Abstract
Control of CD8α transcription during development of α/β T cell receptor (TCR) T lymphocytes is mediated by at least two distinct stage-specific cis-acting transcriptional mechanisms (i.e., enhancers). On the CD8α−/−knockout (KO) background, cis-mechanism I and cis-mechanism II together mediate appropriate stage- and sublineage-specific transgenic (Tg) CD8α expression and “rescue” development of peripheral CD8+ single-positive (SP) cytotoxic T lymphocytes (CTLs). In contrast, on the wild-type (WT)/CD8+/+ or CD8α−/−KO backgrounds, a CD8α Tg directed by cis-mechanism I alone is activated during the double negative [DN] to double positive [DP] transition and expressed up to the CD3low/intermediate DP stage but not in more mature DP or SP thymocytes or peripheral T cells. As loss of cis mechanism I activity occurs around the onset of positive selection, it is possible that events associated with TCR/major histocompatibility complex (MHC) interactions and selection are involved in initiating these changes in CD8α transcription. To examine this issue, phenotypic and functional studies were performed for thymocytes and T cells of CD8α−/−KO mice that expressed a CD8α Tg under control of cis-mechanism I only. Despite loss of CD8α expression at the DP CD3low/intermediate stage, increased populations of mature CD3hiCD4−CD8− thymocytes and CD3+CD4−CD8− peripheral T cells were detected. By several criteria, including MHC class I–restricted antigen recognition, these cells have at least partially undergone positive and negative selection. Therefore, initiation of selection and sublineage commitment are determined before loss of cis-mechanism I–mediated control of CD8α transcription. Further, CD8 expression beyond the CD3low/intermediate DP thymic stage is not essential for CTL development in vivo or function.
Keywords: CD8, gene expression, stage-specific regulation, thymic selection, sublineage commitment
Introduction
The strict correlation among peripheral α/β TCR T lymphocytes of CD8 expression with specificity for MHC class I, and CD4 expression with specificity for MHC class II, is established during thymic development 1 2. As thymocytes mature, they go through progressive stages of CD4 and CD8 expression, from initially expressing neither (i.e., the double-negative [DN] stage), to coexpressing both (the double-positive [DP] stage), to expressing one or the other (the single positive [SP] stage) 3 4. The DP to SP transition depends in part on TCR/MHC interactions and is characterized by positive and negative selection of the TCR repertoire, loss of one or the other coreceptor, and commitment to the cytotoxic or helper sublineage 1 2 3 4. Although some progress has been made in defining intermediate subpopulations 5 6 and signaling pathways 7 in the DP to SP transition, their relationships and contributions to T cell development at the transcriptional level are not yet clear. As CD8 and CD4 regulation are so tightly coupled to events of thymic differentiation, identification of their transcriptional control mechanisms will contribute to resolving these issues.
CD8 and CD4 are known to function during thymic selection and in the peripheral response to antigen 1 2 3 4 5 6. By binding conserved regions on class I and class II proteins respectively, these molecules stabilize cell interactions and contribute to signaling 3 4 8. Their importance in selection is illustrated by impaired development of cytotoxic and helper T cells in CD8- and CD4-deficient (i.e., knock-out [KO]) mice 9. Aside from the above, it is not known if either coreceptor serves additional functions before or after selection in the thymus or in peripheral T cells before encounter with antigen.
With these issues in mind, we have investigated transcriptional regulation of CD8 by assessing the patterns of expression and function of a series of mouse CD8α gene constructs in thymocytes and T cells of transgenic (Tg) mice that are wild type (WT) or deficient (i.e., KO) for endogenous CD8α 10. The CD8α Tg constructs contained varying amounts of 5′ and 3′ flanking DNA to permit identification of important transcriptional control sequences (i.e., enhancers) within the CD8 locus. Although CD8α (Lyt2.1) constructs with up to 12 kb of 5′ and 4.5 kb of 3′ flanking DNA were not expressed in T or non-T cells of Tg mice, inclusion of a 40-kb upstream segment led to expression in thymocytes and peripheral T cells in a pattern paralleling endogenous CD8α (Lyt 2.2) 10. Thus, this 40-kb region contains transcriptional enhancers able to direct stage- and sublineage-specific expression of CD8α in developing and mature α/β TCR T cells 10 11. Studies to characterize these cis-sequences showed that a 17-kb 5′ subfragment directs Tg CD8α expression during the DN to DP transition and up to the CD3low/intermediate DP stage but not in more mature DP or SP thymocytes or T cells 10. These findings, with results from others 11 12, suggest that thymic CD8α expression is mediated by at least two distinct stage-specific cis-acting transcriptional control mechanisms: cis-mechanism I resides within the 17-kb subfragment and is important for directing CD8α expression up to an early/intermediate DP stage; cis-mechanism II is located downstream of this region and is important for expression after this stage in more mature DP and SP thymocytes and CD8+ SP T cells 10 11 12 13 14. As the stage when cis-mechanism I is no longer active is around the onset of positive selection, it is possible that these changes in CD8α transcriptional control are causally linked to events of selection. To investigate this issue, we performed phenotypic and functional analyses of thymic subpopulations and peripheral T cells of mice expressing Tg CD8α under control of cis-mechanism I alone (and in the absence of endogenous CD8α). Our results indicate that at least some aspects of selection and sublineage commitment precede loss of cis-mechanism I activity.
Materials and Methods
CD8α Tg Mice, Inbred Mice, and KO Mice.
