Abstract
Reduced mechanical stress to bone in bedridden patients and astronauts leads to bone loss and increase in fracture risk which is one of the major medical and health issues in modern aging society and space medicine. However, no molecule involved in the mechanisms underlying this phenomenon has been identified to date. Osteopontin (OPN) is one of the major noncollagenous proteins in bone matrix, but its function in mediating physical-force effects on bone in vivo has not been known. To investigate the possible requirement for OPN in the transduction of mechanical signaling in bone metabolism in vivo, we examined the effect of unloading on the bones of OPN−/− mice using a tail suspension model. In contrast to the tail suspension–induced bone loss in wild-type mice, OPN−/− mice did not lose bone. Elevation of urinary deoxypyridinoline levels due to unloading was observed in wild-type but not in OPN−/− mice. Analysis of the mechanisms of OPN deficiency–dependent reduction in bone on the cellular basis resulted in two unexpected findings. First, osteoclasts, which were increased by unloading in wild-type mice, were not increased by tail suspension in OPN−/− mice. Second, measures of osteoblastic bone formation, which were decreased in wild-type mice by unloading, were not altered in OPN−/− mice. These observations indicate that the presence of OPN is a prerequisite for the activation of osteoclastic bone resorption and for the reduction in osteoblastic bone formation in unloaded mice. Thus, OPN is a molecule required for the bone loss induced by mechanical stress that regulates the functions of osteoblasts and osteoclasts.
Keywords: osteopontin, mechanical stress, osteoblasts, osteoclasts, tail suspension
Introduction
Bone is a highly dynamic tissue that has evolved over millions of years on earth under gravitational stress to provide mechanical support for both locomotion and protection, to serve as a calcium reservoir for mineral homeostasis, and to support hemopoiesis 1. The structure, organization, and remodeling of bone are sensitive to the mechanical environment evidenced by the bone loss in bedridden patients or space flight, as well as the increase in bone mass in athletes participating in high impact sports 2. Especially in the modern aging society, where the incidence of osteoporosis is soaring, bone loss due to reduced physical activity in aged people is one of the major risk factors for this disease. In contrast to the presence of these valid observations on the effects of physical force on bone metabolism, no molecule has been identified to date to mediate the effects of physical force on bone in vivo.
Integrins mediate extracellular signals to the cells and are involved in several physiological and pathological events 3 4. Integrins have been suggested also to transduce signals to the cells in response to mechanical stimuli 5 6 7. One of the integrin ligands present in bone is osteopontin (OPN). OPN is an arginine-glycine-aspartate–containing noncollagenous protein abundant in the bone matrix that is produced by osteoblasts and osteoclasts. It may facilitate osteoclast attachment to the mineralized extracellular matrix during bone resorption 8 9 10 11. Although OPN does not appear to be required for normal development of bones 12 13 14, the absence of OPN makes the animal less sensitive to ovariectomy-induced bone resorption 15.
OPN expression is regulated by calcitropic cytokines and hormones as well as mechanical stress both in vitro and in vivo. Intermittent hydrostatic compression, four point bending, and experimental tooth movement enhance OPN expression in osteoblasts or osteoclasts in vitro or in vivo 16 17 18 19 20. Mechanical forces have been suggested to act at the sites of cell attachment, possibly involving extracellular matrix proteins including OPN, to generate a shear stress at adhesion plaques, to transmit signals via integrins to the cytoskeleton, and to modify cell shape and gene expression 21. These observations suggest that OPN is involved in the alterations in bone metabolism induced by mechanical stress; however, there is as yet no direct evidence for this hypothesis either in vitro or in vivo.
The aim of the work reported here is to investigate the role of OPN in bone loss induced by the reduction of mechanical stress by using OPN knockout mice subjected to tail suspension.
Materials and Methods
Animals.
Female wild-type and OPN−/− mice in a 129/S3XC57BL/6F2 background 12 derived from the original heterozygous crosses were maintained as separate colonies. 12-wk-old female OPN−/− and wild-type mice (48 mice in total), 18–23 g in body weight, were used in the experiments and randomly assigned in equal numbers to loaded control and unloaded tail suspension groups.
Tail Suspension Model.
For tail suspension, a tape was applied to the surface of the tail to set a metal clip. The end of the clip was fixed to an overhead bar and the height of the bar was adjusted to maintain the mice at an ∼30° headdown tilt with the hindlimbs elevated above the floor of the cage. One-half of the mice in the tail suspension group was subjected to tail suspension for 2 wk and the other half for 4 wk (n = 6 per group). Loaded control mice were also housed individually under the same condition except for tail suspension for the same duration (2 or 4 wk). The mice were injected intraperitoneally with calcein at 4 mg/kg 4 and 2 d before killing at 2 wk. After 2 or 4 wk of tail suspension, mice were anesthetized with pentobarbital and were killed by cervical dislocation.
