Abstract
Lipoteichoic acids (LTAs) have been proposed as putative Gram-positive immunostimulatory counterparts to Gram-negative lipopolysaccharides. However, LTA from Staphylococcus aureus, the clinically most frequent Gram-positive pathogen, was inactive after purification. Here, a novel isolation procedure to prepare pure (>99%) biologically active LTA, allowing the first structural analysis by nuclear magnetic resonance and mass spectrometry, is described. A comparison with LTA purified by standard techniques revealed that alanine substituents are lost during standard purification, resulting in attenuated cytokine induction activity. In line with this finding, hydrolysis of alanine substituents of active LTA decimated cytokine induction. LTA represents a major immunostimulatory component of S. aureus.
Keywords: magnetic resonance spectroscopy; tumor necrosis factor; gram-positive bacteria; isolation and purification; immunity, natural
Introduction
LPS from Gram-negative bacteria is well established as the crucial stimulus of inflammatory responses in vivo and in vitro 1 2. A functional equivalent to LPS has not been identified for Gram-positive bacteria in general, although the inflammatory immune response to Gram-negative and Gram-positive bacteria cannot be distinguished clinically. Staphylococcus aureus is a predominant cause of hospital infections 3 4, and in recent years the importance of Gram-positive bacteria in general in the pathogenesis of sepsis has been emphasized 5 6. Lipoteichoic acids (LTAs) from Gram-positive bacteria have been suggested to represent immunostimulatory principles 7 that are still under debate 8 9. In particular, while crude commercial LTA preparations from Staphylococcus aureus were potent immune stimuli 10 11, the same LTA isolated and purified by methods adopted from LPS purification did not induce cytokine release by monocytes 12.
Materials and Methods
Bacteria Culture and Standard LTA Purification.
S. aureus (DSM 20233) was cultured aerobically in a 42-liter fermentor (MBR Bio Reactor) at 37°C and harvested at an OD578 of 15 (extrapolated) in a continuous flow centrifuge, resuspended in 0.1 M citrate buffer, pH 4.7, and disrupted with glass beads in a Braun disintegrator. Standard hot phenol/water extractions followed by fast performance liquid chromatography (FPLC) of aqueous extracts on octyl-Sepharose (Amersham Pharmacia Biotech) and DEAE–Sepharose (Amersham Pharmacia Biotech) were performed according to the procedure described in reference 13.
Improved LTA Purification Procedure.
A defrosted aliquot of bacteria was mixed with an equal volume of n-butanol (Merck) under stirring for 30 min at room temperature (RT). After centri-fugation at 13,000 g for 20 min, the aquatic phase was lyophilized, resuspended with chromatography start buffer (15% n-propanol in 0.1 M ammonium acetate, pH 4.7), and centrifuged at 45,000 g for 15 min. The supernatant was subjected to hydrophobic interaction chromatography (HIC) on octyl-Sepharose.
Cytokine Induction Assay.
Cytokine release by human whole blood was determined as described 14, incubating 800 μl of isotonic sodium chloride solution, 200 μl of human heparinized whole blood, and 10 μl of chromatography fraction, which was evaporated, resuspended in 10 μl of distilled water, and sonified. TNF-α was measured by sandwich ELISA (Endogen).
LTA Structure Analysis.
Carbohydrates, d-alanine, glycerol, and phosphorus were determined by established procedures 15 16. Fatty acids of LTA were determined by gas chromatography–mass spectrometry (GC–MS; Hewlett-Packard) as the respective methyl esters after methanolysis using 2 M HCl in methanol for 7 h at 85°C.
Nuclear magnetic resonance (NMR) experiments were performed at 600.13 MHz (1H) and 300 K. The NMR spectra were related to 3-(trimethylsilyl) 3,3,2,2-tetradeuteropropionic acid Na salt (d4-TSPA). Homonuclear assignments were taken from double-quantum filtered correlation spectroscopy (DQF-COSY), total correlation spectroscopy (TOCSY), rotating frame Overhauser enhancement spectroscopy (ROESY), and nuclear Overhauser effect spectroscopy (NOESY) spectra. 13C assignments were based on heteronuclear multiple-quantum correlation (HMQC).
