While a main feature of HIV-1 pathogenesis is the death of CD4+ T cells due to apoptosis, the mechanisms of apoptosis are highly controversial 1 2. Several HIV-1 proteins have been implicated in apoptosis regulation, among them viral protein R (Vpr), a small (∼14 kD) HIV-1 accessory protein that is packed in the nucleocapsid through its interaction with the Pr55 Gag precursor 3. In addition to its roles in apoptosis and virus assembly, several other activities have been ascribed to Vpr–protein interaction, including: (a) translocation of the HIV-1 preintegration complex through the nuclear pore, a necessary step for the replication of HIV-1 in nondividing cells—Vpr appears to participate in this process by binding to kariopherin α; (b) induction of cell cycle arrest, likely by Vpr binding to and inactivating MOV34, an upstream positive regulator of the p34–cyclin B complex shown to be essential for the G2–M phase transition; (c) stimulation of viral gene expression through physical interaction of Vpr with transcription factors and/or as a consequence of its effect on cell cycle. The ability of Vpr to exert so many effects through direct protein–protein interactions, followed by changes in target protein activity, can be explained by thinking of Vpr as a chaperone, as recently suggested by the fact that Vpr can substitute for hsp70, a cellular chaperone 4. Thus, Vpr seems to possess structural features that allow for binding to more than one protein with sufficient energy to cause changes in activity (presumably in conformation) of target proteins.
In this issue 5, Jacotot et al. extend their previous finding 6 that the mitochondrial adenine nucleotide translocator (ANT), a proposed component of the permeability transition pore, constitutes a novel Vpr target. Here, they present a large number of experiments to test the idea that this physical interaction of Vpr and ANT is central to Vpr-induced apoptosis. First, in pure lipid bilayer membranes, they demonstrate that adding an apoptogenic peptide derived from Vpr (Vpr 52–96) and ANT together leads to channel formation. The channels they measure could easily be large enough to permeabilize (although channel selectivity remains to be determined) the inner mitochondrial membrane, leading to uncoupling of mitochondrial respiration, loss of transmembrane potential, swelling of the matrix, and release of intermembrane proteins, activities of Vpr 52–96 that they independently demonstrate on isolated mitochondria. Second, based on the ability of PA10, a voltage-dependent anion channel (VDAC) inhibitor to impair Vpr binding to mitochondria, they argue that Vpr targets ANT by passing through VDAC. Third, they show that Bcl-2 can displace Vpr 52–96 from ANT with recombinant proteins and that thereby, Bcl-2 can inhibit both the binding of Vpr to ANT in mitochondrial membranes and the effects of Vpr on mitochondria. According to Jacotot et al., the mechanism of Vpr-induced apoptosis that emerges from this multidisciplinary approach is as follows (Fig. 1, top row: Vpr enters the intermembrane space through VDAC, binds to the intermembrane face of ANT (this is the stage at which Bcl-2 would inhibit apoptosis), and opens ANT to permeabilize the inner mitochondrial membrane. This leads to inner membrane swelling and rupture of the outer membrane to release apoptogenic factors.
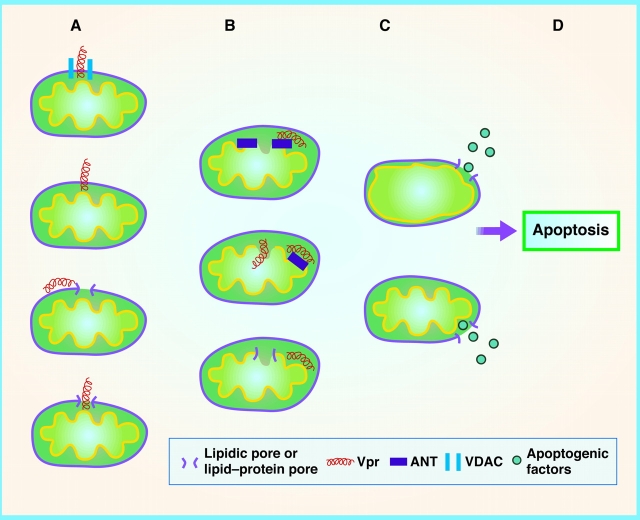
Figure 1.
