Abstract
Polyclonal CD4+ T cell activation is characteristic of spontaneous lupus. As a potential explanation for this phenotype, we hypothesized that T cells from lupus-prone mice are intrinsically hyperresponsive to stimulation with antigen, particularly to those peptide ligands having a low affinity for the T cell receptor (TCR). To test this hypothesis, we backcrossed the α and β chain genes of the AND TCR specific for amino acids 88–104 of pigeon cytochrome C (PCC) to the Fas-intact MRL/Mp+ Fas-lpr and to the H-2k–matched control backgrounds B10.BR and CBA/CaJ (MRL.AND, B10.AND, and CBA.AND, respectively), and assessed naive CD4+ TCR transgenic T cell activation in vitro after its encounter with cognate antigen and lower affinity altered peptide ligands (APLs). MRL.AND T cells, compared with control B10.AND and CBA.AND cells, proliferated more when stimulated with agonist antigen. More strikingly, MRL.AND T cells proliferated significantly more and produced more interleukin 2 when stimulated with the APLs of PCC 88–104, having lower affinity for the transgenic TCR. These results imply that one of the forces driving polyclonal activation of α/β T cells in lupus is an intrinsically heightened response to peptide antigen, particularly those with low affinity for the TCR, independent of the nature of the antigen-presenting cell and degree of costimulation.
Keywords: autoreactive T cells, autoimmunity, systemic lupus erythematosus, tolerance, murine lupus
Introduction
Activated CD4+ α/β T cells are necessary for the production of high affinity, isotype-switched pathogenic autoantibodies, and full disease penetrance in humans and mice with spontaneous lupus 1 2. Polyclonal CD4+ T cell activation is a hallmark of human and murine lupus 3, suggesting a global defect in the maintenance of T cell tolerance to self; however, the mechanism(s) of activation of T cells responsive to self-peptides in lupus are unknown, as are the source(s) of such peptides and the precise events leading to autoreactive CD4+ T cell–B cell collaboration that appear critical for pathogenic autoantibody production.
Negative selection in the thymus appears intact in lupus-prone mice 4, suggesting that relevant autoreactive T cells are of low enough affinity to escape to the periphery, where they must bypass normal tolerance mechanisms. T cell tolerance in the periphery may be maintained at least in part through the interaction of low affinity self-antigens with autoreactive T cells, resulting in anergy 5. Whereas peripheral T cell tolerance in lupus may be lost because of abnormalities in self-antigen presentation or aberrant costimulation, an intrinsic T cell abnormality in the ability to respond to tolerogenic signals may also contribute. Indeed, there is growing evidence that T cell activation is abnormal in lupus. Studies in lupus-prone NZM mice have identified a locus on chromosome 7 associated with generalized activation, increased proliferation, and reduced activation-induced cell death (AICD) of CD4+ T cells 6. In addition, abnormalities in TCR signaling in human lupus T cells have been identified 7 8, although it is not clear if these abnormalities contribute to the global T cell activation characteristic of the disease.
Since T cell tolerance in the periphery may be maintained by the interaction of self-antigens with low affinity for the TCR, we hypothesized that naive CD4+ T cells in lupus would be more likely than T cells from nonautoimmune individuals to become activated when stimulated with such antigens. This would help explain the polyclonal T cell activation characteristic of human and murine lupus. Since it is difficult to study antigen-specific responses of human lupus CD4+ T cells because of the heterogeneity of human peripheral blood lymphocytes 9 and the potential confounding effect of immunosuppressive therapy, we chose to address our hypothesis using a mouse model in which antigen specificity and the activation state of the T cells could be rigorously controlled. To achieve this, we backcrossed the α and β chain genes of the AND TCR specific for amino acids 88–104 of the pigeon cytochrome C (PCC) to the Fas-intact MRL/Mp+ Fas-lpr (MRL/+ Fas-lpr) and to the H-2k–matched control backgrounds B10.BR and CBA/CaJ (MRL.AND, B10.AND, and CBA.AND, respectively [references 10, 11]). Naive CD4+ TCR transgenic T cells from these mice were then stimulated in vitro using either agonist peptide 88–104 of PCC or altered peptide ligands (APLs) of PCC 88–104 having equivalent affinity for the selecting I-Ek molecules, but lower affinity for the transgenic TCR 12 13. Our results demonstrate that naive CD4+ T cells from lupus-prone mice compared with controls are hyperproliferative, more readily enter the cell cycle, have a higher probability of displaying an activated phenotype, and secrete more IL-2, especially when stimulated with low affinity antigens. These data imply that a lower threshold for activation and/or a more vigorous response to the same degree of TCR activation contributes to the loss of peripheral T cell tolerance in lupus.
Materials and Methods
Mice.
AND transgenic mice, expressing an α/β TCR (Vα11+, Vβ3+) recognizing PCC, were originally provided on the B10.BR background by S. Hedrick (University of California at San Diego, San Diego, CA; reference 10). The transgenic Vα and Vβ chains, which cosegregated in our hands, were serially backcrossed to the MRL/+ Fas-lpr (MRL.AND) and to the H-2k–matched control background B10.BR (B10.AND) for >15 generations, and to the control H-2k CBA/CaJ strain (CBA.AND) for >7 generations. The two control strains lack endogenous (viral) superantigens, products of murine mammary tumor viruses (MMTVs), that bind the transgenic Vβ3 chain, with resultant central deletion in the context of I-E 14. Levels of expression of the transgenic Vα and Vβ chains and the CD4 coreceptor were equivalent among the three strains, as determined by flow cytometry. Transgenic mice were maintained as heterozygotes, with screening performed using the PCR of tail DNA as described previously 11 and phenotypic confirmation by flow cytometry. The animals were identically housed in specific pathogen-free facilities at the Yale Animal Resources Center.
T Cell Preparation.