Body Weight.
Body weights of either loaded or tail-suspended mice, measured every day, were not altered during the 2- and 4-wk experiments in both genotypes (data not shown). This confirms that stress can be considered minimal in our experiments, as previously concluded 22 23.
Analysis of Bone Length.
The lengths of the femora and tibiae measured on x-ray film were not altered in tail-suspended animals compared with the loaded control in both genotypes after 4 wk of tail suspension (data not shown).
μ-CT Analysis of Bone.
For measurements of the bone volume (BV/tissue volume [TV]), the bones were subjected to micro-x-ray computed tomography (μ-CT) analysis, using Musashi (Nittetsu-ELEX). The data were subsequently quantified by using a Luzex-F automated image analysis system (Nireco). The fractional bone volume (BV/TV) was measured in the area of 0.39 mm2 with its closest and furthest edges at 0.34- and 0.62-mm distal to the growth plate of the proximal ends of the tibiae. Threshold for the measurements was set at 110 for the analyses.
Deoxypyridinoline Measurement.
Urinary deoxypyridinoline (Dpyd) levels on day 14 of the tail suspension were measured by ELISA (Metra Biosystems; reference 24). Urine collected from two mice in a metabolic cage during the last 24 h (on day 14 of tail suspension) was combined and three independent samples from each group were analyzed.
Histomorphometric Analysis of Bone.
For decalcified sections (left tibiae), serial 5-μm-thick sagittal sections were made using a microtome and stained for tartrate-resistant acid phosphatase (TRAP) followed by staining with toluidine blue. TRAP-positive multinucleated cells attached to bone were scored as osteoclasts. Measurements were made within an area of 0.32 mm2 with its closest and furthest edges at 0.35- and 0.60-mm distal to the growth plate of the proximal ends of the tibiae. Histomorphometry was conducted to quantify the number of osteoclasts (N.Oc/BS) and osteoclast surface (Oc.S/BS) as defined by Parfitt et al. 25.
For undecalcified sections (right tibiae), serial 3-μm-thick frontal sections were made using a microtome. The metaphyseal cancellous bone fraction was measured in an area with its closest and furthest edges at 0.35- and 0.60-mm distal to the growth plate. All the histomorphometric analyses were conducted within this area.
Statistical Evaluations.
The results are presented as mean values ± SEM. Statistical analysis was performed by Mann-Whitney's U test. A P value < 0.05 was considered to be statistically significant.
Results
Unloading Does Not Reduce Bone in OPN-deficient Mice.
In wild-type mice, trabecular bones in the midsagittal plane of the metaphyseas in the tibiae observed in μ-CT analysis became sparse due to unloading as expected (Fig. 1 A). In contrast to wild-type, no deficiency in trabecular bone pattern was observed in OPN−/− mice even after tail suspension (Fig. 1 A). Quantification of the fractional trabecular bone volume (BV/TV) in the μ-CT analysis indicated ∼50% reduction in wild-type after tail suspension (Fig. 1 B, P <0.05), but in OPN−/− mice no significant decrease was observed (Fig. 1 B). The absence of the tail suspension–induced bone loss in OPN−/− mice was similarly observed at 2 wk of tail suspension (data not shown) compared with 4 wk (Fig. 1A and Fig. B). The basal levels of bone volume fraction of the cancellous bones in OPN−/− mice were not different from those in wild-type (Fig. 1 B).
Figure 1.
