Abstract
CR3-mediated endocytosis is a primary mechanism by which Neisseria gonorrhoeae elicits membrane ruffling and cellular invasion of the cervical epithelia. Our data indicate that, upon infection of cervical epithelia, N. gonorrhoeae specifically releases proteins, including a phospholipase D (PLD) homolog, which facilitate membrane ruffling. To elucidate the function of gonococcal PLD in infection of the cervical epithelia, we constructed an N. gonorrhoeae PLD mutant. By comparative association and/or invasion assays, we demonstrated that PLD mutant gonococci are impaired in their ability to adhere to and to invade primary cervical cells. This defect can be rescued by the addition of supernatants obtained from wild-type-infected cell monolayers but not by exogenously added Streptomyces PLD. The decreased level of total cell association (i.e., adherence and invasion) observed for mutant gonococci is, in part, attributed to the inability of these bacteria to recruit CR3 to the cervical cell surface with extended infection. Using electron microscopy, we demonstrate that gonococcal PLD may be necessary to potentiate membrane ruffling and clustering of gonococci on the cervical cell surface. These data may be indicative of the inability of PLD mutant gonococci to recruit CR3 to the cervical cell surface. Alternatively, in the absence of gonococcal PLD, signal transduction events required for CR3 clustering may not be activated. Collectively, our data indicate that PLD augments CR3-mediated gonococcus invasion of and survival within cervical epithelia.
Neisseria gonorrhoeae is a strict human pathogen, which causes the sexually transmitted disease gonorrhea. This organism possesses multiple mechanisms by which it is able to colonize its sole human host and which are dependent upon the particular microenvironment of the infection site. In this respect, the gonococcus senses its particular microenvironment and adjusts its mode of pathogenicity accordingly. Several gonococcal constituents are implicated to play a role in its pathogenicity, including lipooligosaccharide (LOS), porin, pilus, and the opacity-associated outer membrane proteins. Invasion of male urethral epithelial cells is mediated by LOS, the terminal galactose of which serves as a ligand for the asialoglycoprotein receptor (ASGP-R) (15). An intimate association between the gonococcal and host cell membranes precedes clathrin-dependent endocytosis (15). In contrast, complement (C′) receptor type 3 (CR3)-mediated endocytosis is a primary mechanism by which N. gonorrhoeae invades primary human cervical epithelial cells (8). This process is dependent upon the cooperative binding of (gonococcus-bound, host-derived) iC3b, gonococcal porin, and gonococcal pilus to the I domain of CR3 (7, 9). Engagement of CR3 results in membrane ruffling (8) and internalization of gonococci in macropinosomes (10).
Our studies show that ruffling induced by gonococci during cervical cell infection is delayed from the onset of infection by 60 to 90 min (10). The onset of ruffling can be accelerated to 30 min by the addition of filtered, preconditioned (i.e., derived from a previous infection) medium. This prompted a search for factors which might be responsible for expediting the cytoskeletal changes induced by gonococcal infection. Here we describe the identification of gonococcal phospholipase D (PLD), which is specifically released upon infection of cervical epithelia. We further demonstrate a role for gonococcal PLD in membrane ruffling, CR3 recruitment to the cervical cell surface, and, consequently, in gonococcal invasion of the cervical epithelia. We propose that this secreted protein is a novel neisserial virulence factor, capable of modulating effector functions within host cells.
MATERIALS AND METHODS
Cell culture.
Surgical biopsies derived from the ecto- and the endocervix that were used to seed primary cervical epithelial cell systems were procured and maintained as described previously (10) in defined keratinocyte serum-free medium (Life Technologies, Rockville, Md.). The primary (16) and immortal (17) male urethral epithelial cells used in these studies have been described and were maintained according to the methods of Harvey et al. (15, 16). Pharmacological agents used, as described in the studies outlined below, were not cytotoxic at the indicated concentrations, as determined by trypan blue exclusion.
Bacteria and infection studies.
N. gonorrhoeae strains 1291 (1, 6), FA1090 (4), and MS11 (28, 29) were used in the infection studies outlined below, which were performed as previously described (10). Briefly, overnight cultures of gonococci were harvested from GC-IsoVitaleX agar plates and suspended in sterile physiological saline. The optical density of the bacterial suspension was determined spectrophotometrically, where an optical density of 1 at 600 nm was equivalent to 109 bacteria ml−1, and 107 gonococci were used to infect cervical cell monolayers at a multiplicity of infection of 100. Primary cervical cells were challenged with gonococci for variable time periods (as noted), after which the infection medium was removed, and the cell monolayers were extensively washed with phosphate-buffered saline (PBS). Uninfected control cell monolayers were simultaneously processed with challenged cell monolayers. Infected and uninfected (control) cell monolayers were subsequently harvested for cellular fractionation, quantitative association (i.e., adherence and invasion), or invasion assays, or they were processed for microscopic analyses. Alternatively (as noted), infection supernatants were harvested and immediately transferred to ice, and gonococci were removed by filtration through a 0.22-μm low-protein-binding syringe filter. For PLD activity assays, supernatants depleted of gonococci were filtered with Centricon YM-30 centrifugal filter units (Millipore Corporation, Bedford, Mass.). Protein products were then collected with an equal volume of PLD assay buffer.
N. gonorrhoeae strain 1291ΔPLD was constructed by the insertion of a kanamycin resistance cassette with the EZ::TN <KAN-2> insertion kit (Epicentre, Madison, Wis.). PCR of full-length gonococcal PLD, with the primer pair of 5′-GGT GGT CAT ATG ATG CAT ACA GAC CCC AAA AT-3′ and 5′-GGT GGT TGCTCT TCC GCA TAA TAA ACC TTC TTC GAT GGG CAG-3′ , suggested the insertion of the kanamycin resistance cassette within the pld gene, which was then confirmed by sequence analysis performed at the University of Iowa DNA Sequencing Facility (Iowa City, Iowa).
Radiolabeling and collection of gonococcal products released with infection of primary cervical cells.
Gonococci allowed to grow overnight on GC agar were harvested with a sterile swab and used to inoculate 5-ml cultures of Morse's defined medium (MDM) (26). MDM was prepared such that half the recommended methionine and cysteine was replaced with 125 μCi of Redivue Pro-mix L-[35S] in vitro cell labeling mix (Amersham Pharmacia Biotech Inc., Piscataway, N.J.). After approximately 4 h, gonococci were collected by centrifugation (4,000 rpm, 5 min), rinsed with sterile physiological saline to remove excess label, and resuspended in cold MDM such that a culture density of 107 bacteria ml−1 was obtained. MDM containing the 35S-labeled gonococci was then used to challenge approximately 105 primary human ecto- (PEX) and endocervical (PEN) cells, or it was incubated in 35-mm tissue culture dishes devoid of cervical cells. Alternatively, gonococci were labeled during the course of infection by a 30-min pulse with 35S-labeled MDM at 1 h and 2.5 h postinfection. Before infection, PEX and PEN cells were treated (30 min, 37°C) with 250 μM cycloheximide to inhibit cervical cell protein synthesis. Cycloheximide was maintained in the culture medium throughout the course of the infection.
Cervical cells and tissue culture plates lacking cervical cells were challenged with gonococci for 90 min and 3 h, after which the culture supernatants were collected. Gonococci were removed from the culture supernatants by filtration through low-protein-binding 0.22-μm syringe filter units. Supernatant filtrates were concentrated with Centricon YM-3 centrifugal filter units (Millipore) before suspension in 0.1 M Tris-0.1% sodium dodecyl sulfate. Concentrated supernatants were separated on a sodium dodecyl sulfate-4% to 12% polyacrylamide gel before autoradiography or gel extraction for mass spectrometry at the Mass Spectrometry Facility located at the University of California (San Francisco).
Analysis of mass data was performed with the Protein Prospector (University of California, San Francisco) and the ProFound (Rockefeller University, New York, N.Y.) mass analysis databases. In separate assays, cycloheximide-treated PEX cells were incubated (30 min, 4°C) with 100 ng of recombinant I domain (generously provided by E. Brown, University of California, San Francisco), 20 μg of anti-CD46 (E4.3; Santa Cruz Biotechnology, Santa Cruz, Calif.), 20 μg of anti-CD66 (N-19, Santa Cruz), 20 μg of anti-CD11b (H5A4; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City), 10 μg of lipid A (isolated as described previously [7]), 1 μg of gonococcal porin (PI.B), or 1 μg of gonococcal pilin per ml. Gonococcal porin and pilin were a gift from M. Blake. Radiolabeled gonococci were then added to PEX cells and infection was allowed to proceed for 3 h, after which culture supernatants were collected and secreted gonococcal products were harvested and detected as outlined above.