The CD8α (Lyt2.1) DNA constructs and Tg mice were described previously 10. Briefly, fragment C was 22 kb and contained the CD8α gene with 12 and 4.5 kb of 5′ and 3′ flanking DNA. As this CD8α gene was a hybrid of the first three exons of Lyt-2.1 and the last two exons of Lyt-2.2, the encoded protein is recognized by anti–Lyt-2.1 mAb 10. Fragment D was an upstream 40-kb fragment that overlapped the 5′ end of fragment C. Coinjection of fragments C and D led to cointegration at a single chromosomal site and appropriate expression of TgCD8α in the thymus and periphery 10. These mice are called TgCD8α(C+D). Fragment F was a 17-kb fragment encompassing the 5′ region of fragment D. Tg mice carrying fragments C and F at a single site are TgCD8α(C+F) 10. Tg lines were established by 8–10 backcrosses with C57BL/6J (H-2b; Lyt2.2) mice.
To establish each Tg strain on CD8α−/−KO, TgCD8α (C+D), or TgCD8α(C+F), offspring (on B6) were backcrossed 6–8 times with homozygous CD8α(Lyt2.2) KO mice (on B6) 9. The resulting mice are called TgCD8α(C+D)/CD8α−/−KO and TgCD8α(C+F)/CD8α−/−KO.
C57BL/6J (H-2b; B6), DBA/2J (H-2d), and B6.CH-2bm1(H-2Kbm1) 15 mice were from The Jackson Laboratory. CD8α−/−KO mice 9 were from Dr. T. Mak (Amgen Institute and Ontario Cancer Institute, Ontario, Canada). Mice were housed in a pathogen-free facility at The Hospital For Sick Children according to the Canadian Council of Animal Care.
Flow Cytometry.
Cell suspensions from thymii, spleens, or LNs from 6–8-wk-old mice were used for flow cytometry as described 10. mAbs included: anti–Lyt2.1 and anti–Lyt-2.2 10; anti-CD8α/Lyt2-biotin (BD PharMingen; reacts with Lyt2.1 and Lyt2.2); anti–CD4-PE or -allophycocyanin (APC) (BD PharMingen); anti–CD3ε-biotin, -PerCP (BD PharMingen), or -QR (Sigma-Aldrich). Staining with unconjugated mAbs was detected with FITC-secondary immunoglobulins (Accurate Scientific Corp.) as indicated. Biotinylated antibodies were detected with streptavidin (SA)-FITC (BD PharMingen) or SA-QR (Sigma-Aldrich). The tetramer for flu nucleoprotein (NP) peptide 366–374 with H-2Db was from the National Institutes of Health/National Institute of Allergy and Infectious Diseases Tetramer Facility. Lymphocytes were identified by forward and side scatter and 10,000 gated events were collected on a FACScan™ flow cytometer or a FACSCalibur™. Data analysis was with CELLQuest™ software (Becton Dickinson).
Cell-mediated Lympholysis Assay.
Cell-mediated lympholysis (CML) assays were performed as described 16. Responder LN cells (LNCs) were stimulated with irradiated DBA (H-2d) spleen cells for 5 d and used in 51Cr-release assays with concanavalin A–stimulated spleen cell targets 16. Specific lysis was [(experimental − spontaneous release)/(maximal − spontaneous release)] × 100%.
Skin Grafts.
8–10-wk-old mice were given full thickness skin grafts as described 16 from tails of adult sex-matched bm1 or control B6 mice. After removal of bandages on day 7, grafts were evaluated daily for evidence of rejection. Grafts were considered rejected when less than 10% of the graft bed contained viable grafted skin.
Influenza A Virus, Peptides, and Cytotoxicity Assays.
Mice were infected by intraperitoneal injection of 300 HAU of influenza A virus X-31 (Charles River Laboratories [Spafas]) in PBS. In H-2b mice, the dominant anti-flu CTL response is directed at NP peptide amino acid 366–374 (NP366–374) with H-2Db 17. Peptides were from Research Genetics.
Spleen cells from mice infected 3 wk earlier were restimulated in vitro for two 6–7-d periods with 0.1 μM NP366–374. Autologous peptide-pulsed spleen cells were the source of APCs and T cells for the first period. For the second period, peptide-pulsed irradiated (2,000 rads) H-2b/CD8−/−KO spleen cells served as APCs. For initial stimulation, ∼70 × 106 cells were cultured in 10 ml α-MEM (GIBCO BRL) containing 10% FCS (Sigma-Aldrich), 10 mM Hepes, 5 × 10−5 M β-mercaptoethanol, penicillin/streptomycin (GIBCO BRL), and 5 U/ml of mouse IL-2 (CTL medium). After 6–7 d, viable cells were harvested and restimulated. Cell aliquots were stained on the indicated day for CD3 and CD4. On the day of 51Cr-release assay, 106 EL4 cells (H-2b) were labeled with Na51CrO4 16, then pulsed with 0.1 μM of NP366–374 or a control peptide. Effectors were incubated with targets at various E/T ratios for 4 h. Supernatants were harvested and counted. Specific lysis was [(experimental − spontaneous release)/(maximal − spontaneous release)] × 100% (reference 16).
Results
Stage-specific Expression of CD8 in Developing Thymocytes.
