Abstract
The hippocampus is involved in the detection of novelty and is essential for certain forms of learning about environmental events and relationships. The cellular and molecular mechanisms of one form of hippocampal synaptic plasticity, long-term potentiation (LTP), are thought to overlap significantly with the neural mechanisms of learning. In this study changes in hippocampal synaptic efficacy were measured in awake, freely behaving rats during exploration of novel environments. Because hippocampal physiology is modulated by on-going behavior, evoked potentials collected during Type 1 vs Type 2 behavior were evaluated separately. The effect of prior LTP induction at perforant path-dentate synapses on exploration-induced changes was evaluated. The results show that exploration causes an increase in population spike amplitude with no change in excitatory postsynaptic potential during Type 1 behavior that lasts longer than 5 minutes. Prior induction of hippocampal LTP occludes the change induced by exploration. This change is not likely to be due to a reduction of GABAergic inhibition induced by novelty.
Keywords: Hippocampus, LTP, Type 1 Behavior, Type 2 Behavior, Novelty Learning, Memory
1. Introduction
The specific role for the hippocampal formation in various aspects of memory has been a source of continuing debate. A widely accepted and well-studied role of the hippocampal formation is in certain forms of spatial memory. According to the cognitive map theory [23], the hippocampus constructs an internal representation of the spatial properties of an environment. As an animal moves around an environment, exploring or sampling cues, the hippocampus constructs and stores a cognitive map that contains information about direction and distance between places in that environment. Rats with hippocampal damage are impaired in learning the radial arm maze [10, 24], the T-maze [2, 27] and the hidden platform version of the Morris water task [15, 16, 36, 37]. Recent functional neuroimaging studies in humans have also provided evidence that the hippocampus activated during computerized spatial navigation [1, 13].
The hippocampus is a key structure in the detection of novelty or familiarity. Evidence that hippocampus is involved in novelty detection comes from hippocampal lesion, electrophysiological and biochemical studies in animals and neuroimaging studies in humans [8, 20, 25].
The phenomenon of hippocampal long-term potentiation (LTP) provides a well-described physiological model of synaptic plasticity that may underly learning and memory [4]. If LTP and learning are based upon a significant degree of the same cellular processes, then a mutual interference or occlusion would be expected to take place. It has been postulated that under natural conditions, experience changes hippocampal synaptic strength, which is the basis for certain forms of learning and memory. LTP-like changes could be reflected in hippocampal evoked potentials as a result of natural learning or exploration [38]. A number of researchers have observed LTP-like increases in the EPSP slope and population spike amplitude of perforant path-dentate gyrus evoked potentials as a result training compared to those evoked before training [28, 35, 42]. Active locomotor exploration was shown to be accompanied by a gradual increase in the EPSP and a decrease in the population spike (PS) while animals acquired information about novel environments [3, 33, 34]. However, this relatively short term modulation of evoked potentials during learning through exploration exploratory is in part due to increases in brain temperature caused by increased movement [18]. Studies in area CA1 of the hippocampus [7, 44] have shown that expression of prior electrical stimulation induced LTP was reversed by entering and exploring a novel environment.
Based on recorded hippocampal spontaneous electrical activity, two kinds of wave forms were found by Vanderwolf [40] to correlate strongly with on-going movements. Rhythmical slow activity (RSA or theta waves) was recorded during Type I behavior: walking, changing in posture, turning or raising the head, standing up, rearing, or manipulating objects with the forepaws. Large amplitude irregular activity (LIA) was recorded during Type 2 behavior: alert immobility, face washing, licking, chewing, sniffing, or gnashing the teeth. Hippocampal evoked potentials (EPs) also vary systematically with ongoing behaviors [6, 12, 32, 43]. Entorhinal-dentate gyrus EPs recorded during Type 1 behaviors differ in shape and amplitude from those recorded during Type 2 behaviors. These correlations between hippocampal activity and motor activity are generally not changed by behavioral training [41]. Thus, the RSA accompanying walking has the same frequency and amplitude regardless of whether the walking occurs spontaneously or as a result of previous training [41]. EP amplitudes in the dentate gyrus are greater during grooming, which is typically accompanied by LIA, than running, which is typically accompanied by RSA [6]. In the area CA1, the amplitude of PS is smaller during behaviors associated with RSA than during those associated with LIA [12].
In this study, one method used if called the behavioral clamping method. In this method, EPs are compared only when the animal is in the same behavioral state at the time that EPs are recorded. The most important behavioral dimension is Type 1 vs Type 2 behaviors [40]. This dichotomy is correlated with the presence of RSA vs LIA EEG states and at the initial transition into a novel environment there is a clear shift in the proportion of each type of behavior. Furthermore, existing data [6, 12, 32, 41, 43] demonstrate that important aspects of the dentate evoked potential are modulated by RSA vs LIA states. Thus, the difference caused by the behavioral states is minimized and learning-related effects can be separated from movement-related effects.
The primary purpose of this study is to examine the physiological interaction between natural learning and electrically-induced LTP. In this study, evoked potentials were recorded in the dentate gyrus in freely-moving rats when rats entered a novel environment with behavioral clamping method. If LTP underlies learning then within a relevant set of synapses they should share many of the same cellular processes. Prior LTP induction will therefore interact with learning-induced physiological changes. Brain temperatures in both hemispheres were also recorded to check whether brain temperature did increase in this study in the manner expected by prior experimentation [18, 19].
2. Materials and methods
2.1. Animals
The subjects were male hooded rats of the Long-Evans strain (300–400 g) obtained from Harlan Laboratories. They were housed in hanging wire single mesh cages on a 12:12 light/dark cycle with free access to food and water.
2.2. Surgery
Rats were anesthetized with halothane (O2 flow rate 1.5 l/min at 1.5–2% halothane) for chronic implantation. Rats were implanted, using aseptic surgical techniques, with a single stimulation electrode and a single recording electrode. In the case of hippocampal electrophysiology the recording electrode was aimed at the hilar region of the hippocampal dentate gyrus (Paxinos & Watson, 1986; coordinates: 3.5 mm posterior to the bregma, 1.8 mm lateral to the midline, and 3.6 mm below the top of the skull) and the stimulating electrode was aimed at the ipsilateral perforant path (Paxinos & Watson, 1986; coordinates: 8.1 mm posterior to the bregma, 4.3 mm lateral to the midline, and 3.0 mm below the top of the skull). Two stainless-steel, jewelers’ screws tapped into the skull served as reference and ground components of the differential recording circuit. One jewelers’ screw tapped into the skull served as the return portion of the stimulation circuit. An additional jewelers’ screw tapped into the skull provided structural support for the electrode assembly held in place by dental acrylic cement. The skin incision was closed with a veterinary glue and the rats received injections of penicillin G (60,000 I.U. i. m.) and buprenorphine (0.07 ml, i. p.)