Western blot analysis.
Infection supernatants depleted of gonococci (as described above) were separated on 4% to 12% denaturing polyacrylamide gradient gels and transferred to Immobilon-P membranes (Millipore). Membranes were incubated (2 h, 37°C) with 50 μU of neuraminidase per ml (Roche Diagnostics, Indianapolis, Ind.) prior to immunodetection. Western blotting was subsequently performed according to standard protocols with the anti-LOS monoclonal antibody 6B4. This antibody recognizes the conserved Gal(β1-4)GlcNAc epitope of gonococcal LOS. Chemiluminescent detection was used to visualize labeled LOS.
Determination of PLD activity.
PLD activity was accessed with the Amplex Red phospholipase D assay kit (Molecular Probes, Eugene, Oreg.). Wild-type and PLD mutant gonococci were suspended in PLD assay buffer to a final concentration of 107 bacteria ml−1, and activity was determined according to the manufacturer's protocol. Assessment of gonococcal PLD activity at acidic pH was determined in a two-step assay according to the manufacturer's protocol. For the first step, 108 gonococci were suspended in PBS with the pH adjusted to 3.0, 4.5, or 6.0. Gonococcal suspensions were diluted 10-fold in PLD assay buffer for the second step of the reaction. Cervical cell fractions were prepared as outlined below, and PLD activity was assessed at neutral pH according to the manufacturer's protocol.
Fractionation of primary cervical cells.
Uninfected (control) and infected cervical cell monolayers were lysed in buffer A (50 mM Tris, pH 7.5; 10 mM NaCl; 1 mM KCl; 2 mM MgCl2; 1 mM phenylmethylsulfonyl fluoride) by scraping cervical cells from tissue culture dishes placed on ice. The cell lysate was sonicated (two bursts of 20 s each). Cells that did not lyse and the nuclear fraction were removed by centrifugation (750 × g, 10 min), and the supernatant from this spin was then subjected to filtration through a low-protein-binding 0.22-μm syringe filter to ensure removal of gonococci. Sucrose was added to the resulting gonococcus-depleted supernatant (S1) to a final concentration of 300 mM. Ultracentrifugation (150,000 × g, 90 min) was then performed to produce the plasma membrane- (pellet) and cytosol (supernatant 2, S2)-enriched fractions. The membrane-enriched fraction was resuspended in PLD assay buffer (50 mM Tris, 5 mM CaCl2, pH 7.4). S2 was concentrated by filtration through Centricon YM-30 centrifugal filter units (Millipore), after which cytosolic constituents were recovered in PLD assay buffer. Where indicated, before infection studies, primary PEX and PEN cell monolayers were treated with 300 nM wortmannin (Sigma, St. Louis, Mo.) (2 h, 37°C) or 1 μM cytochalasin D (Sigma) (30 min, 37°C) to inhibit macropinocytosis of gonococci. Wortmannin and cytochalasin D were maintained in cervical cell cultures during the course of infection.
RNA isolation and RT-PCR of gonococci and primary human cervical epithelial cells.
Primary PEX and PEN cell monolayers were challenged with N. gonorrhoeae 1291 or 1291ΔPLD, or they were left uninfected. After 3 h, infection supernatants were removed and cell monolayers were extensively rinsed with PBS. Total RNA (intracellular gonococcal and cervical cell RNA) was isolated with the RNAqueous-4PCR kit (Ambion, Inc., Austin, Tex.) according to the manufacturer's protocol. rRNA was removed from the total RNA with the MicrobExpress kit (Ambion) according to the manufacturer's protocol, yielding message-enriched bacterial and cervical cell RNA. Cervical cell RNA was then separated from intracellular bacterial RNA with the Poly(A)Purist kit (Ambion) according to the manufacturer's protocol with slight modification. Supernatants from the capture and wash steps, which contained gonococcal RNA, were saved and pooled. Gonococcal RNA was recovered by ethanol precipitation.
cDNA was synthesized with the Retroscript First Strand synthesis kit for reverse transcription (RT)-PCR (Ambion); reactions lacking the reverse transcriptase (negative control) were run simultaneously with reactions containing RT. PCR analysis of reverse-transcribed and of mock reactions with primers to β-actin and to gonococcal reduction-modifiable protein (Rmp) demonstrated the absence of contaminating DNA and of gonococcal DNA and RNA in the isolated cervical cell RNA. PCR of reverse-transcribed cervical cell RNA was performed with the primer pairs 5′-TCC ATG CAA GAA TCT GGT TTC-3′ and 5′-CGA CAA TGA GCA CAG ACT CAC A-3′ for human PLD1 to yield a 462-bp product and 5′-CCT TCA GGA TTC TGT CCA CAA-3′ and 5′-CCT CTC TCA CAA CCA ATT CTT C-3′ for human PLD2 to yield a 508-bp product.
Isolation of gonococcal RNA and RT-PCR analysis of gonococcal PLD message levels were performed as outlined above with the RNAqueous-4PCR and MICROBExpress kits (Ambion). Strain 1291 wild-type gonococci were suspended in tissue culture medium to a final concentration of 107 bacteria ml−1. The cell suspension was used to challenge approximately 105 cervical cells or was incubated in the absence of cervical cells. After 90 min and 3 h the infection supernatants were harvested, and bacteria were collected by centrifugation. RT-PCR analysis was performed with primer pairs for gonococcal PLD (5′-CGA AAC CGT CGA ACA GTC GCC-3′ and 5′-GTG CGC CTC CAT CTG TTC TGC-3′) or Rmp (5′-GGG AAT AAA ATG ACC AAA CAG-3′ and 5′-AAC CGA AAA GGG ATG ATG ATA-3′) to yield 569- and 1,239-bp products, respectively. Rmp was selected as an internal control for RT-PCR analysis based on gene chip analysis (our unpublished data), indicating that message levels of this protein do not change upon cervical cell infection.
Determination of CR3 surface expression on primary cervical cells.
PEX and PEN cells were passed to 96-well microtiter plates and allowed to grow to confluence. Cervical cells were then challenged with wild-type or PLD mutant gonococci, after which the infection medium was removed and cells were rinsed thrice with PBS. Cells were fixed with 2% paraformaldehyde. Prior to immunoanalysis of CR3 surface level expression, cells were again rinsed with PBS. Immunoassays were then performed according to standard enzyme-linked immunosorbent assay protocols with the H5A4 anti-CD11b (i.e., CR3) primary and peroxidase-conjugated secondary antibodies. Absorbance of the o-phenylenediamine dihydrochloride peroxidase substrate was determined spectrophotometrically at 490 nm. Primary antibody was omitted from one well, and the secondary antibody was omitted from a second well, which served as controls for nonspecific binding and endogenous peroxidase activity, respectively. Where indicated, gonococcus-depleted supernatants collected from wild-type or PLD mutant infection studies were included in the infection studies, performed as outlined below.
N. gonorrhoeae attachment to and invasion of primary human cervical cells.
Primary cervical cell monolayers were infected with wild-type or PLD mutant gonococci as outlined above. Variable concentrations of phosphatidylcholine (Sigma), ethanol, 2-butanol, or PLD from Streptomyces spp. (SsPLD; Sigma) were included in association (adherence and invasion) or invasion assays, as noted. Phosphatidylcholine, ethanol, 2-butanol, or 10 U of SsPLD ml−1 was added simultaneously with gonococci.
In separate assays, infection supernatants were collected from wild-type- or PLD mutant-infected cervical cell monolayers, gonococci were removed by filtration through a 0.22-μm syringe filter (to yield primed wild-type or mutant supernatants), which were then added to association and/or invasion assays, as noted. The ability of gonococci to adhere to and/or invade PEX and PEN cells was quantitatively determined with standard gentamicin resistance assays, performed as described previously (10) and in which chemical or protein additives were included in or excluded from the invasion assay as described above. The total association (i.e., adherence and invasion) of gonococci with PEX and PEN cells was quantitated by the omission of gentamicin from the above-described invasion assay.
Percent invasion of N. gonorrhoeae 1291 or 1291ΔPLD in the presence or absence of experimental additives was determined as a function of the original inoculum and the number of colonies formed with subsequent plating of the cellular lysate. Inhibition of gonococcal attachment and/or invasion by exogenous phosphatidylcholine, ethanol, or 2-butanol was determined as a normalized function of the ability of gonococci to attach to and/or invade primary cervical cells in the absence of the competimer inhibitor. A Kruskal-Wallis nonparametric analysis of variance was used to determine the statistical significance of the association and invasion assays described above.