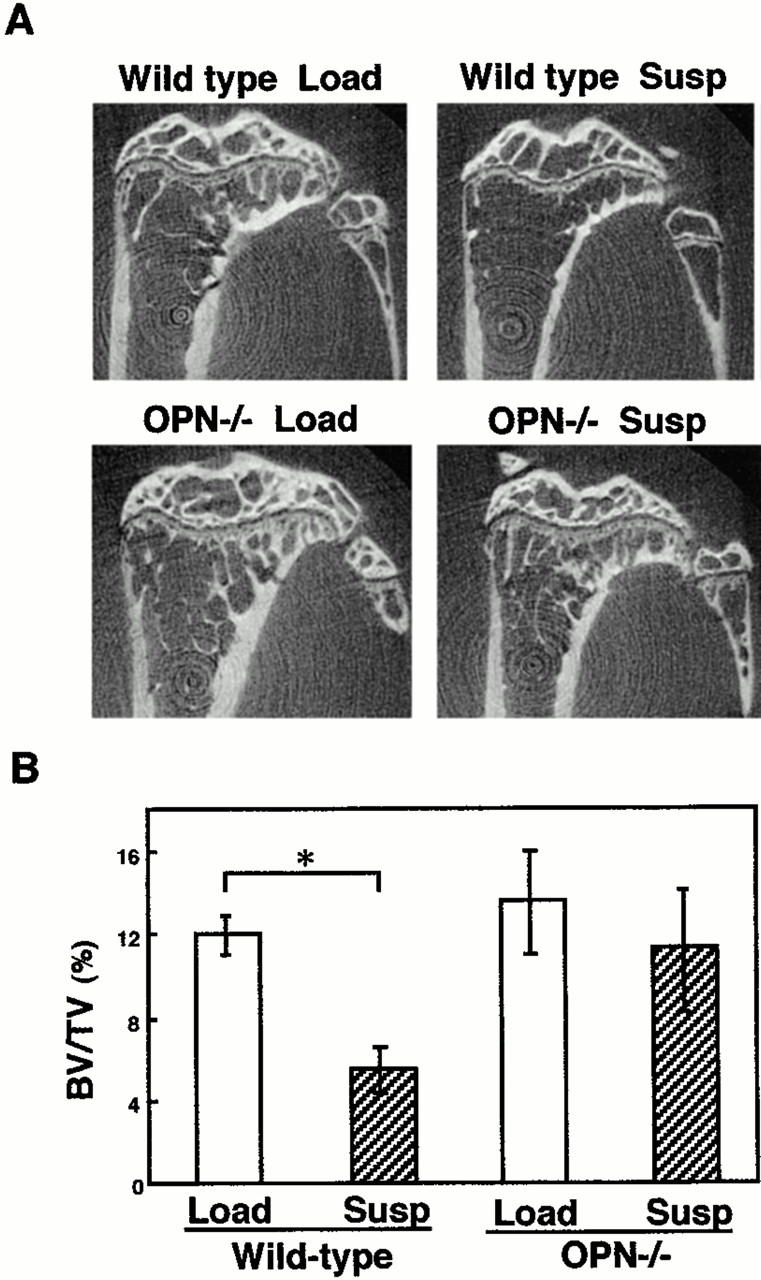
μ-CT tomographs and trabecular BV/TV of the tibiae of loaded and unloaded mice. (A) μ-CT pictures of the midsagittal planes of the proximal regions of the tibiae after 4 wk of tail suspension (Susp) or loading (Load) in wild-type or OPN−/− mice. μ-CT analyses were conducted as described in Materials and Methods. (B) Fractional trabecular BV/TV was quantified based on the image analysis of μ-CT pictures of the tibiae after 4 wk of either tail suspension (Susp) or loading (Load) in wild-type or OPN−/− mice shown in A. Analyses were conducted in the rectangular 280 × 1,400 μm area of 340–620 μm distal to the growth plate of the proximal ends of the tibiae. Each of the four groups consisted of six mice. Data are expressed as means and standard errors. *Statistically significant difference from respective control (P < 0.05).
OPN Prevents the Unloading-induced Increase in the Levels of Urinary Dpyd.
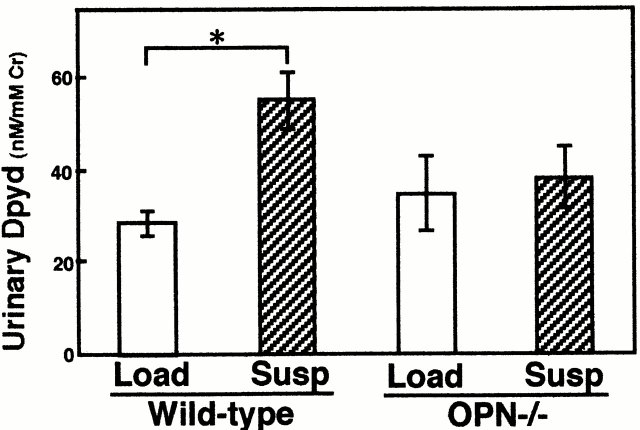
As an alternative method to evaluate bone metabolism in OPN−/− mice, we have evaluated a systemic biochemical marker of bone resorption. Dpyd appearance in urine as a by-product of collagen degradation is a biochemical marker of bone resorption that reflects systemic bone resorption 24. Dpyd excretion on day 14 of the tail suspension was increased about twofold compared with the loaded group in wild-type animals as expected (Fig. 2; P < 0.05). In contrast, no significant increase was observed in the tail-suspended OPN−/− mice compared with loaded OPN−/− mice (Fig. 2). The basal levels of Dpyd in loaded OPN−/− mice were not different from those in loaded wild-type mice. These results indicate that in the absence of OPN there was no increase in the levels of a systemic bone resorption marker from unloading.