Hypothetical interactions of Vpr with outer and inner mitochondrial membranes in the mechanism of Vpr-induced apoptosis. Column A: Outer membrane mechanisms. First, Vpr is proposed to pass through VDAC 5. Second, Vpr is known to traverse biological membranes autonomously 7. Third, Vpr may induce lipidic pores, local discontinuities in the lipid bilayer structure of the membrane composed of lipids or lipid and proteins 13. Fourth, Vpr may traverse a lipidic pore of its own induction. Column B: Inner membrane mechanisms. First, Vpr is shown to bind to ANT and proposed to cause ANT to change conformation to create a channel in the inner membrane 5. Second, while binding to ANT, Vpr may also interact with inner membrane or matrix components in as yet unknown ways to cause depolarization and inner membrane swelling. Third, Vpr may induce a lipidic pore in the inner membrane. Column C: Release of apoptogenic factors. First, inner membrane swelling (secondary to inner membrane permeabilization) ruptures the outer mitochondrial membrane, releasing cytochrome c, apoptosis-inducing factor (AIF), second mitochondria-derived activator of caspase/direct inhibitor of apoptosis proteins (IAP)-binding protein with low pI (Smac/DIABLO), and other intermembrane factors that cause apoptosis 16 17. Second, Vpr-induced lipidic pores in the outer membrane can widen, depending on outer membrane surface tension and spontaneous curvature 13. Note that these putative interactions are not mutually exclusive and may be synergistic. For example, any increased swelling of the inner membrane could increase surface tension in the outer membrane and widen lipidic pores. Also, the local rupture of the outer membrane caused by swelling-induced tension is probably a lipidic pore, albeit a wide one.
While this scheme is attractive, there are alternative hypotheses and many details to worry about. If Vpr crosses the outer mitochondrial membrane through VDAC, this is very important because Vpr oligomerizes at very low (as yet undetectable) concentrations, and the lumen of VDAC is already close to that of an α helix 7 8. More work is needed to confirm their proposal for the following reasons: (a) since Vpr binds mitochondrial VDAC 6, the inhibitory effect of PA10 on Vpr binding to mitochondria may reflect direct binding to VDAC; (b) PA10 generally decreases mitochondrial membrane permeability, and PA10 efficiently inhibits pores other than VDAC (our unpublished observations), so PA10 is not specific for VDAC; (c) NADH does not impair Vpr 52–96 from reaching ANT despite the fact that NADH induces VDAC closure in ways similar to those of PA10 9. As extracellular Vpr crosses the plasma membrane before gaining access to the mitochondria, Vpr can cross membranes through alternative, VDAC-independent pathways, and these transport mechanisms may be at work in the outer mitochondrial membrane as well (Fig. 1 A, second from top).
The fact that Vpr can cross membranes may be related to the fact that Vpr is membrane active and perforates lipid bilayers and cell membranes. The electrophysiological recordings of Vpr-induced conductance changes 10 11 12, being continuously variable, are more consistent with the hypothesis that Vpr causes local disturbances of the lipid bilayer (lipidic pores; reference 13) by interacting with membrane lipids and proteins (Fig. 1 A, third from top) than the hypothesis that Vpr forms channels. Vpr could then move across the monolayer of the lipidic pore (Fig. 1 A, bottom). As anionic lipids and high negative transmembrane potentials increase poration by a COOH-terminal peptide of Vpr 12, and since these are features of the inner mitochondrial membrane, Vpr may itself induce inner mitochondrial membrane permeabilization, explaining the effects of Vpr on respiration (if the two inhibitors of Vpr's action also act on Vpr's alternate target) (Fig. 1 B, bottom). One might think that the ability of Bcl-2 to block Vpr 52–96/ANT channel formation without binding to the viral peptide supports the idea that ANT is the essential channel component, but perhaps Bcl-2 prevents ANT from promoting Vpr's intrinsic poration activity. Jacotot et al. find that Bax enhances the channel activity of Vpr–ANT, and Bax localizes to the outer mitochondrial membrane, so Vpr and endogenous Bax may cooperatively induce outer mitochondrial membrane permeabilization. We have recently proposed that Bax, in association with lipid molecules, forms pores of variable size in the outer mitochondrial membrane 13 14, and this proposal has been extended to t-Bid 15. Formation and widening of such lipidic pores should increase with increasing surface tension in the outer mitochondrial membrane, which is expected as a result of matrix swelling. In other words, ANT opening would always facilitate the autonomous pore-forming activity of proapoptotic Bcl-2 family proteins. The proposal by Jacotot et al. that Vpr affects the permeability of the inner before the outer mitochondrial membrane is hard to judge, because it is based in the comparison of permeability to molecules of very different sizes (NADH and H3O+ versus cytochrome c). Moreover, at low ionic strength, cytochrome c tends to remain attached to inner membranes, not readily releasable upon outer membrane permeabilization. However, all things being equal, and assuming that Vpr has access to all membranes through its translocation activity, the membrane-perturbing effects of Vpr will be detected first by the membrane that is tight, the inner membrane, rather than the outer membrane, which is already comparatively leaky. Thus, inner membrane permeabilization may be the dominant effect in Vpr-induced apoptosis as well (though not for other apoptogenic stimuli that deplete intermembrane cytochrome c without permeabilizing the inner membrane) 16 17. Electrophysiological studies of the effect of Vpr on wild-type and VDAC/ANT-deficient mitochondrial membranes might provide further insight on the exact role of these proteins on mitochondrial membrane permeabilization.