Young (1–2-mo-old) age- and sex-matched mice were used for purification of CD4+ T cells by negative selection to avoid preactivation of the cells before in vitro stimulation. Splenocyte suspensions were first treated with RBC lysing buffer and then passed through Ficoll-Paque™ lymphocyte separation medium. Cell suspensions from lymph nodes were prepared from axillary, brachial, and mesenteric lymph nodes. CD8+ T cells, B cells, and APCs were removed by labeling with the following biotinylated antibodies: anti-CD8 (clone 53-6.7); anti-CD45R/B220 (RA3-6B2); anti-CD16/32 (2.4G2); anti–I-Ak (11-5.2); and anti-CD11b (M1-70) (all from BD PharMingen). The cells were washed and then incubated with streptavidin microbeads (Miltenyi Biotec) with magnetic removal by passage through a column using the protocol of the manufacturer. The purity of transgenic CD4+ T cells was consistently >93%, as confirmed by flow cytometric analysis for cells that were CD4+ and expressed both Vα11+ and Vβ3+. Transgenic TCR density and CD4 coreceptor density were equivalent among the three groups (data not shown). Transgenic cells were also >93% naive as determined by surface markers, CD44low, and CD62Lhi 15. To ensure that only transgenic cells were included in our analyses, CD4+Vα11hi cells were gated on for studies of activation marker expression and division of individual cells 16. The percentage of transgenic CD4+Vα11+Vβ3+ T cells in the periphery was not different among the three strains.
Flow Cytometric Analysis.
The following antibodies were used for flow cytometry: anti-CD44–FITC (Pgp-1); anti-CD62L–PE (MEL-14); anti-CD4–Cychrome (H219.19); biotinylated and FITC–anti-Vα11 (RR8-1); anti-Vβ3–PE (KJ25); and streptavidin-APC (BD PharMingen). Fluorescence analysis was done using a FACSCalibur™ flow cytometer using CELLQuest™ software (Becton Dickinson).
T Cell Stimulation Assays.
TCR transgenic CD4+ T cells (105 cells) were cultured in 96-well round bottomed tissue culture plates in Click's medium containing 10% FCS, 0.2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. T cells were stimulated either with the dominant agonist peptide of PCC (PCC 88–104) KAERADLIAYLKQATAK (peptide purity >90% by HPLC analysis; American Peptide Company) or with APLs of PCC 88–104. These APLs differed from PCC 88–104 by either glutamine (K99Q), arginine (K99R), or isoleucine (A96I) substitutions for lysine at position 99 and alanine at position 96, respectively (peptide purity >90%; Keck Foundation Biomedical Resource Laboratory, Yale University). A96I, K99R, and K99Q are well studied as APLs of the AND transgenic TCR. A96I is an agonist for AND T cells in the C57BL background (AND T cells are positively selected on either H-2k or H-2b backgrounds; references 13, 17 18 19). K99Q functions as a weak antagonist, with minimal evidence of TCR engagement and IL-2 production due to decreased affinity of the K99Q-MHC complex for the PCC-specific TCR 12 13. K99R is an antagonist for the AD10 TCR transgene which also recognizes PCC 18. A96I, K99Q, and K99R bind to I-Ek approximately as well as the agonist peptide PCC 88–104; the relative binding of A96I and K99R to the AND TCR is unknown.
CH27 B lymphoblastoid cells (provided by Dr. C. Janeway, Jr., Yale School of Medicine) expressing I-Ek, B7.1, and B7.2 20 were used as APCs and confirmed by flow cytometry to express these surface molecules. T cells were cocultured for 72 h with mitomycin C–treated APCs (APC/T ratio 2:1) at 37°C. Non–TCR-mediated proliferation was induced by culture with 10 ng/ml PMA (Sigma-Aldrich) and 500 ng/ml ionomycin (Calbiochem). [3H]Thymidine was added 18 h before harvesting. For some experiments, T cells were labeled with the intracellular fluorescent dye 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) to determine their proliferative history after in vitro activation 21. T cells (5–10 × 106/200 μl PBS) were labeled with 5 μM CFSE for 10 min at room temperature. Unbound CFSE was quenched by the addition of 500 μl of fetal bovine serum 21. Transgenic cells were identified by gating on CD4+Vα11hi cells 16. In some experiments, propidium iodide (PI) staining was used to differentiate between living and dead transgenic T cells after gating on CD4+Vα11hi cells.
Cytokine Production.
IL-2 production by transgenic T cells was quantified by indirect ELISA of culture supernatants 24 h after stimulation in vitro using triplicate wells in 96-well round bottomed plates. ELISA was performed according to established methods using a murine IL-2 standard and antimurine IL-2 capture antibody (BD PharMingen). Background production of IL-2 seen with culture of T cells and APCs alone was similar among the three test strains and was subtracted from the OD readings of wells containing antigens to assess antigen-specific production of IL-2. The production of IL-2 by transgenic T cells was also determined by intracellular cytokine staining using anti–IL-2–PE (BD PharMingen) using the manufacturer's recommended protocol. The percentage of transgenic cells producing IL-2 was determined by gating on the CD4+Vα11hi population, as described above.
Statistical Analysis.
Comparative data were analyzed using the unpaired Student's t test with two-tailed P value calculations using InStat software (GraphPad Software). Data shown are representative of at least three separate experiments. Error bars indicate SEM. Results of in vitro experiments are representative of three separate experiments using two to three age- and sex-matched animals in each group.
Results
We elected to use Fas-intact MRL/+ Fas-lpr mice as the experimental autoimmune strain for these studies. These mice develop lupus and their use removed the confounding effect of Fas deficiency on T cell activation and death found in MRL/Faslpr mice 22 and avoids the CD4−CD8− (double negative) T cell population that accumulates in the peripheral lymphoid organs of these latter animals, cells that have defective responses to mitogens and are difficult to remove from in vitro assays.
MRL.AND TCR Transgenic T Cells Proliferate More in Response to Agonist Peptide Than Control Transgenic T Cells.
