Abstract
Infection with the protozoan parasite Leishmania amazonensis can cause diverse clinical forms of leishmaniasis. Immunization with purified P4 nuclease protein has been shown to elicit a protective response in mice challenged with L. amazonensis and L. pifanoi. To explore the potential of a DNA-based vaccine, we tested the L. amazonensis gene encoding P4 nuclease as well as adjuvant constructs encoding murine interleukin-12 (IL-12) and L. amazonensis HSP70. Susceptible BALB/c mice were immunized with the DNA encoding P4 alone, P4/IL-12, or P4/HSP70 prior to challenge with L. amazonensis promastigotes. Mice given P4/IL-12 exhibited no lesion development and had a 3- to 4-log reduction in tissue parasite burdens compared to controls. This protection corresponded to significant increases in gamma interferon and tumor necrosis factor alpha production and a reduction in parasite-specific immunoglobulin G1, suggesting an enhancement in Th1 responses. Moreover, we immunized mice with the L. amazonensis vaccines to determine if this vaccine regimen could provide cross-protection against a genetically diverse species, L. major. While the P4/HSP70 vaccine led to self-healing lesions, the P4/IL-12 vaccine provided negligible protection against L. major infection. This is the first report of successful use of a DNA vaccine to induce protection against L. amazonensis infection. Additionally, our results indicate that different vaccine combinations, including DNA encoding P4, HSP70, or IL-12, can provide significant protection against both Old World and New World cutaneous leishmaniasis.
Leishmaniasis is widespread in over 88 countries. It is estimated that 350 million people live in areas where it is endemic, with 12 million people infected, and that approximately 1.5 million new cases occur each year (65). Current control measures rely on chemotherapy, vector control, and control of reservoir host populations. The chemotherapeutic agents used presently are inadequate, expensive, and often toxic. Due to the existing problems associated with leishmaniasis and the high incidence of infection, the World Health Organization has made it a major goal to develop an effective and affordable vaccine against leishmaniasis.
The different Leishmania species cause a broad spectrum of human diseases. L. amazonensis is known to be associated with cutaneous, diffuse cutaneous, and visceral leishmaniasis in South and Central America. The pathological mechanisms responsible for the variable outcomes of infection in humans are not fully understood; however, it is generally agreed that long-lasting immunity against reinfection can be developed in cutaneous leishmaniasis patients. Several vaccination trials have demonstrated that killed L. amazonensis can induce protection from natural infection (3, 18, 42, 46, 63). However, the efficacy of heat-killed vaccines against Leishmania has been extremely low (36) or highly variable within the same study (47, 55). Live parasites have been used as a vaccine strategy, and although they are highly effective in inducing immunity (24), this strategy has been virtually abandoned due to safety issues associated with injecting virulent organisms.
Leishmania parasites are dimorphic and cycle between promastigotes, which reside extracellularly in the sandfly midgut, and amastigotes, which exist intracellularly in the phagolysosomes of macrophages. This complex life cycle of Leishmania parasites and the antigenic heterogeneity among the different species have greatly impeded vaccine development through conventional immunological methods. DNA vaccination is a relatively new technology that is especially promising when applied to intracellular pathogens, since they can elicit cellular responses which are necessary to clear the infection. Furthermore, DNA vaccines are attractive because they are flexible and low in cost, ensure proper folding of the protein, produce the antigen over a period of time for constant immune stimulation (62), and have the potential for long-lasting immunity (27).
Although DNA vaccination has been pursued for other Leishmania species (6, 9, 19), it has not been reported for protection against L. amazonensis. For our DNA vaccination studies, we decided to focus on nuclease P4 (34). Previously, purified P4 protein has been shown to protect against L. pifanoi infection and against cross-species challenge with L. amazonensis in BALB/c mice (59). In the present study, we tested the efficacy of DNA immunization with P4 along with the adjuvants HSP70 and interleukin-12 (IL-12) in eliciting protective immunity in BALB/c mice against L. amazonensis and L. major. Interestingly, we found that different vaccine regimens (P4/IL-12 versus P4/HSP70) displayed different efficacies in mice challenged with L. amazonensis as opposed to L. major. Mice that received the P4/IL-12 vaccine were completely protected against infection with L. amazonensis but not against L. major, while mice that received the P4/HSP70 vaccine were protected against L. major and only partially protected against L. amazonensis. This study indicates that although DNA vaccination against L. amazonensis is a promising method of protection, different immunization regimens need to be optimally formulated for New World and Old World cutaneous leishmaniases.
MATERIALS AND METHODS
Mice.
Female BALB/c mice were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.). All mice were maintained under specific-pathogen-free conditions and were at 4 weeks of age when immunizations were initiated (4). Animal protocols were approved by the Animal Care and Use Committee of the University of Texas Medical Branch (Galveston, Tex.).
Parasite culture and antigen preparation.
L. amazonensis (MHOM/BR/77/LTB0016) and L. major (MRHO/SU/P/LV39) parasites were maintained by regular passage through BALB/c mice. Promastigotes were cultured at 23°C in 20% fetal bovine serum-supplemented Schneider's Drosophila medium (Invitrogen Corporation, Carlsbad, Calif.). Stationary-phase promastigotes of less than three in vitro passages were used for animal infection. L. amazonensis metacyclics were purified by negative selection with the 3A1 monoclonal antibody (gift of David Sacks, National Institute of Allergy and Infectious Diseases, Bethesda, Md.), according to a previous report (16). L. major metacyclics were purified by peanut agglutinin (53). L. amazonensis amastigotes were cultured at 32°C in 20% fetal bovine serum-supplemented complete Schneider's medium (pH 5.0), according to our previous report (30). To prepare promastigote and amastigote lysates, parasites were suspended in phosphate-buffered saline (PBS) and subjected to three freeze-thaw cycles and a 15-min sonication in an ice bath prior to storage at −70°C.
DNA constructs.
The full-length open reading frame (1 kb) of L. amazonensis P4 was amplified by reverse transcription-PCR. The amastigote cDNA was generated from amastigote total RNA (isolated by Tri-reagent; Sigma, St. Louis, Mo.) and oligo(dT) primers at 47°C with Superscript II (Invitrogen). The full-length P4 cDNA was amplified with P4-specific primers, based on the L. pifanoi sequence (GenBank accession number AF057351) (34): sense, 5′-CGAAGCTTGCCCATCATGCCTGC-3′, and antisense, 5′-GGCTGTTGGCCACCTCGAGTTACG-3′. The italic letters represent the first Met and the stop codon, respectively. Cycling conditions were 95°C for 2 min, followed by 30 cycles at 94°C for 1 min, 50°C for 30 s, and 72°C for 1 min. PCR products of the expected size were ligated into vector pCR (Invitrogen) provided in a TA cloning kit and then transformed into Escherichia coli. Positive clones were sequenced at the University of Texas Medical Branch Protein Chemistry Laboratory with a 373XL automated DNA sequencer (ABI Prism PE Biosystems). The full-length gene was subcloned into pcDNA3.1/Hygro(-) (Invitrogen) at the BamHI and HindIII restriction sites for DNA vaccination.