Figure 2.
Urinary Dpyd levels of loaded and unloaded mice. Urine of either tail suspension (Susp) or loading (Load) from both wild-type or OPN−/− mice was collected during the last 24 h (on day 14). Urine from two mice was combined and three independent samples per group were analyzed by ELISA. Data are expressed as means and standard errors. *Statistically significant difference from respective control (P < 0.05).
OPN Is Required for the Unloading-induced Increase in the Osteoclast Number.
To obtain insights into the cellular basis for the mechanism of OPN action on unloading-induced bone loss, we examined osteoclasts and osteoblasts histologically in the bones. In contrast to the unloading-induced increase in the osteoclast number (N.Oc/BS) by ∼150% (P < 0.05; Fig. 3 A) and the osteoclast surface (Oc.S/BS) by ∼90% (P < 0.05; Fig. 3 B) in the proximal ends of the tibiae after 2 wk in wild-type (Fig. 3 C), no difference in N.Oc/BS and Oc.S/BS was observed in unloaded OPN−/− mice compared with loaded OPN−/− mice (Fig. 3A and Fig. B). After 4 wk, the N.Oc/BS and Oc.S/BS became similar between the tail-suspended and control loaded mice in wild-type animals as reported previously, whereas again no difference was observed in OPN−/− mice (data not shown). These observations indicate that OPN is required for the increase in the osteoclast number caused by unloading.
Figure 3.
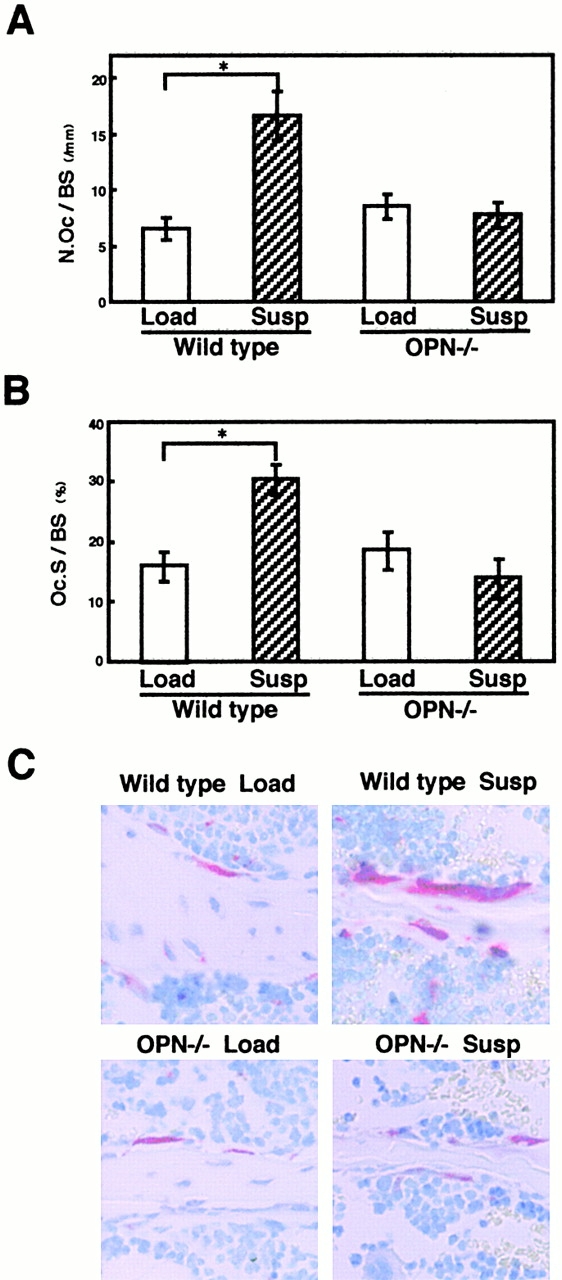
An unloading-induced increase in the N.Oc/BS does not occur in OPN−/− mice. (A and B) In the decalcified sections at the ends of the tibiae, the N.Oc/BS (A) and the Oc.S/BS (B) were measured within an area of 350–600 μm distal to the growth plate. The N.Oc/BS was calculated as the number of osteoclasts per bone surface, and the Oc.S/BS was calculated as the percentage of bone surface covered by osteoclast per total bone surface. Data are expressed as means and standard errors for six bones from each of the wild-type and OPN−/− mice. *Statistically significant difference from respective control (P < 0.05). (C) Osteoclasts on the cancellous bones in the decalcified 5-μm-thick midsagittal sections of the ends of the tibiae after tail suspension (Susp) or loading (Load) in wild-type or OPN−/− mice. TRAP-positive multinucleated cells (red cells) attached to cancerous bone were counted as osteoclasts to obtain data shown in A and B.