The average chain length of the phosphoglycerol backbone and the degree of substitution were quantified directly from the 1H NMR integrals of native LTA. The integral ratio of chemical shift (δ)H 5.4 and δH 5.08 as well as the integral ratio of δH 1.62 and δH 2.1 yielded the ratio of d-alanine to α-d-N-acetylglucosamine (GN). The total amount of glycerol was determined from the integral δH 3.7-4.2 (corrected for the underlying five GN resonances) plus the glycerol methine resonance at δH 5.4.
After hydrolyzing 220 mg of LTA with 5 ml of HF (48%) at 2°C for 42 h, 150 ml of distilled water and 35 ml of saturated NaHCO3 were added. The lyophilisate was resuspended in 70 ml H2O/CH2Cl2/MeOH (3:3:1) and centrifuged at 3,200 rpm for 7 min. The pellet was washed with CH2Cl2 and the water layer with CH2Cl2/MeOH (3:1). The collected organic phases were evaporated under vacuum at 40°C to a volume of ∼15 ml and lyophilized. An aliquot of the lyophilisate (10 mg) was resolved in 200 μl methanol and subjected to a high performance thin layer chromatography plate silica gel 60 without fluorescent indicator using the solvent system CHCl3/MeOH/H2O (65:25:4). The retention factor (Rf) of the intact glycolipid was 0.68. The substance line was scratched off and filtered over a glass filter (pore size 4) under vacuum after suspending with thin layer chromatography solvent. Finally, the solution was evaporated and lyophilized.
Cleavage of phosphodiester bonds by HF was accompanied by a migration of alanine to the primary hydroxy group of glycerol. The NMR analysis of the HF cleavage products (collected water phases after lyophilization) confirmed α-d-N-acetylglucosamine and d-alanine as the only substituents of the phosphoglycerol backbone. The membrane anchor bearing the β-linked gentiobiose disaccharide was assigned independently after HF cleavage. Transglycosidic nuclear Overhauser effects in the rotating frame NOESY spectrum identified the connectivity. The composition of the fatty acids was additionally investigated by GC–MS electron impact–induced fragment generation and pattern comparison with fragment patterns of reference fatty acids.
Results and Discussion
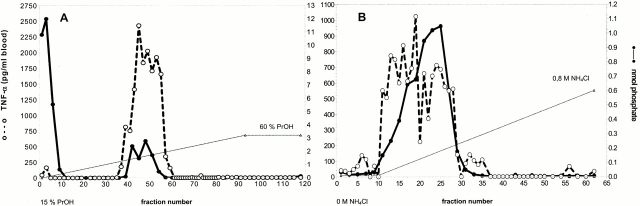
To test whether standard purification techniques for LTA 13 inactivate LTA from S. aureus during the purification process, the molecular structure of LTA and its biological activity was studied after modifications of the preparation procedure, i.e., replacing phenol by butanol extraction, extracting at RT, omitting dialysis, and using an ammonium acetate buffer for HIC on FPLC. Induction of TNF-α in human whole blood 14 was measured as lead activity (Fig. 1A and Fig. B). The cytokine-inducing activity essentially coeluted with the phosphate, which represents a measure for LTA, which comprises a polyglycerol-phosphate backbone. The fact that LTA and cytokine-inducing activity still coeluted after a subsequent DEAE–Sepharose anion exchange chromatography (Fig. 1 B) used as an orthogonal purification method makes contamination by other bacterial components unlikely. The cytokine releasing fractions were characterized by means of phosphate determination, NMR, MS, GC–MS, and carbohydrate, glycerol, and alanine analysis 15 16. Any contamination by Gram-negative LPS was excluded by negative Limulus assay (i.e., <6 pg LPS/mg LTA; QCL-1000; Biowhittaker), distinct pattern of cytokines induced (e.g., failure of LTA to induce IL-12 and IFN-γ; data not shown) in contrast to LPS, and some anti-CD14 antibodies (e.g., biG 3 obtained from Biometec and Leu M3 from Becton Dickinson), which inhibited LTA- but not LPS-inducible cytokine release, while other anti-CD14 antibodies (e.g., biG10; Biometec) blocked cytokine induction by both stimuli, suggesting an overlapping but distinct binding site.