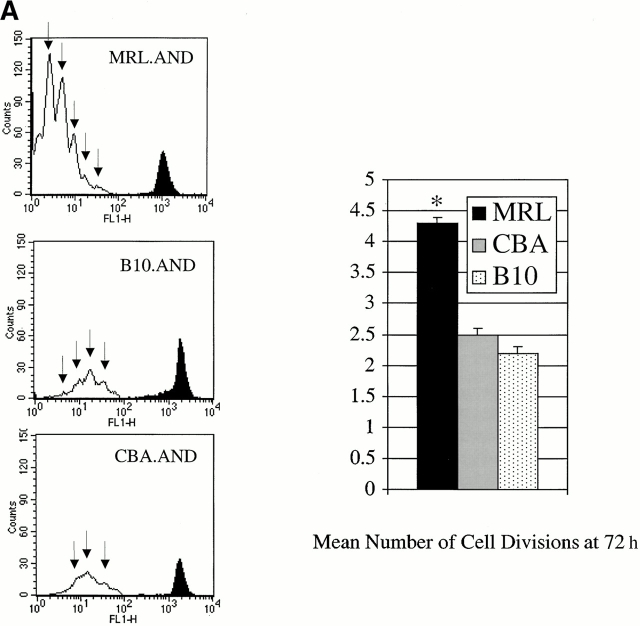
We initially studied the response of AND T cells to peptide PCC 88–104, a known agonist of the AND TCR 12 18. Negatively selected naive AND T cells from the spleens of 1–2-mo-old TCR transgenic mice were identified by flow cytometry as CD4+Vα11hi 16, and the number of cell divisions was estimated using the technique of CFSE dilution. Using agonist PCC 88–104 antigen (0.05 μM) presented by CH27 cells, there was a significant increase in the mean number of cell divisions in MRL.AND T cells 72 h later (Fig. 1 A). We also observed increased proliferation at 72 h across a range of concentrations of PCC 88–104 in a proliferation assay using [3H]thymidine uptake (Fig. 1 B), with proliferation noted beginning at 0.001 μM.
Figure 1.
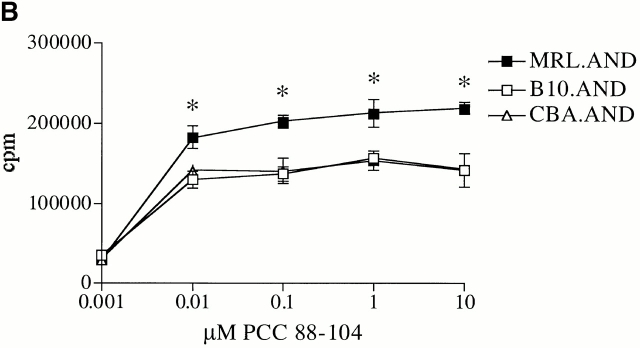
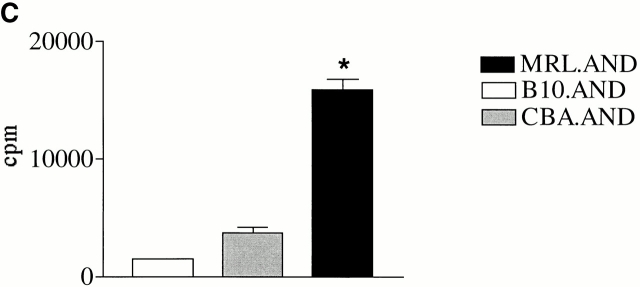
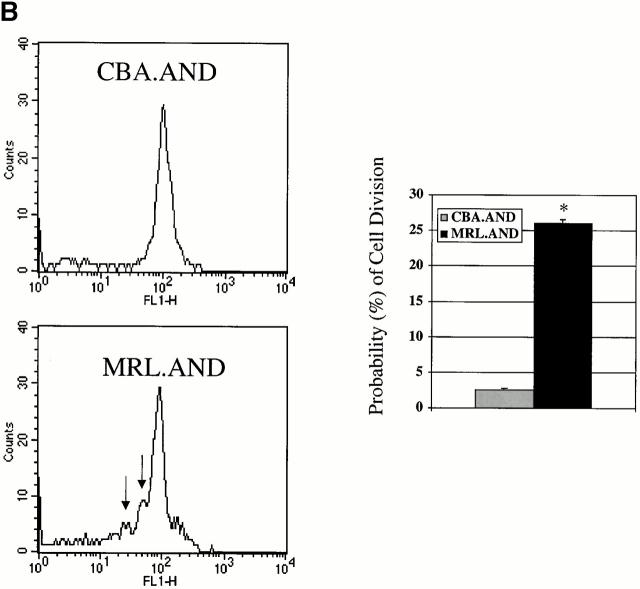
CD4+ AND transgenic T cells from MRL mice proliferate more after agonist peptide and mitogenic stimulation than CD4+ AND transgenic T cells from nonautoimmune strains. (A) Naive CD4+ transgenic T cells were labeled with CFSE and stimulated with 0.05 μM PCC 88–104 agonist peptide using CH27 cells as APCs. The number of cell divisions at 72 h was determined by CFSE labeling. CH27 and dead T cells were excluded from the analysis on the basis of forward/side scatter. The mean number of cell divisions at 72 h is indicated in the bar graph at the right. *P < 0.005 for comparison of MRL.AND to B10.AND or CBA.AND cells. Arrows indicate evidence of cell division. (B) Naive AND transgenic T cells were stimulated in vitro by I-Ek bearing B7.1+/B7.2+ CH27 cells pulsed with varying concentrations of PCC 88–104. Proliferation was measured at 72 h by [3H]thymidine incorporation. Results are means of quadruplicate measurements. Error bars represent SEM. *P < 0.05 for comparison of MRL.AND to B10.AND or CBA.AND cells. (C) Naive AND transgenic T cells were stimulated in vitro with 10 ng/ml of PMA and 500 ng/ml of ionomycin. Proliferation was measured at 72 h by [3H]thymidine incorporation. Results are means of quadruplicate measurements. *P < 0.05 for comparison of MRL.AND to B10.AND or CBA.AND cells.
To test whether the hyperresponsive phenotype of MRL.AND T cells we observed with agonist peptide was dependent on activation through the TCR, we measured the proliferation of AND T cells stimulated with the mitogens PMA (10 ng/ml) and ionomycin (500 ng/ml). MRL.AND T cells proliferated significantly more in response to stimulation with PMA and ionomycin at 72 h compared with control B10.AND and CBA.AND T cells (Fig. 1 C).
AND TCR Transgenic T Cells Do Not Spontaneously Proliferate and Are Not Spontaneously Activated In Vivo.