2.3. Electrophysiology apparatus
All electrodes were constructed of stainless-steel, insulated with Teflon, and had an outside diameter of 114 μm. Gold-plated Amphenol pins on the rat’s head served as connectors to the recording leads. The recording leads passed through a commutator and into a differential preamplifier (Grass model P15D) and thence to a Neurolog filter and amplifier. The signal was filtered (1/2 amplitude low frequency - 1 HZ; 1/2 amplitude high frequency = 10 kHz) and the total amplification was 200X. Signals were displayed on a Nicolet digital storage scope, continuously monitored on a Grass audio monitor, and sent to a computer running the DataWave PSW software package (version 6.0, DataWave Technologies, Longmont, CO) data acquisition and storage system (Delay: 5 ms; Duration: 60 ms; Sampling Rate: 25000 Hz). The Workbench analysis package was used to measure the amplitude and slope of the dentate gyrus evoked potential. The positions of both depth electrodes were optimized under electrophysiological guidance using single pulse stimulation (pulse duration = 100 μs, amplitude = 300 μA, frequency = 1 per 20 s). Stimulation was provided by an AMPI Master 8 pulse former and an Isoflex optically-isolated constant current stimulator (AMPI Company, Jerusalem, Israel).
2.4. Two novel environments
During the time that electrophysiological and behavioral recordings were taken, the freely moving, awake rats were always confined to one of three different recording chambers.
Chamber 1 was the recording chamber that was most familiar to all rats. It was a Plexiglas square box (25 cm long × 18 cm wide) with a floor composed of small stainless steel bars. It was placed inside a 100cm diameter cylindrical, white fibreglass enclosure.
Chamber 2 was a yellow, plastic, square-bottom bucket (height 28 cm, length 30 cm, width 20 cm). Chamber 3 was an iron, circular-bottom popcorn container (height 28 cm; diameter 20 cm). Both chamber 2 and 3 were quite different from the recording chamber.
2.5. Behavioral Observations
During all the recording sessions, behavioral observations were taken at the time of each stimulus delivery. Vanderwolf and his students named these groupings of behaviors Type 1, RSA- or theta-associated, and Type 2, LIA-associated [40]. Behaviors were divided into two categories: 1. walking, leaning forepaws against a wall, head scanning, moving paws, jumping, rearing, sniffing, all four feet still but head moving. 2. freezing or awake immobility (no movement of head, trunk, or paws), grooming, licking, scratching. The first behavior category has been shown to be associated with hippocampal rhythmical slow-EEG activity (RSA or theta) in the rat [40]. The latter behavior category is associated with hippocampal large amplitude irregular- EEG activity (LIA) [40]. Behavioral clamping was accomplished for each rat in the data evaluation by comparing EEG changes before and after introduction into the novel environment during periods of the same behavioral type (i.e. RSA or LIA related categories).
2.6. Experimental Procedures
One week after implantation, each rat received daily 30 min habituation sessions in the recording chamber for 7 consecutive days. In all habituation sessions, each rat was connected to the commutator and was permitted to freely explore the recording chamber. At the same time, single pulse stimulation (pulse duration = 100 μs, amplitude = 100 μA, frequency = 1 per 20 s) was delivered and no recording was made throughout the whole session.
In the control group, 15 rats were used. The size of the evoked potential was measured at each of 6 stimulus pulse intensities (100, 200, 300, 400, 500 μA) - each intensity was repeated 10 times at the rate of 1 pulse per 20 s for each rat. A stimulation intensity was selected by finding the intensity which produced an evoked response 70–75% of the maximal response. A submaximal response was selected to ensure that enhancement and inhibition of the evoked response could be observed. Two measures were taken of each evoked potential: 1. the amplitude of the field EPSP measured at approximately the middle of the rising slope at a fixed time after the stimulus artifact (fEPSP), and 2. the amplitude of the trough using the tangent method (voltage difference between a tangent to the two positive peaks and the trough of the negative-going wave component) (PS). Rats received one-hour baseline recording in the recording chamber. Then rats were gently picked up and placed into one of the novel environments. Recordings continued for another hour in the novel environment.
In the LTP induction group, 15 rats received 20 pulses baseline recording at the optimal intensity in the recording chamber. Then 10 trials of high frequency stimuli (400 Hz, 10 pulses) at 500 μA were delivered. After that, rats received one pulse at the optimal intensity for an hour in the recording chamber. Then rats were gently picked up and placed in a novel environment. Recording continued for a second one hour in the novel environment. The increase in LTP was expressed as a fractional change in which the average value of baseline was divided by the difference between the average value of post-induction minus the average value of baseline. The criterion of LTP was defined as: more than 50% increase in the amplitude of PS and more than 15% increase in the slope of EPSP and the enhancement must last for one hour.
In the paired-pulse control group, 8 rats received paired-pulse stimulation at a constant frequency of 0.1 Hz with varying inter-pulse intervals of ten each at 10, 15, 20, 25, 30, 40, 60, and 100 ms in the familiar and novel chamber. In the paired-pulse LTP group, 5 rats received LTP induction in the familiar chamber, then received paired-pulse stimulation the familiar and novel chamber. The amplitude of the population spike was measured using the tangent method. Data were collected in the recording chamber and in the novel environments. Fractional change at varying inter-pulse intervals was plotted by subtracting the second population spike amplitude from the first and dividing by the first. Differences between fractional change curves were then calculated for familiar and novel environments at each inter-pulse interval. This gave a measure of degree of modulation for novel effect.
The rats’ behavior during all recording sessions was recorded on video tape. At no time in the recording sessions did the rats show behavioral signs of sleep. The electrophysiological data were stored on the microcomputer hard drive.
Bilateral temperature recording was carried out on 2 rats. All of the surgical procedures were the same as the above experiment except two temperature probes were implanted at the hilar regions of the bilateral dentate gyri. The temperature probes were 5cm long and 1mm in diameter (TH-107, PhysiTemp Instruments INC). They were connected to a monitor (Thermalert TH-8, 0.1 °C) through a flexible cable. The entire procedure was the same as the above experiment.
2.7. Histology
Rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.). They were perfused intracardially with 50 ml 0.1M phosphate buffered saline solution followed by 4% formalin/phosphate buffered saline solution (100 ml). The brains were transferred to a 20% sucrose in 4% formalin/phosphate buffered saline solution 48 hr before frozen sectioning in a cryostat. The brains were cut at 30 μ. Every section through the electrode tracks was mounted on gelatin-coated glass slides and stained with cresyl violet. They were examined microscopically and the location of the electrode tips were determined in relation to the atlas of Paxinos & Watson (1986).
2.8. Data Analysis
All recorded evoked potentials were divided into two categories based on behavior Types 1 and 2 and were compared at the same behavior type.
PS amplitude and EPSP slope were measured automatically using the DataWave PSW software package (version 6.0). In order to evaluate the effect of transfer from the familiar environment to a novel one for each rat, six recording intervals were defined. They included the last 5 minutes in the familiar environment (last5minF), the first 5 minutes in the novel environment (first5minN), the second 5 minutes (second5minN), the third 5 minutes (third5minN), then the next 15 minutes in the novel environment (next5minN) and the last 30 minutes in the novel environment (last30minN). The ratio of the average of each these six durations to the average of the whole 60 minutes in the baseline recording in the familiar environment were calculated and the ratios were submitted to repeated-measures ANOVA. Post hoc LSD tests were applied to statistically test the differences between each of the five intervals in the novel environment and the last 5 minutes in the familiar environment when the interactions were statistically significant (p < 0.05).