The gene encoding HSP70 of L. amazonensis (open reading frame = 2 kb) (a generous gift from P. Langer, University of Wyoming; GenBank accession number L14604) was subcloned from pBluescript into pcDNA3.1/Hygro(-) at the XhoI and BamHI restriction sites. The IL-12-encoding plasmid (generously provided by Hua Yu, Moffitt Cancer Center, Tampa, Fla.) is a modified pVR1012x/s plasmid (Vical, Inc., San Diego, Calif.) that encodes the two subunits of murine IL-12, p40 and p35. Transcription of the two subunits is under separate cytomegalovirus promoter regions and bovine growth hormone polyadenylation signals.
Large-scale DNA purification of each plasmid was done by two cycles of CsCl gradient purification. Each sample was tested for lipopolysaccharide contamination by the Limulus amebocyte lysate test (BioWhittaker, Walkersville, Md.). All samples had <3 endotoxin units per 100 μg of DNA. DNA purity was also determined by measuring the spectrophotometric 260/280 absorbance ratio; all samples had a ratio of 1.9 or higher.
To generate the recombinant P4 protein, the entire open reading frame was subcloned into the BamHI and HindIII sites of a pET32a vector (Novagen). E. coli BL21 (Stratagene, La Jolla, Calif.) transformed with the pET32a-P4 vector was grown at 22°C for 6 h in the presence of 1 mM isopropylthiogalactopyranoside (IPTG) prior to purification of the His-tagged P4 protein via Ni2+-charged agarose (Sigma).
Expression in Cos-7 cells.
Cos-7 cells were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum. Approximately 104 cells were seeded in each well of four-chamber glass slides. When the cells reached 75% confluency, they were transfected with 0.1 μg of the appropriate plasmid DNA with Lipofectamine (Invitrogen) and Opti-MEM reduced-serum medium (Invitrogen). The pcDNA3.1/Hygro(-) vector without any insert was used as an experimental control, in addition to cells that were not transfected. For detection of HSP70 expression, cells were fixed in a 1:1 methanol-acetone solution for 10 min, washed, and incubated with a polyclonal anti-L. infantum HSP70 (1:350; a generous gift of Jose Requena, Universidad Autonoma de Madrid, Madrid, Spain) for 2 h at 37°C. The cells were then washed and incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (IgG) (1:64; Sigma) for 1 h at 37°C. Slides were mounted with Vectashield mounting buffer (Vector, Burlingame, Calif.) and viewed on a Zeiss fluorescent microscope. For staining, normal rabbit serum was used as a control.
For the detection of P4 expression, cells were fixed with 100% acetone for 5 min, rehydrated with 0.05% Tween-PBS, and then incubated with anti-P4 monoclonal antibody (1:200) for 30 min at room temperature. The P4 monoclonal antibody (a generous gift from Diane McMahon-Pratt, Yale University School of Medicine, New Haven, Conn.) was reported previously (50). After depletion of endogenous peroxidase activity with 1% H2O2-0.1% sodium azide in 0.05% Tween-PBS, cells were incubated with the horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (1:200) for 30 min at room temperature. Color was developed with 3,3′-diaminobenzidine (Vector) according to the standard protocol. Normal mouse serum was used as a staining control, and other experimental controls were similar to those in the HSP70 experiments described above.
Immunization and challenge.
Mice (four to eight per group) were immunized in five locations with a total of 100 μg of DNA per mouse: four injections in both sides of the inner and outer thigh muscles of the hind legs (≈50 μl/site), and one subcutaneous injection in the left hind foot (≈5 μl/site). The mice were boosted twice at 3-week intervals and then challenged 3 weeks after the last immunization with 2 × 105 metacyclic promastigotes in the right hind foot. A group of age- and sex-matched mice were included as infection controls. Additionally, mice injected with the empty vector only were also included as controls. Lesion development was monitored with a digital caliper (Control Company, Friendswood, Tex.).
Evaluation.
Tissue parasite burdens were measured via a limiting dilution assay as previously described (58). To examine parasite-specific cytokine production, draining lymph node cells were harvested and plated at 5 × 106/ml/well for TNF-α or 2 × 106/ml/well for all other cytokines. Cells were stimulated with concanavalin A (5 μg/ml), P4 recombinant protein (5 μg/ml), or parasite lysates (equivalent to 4 × 106 amastigotes/ml). Supernatants were harvested at 24 to 72 h to determine the levels of IL-2, IL-4, IL-10, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) by sandwich enzyme-linked immunosorbent assay (ELISA) with mouse cytokine-specific OptEIA kits and their standard protocols (BD Biosciences).
To assess parasite-specific antibody titers, Immulon 4 microplates (Dynatech Labs) were coated with amastigote lysate (50 μg/ml) overnight at 4°C. After blocking, plates were incubated with individual mouse serum samples (1:250 to 1:2,000) for 1 h at 37°C. Next, plates were incubated with goat anti-mouse IgG1 or IgG2a (1:1,000; Sigma) for 1 h at 37°C and then incubated with horseradish peroxidase-conjugated rabbit anti-goat IgG (1:1,000; Pierce, Rockford, Ill.). Color was developed with ImmunoPure ABTS tablets and hydrogen peroxide (Pierce) as per the standard protocol.
Statistical analysis.
Data are presented as the means ± standard deviations and were considered significant at P ≤ 0.05. Data were evaluated for statistical significance by one-way analysis of variance. When overall effects were detected, differences among groups were further evaluated for significance with Tukey's honestly significantly different procedure. Data transformations were applied to the original measurements prior to the one-way analysis of variance in order to achieve reasonable normality and similar interanimal variability within groups. Square root transformations were applied to lesion measurement data, while logarithmic transformations were applied to parasite burden and cytokine data. Although significance was determined based on the analysis of transformed measures, results are summarized in the original units.
Nucleotide sequence accession number.
The L. amazonensis P4 gene was reported to GenBank and given accession number AY219923.
RESULTS
DNA-vaccinated mice are protected against challenge with L. amazonensis.
For our DNA vaccine study, we focused on the P4 antigen, which is a differentially regulated nuclease originally isolated from L. pifanoi amastigotes (34). Immunization with affinity column-purified P4 and a killed bacterial adjuvant provided partial to complete protection for BALB/c mice against subsequent infections with L. pifanoi and L. amazonensis (58, 59). L. pifanoi P4 protein was also found to elicit a T-cell response in patients infected with L. braziliensis, demonstrating cross-reactivity between Leishmania species (17).
Subsequent to confirmation of proper expression of our vaccine components (Fig. 1), we divided mice into the following five groups to determine the vaccine potential of L. amazonensis P4 and HSP70 genes: infection control (no immunization); vector control (pcDNA3.1); P4 alone; P4/HSP70; and P4/IL-12. As shown in Fig. 2A, the vector control group exhibited a pace of lesion progression similar to that of the infection control group. Mice immunized with P4 alone or P4/HSP70 had a delayed onset of lesion development and significantly smaller lesions than the infection control group at 11 weeks postinfection (P < 0.05 and P < 0.01, respectively). The protective effect of the vaccine was even more striking in mice that received P4- and IL-12-encoding constructs. These animals showed no sign of lesion development in three independent experiments (P < 0.01).