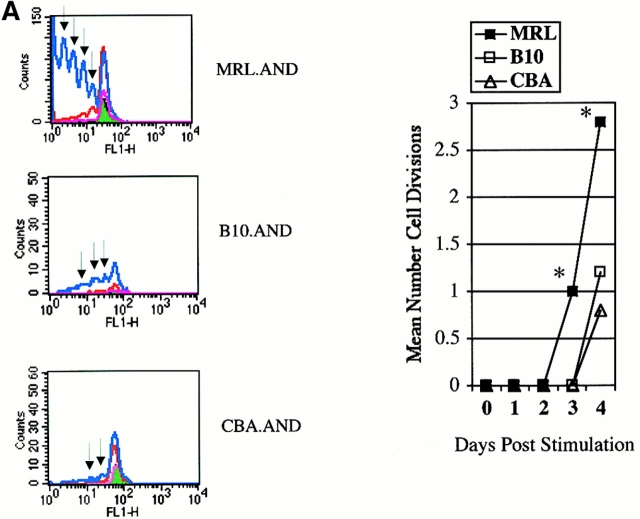
To investigate the possibility that any differences in cell division observed in MRL.AND T cells were a consequence of aberrant cell function attributable to the expression of the TCR transgene itself, and not due to the lupus genotype, we studied the proliferation of negatively selected AND TCR transgenic splenic T cells cultured either without APCs or with unpulsed APCs. In our experimental system, PCC-specific MRL.AND TCR transgenic T cells failed to proliferate when cultured without APCs (data not shown). Moreover, TCR transgenic T cells from all three strains did not undergo cell division, as measured by CFSE dilution, to a significant degree without peptide stimulation from APCs (see Fig. 3 A, data not shown). To further address this question in a physiologic manner, we also asked if there was spontaneous activation of transgenic T cells in vivo. Here, we compared activation markers on splenic T cells in both young (1–2-mo-old) and older (8–9-mo-old) AND transgenic animals (Table ). Less than 2% of B10.AND, CBA.AND, and MRL.AND TCR transgenic T cells from young mice, and <10% of AND T cells from older mice, spontaneously (i.e., without immunization) developed evidence of an activated phenotype in vivo (CD44hiCD62Llo) or a phenotype of recent activation (CD69+CD25+). These data establish that activation and proliferation of AND TCR transgenic T cells are only seen to a significant degree with stimulation by APCs plus peptide antigen and are not a byproduct or artifact of the expression of the TCR itself.
Figure 3.
(A) AND transgenic T cells labeled with CFSE and stimulated in vitro with CH27 cells alone (pink line) or pulsed with either 5 μM (red line) or 25 μM (blue line) K99Q. Fluorescence of T cells cultured without APCs is indicated in green with analysis performed at 96 h. Arrows indicate evidence of cell division. The mean number of cell divisions at 96 h is indicated in the bar graph at the right. *P < 0.005 for comparison of MRL.AND to B10.AND or CBA.AND cells. The change in scale used in MRL plot is indicative of the increased number of T cells. CH27 and dead T cells were excluded from the analysis on the basis of forward/side scatter. (B) AND transgenic T cells were labeled with CFSE and stimulated with 25 μM antagonist K99R. Arrows indicate cell division at 48 h. The probability of cell division is shown in the bar graph at right. Error bar represents SEM. *P < 0.0001 for comparison of MRL.AND to CBA.AND T cells.
Table 1.
AND TCR Transgenic T Cells Are Not Spontaneously Activated In Vivo
| Percentage CD25+ | Percentage CD69+ | Percentage activated | |
|---|---|---|---|
| Young B10.AND | 1.7 | 2.1 | 1.6 |
| Young CBA.AND | 2.0 | 1.0 | 1.9 |
| Young MRL.AND | 1.7 | 1.8 | 1.4 |
| Older B10.AND | 5.9 | 10.4 | 4.6 |
| Older CBA.AND | 3.3 | 5.3 | 6.8 |
| Older MRL.AND | 3.8 | 9.8 | 4.5 |
Flow cytometry was performed on CD4+Vα11hi T cells for the activation markers CD69, CD25, CD44, and CD62L in both young (1–2 mo) and older (8–9 mo) AND TCR transgenic mice. Activated T cells were defined as the CD44hi CD62Llo population (reference 15). Results are representative of two separate experiments involving cohorts of two to three transgenic animals from each group (MRL.AND, B10.AND, and CBA.AND).
MRL.AND TCR Transgenic T Cells Proliferate More in Response to Low Affinity APLs of PCC Than Control TCR Transgenic T Cells.
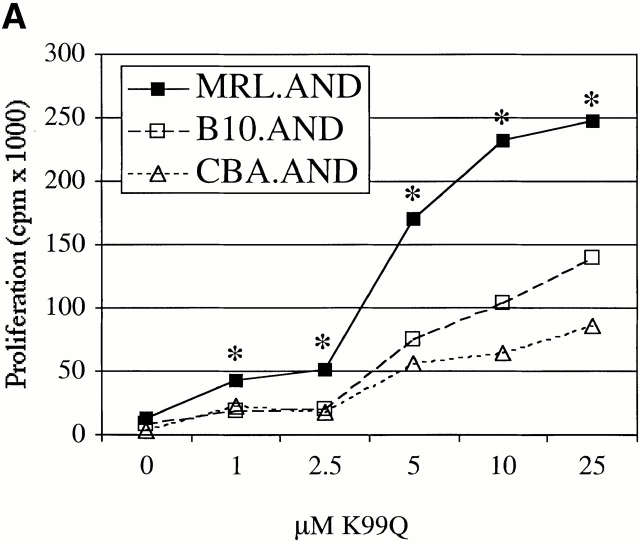
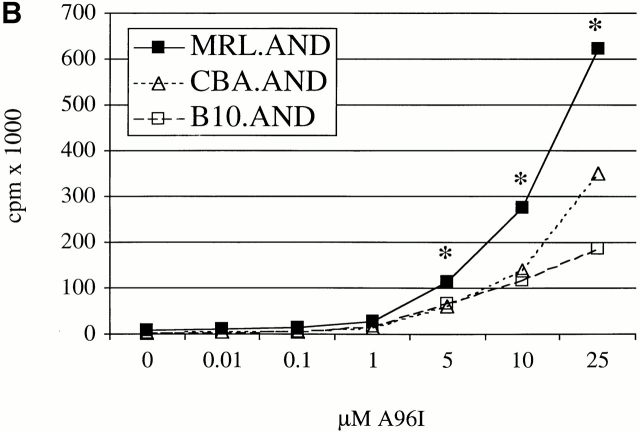
We next studied the proliferative response and activation of MRL.AND T cells to a previously defined APL of the agonist peptide PCC 88–104 in which glutamine is substituted for lysine at position 99 (K99Q; reference 13). This peptide and native PCC 88–104 bind equally well to I-Ek 12 18, but the former peptide–MHC complexes bind with ∼1/10 the affinity to the transgenic TCR 12. Because of low affinity of the K99Q–MHC complex for the TCR, naive mature peripheral CD4+ AND T cells from nonautoimmune mice normally proliferate poorly in response to this peptide and secrete minimal amounts of IL-2, in contrast to the outcome after contact with the cognate peptide PCC 88–104 12 18.