In the paired-pulse experiment the differences between PS amplitude in the familiar and novel environment at 8 different intervals during Type 1 and Type 2 behaviors were submitted to repeated-measures ANOVA.
In order to test the interaction between prior LTP induction and the changes induced by novelty, the correlation of the proportional changes in PS amplitude and in EPSP slope with the proportional changes of all the five intervals in the novel environments {(post-pre)/pre} was analyzed by Pearson’s correlation coefficient.
In the temperature recording experiment, the changes in brain temperature at both hipocampi were measured by the fractional ratio [(value-the average of first 15min)/value]. The correlation between both hippocampi was calculated for each rat to assess how similar the temperature changes throughout the recoding session are in two hippocampi.
3. Results
Changes in behavior types
Both groups showed a burst in exploratory behavior upon transfer into a novel chamber. Type 1 behavior decreased in frequency and Type 2 behavior increased until they reattained the levels shown in the familiar environment (Fig. 1 & 2).
Figure 1.
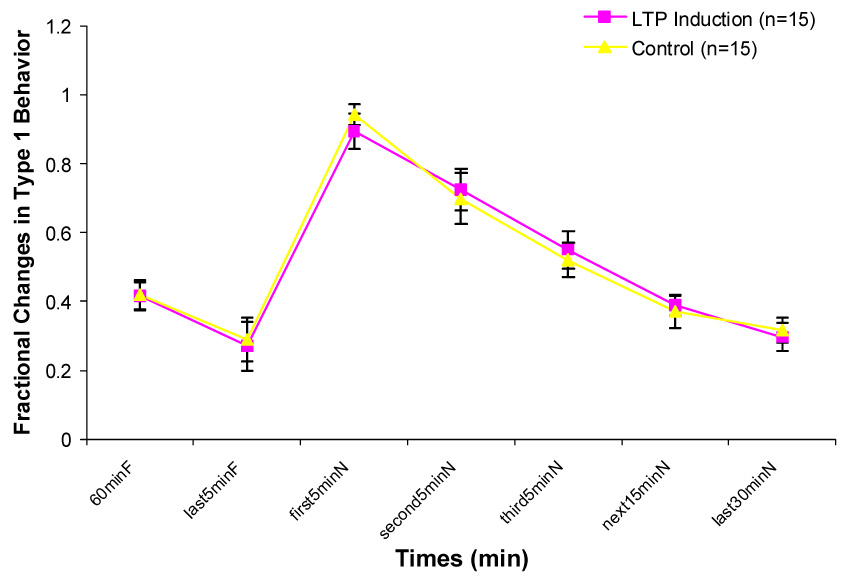
Changes in Type 1 behavior during one hour in the familiar chamber (60minF), last 5minutes in the familiar chamber (last5minF) and 5 different durations in the novel chamber (first 5minN, second5minN, third5minN, next15minN, last30minN). No significant differences exist between two groups.
Figure 2.
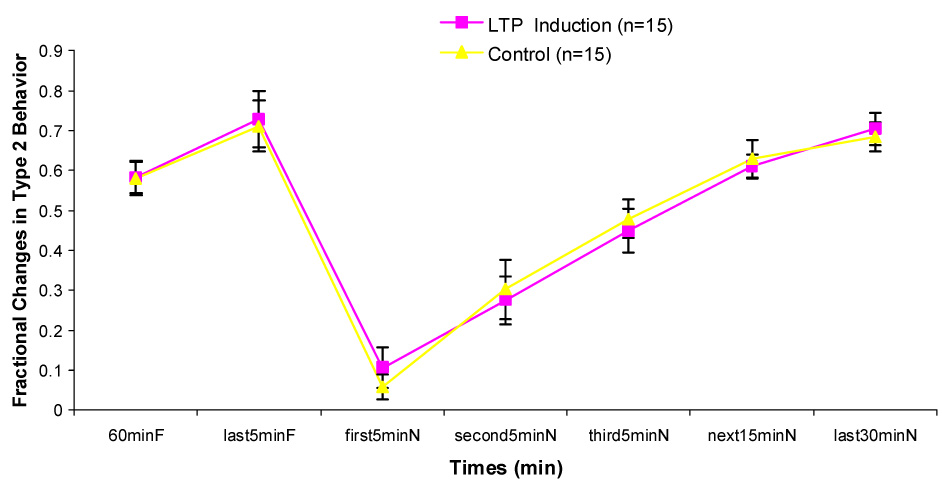
Changes in Type 2 behavior during one hour in the familiar chamber (60minF), last 5minutes in the familiar chamber (last5minF) and 5 different durations in the novel chamber (first 5minN, second5minN, third5minN, next15minN, last30minN). No significant differences exist between two groups.
No significant difference was found on the number of Type 1 behavior between control and LTP induction groups at any of the 7 intervals.
Transfer from the recording chamber to the novel chamber
In the control group, there was a significant increase in PS amplitude ratio immediately after being transferred from the recording chamber to the novel chamber (Fig. 3). This increase was statistically significant only during Type 1 behavior. The enhancement of the population spike persisted in the novel environment (P < 0.01). PS amplitude ratio decreased gradually over the 60 min interval. But it remained insignificantly larger during the second 5 min, the third 5 min, the next 15 min interval and the last 30 min in the novel chamber than that during the last 5 min in the familiar recording chamber. During Type 2 behavior, no significant difference in PS amplitude ratio between any interval in the novel chamber and the last 5 min in the familiar chamber was found (Fig. 4). No significant differences on EPSP slope ratio between any intervals in the novel chamber and the last 5 min in the familiar chamber during Type 1 and Type 2 behaviors were found (Fig. 5 & 6).
Figure 3.
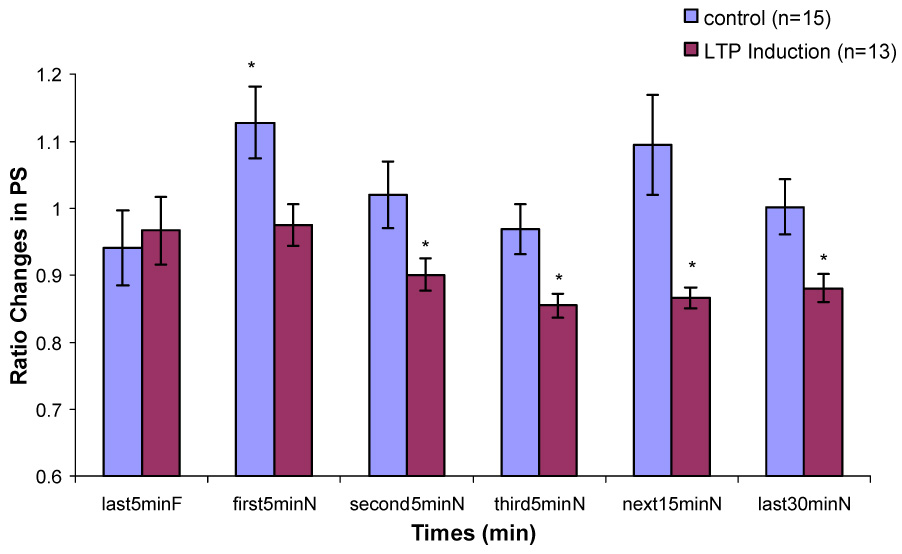
Changes in population spike amplitude during Type 1 behavior in control and LTP induction groups when the rats are transferred from a familiar chamber to a novel chamber. (*, significant differences in comparison to last5minF, p < 0.05)
Figure 4.