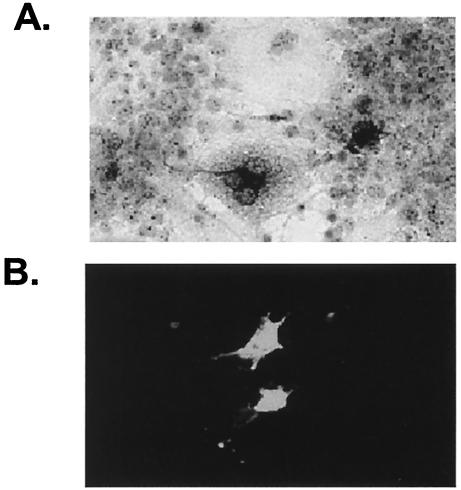
FIG. 1.
Expression of L. amazonensis P4 and HSP70 proteins. Cos-7 cells were transfected with the pcDNA3.1-P4 (A) and pcDNA3.1-HSP70 (B) constructs. After the cells were fixed, expression of the desired proteins in the cytoplasm was visualized by immunohistochemical (A) and immunofluorescent (B) staining. Control mouse and rabbit sera produced background staining (data not shown). Magnification, ×400.
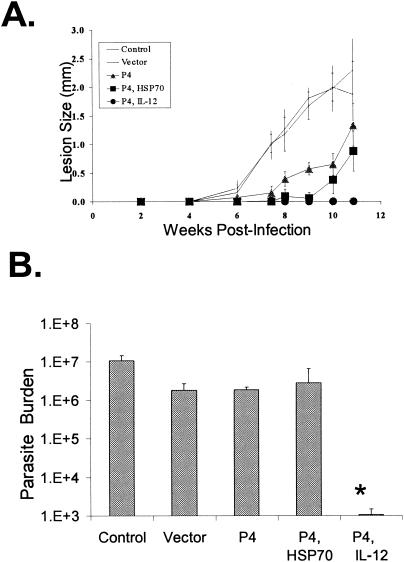
FIG. 2.
Protection of BALB/c mice against L. amazonensis infection. Mice (four to five per group) were left untreated or immunized with the indicated DNA constructs (100 μg total) three times at 3-week intervals prior to challenge with 2 × 105 metacyclic promastigotes. (A) Lesion development was monitored by measurement of footpad thickness and is expressed as the mean difference between the infected and uninfected footpad for each group. Shown is a representative of three independent repeats with similar results. (B) Parasite loads in each infected foot were determined at 11 weeks postinfection and are expressed as means ± standard deviations. This experiment is representative of two independent repeats (*, P < 0.01 in comparison to the infection control).
Parasite burdens (Fig. 2B) for each group were measured to ensure that lesion size corresponded with protection rather than variable levels of inflammation. Mice that received the P4/IL-12 vaccine consistently had much smaller parasite burdens compared to both vector and infection controls (P < 0.01). The infection control group uniformly had the highest numbers of parasites per foot. Although the P4/HSP70 group had delayed lesion development, and these lesions were significantly different in size from those of both control groups (P < 0.01), the parasite burden by the end of the 11-week experiment was only significantly different compared to the infection control group (P < 0.01).
As shown in Fig. 2B, mice given the vector or P4 alone had slightly lower numbers of parasites than the infection control (P < 0.01), but these groups were not significantly different from each other. In separate experiments with a 10-week termination time point, parasite burdens correlated well with the level of protection determined by lesion development; mice that received the P4/HSP70 and P4/IL-12 vaccines had significantly lower parasite burdens than both control groups at this time point (data not shown). These studies indicate significant protection in mice given the P4/IL-12 vaccine, while an appreciable level of protection was seen in mice given P4/HSP70.
To test the protective effects of IL-12 in the absence of parasite-specific antigens, we also included a control group that was vaccinated with the vector construct and the IL-12-encoding plasmid (data not shown). The mice in this control group had slight reductions in parasite burdens, similar to what was reported here for the vector-only group (Fig. 2B). Additionally, trial experiments included coinjection of plasmids encoding the L. amazonensis HSP70 and IL-12 genes. Mice given this vaccine had lesions that were comparable in size to those of the infection control group, and the number of parasites was similar to that in the vector/IL-12 group (data not shown). It appears that this HSP70/IL-12 vaccine regimen may not be sufficient to activate the parasite-specific T cells necessary for the control of disease.
Cytokine production in vaccine groups following L. amazonensis infection.
A Th1 response has previously been associated with protection from L. amazonensis infection (37). To determine if the degree of protection provided by the various vaccine regimens (seen in Fig. 1 and 2) correlated with the expression of a specific cytokine profile, spleen and draining lymph node cells were harvested at 3 days and 11 weeks postinfection and exposed to parasite antigen to evaluate cytokine profiles. Supernatants were harvested at 24 h for IL-2 and TNF-α and 72 h for IL-4, IL-10, and IFN-γ measurement. As shown in Fig. 3, draining lymph node cells produced high levels of IFN-γ in all of the vaccine groups, with the highest levels in P4/HSP70- and P4/IL-12-vaccinated groups, compared with levels in both the infection and vector control groups (P < 0.01). The levels of TNF-α production in stimulated splenocytes also correlated with protection. There was a significant increase in TNF-α production in P4/HSP70 and P4/IL-12 groups compared with both of the control groups (P < 0.01). Furthermore, mice that were not protected (control groups) or had delayed lesion development (P4, P4/HSP70) displayed mixed Th1/Th2 cytokine responses at 11 weeks postinfection, as determined by IL-10 and IFN-γ production in draining lymph node cells. However, the P4/IL-12 group had significantly lower IL-10 production compared with the infection control (P < 0.01). Protection did not correspond to IL-2 or IL-4 levels (data not shown).
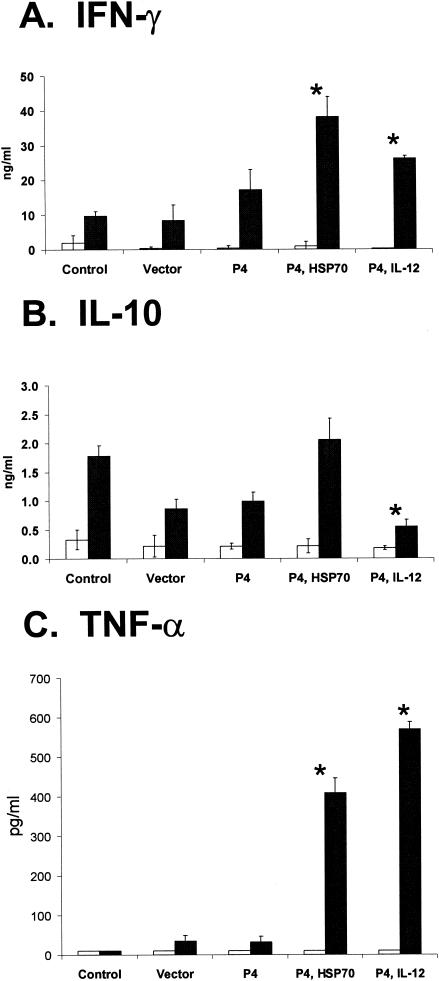
FIG. 3.