Figure 1.
TNF-α release induced by eluate fractions after HIC of a butanol extract (A) and after anion exchange rechromatography on DEAE–Sepharose of HIC-purified LTA (B) from S. aureus. Broken line (○) indicates the TNF-α concentration in picograms per milliliter of human blood, and solid line (•) displays the amount of phosphate-containing substances (including LTA). The gradients (dotted lines) indicate the increasing percentage of propanol in 0.1 M ammonium acetate buffer in the case of HIC and the increasing ammonium chloride concentration in 35% n-propanol/0.1 M ammonium acetate buffer in the case of anion exchange rechromatography, pH = 4.7, respectively.
The highly purified LTA induced a whole blood cytokine response at as little as 10 ng/ml (see Fig. 3 A), i.e., exhibited an immunostimulatory potency similar to that of Pseudomonas aeruginosa LPS (≥10 ng/ml was required to induce cytokine release in human whole blood); at high concentrations of 10 μg/ml LTA, TNF-α levels similar to 10 μg/ml LPS were induced.
Figure 3.

(A) Concentration dependence of TNF-α response by human whole blood to S. aureus LTA. Data are mean ± SD of three donors, 10, 100, and 1000 ng/ml LTA, significantly different (P < 0.05) from 1 ng/ml LTA and control (paired Student's t test) (B) Comparison of TNF-α induction by intact LTA (solid line, •) and dealanylated LTA fractions (broken line, ○) after HIC. Dealanylation of LTA was achieved by increasing the pH of the water phase after butanol extraction under stirring at pH ≈ 8.5 with Tris buffer at RT (21°C) for 24 h. (C) Time course of alkaline hydrolysis of intact LTA, i.e., loss in d-alanine substitution and TNF-α induction capacity.
The molecular structure of LTA was investigated by NMR spectroscopy (Fig. 2). The 1H NMR resonances of genuine LTA were broadened due to the microheterogeneity of the isolated material. Yet after selective hydrolysis of the alanyl esters, signal resolution improved considerably. The doublet (3J = 3.6 Hz) at δH 5.08 was identified as the anomeric proton of α-d-N-acetylglucosamine with the corresponding anomeric carbon resonating at δC 99.7. The well resolved resonances after alanine ester hydrolysis resulted from a very homogeneous molecular environment around the α-d-N-acetylglucosamine moieties, i.e., their nearly equal spacing along the phosphoglycerol backbone. The integral ratio of glycerol and the broad but well separated α-methylene group of the membrane anchor at δH 2.2–2.5 identified an average chain length of n = 45–50. 70% of the glycerophosphate units were esterified with d-alanine, 15% bore α-d-N-acetylglucosamine, and 15% had no substituent. After HF cleavage, gentiobiose (glucose [Glc] α1-6Glcβ) was identified to link the polyglycero-phosphate chain to the diacylglycerol membrane anchor. The methyl signal (δH 0.9) of the gentiobiosyl-sn-diacylglycerol resolved to four carbon resonances in the indirect dimension of the inverse HMQC, indicating a high percentage of ω-branched fatty acids. No olefinic bonds were identified.
Figure 2.