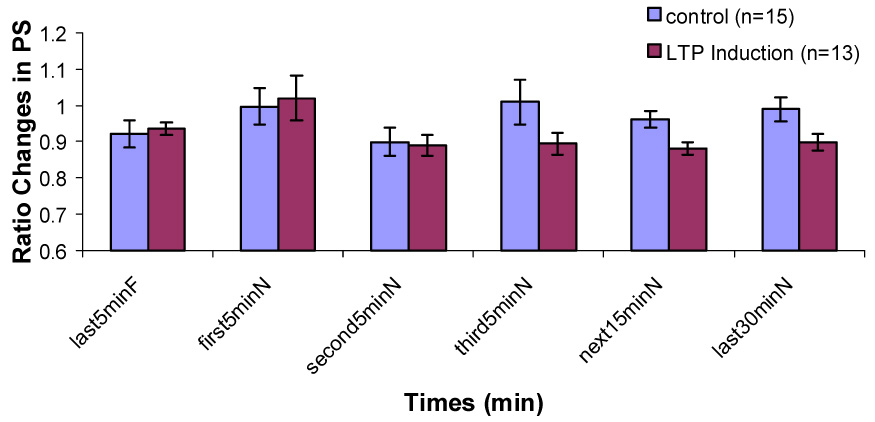
Changes in population spike amplitude during Type 2 behavior in control and LTP induction groups when the rats are transferred from a familiar chamber to a novel chamber.
Figure 5.
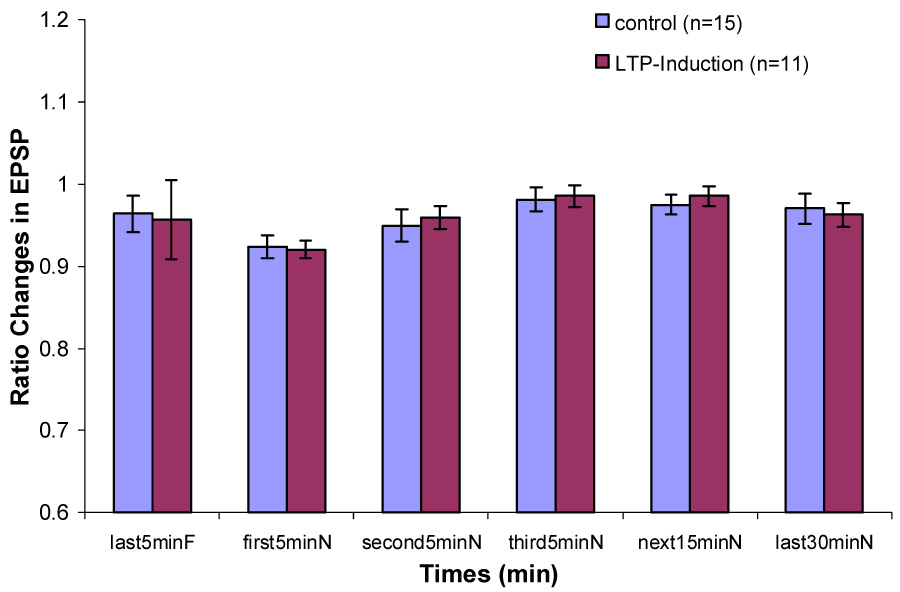
Changes in EPSP slope during Type 1 behavior in control and LTP induction groups when the rats are transferred from a familiar chamber to a novel chamber.
Figure 6.
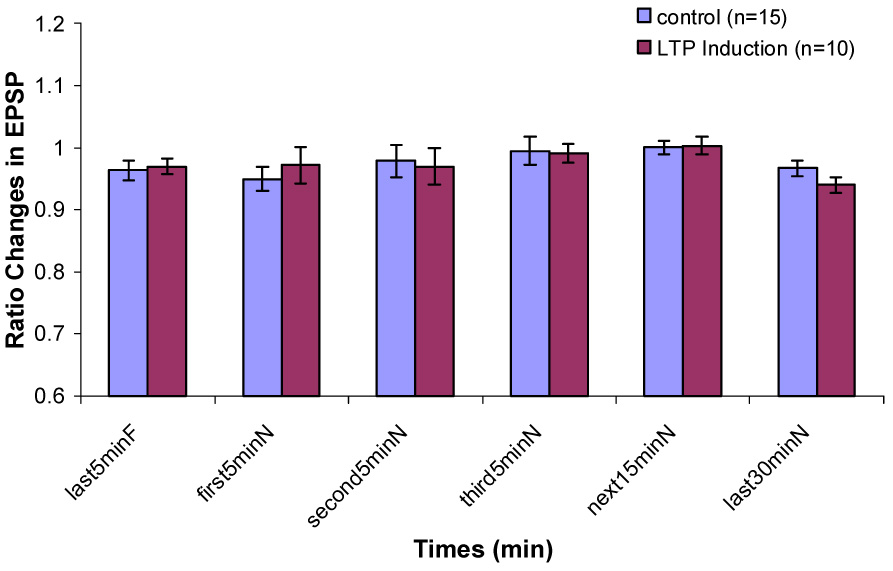
Changes in EPSP slope during Type 2 behavior in control and LTP induction groups when the rats are transferred from a familiar chamber to a novel chamber.
In the LTP induction group, during Type 1 behavior, no significant difference on PS amplitude ratio between the last 5 min in the familiar chamber and the first 5 min in the novel chamber was found. Significant decreases on PS amplitude ratio were found during the second 5 min, the third 5 min, the next 15 min and the last 30 min in the novel chamber (Fig. 3). During Type 2 behavior, no significant decreases were found between any intervals in the novel chamber and the last 5 min in the familiar chamber (Fig. 4). No significant difference on EPSP slope ratio between the last 5 min in the recording chamber and any intervals in the novel chamber during Type 1 and Type 2 behaviors was found (Fig. 5 & 6).
During Type 1 behavior, the proportional change in PS amplitude was significantly negatively correlated with the proportional changes in the third 5 min (r15=−0.554, p=0.032) and the last 30 min (r15=−0.520, p=0.047) in the novel environment. The present data showed that the larger magnitude of LTP in PS amplitude, the less increase in PS amplitude induced by novelty effect. Thus, prior LTP induction significantly interacts with novelty-induced changes.
During Type 2 behavior, the Pearson correlation between the proportional change in PS amplitude and all 5 intervals in the novel environment were not significant. During Type 1 behavior, Pearson correlations between the proportional change in EPSP slope induced by high-frequency stimulation and EPSP slope in the 5 intervals in the novel environment were not significant. During Type 2 behavior, the proportional change in EPSP slope was significantly negatively correlated with the proportional changes of the first 5 min in the novel chamber.