Cytokine production in the different vaccine groups. Mice were immunized and challenged as in Fig. 1. At 11 weeks postinfection, draining lymph node and spleen cells were collected and cultured in medium (open bars) or in the presence of amastigote lysate (solid bars). Culture supernatants were harvested at 24 to 72 h to measure the concentrations of IFN-γ, IL-10, and TNF-α by sandwich ELISA. For each cytokine, the mean concentration ± standard deviation is shown for all vaccine groups. These results are representative of two independent experiments (*, P < 0.01 in comparison to both control groups).
Additionally, we measured cytokine responses at 3 days postinfection. While there was no significant correlation with IL-10 production, the groups with the highest IFN-γ responses were those with the greatest levels of protection (data not shown). Together, these studies indicate that the well-protected group (P4/IL-12) produced the highest levels of IFN-γ and TNF-α but low levels of IL-10, while the partially protected groups (P4 only and P4/HSP70) exhibited mixed Th1/Th2 responses, as represented by the production of both IFN-γ and IL-10.
Serum IgG profiles.
It has been reported that the levels of IgG1 antibodies correlate with an overall Th2 profile, while IgG2a antibodies indicate an overall Th1 profile (60). To verify the cytokine responses and to understand the possible mechanism of protection, we measured serum levels of parasite-specific IgG isotypes (Fig. 4) as well as total IgG (data not shown) by direct ELISA. Naïve mouse sera were included for comparison. Interestingly, mice given P4/IL-12 had very low levels of antibody production that were comparable to those of naïve mice. Mice vaccinated with P4/IL-12 had significantly fewer IgG1 antibodies than both the control groups (P < 0.01). Control mice that were not protected at all had high levels of IgG1, while mice given P4 and P4/HSP70 had significantly lower levels of IgG1 antibodies than the infection control (P < 0.01). These results suggest not only a correlation between a robust humoral response and disease, but also a relationship between the absence of a Th2 response and protection in L. amazonensis-infected mice. The higher antibody production in the unprotected mice may also be significant in that high antibody titers may mediate parasite entry into the macrophages via the Fc receptor and thereby exacerbate the disease (11, 38, 40).
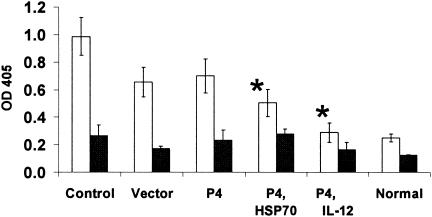
FIG. 4.
Parasite-specific serum IgG isotype profile. Mice were immunized and challenged as in Fig. 1. At 11 weeks postinfection, serum samples were collected from each group and measured for parasite-specific antibodies by direct ELISA. Open and solid bars represent IgG1 and IgG2a, respectively. For both IgG isotypes, the mean ± standard deviation is shown for each group. Normal mouse sera were included for comparison. These results are representative of two independent experiments (*, P < 0.01 in comparison to the control groups).
Lesion development and parasite burdens in L. major-challenged BALB/c mice.
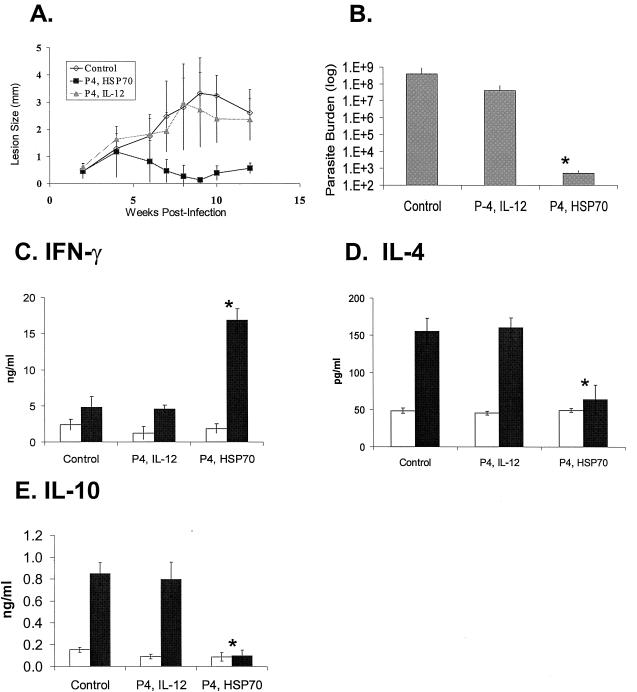
The above protection studies were conducted with genes encoding L. amazonensis proteins following challenge with homologous parasite species. We next assessed possible cross-protection with the same DNA vaccines against L. major, an Old World species. Following the same immunization protocol, we challenged BALB/c mice with 2 × 105 L. major metacyclic promastigotes. Mice given P4/IL-12 developed lesions that were comparable in size to those of the infection control group (Fig. 5A) and contained high numbers of parasites (Fig. 5B). However, in a repeat experiment, mice given P4/IL-12 had smaller lesions and lower parasite burdens, but there was no significant difference from the vector control group (data not shown). Curiously, mice given P4/HSP70 did show signs of lesion development in the first 6 weeks, but lesions appeared to be self-healing. At 10 weeks, these mice had significantly smaller lesions (P < 0.01) and significantly lower parasite burdens than the infection control mice (P < 0.01).
FIG. 5.
Protection of BALB/c mice against L. major infection. Mice (five per group) were immunized with the indicated DNA (100 μg total) three times at 3-week intervals prior to challenge with 2 × 105 metacyclic promastigotes. (A) Lesion development was monitored by measurements of footpad thickness and is shown as the mean difference between the infected and uninfected foot for each group. (B) Tissue parasite loads were assessed by a limiting dilution assay at 10 weeks postinfection, and results are expressed as the mean ± standard deviation for each group. The difference between the P4/HSP70 group and the other two groups was significant (*, P < 0.01). Cytokine levels for IFN-γ (C), IL-4 (D), and IL-10 (E) were determined at 10 weeks postinfection. Draining lymph node cells were collected and cultured in the presence of medium (open bars) or parasite lysate (solid bars). Culture supernatants were harvested at 72 h to measure concentrations of cytokines by sandwich ELISA. For each cytokine, the mean ± standard deviation is shown for each group. The differences between the P4/HSP70 and the other two groups were significant (*, P < 0.01) for all three cytokines. Results are representative of two independent experiments.
