dot plots show staining with combinations of anti-B220, anti-CD43, anti-IgM, and anti-CD25 antibodies in bone marrow and spleen. In the B220 vs. CD43 and B220 vs. IgM plots, numbers indicate percentages of lymphocytes as determined by forward vs. side scatter parameters. In the IgM vs. CD25 plots, numbers indicate percentages of B220+ cells in the boxed region. Wt, wild-type. (B) Intracytoplasmic Igμ expression in IgαΔC/IgβΔC, Igβ−/−, μMT, RAG−/−, and wild-type mice. Dot plots show staining with anti-B220 and anti-CD43, and the boxed region was used in further gating as indicated. Histograms show intracytoplasmic Igμ staining in surface B220+CD43+IgM− pro- and pre-B cells and B220+CD43+IgM−CD25− pre-BI cells. Numbers indicate the percentages of intracellular Igμ+ surface B220+CD43+IgM− pro- and pre-B cells and B220+CD43+IgM−CD25− pre-BI cells.
Mixtures of B220+CD43+ pro- and pre-B cells purified from Igβ−/− mice have fewer complete VDJH genes than wild-type B220+CD43+IgM− pro- and pre-B cells 24. This effect could be due to inefficient V to DJH recombination in pre-BI cells lacking Igβ, or to lack of positive selection and amplification of pre-BII cells with in-frame Ig H chains 24. To determine whether the cytoplasmic domains of Igα and Igβ are required for Ig H chain recombination and expression, we stained for intracellular Igμ. Developing B cells in IgαΔC/IgβΔC, Igβ−/−, μMT, recombination activating gene (RAG)−/−, and wild-type mice were compared after cell surface staining with anti-B220, anti-CD43, anti-CD25, and anti-IgM to separate pre-BI and pre-BII cell subpopulations, and intracellular staining for Igμ to measure H chain expression. Consistent with previous reports, intracellular Igμ levels in B220+CD43+IgM− mixtures of pre-BI and pre-BII cells were decreased in Igβ−/− mice compared with wild-type controls (Fig. 6 B). IgαΔC/IgβΔC and μMT mice resembled Igβ−/− mice in that their B220+CD43+ cells also showed lower levels of intracellular Igμ expression than controls. However, intracellular Igμ levels in pre-BI cells in IgαΔC/IgβΔC mice were similar to Igβ−/−, μMT, and wild-type controls (B220+CD43+IgM−CD25− cells; Fig. 6 B). Therefore, the decreased Igμ expression in the developing B cells in these mutant strains is due to arrest at the pre-BI stage and lack of positive selection for B cells with an in-frame Ig H chain during pre-BII cell expansion. We conclude that the cytoplasmic domains of Igα and Igβ are essential for B cell development past the pre-BI stage, and that IgαΔC/IgβΔC, Igβ−/−, and μMT are all arrested at a similar stage in development.
Discussion
Igα or Igβ Signaling Is Essential for Pre-B Cell Development.
We have shown that the cytoplasmic domain of either Igα or Igβ is essential for B cells to develop beyond the pre-BI (fraction B/C) stage. In the absence of BCR assembly, RAG−/− 43 44, Igβ−/− 24, and μMT 33 B cells all fail to progress beyond the pre-BI stage. Although it has been assumed that this early block in development is due to failure to activate a BCR-dependent checkpoint, it might also be due to aberrant expression of BCR components. For example, expression of Igμ and Igα in the absence of Igβ in Igβ−/− mice produces an incomplete receptor that is not transported to the cell surface and might be toxic for developing pre-B cells. Similarly, only very low levels of Igα–Igβ are expressed on the cell surface in the absence of mIgμ in RAG−/− B cells 45. In contrast, the combination of IgαΔC and IgβΔC mutations produces surface BCRs that are simply unable to signal. Therefore, the finding that IgαΔC/IgβΔC B cells arrest at the pre-BI stage shows that the cytoplasmic domain of either IgαΔC or IgβΔC is essential for early B cell development, and that BCR signaling as opposed to assembly is required for later stages of B cell development.
B Cell Development Differs in IgαΔC and IgβΔC Mice.