Effects on paired-pulse inhibition and facilitation
During both types of behaviors in the familiar and novel chambers, paired-pulse inhibition was evident when the interpulse interval is less than 30 ms and paired-pulse facilitation was evident when IPI is from 30 ms to 60 ms. Paired-pulse facilitation was also seen at 100 ms IPI during Type 2 behavior in the novel chamber.
No significant differences on paired-pulse inhibition or paired-pulse facilitation between familiar and novel environments were found at any interpulse interval during either Type 1 or 2 behavior in the paired-pulse control and LTP groups (Fig. 7, 8, 9, 10)
Figure 7.
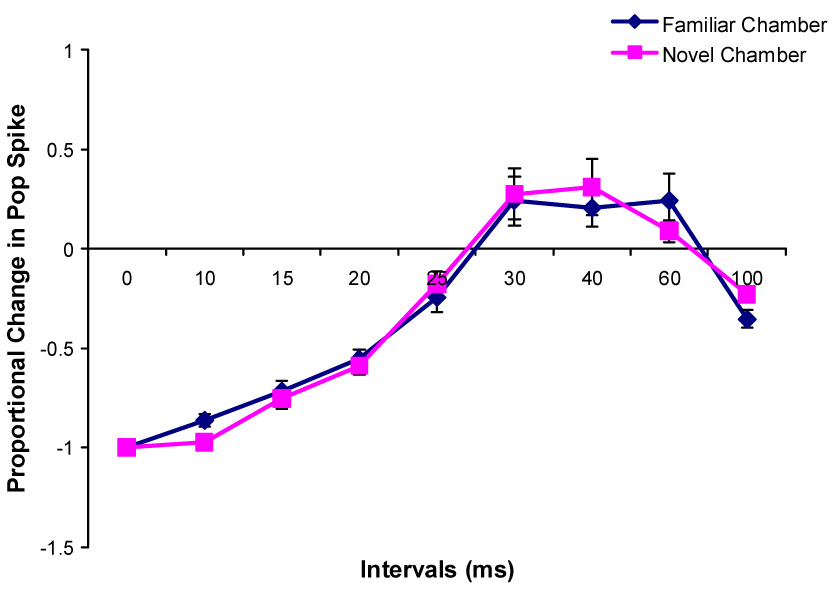
Changes in the paired-pulse effects on the population spike amplitude during Type 1 behavior after rats were transferred into a novel chamber (n=8).
Figure 8.
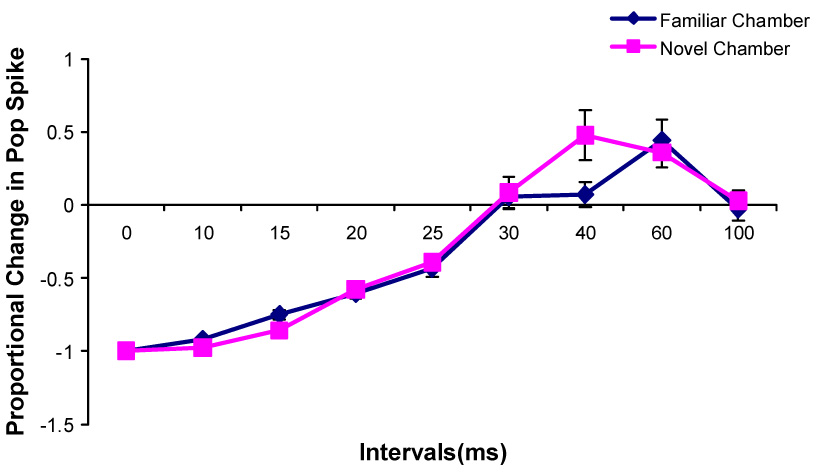
Changes in the paired-pulse effects on the population spike amplitude during Type 2 behavior after rats were transferred into a novel chamber (n=8).
Figure 9.
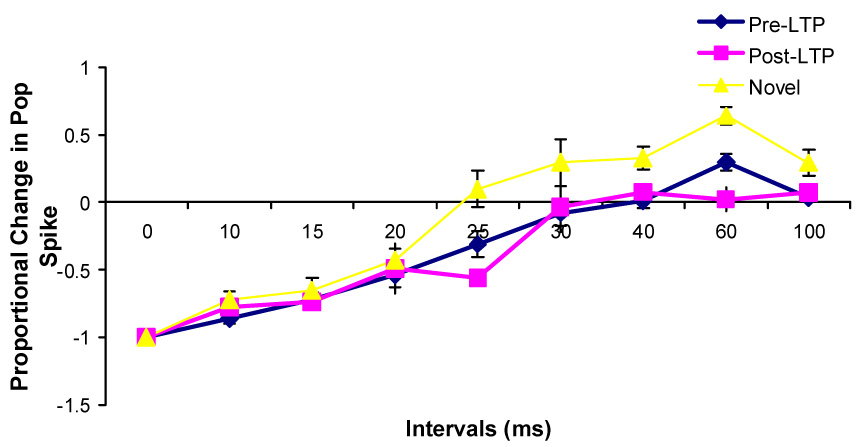
Changes in the paired-pulse effects on the population spike amplitude during Type 1 behavior at the unilaterally implanted rats after the high frequency stimulations and after being transferred into a novel chamber (n=5).
Figure 10.
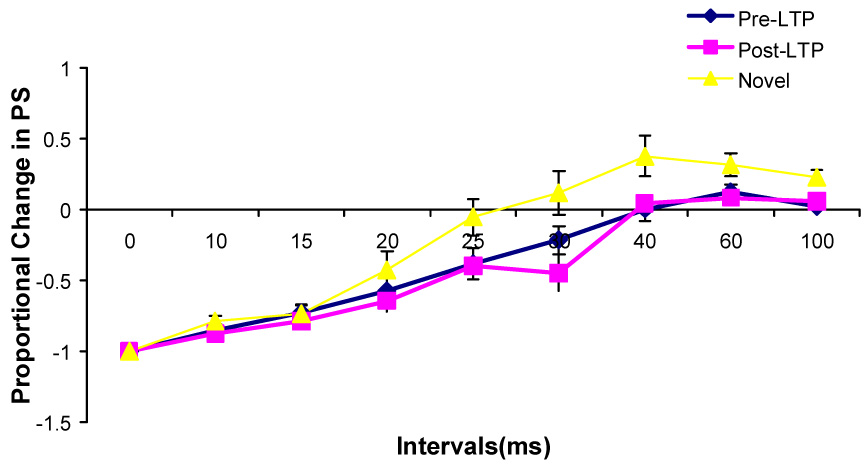
Changes in the paired-pulse effects on the population spike amplitude during Type 2 behavior at the unilaterally implanted rats after the high frequency stimulations and after being transferred into a novel chamber (n=5).
Temperature changes (Fig. 11 & Fig. 12)
Figure 11.