Strikingly, Vpr can not only cause apoptosis but can also inhibits apoptosis 18 19 20! It has been proposed that Vpr-induced apoptosis occurs at the relatively large concentrations of Vpr seen during active viral replication, while low Vpr concentrations at the onset of infection inhibit the host apoptotic response to help ensure prolonged viral replication, as in many other viruses 21. Perhaps the antiapoptotic activity exerted by Vpr is also due to its mitochondrial localization. Vpr may maintain VDAC in an open configuration to facilitate nucleotide exchange across the outer mitochondrial membrane, similar to that proposed for Bcl-2 and Bcl-xL 22. Thus, the antiapoptotic effects of Vpr may be exerted through a VDAC-mediated mitochondrioprotective effect, while the proapoptotic effects of Vpr may be mediated through both its ability to perturb mitochondrial membranes and its more specific ANT-mediated effects. The work of Jacotot et al. provides a framework for further testing these mechanisms.
Whatever the in vitro mechanisms of apoptosis, it is the in vivo mechanisms that are the most important. While isolated PBMCs from HIV-1–infected individuals do not undergo apoptosis at either an increased or a decreased rate, they are much more susceptible to apoptotic stimuli 1 2. This is understandable in terms of the properties of Vpr discussed above, which would be synergistic with other stimuli to promote apoptosis. But why should HIV-1 want to promote apoptosis? It is possible that Vpr acts as a necessary brake on HIV-1 replication by removing productively infected T cells through apoptosis induction, to prevent premature T cell burnout and rapid disease progression to host death. The antiapoptotic function of Vpr is easier to understand, as it ensures continuance of viral production by infected cells. Also, the antiapoptotic function of Vpr may play a role in the incomplete eradication of HIV by current therapies. In spite of highly active antiretroviral therapy (HAART), HIV-1 continues to reside in resting memory CD4+ T cells and to replicate at low levels in undefined cells 23 24 25. Perhaps the antiapoptotic effects of Vpr lead to the preservation of a pool of infected cells constitutively expressing Vpr at low levels. In support of this idea, it has recently been found that Vpr is not structurally or functionally inactivated in latently infected CD4+ T cells of patients on HAART 26. We propose that Vpr plays a prominent role in maintaining HIV-1 reservoirs through its antiapoptotic activity, and the idea of reversing this activity to promote the proapoptotic activity of Vpr discussed in this issue might make the reservoir cells more vulnerable to therapeutic attack.
Acknowledgments
We wish to thank Drs. Svetlana Glushakova, Marco Colombini, Leonid Margolis, and Michael Bukrinsky for informative discussions and helpful comments.
References
- Badley A.D., Pilon A.A., Landay A., Lynch D.H. Mechanisms of HIV-associated lymphocyte apoptosis. Blood. 2000;96:2951–2964. [PubMed] [Google Scholar]
- Jaworosky A., Crowe S.M. Does HIV cause depletion of CD4+ T cells in vivo by the induction of apoptosis? Immunol. Cell Biol. 1999;77:90–98. doi: 10.1046/j.1440-1711.1999.00798.x. [DOI] [PubMed] [Google Scholar]
- Bukrinsky M., Adzhubei A. Viral protein R of HIV-1. Rev. Med. Virol. 1999;9:39–49. doi: 10.1002/(sici)1099-1654(199901/03)9:1<39::aid-rmv235>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Agostini I., Popov S., Li J., Dubrovsky L., Hao T., Bukrinsky M. Heat-shock protein 70 can replace viral protein R of HIV-1 during nuclear import of the viral preintegration complex. Exp. Cell Res. 2000;259:398–403. doi: 10.1006/excr.2000.4992. [DOI] [PubMed] [Google Scholar]
- Jacotot E., Ferri K.F., El Hammel C., Brenner C., Druillennec S., Hoebeke J., Rustin P., Métivier D., Lenoir C., Geuskens M. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 Viral Protein R and Bcl-2. J. Exp. Med. 2001;193:509–519. doi: 10.1084/jem.193.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E., Ravagnan L., Loeffler M., Ferri K.F., Vieira H.L.A., Zamzani N., Constantini P., Druillennec S., Hoebeke J., Briand J.P. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 2000;191:33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henklein P., Brun K., Sherman M.P., Tessmer U., Licha K., Kopp J., de Noronha C.M.C., Greene W.C., Wray V., Schubert U. Functional and structural characterization of synthetic HIV-1 Vpr that transduces cells, localizes to the nucleus, and induces G2 arrest. J. Biol. Chem. 2000;275:32016–32026. doi: 10.1074/jbc.M004044200. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Parsegian V.A. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 1986;323:36–39. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]
