Abstract
The diphtheria toxin A chain (DTA) was gene targeted into the Joining chain (J chain) locus to create a mouse strain selecting against J chain–expressing cells, JDTA mice. Serum immunoglobulin (Ig)M and serum IgG were reduced six to eightfold, while serum IgA was elevated 14-fold in these mice. JDTA mice were immune competent although the serum Ig response compared with wild-type mice was reduced sixfold at day 14 but only fourfold at day 45 after immunization. Exchanging the DTA gene with a cDNA for c-myc resulted in mice with a distinct phenotype with increased Ig production and enhanced humoral immune responses. Analysis of single B cells stimulated by lipopolysaccharide in vitro using reverse transcription–polymerase chain reaction showed that J chain–nonexpressing B cells could be detected that had a secretory phenotype as determined by an abundance of transcript for secretory IgM. Finally, limiting dilution analysis of peripheral B cells showed that J chain expression was a clonal property already established in naive, peripheral B lymphocytes.
Keywords: B cells, plasma cells, mice, ES cells, J chain
Introduction
Humoral immunity is mediated by Igs that are secreted into the body fluids and actively transported onto mucosal surfaces. In the serum, two types of Ig can be detected, so-called natural antibodies and antigen-induced antibodies. Natural antibodies 1 2 3 are mostly of IgM but also of IgG or IgA isotype and the serum levels of these seems to be controlled via a feedback mechanism 4 5. Antigen-induced antibodies are the end product of a humoral immune response where antibodies of a given affinity and specificity have been selected through intricate cellular mechanisms 6 7. Long-lasting serum Ig levels is an important component in immunological memory through their ability to neutralize viruses and other antigens on immediate contact 8. The effector cell responsible for Ig production into the body fluids is the plasma cell. Plasma cells can be found in all secondary lymphoid organs, secreting Ig at a high rate 9 and most of the serum Ig is derived from plasma cells residing in the bone marrow 10 11 12. Plasma cells have been believed to be short-lived, and to maintain long-lasting serum antibody levels there consequently had to be a continuous proliferation and differentiation of memory B cells into plasma cells 8 13 14. However, other reports have shown that there are two distinct populations of plasma cells that act as effectors of the humoral immune response, short-lived and long-lived plasma cells 11 12 15 16. Mucosal antibody responses are relatively short-lived compared with serum antibody responses, which may be due to either migration of plasma cells away from this site or that plasma cells in these sites are short-lived 11 17.
The Joining chain (J chain) is a glycoprotein that is associated with pentameric IgM and dimeric IgA 18 19. The J chain is required to generate stable pentameric IgM and in its absence IgM will be assembled into hexamers 18 20. In the case of IgA J chain is essential for the generation of dimeric IgA and its transport onto mucosal surfaces, while serum IgA is also present in a monomeric form that does not contain the J chain protein 19. In the absence of J chain, IgA will neither interact with the poly-Ig receptor nor form stable complexes with secretory component 21 22 23. Mice where the J chain locus has been inactivated by homologous recombination 24 25 have an impaired transport of IgA into the bile and over the intestinal mucosa, while at other mucosal sites the transport of IgA seemed unaffected 24 26 27. Furthermore, J−/− mice have elevated levels of IgA and unchanged IgG levels in serum, while serum IgM levels are reduced and IgM immune responses are compromised 25. Also, poly-Ig receptor–deficient mice show an accumulation of serum IgA as well as severely blocked transport of dimeric IgA across the intestinal mucosa 28 29, further illustrating the biological role for the J chain and the poly-Ig receptor.
In the mouse, the J chain is believed to be expressed in plasma cells only, irrespective of the Ig isotype produced 30 31. However, several reports have indicated that human B cells initiate J chain expression at an earlier developmental stage than shown for murine B cells. The J chain gene was shown to be transcribed during both B and T lymphopoiesis, but increased its expression as a function of plasma cell differentiation. Similar results have been obtained both in transformed B cell lines representing different stages of B cell differentiation 32 33 34, and in sorted hematopoetic subpopulations from human fetal and adult tissues 35. Interestingly, J chain–like transcripts have also been detected in species that lack Ig 36.
In humans, there has been reports indicating the presence of J chain-negative plasma cell populations. Thus, only a fraction of IgA- and IgG-producing human B cells stain positive for J chain using immunofluorescence 37 38 39. In addition, a positive correlation between the production of polymeric IgA and the presence of cytoplasmic J chain was seen; tissues that produced predominantly monomeric IgA, like the bone marrow, generally displayed fewer J chain-positive cells 37 40. It has also been shown that in secretory tissues such as salivary, mammary, and lacrimal glands, nasal and intestinal mucosa >90% of the IgA− and 50–70% of the IgG-secreting cells were J chain-positive 39. We show here also in the mouse the presence of two separate populations of B cells in vivo; one expressing J chain and one that does not. The described experimental system can be used to further explore the function of these two B cell subsets.