Many aspects of B cell development are similar in IgαΔC and IgβΔC mice. For example, pre-B cell development and allelic exclusion are activated in both strains, and there are few peripheral B cells in either. However, the two strains differ in that B cells are lost throughout development in IgαΔC mice, whereas significant B cell loss is not apparent in IgβΔC mice until the late stages of B cell maturation. IgβΔC B cell development is arrested before high level surface IgM expression and acquisition of surface IgD. Low surface IgM expression is not characteristic of IgαΔC mice and appears to be specific for IgβΔC, suggesting that the cytoplasmic domain of Igβ plays an important role in regulating surface BCR expression. Alternatively, the single Igα molecule may interfere with receptor assembly or enhance receptor degradation in IgβΔC mice. Failure to acquire high levels of surface IgM is not due to an intrinsic defect in BCR expression, as there is a broad spectrum of IgM expression in the selected B cells found in the spleen of IgβΔC mice including B cells that express high levels of surface IgM. Indeed, the heterogeneity of BCR surface expression suggests that antibody specificity contributes to setting the level of BCR expression in IgβΔC mice. We would like to speculate that decreased surface BCR expression is a consequence of altered BCR signaling in IgβΔC B cells.
The differences in B cell development between IgαΔC and IgβΔC mice are reminiscent of the differences in signaling between Igα and Igβ chimeras in transfected cell lines. B and T cell lines transfected with Igα chimeras showed higher levels of signaling than those transfected with Igβ chimeras 6 7 10 11 12 13 14. Furthermore, in some cell lines, chimeric receptors required both Igα and Igβ cytoplasmic domains to trigger cell death 13. However, the differences in the IgαΔC and IgβΔC mice were unexpected because transgenic mice that carry Igμ–Igα or Igμ–Igβ chimeric receptors showed equivalent function in early 8 22 and late stages of development 23. Furthermore, in both Igμ–Igα or Igμ–Igβ transgenics, B cells developed fully and left the bone marrow whereas IgαΔC and IgβΔC mice show few mature B cells in the spleen 23. Several differences between the chimeric antibody transgenics and IgαΔC and IgβΔC mice could account for these apparent discrepancies. First, the transgenic mice carried artificial receptors in which the tails of Igα or Igβ were grafted onto heterologous transmembrane and external domains 8 22 23. Second, the genes coding for the transgenic receptors were controlled by Ig regulatory elements in multicopy randomly integrated loci and therefore the regulation of expression was not that of endogenous Igα and Igβ. Finally, the transgenic receptors carried dimers of Igα or Igβ tails instead of the normal monomers and therefore had twice as many signaling ITAMs as the BCRs in IgαΔC and IgβΔC mice.
Experiments performed on TCR CD3 proteins suggest that the ITAM-containing cytoplasmic domains of γ, ε, δ, and ζ proteins are functionally equivalent and that multiple ITAMs merely amplify signal strength 15 16 17 18 19 20 21. However, the TCR is a complex with 4 signaling proteins containing 10 ITAMs, and the role of individual ITAMs in T cell function has not been fully explored. In contrast to the TCR, the BCR has only two transducers, each with a single ITAM, and therefore differences between IgαΔC and IgβΔC mice cannot simply be due to a difference in the number of ITAMs 46.
These differences in signaling between Igα and Igβ may be attributed to the two additional non-ITAM tyrosines in the cytoplasmic domain of Igα (nos. 204 and 176; references 2 and 46). Neither of these tyrosine residues is known to be phosphorylated upon BCR cross-linking. Nevertheless, the sequence around tyrosine 204, YDQV, conforms to a consensus src homology 2 (SH2) docking site 47, and the acidic residues surrounding tyrosine 176 resemble those found in the cytoplasmic domain of erythrocyte band 3 protein, a target of ptk72 48. Therefore, tyrosine 204 and 176 in Igα may recruit a distinct set of SH2 domain–containing signaling proteins, or simply enhance signaling through Igα by increasing the number of SH2 docking sites on Igα. Other differences between Igα and Igβ that could account for the differences in signaling include higher levels of serine and threonine phosphorylation on Igβ 9 and nonconserved residues between the tyrosines in the ITAMs of Igα and Igβ that appear to modulate src kinase binding 49.