We have gone on to ask whether the failure of the osteoclast number to increase after unloading is intrinsic to osteoclastogenesis in the bone marrow cells in OPN−/− mice. Bone marrow cells obtained from the femora of unloaded and loaded mice were cultured in the presence of soluble receptor activator of nuclear factor-κB ligand (RANKL) and M-CSF (macrophage-CSF) for 9 d. The efficiency of the formation of TRAP-positive multinucleated cells in these cultures was similar regardless of unloading or loading in either wild-type or OPN−/− mice (data not shown). These results suggest that OPN deficiency does not affect the proportions of bone marrow cells able to differentiate into osteoclasts in vitro, at least under these conditions.
OPN Is Required for the Unloading-induced Reduction in Osteoblastic Activity In Vivo.
Bone loss occurs as the result of an imbalance between bone resorption and bone formation. This imbalance could result from a relative increase of osteoclastic activity and/or a relative decrease in osteoblastic activity. Therefore, in addition to the analyses on osteoclastic aspects, we also examined the effects of OPN deficiency on unloading-induced bone loss with respect to osteoblastic features by using calcein double labeling. In loaded mice, basal levels of bone formation rate (BFR) and mineral apposition rate (MAR) were similar regardless of the genotypes (Fig. 4A and Fig. B) based on the analyses of calcein labeling (Fig. 4 C). In contrast to the reduction in BFR and MAR in wild-type mice after unloading (∼50 and 30%, respectively, P < 0.05; Fig. 4A and Fig. B), no alteration in BFR and MAR was observed in OPN−/− mice after unloading (Fig. 4A and Fig. B).
Figure 4.
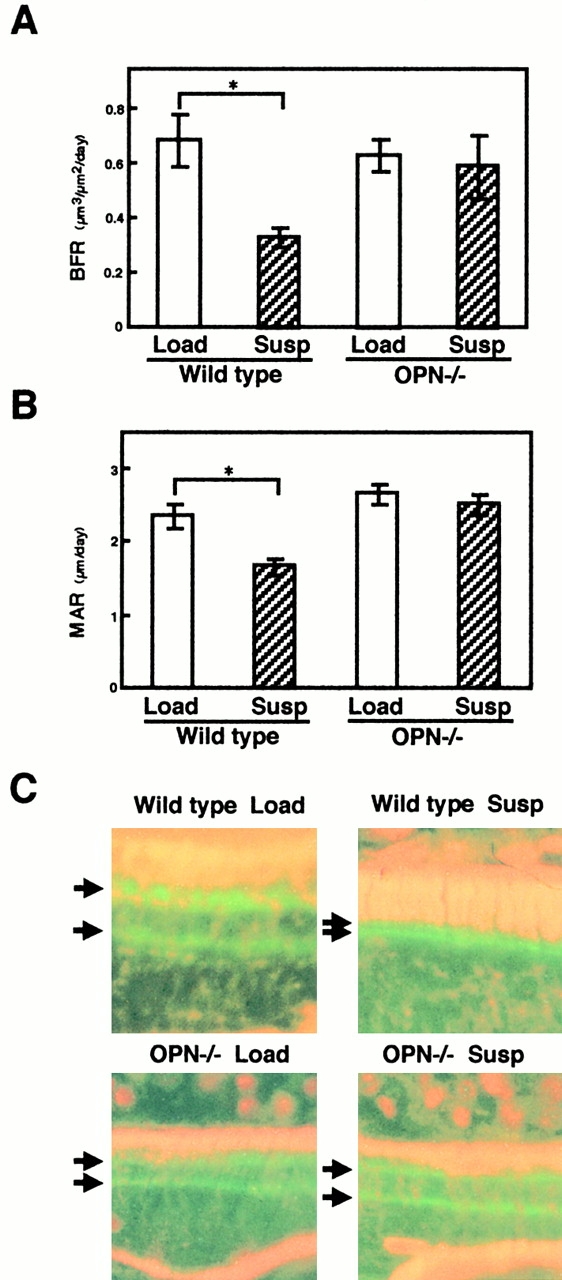
An unloading-induced reduction in osteoblastic activity in vivo does not occur in OPN−/− mice. (A and B) In the undecalcified sections of the proximal ends of the tibiae, (A) BFR and (B) MAR at 350–600 μm distal to the growth plate in the metaphyseal region was measured as described in Materials and Methods. The mice were injected intraperitoneally with calcein at 4 mg/kg 4 and 2 d before killing at 2 wk. Data are expressed as means and standard errors for six bones from each of the wild-type and OPN−/− mice groups. *Statistically significant difference from respective control (P < 0.05). (C) Calcein double-labeled surfaces of the bones at the ends of the tibiae after tail suspension (Susp) or loading (Load) in wild-type or OPN−/− mice. Arrows indicate the lines of calcein labeling (light green) used to obtain data shown in A and B.