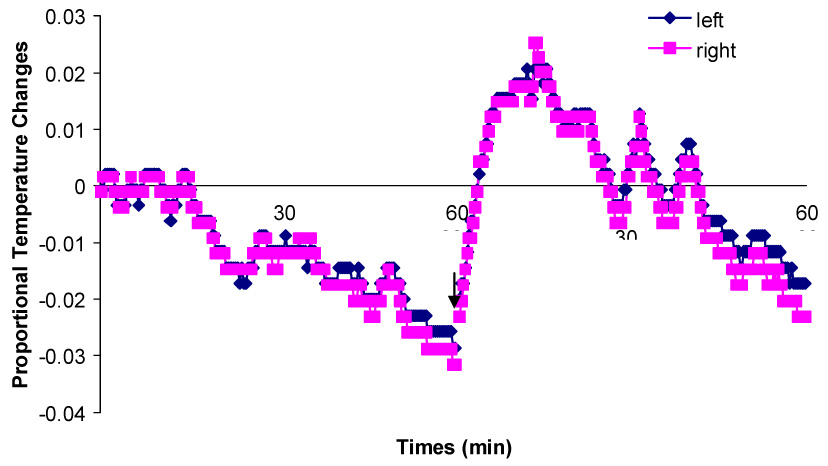
Proportional temperature changes at the left and right sides of a bilaterally implanted rat during one hour in the familiar chamber and after being transferred into the novel chamber. The arrow indicates the moment that the rat is transferred from the familiar chamber into the novel chamber.
Figure 12.
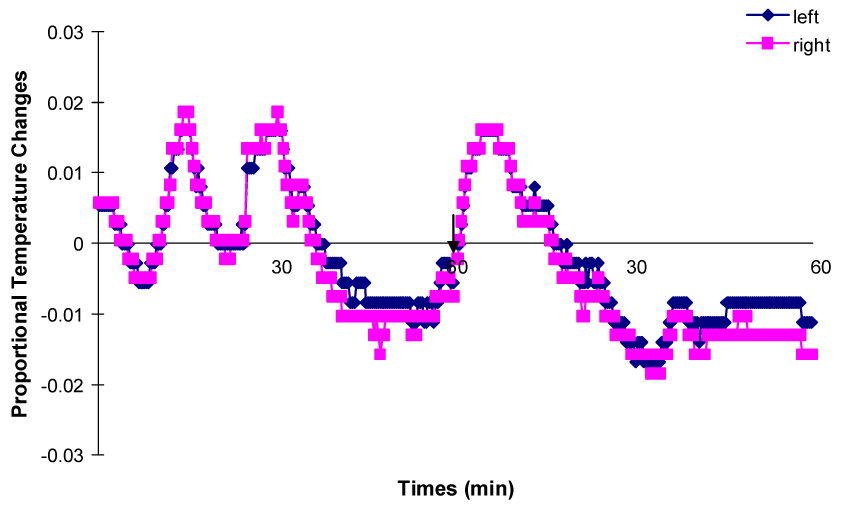
Proportional temperature changes at the left and right sides of another bilaterally implanted rat during one hour in the familiar chamber and after being transferred into the novel chamber. The arrow indicates the moment that the rat is transferred from the familiar chamber into the novel chamber.
Brain temperature was highly associated with rats’ movement. It slowly decreased in both hippocampi as rats reduced their activity in the recording chamber. When the rats were moving around, the same increases in temperature were recorded from both hippocampi. After the rats were transferred into the novel chambers, brain temperature for both hippocampi increased about 1–2 °C in 10min as a result of increased movements. Then it started decreasing as the rats habituated to the novel chamber.
No difference was found in temperature variations between the two hippocampi. For both rats, the changes in brain temperature at both hippocampi were extremely highly positively correlated (r356 = 0.99, p < 0.0001; r360 = 0.986, p < 0.0001).
4. Discussion
Exploratory activity, marked by locomotion, rearing, and head scanning, accounted for less than 50% of behavior during a 60 min baseline recording period in a familiar environment. During the first 15 min in a novel environment, rats exhibited extensive exploratory activities. Although all rats showed large increases in novelty-induced exploration, this experiment revealed an increase in PS amplitude and no change in EPSP slope during walking, posture changing, turning or raising the head, standing up or rearing, which are termed as Type 1 behavior. While no changes were found in PS amplitude and EPSP slope during immobility, face washing, licking, sniffing, or gnashing the teeth, which are termed as Type 2 behavior. These results differ with results from some previous studies [9, 33, 34]. In those studies, exploration was accompanied by a gradual increase in the EPSP and a decreased PS amplitude. The increase in EPSP reached a maximum within 10–15 min, after which it decayed slowly within the succeeding hour. These changes were interpreted as a result of spatial learning which occurs during the exploration of a novel environment. In subsequent work, this exploration-induced increase in EPSP slope and a decrease in PS amplitude were shown to be related to exploration-induced increases in brain temperature [18].
Moser and his colleagues provided evidence that changes in brain temperature induced by exploration are a very important factor, but not the only one, causing changes in hippocampal evoked potentials. They carried out a specific experiment to investigate changes in evoked potentials induced by exploration after brain temperature was controlled [19]. When rats explored novel objects or familiar objects in a new spatial arrangement, changes in entorhinal-dentate evoked potentials induced by exploration showed an increase in EPSP slope and a decrease in PS amplitude. But when brain temperature was manually increased to the same level as that increased by exploration, a smaller increase in EPSP slope and a bigger decrease in PS amplitude were found compared to those induced by exploration. Thus, they argue, if one adjusts for the temperature effect, then the change induced by exploration is an increase in both PS amplitude and EPSP slope. In the present experiment, brain temperature was measured not maintained about the same level. The present data are consistent with Moser’s data that brain temperature is highly positively associated with the rat’s motor activities. Furthermore, the present data showed that brain temperatures measured from both hippocampi change during behavior in a very similar way. According to Moser, an increase in EPSP slope and a decrease in PS amplitude would be expected if the burst of Type 1 activity increased brain temperature. Our data, though, showed the opposite effect, an increase in PS amplitude with no change in EPSP slope related to the change from the recording chamber to the novel environments. If brain temperature was the key to our results, we would expect an increase in brain temperature during intense exploration with a resulting decrease in PS amplitude and an increase in EPSP slope, in both Type 1 and Type 2 behavioral states. We consistently observed an increase in PS amplitude, lasting more than 5 minutes only during Type 1 behavior in the novel environment, with no change in EPSP. The fact is, that the changes we observed in PS amplitude are in the opposite direction from those predicted by an increase in temperature, but in the same direction as those observed by exploration minus temperature in the Moser et al’s study [19]. Furthermore, the changes are specific to evoked potentials recorded during Type 1 behavior; they are absent during all intervals of Type 2 behavior. This fact certainly does not fit with relatively slow brain temperature changes lasting minutes. Furthermore, there was no evidence in this experiment for an increase in EPSP slope. This is another phenomenon that is inconsistent with an effect of brain temperature increases.