Immunolabeling and microscopy.
Immunolabeling of N. gonorrhoeae 1291- or 1291ΔPLD-infected PEX cell monolayers was performed as described previously (10). Primary antibodies used for immunolabeling were specific for the CR3 β-subunit, CD18 (anti-CD18 CTB104 [Santa Cruz]), or for the gonococcal H.8 outer membrane protein (antibody 2C3). Fluorescein isothiocyanate- and tetramethylrhodamine isothiocyanate-conjugated secondary antibodies were applied to cell monolayers, as noted. Infected and uninfected (control) cell monolayers were viewed with the Bio-Rad MRC-1024 laser scanning confocal viewing system. Primary cervical cell monolayers were processed for scanning electron microscopy (10) and viewed with the Hitachi S-4000 scanning electron microscope. All the microscopes used in these studies are located at the Central Microscopy Research Facility at the University of Iowa (Iowa City).
RESULTS
N. gonorrhoeae specifically releases protein products upon infection of cervical epithelia.
Our previous studies show that membrane ruffling of N. gonorrhoeae-infected primary human cervical cells occurs by approximately 90 min postinfection (10). The same studies also demonstrated that the onset of membrane ruffling in response to gonococcal infection can be expedited by the use of a primed infection inoculum, that is, an inoculum derived from a previous infection. Based on these studies, we reasoned that gonococcal products were being released upon infection that facilitated membrane ruffling.
Autoradiography of infection supernatants demonstrated that gonococcal products were, in fact, being released with infection of primary human ecto- and endocervical epithelium. Release of these gonococcal products was not strain specific in that an identical protein pattern was observed with autoradiography of infection supernatants obtained from N. gonorrhoeae strain 1291- (Fig. 1), FA1090- (data not shown), or MS11- (data not shown) infected primary cervical cells. In contrast, analysis of supernatants derived from an identical time course of infection of urethral epithelial cells revealed that only a small amount of gonococcal products were released by 90 min postinfection, and by 3 h of infection no products could be detected (Fig. 1).
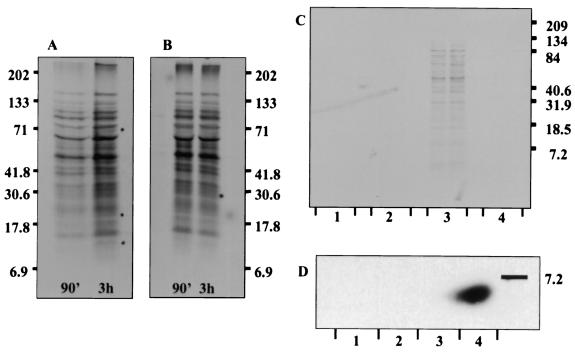
FIG. 1.
Analysis of infection supernatants demonstrates that gonococcal products are released upon infection of cervical epithelia. Similar results are observed upon analysis of supernatants obtained from 90-min and 3-h infections, from PEX (A) and PEN (B) cells, and from the same cells obtained from different tissue donors. An identical protein pattern is observed with N. gonorrhoeae strains 1291 (shown), FA1090, or MS11, indicating that protein release is not strain dependent. To determine if gonococcal proteins released upon cervical infection were specific to cervical cell invasion, we repeated these studies with primary male urethral epithelial cells. Autoradiography revealed that while a minimal amount of protein products are released by 90 min postinfection, these proteins are not present by 3 h of infection of urethral epithelial cells (C). Collectively, these data suggest that the continued release of gonococcal products was specific to gonococcal infection of cervical epithelial cells. (D) Western blot analysis failed to reveal the presence of gonococcal LOS in culture supernatants, indicating that the protein products identified were not present as the result of bacterial lysis or of membrane blebbing. Lanes: C1, gonococci incubated in tissue culture dishes devoid of cervical cells; C2, uninfected cervical cells; C3, 90-min infection of urethral epithelial cells; C4, 3-h infection of urethral epithelial cells; D1, 3-h infection of PEN cells; D2, 90-min infection of PEN; D3, uninfected PEN cells; and D4, N. gonorrhoeae LOS.
Western blot analysis of infection supernatants with the anti-LOS 6B4 antibody probe did not reveal the presence of gonococcal LOS. This indicated that the release of gonococcal products with cervical cell infection was the result of bacterial secretion and not of bacterial lysis or of membrane blebbing (Fig. 1). Release of gonococcal proteins was inhibited by the addition of the anti-CD11b monoclonal antibody H5A4 or recombinant I domain and by purified gonococcal porin, pilin, and lipid A to PEX cell infection studies (Fig. 2). Antibodies to CD66 and CD46 did not inhibit protein release (Fig. 2). Our previous data indicate that at 90 min of infection de novo protein synthesis is not required for invasion of primary cervical cells. RT-PCR analysis of PLD message levels was consistent with these data in that message levels were not increased at 90 min postinfection, but message levels did appear to increase by 3 h postinfection (Fig. 3). These data may indicate that a basal level of PLD is found constitutively within gonococci, but also that upon cervical cell infection PLD transcription is increased.
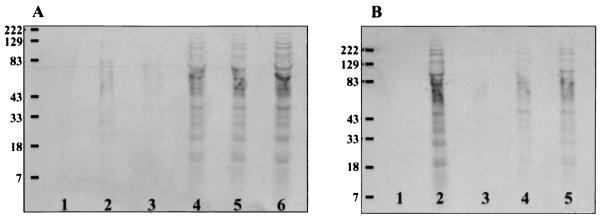
FIG. 2.
35S-labeled strain 1291 gonococci were used to challenge PEX cells in the presence or absence of competimers of CR3 I domain adherence (A) or gonococcal constituents involved in CR3-mediated adherence to cervical epithelia (B), as described in the text. Lanes A1 and B1, Gonococci incubated in the absence of cervical cells. Lanes A6 and B2, no competimer addition. Panel A lanes: 2, anti-CR3 antibody; 3, recombinant I domain; 4, anti-CD66 antibody; or 5, anti-CD46 antibody competimers to infection studies. Panel B lanes: 3, unlabeled gonococcal LOS; 4, porin; 5, pilin. Protein secretion is reduced in the presence of anti-CR3 antibody and recombinant I domain and gonococcal pilus, porin, and lipid A. Collectively these data suggest that the release of gonococcal products occurs in a contact-dependent manner in which engagement of the CR3 I domain by gonococcal pilus, porin, and internal C3b-opsonized lipid A triggers secretion.
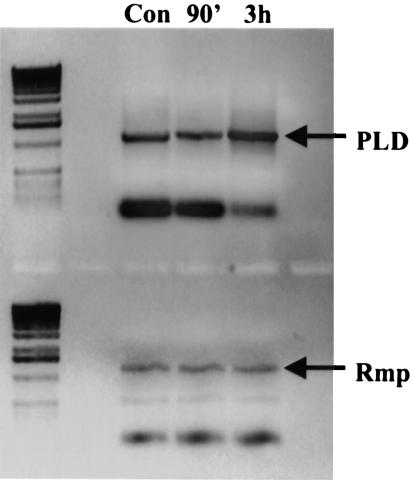
FIG. 3.
RT-PCR analysis of PLD transcription levels in gonococci was performed as described in the text. RNA was isolated from gonococci collected from 90-min and 3-h infections of PEX cells. Alternatively, RNA was harvested from gonococci in parallel (mock) experiments performed in the absence of PEX cells (control). These data demonstrate that transcription levels of PLD are not increased by 90 min but are increased by 3 h of infection.
Characterization of an N. gonorrhoeae PLD homolog.
With mass spectroscopy, we identified a subset of gonococcal products that are secreted upon cervical cell infection. One secreted product, p55, was identified by mass analysis with the Protein Prospector and Profound databases as sharing significant homology to a Neisseria meningitidis hypothetical PLD homolog. Primers for PCR were designed based on the N. meningitidis serogroup B sequence and used to amplify pld from N. gonorrhoeae strains 1291, FA1090, and MS11. Sequences obtained from cloning of the PCR amplicons were then used in a Blast search of the N. gonorrhoeae genome database to identify p55 as a N. gonorrhoeae PLD homolog (GenBank accession number AY307929). We also used p55 sequence to perform a Blast search of the National Center for Biotechnology Information (NCBI) database, which revealed significant sequence homology of p55 to Neisseria meningitidis serogroups A and B hypothetical PLD homologs, hypothetical and/or putative synthases of Escherichia coli and Shigella flexneri, and a putative phospholipase of Salmonella.