Materials and Methods
Targeting Constructs and Embryonic Stem Cell Transfection.
The mouse J chain gene locus was cloned from an I129 genomic phage library and a targeting vector for gene replacement was constructed as shown in Fig. 1 A and 6 A. The diphtheria toxin A chain (DTA) subunit in a pUC plasmid 41 was given to us by Ian H. Maxwell, University of Colorado Health Services Center, Denver, Colorado. A 795-bp BglII fragment containing the DTA gene (without its polyA sequence) or a 1.4-kb murine c-myc cDNA fragment (a gift from Dr. Richard H. Scheuermann, University of Texas) was cloned 3′ of a 5-kb genomic fragment containing the J chain promoter. An EcoRI/XhoI fragment from the ploxPneo-1 plasmid containing the neomycin resistance gene (neor) under the control of the phosphoglycerate kinase promoter and flanked by loxP sites was cloned 3′ of the DTA gene. A J chain splice site was synthesized and cloned 3′ of the neor gene after which a 10-kb KpnI/SalI fragment 3′ of exon 1 was cloned. The result was a construct where exon 1 of the J chain gene was replaced by the DTA or the cMyc together with the neor gene. The phosphoglycerate kinase promoter Herpes simplex thymidine kinase gene was cloned into the polylinker 3′ of the J chain construct. The whole construct was linearized by a unique NotI site 3′ of the thymidine kinase gene and transfected into the embryonic stem (ES) cell line E14 provided by Dr. Werner Muller, Institute for Genetics, Cologne, Germany. Colonies were screened by Southern blotting for homologous recombination events after KpnI digestion and probed with an outside probe (see Fig. 1 A and 5 A). A targeted JDTAneo clone was subsequently transiently transfected with the plasmid pBS.Cre provided by Dr. Reinhard Fassler, Lund University, to remove the neor gene, leaving only one loxP sequence in the locus. Colonies that had lost their resistance to G418 were expanded, DNA prepared and screened by Southern blotting for removal of the neor gene (see Fig. 1 A). A targeted JcMycneo clone was used to generate a mouse strain which subsequently was bred to mice constitutively expressing the Cre enzyme 42, enabling in vivo excision of the neor gene. The thereby generated JcMyc mice were identified by Southern blot analysis on DNA from tail biopsies (see Fig. 5 A).
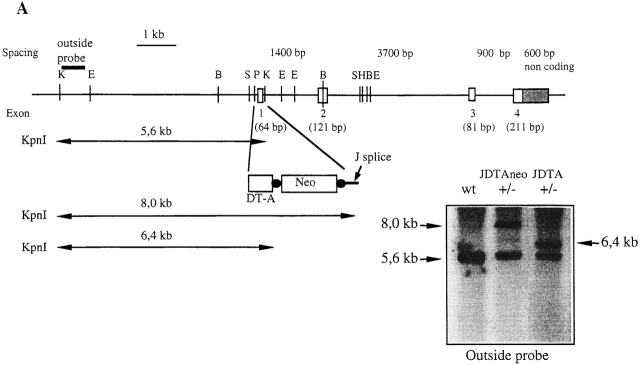
Figure 1.
Replacement of the J chain exon 1 with the DTA gene. (A) Disruption of the J chain gene and gene replacement by homologous recombination. Genomic map of J chain locus before and after homologous recombination. Digestion with KpnI gives an endogenous band of 5.6 kb while the recombined locus gives a band of 8.0 kb for JDTAneo and 6.4 kb for JDTA after removal of the neor gene. Southern blot analysis of the recombined J chain locus showing wt, heterozygous (+/−) JDTAneo, and heterozygous (+/−) JDTA using an outside probe (not present in the construct used for targeting). E, EcoRI; B, BamHI; S, SacI; P, PstI; K, KpnI; and H, HindIII. (B) Expression analysis by RT-PCR for J chain and DTA transcripts in wt, JDTA heterozygous (JDTA+/−), and JDTA mice. Primers for J chain were located in exon 1 and in exon 4 of the J chain locus. Primers for DTA transcripts were located in the 3′ end of the DTA gene and in the exon 4 of the J chain locus. Shown is also analysis for HPRT transcripts as a cDNA control.
Figure 5.