It is well accepted that mice susceptible to L. major produce high levels of IL-4 and IL-10 but very little IFN-γ (29). We found that the self-healing pattern seen in mice given P4/HSP70 corresponded with prominent Th1 cytokine responses in lymph node cells (Fig. 5C to E), which was characterized by high levels of IFN-γ compared to the other two groups (P < 0.01) but background levels of IL-4 and IL-10. These results suggest that cross-species protection against Leishmania infection can be induced by a DNA vaccine and that vaccine-protected mice show Th1-dominant responses, while diseased mice show Th2-biased responses.
DISCUSSION
This study describes a DNA vaccine effective in protecting BALB/c mice against cutaneous leishmaniasis. The L. amazonensis P4 gene in conjunction with the IL-12 gene proved unambiguously effective in preventing lesion development after L. amazonensis challenge. While many Leishmania genes have been tested by DNA immunization against different Leishmania species, with varying success (6, 9, 19, 22, 45, 51, 54, 56, 66), information on DNA vaccination against L. amazonensis has been exiguous. To our knowledge, this is the first report of a DNA vaccine that is capable of protecting against this species. Additionally, we have provided evidence that DNA vaccines have the potential to protect against cutaneous leishmaniasis caused by genetically diverse species.
The underlying mechanisms of pathogenesis for L. amazonensis are seemingly complex, but protective mechanisms are considered to involve Th1 responses (5, 37, 59). The superior protection against L. amazonensis provided by the P4/IL-12 DNA vaccine correlated nicely with a robust IFN-γ response in the absence of a considerable IL-10 response (Fig. 3). Although the partially protected P4/HSP70 vaccine group had high levels of IFN-γ as well, this was in the presence of a very substantial IL-10 response. Given this, there is an evident correlation between protection and the cytokine profile when IFN-γ and IL-10 are compared. Additionally, the level of protection corresponded with increasing amounts of TNF-α, which is also associated with a Th1 response. Conceivably, TNF-α production in conjunction with IFN-γ provides the optimal environment for macrophage activation and subsequent killing of the parasite (13-15).
Overall, the cytokine data presented herein correspond with previous reports of mixed Th1/Th2 responses being associated with the pathogenesis of L. amazonensis infection in BALB/c mice (32) and with the notion that production of IFN-γ and TNF-α is associated with protection (5, 12, 37, 59). The cell types responsible for the production of these cytokines remain unknown. However, a recent report on L. amazonensis-challenged C57BL/6 mice vaccinated with P-8 antigen cited both CD4+ and CD8+ T cells that produced IFN-γ as correlating with protection (12). Further investigations are needed to define the role of CD4+- and CD8+-mediated T-cell responses in the vaccine-induced protection reported here for L. amazonensis-challenged BALB/c mice, as well as the events underlying these responses.
For L. major, protection is known to be associated with the activation of Th1 cells that produce IFN-γ but not IL-4 (39); however, protective mechanisms in L. amazonensis are unclear. While IL-4 plays a crucial role in the pathogenesis of L. major in BALB/c mice (7), it is not essential for the pathogenesis in L. amazonensis infection (2). During L. amazonensis infection, BALB/c mice display a mixed profile of Th1 and Th2 responses (32), similar to those seen in our vaccine groups that were not protected or only partially protected (Fig. 3). A mixed Th1/Th2 response has also been implicated for the failure in an L. major vaccine study (57). The need for vaccines to induce a Th1-dominant response for these species of Leishmania appears consequential for protection, and it is notable that different P4 DNA vaccines have the capacity to induce such reactions.
At present, it is unclear how this balanced Th1/Th2 response is maintained during L. amazonensis infection. Recent studies in our laboratory indicate that impaired production of inflammatory mediators, especially during the first few days of infection, contributes to the pathogenesis of L. amazonensis infection. We have found that when L. major-infected (healing model) and L. amazonensis-infected (disease model) C57BL/6 mice are compared, foot tissues and draining lymph node cells express significantly lower levels of both inflammatory cytokines and CC chemokines in the disease model (33). Inherent differences between these two species of Leishmania are also indicated by the contrasting responses of IFN-γ-treated macrophages to infection with L. major (23) and L. amazonensis (H. Qi, J. Ji, and L. Soong, submitted for publication) amastigotes. Upon IFN-γ treatment, macrophages are activated to kill L. major parasites; however, IFN-γ-treated macrophages are unable to kill L. amazonensis amastigotes, and replication of these parasites is even abetted. Such obvious fundamental differences in these parasites as well as the diverse immune responses to the parasite create interesting levels of complexity that need to be considered for cross-species vaccine design.
A vaccine that could offer cross-protection against diverse Leishmania species would be the most beneficial. DNA vaccination has proven a successful vehicle for protection against L. major (6, 26, 45) but has yet to be fine-tuned for other Leishmania species (19, 20, 22, 44). Vaccines that invoke Th1 cytokine responses have a greater likelihood of success, and DNA vaccination by design has inherent Th-stimulatory effects due to the presence of CpG motifs in the plasmid DNA. Additionally, gene adjuvants can be used alone or in combination to enhance or develop a desired immune response.
IL-12 plays a pivotal role in the development of a Th1 response (43) and has been used as an effective adjuvant in Leishmania vaccine studies employing both proteins (10, 35, 48) and DNA (1, 6, 25). Similar to IL-12, heat shock proteins have been reported to stimulate Th1-type responses and have remarkable immunostimulatory properties (8, 21, 52, 64). Heat shock proteins in general are enticing vaccine candidates because they have been used successfully in vaccines for other microorganisms (41, 61) and are known to be highly conserved among diverse Leishmania species (e.g., L. amazonensis HSP70 is >90% homologous to L. major HSP70). However, the members of the heat shock protein families have only begun to be exploited as vaccine candidates for Leishmania (6, 21, 31). An ideal Leishmania DNA vaccine should confer protection against at least several Leishmania species, most likely by using a combination of several parasite genes and an appropriate adjuvant. Whether Leishmania antigens such as heat shock proteins can be used in vaccine design to replace more commonly investigated adjuvants remains to be elucidated.
Although the P4/IL-12 vaccine provided unequivocal protection against L. amazonensis challenge (Fig. 2), it did not alter the course of disease in L. major-challenged mice (Fig. 5A). In an attempt to understand this lack of protection, we inspected the P4 protein sequence and detected a 70% overall identity between L. major and L. amazonensis (GenBank accession numbers AY079097 and AY219923, respectively). Protein alignment revealed four regions (each about 16 to 27 amino acids in length) that are highly variable, showing ≈50 to 67% homology. Of note, two of these regions corresponded with the area of the two computer-predicted major histocompatibility complex class II epitopes previously reported by Haberer et al. for L. pifanoi (28). The L. pifanoi and L. amazonensis P4 proteins have an overall homology of 88%, with less than 25% dissimilarity within these four highly variable regions. It is therefore possible that the antigenic regions of the L. amazonensis P4 protein may not be homologous enough with those of L. major to elicit protective immunity in mice immunized with the P4/IL-12 vaccine and challenged with L. major.