An additional distinction between IgαΔC and IgβΔC mice is that the Igα tail truncation created by Torres et al. 25 shortened the cytoplasmic tail of Igα by 40 amino acids leaving 21 amino acids, including one non-ITAM tyrosine intact. Our strategy shortened the Igβ cytoplasmic tail by 45 amino acids, leaving a 3 amino acid anchor, DKD. The considerably longer remaining cytoplasmic sequence in the Igα tail truncation may have some signaling function beyond that attributable to the ITAM sequence. Thus, there may be an even greater difference between a complete Igα and Igβ tail truncation.
Hyperresponsive BCRs in IgβΔC IgHEL Transgenic B Cells.
The hyperresponsive phenotype found in IgβΔC IgHEL transgenic mice resembles the effects found in IgHEL transgenic Src homology 2 domain–containing phosphatase 1 (SHP1) and lyn-deficient mice 50 51. In the absence of these negative regulators, B cells are hyperresponsive to BCR cross-linking. Therefore, one explanation for the hyperreactive phenotype in IgαΔC and IgβΔC IgHEL transgenic B cells might be that their BCRs are unable to recruit negative regulators of signal transduction such as SHP1 and lyn.
In contrast to IgβΔC IgHEL transgenic B cells, nontransgenic IgβΔC B cells are indistinguishable from controls in Ca2+ flux experiments. Thus, the hyperactive phenotype appears to be Ig transgene specific. The discrepancy between IgβΔC IgHEL transgenic B cells and nontransgenic IgβΔC B cells could be due to partial compensation for abnormal B cell development in IgβΔC mice by the IgHEL transgene. Alternatively, the difference between transgenic and nontransgenic B cells could be due to artificially accelerated and altered B cell development in the transgenic mice.
A unique negative regulatory role for Igα was suggested by experiments with IgαΔC mice 26 27. However, IgβΔC IgHEL transgenic B cells resemble IgαΔC IgHEL transgenic B cells in that they too were hyperresponsive compared with IgHEL controls in Ca2+ flux experiments. Thus, the absence of either Igα or Igβ produces a hyperreactive IgHEL transgenic B cell and this negative regulatory effect is not specific for Igα or Igβ.
Arrested B Cell Development in IgβΔC Mice.
Several mutations in signaling molecules and B cell coactivators have phenotypes similar to IgβΔC. In humans, Btk mutation interferes with B cell development at several stages, beginning at the pre-B cell stage resulting in a near absence of peripheral B cells (X-linked agammaglobulinemia 52 53 54). In mice, Btk mutation results in a four- to fivefold decrease in the number of recent bone marrow emigrants. Although the number of mature B cells is near normal, T cell–independent responses are severely diminished in these mice 55 56 57 58. Phosphoinositide 3-kinase deficiency in mice resembles Btk mutation in that there are decreased numbers of mature peripheral B cells and decreased levels of serum Ig 59 60. Mouse mutations in B cell coreceptors CD22 61 62, CD19 63 64, the lyn kinase 65, and the CD45 phosphatase 66 all interfere with B cell development at the immature to mature B cell transition, but these effects are more subtle and less specific than the block in B cell development seen in IgβΔC mice.
Immature B cells are highly susceptible to deletion induced by BCR cross-linking, a feature which is likely to contribute to B cell tolerance by removing cells with self-reactive receptors 67 68. Our work shows that this checkpoint is regulated by Igα–Igβ and that Igβ plays a particularly important role in setting the threshold for B cell development beyond the immature B cell stage.
Acknowledgments
We thank H. Karasuyama for the Igβ monoclonal antibody, Dr. A. Rolink for anti-493 monoclonal antibody, members of the Nussenzweig lab for helpful discussions, and F. Isdell and M. Genova for flow cytometry.
A. Reichlin was supported by a National Institutes of Health KO8 training grant. This work was funded in part by a grant to K. Rajewsky from Deutsche Forschungsgemeinschaft through SFB 243, The Max Planck Research Award to K. Rajewsky, and grants from the National Institutes of Health to M.C. Nussenzweig. M.C. Nussenzweig is an Investigator in the Howard Hughes Medical Institute.
