Abstract
Hematogenous precursors repopulate the thymus of normal adult mice, but it is not known whether this process is continuous or intermittent. Here, two approaches were used to demonstrate that the importation of prothymocytes in adult life is a gated phenomenon. In the first, age-dependent receptivity to thymic chimerism was studied in nonirradiated Ly 5 congenic mice by quantitative intrathymic and intravenous bone marrow (BM) adoptive transfer assays. In the second, the kinetics of importation of blood-borne prothymocytes was determined by timed separation of parabiotic mice. The results showed that >60% of 3–18-wk-old mice developed thymic chimerism after intrathymic injection of BM cells, and that the levels of chimerism (range, 5–90% donor-origin cells) varied cyclically (periodicity, 3 to 5 wk). In contrast, only 11–14% of intravenously injected recipients became chimeric, and chimerism occurred intermittently (receptive period ∼1 wk; refractory period ∼3 wk). In the intravenously injected mice, chimerism occurred simultaneously in both thymic lobes; gate opening occurred only after most intrathymic niches for prothymocytes had emptied; and the ensuing wave of thymocytopoiesis encompassed two periods of gating. These kinetics were confirmed in parabiotic mice, and in cohorts of mice in whom gating was synchronized by an initial intrathymic injection of BM cells. In addition, a protocol was developed by which sequential intravenous injections of BM cells over a 3 to 4 wk period routinely induces thymic chimerism in the apparent absence of stem cell chimerism. Hence, the results not only provide a new paradigm for the regulation of prothymocyte importation during adult life, but may also have applied implications for the selective induction of thymocytopoiesis in nonmyeloablated hosts.
Keywords: thymus, bone marrow, lymphoid organization, lymphoid migration, ontogeny
Introduction
The importation of hematogenous thymocyte precursors (prothymocytes) in late fetal and early postnatal life in birds and mice (as well as in larval and postmetamorphic frogs) appears to be a gated phenomenon characterized by brief periods of receptivity interspersed by longer periods of refractivity 1 2 3. It has been postulated that prothymocyte gating early in ontogeny permits the sequential generation, selection, and exportation of developmentally and functionally discrete populations of thymocytes, and that differential prothymocyte generation and intrathymic processing serves to coordinate both the establishment of the immunological repertoire and the distribution of specialized populations of T cells to skin, mucosa, liver, and peripheral lymphoid tissues (for reviews, see references 4 and 5).
Unfortunately, the serial transplantation/explantation procedures used in the preceding studies cannot be conducted with adult thymic lobes, even using vascular transplantation procedures 6. Therefore, it has not been possible, until now, to experimentally assess the possibility of continued prothymocyte gating beyond the neonatal period. In addition, several cogent arguments have been advanced against this proposition. These include the: (a) possible role of intrathymic precursors in maintaining thymocytopoiesis in adult animals (for a review, see reference 7); (b) failure of large doses of bone marrow (BM) cells to establish significant thymic chimerism in nonirradiated adult recipients 8 9 10; (c) absence of evidence for the sequential production of specialized populations of thymocytes in adult life 11 12; and (d) occurrence of age-related thymic involution and decreased T cell export 13. Yet despite these arguments, evidence exists that hematogenous precursors continue to maintain thymocytopoiesis throughout adult life 7; repetitive intravenous injections of BM cells can induce lymphoid chimerism in nonmyeloablated recipients 14; the developmental potential of thymocyte precursors continues to change postnatally 15; and recent thymic emigrants continue to contribute to the diversification of the TCR repertoire 16.
Based on the kinetics of induction of thymic chimerism in parabiotic mice, we have estimated that the replacement rate for prothymocytes in the adult thymus averages 2–3% per day 7. However, we could not discern whether this replacement was continuous or episodic. In this study, we have modified our previously described intrathymic and intravenous BM adoptive transfer systems 17 to determine the kinetics of prothymocyte recruitment in the thymus of nonmyeloablated adult mice. We also conducted timed separation experiments in parabiotic mice to detect the progeny of recently imported donor-origin prothymocytes. In both instances the results indicated that: (a) the importation of blood-borne prothymocytes is a gated phenomenon during at least the first 4 mo of normal postnatal life; (b) the receptive period (open gate) lasts ∼1 wk and coincides with the period of maximum availability of putative intrathymic niches for prothymocytes; and (c) the refractory period (closed gate) lasts ∼3 wk and includes the period of progressive emptying of these niches. Furthermore, using the periodicity of prothymocyte gating rather than chronological age, a protocol was devised by which repetitive intravenous injections of BM cells over a 3 to 4 wk period uniformly induces a wave of thymocyte chimerism in normal adult mice, but appears not to establish hemopoietic stem cell engraftment.
Materials and Methods
Animals.
Cohorts of 4 to 6-wk-old (± 3 d) male and female Ly 5 congeneic C57BL/6NCR(B6) mice, obtained from the National Cancer Institute, were housed in the Center for Laboratory Animal Care (The University of Connecticut Health Center) until they reached the designated ages. Animals were maintained on commercial mouse chow and water ad libitum. In some experiments, breeding pairs were established to generate younger progeny or progeny of timed matings. Cell transfer was carried out only in sex-matched combinations and, as no gender differences were noted, the data were pooled.
Preparation of Cell Suspensions.
BM cell suspensions were prepared by flushing the marrow from tibia and femur of 4 to 5-wk-old donors with cold RPMI 1640 (GIBCO BRL) supplemented with sodium bicarbonate (2 mg/ml) and 1% Hepes (1.5 M), as described 17. Repeated gentle pipetting further dispersed the cells, which were then washed in cold medium and centrifuged at 4°C for 5 min at 1,500 rpm. Thymocyte cell suspensions were prepared by gently pressing thymus lobes, stripped of attached lymph nodes, through a stainless steel screen (50 mesh), followed by washing in cold medium. Nucleated cells were counted on a Z1 Coulter Counter (Beckman Coulter).
Intrathymic Adoptive Transfer Assay for Prothymocytes.
The thymus was surgically exposed and one-half of the indicated number of BM cells were injected into the anterior superior portion of each lobe (10 μl/site) using a 1-ml syringe (with attached 28 gauge needle) mounted on a Tridek Stepper (Indicon Inc.), as described 17. The incision was closed with Nexaband Liquid (Veterinary Products Lab.). Control mice were injected intrathymically with RPMI alone, or one lobe was injected with BM cells and the contralateral lobe with RPMI, as indicated.
Intravenous Adoptive Transfer Assay for Prothymocytes.
The indicated number of BM cells suspended in 0.5 ml RPMI were injected through a 28 gauge needle into the lateral tail veins of unanesthetized recipient mice. Control mice were injected intravenously with RPMI alone.