Further sequence analysis of p55 revealed that this protein contains two HKD motifs (amino acids 184 to 201 and 422 to 439), which are required for PLD activity. p55 also possesses an N-terminal signal sequence (amino acids 1 to 38) that harbors a short region (amino acids 24 to 34) of hydrophobicity, consistent with a sec-dependent mechanism of secretion. Two additional regions of hydrophobicity (amino acids 217 to 231 and 512 to 523) might potentially serve as lipid association domains. Comparative assessment of PLD activity in N. gonorrhoeae strains 1291 and 1291ΔPLD (performed at pH 7.4) demonstrated PLD activity in wild-type but not mutant gonococci (Table 1), indicating that p55 functions as a phospholipase D. In separate assays, gonococcal PLD exhibited characteristic PLD activity at pH 3.0, 4.5, and 6.0 (Table 1), consistent with the ability of this enzyme to function within the lower female genital tract under normal conditions or during bacterial vaginosis and/or cervicitis.
TABLE 1.
PLD activity in N. gonorrhoeae cell lysatesa
| Strain | PLD activity, mean fluorescence units (variance)
|
|||
|---|---|---|---|---|
| pH 7.4 | pH 6.0 | pH 4.5 | pH 3.0 | |
| 1291 | 1.00 (0.12) | 1.49 (0.01) | 1.52 (0.06) | 1.54 (0.04) |
| 1291ΔPLD | 0.17 (0.03) | 0.10 (0.01) | 0.10 (0.01) | 0.11 (0.01) |
| Positive control | 0.93 (0.12) | 1.53 (0.03) | 1.47 (0.02) | 1.47 (0.01) |
| Negative control | 0.20 (0.01) | 0.10 (0.01) | 0.09 (0.01) | 0.11 (0.01) |
PLD activity was determined as described in the text. Values given are the mean values of three trials.
N. gonorrhoeae PLD augments gonococcal infection of cervical epithelial cells.
To determine if gonococcal PLD plays a role in infection of cervical epithelia, quantitative association and/or invasion assays were performed with N. gonorrhoeae strains 1291 and 1291ΔPLD. Association and invasion assays demonstrated a role for gonococcal PLD in gonococcal cervicitis, as indicated by the decreased levels of association and of invasion observed with infection of primary cervical cells with the PLD mutant upon comparison to the wild-type bacteria (Table 2). The addition of SsPLD to association and/or invasion assays performed with mutant gonococci could not rescue the decreased levels of association and of invasion observed in the absence of gonococcal PLD.
TABLE 2.
Adherence to and invasion of primary cervical cells by gonococci in the presence and absence of primed medium or exogenous PLDa
| Strain | Ectocervical cells
|
Endocervical cells
|
||
|---|---|---|---|---|
| % Association | % Invasion | % Association | % Invasion | |
| 1291 | 28.66 ± 1.35 | 2.91 ± 0.22 | 16.79 ± 0.54 | 1.59 ± 0.02 |
| 1291ΔPLD | 15.93 ± 0.80 (<0.05) | 0.35 ± 0.04 (<0.05) | 6.98 ± 0.83 (<0.05) | 0.24 ± 0.02 (<0.05) |
| 1291ΔPLD + wt sup | 23.99 ± 3.06 (<0.05) | 2.10 ± 0.15 (<0.05) | 11.90 ± 1.28 (<0.05) | 1.34 ± 0.21 (<0.05) |
| 1291 | 27.76 ± 0.14 | 2.65 ± 0.04 | 15.55 ± 0.80 | 1.64 ± 0.12 |
| 1291ΔPLD | 15.39 ± 0.99 (<0.05) | 0.32 ± 0.02 (<0.05) | 6.85 ± 0.67 (<0.05) | 0.24 ± 0.01 (<0.05) |
| 1291ΔPLD + pld sup | 15.62 ± 0.87 (<0.05) | 0.31 ± 0.03 (<0.05) | 6.36 ± 0.57 (<0.05) | 0.24 ± 0.02 (<0.05) |
| 1291 | 30.64 ± 1.69 | 2.91 ± 0.39 | 17.08 ± 1.43 | 1.64 ± 0.11 |
| 1291ΔPLD | 13.63 ± 0.63 (<0.05) | 0.37 ± 0.03 (<0.05) | 7.28 ± 0.19 (<0.05) | 0.20 ± 0.03 (<0.05) |
| 1291ΔPLD + SsPLD | 14.70 ± 2.16 (<0.05) | 0.39 ± 0.05 (<0.05) | 6.81 ± 0.41 (<0.05) | 0.17 ± 0.01 (<0.05) |
Values given are the mean values in which total association (adherence and/or invasion) and invasion were determined as a function of the original inoculum and the subsequent number of colony-forming units formed with subsequent plating of the ecto- or endocervical cell lysates. Data are the mean values obtained from at least three trials performed in triplicate. P values (noted parenthetically) were determined with a Kruskal-Wallis k-sample analysis of variance calculated for association and/or invasion of wild-type (wt) or mutant gonococci in the presence of wild-type (wt)- or PLD mutant (pld)-primed supernatant (sup) or 10 U of SsPLD in comparison to the absence of primed medium or exogenous SsPLD, as outlined in the text.
Although efforts to isolate gonococcal PLD have, to date, been unsuccessful, the addition of primed wild-type supernatants to infection assays performed with mutant gonococci restored association and invasion to nearly wild-type levels. However, the addition of primed mutant supernatants had no effect on the ability of PLD mutant gonococci to adhere to or to invade primary cervical cells (Table 2). Similar studies in which immortal urethral epithelial cells were challenged with N. gonorrhoeae strains 1291 or 1291ΔPLD revealed that gonococcal PLD does not play a role in the association of gonococci with the urethral epithelium but may promote the intracellular survival of these organisms. The association of 1291ΔPLD with immortal urethral epithelial cells (25.11% ± 1.01%) was comparable to that of wild-type gonococci (25.16% ± 1.69%, P < 0.75), whereas invasion levels were decreased in the absence of gonococcal PLD (for strain 1291, 2.43 ± 0.05%; for strain 1291ΔPLD, 1.16% ± 0.11%, P < 0.05).
N. gonorrhoeae PLD plays a role in CR3 recruitment to the cervical cell surface.
CR3 serves as the primary receptor for gonococcal adherence to and invasion of the cervical epithelium (8). Our previous studies also indicate that surface levels of CR3 increase with gonococcal infection (8). We performed laser scanning confocal microscopy to examine the gonococcus-CR3 association in mutant gonococci to determine if the decrease in gonococcal cervical cell association observed with use of the PLD mutant was because of the inability of mutant gonococci to recruit CR3 to the cervical cell surface.
Laser scanning confocal microscopy revealed that in comparison to wild-type-infected PEX cells, which exhibited abundant CR3 on the monolayer surface, PEX cells infected with PLD mutant gonococci exhibited decreased fluorescence, indicative of a decreased level of CR3 on their cell surface (data not shown). To quantitate these findings, we developed an enzyme-linked immunosorbent assay to measure cervical cell surface expression of CR3 in uninfected PEX and PEN cells and cells challenged with N. gonorrhoeae strains 1291 and 1291ΔPLD (Table 3). Immunoanalysis of the presence of CR3 on the surface of PEX and PEN cells confirmed our microscopy data. The amount of CR3 present on the surface of wild-type-infected cervical cells was significantly greater than the levels of CR3 measured on either the PLD mutant-infected or uninfected cervical cells. The addition of primed wild-type supernatants to PLD mutant-infected and uninfected cells increased CR3 recruitment to the cervical cell surface. However, the addition of primed supernatants from the PLD mutant had no effect on CR3 recruitment to the cervical cell surface of PLD mutant-infected or uninfected cells.
TABLE 3.