At this stage we cannot exclude an alternative possibility, that our immunization regimen for P4/IL-12 was not optimal for L. major challenge. It has been reported for the PSA-2 DNA vaccine that IL-12 had paradoxical effects: while DNA vaccination with either PSA-2 or the IL-12 gene alone protected mice (57), coadministration of the PSA-2 and IL-12 genes resulted in inferior protection compared to either gene alone (49). Delayed administration of IL-12 markedly enhanced the efficacy of the vaccine (49), suggesting that timing of adjuvant administration may be a critical consideration in vaccine design.
We unexpectedly found that mice immunized with the P4/HSP70 vaccine were significantly protected against L. major, showing a self-healing phenotype (Fig. 5A and B). Protection in these mice correlated well with the enhanced activation of Th1 cells and reduced production of IL-4 and IL-10 compared to infection control mice (Fig. 5C to E). Although the specific phenomenon underlying the induction of strong Th1-type responses in this group of mice is not known, it appears that HSP70 may play a major role in the protection against L. major. At this stage, it is unclear whether HSP70 serves as an adjuvant, antigen, or protein chaperone for antigen presentation and which of these mechanisms contribute to protection for the P4/HSP70 vaccine in L. major infection. The elucidation of this mechanism might also reveal why HSP70 seemingly plays a major role in protection against L. major but not L. amazonensis.
In summary, this study indicates that the P4 and IL-12 genes can provide potent immune protection against challenge with L. amazonensis, but they are insufficient in protection against L. major challenge. On the other hand, while the P4/HSP70 vaccine only delayed lesion development in L. amazonensis-infected mice, it led to a self-healing phenotype in L. major-infected mice. Thus, it appears that P4 and HSP70 are candidates that could potentially be useful in DNA-based vaccines for both New and Old World Leishmania species.
Acknowledgments
This study was supported in part by James W. McLaughlin Fellowships to K.C. and J.J., the NIAID T32 “Emerging and Tropical Infectious Diseases” training grant to K.C., as well as NIH grant AI43003 and the Sealy Memorial Endowment Fund to L.S.
We thank Nisha Garg, Hai Qi, Jiaren Sun, and Elisa Fleming for valuable scientific discussion, Elbert Whorton for guidance on statistical analysis, and Mardelle Susman for comments on the manuscript.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aebischer, T., M. Wolfram, S. I. Patzer, T. Ilg, M. Wiese, and P. Overath. 2000. Subunit vaccination of mice against New World cutaneous leishmaniasis: comparison of three proteins expressed in amastigotes and six adjuvants. Infect. Immun. 68:1328-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonso, L. C., and P. Scott. 1993. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect. Immun. 61:2952-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armijos, R. X., M. M. Weigel, H. Aviles, R. Maldonado, and J. Racines. 1998. Field trial of a vaccine against New World cutaneous leishmaniasis in an at-risk child population: safety, immunogenicity, and efficacy during the first 12 months of follow-up. J. Infect. Dis. 177:1352-1357. [DOI] [PubMed] [Google Scholar]
- 4.Barry, M. A., and S. A. Johnston. 1997. Biological features of genetic immunization. Vaccine 15:788-791. [DOI] [PubMed] [Google Scholar]
- 5.Beyrodt, C. G., A. R. Pinto, E. Freymuller, and C. L. Barbieri. 1997. Characterization of an antigen from Leishmania amazonensis amastigotes able to elicit protective responses in a murine model. Infect. Immun. 65:2052-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos-Neto, A., J. R. Webb, K. Greeson, R. N. Coler, Y. A. Skeiky, and S. G. Reed. 2002. Vaccination with plasmid DNA encoding TSA/LmSTI1 leishmanial fusion proteins confers protection against Leishmania major infection in susceptible BALB/c mice. Infect. Immun. 70:2828-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatelain, R., K. Varkila, and R. L. Coffman. 1992. IL-4 induces a Th2 response in Leishmania major-infected mice. J. Immunol. 148:1182-1187. [PubMed] [Google Scholar]
- 8.Chopra, U., H. Vohra, S. Chhibber, N. K. Ganguly, and S. Sharma. 1997. TH1 pattern of cytokine secretion by splenic cells from pyelonephritic mice after in-vitro stimulation with HSP-65 of Escherichia coli. J. Med. Microbiol. 46:139-144. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, C. B., L. Jorgensen, A. T. Jensen, S. Gasim, M. Chen, A. Kharazmi, T. G. Theander, and K. Andresen. 2000. Molecular characterization of a Leishmania donovani cDNA clone with similarity to human 20S proteasome a-type subunit. Biochim. Biophys. Acta 1500:77-87. [DOI] [PubMed] [Google Scholar]
- 10.Coler, R. N., Y. A. Skeiky, K. Bernards, K. Greeson, D. Carter, C. D. Cornellison, F. Modabber, A. Campos-Neto, and S. G. Reed. 2002. Immunization with a polyprotein vaccine consisting of the T-cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infect. Immun. 70:4215-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colmenares, M., S. L. Constant, P. E. Kima, and D. McMahon-Pratt. 2002. Leishmania pifanoi pathogenesis: selective lack of a local cutaneous response in the absence of circulating antibody. Infect. Immun. 70:6597-6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colmenares, M., P. E. Kima, E. Samoff, L. Soong, and D. McMahon-Pratt. 2003. Perforin and gamma interferon are critical CD8+ T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection. Infect. Immun. 71:3172-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corradin, S. B., Y. Buchmuller-Rouiller, and J. Mauel. 1991. Phagocytosis enhances murine macrophage activation by interferon-gamma and tumor necrosis factor-alpha. Eur. J. Immunol. 21:2553-2558. [DOI] [PubMed] [Google Scholar]
- 14.Corradin, S. B., Y. Buchmuller-Rouiller, J. Smith, L. Suardet, and J. Mauel. 1993. Transforming growth factor beta 1 regulation of macrophage activation depends on the triggering stimulus. J. Leukoc. Biol. 54:423-429. [DOI] [PubMed] [Google Scholar]