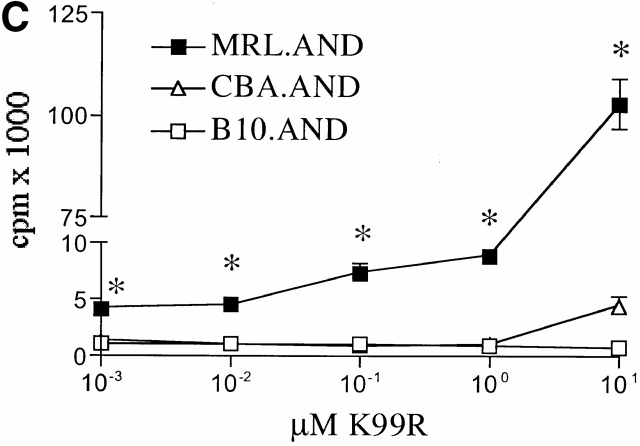
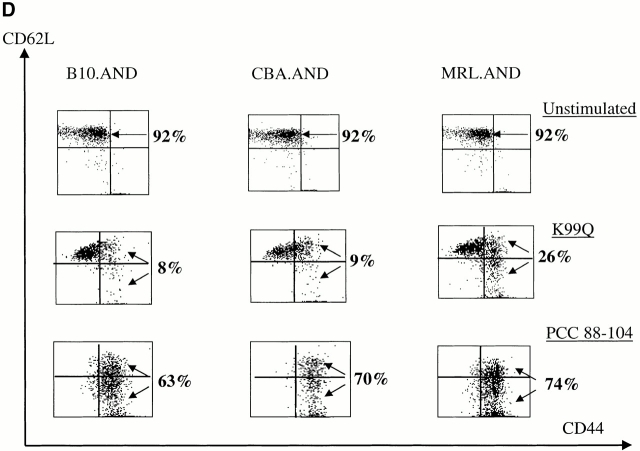
Naive AND T cells were stimulated with APL K99Q presented by H-2k on CH27 cells at concentrations >100-fold higher than is necessary to stimulate the cells with full agonist peptide 13. MRL.AND T cells proliferated significantly more than B10.AND and CBA.AND T cells across a range of concentrations of the K99Q peptide (P < 0.005; Fig. 2 A). A different APL (A96I) yielded results similar to K99Q (Fig. 2 B). To see if the hyperresponsive phenotype to APL stimulation we observed in splenic T cells was also true for lymph node T cells, we stimulated CD4+ transgenic T cells from lymph nodes with varying concentrations of the APL K99R presented by CH27 cells. Little to no proliferation was noted in B10.AND and CBA.AND T cells at the highest concentration of K99R tested (Fig. 2 C). In contrast, MRL.AND T cells proliferated >20-fold more at the highest concentration of K99R. In addition, MRL.AND T cells began to proliferate at three logs lower concentration of K99R (0.1 μM) than needed to stimulate control T cells. In concordance with these findings, there was a striking difference in the effector phenotype of splenic MRL.AND T cells compared with the other two strains, with an approximately threefold increase in the percentage of activated CD44hi cells from the lupus background 48 h after stimulation with 25 μM K99Q (Fig. 2 D, middle; for comparison, the baseline phenotype of unstimulated cells is shown at the top). In comparison, there was no significant difference in the percentage of activated AND cells when stimulated with 0.05 μM agonist peptide PCC 88–104 (Fig. 2 D, bottom).
Figure 2.
CD4+ AND transgenic T cells from MRL mice proliferate more and differentially express activation markers in response to low affinity TCR ligands compared with CD4+ transgenic T cells from nonautoimmune mice. (A and B) Naive splenic CD4+ transgenic T cells were stimulated with varying concentrations of K99Q (A) or A96I (B) presented by I-Ek bearing B7.1+/B7.2+ CH27 cells. (C) Naive CD44loCD62Lhi AND transgenic T cells from lymph nodes were stimulated with varying concentrations of K99R presented by CH27 cells. Proliferation was measured at 72 h by [3H]thymidine incorporation. Results are means of quadruplicate measurements. *P < 0.005 for comparison of MRL.AND to B10.AND or CBA.AND cells in A–C. (D) Naive CD44loCD62Lhi splenic CD4+ transgenic T cells were stimulated with 25 μM K99Q or 0.05 μM PCC 88–104 presented by CH27 cells. CD44 and CD62L expression were determined by flow cytometry 48 h after stimulation, with CD44hi cells designated as activated cells and CD44hiCD62Llo cells as memory cells. Arrows indicate the population of activated T cells that have upregulated CD44.
MRL.AND T Cells Undergo More Cell Divisions in Response to Stimulation with Low Affinity Ligands Than Nonautoimmune AND T Cells.
The differences in overall [3H]thymidine incorporation noted above with APL stimulation could be due to decreased cell death as well as increased proliferation of individual T cells. To address this question, we studied the proliferative history of individual transgenic T cells using CFSE labeling. This also allowed us to specifically gate on CD4+Vα11hi TCR transgenic cells 16. TCR transgenic cells stimulated with 25 μM APL K99Q resulted in a significant increase in the mean number of cell divisions in MRL.AND T cells at 72 h compared with CBA.AND and B10.AND cells (Fig. 3 A). This concentration of K99Q was 50-fold higher than used to stimulate cells with agonist peptide for cell division (Fig. 1 A). A small fraction of control B10.AND and CBA.AND T cells did undergo two to three rounds of cell division in response to stimulation with APL K99Q. Likewise, a significantly higher proportion of MRL.AND T cells proliferated in response to stimulation with a 50-fold higher concentration (25 μM) of antagonist APL K99R compared with control CBA.AND T cells (Fig. 3 B). In this latter case, the probability of cell division is shown, as the mean number of cell divisions in all groups was <1. Although an increased number of MRL.AND T cells, including cells that divided and those that did not, were available for analysis in the data shown in Fig. 3 A, staining of transgenic T cells with PI demonstrated an identical percentage of live (PI-negative) cells among all three strains upon stimulation with these APLs (data not shown). This finding argues against enhanced cell survival as the reason for the comparative increase in proliferation seen in MRL.AND T cells.