Footnotes
A. Reichlin and Y. Hu contributed equally to this work.
Abbreviations used in this paper: BCR, B cell receptor; CGG, chicken gamma globulin; ITAM, immunoreceptor tyrosine activation motif; RAG, recombination activating gene; SH2, src homology 2.
References
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Kashiwamura S., Kimoto M., Thalmann P., Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO (Eur. Mol. Biol. Organ.) J. 1988;7:3457–3464. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach J., Leclercq L., Radbruch A., Rajewsky K., Reth M. A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO (Eur. Mol. Biol. Organ.) J. 1988;7:3451–3456. doi: 10.1002/j.1460-2075.1988.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson G.G., Eisenberg D., Kincade P.W., Wall R. B29a member of the immunoglobulin gene superfamily exclusively expressed on B-lineage cells. Proc. Natl. Acad. Sci. USA. 1988;85:6890–6894. doi: 10.1073/pnas.85.18.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A.C., Mitchell R.N., Weaver Y.K., Campos T.J., Abbas A.K., Leder P. Mutations of immunoglobulin transmembrane and cytoplasmic domainseffects on intracellular signaling and antigen presentation. Cell. 1990;63:381–392. doi: 10.1016/0092-8674(90)90171-a. [DOI] [PubMed] [Google Scholar]
- Sanchez M., Misulovin Z., Burkhardt A.L., Mahajan S., Costa T., Franke R., Bolen J.B., Nussenzweig M. Signal transduction by immunoglobulin is mediated through Igα and Igβ. J. Exp. Med. 1993;178:1049–1056. doi: 10.1084/jem.178.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G.T., Peaker C.J.G., Patel K.J., Neuberger M.S. The α/β sheath and its cytoplasmic tyrosines are required for signaling by the B-cell antigen receptor but not for capping of for serine/threonine-kinase recruitment. Proc. Natl. Acad. Sci. USA. 1994;91:471–478. doi: 10.1073/pnas.91.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavasiliou F., Misulovin Z., Suh H., Nussenzweig M.C. The role of Igβ in precursor B cell transition and allelic exclusion. Science. 1995;268:408–411. doi: 10.1126/science.7716544. [DOI] [PubMed] [Google Scholar]
- Clark M.R., Campbell K.S., Kazlauska A., Johnson S.A., Hertz M., Potter T.A., Pleiman C., Cambier J.C. The B cell antigen receptor complexassociation of Ig-alpha and Ig-beta with distinct cytoplasmic effectors. Science. 1992;258:123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- Kim K.M., Alber G., Weiser P., Reth M. Differential signaling through the Ig-alpha and Ig-beta components of the B cell antigen receptor. Eur. J. Immunol. 1993;23:911–916. doi: 10.1002/eji.1830230422. [DOI] [PubMed] [Google Scholar]
- Taddie J., Hurley T.R., Hardwick B.S., Sefton B.M. Activation of B- and T-cells by the cytoplasmic domains of the B-cell antigen receptor proteins Ig-α and Igβ. J. Biol. Chem. 1994;269:13529–13535. [PubMed] [Google Scholar]
- Luisiri P., Lee Y.J., Eisfelder B.J., Clark M.R. Cooperativity and segregation of function within the Ig-alpha/beta heterodimer of the B cell antigen receptor complex. J. Biol. Chem. 1996;271:5158–5163. doi: 10.1074/jbc.271.9.5158. [DOI] [PubMed] [Google Scholar]
- Tseng J., Eisfelder B.J., Clark M.R. B-cell antigen receptor-induced apoptosis requires both Ig alpha and Ig beta. Blood. 1997;89:1513–1520. [PubMed] [Google Scholar]
- Pao L., Famiglietti S.J., Cambier J.C. Asymmetrical phosphorylation and function of the immunoreceptor tyrosine-based activation motif tyrosines in B cell antigen receptor signal transduction. J. Immunol. 1998;160:3305–3314. [PubMed] [Google Scholar]
- Romeo C., Amiot M., Seed B. Sequence requirements for the induction of cytolisis by the T cell antigen/Fc receptor ζ chain. Cell. 1992;68:889–897. doi: 10.1016/0092-8674(92)90032-8. [DOI] [PubMed] [Google Scholar]