Flow Immunocytometric Analysis.
Thymocytes were harvested 28 d after BM cell transfer, except as indicated. The percentages of donor and host-origin cells were determined by flow immunocytometric (FCM) analysis (FACScan™; Becton Dickinson) after development for immunofluorescence with anti-Ly 5.1 and anti-Ly 5.2 monoclonal antibodies (The Jackson Laboratory), and the expression of CD3, CD4, and/or CD8 was determined by multicolor analysis. Dead cells and nonlymphoid cells were excluded by gating for forward and side angle light scatter, and 10,000 viable cells were collected in each file. Specificity and sensitivity of staining were controlled by checkerboard analysis against normal Ly 5.1 and Ly 5.2 thymocytes and purposeful mixtures thereof. The percentage of positive cells was calculated by using the intersection of the fluorescence histogram with its control profile to determine the cutoff point.
Parabiosis.
Pairs of 4 to 5-wk-old, sex- and weight-matched, Ly 5 congenic mice were surgically joined by cutaneous vascular anastamosis as described previously 7. Parabiotic mice were maintained for periods of 1–9 wk before sacrifice, or were surgically separated at weekly intervals and killed 28 d later. Thymi from 4–6 pairs of nonseparated or separated parabiotic partners were harvested at the indicated time points and the respective degrees of chimerism were determined by FCM analysis.
Results
Frequency and Range of Thymic Chimerism Induced by Intrathymic and Intravenous Injection of BM Cells.
If, as is generally assumed, the kinetics of importation of thymocyte precursors in adult mice is constant under steady-state conditions, <5% of sites (presumptive microenvironmental niches) for prothymocyte engraftment should be available in the thymus at any given time 7 9. To test this prediction, suspensions of Ly 5.2 BM cells were injected intrathymically or intravenously into groups of nonirradiated Ly 5.1 mice of mixed ages (7–12 wk), using doses previously found to be saturating in irradiated recipients (2 × 106 cells intrathymically and 20 × 106 cells intravenously; reference 17). Thymocytes were harvested 28 d later, when peak levels of chimerism occur (reference 17; also see below).
As shown in Fig. 1, 86% of recipients displayed thymic chimerism after intrathymic injection, and the levels of chimerism attained were essentially random over a range of 5–90% donor origin cells. In contrast, only 13% of recipients developed significant chimerism after intravenous injection, and, although the levels of chimerism attained were broad (5–50% donor-origin cells), there was marked skewing towards the lower end of the scale. In both instances, >95% of the donor origin cells expressed CD3 (data not shown), formally demonstrating that they were thymocytes.
Figure 1.
Induction of thymic chimerism in normal adult mice by intravenous and intrathymic injection of BM cells. Groups of normal adult Ly 5.1 mice, obtained from multiple cohorts, were randomized for age (7–12 wk) and sex, and injected intravenously (black bars) or intrathymically (hatched bars) with suspensions of sex-matched Ly 5.2 BM cells (20 × 106 intravenously or 2 × 106 intrathymically) from 6-wk-old donors. The frequency and levels of thymic chimerism attained 28 d later were determined by FCM analysis. Data for male and female recipients are pooled, as no differences were observed. Percentage of chimeric mice (≥5% donor-origin cells): intravenous injection = 12.7% (21 of 165); intrathymic injection = 85.9% (122 of 142).
These results suggested that thymocytopoiesis in normal adult mice was most likely to be maintained by the periodic influx of saturating numbers of blood-borne prothymocytes. This hypothesis is formally confirmed below by age-response experiments conducted in individual cohorts of intrathymically or intravenously injected normal mice.
Cyclical Induction of Thymic Chimerism by Intrathymically Injected BM Cells.