- 15.Corradin, S. B., and J. Mauel. 1991. Phagocytosis of Leishmania enhances macrophage activation by IFN-gamma and lipopolysaccharide. J. Immunol. 146:279-285. [PubMed] [Google Scholar]
- 16.Courret, N., E. Prina, E. Mougneau, E. M. Saraiva, D. L. Sacks, N. Glaichenhaus, and J. C. Antoine. 1999. Presentation of the Leishmania antigen LACK by infected macrophages is dependent upon the virulence of the phagocytosed parasites. Eur. J. Immunol. 29:762-773. [DOI] [PubMed] [Google Scholar]
- 17.Coutinho, S. G., M. P. Oliveira, A. M. Da-Cruz, P. M. De Luca, S. C. Mendonca, A. L. Bertho, L. Soong, and D. McMahon-Pratt. 1996. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp. Parasitol. 84:144-155. [DOI] [PubMed] [Google Scholar]
- 18.De Luca, P. M., W. Mayrink, C. R. Alves, S. G. Coutinho, M. P. Oliveira, A. L. Bertho, V. P. Toledo, C. A. Costa, O. Genaro, and S. C. Mendonca. 1999. Evaluation of the stability and immunogenicity of autoclaved and nonautoclaved preparations of a vaccine against American tegumentary leishmaniasis. Vaccine 17:1179-1185. [DOI] [PubMed] [Google Scholar]
- 19.Dumonteil, E., F. Andrade-Narvarez, J. Escobedo-Ortegon, M. J. Ramirez-Sierra, G. Valencia-Pacheco, A. Flores-Serrano, S. Canto-Lara, and A. Arjona-Torres. 2000. Comparative study of DNA vaccines encoding various antigens against Leishmania mexicana. Dev. Biol. (Basel) 104:135-141. [PubMed] [Google Scholar]
- 20.Dumonteil, E., R. S. Maria Jesus, E. O. Javier, and G. M. Maria del Rosario. 2003. DNA vaccines induce partial protection against Leishmania mexicana. Vaccine 21:2170-2177. [DOI] [PubMed] [Google Scholar]
- 21.Echeverria, P., G. Dran, G. Pereda, A. I. Rico, J. M. Requena, C. Alonso, E. Guarnera, and S. O. Angel. 2001. Analysis of the adjuvant effect of recombinant Leishmania infantum Hsp83 protein as a tool for vaccination. Immunol. Lett. 76:107-110. [DOI] [PubMed] [Google Scholar]
- 22.Fragaki, K., I. Suffia, B. Ferrua, D. Rousseau, Y. Le Fichoux, and J. Kubar. 2001. Immunisation with DNA encoding Leishmania infantum protein papLe22 decreases the frequency of parasitemic episodes in infected hamsters. Vaccine 19:1701-1709. [DOI] [PubMed] [Google Scholar]
- 23.Green, S. J., R. M. Crawford, J. T. Hockmeyer, M. S. Meltzer, and C. A. Nacy. 1990. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J. Immunol. 145:4290-4297. [PubMed] [Google Scholar]
- 24.Guirges, S. Y. 1971. Natural and experimental re-infection of man with Oriental sore. Ann. Trop. Med. Parasitol. 65:197-205. [DOI] [PubMed] [Google Scholar]
- 25.Gurunathan, S., C. Prussin, D. L. Sacks, and R. A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409-1415. [DOI] [PubMed] [Google Scholar]
- 26.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 186:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurunathan, S., C. Y. Wu, B. L. Freidag, and R. A. Seder. 2000. DNA vaccines: a key for inducing long-term cellular immunity. Curr. Opin. Immunol. 12:442-447. [DOI] [PubMed] [Google Scholar]
- 28.Haberer, J. E., A. M. Da-Cruz, L. Soong, M. P. Oliveira-Neto, L. Rivas, D. McMahon-Pratt, and S. G. Coutinho. 1998. Leishmania pifanoi amastigote antigen P-4: epitopes involved in T-cell responsiveness in human cutaneous leishmaniasis. Infect. Immun. 66:3100-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinzel, F. P., M. D. Sadick, S. S. Mutha, and R. M. Locksley. 1991. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc. Natl. Acad. Sci. 88:7011-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodgkinson, V. H., L. Soong, S. M. Duboise, and D. McMahon-Pratt. 1996. Leishmania amazonensis: cultivation and characterization of axenic amastigote-like organisms. Exp. Parasitol. 83:94-105. [DOI] [PubMed] [Google Scholar]
- 31.Jensen, A. T., J. Curtis, J. Montgomery, E. Handman, and T. G. Theander. 2001. Molecular and immunological characterisation of the glucose regulated protein 78 of Leishmania donovani. Biochim. Biophys. Acta 1549:73-87. [DOI] [PubMed] [Google Scholar]
- 32.Ji, J., J. Sun, H. Qi, and L. Soong. 2002. Analysis of T helper cell responses during infection with Leishmania amazonensis. Am. J. Trop. Med. Hyg. 66:338-345. [DOI] [PubMed] [Google Scholar]
- 33.Ji, J., J. Sun, and L. Soong. 2003. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect. Immun. 71:4278-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kar, S., L. Soong, M. Colmenares, K. Goldsmith-Pestana, and D. McMahon-Pratt. 2000. The immunologically protective P-4 antigen of Leishmania amastigotes. A developmentally regulated single strand-specific nuclease associated with the endoplasmic reticulum. J. Biol. Chem. 275:37789-37797. [DOI] [PubMed] [Google Scholar]
- 35.Katae, M., Y. Miyahira, K. Takeda, H. Matsuda, H. Yagita, K. Okumura, T. Takeuchi, T. Kamiyama, A. Ohwada, Y. Fukuchi, and T. Aoki. 2002. Coadministration of an interleukin-12 gene and a Trypanosoma cruzi gene improves vaccine efficacy. Infect. Immun. 70:4833-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khalil, E. A., A. M. El Hassan, E. E. Zijlstra, M. M. Mukhtar, H. W. Ghalib, B. Musa, M. E. Ibrahim, A. A. Kamil, M. Elsheikh, A. Babiker, and F. Modabber. 2000. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double-blind, BCG-controlled trial in Sudan. Lancet 356:1565-1569. [DOI] [PubMed] [Google Scholar]
- 37.Khaskhely, N. M., M. Maruno, A. Takamiyagi, H. Uezato, K. M. Kasem, A. Hosokawa, K. Kariya, Y. Hashiguchi, E. A. Landires, and S. Nonaka. 2001. Pre-exposure with low-dose UVA suppresses lesion development and enhances Th1 responses in BALB/c mice infected with Leishmania (Leishmania) amazonensis. J. Derm. Sci. 26:217-232. [DOI] [PubMed] [Google Scholar]
- 38.Kima, P. E., S. L. Constant, L. Hannum, M. Colmenares, K. S. Lee, A. M. Haberman, M. J. Shlomchik, and D. McMahon-Pratt. 2000. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J. Exp. Med. 191:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locksley, R. M., and J. A. Louis. 1992. Immunology of leishmaniasis. Curr. Opin. Immunol. 4:413-418. [DOI] [PubMed] [Google Scholar]
- 40.Love, D. C., M. Mentink Kane, and D. M. Mosser. 1998. Leishmania amazonensis: the phagocytosis of amastigotes by macrophages. Exp. Parasitol. 88:161-171. [DOI] [PubMed] [Google Scholar]