Our previous studies showed that, although mouse CD8α gene constructs with 12 kb of 5′ and 4.5 kb of 3′ flanking DNA (fragment C in reference 10) were not expressed in T cells of Tg mice, inclusion of the adjacent 40-kb upstream segment from the chromosomal locus (fragment D) gave rise to appropriate TgCD8α expression in DP and SP thymocytes and peripheral CD8+CD4− T cells (TgCD8α[C+D]; reference 10). Thus, this 40-kb region contains transcriptional enhancers able to direct stage- and sublineage expression of CD8α in developing and mature α/β TCR T cells, consistent with results of others 11. A 17-kb 5′ subfragment of D (i.e., F) was subsequently found to direct expression of CD8α fragment C (TgCD8α[C+F]) in the thymus up to the CD3low/intermediate DP stage, but not in more mature DP or SP thymocytes or peripheral T cells 10. These results indicate that distinct cis-elements direct expression of CD8α before versus after this CD3low/intermediate DP stage 10. Although these initial studies with the (C+F) Tg were in Tg mice WT for endogenous CD8α (Lyt2.2 allele), a similar distribution of thymic expression was observed for this Tg bred onto the CD8α−/−KO background which is deficient for surface expression of CD8α and CD8β in the thymus and periphery and lacks detectable CTLs 9 (not shown). Therefore, our results show the CD8(C+F) construct directs expression of surface protein up to the CD3inter stage but not beyond, and coexpression of endogenous CD8 does not influence this distribution. Due to the unknown time-lag between changes in transcription being represented at the cell surface, the loss of TgCD8(C+F) surface protein after the CD3inter stage implies downregulation of transcription occurs within the CD3inter or CD3low population.
Fate of DP Thymocytes After Loss of Expression of TgCD8.
While thymocytes which express TgCD8(C+F) on the WT background will continue development beyond the CD3low/intermediate stage due to endogenous CD8, on the KO background there appeared to be a couple of possible fates depending whether positive selection has occurred by the time TgCD8α expression is lost at the CD3low/intermediate stage, and continued CD8 expression is required beyond this stage (i.e., independent of its role in selection). The left panels of Fig. 1 show CD3 expression for thymocytes of (Fig. 1 A) WT B6 [CD8++], (Fig. 1 B) CD8−/−KO, (Fig. 1 C) TgCD8[C+D]/CD8−/−KO, and (Fig. 1 D) TgCD8(C+F)/CD8−/−KO mice. The right panels show expression of CD4 versus CD8 for CD3hi thymocytes (identified by the bar in the CD3 histograms) 10. Compared with the low levels of CD4−CD8− cells in WT, CD8−/−, and TgCD8(C+D)/CD8−/−KO mice (1.5, 2.1, and 2.2% respectively), there was a 2.5–3-fold increase in this population in TgCD8(C+F)/CD8−/−KO mice (Fig. 1 D; 4.6%). This was a reproducible observation and demonstrates that expression of CD8α up to the DP CD3low/ intermediate stage is associated with an increased proportion of cells that express high levels of CD3 yet are CD4−CD8−. A possible explanation for their origin is that selection begins before loss of TgCD8(C+F) expression and that the selected cells continue development to “CD8+SP.” However, as CD8 is unable to be expressed in these TgCD8(C+F)/CD8−/−KO cells, they are in fact not CD8+SP but appear as CD4−CD8− DN.
Figure 1.
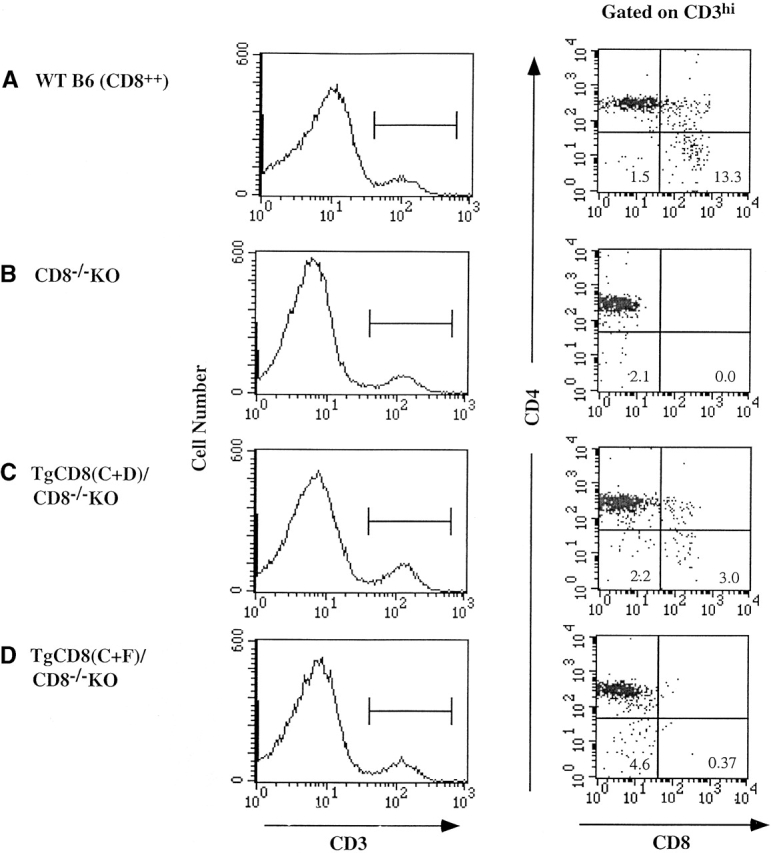
Three-color flow cytometry of CD4 and CD8 expression on CD3hi thymocytes of TgCD8α(C+F) mice in the absence of endogenous CD8α. Thymocytes from (A) WT (C57BL/6; CD8++), (B) CD8−/−KO, (C) TgCD8(C+D)/CD8−/−KO, and (D) TgCD8(C+F)/CD8−/−KO mice were stained for CD3, CD4, and CD8. The anti-CD8α mAb reacts with endogenous Lyt2.2 expressed by WT mice (A) and Tg Lyt2.1 expressed by TgCD8(C+D) and (C+F) mice (C and D). Endogenous CD8α (Lyt2.2) is not expressed by the mice in B, C, or D. The left panels (A–D) show CD3 expression for each strain with the bar identifying CD3hi cells. The right panels (A–D) show CD4/CD8 plots for CD3hi cells. The numbers in specific quadrants represent the percentage of cells with the indicated phenotype.
Peripheral CD3+CD4− T Cells in the Absence of CD8 Expression Beyond the DP CD3intermediate Thymic Stage.