In these experiments, age-matched (± 3 d) groups of 5 to 16-wk-old nonablated Ly 5.1 mice obtained from a single cohort were injected intrathymically with 2 × 106 Ly 5.2 BM cells. Results in Fig. 2 show that the frequency of thymic chimerism and mean level and number of donor origin thymocytes attained 28 d after injection varied cyclically, with peaks occurring at 5, 9, and 13 wk of age. The deepest valley (week 15) occurred after the onset of physiological thymus involution. Again, the range of donor origin thymocytes varied from <5 to >75% (data not shown). Furthermore, the maximal numbers of donor-origin thymocytes generated in nonirradiated recipients (120 × 106) were similar to those obtained in sublethally irradiated recipients 17. These results suggested that the age-related waves of thymic chimerism detected by intrathymic injection represented changes in the proportion of niches available for prothymocyte engraftment.
Figure 2.
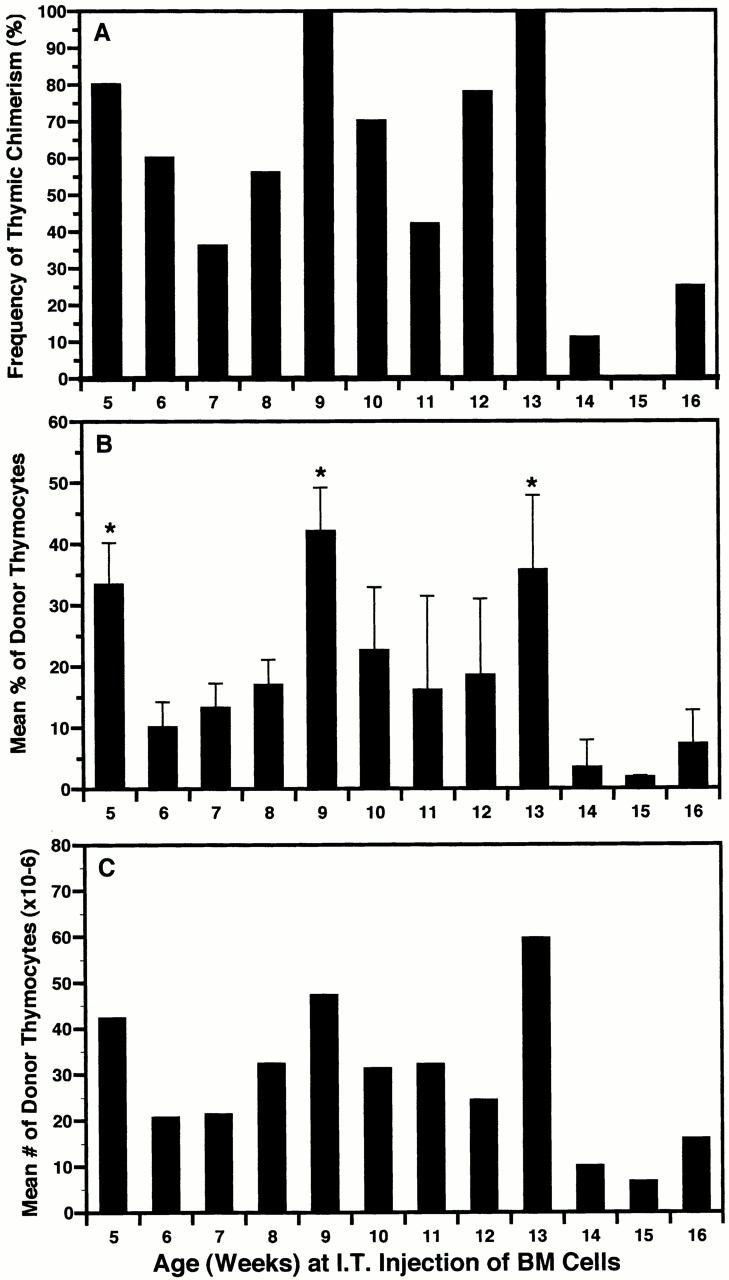
The induction of thymic chimerism in adult mice is cyclical after intrathymic (I.T.) injection of BM cells. A cohort of 5-wk-old (± 3 d) Ly 5.1 mice was divided into 12 groups (9–12 mice each) and, at weekly intervals, a different group was injected intrathymically with a saturating dose (2 × 106) of Ly 5.2 BM cells. The percentage of Ly 5.2 thymocytes present 28 d later was determined by FCM analysis. Results for total mice in each group (5–16 wk of age) are presented as: (A) frequency of thymic chimerism (≥5% donor-origin cells); (B) mean percentage of donor-origin thymocytes (± SD); and (C) mean number of donor-origin thymocytes. Percentage of chimeric mice = 62.5%. Maximum level of thymic chimerism = 88% (120 × 106 donor-origin cells). *P < 0.05 between highest and lowest values in each cycle. This experiment was repeated in part on two occasions using cohorts of mice varying in age from 5–8, 7–12, and 12–16 wk. Similar results were obtained, with peaks and valleys shifting by no more than 1 wk from those illustrated.
Periodic Induction of Thymic Chimerism by Intravenously Injected BM Cells.
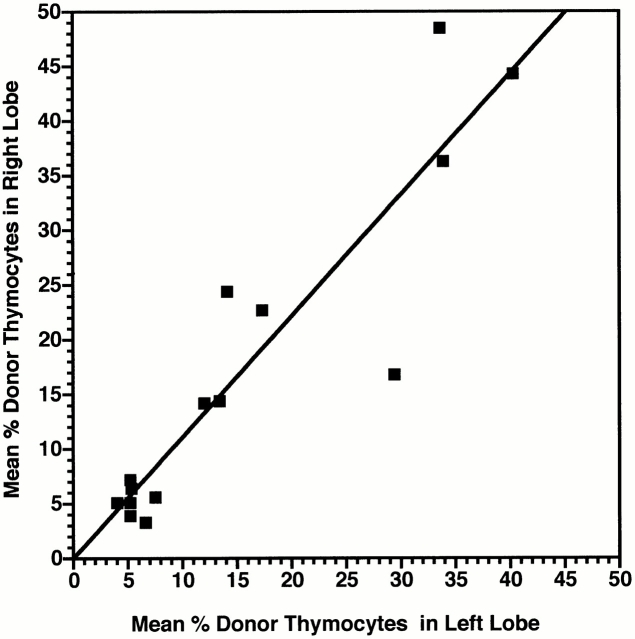
In these experiments, age-matched (± 3 d) groups of 3 to 18-wk-old nonablated Ly 5.1 mice were injected intravenously with 20 × 106 Ly 5.2 BM cells. Results in Fig. 3 show that the ability of intravenously injected BM cells to induce thymic chimerism was intermittent (periodicity 3–6 wk) rather than cyclical. Hence, although the spikes of receptivity in intravenously injected mice coincided roughly with the peaks of receptivity in intrathymically injected mice (Fig. 2), and the maximal percentage (78%) and number (98 × 106) of donor-origin thymocytes generated approximated those by intrathymic injection, the differential receptivity to intravenous and intrathymic injections at most other time points indicated that the importation of blood-borne prothymocytes was gated. Furthermore, paired analysis of individual thymic lobes in intravenously injected mice (Fig. 4) suggested that gate opening was tightly coordinated between both lobes of individual thymi.
Figure 3.
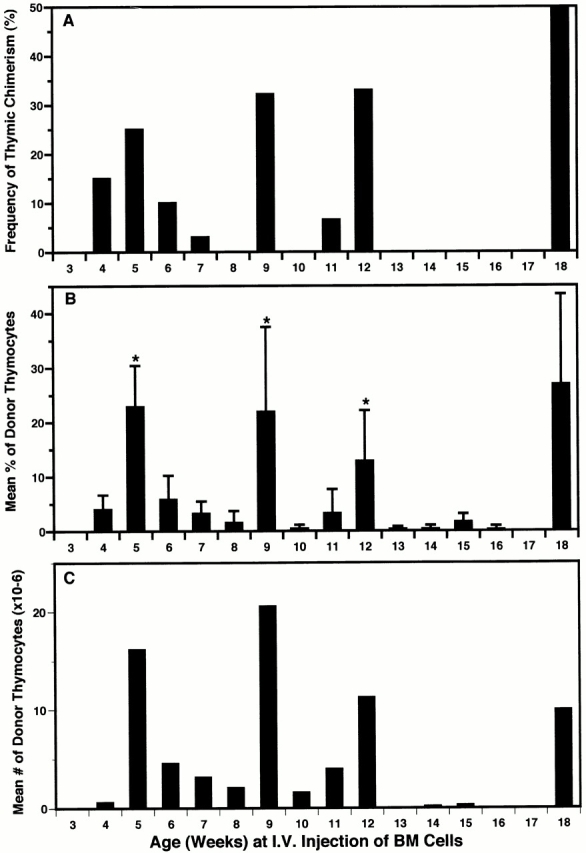
The induction of thymic chimerism in adult mice is periodic after intravenous (I.V.) injection of BM cells. A cohort of 3-wk-old (± 3 d) Ly 5.1 mice was divided into 16 groups (15–20 mice each) and, at weekly intervals, a different group was injected intravenously with 20 × 106 Ly 5.2 BM cells. The percentage of Ly 5.2 thymocytes present 28 d later was determined by FCM analysis. Results for total mice in each group (3–18 wk of age) are presented as: (A) frequency of thymic chimerism (≥5% donor-origin cells); (B) mean percentage of donor-origin thymocytes (± SD); and (C) mean number of donor-origin thymocytes. Percentage of chimeric mice = 10.9% (30 of 275). Maximum level of thymic chimerism = 78% (98 × 106 donor-origin cells). *P < 0.05 between highest and lowest values in sequential periods of receptivity and refractivity. This experiment was repeated in part on two occasions using cohorts of mice varying in age from 4–13, 7–12, and 12–18 wk. Similar results were obtained (± 1 wk).
