Abstract
Interleukin-12 (IL-12) has been inversely associated with disease severity in human and murine malaria, and a polymorphism in the IL-12 p40 subunit gene (IL12B) has been associated with susceptibility to human cerebral malaria and reduced nitric oxide (NO) production. To better define the relationships between IL-12, NO, malaria parasitemia, and IL12B polymorphisms during malarial tolerance, plasma IL-12 levels and peripheral blood mononuclear cell NO synthase (NOS) activity were measured in asymptomatic Papua New Guineans exposed to intense malaria transmission. The IL-12 level was strongly inversely correlated with the density of Plasmodium falciparum parasitemia (ρ = −0.45; P < 0.001) and was predicted to decrease by 19% (95% confidence interval [CI], 10 to 27%) for each twofold increase in P. falciparum parasitemia. This is consistent with a suppressive effect of parasitemia on IL-12 production, an effect previously shown in vitro and in rodent models of disease. The IL-12 level was inversely correlated with NOS activity (r = −0.22; P = 0.007), with each twofold increase in NOS activity being predictive of a 25% (95% CI, 7 to 38%) decrease in plasma IL-12 levels. This probably reflects additional down-regulation of IL-12 by the high basal NO production and monocyte NOS expression found in the malaria-tolerant state. Neither the IL-12 level nor NOS activity was associated with either of two IL12B polymorphisms, reflecting the diversity of genetic control over immune responses in different populations.
There is increasing recognition that interleukin-12 (IL-12) plays an important regulatory role in both the cell-mediated (7, 8, 10, 11, 17-20) and antibody-mediated (21) immune responses to malaria. In murine malaria models, IL-12 administration decreases mortality in association with a reduction in peak parasitemia that appears to be critically dependent on gamma interferon (IFN-γ) and partially dependent on nitric oxide (NO) (19). In clinical studies, the plasma IL-12 level and its ratio to that of anti-inflammatory mediators such as transforming growth factor β (TGF-β) have been inversely correlated with disease severity (6, 10, 11, 17), although it has been both positively (11) and negatively (10) correlated with Plasmodium falciparum density. The relative contributions of malaria parasitemia, other infections, cross-regulatory cytokines, NO, and genetic determinants of IL-12 expression in the setting of human malaria require further elucidation.
Innate and adaptive cell-mediated immune responses are thought to be important in regulating “either the production or downstream effects of endogenous pyrogens” (7) in individuals from regions of highly endemic infection who are tolerant of circulating malaria parasitemia, although the precise roles of specific cytokines (including IL-12) are presently unknown. Understanding the role of IL-12 and other mediators such as NO in this setting is critical in fully informing the application of novel immunopreventive and therapeutic strategies to populations living under conditions of intense malaria transmission. We have previously defined two polymorphisms within IL12B, the gene encoding the p40 subunit of IL-12. The point mutation giving rise to the IL12B-3′UTR polymorphism (designated allele 1) was associated with higher IL-12 production and susceptibility to the autoimmune disorder type I diabetes (13), whereas homozygosity for IL12B-pro allele 1 (an insertion allele 4 bp longer than allele 2) was associated with increased mortality from cerebral malaria and reduced NO production in Tanzanian children (12). To our knowledge, the relationship between IL-12 production and NO production and monocyte NO synthase (NOS) activity has not been examined previously in human malaria. In this study, we hypothesized that basal IL-12 and NO production during good health in a population chronically exposed to high-level malaria transmission would be positively correlated, in line with the general observation that IL-12 is a potent stimulator of NO expression (16) and with the association shown in our previous study (12). Further, we hypothesized that IL-12 production would be increased in subjects with an IL12B-pro allele 2 and/or and IL12B-3′UTR allele 1 and that both the presence and level of malaria parasitemia would be inversely correlated with IL-12 as previously shown in clinical disease (7, 10).
MATERIALS AND METHODS
The subjects were coastal villagers from Madang, Papua New Guinea, where malaria transmission is intense and all four parasite species infecting humans are found (3). Informed consent was obtained in accordance with the ethical guidelines of the Human Research Ethics Committee of the Northern Territory Department of Health and Community Services and Menzies School of Health Research, Northern Territory, Australia, and the Papua New Guinea Medical Research Advisory Committee.