Interestingly, in rats who had received prior LTP induction one hour before transfer to a novel environment, there was no rapid change in PS amplitude. This phenomenon is not due to an effect of prior LTP induction on behavioral states in the novel environment because no significant difference on the proportions of Type 1 and 2 behaviors between control groups and prior LTP group. The present data also showed that during Type 1 behavior, the greater was the LTP induction, the lower was PS amplitude in the novel environment. This supports the conclusion that there is an important interaction between processes underlying LTP induction and exploration-induced changes. The nature of the interaction implies that LTP and exploration induced enhancement may share common mechanisms.
In Xu’s study, it showed an immediate decrease in the population EPSP to prior LTP induction level in area CA1 when animals started exploration after LTP had been induced for an hour [44]. No evidence showed that erasure effect was found in this experiment. EPSP slope did not change significantly after rats were transferred to the novel environments. Even though PS amplitude during both type of behaviors decreased 5 min after exploration started, it did not return to baseline value. It disagrees with some studies which have examined the effect of exploration on electrically induced LTP in area CA1 of the hippocampus [7, 14, 44]. In these studies, they showed that the expression of prior electrically induced LTP was unaffected when rats explored a familiar environment, but LTP was reversed and returned towards baseline values when animals were transferred to a novel environment one hour after the induction. They inferred that exposure to novelty, and the attendent synaptic plasticity underlying acquisition of new information, had the effect of interfering with or erasing the synaptic enhancement produced by prior LTP induction. A possible explanation for the different outcomes in the present experiment may be that different hippocampal regions received LTP induction. Even though LTP in both CA1 area and DG is NMDA-dependent, these two regions have different roles in spatial memory. It has been reported that rat behavioral performance in a Y-shaped maze task showed a significant correlation with LTP in the DG, but not in CA1 area [21]. In the Morris water maze task, increased synaptic efficacy in CA1 area facilitates learning while increased synaptic efficacy in the DG impairs learning [22]. These studies clearly show that area CA1 and the DG have different roles in different spatial behavioral tasks. Thus, it is not surprising that the interaction between prior LTP induction and exploration induced enhancement are different.
A study with a fear conditioning task in the amygdala showed that prior experience (learned fear) actually occludes electrically induced LTP in the cortico-amygdala pathway. It indicates that this kind of learning-inducing enhancement shared some steps with electrically induced LTP [39]. Another study with contextual fear conditioning in the hippocampal CA1 area reported that after rats freely explored the experimental apparatus without any adverse stimulation, the increase in EPSP amplitude was decreased significantly compared to naïve rats [29]. These studies show prior experience occludes the changes induced by high frequency stimulations in the amygdala. The present data show that prior LTP induction in the hippocampus occludes the changes induced by exploration. It implies that LTP and the changes in evoked potentials induced by exploration share the same mechanism.
In Moser’s study [19], the effect of sensory stimulus-elicited arousal on entorhinal-dentate evoked potentials was also investigated by presenting an unexpected high frequency tone. The results showed that the tone caused an immediate increase of the EPSP slope and a parallel reduction of the PS amplitude. Four minutes after tone onset, this effect disappeared. In our study, novelty-induced changes in evoked potentials are different from this, but the time course is similar. It cannot be denied that when rats enter a novel environment they have an initial arousal or alerting response at the beginning. It is possible that the level of arousal in our study is different from Moser’s study. Thus, even though our present data are quite different from Moser’s data, a hypothesis that cannot be ruled out is that the evoked potential changes reported here reflect a kind of arousal-related modulation of dentate gyrus excitability.
In a previous study, Kitchigina and his colleagues [11] reported that when rats explored a novel object in an open field, noradrenergic activity arising from cells in the locus coeruleus was increased and resulted in a large and transient (about 75 s) potentiation of PS amplitude with no change in EPSP slope in the dentate gyrus. After a intraperitoneal injection of propranolol (noradrenergic receptor blocker), this phenomenon disappeared. They suggest that novelty-induced exploration can temporarily increase information transmission through the hippocampus via the activation of the noradrenergic neurons. In the present experiment, the increase in PS amplitude during Type 1 behavior lasts longer than 5 min. If a noradrenergic modulatory effect induced by novelty only lasts 75s, the remaining period (about 225s) of increase in PS amplitude could be due to different processes.
Another possible factor involved in the present novelty-induced effect is GABAergic interneurons. Moser [17] reported a decreased PS inhibition via GABAergic modulation during exploration of new objects. The present data cannot exclude the possibility that the increase in PS amplitude reflects a temporary reduction in GABA-related inhibition of dentate gyrus activation. A decrease in GABA inhibition would lead to a larger PS but not EPSP via reducing current shunting through the GABA-activated Cl− channel. In our study, the novelty effect which exploration of a novel environment caused an increase in PS amplitude over 5 min and no change in EPSP slope is not likely to be explained by a reduction of GABAergic inhibition because the data show no evidence for a reduction in paired-pulse inhibition induced by novelty.
In the present experiment, the increase in PS amplitude during Type 1 behavior lasts longer than 5 min with no increase in PS amplitude during Type 2 behavior. It is known that Type 1 behavior is associated with the rhythmical slow activity (RSA). RSA has been considered to be related to aspects of the learning process [5]. It has been suggested that natural patterns of bursts of presynaptic activity that are locked to a certain phase of the theta rhythm are especially able to generate long-lasting synaptic enhancement. A few studies have shown that behavioral performance is related to the hippocampal theta rhythm. In an eyeblink classical conditioning study [31], rabbits who showed a large portion of theta rhythm during the acquisition phase were found to learn this task at a faster rate than those had higher frequencies. When hippocampal theta rhythm is disrupted by lesion or drug application, acquisition of this task is delayed [30]. In this study, during the first 5 min in the novel chamber, each animal was engaged in Type 1 behavior. It is possible that information about the novel environments is acquired more efficiently during this burst of Type 1 behavior.