Figure 4.
The induction of thymic chimerism in adult mice occurs in both lobes after intravenous injection of BM cells. Three cohorts of 5-wk-old (± 3 d) Ly 5.1 mice (total 30) were injected intravenously with a saturating dose of Ly 5.2 BM cells. The percentage of Ly 5.2 thymocytes present 28 d later in the left and right lobes of each thymus was determined by FCM analysis. Squares represent paired data for each animal in which at least one thymic lobe had ≥5% Ly 5.2+ cells. Line of best fit was determined by regression analysis (r 2 = 0.84; slope = 1.11; x and y intercepts = 0). Mean levels of thymic chimerism: left lobe = 16 ± 13%; right lobe = 17 ± 15%; P > 0.1.
Synchronization of Intrathymic Gating.
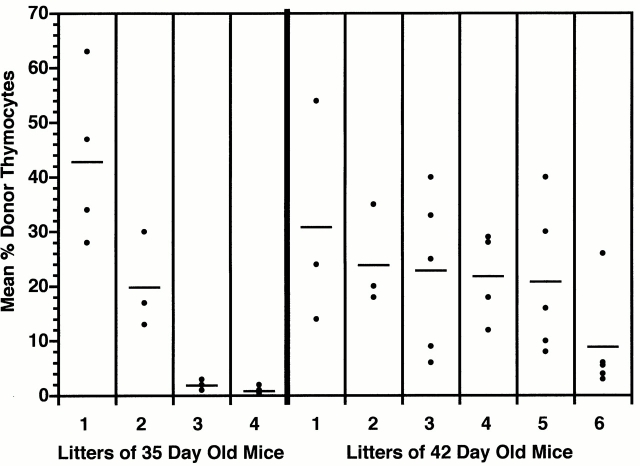
Despite the general correlation between maximal receptivity for the induction of thymic chimerism after intravenous and intrathymic injection of BM cells, the asynchrony within a given cohort of mice made it difficult to determine the precise relationship between these parameters. As shown in Fig. 5, this problem was not overcome by using litters of age- and sex-matched mice from timed matings. Thus, although the levels of chimerism attained after intrathymic injection were fairly uniform within individual litters, there was considerable asynchrony between litters. This was especially well illustrated in 35-d-old mice, in which the wide differences in mean levels of thymic chimerism between litters presumably reflected minor differences in the timing of gate opening for circulating host prothymocytes.
Figure 5.
Thymic chimerism is heterogeneous between age-matched litters of mice injected intrathymically with BM cells. Litters of age-matched male Ly 5.1 mice from timed matings were injected intrathymically with 2 × 106 Ly 5.2 BM cells on days 35 or 42. The percentage of Ly 5.2 thymocytes present 28 d later was determined by FCM analysis. Dots represent results for individual animals. Bars indicate mean level of thymic chimerism for each litter.
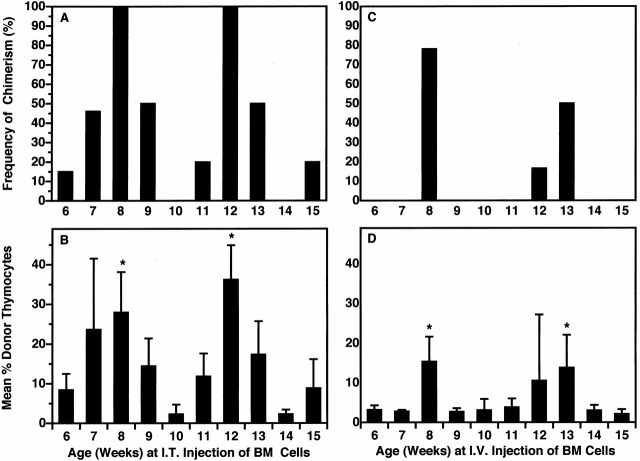
We therefore attempted to synchronize intrathymic gating in a cohort of 5-wk-old (± 3 d) Ly 5.1 mice by first filling all available niches for prothymocytes by intrathymic injection of host allotype BM cells. Subsequent intrathymic or intravenous injections of Ly 5.2 BM cells documented the resulting synchronization of chimerism with time. As shown in Fig. 6A and Fig. B, the frequencies and mean levels of thymic chimerism after intrathymic injection occurred in two clearly defined cycles. Each peak was preceded by an ∼2-wk period of increasing availability of putative niches for prothymocytes, followed by a 2-wk period of decreasing availability of niches (presumably due to occupation by recently imported host-origin precursors).
Figure 6.
Intrathymic gating is synchronized by initial intrathymic (I.T.) injection of BM cells. A cohort of 5-wk-old (± 3 d) Ly 5.1 mice was injected intrathymically with a saturating dose of Ly 5.1 BM cells, and at weekly intervals thereafter a separate group of 9–12 of these mice was reinjected with saturating doses of Ly 5.2 BM cells (A and B) intrathymically or (C and D) intravenously. The percentage of Ly 5.2 thymocytes present 28 d later was determined by FCM analysis. The frequencies (A and C) and mean levels (B and D) of thymic chimerism within each group were plotted as a function of age. Percentage of chimeric mice (≥5% donor-origin cells) after intrathymic injection = 62. Percentage of chimeric mice after intravenous (I.V.) injection = 13.8.
Similarly, results in Fig. 6C and Fig. D, formally documented the gated entry of intravenously injected precursors at times corresponding to maximal availability of niches for intrathymically injected precursors (weeks 8 and 12 to 13). These results also suggested that gate closure (refractory period) is initiated by the occupation of these niches (descending limbs; Fig. 6A and Fig. B), and that it persists through the subsequent period of increasing availability (emptying) of niches (ascending limbs; Fig. 6A and Fig. B).
As anticipated, synchronization of the importation of blood-borne precursors permitted a more precise analysis of the kinetics of thymocytopoiesis during the establishment of chimerism. Thus, although a single wave of thymocytopoiesis spanning two periods of prothymocyte gating (8–10 wk) was observed after intravenous injection of BM cells into either synchronized or nonsynchronized mice (Fig. 7), peak levels of thymocytopoiesis appeared to be reached ∼1 wk earlier and to persist for 1 wk less in the synchronized mice. Intermediate kinetics were observed after intrathymic injection of BM (data not shown). In addition, phenotypic analysis of the donor-origin thymocytes in intrathymically injected mice showed a progression between weeks 1 and 5 from double-negative (CD4−CD8−) to double-positive (CD4+CD8+) to single-positive (CD4+CD8− or CD4−CD8+) cells (Table ). Similar results were obtained after intravenous injection (data not shown). Hence, most of the donor-origin thymocytes in these assay systems appear to be generated by early lymphoid precursors in the BM cell inoculum.