Semiquantitative immunoanalysis of CR3 expression on the surface of primary cervical cellsa
| Sample | Absorbance at 490 nm
|
|
|---|---|---|
| Ectocervical cells | Endocervical cells | |
| Uninfected control | 0.53 ± 0.13 | 0.30 ± 0.03 |
| Strain 1291 | 2.37 ± 0.18 | 1.65 ± 0.17 |
| Strain 1291ΔPLD | 0.68 ± 0.12 | 0.34 ± 0.11 |
| Uninfected control + primed wt sup | 1.08 ± 0.36 | 0.73 ± 0.06 |
| Uninfected control + primed pld sup | 0.44 ± 0.14 | 0.38 ± 0.05 |
| 1291ΔPLD + primed wt sup | 1.36 ± 0.43 | 1.13 ± 0.28 |
| 1291ΔPLD + primed pld sup | 0.39 ± 0.13 | 0.31 ± 0.08 |
| No primary antibody | 0.06 ± 0.01 | 0.08 ± 0.01 |
Values given are the mean values in which the presence of CR3 on the cervical cell surface was measured by an immunoassay with monoclonal antibody H5A4 as outlined in the text. See Table 2, footnote a, for abbreviations.
N. gonorrhoeae PLD plays a role in membrane ruffling of the cervical epithelium.
PLD activation in mammalian cells is thought to occur early in the phagocytic process, before the onset of actin reorganization. To determine if gonococcal PLD plays a role in the cytoskeletal rearrangements leading to membrane ruffling of the cervical epithelium, we performed scanning electron microscopy. Scanning electron microscopy analysis demonstrated that aberrant cytoskeletal rearrangements occur upon infection of cervical epithelia with PLD mutant gonococci, compared to infection with wild-type gonococci. At 15 min postinfection no significant difference was observed between PLD mutant- and wild-type-infected PEX cells (data not shown). Small bacterial clusters were evident, as were microvilli and filopodia. However, by 3 h postinfection bacterial clusters and membrane ruffles were not readily evident on cell monolayers infected with mutant gonococci, but were characteristically prevalent on wild-type infected cell monolayers (Fig. 4). The addition of primed wild-type supernatants but not primed PLD mutant supernatants to N. gonorrhoeae 1291ΔPLD infection studies restored bacterial clustering and membrane ruffling (Fig. 4), suggesting that gonococcal PLD plays a role in signal transduction events leading to CR3 clustering and membrane ruffling.
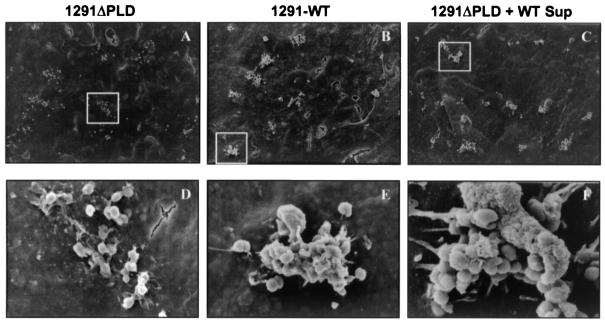
FIG. 4.
To determine if gonococcal PLD plays a role in the cytoskeletal rearrangements leading to membrane ruffling of the cervical epithelium, we performed scanning electron microscopy. Scanning electron microscopy analysis demonstrates that aberrant cytoskeletal rearrangements occur upon infection of cervical epithelia with PLD mutant gonococci compared to infection with wild-type gonococci. Endocytosis mediated by CR3 requires receptor clustering. The absence of bacterial clusters in electron micrographs taken of mutant gonococci at 3 h postinfection (upper panel) may be reflective of the inability of these bacteria to elicit up-regulation of CR3 or of their inability to initiate signaling cascades required for CR3 clustering. Similarly, the absence of membrane ruffles (lower panel) in PLD-infected cells suggests that gonococcal PLD may be required to potentiate the cytoskeletal rearrangements required to form membrane ruffles. These processes are restored when assays are performed with PLD mutant gonococci in the presence of primed wild-type supernatants. No observable differences between mutant or wild-type gonococci were noted in the ability of gonococci to interact with each other or with cervical cells at earlier points of infection. Electron micrographs shown in the lower panel correspond to the respective boxed areas shown in the upper panel. Magnifications: A, ×1,000; B, ×1.1,000; C, ×800,000; D, ×9,000; E, ×10,000; and F, ×15,000.
Activity and subcellular localization of N. gonorrhoeae PLD in cervical epithelia.
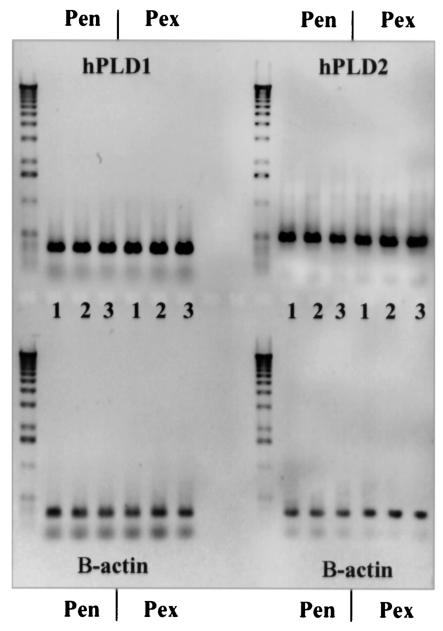
To determine if PLD activity is increased in infected cervical cells, we measured PLD activity in infected and uninfected cervical cell lysates. Comparison of wild-type-infected cervical cells to that of the PLD mutant-infected or uninfected cells demonstrated that total PLD activity is increased in wild-type-infected PEX and PEN cells. There was no significant difference between uninfected cells and mutant-infected cervical cells, suggesting that the observed increase in PLD activity is primarily due to gonococcal PLD. RT-PCR analysis of human PLD1 and PLD2 in PEX and PEN cells demonstrated that endogenous cervical cell PLD message levels are not up-regulated in cells infected with N. gonorrhoeae strains 1291 and 1291ΔPLD compared to uninfected cells (Fig. 5). These data support a role for gonococcal PLD, rather than endogenous cervical cell PLD, in the observed increase in PLD activity described above.
FIG. 5.
RT-PCR analysis demonstrates that endogenous cervical cell PLD activity does not account for the observed increased in total PLD activity in gonococcus-infected cervical cells. Lanes: 1, uninfected PEX or PEN cells; 2, 3-h infection of PEX or PEN cells with wild-type N. gonorrhoeae strain 1291; and 3, 3-h infection of PEX or PEN cells with N. gonorrhoeae strain 1291ΔPLD.
Analysis of PLD activity in infected and uninfected cervical cell fractions revealed that a significant portion of gonococcal and cervical cell PLD activity lies within the membrane-enriched portion of cervical cell lysates. However, PLD activity was also observed in the cytosol-enriched cell fraction. No significant difference was observed between PLD activity in uninfected cervical cells and the PLD mutant-infected cells.
Membrane ruffling followed by macropinocytosis of gonococci serves as a primary mechanism by which these bacteria invade the cervical epithelium. To determine if gonococcal PLD nonspecifically gains access to the cervical cell cytosol during macropinocytosis of gonococci, we performed cell fractionation studies of infected and uninfected PEX cells treated or untreated with wortmannin or cytochalasin D (Table 4). PLD activity was significantly reduced in membrane- and cytosol-enriched cell fractions when wortmannin and cytochalasin D were included in wild-type infection studies. No significant difference was observed in PLD activity in uninfected or 1291ΔPLD-infected PEX cells when the same cytoskeletal inhibitors were included or excluded from the assay. Collectively, these data indicate that macropinocytosis of invasive gonococci allows gonococcal PLD to enter primary cervical cells.
TABLE 4.
PLD activity in cervical cells treated with cytoskeletal inhibitorsa
| Cell fraction | PLD activity in ectocervical cell fractions, mean fluorescence units (variance)
|
|||
|---|---|---|---|---|
| Wortmannin treatment
|
Cytochalasin D treatment
|
|||
| Membrane | Cytosolic | Membrane | Cytosolic | |
| Uninfected, untreated | 0.58 (0.04) | 0.39 (0.02) | 0.37 (0.04) | 0.48 (0.05) |
| Uninfected, treated | 0.47 (0.05) | 0.40 (0.01) | 0.39 (0.01) | 0.40 (0.03) |
| 1291, untreated | 0.99 (0.10) | 0.86 (0.08) | 1.04 (0.06) | 0.85 (0.09) |
| 1291, treated | 0.78 (0.06) | 0.41 (0.03) | 0.44 (0.03) | 0.46 ((0.03) |
| 1291ΔPLD, untreated | 0.41 (0.01) | 0.41 (0.02) | 0.34 (0.01) | 0.39 (0.01) |
| 1291ΔPLD, treated | 0.42 (0.02) | 0.41 (0.01) | 0.39 (0.01) | 0.33 (0.07) |
Cellular fractionation and PLD activity were assayed as described in the text. Data given are the mean values obtained from three trials in which gonococci were removed by filtration through a 0.22-mm syringe filter (see Materials and Methods). Positive and negative control values were 1.13 ± 0.29 and 0.18 ± 0.04, respectively.