- Shores E.W., Huang K., Tran T., Lee E., Grinberg A., Love P.E. Role of TCR zeta chain in T cell development and selection. Science. 1994;266:1047–1050. doi: 10.1126/science.7526464. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Ma A., Cheng H.L., Alt F.W. CD3 epsilon and CD3 zeta cytoplasmic domains can independently generate signals for T cell development and function. Immunity. 1995;2:401–411. doi: 10.1016/1074-7613(95)90148-5. [DOI] [PubMed] [Google Scholar]
- Malissen M., Gillet A., Ardouin L., Bouvier G., Trucy J., Ferrier P., Vivier E., Malissen B. Altered T cell development in mice with a targeted mutation of the CD3-epsilon gene. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:4641–4653. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave V.P., Cao Z., Browne C., Alarcon B., Fernandez-Miguel G., Lafaille J., de la Hera A., Tonegawa S., Kappes D.J. CD3 delta deficiency arrests development of the alpha beta but not the gamma delta T cell lineage. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:1360–1370. doi: 10.1093/emboj/16.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haks M.C., Krimpenfort P., Borst J., Kruisbeek A.M. The CD3gamma chain is essential for development of both the TCRalphabeta and TCRgammadelta lineages. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1871–1882. doi: 10.1093/emboj/17.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen B., Ardouin L., Lin S.Y., Gillet A., Malissen M. Function of the CD3 subunits of the pre-TCR and TCR complexes during T cell development. Adv. Immunol. 1999;72:103–148. doi: 10.1016/s0065-2776(08)60018-8. [DOI] [PubMed] [Google Scholar]
- Papavasiliou F., Jankovic M., Suh H., Nussenzweig M.C. The cytoplasmic domains of Igα and Igb can independently induce the pre-B cell transition and allelic exclusion. J. Exp. Med. 1995;182:1389–1394. doi: 10.1084/jem.182.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh Y., Neuberger M.S. The immunoglobulin Igα and Igβ cytoplasmic domains are independently sufficient to signal B cell maturation and activationin transgenic mice. J. Exp. Med. 1997;185:1753–1768. doi: 10.1084/jem.185.10.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S., Nussenzweig M.C. Regulation of an early developmental checkpoint in the B cell pathway by Igβ. Science. 1996;272:411–414. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- Torres R.M., Flaswinkel H., Reth M., Rajewsky K. Aberrant B cell development and immune response in mice with a compromised BCR complex. Science. 1996;272:1802–1804. doi: 10.1126/science.272.5269.1804. [DOI] [PubMed] [Google Scholar]
- Torres R.M., Hafen K. A negative regulatory role for Ig-alpha during B cell development. Immunity. 1999;11:527–536. doi: 10.1016/s1074-7613(00)80128-4. [DOI] [PubMed] [Google Scholar]
- Kraus M., Saijo K., Torres R.M., Rajewsky K. Ig-alpha cytoplasmic truncation renders immature B cells more sensitive to antigen contact. Immunity. 1999;11:537–545. doi: 10.1016/s1074-7613(00)80129-6. [DOI] [PubMed] [Google Scholar]
- Yagi Y., Ikawa Y., Yoshida K., Shigetani Y., Takeda N., Mabuchi I., Yamamoto T., Aizawa S. Homologous recombination at c-fyn locus of mouse embryonic stem cells with use of diptheria toxin A-fragment gene in negative selection. Proc. Nat. Acad. Sci. USA. 1990;87:9918–9922. doi: 10.1073/pnas.87.24.9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M., Pichel J.G., Gorman J.R., Sauer B., Okamoto Y., Lee E., Alt F.W., Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C.C., Crosbie J., Adelstein S., Lavoie T.B., Smith-Gill S.J., Brink R.A., Pritchard-Briscoe H., Wotherspoon J.S., Loblay R.H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Cumano A., Dorshkind K., Gillis S., Paige C.J. The influence of S17 stromal cells and interleukin 7 on B cell development. Eur. J. Immunol. 1990;20:2183–2189. doi: 10.1002/eji.1830201006. [DOI] [PubMed] [Google Scholar]