MRL.AND T Cells Produce More IL-2 upon Stimulation with Low Affinity APLs Than Nonautoimmune T Cells.
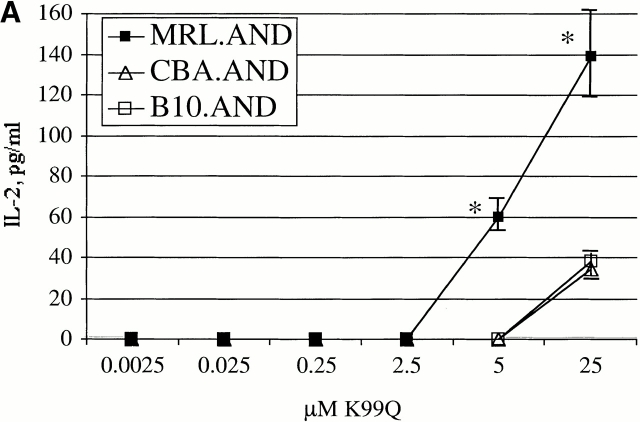
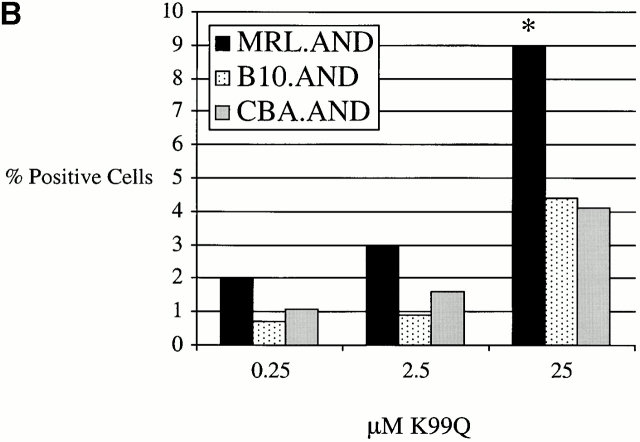
Only minimal, if any, IL-2 is produced when AND TCR transgenic T cells encounter low affinity APL K99Q 13. Given our observation of enhanced proliferation by MRL T cells in response to TCR stimulation with such ligands, we next examined IL-2 production in response to APL stimulation. Production of higher amounts of IL-2 could also be necessary for the clonal expansion of autoreactive T cells observed in disease. Hence, we measured the production of IL-2 in culture supernatants by indirect ELISA. There was a clear increase in the production of IL-2 from MRL T cells after stimulation with K99Q compared with control T cells (Fig. 4 A). This was confirmed by intracellular cytokine staining for IL-2 by TCR transgenic CD4+Vα11hi T cells (Fig. 4 B).
Figure 4.
MRL.AND transgenic T cells produce more IL-2 after stimulation with APLs than control transgenic T cells. (A) Negatively selected naive CD4+ AND transgenic T cells were stimulated in vitro with varying concentrations of a low affinity APL of PCC 88–104 (K99Q) presented by CH27 cells. IL-2 was determined by indirect ELISA from culture supernatant 24 h after stimulation. Error bars represent SEM of triplicate wells. *P < 0.05 for comparison of MRL.AND to control nonautoimmune transgenic strains. (B) The percentage of transgenic cells producing IL-2 was estimated using intracellular cytokine staining with anti–IL-2 by gating on CD4+Vα11hi double-positive cells. *P < 0.05 for comparison of MRL.AND T cells to CBA.AND and B10.AND T cells.
Discussion
T cell tolerance to self-antigens is maintained in the periphery through the induction of anergy, partial activation, or cell death 13 23. Since antigens with a low affinity for binding to TCRs induce T cell anergy 5 24, it is logical to posit that the interaction of self-reactive T cells with peptides having a low affinity for TCRs should normally result in peripheral tolerance rather than activation. We hypothesized that naive CD4+ T cells from mice with a genetic predisposition to lupus would become more activated compared with T cells with the identical TCR from nonautoimmune control strains in response to presentation of such antigens by APCs. Moreover, this phenotype could contribute to the abrogation of peripheral T cell tolerance in lupus, potentially leading to the polyclonal T cell activation characteristic of disease.
Indeed, we found that naive CD4+ AND transgenic T cells specific for PCC from the lupus-prone, Fas-intact MRL background divided more than control transgenic T cells when stimulated in vitro with the full agonist peptide PCC 88–104. This phenotype was not strictly dependent on proximal TCR signaling, as MRL T cells were still hyperresponsive even when TCR signaling was bypassed with PMA and ionomycin. Perhaps of greater importance, the difference between T cells from MRL mice and control strains was even more apparent using APLs of this ligand having lower affinity for the transgenic TCR. MRL T cells divided more and more readily entered the cell cycle. In particular, they produced more IL-2 when stimulated with a low affinity ligand that results in minimal IL-2 synthesis by nonautoimmune CD4+ T cells, despite evidence of equivalent TCR engagement (data not shown, reference 13). While it is possible that the proliferative alterations we identified are a consequence of decreased cell death, as has been reported for CD4+ T cells from C57BL mice congenic for lupus susceptibility loci 6 25, the augmented response of AND TCR transgenic T cells to the antagonist peptide K99Q suggests an intrinsic T cell abnormality, since this peptide does not induce AICD in control backgrounds 13. In addition, equivalent survival was seen among the three groups of transgenic T cells as determined by concurrent staining with PI. Finally, the hyperresponsive phenotype we have observed in AND transgenic T cells was not attributable to aberrant cell function due to the expression of the transgene itself, as TCR transgenic T cells from all three strains failed to proliferate when cultured without APCs, and >90% of the transgenic T cells in vivo remained in the naive state in all older transgenic mice studied.