- Zizi M., Forte M., Blachly-Dyson E., Colombini M. NADH regulates the gating of VDAC, the mitochondrial outer membrane channel. J. Biol. Chem. 1994;21:1614–1616. [PubMed] [Google Scholar]
- Piller S.C., Ewart G.D., Premkumar A., Cox G.B., Gage P.W. Vpr protein of human immunodeficiency virus type 1 forms cation-selective channels in planar lipid bilayers. Proc. Natl. Acad. Sci. USA. 1996;93:111–115. doi: 10.1073/pnas.93.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller S.C., Jans P., Gage P.W., Jans D.A. Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neuronsimplications for AIDS pathology. Proc. Natl. Acad. Sci. USA. 1998;95:4595–4600. doi: 10.1073/pnas.95.8.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller S.C., Ewart G.D., Jans D.A., Gage P.W., Cox G.B. The amino-terminal region of Vpr from human immunodeficiency virus type 1 forms ion channels and kills neurons. J. Virol. 1999;73:4230–4238. doi: 10.1128/jvi.73.5.4230-4238.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J. Hole-istic medicine. Science. 1999;284:1475–1477. doi: 10.1126/science.284.5419.1475. [DOI] [PubMed] [Google Scholar]
- Basañez G., Nechushtan A., Drozhinin O., Chanturiya A., Choe E., Tutt S., Wood K.A., Hsu Y.-T., Zimmerberg J., Youle R.J. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. USA. 1999;96:5492–5497. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzegorz K., Montessuit S., Eskes R., Berrier C., Martinou J.-C., Ghazi A., Antonsson B. The destabilization of lipid membranes induced by the C-terminal fragment of caspase 8-cleaved Bid is inhibited by the N-terminal fragment. J. Biol. Chem. 2000;275:22713–22718. doi: 10.1074/jbc.M003807200. [DOI] [PubMed] [Google Scholar]
- Martinou J.-C., Green D. Breaking the mitochondrial barrier. Nat. Rev. 2001;2:63–67. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- Zamzami G., Kroemer G. The mitochondrion in apoptosishow Pandora's box opens. Nat. Rev. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- Ayyavoo V., Mahboubi A., Mahalingam S., Ramalingam R., Kudchodkar S., Williams W.V., Green D.R., Weiner D.B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat. Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- Conti L., Rainaldi G., Matarrese P., Varano B., Rivabene R., Columba S., Sato A., Belardelli F., Malorni W., Gessani S. The HIV-1 Vpr protein acts as a negative regulator of apoptosis in human lymphoblastoid T cell linepossible implications for the pathogenesis of AIDS. J. Exp. Med. 1998;187:403–413. doi: 10.1084/jem.187.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumori T., Akari H., Iida S., Hata S., Kagawa S., Aida Y., Koyama A.H., Adachi A. The HIV-1 Vpr displays strong anti-apoptosis activity. FEBS Lett. 1998;432:17–20. doi: 10.1016/s0014-5793(98)00824-2. [DOI] [PubMed] [Google Scholar]
- Conti L., Matarrese P., Varano B., Gauzzi M.C., Saito A., Malorni W., Belardelli F., Gessani S. Dual role of the HIV-1 Vpr protein in the modulation of the apoptotic response of T cells. J. Immunol. 2000;165:3293–3300. doi: 10.4049/jimmunol.165.6.3293. [DOI] [PubMed] [Google Scholar]
- Vander Heiden M.G., Chandel N.S., Li X.X., Schumacker P.T., Colombini M., Thompson C.B. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc. Natl. Acad. Sci. USA. 2000;25:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun T.W., Fauci A.S. Latent reservoirs of HIVobstacles to the eradication of virus. Proc. Natl. Acad. Sci. USA. 1999;96:10958–10961. doi: 10.1073/pnas.96.20.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson T., McArthur J., Siliciano R.F. Reservoirs for HIV-1mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu. Rev. Immunol. 2000;18:665–708. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- Ramratnam B., Mittler J.E., Zhang L., Boden D., Hurley A., Fang F., Macken C.A., Perelson A.S., Markowitz M., Ho D.D. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat. Med. 2000;6:82–85. doi: 10.1038/71577. [DOI] [PubMed] [Google Scholar]
- Shen A., Siliciano J.D., Pierson T.C., Buck C.B., Siliciano R.F. Establishment of latent HIV-1 infection of resting CD4+ T lymphocytes does not require inactivation of Vpr. Virology. 2000;278:227–233. doi: 10.1006/viro.2000.0650. [DOI] [PubMed] [Google Scholar]