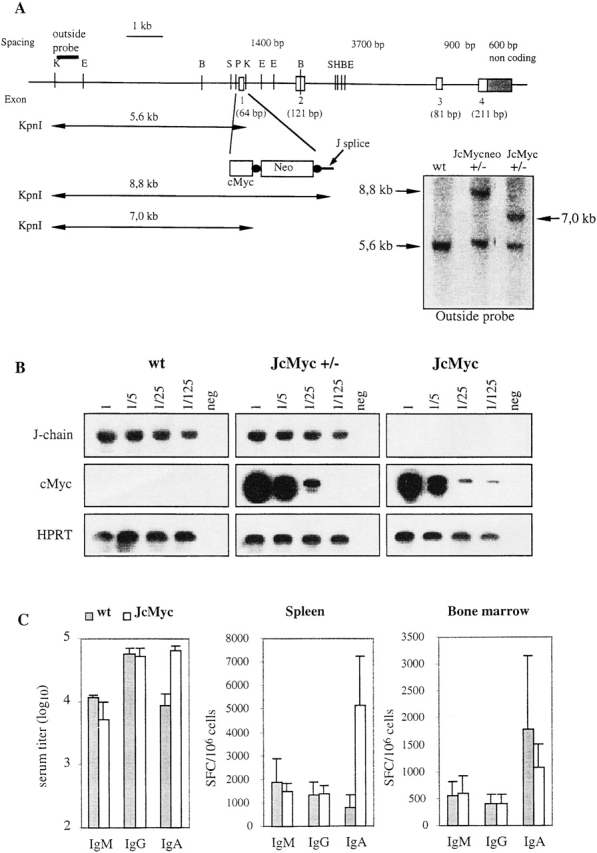
Replacement of the J chain exon 1 with the cMyc gene and Ig production in the resulting JcMyc mice. (A) Disruption of the J chain gene and gene replacement by homologous recombination. Genomic map of J chain locus before and after homologous recombination. Digestion with KpnI gives an endogenous band of 5.6 kb while the recombined locus gives a band of 8.8 kb for JcMycneo and 7.0 kb for JcMyc after removal of the neor gene. Southern blot analysis of the recombined J chain locus showing wt, heterozygous (+/−) JcMycneo, and heterozygous (+/−) JcMyc using an outside probe (not present in the construct used for targeting). E, EcoRI; B, BamHI; S, SacI; P, PstI; K, KpnI; and H, HindIII. (B) Expression analysis by RT-PCR for J chain and cMyc transcripts in wt, JcMyc heterozygous (JcMyc+/−), and JcMyc mice. Primers for J chain were located in exon 1 and in exon 4 of the J chain locus. Primers for cMyc transcripts were located in the 3′ end of the cMyc gene and in the exon 4 of the J chain locus. Shown is also analysis for HPRT transcripts as a cDNA control. (C) Serum IgM, IgG, and IgA levels in wt and JcMyc mice at the age of 4 mo (n = 3) and number of Ig-secreting cells per 106 cells from wt and JcMyc spleen and bone marrow as detected by ELISPOT assay (n = 3). Data shown from one out of two experiments giving similar results. SFC, spot forming cell.
ELISA.
To determine serum levels of the different Ig isotypes we used goat anti–mouse IgA, IgM, or IgG antibodies as coating antibodies (Southern Biotechnology Associates, Inc.) and an horseradish peroxidase (HRP)-conjugated rabbit anti–mouse Ig antibody (Dako) as secondary antibody. As a substrate we used TMB (3,3′, 5,5′-tetramethylbenzidine; Sigma-Aldrich). The isotype-specific antisera were highly specific and did not cross-react with purified proteins of other isotypes. To determine total serum Ig levels we used a rabbit anti–mouse Ig antibody as a coating antibody and an HRP-conjugated rabbit anti–mouse Ig antibody (Dako) as secondary antibody.
Enzyme-linked Immunospot Assay.