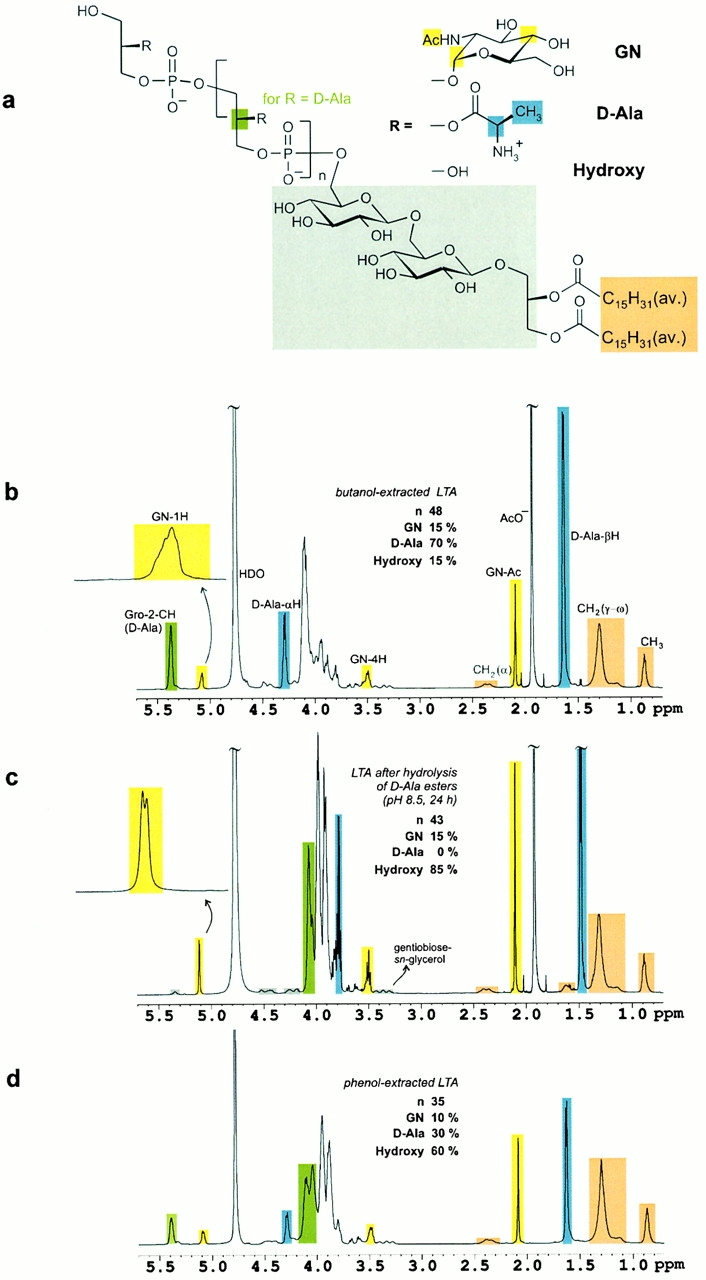
(A) The structure of S. aureus LTA as determined from NMR spectroscopic analysis. Color coding identifies the methine group of the sn-glycerol repeating unit (green), three resonance signals of d-N-acetylglucosamine (GN, yellow), d-alanine (d-Ala, blue), gentiobiose-sn-glycerol (gray), and fatty acids (orange). (B) 1H NMR spectrum (600 MHz, 300 K) of LTA obtained after butanol extraction. The resonance signal intensities allow for the quantification of the substituents and the average glycerophosphate chain length (45 < n < 50). Microheterogeneity leads to signal broadening as visible in the expansion of the anomeric proton of d-N-acetylglucosamine. (C) Hydrolysis of the d-alanine esters was performed at pH 8.5 in the NMR tube overnight. No cleavage of the fatty acids was observed under these conditions. The anomeric proton of N-acetylglucosamine resolved to a doublet with a vicinal coupling constant of 3.6 Hz as expected for an α-glycosidic bond. The gentiobiose resonances are highlighted in gray. (D) S. aureus LTA obtained after standard phenol extraction was characterized by a reduced chain length and significant hydrolysis of d-alanine esters. The alkyl chains of the fatty acids served as reference intensities for comparison of the three 1H NMR spectra, B–D.