Based on the above, we examined whether expression of the CD8α(C+F) Tg influenced the distribution of peripheral T lymphocytes. The left panels of Fig. 2 show that CD3+CD8+ cells were not detected in LNCs of TgCD8(C+F)/CD8−/−KO (Fig. 2 D) or CD8−/−KO (Fig. 2 B) mice but were detected in both WT (Fig. 2 A) and CD8α−/−KO mice Tg for the CD8(C+D) construct [TgCD8(C+D)/CD8−/−KO] (Fig. 2 C). The lower level of CD8+ cells for Tg(C+D) (5.7%) relative to WT (18.9%) is due to the lower level of expression of Tg CD8 compared with endogenous CD8 in this line 10.
Figure 2.
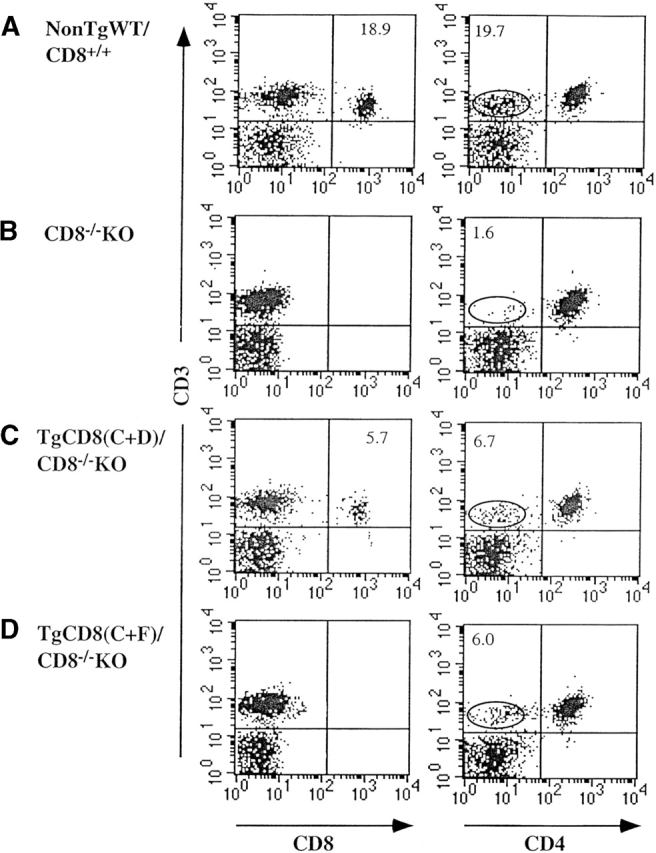
Three-color flow cytometry of peripheral T lymphocytes of TgCD8α(C+F) mice. LNCs of (A) non-Tg WT/CD8++, (B) CD8−/−KO, (C) TgCD8(C+D)/CD8−/−KO, and (D) TgCD8(C+F)/ CD8−/−KO mice were stained for CD3, CD8, and CD4. The anti-CD8α mAb reacts with Lyt2.2 expressed by WT mice (A) and Tg Lyt2.1 expressed by TgCD8(C+D) and (C+F) mice (C and D). Endogenous CD8α (Lyt2.2) is not expressed by the mice in B, C, or D. The left and right panels (A–D) show CD3 vs. CD8 and CD3 vs. CD4 profiles. The CD3+CD4− population is identified by the circle. The numbers in specific quadrants represent the percentage of cells with the indicated phenotype.
When analyzed for CD4 expression, a significant CD3+ CD4− population was detected in TgCD8(C+D)/CD8−/− (Fig. 2 C, right; 6.7%) and WT (Fig. 2 A, right; 19.7%) mice but not in CD8−/−KO (Fig. 2 B, right; 1.6%). These CD3+CD4− cells were CD8+ for both the WT (Lyt2.2+) and TgCD8(C+D)/CD8−/− (Lyt2.1+) samples (not shown). This, together with the similar percentages of CD3+CD8+ and CD3+CD4− cells for both WT and TgCD8(C+D) strains (18.9 vs. 19.7% for WT; 5.7 vs. 6.7% for TgCD8(C+D)/CD8−/−), indicates these are the same populations. Interestingly, TgCD8(C+F)CD8−/−KO LN cells also contained a population of CD3+CD4− cells (Fig. 2 D, right) similar in size to TgCD8(C+D) mice (6.0 vs. 6.7%). The appearance of these cells in the periphery of TgCD8(C+F)/CD8−/−, but not CD8−/− KO mice, indicates that they are the result of expression of the CD8(C+F) Tg, in spite of the demonstration that this Tg is only expressed up to the CD3low/intermediate DP thymic stage. Examination of the relative abundance of several TCR Vβ subfamilies in this population showed no major distortions in the repertoire (not shown).
Selection of “Rescued” Peripheral CD3+CD4−CD8− Cells.
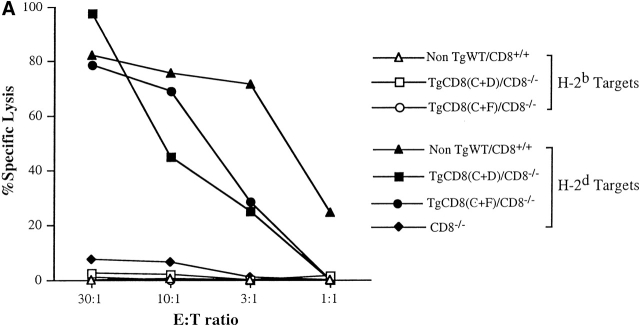
It was important to determine whether CD3+CD4−CD8− cells in the periphery of CD8(C+F) Tg mice had undergone selection for self-MHC restriction and tolerance. To examine the alloreactive cytotoxic response typical of CD8+ CTLs, primary in vitro cell–mediated lympholysis assays were performed. LNCs from each of the four groups were stimulated with H-2d (DBA/2J) spleen cells and tested in 51Cr-release assays (Fig. 3 A). Due to the absence of CTLs, there was no killing of H-2d targets by CD8−/− KO LNCs. In contrast, there was a high level of killing of these targets by TgCD8(C+F)/CD8−/−KO LNCs, similar to TgCD8(C+D)/CD8−/−KO and WT LNCs. None of these cultures lysed H-2b (self) targets. Thus, LNCs from TgCD8(C+F)/CD8−/−KO mice demonstrate alloreactive cytotoxicity to non–self-MHC and tolerance to self-MHC, implying that they have at least partially undergone positive and negative selection.