To analyze the number of cells secreting a specific isotype in different cell preparations we performed an enzyme-linked immunospot assay (ELISPOT). We used goat anti–mouse IgM, IgG, or IgA antibody as coating antibody at 10 μg/ml (Southern Biotechnology Associates, Inc.). After blocking, the cells were added to duplicate wells and incubated for 4–5 h at 37°C. Cells were lysed and the debris removed by washing in PBS/0.5% Tween. Biotinylated rabbit anti–mouse IgM, IgG, or IgA was used as secondary antibody (Southern Biotechnology Associates, Inc.) and the spots were developed with AP-conjugated avidin (ExtrAvidin; Sigma-Aldrich) followed by the substrate BCIP (5-bromo-4-chloro-3-indolylphosphate; Sigma-Aldrich). To analyze the number of Cholera toxin (CT)-specific IgA-secreting cells after oral CT immunization we performed a CT-specific ELISPOT assay 27. In brief, polystyrene petri dishes (Nunc A/S; Nunclon) were coated with 3 nmol/ml of GM1 ganglioside (Sigma-Aldrich) followed by an additional incubation with 3 μg/ml CT (List Biological Laboratories) in PBS. After blocking, lamina propria (LP) lymphocytes at 4 × 105 were added to each petri dish and incubated for 4 h at 37°C. The cells were lysed and the debris removed by washing in PBS/0.5% Tween. Single LP anti-CT–secreting cells were visualized by adding goat anti–mouse IgA as a primary antibody (Cappel) and then a HRP-conjugated rabbit anti–goat Ig as a secondary antibody (Dako A/S) followed by the substrate paraphenylenediamine (Sigma-Aldrich). Spots were counted under low magnification.
Western Blot Analysis.
Serum from wild-type (wt), JDTA heterozygote (JDTA+/−), and homozygote (JDTA+/+) mice were collected and IgA were purified with biotinylated goat anti–mouse IgA (Southern Biotechnology Associates, Inc.) and streptavidine Dynabeads (Dynal) according to the manufacturer's protocol. Electrophoresis was performed using a 5%, nondenaturating polyacrylamide gel. Equal amounts of protein (0.2 μg) was loaded in each lane. The protein was transferred to a nylon membrane (Hybond-C extra; Amersham Pharmacia Biotech) using a Trans-blot SD system (Bio-Rad Laboratories). The filters were blocked overnight in 5% dry fat-free milk in PBS-T, followed by incubation with a rabbit anti–mouse IgA antibody (Zymed Laboratories). HRP-conjugated donkey anti–rabbit Ig was used as secondary antibody (Southern Biotechnology Associates, Inc.), and the filters were developed with ELC-reagent (Amersham Pharmacia Biotech) according to the manufacturer's protocol.
Cell and Intestinal Sample Preparations.
Spleen cells were removed aseptically, single cell preparations made, counted, and depleted of erythrocytes by treatment with Gey's solution. LP lymphocytes were prepared as described previously 27. In brief, after thorough washing in Ca2+- and Mg2+-free HBSS (CMF-HBSS; GIBCO BRL) the tissue pieces were incubated in CMF-HBSS containing 5 mM EDTA to remove epithelial cells and intraepithelial lymphocytes. The intestinal pieces were then incubated in RPMI (GIBCO BRL) containing 300 U/ml collagenase type C-2139 (Sigma-Aldrich) in order to enzymatically extract the LP lymphocytes. The single cell suspension was washed three times in CMF-HBSS and diluted to a final concentration of 2 × 106 cells per milliliter in Iscove's medium (GIBCO BRL) containing 5% FCS (GIBCO BRL). Intestinal lavage samples for antibody determinations were collected by a method adopted from Elson et al. 43 described previously 27, and analyzed by ELISA as described previously.
Mitogen Stimulation and Cell Fusion.
B cells were cultured in RPMI (GIBCO BRL) supplemented with 7.5% FCS (Sigma-Aldrich). LPS (Escherichia coli 055:B5; Difco) was added at a final concentration of 25 μg/ml. Cells for preparation of RNA were harvested after 72 h of LPS stimulation. Fusion with Sp2/0 was performed on day 2 LPS cultures, using 3.3 × 106 Sp2/0 cells and 16.5 × 106 LPS-stimulated B lymphocytes. The fused cells were resuspended carefully and plated on 96-well plates in three different cell densities with regard to Sp2/0 cells; 20,000, 5,000, and 1,250 Sp2/0 cells per well. Conventional selection procedures were used.
Reverse Transcription PCR Analysis.