Discussion
In this study, we demonstrated that OPN is required for the unloading-induced enhancement of osteoclastic bone resorption as well as the unloading-induced suppression of osteoblastic bone formation, two major cellular events that lead to bone loss. As a consequence, μ-CT examinations revealed preservation of trabecular patterns and fractional BV/TV in OPN−/− mice even after unloading. Moreover, evaluation of the systemic biochemical parameters using urinary Dpyd further confirmed that OPN deficiency protects bone against enhanced bone resorption in tail-suspended mice. These observations clearly indicate that OPN plays a critical role in the regulation of events that cause unloading-induced bone loss.
The suppression of the increase in the osteoclast number and osteoclast surface in OPN−/− mice after unloading was unexpected. It does not appear to be due to an intrinsic deficit of osteoclastogenesis in OPN−/− cells, at least in vitro, since the bone marrow cells recovered from tail-suspended OPN−/− mice exhibited osteoclastogenesis at a similar efficiency to the cells from tail-suspended wild-type mice in vitro, in the presence of soluble RANKL and M-CSF. Therefore, in the case of unloading-induced bone resorption, OPN may be involved in the mechanisms sensing the physical force that induces the increase in the number of osteoclasts rather than osteoclastogenesis per se.
Although hindlimb suspension has been widely used as a model of unloading, whether it enhances bone resorption in the rodent, such as rat, bone is still a matter of dispute 26. Our data clearly indicated an increase in bone resorption in the hindlimbs of the wild-type mice. The difference in the efficiencies in osteoclastogenesis from OPN-null cells in this and our previous paper 12 could be due to the culture conditions, including the types of stimulators used for the osteoclast development.
It is known that mechanical stress induces OPN production, and this phenomenon has been suggested to trigger events in mechanical stress–dependent bone formation and bone resorption. However, no evidence has been obtained to show that OPN really plays a role in vivo. Our data in this paper indicate for the first time that the presence of OPN is critical in mediating the effect of physical force to change the metabolism of bone. With regard to unloading-induced bone resorption, OPN could be required to modulate the development or recruitment of osteoclast precursors upon unloading to augment the number of osteoclasts and promote bone resorption, even though not required for base line of osteoclastogenesis. OPN could act as a chemoattractant as observed in other types of cells 27. Thus, the role of OPN would be to provide critical regulatory information for the recruitment and/or development of osteoclasts in a situation of acute bone resorption in vivo. In this sense, OPN appears to be the bone resorption–inducing mechanical stress sensor that contributes to unloading-induced bone resorption. Alternatively, OPN functions downstream of the sensor and modulates cell function as a consequence of signals generated by this sensor in response to mechanical forces.
Our data also indicate that OPN is required to convey the effect of mechanical stress to osteoblasts as shown by the absence of a reduction of BFR and MAR in OPN−/− mice subjected to tail suspension. This effect on bone formation parameters might be secondary to the lack of increase in the number of osteoclasts in unloaded OPN−/− bones, and hence might be regarded as an indirect phenomenon. However, this possibility is contradicted by the fact that reduction in bone formation parameters has been observed in tail-suspended animals even when bone resorption was completely blocked by the treatment of animals with bisphosphonates 28 29. Therefore, we conclude that OPN directly modulates bone formation in response to mechanical stress independent of its effect on osteoclasts. Although OPN-null mice may compensate for weight deprivation in 2 wk, the fact that no alteration in BFR was observed at a 2-wk time point suggests that this possibility is less likely. The fact that OPN−/− mice develop normal skeletal structures and do not exhibit major morphological defects 12 indicates that OPN is not required as a basic component for bone formation. Thus, the observations on bone formation presented in this paper highlight the point that OPN is a molecule which is required specifically to convey loss of mechanical stress–dependent events to osteoblasts to negatively modulate basic bone-producing functions.