Recruitment of study participants.
Volunteers aged ≥1 year were screened for enrolment by a single blood smear between February and May 2000, with the aim of positively selecting for parasitemic subjects in addition to recruiting negative controls. Participants were excluded from the analysis if they were febrile (axillary temperature, ≥37.5°C) on any of three occasions throughout a 24-h period, had taken antimalarial or anti-inflammatory medication within 1 week, had symptoms suggestive of recent (≤1 week) malaria infection (3), or had diarrhea (since gastroenteritis may induce NO production). Subjects and controls were fasted and supervised overnight for >12 h in their villages by a member of the study team after a low-nitrate meal of chicken and rice to control for the confounding effect of dietary nitrite and nitrate ingestion in quantitating NO metabolites (1).
Specimen collection and assays.
Venous blood was collected following the fast, and a second blood smear was made (24 h after the first), plasma was cryopreserved at −70°C, and peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation. Thick and thin blood smears were prepared with 4% Giemsa stain and examined by microscopists with >15 years experience, and leukocyte counts were calculated manually for estimation of parasitemia (3). Systemic NO production was determined by measuring the concentration of nitrite plus nitrate (NOx) in urine and plasma by using Aspergillus nitrate reductase coupled with the Griess reaction (1). NOS activity was measured in PBMC pellet lysates by measuring the amount of [14C]arginine that was converted to citrulline per milligram of total cellular protein per hour (23). IL-12 levels were measured with a commercial sandwich enzyme-linked immunosorbent assay kit (OptEIA; BD Pharmingen, San Diego, Calif.) as specified by the manufacturer. The IL12B-pro and IL12B-3′UTR genotypes were determined by PCR and resolved on polyacrylamide gels as previously described (12).
Statistical analysis.
The continuous variables plasma NOx, PBMC NOS activity, IL-12 level, and P. falciparum parasite density were logarithmically transformed for use in correlation analysis (testing Pearson's r against 0 [and Spearman's ρ against 0 for comparison to an earlier study; see Discussion]), multiple linear-regression models, and Student's t tests appropriate for variance. In separate models, P. falciparum density and IL-12 were transformed to log 2 purely for explanatory purposes; this makes no difference to the interpretation of statistical significance. The IL12B-pro and IL12B-3′UTR polymorphisms were considered in analysis of variance and linear-regression models categorized according to separate genotypes (i.e., alleles 1-1, 1-2, and 2-2) and also according to the following a priori hypotheses: for IL12B-pro, allele 1-1 versus 1-2 and 2-2 combined, and for IL12B-3′UTR, allele 2-2 versus 1-1 and 1-2 combined. All statistical analyses were performed using Stata 7.0 software (StataCorp), with P values of <0.05 understood to indicate statistical significance. Since it was preplanned to make three pairwise comparisons in relation to the effect of parasitemia on IL-12 levels, consideration has been given in the discussion to how this should be interpreted statistically.
RESULTS
Baseline characteristics.
In the 424 villagers screened for enrolment (median age, 16 years; interquartile range, 7 to 26 years), the prevalence of any malaria parasite was 45% and that of P. falciparum was 30%. Plasma was available to test for IL-12 in 161 enrolled subjects (median age, 15 years; IQR, 9 to 25 years), and PBMCs were collected and available for testing in 142 subjects. The basic demographics and malaria status of this cohort are shown in Table 1. No subjects were observed to have breached the fasting protocol, and all were afebrile on up to three occasions during a 24-h period. DNA amplification for the IL12B-pro and IL12B-3′UTR genotypes was successful in 157 and 154 subjects respectively. The number of subjects with alleles 1-1, 1-2, and 2-2 was 110, 42, and 5, respectively, for IL12B-pro and 33, 54, and 67, respectively, for IL12B-3′UTR.
TABLE 1.