- 41.Lowrie, D. B., R. E. Tascon, V. L. Bonato, V. M. Lima, L. H. Faccioli, E. Stavropoulos, M. J. Colston, R. G. Hewinson, K. Moelling, and C. L. Silva. 1999. Therapy of tuberculosis in mice by DNA vaccination. Nature 400:269-271. [DOI] [PubMed] [Google Scholar]
- 42.Marzochi, K. B., M. A. Marzochi, A. F. Silva, N. Grativol, R. Duarte, E. M. Confort, and F. Modabber. 1998. Phase 1 study of an inactivated vaccine against American tegumentary leishmaniasis in normal volunteers in Brazil. Mem. Inst. Oswaldo Cruz 93:205-212. [DOI] [PubMed] [Google Scholar]
- 43.Mattner, F., J. Magram, J. Ferrante, P. Launois, K. Di Padova, R. Behin, M. K. Gately, J. A. Louis, and G. Alber. 1996. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 26:1553-1559. [DOI] [PubMed] [Google Scholar]
- 44.Melby, P. C., J. Yang, W. Zhao, L. E. Perez, and J. Cheng. 2001. Leishmania donovani p36(LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis. Infect. Immun. 69:4719-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendez, S., S. Gurunathan, S. Kamhawi, Y. Belkaid, M. A. Moga, Y. A. Skeiky, A. Campos-Neto, S. Reed, R. A. Seder, and D. Sacks. 2001. The potency and durability of DNA- and protein-based vaccines against Leishmania major evaluated with low-dose, intradermal challenge. J. Immunol. 166:5122-5128. [DOI] [PubMed] [Google Scholar]
- 46.Modabber, F. 1995. Vaccines against leishmaniasis. Ann. Trop. Med. Parasitol. 89(Suppl. 1):83-88. [DOI] [PubMed] [Google Scholar]
- 47.Momeni, A. Z., T. Jalayer, M. Emamjomeh, A. Khamesipour, F. Zicker, R. L. Ghassemi, Y. Dowlati, I. Sharifi, M. Aminjavaheri, A. Shafiei, M. H. Alimohammadian, R. Hashemi-Fesharki, K. Nasseri, T. Godal, P. G. Smith, and F. Modabber. 1999. A randomised, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine 17:466-472. [DOI] [PubMed] [Google Scholar]
- 48.Moore, A. C., W. P. Kong, B. K. Chakrabarti, and G. J. Nabel. 2002. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J. Virol. 76:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noormohammadi, A. H., H. Hochrein, J. M. Curtis, T. M. Baldwin, and E. Handman. 2001. Paradoxical effects of IL-12 in leishmaniasis in the presence and absence of vaccinating antigen. Vaccine 19:4043-4052. [DOI] [PubMed] [Google Scholar]
- 50.Pan, A. A., and D. McMahon-Pratt. 1988. Monoclonal antibodies specific for the amastigote stage of Leishmania pifanoi. I. Characterization of antigens associated with stage- and species-specific determinants. J. Immunol. 140:2406-2414. [PubMed] [Google Scholar]
- 51.Rafati, S., A. H. Salmanian, T. Taheri, M. Vafa, and N. Fasel. 2001. A protective cocktail vaccine against murine cutaneous leishmaniasis with DNA encoding cysteine proteinases of Leishmania major. Vaccine 19:3369-3375. [DOI] [PubMed] [Google Scholar]
- 52.Rico, A. I., G. Del Real, M. Soto, L. Quijada, A. C. Martinez, C. Alonso, and J. M. Requena. 1998. Characterization of the immunostimulatory properties of Leishmania infantum HSP70 by fusion to the Escherichia coli maltose-binding protein in normal and nu/nu BALB/c mice. Infect. Immun. 66:347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sacks, D. L., S. Hieny, and A. Sher. 1985. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J. Immunol. 135:564-569. [PubMed] [Google Scholar]
- 54.Serezani, C. H., A. R. Franco, M. Wajc, J. K. Umada Yokoyama-Yasunaka, G. Wunderlich, M. M. Borges, and S. R. Uliana. 2002. Evaluation of the murine immune response to Leishmania meta 1 antigen delivered as recombinant protein or DNA vaccine. Vaccine 20:3755-3763. [DOI] [PubMed] [Google Scholar]
- 55.Sharifi, I., A. R. FeKri, M. R. Aflatonian, A. Khamesipour, A. Nadim, M. R. Mousavi, A. Z. Momeni, Y. Dowlati, T. Godal, F. Zicker, P. G. Smith, and F. Modabber. 1998. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet 351:1540-1543. [DOI] [PubMed] [Google Scholar]
- 56.Sjolander, A., T. M. Baldwin, J. M. Curtis, K. L. Bengtsson, and E. Handman. 1998. Vaccination with recombinant parasite surface antigen 2 from Leishmania major induces a Th1 type of immune response but does not protect against infection. Vaccine 16:2077-2084. [DOI] [PubMed] [Google Scholar]
- 57.Sjolander, A., T. M. Baldwin, J. M. Curtis, and E. Handman. 1998. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response are required for generation of immunity to leishmaniasis. J. Immunol. 160:3949-3957. [PubMed] [Google Scholar]
- 58.Soong, L., C. H. Chang, J. Sun, B. J. Longley, Jr., N. H. Ruddle, R. A. Flavell, and D. McMahon-Pratt. 1997. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J. Immunol. 158:5374-5383. [PubMed] [Google Scholar]
- 59.Soong, L., S. M. Duboise, P. Kima, and D. McMahon-Pratt. 1995. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect. Immun. 63:3559-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevens, T. L., A. Bossie, V. M. Sanders, R. Fernandez-Botran, R. L. Coffman, T. R. Mosmann, and E. S. Vitetta. 1988. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334:255-258. [DOI] [PubMed] [Google Scholar]
- 61.Todoroki, I., T. Joh, K. Watanabe, M. Miyashita, K. Seno, T. Nomura, H. Ohara, Y. Yokoyama, K. Tochikubo, and M. Itoh. 2000. Suppressive effects of DNA vaccines encoding heat shock protein on Helicobacter pylori-induced gastritis in mice. Biochem. Biophys. Res. Commun. 277:159-163. [DOI] [PubMed] [Google Scholar]
- 62.Ulmer, J. B., J. J. Donnelly, and M. A. Liu. 1996. DNA vaccines: promising a new approach to inducing protective immunity. ASM News 62:476. [Google Scholar]
- 63.Velez, I. D., S. del Pilar Agudelo, M. P. Arbelaez, K. Gilchrist, S. M. Robledo, J. A. Puerta, F. Zicker, J. Berman, and F. Modabber. 2000. Safety and immunogenicity of a killed Leishmania (L.) amazonensis vaccine against cutaneous leishmaniasis in Colombia: a randomized controlled trial. Trans. R. Soc. Trop. Med. Hyg. 94:698-703. [DOI] [PubMed] [Google Scholar]
- 64.Wang, Y., C. G. Kelly, M. Singh, E. G. McGowan, A. S. Carrara, L. A. Bergmeier, and T. Lehner. 2002. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J. Immunol. 169:2422-2429. [DOI] [PubMed] [Google Scholar]
- 65.World Health Organization. 1994. Leishmaniasis in the Americas. W.H.O. Rep. 15:8-11. [Google Scholar]
- 66.Xu, D., and F. Y. Liew. 1995. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. major. Immunology 84:173-176. [PMC free article] [PubMed] [Google Scholar]