Gonococcal PLD acts at multiple levels in cervical cell infection.
Studies with Streptomyces PLD indicate that the exogenous addition of PLD to vascular smooth muscle cells mimics endogenous PLD activity within these cells (21). Consequently, we wanted to determine if exogenously added PLD substrates could compete with cervical cell constituents for gonococcal PLD activity and in doing so interfere with the role of gonococcal PLD in gonococcal invasion. The addition of 10, 1, 0.1, or 0.01 μg of phosphatidylcholine per ml or 1.0%, 0.1%, or 0.01% ethanol to infection studies impaired the ability of gonococci to invade PEX cells in a dose-dependent manner (Table 5). In contrast, no effect was observed in gonococcal association with and/or their invasion of primary cervical cells in the presence of 1.0%, 0.1%, or 0.01% 2-butanol (Table 5), which cannot serve as a substrate in PLD-catalyzed transphosphatidylation. There was no significant difference in survival observed between gonococci incubated in the presence or absence of 1.0%, 0.1%, or 0.01% ethanol or 2-butanol in the absence of cervical cells (data not shown). These data suggest a role for gonococcal PLD in modulating cervical cell signaling events (e.g., through phosphatidic acid generation) and suggest that gonococcal PLD may function at several different levels in gonococcal invasion of cervical epithelia.
TABLE 5.
Adherence to and/or invasion of primary cervical cells by gonococci in the presence and absence of PLD substrate competimersa
| Competimer | 1291b
|
1291ΔPLD, % invasionc | |
|---|---|---|---|
| % Inhibition | % Invasion | ||
| None | NA | 2.78 (0.16) | 0.34 (0.02) |
| Ethanol | |||
| 1.0% | 90.73 (0.92) (P < 0.05) | 0.26 (0.01) (P < 0.05) | 0.33 (0.03) (P > 0.75) |
| 0.1% | 80.92 (1.41) (P < 0.05) | 0.53 (0.01) (P < 0.05) | 0.34 (0.04) (P > 0.90) |
| 0.01% | 74.96 (0.87) (P < 0.05) | 0.70 (0.02) (P < 0.05) | 0.34 (0.04) (P > 0.90) |
| 2-Butanol | |||
| 1.0% | ND | 2.70 (0.47) (P > 0.90) | 0.30 (0.05) (P > 0.25) |
| 0.1% | ND | 2.71 (0.47) (P > 0.75) | 0.32 (0.03) (P > 0.25) |
| 0.01% | ND | 2.87 (0.53) (P > 0.75) | 0.28 (0.02) (P > 0.25) |
| Phosphatyacholine (μg/ml) | |||
| 10.0 | 98.78 (0.08) (P < 0.05) | 0.03 (0.01) (P < 0.05) | |
| 1.0 | 92.47 (0.38) (P < 0.05) | 0.20 (0.01) (P < 0.05) | |
| 0.1 | 87.36 (0.28) (P < 0.05) | 0.34 (0.01) (P < 0.05) | |
| 0.01 | 68.81 (2.15) (P < 0.05) | 0.84 (0.03) (P < 0.05) | |
| 0.0 | NA | 2.71 (0.11) (P < 0.05) | |
Values given are the mean values for percent invasion determined as a function of the original inoculum and the subsequent number of colony-forming units formed with subsequent plating of the ecto- or endocervical cell lysates. Inhibition values given were determined as a normalized function of the ability of the gonococcus to invade primary ectocervical cells in the presence of, in comparison to the absence of, alcohol or phosphatidylcholine competimers, as outlined in the text. Data given are the mean values obtained from at least three trials performed in triplicate. Variances are noted parenthetically. P values were determined with a Kruskal-Wallis k-sample analysis of variance. NA, not applicable. ND, not determined.
P values were calculated for wild-type gonococci in the presence of competimer compared to the absence of the competimer.
P values were calculated for PLD mutant gonococci in the presence of competimer compared to the absence of the competimer.
DISCUSSION
Phospholipases are a diverse group of hydrolytic enzymes, which are classified by the specificity they exert for the site of phospholipid cleavage. In eukaryotic systems, homologs of PLD can be activated by a variety of stimuli (e.g., hormones and growth factors), after which they catalyze the hydrolysis of phosphatidylcholine to choline (Cho) and phosphatidic acid (11, 20, 32). PLDs belong to a large superfamily of proteins, which can be divided into eight classes (27). Included in the PLD superfamily are prokaryotic and eukaryotic PLDs, cardiolipin, and phosphatidylserine synthases, vaccinia and fowlpox viral proteins of unknown function, an Escherichia coli nuclease, and an E. coli helicase (27). All members of the PLD superfamily (usually) contain two HKD motifs, which are thought to associate to form a catalytic center. However, unique to PLD is the ability to catalyze a transphosphatidylation reaction in which a primary alcohol preferentially serves as a nucleophilic acceptor instead of water, resulting in the near-exclusive production of a phosphatidylalcohol at the expense of phosphatidic acid. The resulting phosphatidylalcohol is metabolically stable and thus serves as a specific indicator of PLD activity.
Although eukaryotic PLDs have been well studied, much less is known about bacterial PLDs, and, in fact, only a handful have been identified. Some bacterial PLDs are associated with virulence, e.g., the Yersinia murine toxin (18) and Corynebacterium PLDs (25), pathogens of humans and domestic animals. It was demonstrated in Corynebacterium pseudotuberculosis that PLD mutation results in the attenuation of this microbe (19, 30).
We have described PLD activity in N. gonorrhoeae and have demonstrated a role for this secreted protein in gonococcal pathogenesis of cervical epithelia. Characteristic PLD activity (i.e., removal of Cho by cleavage of the terminal phosphodiester bond of phosphatidylcholine) was observed in gonococcal whole cell lysates but was absent in gonococci in which pld was mutated by the insertion of a kanamycin resistance cassette. PLD activity was observed over a pH range of 3.0 to 7.4, which is consistent with its ability to function as an effector protein within the lower female genital tract under normal (uninfected) or diseased states. The ability of gonococcal PLD activity to promote gonococcal invasion of primary cervical cells was inhibited in the presence of phosphatidylcholine and ethanol (a primary alcohol) but not 2-butanol (a secondary alcohol). These data definitively demonstrate that this gonococcal protein does indeed exhibit characteristic PLD function and argue against a role for endogenous PEX or PEN cell phospholipase C activity in CR3-mediated invasion of cervical epithelia by gonococci. (A Blast search of the N. gonorrhoeae and N. meningitidis genomic databases with sequences to several bacterial phospholipase C proteins failed to reveal the presence of this enzyme in the pathogenic Neisseria spp.) Furthermore, these data indicate that the generation of phosphatidic acid or its catabolic products are required for CR3-mediated macropinocytosis of gonococci.
Our data suggest that the continued release of gonococcal products, including gonococcal PLD, is specific to gonococcal infection of cervical epithelia. Sequence analysis of gonococcal PLD suggests that this protein may be secreted in a sec-dependent manner. Studies to definitively determine the mode of PLD secretion are under way. Although our data indicate that the release of gonococcal products appears to occur in a contact-dependent manner, a type III secretion system has not been described for N. gonorrhoeae. Furthermore, a Blast search of the N. gonorrhoeae and N. meningitidis genomic databases failed to reveal any proteins with significant homology to bacterial type III secretion system-associated proteins. Engagement of the CR3 I domain by gonococcal pilus and porin and by iC3b covalently bound to lipid A appears to act as a trigger for protein release.
The presence of an I domain is not unique to CR3. Some integrins (e.g., the α1 integrin, which is present on urethral epithelial cells) also possess an I domain. Although speculative, binding of pilus to the I domain of integrins other than CR3 (which is not present on male urethral epithelium) (8) may trigger early (i.e., less than 90 min) protein release upon infection of urethral epithelial cells. A secondary, tight association with the I domain might be required for increased PLD transcription and, consequently, sustained PLD release. After 90 min of infection, continued release of gonococcal products may not be observed with urethral epithelial cell infection because the tight association of the gonococcus with the urethral epithelium occurs through the ASGP-R (15).