Baseline characteristics of subjectsa
| Age group (yr) | Total no. of patients | No. of patients negative for parasitemia | No. (%) of patients with parasitemiab | No. of patients with P. falciparum includedc | P. falciparum densityd | No. of patients infected withe:
|
||
|---|---|---|---|---|---|---|---|---|
| P. falciparum only | P. vivax only | Othere | ||||||
| 1-4 | 16 | 3 | 13 (81) | 10 | 862 | 7 | 3 | 3 |
| 5-9 | 29 | 4 | 25 (86) | 21 | 450 | 11 | 4 | 10 |
| 10-14 | 34 | 6 | 28 (82) | 20 | 306 | 9 | 4 | 15 |
| 15-19 | 19 | 6 | 13 (68) | 13 | 130 | 8 | 0 | 5 |
| 20+ | 63 | 26 | 37 (59) | 24 | 103 | 20 | 9 | 8 |
| Totals | 161 | 45 | 116 (72) | 88 | 247 | 55 | 20 | 41 |
The number of subjects with each type of malarial parasitemia is shown.
The number and percentage of patients infected with any malaria parasite.
Number of patients with P. falciparum included in the parasitemia.
Geometric mean highest density of P. falciparum parasitemia from two consecutive daily blood smears (number of trophozoites per microliter).
May include any combination of P. falciparum, P. vivax, P. malariae, and P. ovale.
IL-12 levels and NO production.
The geometric mean IL-12 in all subjects was 207 pg/ml (95% confidence interval [CI], 172 to 248 pg/ml), and there was no significant relationship between IL-12 and sex, age in years, or age when the patients were put into groups aged 1 to 4, 5 to 9, 10 to 14, 15 to 19, and ≥20 years. IL-12 levels were significantly inversely correlated with PBMC NOS activity (r = −0.22; P = 0.007). Each twofold increase in NOS activity was predictive of a 25% (95% CI, 7 to 38%) decrease in plasma IL-12 levels. There was no relationship between IL-12 and plasma NO x level (P = 0.99), and the plasma NO x level was not correlated with PBMC NOS activity. There were no significant relationships between IL-12, NOS activity, or plasma NO x level and either of the IL12B polymorphisms, whether considered in three different allele categories or condensed to two as per the a priori hypothesis.
IL-12 levels and parasitemia.
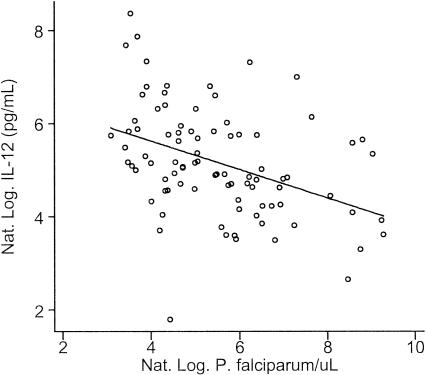
There was no difference in IL-12 levels between subjects with any malaria parasitemia and those who were aparasitemic (P = 0.23) or between subjects whose only parasitemia was due to P. falciparum and those who were aparasitemic (P = 0.35). However, the geometric mean IL-12 was 31% (95% CI, 1 to 52%) lower in subjects with parasitemia, including P. falciparum infection (175 pg/ml), than in the combined group of aparasitemic subjects and subjects infected with other Plasmodium species (252 pg/ml; P = 0.045). Furthermore; P. falciparum density and IL-12 were strongly inversely correlated (r = −0.41; ρ = −0.45; P < 0.001 for both [Fig. 1]), with the IL-12 level predicted to decrease by 19% (95% CI, 10 to 27%) for each twofold increase in P. falciparum parasitemia. The statistical significance of the predictive effect of NOS activity on IL-12 levels was reduced (P = 0.09), but that of P. falciparum density was not (P = 0.001), when included together in the regression model. Controlling for the nonsignificant effect of age made no difference to the magnitude or significance of any of these relationships. Neither measure of NO production was significantly associated with malaria parasitemia (data not shown).
FIG. 1.
Relationship between plasma IL-12 level and P. falciparum asexual parasite density. Individual values are represented by circles. The straight line is the regression line showing a significant negative inverse relationship (r2 = 0.17; P < 0.001).