We believe this is the first example demonstrating that naive lupus T cells are hyperresponsive upon initial encounter with peptide antigen, and in particular, that lupus T cells, compared with nonautoimmune controls, have a lower threshold of activation or an exaggerated response to antigens with a low affinity for TCR engagement. These results imply that one of the forces driving the activation of α/β T cells in lupus is a heightened response to self-antigens, independent of the nature of the APCs and degree of costimulation. Further evidence suggesting an intrinsic abnormality in lupus T cells comes from recent genetic studies in NZM mice that demonstrated a locus on chromosome 7 associated with CD4+ T cell polyclonal activation, enhanced proliferation to anti-CD3, and decreased AICD 6 25 26. This genetic locus is necessary for the development of fully penetrant immune-mediated renal disease in C57BL/6 congenic mice, highlighting the pathologic consequence of global T cell activation 6. This NZM locus overlaps with the lupus susceptibility locus Lbw5, mapped in an (NZB × NZW) F2 cross 27, the MRL locus lmb3, associated with lymphadenopathy, splenomegaly, and anti-DNA autoantibody production 28, and the MRL locus Lrdm2 associated with renal disease 29. These findings suggest the possibility that there is a gene(s) in all lupus-prone mice that confers an intrinsic abnormality in T cells, and taken together, are consistent with the evolving view that excessive, unchecked T cell activation is necessary for the development of systemic autoimmunity. Support for this notion comes from the observations that mice with hyperproliferative CD4+ T cells, due to targeted disruption either of regulators of the cell cycle 30 or TGF-β signaling 31, develop a phenotype very similar to human lupus, with antibodies against native DNA and immune complex glomerulonephritis. Studies in human lupus patients have also suggested that there are abnormalities in lymphocyte signaling 7 8 32 33.
Identification of a lower threshold for activation in lupus T cells does not preclude other intrinsic abnormalities in the immune system from contributing to a predisposition to autoimmunity, such as abnormalities in B cell 26 33 34 or APC function 35, or in clearance of immune complexes 36. Rather, we would argue that the intrinsic T cell defect we describe, in conjunction with other abnormalities in the immune system, would be sufficient to disrupt autoreactive T cell tolerance in the periphery, and is consistent with the polygenic susceptibility model for lupus supported by strong experimental evidence 26 37. While it is possible that the abnormalities we have identified herein are the result of strain differences in TCR stimulation between the MRL and nonautoimmune backgrounds, and not related to the lupus phenotype, our results nevertheless offer a logical explanation of the polyclonal T cell reactivity in lupus. This question will be addressed by determining if the differences in CD4+ T cell responses we identified are associated with lupus susceptibility loci.
Although the above observations are limited to in vitro studies, experiments are underway to compare the activation and survival of both naive and memory transgenic T cells in vivo. Indeed, preliminary work from our laboratory suggests that T cells from lupus-prone mice survive much better in vivo in response to peptide ligands with low TCR affinity, compared with cells from nonautoimmune controls, similar to our in vitro results. These cumulative data suggest the possibility that self-peptides normally displayed by MHC class II molecules in the periphery, and responsible for naive CD4+ T cell survival and homeostasis in normal animals, lead to enhanced (polyclonal) activation of T cells from lupus-prone mice compared with cells from nonautoimmune animals 38 39 40. In addition, studies are underway that begin to address the biochemical mechanism for the defect described in lupus T cells. It will be important to compare the AICD of transgenic T cells when stimulated with peptides having both low and high affinity for the TCR.
Acknowledgments
We thank the members of the Craft laboratory for helpful discussions, especially Elizabeth Ramsburg for her careful review of the manuscript. We also thank Mark Shlomchik and Mark Mamula for their review of the work and for ongoing discussions of its implications.
This work was supported in part by National Institutes of Health grants AR40072 and AR44076, the Arthritis Foundation, and the Lupus Foundation of America and their Connecticut chapters (to J. Craft). G. Vratsanos was supported by a Physician Scientist Development Award from the American College of Rheumatology and the Arthritis Foundation, and by the Hartford Foundation Center of Excellence for Research on Aging.
Footnotes
Abbreviations used in this paper: AICD, activation-induced cell death; APL, altered peptide ligand; PCC, pigeon cytochrome C; PI, propidium iodide.
G.S. Vratsanos and S. Jung contributed equally to this work.
S. Jung's present address is Dept. of Internal Medicine, The Hospital for Rheumatic Diseases, Hanyang University Medical Center, Seoul 133-792, South Korea. Y. Park's present address is Dept. of Microbiology and Immunology, Pusan National University College of Medicine, Pusan 602-739, South Korea.
References
- Wofsy D., Ledbetter J.A., Hendler P.L., Seaman W.E. Treatment of murine lupus with monoclonal anti-T cell antibody. J. Immunol. 1985;134:852–857. [PubMed] [Google Scholar]
- Peng S.L., Madaio M.P., Hughes D.P., Crispe I.N., Owen M.J., Wen L., Hayday A.C., Craft J. Murine lupus in the absence of αβ T cells. J. Immunol. 1996;156:4041–4049. [PubMed] [Google Scholar]
- Rozzo S.J., Drake C.G., Chiang B.L., Gershwin M.E., Kotzin B.L. Evidence for polyclonal T cell activation in murine models of systemic lupus erythematosus. J. Immunol. 1994;153:1340–1351. [PubMed] [Google Scholar]
- Fatenejad S., Peng S.L., Disorbo O., Craft J. Central T cell tolerance in lupus-prone miceinfluence of autoimmune background and the lpr mutation. J. Immunol. 1998;161:6427–6432. [PubMed] [Google Scholar]
- Sloan-Lancaster J., Allen P.M. Altered peptide ligand-induced partial T cell activationmolecular mechanisms and role in T cell biology. Annu. Rev. Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- Mohan C., Yu Y., Morel L., Yang P., Wakeland E.K. Genetic dissection of Sle pathogenesisSle3 on murine chromosome 7 impacts T cell activation, differentiation, and cell death. J. Immunol. 1999;162:6492–6502. [PubMed] [Google Scholar]