The biologically active LTA isolated according to the improved preparation method was compared with a phenol-extracted LTA with minor stimulatory activity. A significant (∼50%) reduction of the d-alanine content was observed for phenol-extracted LTA, the 1H NMR spectrum of which is shown in Fig. 2 D, similar to LTA hydrolyzed at pH 8.5 (Fig. 2 C). In line with this observation, alkaline hydrolysis of the natural LTA resulted in a constant loss of d-alanine, paralleling a reduction in TNF-α induction capacity (Fig. 3 C). After 1 h of hydrolysis of the butanol-extracted LTA, alanine substituents were reduced by 55%, and the concentration response curve of TNF-α formation in human whole blood was shifted by two orders of magnitude (Fig. 3 B).
The amended protocol for the isolation of LTA from S. aureus allowed us to correlate structure and immunostimulatory properties of this biopolymeric molecule. Previously unknown structural details are the chain length of the polyglycerophosphate backbone, which is much longer (45 < n < 50) than reported previously 17 18 19, the α-d-N-acetylglucosamine substitution, which was not described for S. aureus LTA before, and the high degree (85%) of overall substitution with both d-alanine and α-d-N-acetylglucosamine, respectively. The critical role of alanine substituents for the biological activity of LTA from S. aureus was shown by deliberate hydrolysis of alanine as well as by comparison of phenol- and butanol-extracted preparations. Alanine measurements by established photometric methods 15 16 were in line with the reduced signals in NMR. Whether alanine substituents play a decisive role in the immunobiology of LTA from other bacterial species deserves further study. It might be speculated that alanine substituents enable hydrophobic interactions between LTA molecules; dimerization of LTA molecules by antiglycerophosphate antibodies has been shown to amplify cytokine induction by LTA 20.
When adequately purified (>99% pure as indicated by NMR analysis), the LTA represented an immunostimulus of similar potency to LPS from, for example, Pseudomonas aeruginosa. There is evidence for synergy with peptidoglycane and its breakdown products 21 22, which might further contribute to immunostimulation by LTA. In conclusion, LTA from S. aureus represents a distinct and potent immunostimulatory principle deserving further characterization.
Acknowledgments
The excellent technical assistance by Leo Cobianchi, Gregor Pinski, and Gunthard Stuebs, the prompt help by Ingo Spreitzer and Dr. Peter Roethlisberger, as well as the critical reading of the manuscript by Sonja von Aulock, Dr. Mary Ann Foote, and Dr. Stefanie Dimmeler is greatly appreciated.
References
- Zivot J.B., Hoffmann W.D. Pathogenic effects of endotoxin. New Horiz. 1995;3:267–275. [PubMed] [Google Scholar]
- Rietschel E.T., Brade H., Holst O., Brade L., Muller-Loennies S., Mamat U., Zahringer U., Beckmann F., Seydel U., Brandenburg K. Bacterial endotoxinchemical constitution, biological recognition, host response, and immunological detoxification. Curr. Top. Microbiol. Immunol. 1996;216:39–81. doi: 10.1007/978-3-642-80186-0_3. [DOI] [PubMed] [Google Scholar]
- Neely J.L. Staphylococcus aureusa continuing problem. WV Med. J. 1994;90:238–241. [PubMed] [Google Scholar]
- al-Ujayli B., Nafziger D.A., Saravolatz L. Pneumonia due to Staphylococcus aureus infection. Clin. Chest Med. 1995;16:111–120. [PubMed] [Google Scholar]
- Bone R.C. Gram-positive organisms and sepsis. Arch. Intern. Med. 1994;154:26–34. [PubMed] [Google Scholar]