DISCUSSION
This study has demonstrated a strong inverse correlation between P. falciparum parasitemia and plasma IL-12 levels of similar magnitude to that previously found in a study of uncomplicated and severe malaria cases (in which ρ was −0.51) (10). The strength and direction of the relationship in this study increases the validity of the finding that the IL-12 level was also lower in subjects with P. falciparum parasitemia than in those without (P = 0.045), which was made in one of three pairwise comparisons. This also supports the finding from a previous study that background ex vivo IL-12 expression in whole blood was associated with absence of parasitemia in an asymptomatic malaria-exposed population (7), which was significant but did not withstand statistical correction for multiple comparisons.
There are a number of plausible explanations for these results that relate both to cause and effect. P. falciparum (22) and P. yoelii (15) are known to directly suppress the secretion of IL-12 by dendritic cells, and ingested malarial hemozoin has been shown to impair mononuclear cell function (14). Indirect suppression of IL-12 production may occur via induction of IL-10 (25) and/or TGF-β, cytokines proposed to explain the lower production of IL-12 in severe malaria than in mild malaria (17). Alternatively, although protozoan glycosylphosphatidylinositol molecules similar in structure to the proposed glycosylphosphatidylinositol toxin of P. falciparum can induce IL-12 production via binding to monocyte toll-like receptor 2 (5), repetitive stimulation may lead to down-regulation of IL-12 in a process akin to endotoxin tolerance without necessarily relying on IL-10 or other paracrine mediators (24). Since IL-12 has been predominantly associated with antiparasitic effects in murine malaria models (19), suppression of mononuclear IL-12 production by P. falciparum in the malaria-tolerant state may allow greater parasite replication and higher levels of parasitemia.
The inverse association between IL-12 production and PBMC NOS activity that we have shown in the present study was the opposite to what we expected, given that IL-12 is generally thought to up-regulate NO (16); however, it is consistent with the known inhibitory paracrine effect of NO on IL-12 expression (9). Monocyte nitric oxide synthase 2 expression and NO production are known to be higher in the malaria-tolerant state (2, 4), and in this setting NO may also contribute to inhibition of IL-12 production.
The unexplained variance in plasma IL-12 levels in the present study may be accounted for in part by interindividual differences in the genes controlling IL-12 expression and/or regulation. In another study (7), genetic differences were thought more likely to explain the ex vivo correlation between mitogen and P. falciparum antigen-stimulated whole-blood IL-12 activity than was exposure to other infections, and a polymorphism in the IL12B gene has been associated with increased risk of death from cerebral malaria (12). On the other hand, plasma IL-12 levels were lower in Gabonese children with severe malaria than with mild malaria (10), but basal levels in the same children after recovery were similar, which argued against an important effect of host genetics. Increased constitutive expression of IL-12 would be expected to be inversely associated with parasitemia if IL-12 is associated with similar antiparasitic responses in murine and human malaria (19). That plasma IL-12 levels and NO production were not associated with either of the IL12B polymorphisms previously associated with actual or predicted functional differences in IL-12 expression (12) may not be surprising, given the geographical separation of the populations, the possibility that linkage disequilibrium explained the earlier findings, and potential differences in the background stimuli inducing NO and IL-12. Additional studies involving in vitro stimulation of IL-12 response in individuals with different IL12B polymorphisms may be necessary to determine their relevance to IL-12 production in malaria-exposed populations.
Although the interrelationship between IL-12, other immunoregulatory cytokines, and malaria is complex, interpretation of our data in the context of other studies (7, 10, 17) suggests that IL-12 production is highest in asymptomatic malaria-exposed subjects and then decreases in proportion to the level of parasitemia, encompassing both the malaria-tolerant state and clinical disease. If this interpretation is correct, the fact that tolerance is necessarily lost in progressing from the asymptomatic to the diseased state provides further evidence that increasing parasitemia causes lower levels of IL-12, rather than low levels of IL-12 allowing higher parasitemias. Down-regulation of monocyte IL-12 production (24) and constitutive factors may also determine IL-12 production during tolerance (with a resultant effect on parasitemia, possibly via modulation of IFN-γ production [18]), whereas different processes may be operative during disease. While our findings suggest that high levels of monocyte NOS activity and NO production found in the tolerant state may contribute to the down-regulation of IL-12 in malaria tolerance, it is unlikely to do so in clinical disease, where both IL-12 (10, 17) and NOS2 (2) are similarly suppressed in proportion to disease severity.