Figure 7.
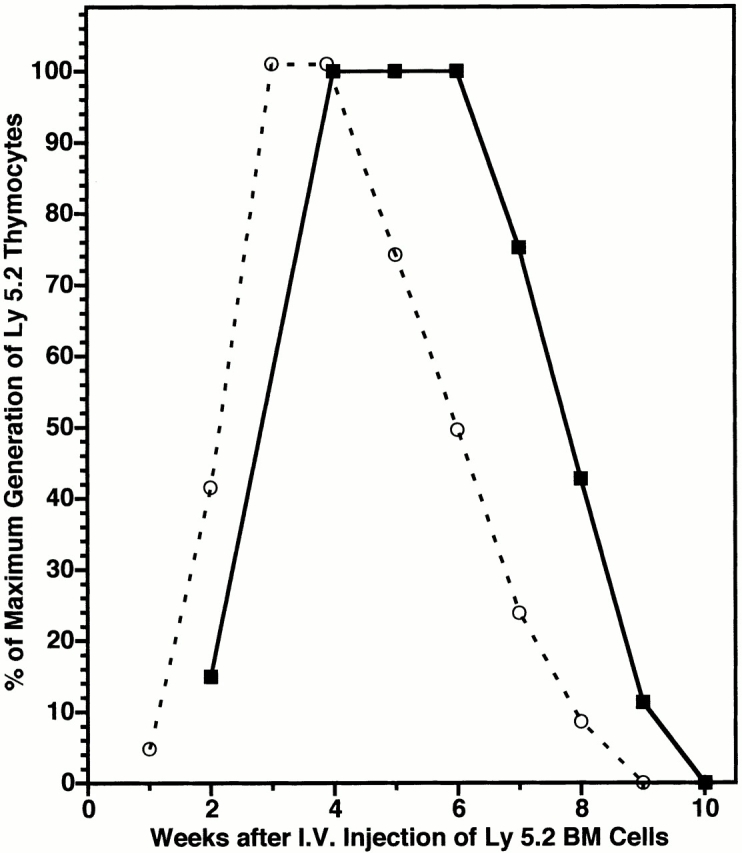
A single wave of thymocytopoiesis is induced by intravenous (I.V.) injection of BM cells into normal or synchronized adult mice. A cohort of 6-wk-old Ly 5.1 mice was injected intravenously (▪) with a saturating dose of Ly 5.2 BM cells. In addition, a cohort of 4-wk-old Ly 5.1 mice was synchronized by intrathymic injection of Ly 5.1 BM cells and reinjected intravenously 3 wk later with Ly 5.2 BM cells (○). Mean numbers of Ly 5.2 thymocytes present in groups of 5–10 mice were determined at weekly intervals thereafter. Results at each time point are expressed as percentage of maximal numbers of Ly 5.2 thymocytes generated to allow for differences in peak levels of chimerism.
Table 1.
Phenotypic Profile of Donor-Origin Thymocytes after Intrathymic Injection
| Percentage of positive cells at indicated weeks after injection | |||||
|---|---|---|---|---|---|
| Phenotype | 1 | 2 | 3 | 4 | 5 |
| CD4−CD8− | 59.9 ± 2.4 | 40.2 ± 6.5 | 3.4 ± 0.9 | 2.1 ± 0.4 | 2.0 ± 0.5 |
| CD4+CD8+ | 25.7 ± 5.1 | 52.5 ± 3.6 | 88.6 ± 1.5 | 87.7 ± 2.5 | 83.0 ± 3.0 |
| CD4+CD8− | 2.6 ± 1.5 | 3.6 ± 1.7 | 4.2 ± 1.3 | 7.7 ± 2.4 | 10.6 ± 2.5 |
| CD4−CD8+ | 11.7 ± 3.9 | 3.7 ± 1.9 | 3.8 ± 1.8 | 2.5 ± 0.1 | 4.4 ± 0.8 |
A cohort of 5-wk-old Ly5.1 mice was injected intrathymically with 2 × 106 Ly5.2 BM cells. Thymocytes were harvested from groups of five mice at the indicated times after injection and subjected to multiparameter FCM analysis. Results represent mean percentage ± SD of Ly5.2+ thymocytes that express the indicated CD4 and/or CD8 phenotypes.
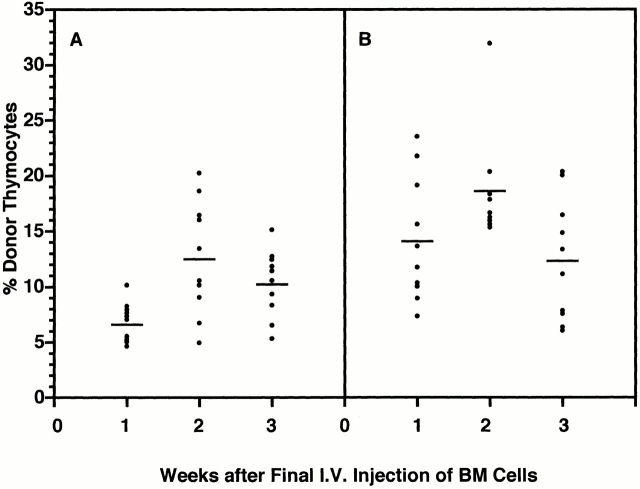
Reproducible Induction of Thymic Chimerism by Sequential Intravenous Injections of BM Cells.