Figure 3.
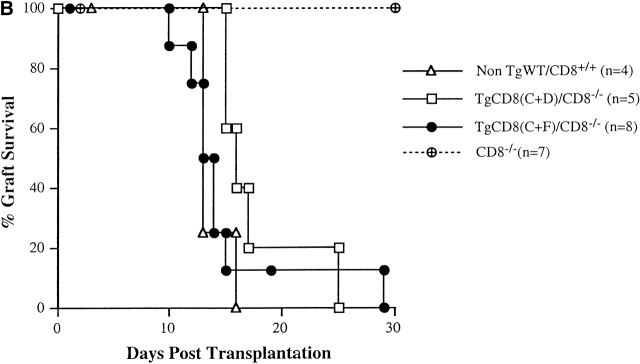
(A) Primary in vitro alloreactive response of TgCD8(C+F)/CD8−/− KO mice against non-self (H-2d) MHC. LNCs from non-TgWT/CD8+/+ (triangles), CD8−/− KO (diamonds), TgCD8(C+D)/CD8−/− KO (squares), and TgCD8(C+F)/CD8−/− KO (round symbols) H-2b mice were stimulated with DBA/2 (H-2d) spleen cells and assayed on Con-A–stimulated H-2b (open symbols) or H-2d (filled symbols) target cells. The effector to target (E:T) ratio and percentage of specific lysis are indicated. (B) Recognition of H-2Kbm1 tail skin allografts by TgCD8(C+F)/CD8−/− KO mice. Groups (n = no. of recipients) of non-Tg WT/CD8+/+ (▵), CD8−/− KO (⊕), TgCD8(C+D)/CD8−/− KO (□) and TgCD8(C+F)/CD8−/− KO (•) H-2b mice were grafted with tail skin from bm1 mice as described. The plot shows the percentage of mice with surviving grafts as a function of days after transplantation. Control grafts from H-2 syngeneic (H-2b) mice were not rejected by any of the groups (not shown).
To test the alloreactive response in vivo, skin grafting was performed using bm1 mice as donor. Relative to H-2b, bm1 mice carry a single class I difference (H-2Kbm1) which is recognized by H-2b CD8+ CTLs as a transplantation antigen 15. While CD8−/−KO (H-2b) mice do not reject bm1 grafts due to lack of CD8+ CTLs (Fig. 3 B), TgCD8(C+F)/CD8−/−KO mice rapidly reject these grafts similar to TgCD8(C+D)/CD8−/−KO and WT C57BL/6 mice. These results indicate the presence of effector cells in the periphery of TgCD8(C+F)/CD8−/−KO mice that respond to MHC class I alloantigens.
“Rescued” Peripheral CD3+CD4−CD8− Population Displays MHC Class I Restriction.
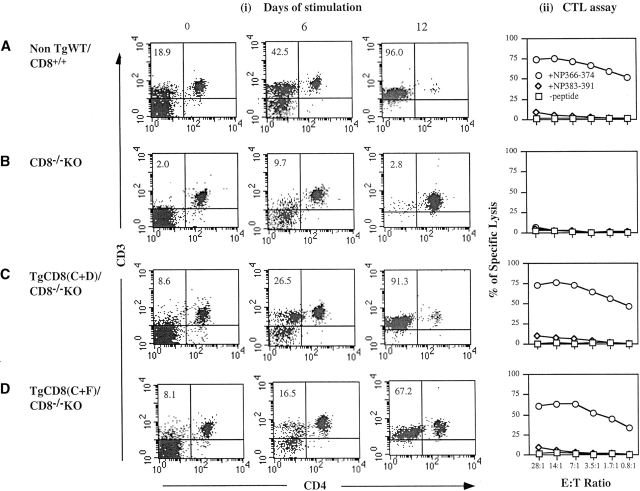
To test whether the peripheral CD3+CD4−CD8− population in TgCD8(C+F)/CD8−/−KO mice is capable of MHC class I–restricted antigen recognition, the response to influenza A infection was examined. The major anti–flu A CTL response in H-2b mice is directed toward flu NP peptide 366–374 (NP366–374) in association with H-2Db 17. To test for this response, spleen cells from infected mice of all four groups were restimulated with H-2b cells pulsed with NP366–374. After two 6–7-d periods of stimulation, responding cells were used in 51Cr-release assays. On days 0, 6, and 12, samples of each culture were stained with anti-CD3 and anti-CD4 mAbs to follow the CD3+CD4− population (Fig. 4A–D). On day 0, 18.9% of WT/CD82+ spleen cells were CD3+CD4− (i.e., CD8+) (Fig. 4 A, i). At 6 and 12 d, this population increased to 42.5 and 96.0% of the total (Fig. 4 A, i). The day 12 culture gave high levels of lysis of NP366–374-pulsed EL4 (H-2b) targets but did not lyse EL4 pulsed with an irrelevent peptide (NP383–391) or no peptide (-peptide) (Fig. 4 A, ii). In contrast, there was no significant expansion of CD3+CD4− cells for cultures from infected CD8−/−KO mice (Fig. 4 B, i) or killing of NP366–374-pulsed EL4 (Fig. 4 B, ii). Spleen cells from infected TgCD8(C+D)/CD8−/−KO mice also showed a NP366–374-specific expansion of the CD3+CD4− population from 8.6% on day 0 to 26.5% on day 6 to 91.3% by day 12 (Fig. 4 C, i). The day 12 culture showed NP366–375-specific killing of EL4 cells (Fig. 4 C, ii).