Acknowledgments
This work was supported by the National Health and Medical Research Council Australia (Scholarship to C.S.B., Fellowship to N.M.A.), National Institutes of Health grant R01 AI-41764, the Tudor Foundation, and the Mark Nicholson and Alice Hill Malaria Research Fund.
We thank the people of Haven and Midiba villages for their participation and assistance; Erwin Ibam, Kerry Lorry, Joseph Slagi, and Ferdinand Baighi for assisting with the field and laboratory work; Andrew Raiko and his staff at the Madang IMR for facilitating the laboratory studies; Joanne Bex and Jocelyn Saunders for assisting with DNA preparation; Deborah Holt for critically appraising the manuscript; and Michael Alpers, Ric Price, Bart Currie, and Altaf Lal for support.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anstey, N. M., C. S. Boutlis, and J. R. Saunders. 2002. Systemic nitric oxide production in human malaria. I. Analysis of NO metabolites in biological fluids. Methods Mol. Med. 72:461-467. [DOI] [PubMed] [Google Scholar]
- 2.Anstey, N. M., J. B. Weinberg, M. Y. Hassanali, E. D. Mwaikambo, D. Manyenga, M. A. Misukonis, D. R. Arnelle, D. Hollis, M. I. McDonald, and D. L. Granger. 1996. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 184:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutlis, C. S., D. C. Gowda, R. S. Naik, G. P. Maguire, C. S. Mgone, M. J. Bockarie, M. Lagog, E. Ibam, K. Lorry, and N. M. Anstey. 2002. Antibodies to Plasmodium falciparum glycosylphosphatidylinositols: inverse association with tolerance of parasitemia in Papua New Guinean children and adults. Infect. Immun. 70:5052-5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutlis, C. S., E. Tjitra, H. Maniboey, M. A. Misukonis, J. R. Saunders, S. Suprianto, J. B. Weinberg, and N. M. Anstey. 2003. Nitric oxide production and mononuclear cell nitric oxide synthase activity in malaria-tolerant Papuan adults. Infect. Immun. 71:3682-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos, M. A., I. C. Almeida, O. Takeuchi, S. Akira, E. P. Valente, D. O. Procopio, L. R. Travassos, J. A. Smith, D. T. Golenbock, and R. T. Gazzinelli. 2001. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167:416-423. [DOI] [PubMed] [Google Scholar]
- 6.Chaisavaneeyakorn, S., C. Othoro, Y. P. Shi, J. Otieno, S. C. Chaiyaroj, A. A. Lal, and V. Udhayakumar. 2003. Plasma levels of interleukin (IL)-12 and IL-18 in relation to severe malaria anemia in a holoendemic area of western Kenya. Clin. Diagn. Lab. Immunol. 10:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodoo, D., F. M. Omer, J. Todd, B. D. Akanmori, K. A. Koram, and E. M. Riley. 2002. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J. Infect. Dis. 185:971-979. [DOI] [PubMed] [Google Scholar]
- 8.Doolan, D. L., and S. L. Hoffman. 1999. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J. Immunol. 163:884-892. [PubMed] [Google Scholar]
- 9.Huang, F. P., W. Niedbala, X. Q. Wei, D. Xu, G. J. Feng, J. H. Robinson, C. Lam, and F. Y. Liew. 1998. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur. J. Immunol. 28:4062-4070. [DOI] [PubMed] [Google Scholar]
- 10.Luty, A. J., D. J. Perkins, B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, J. B. Weinberg, and P. G. Kremsner. 2000. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect. Immun. 68:3909-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malaguarnera, L., R. M. Imbesi, S. Pignatelli, J. Simpore, M. Malaguarnera, and S. Musumeci. 2002. Increased levels of interleukin-12 in Plasmodium falciparum malaria: correlation with the severity of disease. Parasite Immunol. 24:387-389. [DOI] [PubMed] [Google Scholar]