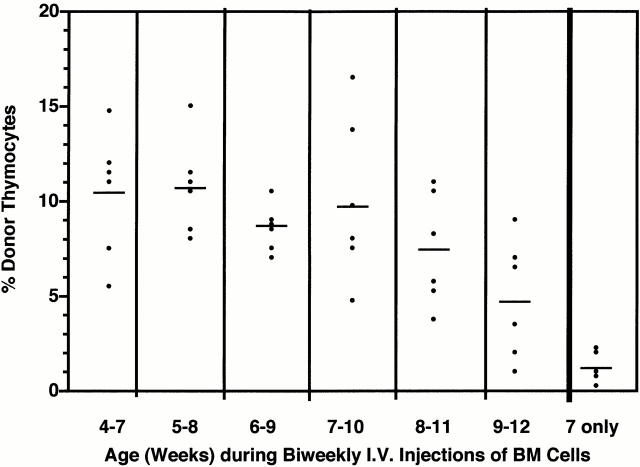
Given the periodicity of prothymocyte gating (∼4 wk) and the duration of the ensuing wave of thymocytopoiesis (∼8 wk), it should be possible to induce thymic chimerism reliably in nonsynchronized adult mice by repetitive intravenous injections of BM cells over a 3 to 4 wk period. This was confirmed in cohorts of 7-wk-old Ly 5.1 mice injected intravenously with 20 × 106 Ly 5.2 BM cells once or twice weekly for 4 wk and killed 1, 2, or 3 wk thereafter. Results in Fig. 8 revealed that: (a) all of the multiply injected mice developed significant thymic chimerism (range of donor-origin cells, 5–32%); (b) the highest levels of chimerism in this experiment occurred at week 2 of harvest; and (c) the mean peak chimerism was significantly higher (P < 0.05) after biweekly (19 ± 5%; Fig. 8 B) than weekly (13 ± 5%; Fig. 8 A) injections. In contrast, only 5% of 7-wk-old control mice that had been injected for 1 wk only developed thymic chimerism 4 to 6 wk later (data not shown). These results suggested that, in most of the animals, the gate for prothymocytes opened during the second week of injection (8 to 9 wk of age), as reflected by the peak of thymocytopoiesis 4 wk later.
Figure 8.
Sequential intravenous (I.V.) injections of BM cells routinely induce thymic chimerism in cohorts of 7-wk-old mice. Groups of 30 7-wk-old (± 3 d) Ly 5.1 mice from a single cohort were sequentially injected intravenously with saturating doses of Ly 5.2 BM cells (A) weekly on four occasions or (B) biweekly on eight occasions. Sets of 10 mice from each group were killed 1, 2, and 3 wk after the final injection, and levels of thymic chimerism were determined by FCM analysis. Dots represent results for individual animals. Bars indicate mean levels of thymic chimerism.
To determine if BM chimerism also occurred in these animals, 20 × 106 BM cells were harvested from each of 20 nonablated mice 1 wk after they had been given a series of four weekly or eight biweekly intravenous injections. These cells were then transferred intravenously into sublethally irradiated (6 Gy) Ly 5.1 recipients, which were analyzed 28 d later for thymic chimerism. Only one of the secondary recipients became chimeric (data not shown). In contrast, all irradiated recipients became chimeric when they were injected with purposeful mixtures of normal BM cells at donor/host ratios as low as 1:40. Hence it appeared that stem cell chimerism capable of generating prothymocytes had not been established in the BM of the primary (nonablated) recipients.
These experiments were then expanded to encompass two gate-openings. Groups of 4 to 9-wk-old mice were injected with BM biweekly for a total of seven injections and killed 2 wk later. As shown in Fig. 9, thymic chimerism was obtained in the majority of multiply injected mice in each age group, but not in control mice injected during week 7 only. Hence, the observed chimerism induced by intravenous injections given before or after week 7 must have resulted from separate gate openings.
Figure 9.
Sequential intravenous (I.V.) injections of BM cells routinely induce thymic chimerism in cohorts of 4 to 9-wk-old mice. A cohort of 4-wk-old (± 3 d) Ly 5.1 mice was divided into seven groups (six mice each) and, at weekly intervals, a different group was enrolled in a course of seven biweekly intravenous injections (3 wk) of Ly 5.2 BM cells. Levels of thymic chimerism for each group were determined 2 wk after the final injection. Dots indicate results for individual animals. Bars indicate mean chimerism for each group (identified by elapsed ages during injections). The group designated “7 only” received two intravenous injections of BM cells during week 7 only and was analyzed for thymic chimerism at week 12 (i.e., at the same time as the 7–10 wk group).
Gated Importation of Prothymocytes in Parabiotic Mice.
In the preceding experiments, mice received single or multiple intravenous injections of large numbers (20 × 106) of BM cells. It therefore was possible that the physiological mechanisms that regulate the importation of prothymocytes were periodically overwhelmed, yielding spikes of thymic chimerism. To exclude this possibility, the kinetics of importation of hematogenous precursors were studied in parabiotic mice, whose thymi presumably are exposed to physiological numbers of blood-borne prothymocytes at physiological intervals. 5-wk-old Ly 5 congeneic mice were parabiosed for periods of 1 to 9 wk and then surgically separated to prevent further exchange of blood-borne prothymocytes, the primary mechanism for maintaining thymic chimerism in such animals 7. Under these circumstances, the occurrence of thymic chimerism 4 wk later was presumed to reflect the entry of donor-origin prothymocytes shortly before the time of separation.
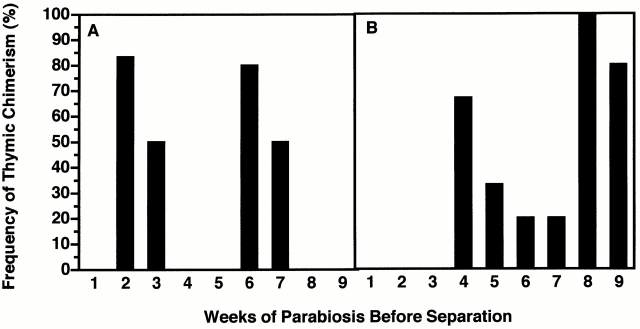
Results in Fig. 10A and Fig. B, showed two windows of receptivity for induction of thymic chimerism in the separated parabiotic partners, the first occurring at week 2 (Ly 5.2 mice) or week 4 (Ly 5.1 mice) of parabiosis, and the second 4 wk later. In addition, the mean levels of thymic chimerism attained after the first windows of receptivity closely approximated those observed 4 wk later in unseparated parabionts (data not shown). This further indicated that the initial waves of prothymocyte importation had occurred at weeks 2 and 4 of parabiosis, respectively.
Figure 10.
The induction of thymic chimerism is periodic in parabiotic mice. Ly 5.1 and Ly 5.2 congenic mice were parabiosed at 5 wk of age, and groups of 4–6 parabiotic pairs were surgically separated at weekly intervals over a 9-wk period. The frequency of thymic chimerism (≥5% donor-origin cells) in the separated (A) Ly 5.2 and (B) Ly 5.1 parabiotic partners was determined 28 d later.
Discussion
Two experimental approaches were used to demonstrate that thymocytopoiesis is a gated phenomenon in normal adult mice. First, age-related transfer of BM cells into nonablated mice revealed a cyclical pattern of engraftment of intrathymically injected thymocyte precursors, and an intermittent pattern of engraftment of intravenously injected precursors. Both patterns had average periodicities of 4 wk, and receptivity for intravenously injected precursors correlated with maximum availability of putative niches for intrathymically injected precursors. Second, timed separation of parabiotic mice also revealed an intermittent pattern of importation of hematogenous precursors into thymus, again with a periodicity of ∼4 wk.