- Friedman G., Silva E., Vincent J.L. Has the mortality of septic shock changed with time. Crit. Care Med. 1998;26:2078–2086. doi: 10.1097/00003246-199812000-00045. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Klonisch T., Nuber P., Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect. Immun. 1991;59:4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Yasuoka J., Suda Y., Takada H., Yoshida T., Kotani S., Kusumoto S. Structural feature of the major but not cytokine-inducing molecular species of lipoteichoic acid. J. Biochem. 1997;121:779–786. doi: 10.1093/oxfordjournals.jbchem.a021653. [DOI] [PubMed] [Google Scholar]
- Suda Y., Tochio H., Kawano K., Takada H., Yoshida T., Kotani S., Kusumoto S. Cytokine-inducing glycolipids in the lipoteichoic acid fraction from Enterococcus hirae ATCC 9790. FEMS Immunol. Med. Microbiol. 1995;12:97–112. doi: 10.1111/j.1574-695X.1995.tb00181.x. [DOI] [PubMed] [Google Scholar]
- De Kimpe S.J., Hunter M.L., Bryant C.E. Delayed circulatory failure due to the induction of nitric oxide synthase by lipoteichoic acid from Staphylococcus aureus in anaesthetized rats. Br. J. Pharmacol. 1995;114:1317–1323. doi: 10.1111/j.1476-5381.1995.tb13349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung T., Docke W.D., Gantner F., Krieger G., Sauer A., Stevens P., Volk H.D., Wendel A. Effect of granulocyte colony-stimulating factor treatment on ex vivo blood cytokine response in human volunteers. Blood. 1995;85:2482–2489. [PubMed] [Google Scholar]
- Kusunoki T., Hailman E., Juan T.S., Lichenstein H.S., Wright S.D. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J. Exp. Med. 1995;182:1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Koch H.U., Haas R. Improved preparation of lipoteichoic acids. Eur. J. Biochem. 1983;133:523–530. doi: 10.1111/j.1432-1033.1983.tb07495.x. [DOI] [PubMed] [Google Scholar]
- Hartung T., Sauer A., Wendel A. Testing of immunomodulatory properties in vitro. Dev. Biol. Stand. 1996;86:85–96. [PubMed] [Google Scholar]
- Nakano M., Fischer W. Trihexosyldiacylglycerol and acyltrihexosyldiacylglycerol as lipid anchors of the lipoteichoic acid of Lactobacillus casei DSM 20021. Hoppe-Seylers Z. Physiol. Chem. 1978;359:1–11. doi: 10.1515/bchm.1978.359.1.1. [DOI] [PubMed] [Google Scholar]
- Fischer W., Koch H.U., Rosel P., Fiedler F. Alanine ester-containing native lipoteichoic acids do not act as lipoteichoic acid carrier. Isolation, structural and functional characterization. J. Biol. Chem. 1980;255:4557–4562. [PubMed] [Google Scholar]
- Fischer W., Markwitz S., Labischinski H. Small-angle X-ray scattering analysis of pneumococcal lipoteichoic acid phase structure. Eur. J. Biochem. 1997;244:913–917. doi: 10.1111/j.1432-1033.1997.00913.x. [DOI] [PubMed] [Google Scholar]
- Ruhland G.J., Fiedler F. Occurrence and structure of lipoteichoic acids in the genus Staphylococcus . Arch. Microbiol. 1990;154:375–379. doi: 10.1007/BF00276534. [DOI] [PubMed] [Google Scholar]
- Fischer W., Mannsfeld T., Hagen G. On the basic structure of poly(glycerophosphate) lipoteichoic acids. Biochem. Cell Biol. 1990;68:33–43. doi: 10.1139/o90-005. [DOI] [PubMed] [Google Scholar]
- Mancuso G., Tomasello F., Ofek I., Teti G. Anti-lipoteichoic acid antibodies enhance release of cytokines by monocytes sensitized with lipoteichoic acid. Infect. Immun. 1994;62:1470–1473. doi: 10.1128/iai.62.4.1470-1473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kengatharan K.M., De Kimpe S.J., Robson C., Foster S.J., Thiemermann C. Mechanism of gram-positive shockidentification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J. Exp. Med. 1998;188:305–315. doi: 10.1084/jem.188.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kimpe S.J., Kengatharan M., Thiemermann C., Vane J.R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]