The basal levels of bone volume fraction of the cancellous bones and osteoclast number in the 14-wk-old OPN−/− mice used in this paper were not different from those in wild-type. This could be due to the age dependence of the phenotype as described in previous papers 12 15, where mild osteopetrosis was observed in older (25 ± 3-wk-old) OPN-null mice 15 but not in the younger mice. We assume that age-dependent suppression of osteoclastic activity could result in mild osteopetrosis and a mild hypocalcemic state which may stimulate osteoclastogenesis in the older null mice for compensation. Age may have also affected in vitro osteoclastogenesis. Age dependence of osteopetrosis was similarly described in β3-integrin knockout mice 30.
Taken together, our data provide evidence that OPN is required for both the increase in osteoclasts to enhance bone resorption and the decrease in osteoblastic function to suppress bone formation, two cellular phenomena in bone that are well characterized in tail-suspended mice. Therefore, OPN plays a key role in conveying the effect of mechanical stress to these two types of bone cells, major regulators that determine bone mass in response to mechanical stress.
Acknowledgments
This research was supported by grants 12557123, 12026212, 12215040, and 0930734 from the Core Research for Evolutional Science and Technology, Japan Science and Technology Corporation, grant JSPS96I00205, National Space Development Agency of Japan (NASDA), Tokyo Biochemistry Research Foundation, and by US National Institutes of Health grants AR44434 and ES06897 to D.T. Denhardt and grant CA72740 to S.R. Rittling.
References
- Einhorn T.A. Biomechanics of bone. In: Bilezikian R.L., Rodan G.A., editors. Principles of Bone Biology. Academic Press, Inc; San Diego: 1996. pp. 25–37. [Google Scholar]
- Bikle D.D., Halloran B.P. The response of bone to unloading. J. Bone Miner. Metab. 1999;17:233–244. doi: 10.1007/s007740050090. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. Integrinsversatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder S.M., Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr. Opin. Cell Biol. 1999;11:274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- Wang N., Butler J.P., Ingber D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Shyy J.Y., Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr. Opin. Cell Biol. 1997;9:707–713. doi: 10.1016/s0955-0674(97)80125-1. [DOI] [PubMed] [Google Scholar]
- Chen K.D., Li Y.S., Kim M., Li S., Yuan S., Chien S., Shyy J.Y. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J. Biol. Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- Rodan G.A. Osteopontin overview. Ann. NY Acad. Sci. 1995;760:1–5. doi: 10.1111/j.1749-6632.1995.tb44614.x. [DOI] [PubMed] [Google Scholar]
- Denhardt D.T., Noda M. Osteopontin expression and functionrole in bone remodeling. J. Cell. Biochem. Suppl. 1998;30–31:92–102. [PubMed] [Google Scholar]
- Denhardt D.T., Guo X. Osteopontina protein with diverse functions. FASEB J. 1993;7:1475–1482. [PubMed] [Google Scholar]
- Hruska K.A., Rolnick F., Huskey M., Alvarez U., Cheresh D. Engagement of the osteoclast integrin αvβ3 by osteopontin stimulates phosphatidylinositol 3-hydroxyl kinase activity. Endocrinology. 1995;136:2984–2992. doi: 10.1210/endo.136.7.7540546. [DOI] [PubMed] [Google Scholar]
- Rittling S.R., Matsumoto H.N., McKee M.D., Nanci A., An X.R., Novick K.E., Kowalski A.J., Noda M., Denhardt D.T. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J. Bone Miner. Res. 1998;13:1101–1111. doi: 10.1359/jbmr.1998.13.7.1101. [DOI] [PubMed] [Google Scholar]
- Liaw L., Birk D.E., Ballas C.B., Whitsitt J.S., Davidson J.M., Hogan B.L. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J. Clin. Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittling S.R., Denhardt D.T. Osteopontin function in pathologylessons from osteopontin-deficient mice. Exp. Nephrol. 1999;7:103–113. doi: 10.1159/000020591. [DOI] [PubMed] [Google Scholar]