Acknowledgments
This work was supported by NIMH MH061460 and AHFMR to RJS
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aguirre GK, D'Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999;122(Pt 9):1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- 2.Bannerman DM, Lemaire M, Beggs S, Rawlins JN, Iversen SD. Cytotoxic lesions of the hippocampus increase social investigation but do not impair social-recognition memory. Exp Brain Res. 2001;138(1):100–109. doi: 10.1007/s002210100687. [DOI] [PubMed] [Google Scholar]
- 3.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 4.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 5.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 6.Buzsaki G, Grastyan E, Czopf J, Kellenyi L, Prohaska O. Changes in neuronal transmission in the rat hippocampus during behavior. Brain Res. 1981;225(2):235–247. doi: 10.1016/0006-8993(81)90833-7. [DOI] [PubMed] [Google Scholar]
- 7.Diamond DM, Fleshner M, Rose GM. Psychological stress repeatedly blocks hippocampal primed burst potentiation in behaving rats. Behav Brain Res. 1994;62(1):1–9. doi: 10.1016/0166-4328(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 8.Eichenbaum H. The hippocampus: The shock of the new. Curr Biol. 1999;9(13):R482–R484. doi: 10.1016/s0960-9822(99)80301-7. [DOI] [PubMed] [Google Scholar]
- 9.Green EJ, McNaughton BL, Barnes CA. Exploration-dependent modulation of evoked responses in fascia dentata: dissociation of motor, EEG, and sensory factors and evidence for a synaptic efficacy change. J Neurosci. 1990;10(5):1455–1471. doi: 10.1523/JNEUROSCI.10-05-01455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarrard LE. Selective hippocampal lesions and behavior: effects of kainic acid lesions on performance of place and cue tasks. Behav Neurosci. 1983;97(6):873–889. doi: 10.1037//0735-7044.97.6.873. [DOI] [PubMed] [Google Scholar]
- 11.Kitchigina V, Vankov A, Harley C, Sara SJ. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 1997;9(1):41–47. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 12.Leung LS. Behavior-dependent evoked potentials in the hippocampal CA1 region of the rat. I. Correlation with behavior and EEG. Brain Res. 1980;198(1):95–117. doi: 10.1016/0006-8993(80)90347-9. [DOI] [PubMed] [Google Scholar]
- 13.Maguire EA, Frackowiak RS, Frith CD. Learning to find your way: a role for the human hippocampal formation. Proc R Soc Lond B Biol Sci. 1996;263(1377):1745–1750. doi: 10.1098/rspb.1996.0255. [DOI] [PubMed] [Google Scholar]
- 14.Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci USA. 1999;96(15):8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 16.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 17.Moser EI. Altered inhibition of dentate granule cells during spatial learning in an exploration task. J Neurosci. 1996;16(3):1247–1259. doi: 10.1523/JNEUROSCI.16-03-01247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moser E, Moser MB, Andersen P. Synaptic potentiation in the rat dentate gyrus during exploratory learning. Neuroreport. 1993;5(3):317–320. doi: 10.1097/00001756-199312000-00035. [DOI] [PubMed] [Google Scholar]
- 19.Moser EI, Moser MB, Andersen P. Potentiation of dentate synapses initiated by exploratory learning in rats: dissociation from brain temperature, motor activity, and arousal. Learn Mem. 1994;1(1):55–73. [PubMed] [Google Scholar]
- 20.Myhrer T. Exploratory behavior and reaction to novelty in rats with hippocampal perforant path systems disrupted. Behav Neurosci. 1988;102(3):356–362. doi: 10.1037//0735-7044.102.3.356. [DOI] [PubMed] [Google Scholar]
- 21.Nakao KIY, Yamada MK, Nishiyama N, Matsuki N. Spatial performance correlates with long-term potentiation of the dentate gyrus but not of the CA1 region in rats with fimbria-fornix lesions. Neurosci Lett. 2001;307(3):159–162. doi: 10.1016/s0304-3940(01)01961-9. [DOI] [PubMed] [Google Scholar]
- 22.Okada T, Yamada N, Tsuzuki K, Horikawa HP, Tanaka K, Ozawa S. Long-term potentiation in the hippocampal CA1 area and dentate gyrus plays different roles in spatial learning. Eur J Neurosci. 2003;17(2):341–349. doi: 10.1046/j.1460-9568.2003.02458.x. [DOI] [PubMed] [Google Scholar]
- 23.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. p. 570. [Google Scholar]
- 24.Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17(6):669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- 25.Parkin AJ. Human memory: novelty, association and the brain. Curr Biol. 1997;7(12):R768–R769. doi: 10.1016/s0960-9822(06)00400-3. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Second Edition. New York: Academic Press; 1986. [Google Scholar]
- 27.Rawlins JN, Olton DS. The septo-hippocampal system and cognitive mapping. Behav Brain Res. 1982;5(4):331–358. doi: 10.1016/0166-4328(82)90039-0. [DOI] [PubMed] [Google Scholar]
- 28.Ruthrich H, Matthies H, Ott T. Long term changes in synaptic excitability of hippocampal cell populations as a result of training. In: M CA, Matthies H, editors. Neuronal plasticity and memory formation. New York: Raven; 1982. pp. 589–594. [Google Scholar]
- 29.Sacchetti B, Lorenzini CA, Baldi E, Bucherelli C, Roberto M, Tassoni G, Brunelli M. Time-dependent inhibition of hippocampal LTP in vitro following contextual fear conditioning in the rat. Eur J Neurosci. 2002;15(1):143–150. doi: 10.1046/j.0953-816x.2001.01844.x. [DOI] [PubMed] [Google Scholar]
- 30.Salvatierra AT, Berry SD. Scopolamine disruption of septo-hippocampal activity and classical conditioning. Behav Neurosci. 1989;103(4):715–721. doi: 10.1037//0735-7044.103.4.715. [DOI] [PubMed] [Google Scholar]
- 31.Seager MA, Johnson LD, Chabot ES, Asaka Y, Berry SD. Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. Proc Natl Acad Sci USA. 2002;99(3):1616–1620. doi: 10.1073/pnas.032662099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segal M. A correlation between hippocampal responses to interhemispheric stimulation, hippocampal slow rhythmic activity and behaviour. Electroencephalogr Clin Neurophysiol. 1978;45(3):409–411. doi: 10.1016/0013-4694(78)90192-x. [DOI] [PubMed] [Google Scholar]
- 33.Sharp PE, McNaughton BL, Barnes CA. Enhancement of hippocampal field potentials in rats exposed to a novel, complex environment. Brain Res. 1985;339(2):361–365. doi: 10.1016/0006-8993(85)90105-2. [DOI] [PubMed] [Google Scholar]
- 34.Sharp PE, McNaughton BL, Barnes CA. Exploration-dependent modulation of evoked responses in fascia dentate: fundamental observations and time course. Psychobiology. 1989;17(3):257–269. [Google Scholar]
- 35.Skelton RW, Scarth AS, Wilkie DM, Miller JJ, Phillips AG. Long-term increases in dentate granule cell responsivity accompany operant conditioning. J Neurosci. 1987;7(10):3081–3087. doi: 10.1523/JNEUROSCI.07-10-03081.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci Lett. 1982;31(3):271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- 37.Sutherland RJ, Whishaw IQ, Kolb B. A behavioural analysis of spatial localization following electrolytic kainate- or colchicine-induced damage to the hippocampal formation in the rat. Behav Brain Res. 1983;7(2):133–153. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- 38.Teyler TJ, Discenna P. Long-term potentiation. Annu Rev Neurosci. 1987;10:131–161. doi: 10.1146/annurev.ne.10.030187.001023. [DOI] [PubMed] [Google Scholar]
- 39.Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34(2):289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- 40.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26(4):407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 41.Vanderwolf CH, Cain DP. The behavioral neurobiology of learning and memory: a conceptual reorientation. Brain Res Brain Res Rev. 1994;19(3):264–297. doi: 10.1016/0165-0173(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 42.Weisz DJ. New York: Plenum; 1982. Activity of the dentate gyrus during nictitating membrane conditioning in the rabbit. Conditioning: Representations of involved neural functions, advances in behavioral biology; pp. 131–146. W. C.D. [Google Scholar]
- 43.Winson J, Abzug C. Neuronal transmission through hippocampal pathways dependent on behavior. J Neurophysiol. 1978;41(3):716–732. doi: 10.1152/jn.1978.41.3.716. [DOI] [PubMed] [Google Scholar]
- 44.Xu L, Anwyl R, Rowan MJ. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature. 1998;394(6696):891–894. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]