Figure 4.
Analysis of the anti–influenza A T cell response of TgCD8(C+F)/CD8−/− KO mice. 3 wk after infection, single cell suspensions of (A) non-Tg WT/CD8++, (B) CD8−/−KO, (C) TgCD8(C+D)/CD8−/−KO, and (D) TgCD8(C+F)/CD8−/−KO H-2b mice were stimulated for two 6-d periods with flu peptide NP366–374 and then tested in a 51Cr-release assay. Targets were EL4 (H-2b) cells pulsed with NP366–374 (+NP366–374), an irrelevant non–H-2b-binding flu peptide NP383–391 (+NP383–391), or no peptide (−peptide) (right panels, (ii) CTL assay]. The E/T ratio and percentage of specific lysis are indicated. On days 0, 6 and 12, cell samples of all groups (A–D) were stained for CD3 and CD4. The profiles in (i) show the results. The numbers in specific quadrants represent the percentage of cells with the indicated phenotype.
Interestingly, there was also a significant expansion of a CD3+CD4− population in NP366–374-stimulated cultures from infected TgCD8(C+F)/CD8−/− mice, from 8.1% on day 0 to 16.5% on day 6 and 67.2% on day 12 (Fig. 4 D, i). The day 12 culture gave a high level of killing of EL4 pulsed with NP366–374 almost similar to infected WT and TgCD8(C+D)/CD8−/−KO mice (Fig. 4 D, ii) but did not kill EL4 in the absence of peptide or pulsed with irrelevant peptide. Although the CD3+CD4−(CD8−) population of 12-d stimulated TgCD8(C+F)/CD8−/−KO cells for the experiment shown was slightly less (67.2%) than WT (96%) and TgCD8(C+D)/CD8−/−KO (91.3%), additional studies showed that a few more days of stimulation resulted in >90% CD3+CD4− cells (not shown).
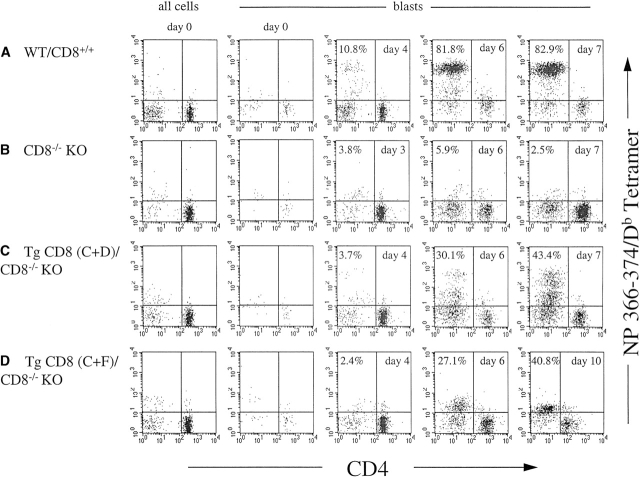
To compare the response of the CD3+CD4− cells of each infected group that were specific for NP366–374-associated H-2Db, MHC-peptide tetramers with this specificity were used in flow cytometry with anti-CD3 and anti-CD4 mAbs in a time-course analysis. The plots in Fig. 5 display the staining for the NP366–374/H-2Db tetramer versus CD4 for CD3+ cells of each culture on the indicated day. On day 0, the results are shown for total CD3+ cells (Fig. 5A–D, left panels) as well as for the CD3+ blast population (blasts). The level of tetramer staining of CD3+CD4− cells in the total population (all cells) for each group on day 0 was only marginally above background (i.e., 2–3%). As the blast population on day 0 was very low, it was difficult to detect any tetramer staining above background. For the WT group (Fig. 5 A), a tetramer-reactive population of CD3+CD4− cells representing ∼6.9% of CD3+ blasts first became evident on day 3 (not shown) and increased on days 4, 6, and 7 to ∼10.8, 81.8, and 82.9%. For the CD8−/−KO group, from day 0 through day 10 there was no tetramer staining above background (Fig. 5 B). For the TgCD8(C+D)/CD8−/−KO group, tetramer+ cells first became apparent on the same day as for WT (day 3) but at a lower level (not shown). This population was still low on day 4 (3.7%) but increased to 30.1 and 43.4% on days 6 and 7. While all tetramer+ cells detected for WT displayed a single high level of staining (Fig. 5 A, days 4, 6, and 7), for the TgCD8(C+D)/CD8−/−KO group there were two populations, one that stained at a high intensity (tet+hi) similar to the WT group, and another that stained at a lower level (tet+low). The percentage of both tet+hi and tet+low populations increased from days 4 to 10 in parallel. For the TgCD8(C+F)/CD8−/−KO group, tetramer+ cells were not detected on day 3 or 4 (Fig. 5 D) and became apparent on day 5, 2 d later than the WT or TgCD8(C+D)/CD8−/−KO groups (not shown). In contrast to both other groups, tetramer-reactive cells of the TgCD8(C+F)/CD8−/−KO group were all of lower intensity and increased from 7% on day 5 (not shown) to 27.1% on day 6 and 40.8% on day 10. Although this proportion increased further on subsequent days, in several experiments to more than 85% tetramer+, all tetramer-reactive cells on all days for the (C+F) group were of low intensity.