Experiments in mice in which gating was synchronized by an initial intrathymic injection of host-allotype BM cells were especially useful in establishing the kinetics of prothymocyte importation. This model demonstrated that the gate for hematogenous precursors opens for ∼1 wk to allow the niches to fill and then closes for 2 to 3 wk to allow them to empty. In addition, the mean duration of a wave of thymocytopoiesis in nonablated recipients of BM, as in parabiotic mice 7, was found to exceed the periodicity of gating by twofold. This is important, as proportionately shorter waves would not generate a steady-state pattern of thymocyte production. Furthermore, the ability of intrathymically injected BM cells both to synchronize and alter the time of gate opening in nonablated mice suggested that, under steady-state conditions, gate closing is initiated by the coordinated filling of most, if not all, niches for prothymocytes. Conversely, the temporal association of prothymocyte importation with the availability (emptying) of most, if not all, of these niches suggested that gate opening may be regulated by a threshold-dependent, downstream signal. An integrated model of thymocytopoiesis and prothymocyte gating based on these considerations is presented in Fig. 11.
Figure 11.
Integrated scheme of the kinetics of prothymocyte gating, occupation of microenvironmental niches, and generation of thymocytes in normal mice. Clusters of vertical arrows represent receptive periods (open gate) and horizontal black bars represent refractory periods (closed gate) for importation of hematogenous prothymocytes. Shaded triangles represent filling/equilibration (up slope) and emptying (down slope) phases of occupation of a finite number of intrathymic niches by prothymocytes and their immediate descendants. Dashed, dotted, and mixed symbol curves represent sequential waves of thymocytopoiesis, each generated by the gated importation of a saturating wave of prothymocytes. The lag period of thymocytopoiesis corresponds roughly to the filling/equilibration phase of occupation of the intrathymic niches. The duration of each wave of thymocytopoiesis exceeds the periodicity of gate-opening by twofold, so as to maintain relatively constant levels of total thymocytes. Gate closing appears to be initiated by occupation of intrathymic niches. Gate opening appears to be regulated in a threshold-dependent (all-or-none) manner by emptying of intrathymic niches, and to be synchronized between thymus lobes. The onset of thymic involution occurs at about week 12 and is partly related to a decrease in the number of available intrathymic niches for prothymocytes (unpublished observations). Although drawn as discrete curves, each idealized wave of thymocytopoiesis may actually consist of a series of partially overlapping waves (reference 9). Similarly, the prothymocyte “gate” may actually be a series of individual microvascular gates. The idealized time scale (weeks) approximates, but is not necessarily identical to, chronological age. The receptivity of normal neonatal mice and rats (week 0) to the induction of thymic chimerism by intravenously injected BM cells has been established in earlier studies (references 23 and 24).
Our ongoing studies suggest that the availability of saturating levels of prothymocytes to the thymus at the time of gate opening normally is assured by a feedback loop that regulates the periodic release of waves of prothymocytes from the BM (unpublished observations). Although it is tempting to speculate that this same feedback loop triggers gate opening itself, neither the nature of the gate(s) nor the signal(s) that regulates its activity is known. Thus, although gate opening occurs simultaneously in both thymic lobes (Fig. 4), the asynchronous development of thymic chimerism in parabiotic partners (Fig. 10) suggests that the signal for gate opening either does not effectively cross-circulate in the blood or is otherwise unable to stimulate the refractory partner.
It might be argued that the differential kinetics of receptivity to the establishment of thymic chimerism after intravenous and intrathymic injection of BM is not due to gating, but to occupation of different sets of binding sites by the intravenously and intrathymically injected precursors. This is highly unlikely, as differential occupation of binding sites would not explain: (a) the ability of intrathymically injected BM cells to synchronize gating for intravenously injected BM cells; (b) the origin of donor thymocytes from CD4−CD8− precursors in both assay systems; or (c) the gated entry of circulating precursors into the thymus of parabiotic mice. It is also unlikely that the periodic importation of prothymocytes in adult life is due to cyclical hormonal changes or to inapparent stress. Thus, gating is not sex-related, and dexamethasone treatment does not predispose recipients to thymocyte chimerism after intravenous or intrathymic injection (reference 9; and our unpublished observations). Rather, the fact that both the intravenous and intrathymic assays obey strict log dose saturation kinetics and generate the same maximum number of thymocytes 17 favors the existence of a finite number of specific binding sites (niches) for prothymocytes. This notion is further supported by our recent demonstration of competitive one-on-one occupancy kinetics for binding sites after combined intravenous and intrathymic injections of BM cells into radioablated mice (unpublished observation).
Pragmatically, single intravenous injections of BM cells into young adult mice of nominally receptive ages may not consistently establish thymic chimerism because of asynchronous gating, and synchronization of gating by initial intrathymic injection does not lend itself to routine use. Instead, reliance on the periodicity of gating, rather than chronological age, appears to be a more efficient approach to inducing thymic chimerism in heterogeneous groups of mice. Thus, as shown in Fig. 8 and Fig. 9, biweekly intravenous injections of BM cells over a period of 3 to 4 wk (7 to 8 injections) reproducibly generates significant thymic chimerism in most recipients. Although more frequent injections or a more prolonged course of injections might further improve this protocol, at some point hemopoietic stem cell chimerism will occur in BM 14, after which the development of thymic chimerism no longer will reflect the direct importation of prothymocytes from the original inoculum.
As mentioned, gated importation of waves of prothymocytes serves to generate discrete populations of T cells in a programmed fashion in late fetal and early neonatal life 1 2 3 4 5. Yet, absent evidence for the continued need to generate developmentally disparate waves of thymocytes, the possible immunobiological benefits of intermittent importation of prothymocytes in adult life can only be surmised. At one extreme, the benefits of continued prothymocyte gating may relate primarily to matters of quantitative efficiency, whereby monthly restocking of the intrathymic “warehouse” with a saturating dose of prothymocytes eliminates the need for constant monitoring of inventory and available space. At the other extreme, prothymocyte gating may relate more to matters of qualitative efficiency, whereby periodic changes in the antigenic milieu can be selectively monitored 18. Such a mechanism could serve to update the immunological repertoire throughout life by optimizing the production and selection of new T cell specificities.
A particularly attractive scenario under the latter rubric would be for each wave of developing thymocytes to be selected against newly processed antigenic peptides presented by a coordinate wave of newly generated and/or imported thymic dendritic cells 19 20. Evidence for parallel generation and interaction of waves of thymocytes and thymic dendritic cells has been provided in the fetal/neonatal period and in adult radiation BM chimeras 21. In addition, we have observed parallel waves of thymocyte and dendritic cell chimerism in parabiotic mice and in nonablated adult mice injected intravenously or intrathymically with BM cells (unpublished observations). A similar, nonexclusive scenario envisions the coordinate selection of waves of developing thymocytes by the developmentally and/or temporally regulated expression of “extrathymic” gene products by thymic epithelial cells 22.