- Kammer G.M., Khan I.U., Malemud C.J. Deficient type I protein kinase A isozyme activity in systemic lupus erythematosus T lymphocytes. J. Clin. Invest. 1994;94:422–430. doi: 10.1172/JCI117340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilopoulos D., Kovacs B., Tsokos G.C. TCR/CD3 complex-mediated signal transduction pathway in T cells and T cell lines from patients with systemic lupus erythematosus. J. Immunol. 1995;155:2269–2281. [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Kaye J., Hsu M.L., Sauron M.E., Jameson S.C., Gascoigne N.R., Hedrick S.M. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- Peng S.L., Fatenejad S., Craft J. Induction of nonpathologic, humoral autoimmunity in lupus-prone mice by a class II-restricted, transgenic αβ T cell. Separation of autoantigen-specific and -nonspecific help. J. Immunol. 1996;157:5225–5230. [PubMed] [Google Scholar]
- Lyons D.S., Lieberman S.A., Hampl J., Boniface J.J., Chien Y., Berg L.J., Davis M.M. A TCR binds to antagonist ligands with lower affinities and faster dissociation rates than to agonists. Immunity. 1996;5:53–61. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- Lucas B., Stefanova I., Yasutomo K., Dautigny N., Germain R.N. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10:367–376. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A.M., Kubo R., Kappler J.W., Marrack P. The V β-specific superantigen staphylococcal enterotoxin Bstimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989;56:27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Tough D.F., Sprent J. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursalian T.E., Bottomly K. Stability of naive and memory phenotypes on resting CD4 T cells in vivo. J. Immunol. 1999;162:9–16. [PubMed] [Google Scholar]
- De Magistris M.T., Alexander J., Coggeshall M., Altman A., Gaeta F.C., Grey H.M., Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- Page D.M., Alexander J., Snoke K., Appella E., Sette A., Hedrick S.M., Grey H.M. Negative selection of CD4+ CD8+ thymocytes by T-cell receptor peptide antagonists. Proc. Natl. Acad. Sci. USA. 1994;91:4057–4061. doi: 10.1073/pnas.91.9.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrenas J., Wange R.L., Wang J.L., Isakov N., Samelson L.E., Germain R.N. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- Haughton G., Arnold L.W., Bishop G.A., Mercolino T.J. The CH series of murine B cell lymphomasneoplastic analogues of Ly-1+ normal B cells. Immunol. Rev. 1986;93:35–51. doi: 10.1111/j.1600-065x.1986.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Wells A.D., Gudmundsdottir H., Turka L.A. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J. Clin. Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu P., Singer G.G., Andres P., Ettinger R., Marshak-Rothstein A., Abbas A.K. Mature CD4+ T lymphocytes from MRL/lpr mice are resistant to receptor-mediated tolerance and apoptosis. J. Immunol. 1993;151:7233–7239. [PubMed] [Google Scholar]
- Alexander J., Snoke J., Ruppert J., Sidney J., Wall M., Southwood S., Oseroff C., Arrhenius T., Gaeta F., Colon S. Functional consequences of engagement of the T cell receptor by low affinity ligands. J. Immunol. 1993;150:1–7. [PubMed] [Google Scholar]
- Sloan-Lancaster J., Shaw A.S., Rothbard J.B., Allen P.M. Partial T cell signalingaltered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Mohan C., Morel L., Yang P., Watanabe H., Croker B., Gilkeson G., Wakeland E.K. Genetic dissection of lupus pathogenesisa recipe for nephrophilic autoantibodies. J. Clin. Invest. 1999;103:1685–1695. doi: 10.1172/JCI5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L., Mohan C., Yu Y., Croker B.P., Tian N., Deng A., Wakeland E.K. Functional dissection of systemic lupus erythematosus using congenic mouse strains. J. Immunol. 1997;158:6019–6028. [PubMed] [Google Scholar]
- Kono D.H., Burlingame R.W., Owens D.G., Kuramochi A., Balderas R.S., Balomenos D., Theofilopoulos A.N. Lupus susceptibility loci in New Zealand mice. Proc. Natl. Acad. Sci. USA. 1994;91:10168–10172. doi: 10.1073/pnas.91.21.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Kono D.H., Theofilopoulos A.N. Loci predisposing to autoimmunity in MRL-Faslpr and C57BL/6-Faslpr mice. J. Clin. Invest. 1998;101:696–702. doi: 10.1172/JCI1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M.L., Rao J.K., Gilkeson G.S., Ruiz P., Eicher E.M., Pisetsky D.S., Matsuzawa A., Rochelle J.M., Seldin M.F. Genetic analysis of MRL-lpr micerelationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J. Exp. Med. 1992;176:1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balomenos D., Martin-Caballero J., Garcia M.I., Prieto I., Flores J.M., Serrano M., Martinez A.C. The cell cycle inhibitor p21 controls T-cell proliferation and sex-linked lupus development. Nat. Med. 2000;6:171–176. doi: 10.1038/72272. [DOI] [PubMed] [Google Scholar]
- Gorelik L., Flavell R. Abrogation of TGF-β signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- Kammer G.M., Khan I.U., Kammer J.A., Olorenshaw I., Mathis D. Deficient type I protein kinase A isozyme activity in systemic lupus erythematosus T lymphocytes. II. Abnormal isozyme kinetics. J. Immunol. 1996;157:2690–2698. [PubMed] [Google Scholar]
- Liossis S.N., Kovacs B., Dennis G., Kammer G.M., Tsokos G.C. B cells from patients with systemic lupus erythematosus display abnormal antigen receptor-mediated early signal transduction events. J. Clin. Invest. 1996;98:2549–2557. doi: 10.1172/JCI119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongstra-Bilen J., Vukusic B., Boras K., Wither J.E. Resting B cells from autoimmune lupus-prone New Zealand Black and (New Zealand Black × New Zealand White) F1 mice are hyper-responsive to T cell-derived stimuli. J. Immunol. 1997;159:5810–5820. [PubMed] [Google Scholar]
- Alleva D.G., Kaser S.B., Beller D.I. Aberrant cytokine expression and autocrine regulation characterize macrophages from young MRL+/+ and NZB/W F1 lupus-prone mice. J. Immunol. 1997;159:5610–5619. [PubMed] [Google Scholar]
- Botto M., Dell'Agnola C., Bygrave A.E., Thompson E.M., Cook H.T., Petry F., Loos M., Pandolfi P.P., Walport M.J. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Morel L., Rudofsky U.H., Longmate J.A., Schiffenbauer J., Wakeland E.K. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1:219–229. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II–expressing dendritic cells. J. Exp. Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viret C., Wong F.S., Janeway C.A., Jr. Designing and maintaining the mature TCR repertoirethe continuum of self-peptide:self-MHC complex recognition. Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- Lee D.S., Ahn C., Ernst B., Sprent J., Surh C.D. Thymic selection by a single MHC/peptide ligandautoreactive T cells are low-affinity cells. Immunity. 1999;10:83–92. doi: 10.1016/s1074-7613(00)80009-6. [DOI] [PubMed] [Google Scholar]