Figure 5.
Time-course analysis of flu NP366–374/H-2Db tetramer-reactive CD3+CD4− T cell response of TgCD8(C+F)/CD8−/− KO mice. 3 wk after infection, cell suspensions of (A) non-Tg WT/CD8+/+, (B) CD8−/−KO, (C) TgCD8(C+D)/CD8−/−KO, and (D) TgCD8(C+F)/CD8−/−KO H-2b mice were stimulated with the H-2Db-binding flu peptide NP366–374 for two 7-d periods. On day 0 and each day from day 3 to day 10, samples of all groups (A–D) were stained with anti-CD3 and anti-CD4 mAbs and the NP366–374/H-2Db tetramer. For day 0, the results are shown for all cells and for blasts. For days 3 to 10 the results for blasts are shown. The numbers in specific quadrants represent the percentage of cells with the indicated surface phenotype.
These results demonstrate that peripheral CD3+ CD4−CD8− cells of TgCD8(C+F)/CD8−/−KO mice respond to a well defined influenza antigen with significant expansion of a NP366–374/Db tetramer-reactive population which appears similar in some, but not all, respects to the CD3+CD4−CD8+ populations induced in WT and TgCD8(C+D)/CD8−/−KO mice. Additional studies showed that, while the TCR Vβ8.3 chain dominates this response among WT CD8+ CTLs 17, there is a significant reduction in usage of this Vβ chain in the induced population of (C+F) mice (results not shown).
Discussion
Recent studies indicate that DP to SP coreceptor regulation is more complex than previously assumed, as DP thymocytes first terminate CD8 expression to yield a CD4+CD8− intermediate that can then progress to SP CD4+, or undergo “coreceptor reversal” to SP CD8+ 5 6. Relevant to this are results from ourselves and others 10 13 14 showing expression of CD8 at the DP versus SP stage depends on distinct transcriptional control sequences (cis-mechanisms I and II). These findings raise the possibility that the CD4+CD8− intermediate 6 corresponds to a transitional stage between inactivation of cis-mechanism I and activation of cis-mechanism II in cells progressing to SP CD8+.
Our objective in this paper was to determine, first, whether cells that have progressed to the stage when CD8 cis-mechanism I activity is lost have begun thymic selection, and second, the fate of these cells in the absence of subsequent CD8 expression. Compared with CD8−/−KO, there was an increased population of CD3hi thymocytes that were negative for both CD4 and CD8 in TgCD8α(C+F)/CD8−/−KO mice (Fig. 1). Peripheral lymphoid tissues of these mice also contained an increased population of CD3+CD4−CD8− cells relative to the other groups. As the only difference between these mice and CD8−/−KO was expression of the CD8(C+F) Tg, the appearance of these cells in the thymus and periphery is dependent on thymic expression of TgCD8 up to the DP CD3low/intermediate stage.
Functional analyses of peripheral lymphocytes of (C+F)/CD8−/−KO mice demonstrated the presence of cells displaying properties typical of MHC class I–selected CTLs, including alloreactivity to class I–mismatched skin grafts, cytotoxicity toward non-self cells in vitro, tolerance toward self, and MHC class I–restricted recognition of foreign antigen. Although we do not have proof that the increased population of CD3+CD4−CD8− T cells in (C+F)/CD8−/− KO lymphoid tissues are responsible for mediating the alloreactive responses, these activities are not detected in CD8−/−KO mice and thus result from thymic expression of TgCD8 up to the DP CD3low/intermediate stage. For the anti-flu response, there was a strong correlation between induction of CD3+CD4−CD8− cells from spleen and the level of antigen-specific killing (Fig. 4). Further, these cells reacted with a tetramer consisting of flu NP366–374 peptide associated with H-2Db, the dominant complex recognized by CTLs in infected H-2b mice 17. Together, these results show that thymic expression of TgCD8 in (C+F)/CD8−/−KO mice is sufficient to give rise to peripheral CD3+CD4−CD8− T cells that possess a number of functional properties typical of SP CD8+ CTLs. As such cells are not detected in CD8−/−KO mice, both positive and negative selection must have at least partially taken place by the stage that cis-mechanism I–mediated control of CD8 expression is lost in (C+F)/CD8−/−KO mice.
Despite the apparent similarity between the CD3+CD4− CD8− peripheral T cells of (C+F)/CD8−/−KO mice and conventional SP CD8+ CTLs, there are also differences. First, although these CD3+CD4−CD8− cells display self-MHC restriction for foreign antigen, the rate at which they respond with increased cell numbers after stimulation in vitro is slightly less than WT or TgCD8(C+D) CTLs and somewhat more variable. We are uncertain whether this is due to a lower starting frequency, a lower rate of proliferation, or some other effect. Second, these stimulated (C+F) cells also stain at a lower intensity with the NP366–374/ H-2Db tetramer, suggesting that they may express a lower level of TCR (Fig. 5). Finally, despite recognizing the same flu peptide/H-2Db complex, the TCR Vβ8.3 chain, which dominates the response of WT mice 17, is expressed by a much lower proportion of responding (C+F) cells. As Vβ8.3+ T cells are present in the naive repertoire (not shown), these results suggest that lack of CD8 expression by responding CTLs influences the choice of TCR Vβ chain.
Two previous reports showing variant antigenic peptides, but not WT peptide, could mediate selection and commitment of Tg TCR/CD8−/−KO thymocytes in vitro indicate that CD8 is not absolutely required for development of the CD8/CTL lineage 18 19. Although this may be so for peptides with increased affinity tested in vitro, the results here show that expression of CD8 is required for development of this lineage in vivo, although only up to the thymic CD3low/intermediate DP stage.