- Qin X.F., Reichlin A., Luo Y., Roeder R.G., Nussenzweig M.C. OCA-B integrates B cell antigen receptor-, CD40L-, and IL4-mediated signals for the germinal center pathway of B cell development. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:5066–5075. doi: 10.1093/emboj/17.17.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kuhn R., Rajewsky K. A B cell deficient mouse by targeted disruption of the membrane exons of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Hardy H., Carmack C.E., Shinton S.A., Kemp J.D., Hayakawa K. Resolution and characterization of pro-B and pre-pro B cell stages in normal mouse bone marrow. J. Exp. Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A., Grawunder U., Winkler T.H., Karasuyama H., Melcher F. IL-2 receptor alpha chain (CD25, TAC) expression defines a crucial stage in pre-B cell development. Int. Immunol. 1994;6:1257–1264. doi: 10.1093/intimm/6.8.1257. [DOI] [PubMed] [Google Scholar]
- Spanopoulou E., Roman C.A.J., Corcoran L.M., Schlissel M.S., Silver D.P., Nemazee D., Nussenzweig M.C., Shinton S.A., Hardy R.R., Baltimore D. Functional immunoglobulin transgenes guide ordered B-cell differentiation in Rag-1-deficient mice. Genes Dev. 1994;8:1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- Rolink A.G., ten Boekel E., Yamagami T., Ceredig R., Andersson J., Melchers F. B cell development in the mouse from early progenitors to mature B cells. Immunol. Lett. 1999;68:89–93. doi: 10.1016/s0165-2478(99)00035-8. [DOI] [PubMed] [Google Scholar]
- Koyama M., Ishihara K., Karasuyama H., Cordell J.L., Iwamoto A., Nakamura T. CD79 alpha/CD79 beta heterodimers are expressed on pro-B cell surfaces without associated mu heavy chain. Int. Immunol. 1997;9:1767–1772. doi: 10.1093/intimm/9.11.1767. [DOI] [PubMed] [Google Scholar]
- Dillon S., Mancini M., Rosen A., Schlissel M.S. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J. Immunol. 2000;164:1322–1332. doi: 10.4049/jimmunol.164.3.1322. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M.C., Shaw A.C., Sinn E., Danner D.B., Holmes K.L., Morse H.C., III, Leder P. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin μ. Science. 1987;236:816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Rajewsky K. Targeted disruption of μ chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992;356:154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- Rolink A.G., Andersson J., Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur. J. Immunol. 1998;28:3738–3748. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R.S., Herrup K., Tonegawa S., Papaioannou V.E. RAG-1 deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K.-P., Oltz E.M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A.M., Alt F.W. RAG-2 deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Nagata K., Nakamura T., Kitamura F., Kuramochi S., Taki S., Campbell K.S., Karasuyama H. The Ig alpha/Ig beta heterodimer on mu-negative proB cells is competent for transducing signals to induce early B cell differentiation. Immunity. 1997;7:559–570. doi: 10.1016/s1074-7613(00)80377-5. [DOI] [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S.E., Chaudhuri M., Gish G., Pawson T., Haser W.G., King F., Roberts T., Ratnofsky S., Lechleider R.J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Wang Q., Smith J.B., Harrison M.L., Geahlen R.L. Identification of tyrosine 67 in bovine brain myelin basic protein as a specific phophorylation site for thymus p56lck. Biochem. Biophys. Res. Commun. 1991;178:1393–1399. doi: 10.1016/0006-291x(91)91048-h. [DOI] [PubMed] [Google Scholar]