Recent evidence indicates that Yersinia murine toxin promotes the survival of Yersinia pestis within the flea midgut from a cytotoxic digestion product present in blood plasma and, consequently, promotes disease transmission (18). Our data indicate that the presence of a functional gonococcal PLD is essential for the survival of gonococci within primary cervical cells; however, the specific mechanism(s) (e.g., alternative trafficking, alternative signaling, and/or inactivation of reactive oxygen species or other antimicrobial agents) by which protection is conferred remains to be determined. Although an interaction with the ASGP-R does not appear to sustain gonococcal PLD secretion in culture supernatants, gonococcal PLD does play a role in gonococcal survival within urethral epithelial cells. This might suggest that intracellular host proteins elicit gonococcal PLD secretion and/or that shared trafficking pathways exist for the gonococcus within cervical and urethral epithelial cells. The addition of SsPLD to association and invasion assays of N. gonorrhoeae 1291ΔPLD-infected cervical cells did not compensate for the absence of gonococcal PLD, suggesting that gonococcal PLD exhibits unique effector functions in addition to sharing structural and functional properties with SsPLD. This idea is supported by the finding that, although all PLDs (usually) contain two HKD motifs, sequences outside these regions are not necessarily highly conserved and may confer specific effector functions to their respective proteins (32).
Total PLD activity was greater in infected PEX and PEN cells upon comparison to uninfected or PLD mutant-infected cells, and we attributed this increase to gonococcal PLD and not endogenous PLD activity. Gonococcal PLD appears to modulate cervical cell function, either directly or indirectly in a cooperative manner with host cell effector molecules, to promote the appropriate targeting of gonococci to permissive host cells (i.e., CR3-expressing ecto- and endocervical cells) and to ensure their intracellular survival. To our knowledge, this makes gonococcal PLD unique among prokaryotic proteins identified to date. This idea is supported by the following observations: the association with and invasion of primary cervical epithelia are impaired in the absence of gonococcal PLD; CR3, the primary receptor by which gonococci invade the cervical epithelia, is not recruited to the cervical cell surface in the absence of gonococcal PLD; and membrane ruffling is not evident in the absence of gonococcal PLD with extended infection.
The ability of PLD to cause the release of secondary granules in neutrophils suggests that this molecule may play a role in the recruitment of CR3 to the surface of these cells. Additionally, products of PLD-catalyzed phospholipid hydrolysis serve as second messengers, eliciting a variety of cellular responses and are thought to function in complement (C′)-mediated endocytosis (12) and in cytoskeletal rearrangements (5, 14, 20). The absence of CR3 recruitment to the cell surface in N. gonorrhoeae 1291ΔPLD-infected primary cervical cells strongly suggests an early role for gonococcal PLD, directly or indirectly, in modulating CR3 effector function. Studies with Streptomyces chromofuscus PLD indicate that it can mimic endogenous PLD activity by triggering cytoskeletal rearrangements, DNA synthesis, and cell proliferation (21, 31). These data provide a precedent for our observations demonstrating the ability of exogenous gonococcal PLD, a secreted bacterial protein, to modulate CR3 effector function in primary cervical epithelial cells. Studies to define the substrate specificity of gonococcal PLD and to further elucidate signal transduction pathways and effector molecules activated upon CR3 engagement of cervical epithelial cells are under way.
Reorganization of the actin cytoskeleton is the result of the activation of a complex network of signal transduction pathways involving many effector molecules. Bacterial, plant, and human PLDs directly bind polymeric F-actin, which in turn increases PLD activity (23). In contrast, monomeric G-actin inhibits PLD activity in a species-specific manner (22) in that, in vitro, G-actin-induced PLD inhibition is 20-fold greater for human PLD1 than it is for Streptomyces PLD (23). The greatest degree of inhibition occurs upon the initiation of PLD activity in the presence of G-actin; less inhibition is observed when G-actin is added to previously activated PLD (23). Phosphatidylinositol 4,5-bisphosphate is a required cofactor in human PLD activity; in contrast, bacterial PLD activity does not exhibit a cofactor requirement. In resting cells, mammalian PLD resides in an inactive state because phosphatidylinositol 4,5-bisphosphate, which remains bound to actin-associated proteins (e.g., vinculin, α-actinin, and fodrin), is unavailable as a required cofactor (24).
Cervical cells infected with the N. gonorrhoeae 121ΔPLD mutant failed to elicit membrane ruffling but did promote microvillus and filopodium formation, suggesting that gonococcal PLD might be required to potentiate the extensive cytoskeletal rearrangements necessary for ruffle formation. In view of the above observations, it is not unreasonable to suggest that gonococcal PLD may act in a synergistic or an additive manner with endogenous cervical cell PLD to potentiate membrane ruffling by stabilizing actin filaments as observed with phalloidin. Our previous data indicate that vinculin, ezrin, and α-actinin colocalize with gonococci and accumulate in focal contacts (10). Therefore, the absence of gonococcal PLD might favor microvillus and filopodium formation because of the negative effects of vinculin and α-actinin on the availability of phosphatidylinositol 4,5-bisphosphate (13) and because of the presence of a (relatively) large pool of monomeric G-actin.
The inhibitory effect of G-actin on bacterial PLD is significantly less than that for human PLD (23), and bacterial PLDs do not require cofactor activity for function; consequently, induction of actin polymerization may be kinetically favored and, thus, be more extensive and sustained. F-actin produced would be anticipated to stimulate directly and indirectly (by depleting intracellular levels of G-actin) both gonococcal and cervical cell PLD activity, ultimately leading to membrane ruffles. Kusner et al. (22) have demonstrated that actin binding to PLD occurs through a conserved region of this protein which is found in all PLDs (including gonococcal PLD), but have also suggested that heterogeneic regions may modulate this interaction. In this regard, it is of interest that PLD homologs exhibiting the highest similarity to gonococcal PLD are found in other bacterial species (i.e., Salmonella, Shigella, and Escherichia) capable of eliciting extensive cytoskeletal rearrangements in their respective target cells.
Future studies may reveal that these novel bacterial proteins represent a new class of bacterial virulence factors capable of sustaining host cell cytoskeletal rearrangements and, consequently, promoting bacterial invasion via macropinocytosis. It is paramount to note, however, that in a previous study we demonstrated that CR3 engagement by anti-CR3 antibodies results in membrane ruffling of cervical epithelial cells and of CR3-expressing CHO cells (8). These data would tend to argue that the contribution of gonococcal PLD to membrane ruffling resides in the signal transduction events triggered by its presence; however, they do not exclude an additional role for gonococcal PLD in actin stabilization after CR3-activation.
The contribution of CR3 to innate immunity at the level of the cervical epithelium and to the potential role of complement in reproductive biology are not known. Although several effector molecules have been implicated in CR3-mediated signal transduction, these studies have primarily involved the use of neutrophils and of macrophages, in which membrane ruffling is mediated through the actions of the Rho family of small G-proteins, but which does not result in membrane ruffling (2). Currently, no studies have addressed C′-mediated signal transduction in epithelial cells; therefore, it cannot be stated or negated that signal transduction pathways analogous to professional phagocytic cells exist in cervical epithelia upon CR3 engagement.
In this work, we have initiated studies to elucidate the signaling pathways that participate in the response of cervical epithelia to N. gonorrhoeae infection. In this respect, we have identified a novel gonococcal virulence factor, gonococcal PLD, which modulates CR3 effector function in conjunction with cervical cell effector molecules to trigger alternative signal transduction pathways. This secreted gonococcal product serves a critical role in ensuring appropriate targeting of the gonococcus to the ecto- and endocervical epithelium by recruiting CR3 to the cervical cell surface and promotes intracellular survival of gonococci following CR3-mediated macropinocytosis. A better understanding of both the human and the bacterial factors that contribute to a diseased state at the level of the mucosal epithelium is greatly needed. It is anticipated that these studies will contribute significantly to the current understanding of signal transduction pathways in epithelial cells, of effector functions elicited by characterized and as yet uncharacterized bacterial PLDs, and of gonococcal infection of the lower female genital tract, which may lead to the development of effective therapeutic strategies against Neisseria and other mucosal pathogens.
Acknowledgments
We thankfully acknowledge K. A. Ault and the Department of Obstetrics and Gynecology at the University of Iowa for allowing us to obtain cervical tissue biopsies used to seed the primary cell cultures used in these studies. We are grateful to B. Gibson and K. Scheffler for mass spectroscopy and D. Kusner, S. Iyer, and J. Barton for technical advice. Antibody H5A4 was developed by J. T. August and J. E. K. Hildreth and was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City.