In summary, the presence of self-MHC–restricted and tolerant CTL-like cells in the periphery of (C+F)/CD8−/−KO mice indicates that positive and negative selection, as well as commitment to the cytotoxic sublineage, must have at least partially taken place before loss of CD8 expression at the DP CD3low/intermediate stage. As such, some event induced by TCR/MHC interactions and selection may be responsible for triggering the stage-specific changes in CD8α transcriptional control involving cis-mechanisms I and II. Furthermore, our results indicate that development beyond the DP CD3low/intermediate stage, as well as thymic export, peripheral survival and cytotoxic function for at least a portion of the CTL repertoire, does not depend on CD8 being expressed beyond the thymic CD3low/intermediate stage. However, CD8 expression up to this stage under control of cis-mechanism I is essential for development of these cells because they are not detected in CD8−/−KO mice.
Acknowledgments
This work was supported by the Medical Research Council of Canada.
Footnotes
X.-L. Zhang and S. Zhao contributed equally to this work.
References
- Goldrath A., Bevan M. Selecting and maintaining a diverse T cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Sebzda E., Mariathason S., Ohteki T., Jones R., Bachmann M.F., Ohashi P.S. Selection of the T cell repertoire. Annu. Rev. Immunol. 1999;17:829–874. doi: 10.1146/annurev.immunol.17.1.829. [DOI] [PubMed] [Google Scholar]
- Miceli M.C., Parnes J.R. Role of CD4 and CD8 in T cell activation and differentiation. Adv. Immunol. 1993;53:59–122. doi: 10.1016/s0065-2776(08)60498-8. [DOI] [PubMed] [Google Scholar]
- Lucas B., Germain R.N. Unexpectedly complex regulation of CD4/CD8 coreceptor expression supports a revised model for CD4+CD8+ thymocyte differentiation. Immunity. 1996;5:461–477. doi: 10.1016/s1074-7613(00)80502-6. [DOI] [PubMed] [Google Scholar]
- Brugnera E., Bhandoola A., Cibotti R., Yu Q., Guinter T., Yamashita Y., Sharrow S., Singer A. Coreceptor reversal in the thymussignaled CD4+CD8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity. 2000;13:59–71. doi: 10.1016/s1074-7613(00)00008-x. [DOI] [PubMed] [Google Scholar]
- Deftos M., He Y., Ojala E., Bevan M.J. Correlating notch signaling with thymocyte maturation. Immunity. 1998;9:777–786. doi: 10.1016/s1074-7613(00)80643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Jakobsen B. Molecular interactions of coreceptor CD8 and MHC class Ithe molecular basis for functional co-ordination with the T cell receptor. Immunol. Today. 2000;21:630–636. doi: 10.1016/s0167-5699(00)01750-3. [DOI] [PubMed] [Google Scholar]
- Fung-Leung W.P., Schillham M.W., Rahemtulla A., Kundig T.M., Vollenweider M., Potter J., van Ewijk W., Mak T. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- Zhang X.-L., Seong R., Piracha R., Larijani M., Heeney M., Parnes J.R., Chamberlain J.W. Distinct stage-specific cis-active transcriptional mechanisms control expression of T cell coreceptor. CD8α at double- and single-positive stages of thymic development. J. Immunol. 1998;161:2254–2266. [PubMed] [Google Scholar]
- Hostert A., Tolaini M., Festenstein R., McNeil L., Malissen B., Willimas O., Zamoyska R., Kioussis D. A CD8 genomic fragment that directs subset-specific expression of CD8 in transgenic mice. J. Immunol. 1997;158:4270–4281. [PubMed] [Google Scholar]
- Ellmeier W., Sunshine M.J., Losos K., Littman D.R. Multiple developmentally stage-specific enhancers regulate CD8 expression in developing thymocytes and in thymus-independent T cells. Immunity. 1998;9:485–496. doi: 10.1016/s1074-7613(00)80632-9. [DOI] [PubMed] [Google Scholar]
- Ellmeier W., Sunshine M., Losos K., Hatam F., Littman D.R. An enhancer that directs lineage-specific expression of CD8 in positively selected thymocytes and mature T cells. Immunity. 1997;7:537–547. doi: 10.1016/s1074-7613(00)80375-1. [DOI] [PubMed] [Google Scholar]
- Hostert A., Tolaini M., Roderick K., Harker N., Norton T., Kioussis D. A region in the CD8 gene locus that directs expression to the mature CD8 T cell subset in transgenic mice. Immunity. 1997;7:525–536. doi: 10.1016/s1074-7613(00)80374-x. [DOI] [PubMed] [Google Scholar]
- Nathenson S.G., Gelieber J., Pfaffenbach G.M., Zeff R.A. Murine major histocompatibility complex class-I mutantsmolecular analysis and structure-function implications. Annu. Rev. Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Borenstein S.H., Graham J., Zhang X.L., Chamberlain J.W. CD8+ T cells are necessary for recognition of allelic, but not locus-mismatched or xeno-, HLA class I transplantation antigens. J. Immunol. 2000;165:2341–2353. doi: 10.4049/jimmunol.165.5.2341. [DOI] [PubMed] [Google Scholar]
- Flynn K.J., Belz G., Altman J.D., Ahmed R., Woodland D.L., Doherty P.C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- Goldrath A., Hogquist K., Bevan M.J. CD8 lineage commitment in the absence of CD8. Immunity. 1997;6:633–642. doi: 10.1016/s1074-7613(00)80351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebzda E., Choi M., Fung-Leung W.P., Mak T.W., Ohashi P.S. Peptide-induced positive selection of TCR transgenic thymocytes in a coreceptor-independent manner. Immunity. 1997;6:643–653. doi: 10.1016/s1074-7613(00)80352-0. [DOI] [PubMed] [Google Scholar]