- 12.Morahan, G., C. S. Boutlis, D. Huang, A. Pain, J. R. Saunders, M. R. Hobbs, D. L. Granger, J. B. Weinberg, N. Peshu, E. D. Mwaikambo, K. Marsh, D. J. Roberts, and N. M. Anstey. 2002. A promoter polymorphism in the gene encoding interleukin-12 p40 (IL12B) is associated with mortality from cerebral malaria and with reduced nitric oxide production. Genes Immun. 3:414-418. [DOI] [PubMed] [Google Scholar]
- 13.Morahan, G., D. Huang, S. I. Ymer, M. R. Cancilla, K. Stephen, P. Dabadghao, G. Werther, B. D. Tait, L. C. Harrison, and P. G. Colman. 2001. Linkage disequilibrium of a type 1 diabetes susceptibility locus with a regulatory IL12B allele. Nat. Genet. 27:218-221. [DOI] [PubMed] [Google Scholar]
- 14.Mordmuller, B., F. Turrini, H. Long, P. G. Kremsner, and P. Arese. 1998. Neutrophils and monocytes from subjects with the Mediterranean G6PD variant: effect of Plasmodium falciparum hemozoin on G6PD activity, oxidative burst and cytokine production. Eur. Cytokine Netw. 9:239-245. [PubMed] [Google Scholar]
- 15.Ocana-Morgner, C., M. M. Mota, and A. Rodriguez. 2003. Malaria blood stage suppression of liver stage immunity by dendritic cells. J. Exp. Med. 197:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkins, D. J., P. G. Kremsner, D. Schmid, M. A. Misukonis, M. A. Kelly, and J. B. Weinberg. 1999. Blood mononuclear cell nitric oxide production and plasma cytokine levels in healthy Gabonese children with prior mild or severe malaria. Infect. Immun. 67:4977-4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perkins, D. J., J. B. Weinberg, and P. G. Kremsner. 2000. Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J. Infect. Dis. 182:988-992. [DOI] [PubMed] [Google Scholar]
- 18.Rhee, M. S., B. D. Akanmori, M. Waterfall, and E. M. Riley. 2001. Changes in cytokine production associated with acquired immunity to Plasmodium falciparum malaria. Clin. Exp. Immunol. 126:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson, M. M., Z. Su, H. Sam, and K. Mohan. 2001. Modulation of host responses to blood-stage malaria by interleukin-12: from therapy to adjuvant activity. Microbes Infect. 3:49-59. [DOI] [PubMed] [Google Scholar]
- 20.Stevenson, M. M., M. F. Tam, S. F. Wolf, and A. Sher. 1995. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J. Immunol. 155:2545-2556. [PubMed] [Google Scholar]
- 21.Su, Z., and M. M. Stevenson. 2002. IL-12 is required for antibody-mediated protective immunity against blood-stage Plasmodium chabaudi AS malaria infection in mice. J. Immunol. 168:1348-1355. [DOI] [PubMed] [Google Scholar]
- 22.Urban, B. C., N. Willcox, and D. J. Roberts. 2001. A role for CD36 in the regulation of dendritic cell function. Proc. Natl. Acad. Sci. USA 98:8750-8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberg, J. B., M. A. Misukonis, P. J. Shami, S. N. Mason, D. L. Sauls, W. A. Dittman, E. R. Wood, G. K. Smith, B. McDonald, and K. E. Bachus. 1995. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood 86:1184-1195. [PubMed] [Google Scholar]
- 24.Wysocka, M., S. Robertson, H. Riemann, J. Caamano, C. Hunter, A. Mackiewicz, L. J. Montaner, G. Trinchieri, and C. L. Karp. 2001. IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J. Immunol. 166:7504-7513. [DOI] [PubMed] [Google Scholar]
- 25.Xu, X., K. Sumita, C. Feng, X. Xiong, H. Shen, S. Maruyama, M. Kanoh, and Y. Asano. 2001. Down-regulation of IL-12 p40 gene in Plasmodium berghei-infected mice. J. Immunol. 167:235-241. [DOI] [PubMed] [Google Scholar]