In sum, the present results provide a potentially new paradigm for thymic function in adult life based on the regulated importation of waves of hematogenous prothymocytes in coordination with the maximal availability of intrathymic binding sites. At a basic level, these observations should expedite the fine analysis of the earliest stages of thymocytopoiesis, allow the identification of microenvironmental niches for prothymocytes, and permit the characterization of the feedback loop(s) that regulates prothymocyte gating. They should also provide a direct approach to determining if the receptor repertoire of a given wave of emerging T cells is biased towards newly introduced and/or newly expressed peptide specificities. In addition, by offering a protocol for inducing individual waves of thymic chimerism on demand in nonmyeloablated recipients, the results may have applied implications for selective prothymocyte engraftment.
Acknowledgments
We would like to thank Dr. Lynn Puddington for her expert assistance in the FCM analyses.
This study was supported in part by National Institutes of Health Grant AI33741.
Footnotes
Abbreviations used in this paper: BM, bone marrow; FCM, flow immunocytometric.
References
- Jotereau F.V., LeDouarin N.M. Demonstration of a cyclic renewal of the lymphocyte precursor cells in the quail thymus during embryonic and perinatal life. J. Immunol. 1982;129:1869–1877. [PubMed] [Google Scholar]
- Jotereau F.V., Heuze F., Salmon-vie V., Gascan H. Cell kinetics in the fetal mouse thymusprecursor cell input, proliferation, and emigration. J. Immunol. 1987;138:1026–1030. [PubMed] [Google Scholar]
- Bechtold T.E., Smith P.B., Turpen J.B. Differential stem cell contributions to thymocyte succession during development of Xenopus laevis . J. Immunol. 1992;148:2975–2982. [PubMed] [Google Scholar]
- Shortman K., Wu L. Early T lymphocyte progenitors. Annu. Rev. Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- Dunon D., Courtois D., Vainio O., Six A., Chen C.H., Cooper M.D., Dangy J.P., Imhof B.A. Ontogeny of the immune systemγ/δ and α/β T cells migrate from thymus to periphery in alternating waves. J. Exp. Med. 1997;186:977–988. doi: 10.1084/jem.186.7.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga J., Pol G.H., Bartels H., Duijvestijn A.M., Marawska M.B., van de Berk J.M.M.M., Nieuwenhuis P. Vascular thymus transplantation in ratsa new method to study thymocyte kinetics. Adv. Exp. Med. Biol. 1988;237:333–338. doi: 10.1007/978-1-4684-5535-9_49. [DOI] [PubMed] [Google Scholar]
- Donskoy E., Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J. Immunol. 1992;148:1604–1612. [PubMed] [Google Scholar]
- Micklem H.S., Clarke C.M., Evans E.P., Ford C.E. Fate of chromosome-marked mouse bone-marrow cells transfused into normal syngeneic recipients. Transplantation. 1968;6:299–302. [PubMed] [Google Scholar]
- Scollay R., Smith J., Stauffer V. Dynamics of early T cellsprothymocyte migration and proliferation in the adult mouse thymus. Immunol. Rev. 1986;91:129–157. doi: 10.1111/j.1600-065x.1986.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Kyewski B.A. Seeding of thymic microenvironments defined by distinct thymocyte-stromal cell interactions is developmentally controlled. J. Exp. Med. 1987;166:520–538. doi: 10.1084/jem.166.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E., Medvinsky A., De Bruijn M. Qualitative and quantitative aspects of hematopoietic cell development in the mammalian embryo. Immunol. Today. 1998;19:228–236. doi: 10.1016/s0167-5699(98)01258-4. [DOI] [PubMed] [Google Scholar]
- Adkins B., Hamilton K. Developmental ages of the thymic epithelium and of the T cell precursors together determine the proportions of peripheral CD4+ cells. J. Immunol. 1994;153:5359–5365. [PubMed] [Google Scholar]
- Scollay R.G., Butcher E.C., Weissman I.L. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur. J. Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- Blomberg M., Rao S., Reilly J., Tiarks C., Peters S., Kittler E., Quesenberry P. Repetitive bone marrow transplantation in nonmyeloablated recipients. Exp. Hematol. 1998;26:320–324. [PubMed] [Google Scholar]
- Ikuta K., Kina T., MacNeil I., Uchida N., Peault B., Chien Y.-H., Weissman I.L. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hemotopoietic stem cells. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- Poulin J.F., Viswanathan M.N., Harris J.M., Komanduri K.V., Wieder E., Ringvette N., Jenkins M., McCune J.M., Sekaly R.P. Direct evidence for thymic function in adult humans. J. Exp. Med. 1999;190:479–488. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Komschlies K.L., Greiner D.L. Studies of thymocytopoiesis in rats and mice. I. Kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. J. Exp. Med. 1986;163:1–17. doi: 10.1084/jem.163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchot C., Rocha B. Peripheral selection of T cell repertoiresthe role of continuous thymus output. J. Exp. Med. 1997;186:1099–1106. doi: 10.1084/jem.186.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardavin C. Thymic dendritic cells. Immunol. Today. 1997;18:350–361. doi: 10.1016/s0167-5699(97)01090-6. [DOI] [PubMed] [Google Scholar]
- Shortman K., Vremec D., Corcoran L.M., Georgopoulos K., Lucas K., Wu L. The linkage between T-cell and dendritic cell development in the mouse thymus. Immunol. Rev. 1998;165:39–46. doi: 10.1111/j.1600-065x.1998.tb01228.x. [DOI] [PubMed] [Google Scholar]
- Kyewski B.A., Rouse R.V., Kaplan H.S. Thymocyte rosettesmulticellular complexes of lymphocytes and bone marrow-derived stromal cells in the mouse thymus. Proc. Natl. Acad. Sci. USA. 1982;79:5646–5650. doi: 10.1073/pnas.79.18.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A.G., Rudensky A. Medullary thymic epitheliuma mosaic of epithelial “self”? J. Exp. Med. 1998;188:1–4. doi: 10.1084/jem.188.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M.A.S., Owen J.J.T. Experimental studies on the development of the thymus. J. Exp. Med. 1967;126:715–726. doi: 10.1084/jem.126.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., McGregor D.D. Development of immunologically competent lymphocytes in the rat. Nature. 1966;232:1433–1435. doi: 10.1038/2121433a0. [DOI] [PubMed] [Google Scholar]