This work was supported by NIH grants AI45728, AI38515, and 5-T32-AI07343-14.
Editor: D. L. Burns
REFERENCES
- 1.Apicella, M. A. 1974. Antigenically distinct populations of Neisseria gonorrhoeae: isolation and characterization of the responsible determinants. J. Infect. Dis. 130:619-625. [DOI] [PubMed] [Google Scholar]
- 2.Caron, E., and A. Hall. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717-1720. [DOI] [PubMed] [Google Scholar]
- 3.Carrea, G., P. D'Arrigo, V. Piergianni, S. Roncaglio. F. Secundo, and S. Servi. 1995. Purification and properties of two phospholipases D from Streptomyces sp. Biochim. Biophys. Acta 1255:273-279. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, M. S., J. G. Cannon, A. E. Jerse, L. M. Charniga, S. F. Isbey, and L. G. Whicker. 1994. Hum. experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532-537. [DOI] [PubMed] [Google Scholar]
- 5.Colley, W. C., T-C. Sung, R. Roll, J. Jenco, S. M. Hammond, Y. Altshuller, D. Bar-Sagi, A. J. Morris, and M. A. Frohman. 1997. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol. 7:191-201. [DOI] [PubMed] [Google Scholar]
- 6.Dudas, K. C., and M. A. Apicella. 1988. Selection and immunochemical analysis of lipooligosaccharide mutants of Neisseria gonorrhoeae. Infect. Immun. 56:499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards, J. L., and M. A. Apicella. 2002. The role of lipooligosaccharide in Neisseria gonorrhoeae pathogenesis of cervical epithelia: lipid A serves as a C3 acceptor molecule. Cell. Microbiol. 4:584-598. [DOI] [PubMed] [Google Scholar]
- 8.Edwards, J. L., E. J. Brown, K. A. Ault, and M. A. Apicella. 2001. The role of complement receptor 3 (CR3) in Neisseria gonorrhoeae infection of human cervical epithelia. Cell. Microbiol. 3:611-622. [DOI] [PubMed] [Google Scholar]
- 9.Edwards, J. L., E. J. Brown, S. Uk-Nham, J. G. Cannon, M. S. Blake, and M. A. Apicella. 2002. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell. Microbiol. 4: 571-584. [DOI] [PubMed] [Google Scholar]
- 10.Edwards, J. L., J. Q. Shao, K. A. Ault, and M. A. Apicella. 2000. Neisseria gonorrhoeae elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect. Immun. 68:5354-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Exton, J. H. 1997. New developments in phospholipase D. J. Biol. Chem. 272:15579-15582. [DOI] [PubMed] [Google Scholar]
- 12.Fällman, M., M. Gullberg, C. Hellberg, and T. Andersson. 1992. Complement receptor-mediated phagocytosis is associated with accumulation of phosphatidylcholine-derived diglyceride in human neutrophils. J. Biol. Chem. 267:2656-2663. [PubMed] [Google Scholar]
- 13.Fukami, K., T. Endo, M. Imamura, and T. Takenawa. 1994. α-Actinin and vinculin are PIP2-binding proteins involved in signaling by tyrosine kinase. J. Biol. Chem. 269:1518-1522. [PubMed] [Google Scholar]
- 14.Ha, K-S. And J. H. Exton. 1993. Activation of actin polymerization by phosphatidic acid derived from phosphatidylcholine in IIC9 fibroblasts. J. Cell Biol. 123:1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey, H. A., M. P. Jennings, C. A. Campbell, R. Williams, and M. A. Apicella. 2001. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol. Microbiol. 42:659-672. [DOI] [PubMed] [Google Scholar]
- 16.Harvey, H. A., M. R. Ketterer, A. Preston, D. Lubaroff, R. Williams, and M. A. Apicella. 1997. Ultrastructure analysis of primary human urethral epithelial cell cultures-infected with Neisseria gonorrhoeae. Infect. Immun. 65:2420-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey, H. A., D. M. Post, and M. A. Apicella. 2002. Immortalization of human urethral epithelial cells: a model for the study of the pathogenesis of and the inflammatory cytokine response to Neisseria gonorrhoeae infection. Infect. Immun. 70:5808-5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinnebusch, B. J., A. E. Rudolph, P. Cherepanov, J. E. Dixon, T. G. Schwan, and A. Forsberg. 2002. Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science 296:733-735. [DOI] [PubMed] [Google Scholar]
- 19.Hodgson, A., L. M., J. Krywult, L. A. Corner, J. S. Rothel, and A. J. Radford. 1992. Rational attenuation of Corynebacterium pseudotuberculosis: potential cheesy gland vaccine and live delivery vehicle. Infect. Immun. 60:2900-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, D., C. Morgan, and S. Cockcroft. 1999. Phospholipase D and membrane traffic potential roles in regulated exocytosis, membrane delivery and vesicle budding. Biochim. Biophys. Acta 1439:229-234. [DOI] [PubMed] [Google Scholar]
- 21.Kondo, T., H. Inui, F. Konishi, and T. Inagami. 1992. Phospholipase D mimics platelet-derived growth factor as a competence factor in vascular smooth muscle cells. J. Biol. Chem. 267:23609-23616. [PubMed] [Google Scholar]
- 22.Kusner, D. J., J. A. Barton, K.-K. Wen, X. Wang, P. A. Rubenstein, and S. S. Iyer. 2002. Regulation of phospholipase D activity by actin. J. Biol. Chem. 277:50683-50692. [DOI] [PubMed] [Google Scholar]
- 23.Kusner, D. J., J. A. Barton, C. Qin, X. Wang, and S. S. Iyer. 2003. Evolutionary conservation of physical and functional interactions between phospholipase D and actin. Arch. Biochem. Biophys. 412:231-241. [DOI] [PubMed] [Google Scholar]
- 24.Lukowski. S., M.-C. Lecomte, J.-P. Mira, P. Marin, H. Gautero, F. Russo-Marie, and B. Geny. 1996. Inhibition of actin activity by fodrin. J. Biol. Chem. 271:24164-24171. [DOI] [PubMed] [Google Scholar]
- 25.McNamara, P. J., W. A. Cuevas, and J. G. Songer. 1995. Toxic phospholipases D of Corynebacterium pseudotuberculosis, C. ulcerans and Arcanobactreium haemolyticum: cloning and sequence homology. Gene 156:113-118. [DOI] [PubMed] [Google Scholar]
- 26.Morse, S. A., and L. Barenstein. 1980. Purine metabolism in Neisseria gonorrhoeae the requirement for hypoxanthine. Can. J. Microbiol. 26:13-20. [DOI] [PubMed] [Google Scholar]
- 27.Ponting, C. P., and I. D. Kerr. 1996. A novel family of phospholipase D homologues that includes phospholipid synthases and putative endonucleases: identification of duplicated repeats and potential active site residues. Protein Sci. 5:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoolnik, G. K., R. Fernandez, J. Y. Tai, J. Rothbard, E. C. Gotschlich. 1984. Gonococcal pili. Primary structure and receptor binding domain. J. Exp. Med. 159:1351-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segal, E., E. Billyard, M. So, S. Storzbach, and T. F. Meyer. 1985. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell 40:293-300. [DOI] [PubMed] [Google Scholar]
- 30.Simmons, C. P., S. J. Dunstan, M. Tachedjian, J. Krywult, A. L. M. Hodgson, and R. A. Strugnell. 1998. Vaccine potential of attenuated mutants of Corynebacterium pseudotuberculosis in sheep. Infect. Immun. 66:474-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dijk, M. C. M., F. Postma, H. Hilkmann, K. Jalink, W. J. van Blitterswijk, and W. H. Moolenaar. 1998. Exogenous phospholipase D generates lysophosphatidic acid and activates Ras, Rho, and Ca2+ signaling pathways. Curr. Biol. 8:386-392. [DOI] [PubMed] [Google Scholar]
- 32.Waite, M. 1999. The PLD superfamily: insights into catalysis. Biochim. Biophys. Acta 1439:187-197. [DOI] [PubMed] [Google Scholar]
- 33.Wen, K.-K., P. C. Giardina, M. S. Blake, J. L. Edwards, M. A. Apicella, and P. A. Rubenstein. 2000. Interaction of the gonococcal porin P. IB with G- and F-actin. Biochemistry 39:8638-8647. [DOI] [PubMed] [Google Scholar]