- Clark M., Johnson S.A., Cambier J.C. Analysis of Iga-tyrosine kinase interaction reveals two levels of binding specificity and tyrosine phosphorylated Ig-stimulation of Fyn activity. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:1911–1919. doi: 10.1002/j.1460-2075.1994.tb06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster J.G., Goodnow C.C. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Cornall R., Cyster J.G., Hibbs M.L., Dunn A.R., Otipoby K.L., Clark E.A., Goodnow C.C. Polygenic autoimmune traitsLyn, CD22, and SHP-1, are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- Vetrie D., Vorechovsky I., Sideras P., Holland J., Davies A., Flinter F., Hammarstrom L., Kinnon C., Levinsky R., Bobrow M. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;364:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- Conley M., Parolini O., Rohrer J., Campana D. X-linked agammaglobulinemianew approaches to old questions based on the identification of the defective gene. Immunol. Rev. 1994;138:5–21. doi: 10.1111/j.1600-065x.1994.tb00844.x. [DOI] [PubMed] [Google Scholar]
- de Weers M., Brouns G.S., Hinshelwood S., Kinnon C., Schuurman R.K., Hendriks R.W., Borst J. B-cell antigen receptor stimulation activates the human Bruton's tyrosine kinase, which is deficient in X-linked agammaglobulinemia. J. Biol. Chem. 1994;269:23857–23860. [PubMed] [Google Scholar]
- Hardy R.R., Hayakawa K., Parks D.R., Hertzenberg L.A. Demonstration of B cell maturation in X-linked immunodeficient mice by simultaneous three-color immunofluorescence. Nature. 1983;306:270–272. doi: 10.1038/306270a0. [DOI] [PubMed] [Google Scholar]
- Khan V., Alt F.W., Gerstein R.M., Malynn B.A., Larsson I., Rathbun G., Davidson L., Muller S., Kantor A.B., Hersenberg L.A. Defective B cell development and function in Btk deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- Oka Y., Rolink A.G., Andersson J., Kananka M., Uchida Y., Yasui T., Kishimoto T., Kikutani H., Melchers F. Profound reduction of mature B cell numbers, reactivities and serum immunoglobulin levels in mice which simultaneously carry the XID and CD40-deficiency genes. Int. Immunol. 1996;8:1675–1685. doi: 10.1093/intimm/8.11.1675. [DOI] [PubMed] [Google Scholar]
- Rolink A., Brocker T., Bluethmann H., Kosco-Vilbois M.H., Andersson J., Melchers F. Mutations affecting either generation or survival of cells influence the pool size of mature B cells. Immunity. 1999;10:619–628. doi: 10.1016/s1074-7613(00)80061-8. [DOI] [PubMed] [Google Scholar]
- Fruman D., Snapper S.B., Yballe C.M., Davidson L., Yu J.Y., Alt F.W., Cantley L.C. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85a. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Terauchi Y., Fujiwara M., Aizawa S., Yazaki Y., Kadowaki T., Koyasu S. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science. 1999;283:390–392. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- O'Keefe T., Williams G.T., Davies S.L., Neuberger M.S. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- Sato S., Miller A.S., Inaoki M., Bock C.B., Jansen P.J., Tang M.L.K., Tedder T.F. CD22 is both a positive and a negative regulator of B lymphocyte antigen receptor signal transductionaltered signaling in CD22-deficient mice. Immunity. 1996;5:551–562. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- Engel P., Zhou L.J., Ord D.C., Sato S., Koller B., Tedder T.F. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39–50. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- Rickert R.C., Rajewsky K., Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- Hibbs M., Tarlinton D.M., Armes J., Grail D., Hodgson G., Maglitto R., Stacker S.A., Dunn A.R. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Kishihara K., Penninger J., Wallace V.A., Kundig T.M., Kawai K., Wakeham A., Timms E., Pfeffer K., Ohashi P.S., Thomas M.L. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- Norvell A., Mandik L., Monroe J.G. Engagement of the antigen-receptor on immature murine B lymphocytes results in death by apoptosis. J. Immunol. 1995;154:4404–4413. [PubMed] [Google Scholar]
- Sandel P.C., Monroe J.G. Negative selection of immature B cells by receptor editing or deletion is determined by site of antigen encounter. Immunity. 1999;10:289–299. doi: 10.1016/s1074-7613(00)80029-1. [DOI] [PubMed] [Google Scholar]