- Yoshitake H., Rittling S.R., Denhardt D.T., Noda M. Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption. Proc. Natl. Acad. Sci. USA. 1999;96:8156–8160. doi: 10.1073/pnas.96.14.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T., Yamauchi M., Onozaki J., Sato S., Suzuki Y., Sodek J. Influence of an intermittent compressive force on matrix protein expression by ROS 17/2.8 cells, with selective stimulation of osteopontin. Arch. Oral Biol. 1993;38:23–30. doi: 10.1016/0003-9969(93)90150-k. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J., Roelofsen J., Semeins C.M., Bronckers A.L., Burger E.H. Mechanical stimulation of osteopontin mRNA expression and synthesis in bone cell cultures. J. Cell. Physiol. 1997;170:174–181. doi: 10.1002/(SICI)1097-4652(199702)170:2<174::AID-JCP9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Toma C.D., Ashkar S., Gray M.L., Schaffer J.L., Gerstenfeld L.C. Signal transduction of mechanical stimuli is dependent on microfilament integrityidentification of osteopontin as a mechanically induced gene in osteoblasts. J. Bone Miner. Res. 1997;12:1626–1636. doi: 10.1359/jbmr.1997.12.10.1626. [DOI] [PubMed] [Google Scholar]
- Miles R.R., Turner C.H., Santerre R., Tu Y., McClelland P., Argot J., DeHoff B.S., Mundy C.W., Rosteck P.R., Jr., Bidwell J. Analysis of differential gene expression in rat tibia after an osteogenic stimulus in vivomechanical loading regulates osteopontin and myeloperoxidase. J. Cell. Biochem. 1998;68:355–365. doi: 10.1002/(sici)1097-4644(19980301)68:3<355::aid-jcb6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Terai K., Takano-Yamamoto T., Ohba Y., Hiura K., Sugimoto M., Sato M., Kawahata H., Inaguma N., Kitamura Y., Nomura S. Role of osteopontin in bone remodeling caused by mechanical stress. J. Bone Miner. Res. 1999;14:839–849. doi: 10.1359/jbmr.1999.14.6.839. [DOI] [PubMed] [Google Scholar]
- Duncan R.L., Turner C.H. Mechanotransduction and the functional response of bone to mechanical strain. Calcif. Tissue Int. 1995;57:344–358. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- Bikle D.D., Halloran B.P., Cone C.M., Morey-Holton E. Bone loss during simulated weightlessnessis it glucocorticoid mediated? Physiologist. 1985;28:S123–S124. [PubMed] [Google Scholar]
- Halloran B.P., Bikle D.D., Cone C.M., Morey-Holton E. Glucocorticoids and inhibition of bone formation induced by skeletal unloading. Am. J. Physiol. 1988;255:E875–E879. doi: 10.1152/ajpendo.1988.255.6.E875. [DOI] [PubMed] [Google Scholar]
- Robins S.P., Woitge H., Hesley R., Ju J., Seyedin S., Seibel M.J. Direct, enzyme-linked immunoassay for urinary deoxypyridinoline as a specific marker for measuring bone resorption. J. Bone Miner. Res. 1994;9:1643–1649. doi: 10.1002/jbmr.5650091019. [DOI] [PubMed] [Google Scholar]
- Parfitt A.M., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R. Bone histomorphometrystandardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Morey-Holton E.R., Globus R.K. Hindlimb unloading of growing ratsa model for predicting skeletal changes during space flight. Bone. 1998;22:83S–88S. doi: 10.1016/s8756-3282(98)00019-2. [DOI] [PubMed] [Google Scholar]
- Liaw L., Skinner M.P., Raines E.W., Ross R., Cheresh D.A., Schwartz S.M., Giachelli C.M. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of α v β 3 in smooth muscle cell migration to osteopontin in vitro. J. Clin Invest. 1995;95:713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D.D., Morey-Holton E.R., Doty S.B., Currier P.A., Tanner S.J., Halloran B.P. Alendronate increases skeletal mass of growing rats during unloading by inhibiting resorption of calcified cartilage. J. Bone Miner. Res. 1994;9:1777–1787. doi: 10.1002/jbmr.5650091115. [DOI] [PubMed] [Google Scholar]
- Kodama Y., Nakayama K., Fuse H., Fukumoto S., Kawahara H., Takahashi H., Kurokawa T., Sekiguchi C., Nakamura T., Matsumoto T. Inhibition of bone resorption by pamidronate cannot restore normal gain in cortical bone mass and strength in tail-suspended rapidly growing rats. J. Bone Miner. Res. 1997;12:1058–1067. doi: 10.1359/jbmr.1997.12.7.1058. [DOI] [PubMed] [Google Scholar]
- McHugh K.P., Hodivala-Dilke K., Zheng M.H., Namba N., Lam J., Novack D., Feng X., Ross F.P., Hynes R.O., Teitelbaum S.L. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]