RNA was prepared from LPS-stimulated wt, JDTA heterozygote (JDTA+/−), JDTA, JcMyc heterozygote (JcMyc+/−), and JcMyc splenocytes by the use of “RNAzol™B” according to the manufacturer's protocol (AMS Biotechnology). cDNA was prepared by the use of “Superscript™ RNase H Reverse Transcriptase” according to the manufacturer's protocol (Life Technologies) and then used in PCR with the following primers. Primers used for detection of HPRT transcripts were 5′-GCT GGT GAA AAG GAC CTC T-3′ located in the 5′ end and 5′-CAC AGG ACT AGA ACA CCT GC-3′ located in the 3′ end giving a PCR product from cDNA of 249 bp. Primers used for detection of J chain transcripts were 5′-ATG AAG ACC CAC CTG CTT CTC TGG-3′ located in exon 1 and 5′-AGG GTA GCA AGA ATC GGG GGT CAA-3′ located in exon 4 giving a PCR product from cDNA of 470 bp. Primers used for detection of transcripts between DTA and exon 4 in the J chain were 5′-TAT ATG GCT CAA GCC TGT G-3′ located in the 3′ end of the DTA gene and 5′-AGG GTA GCA AGA ATC GGG GGT CAA-3′ located in exon 4 of the J-chain gene giving a PCR product from cDNA of 650 bp. Primers used for detection of transcripts between cMyc and exon 4 in the J chain were 5′-GAC GAG CAC AAG CTC ACC TCT GAA-3′ located in the 3′ end of the cMyc gene and 5′-AGG GTA GCA AGA ATC GGG GGT CAA -3′ located in exon 4 of the J chain gene giving a PCR product from cDNA of 560 bp. Primers used for detection of secretory IgM were 5′-TGT GTG TAC TGT GAC TCA CAG GGA-3′ located in exon 3 of the constant part and 5′-AGG GAG ACA TTG TAC AGT GTG GGT-3′ located in the secretory region giving a PCR product of 427 bp. Primers used for detection of membrane bound IgM were 5′-TGT GTG TAC TGT GAC TCA CAG GGA-3′ located in exon 3 of the constant part and 5′-TGT AGA AGA GGC TCA GGA GGA AGA-3′ located in the membrane exon M1 giving a PCR product of 492 bp. PCR products were separated on agarose gels, vacuum blotted to nylon filters (Zeta probe; Bio-Rad Laboratories) and then hybridized with [32P]-labeled probes for HPRT, IgM, and J chain as indicated.
Immunization and Analysis of Immune Responses.
Mice were immunized intraperitoneally with the T cell–dependent antigen 2-phenyl-5-oxazolone coupled to OVA (phOx-OVA), 100 μg alum precipitate per mouse at the age of 4 mo, five mice of each genotype in each of two independent experiments. At indicated days serum samples were collected and antigen-specific ELISAs performed. Coating antigen was phOx coupled to BSA and a HRP-conjugated rabbit anti–mouse Ig antibody (Dako) was used as a secondary antibody. For the antigen-specific IgM, IgG, or IgA ELISA, biotinylated goat anti–mouse IgM, IgG, or IgA (Southern Biotechnology Associates, Inc.) were used as secondary antibodies and developed by HRP-conjugated streptavidin (Sigma-Aldrich). As a substrate we used TMB (3,3′,5,5′-tetramethylbenzidine; Sigma-Aldrich). Six mice of each genotype were given three oral immunizations 10 d apart with 10 μg of CT (List Biological Laboratories) in 6% (wt/vol) NaHCO3 in PBS through a baby feeding tube under light ether anesthesia as described previously 27. A CT-specific ELISA was performed on serum and lavage from immunized mice as follows and as described previously 27; microtiter plates were coated with 0.5 nmol/ml GM1 ganglioside (Sigma-Aldrich) followed by incubation with 0.5 μg/ml CT (List Biological Laboratories). As a secondary antibody we used HRP-conjugated goat anti–mouse Ig or goat anti–IgA antibodies (Southern Biotechnology Associates, Inc.) and as substrate ortho-paraphenylenediamine (Sigma-Aldrich). Analysis of the number of CT-specific IgA-secreting cells in the LP from immunized mice was performed using ELISPOT as described previously.
Single Cell PCR Analysis.
Day 3 LPS-stimulated splenic cell suspensions were incubated with biotinylated anti-B220 antibodies (BD PharMingen), washed twice followed by incubation with streptavidin Dynabeads (Dynal). Positive cells were harvested by picking single cells in 0.8 μl drops of PBS under a light microscope and then transferring them to RNase free tubes. Nested single cell reverse transcription (RT)-PCR was performed by usage of “Titan™ one tube RT-PCR system” according to the manufacturer's protocol (Boehringer).
Limiting Dilution Analysis.
Spleen cells from normal wt C57BL/6 mice were prepared as described previously. Splenic lymphocytes were then seeded at 1, 3, 10, 30, 100, 300, and 1,000 cells per well in 96-well plates, one hole plate for each cell density, in a total of 200 μl RPMI (GIBCO BRL) supplemented with 7.5% FCS (Sigma-Aldrich), 1% IL-6 S/N, and 25 μg/ml LPS (E. coli 055:B5; Difco). After incubation for 7 d, the supernatants were collected and the dry plates frozen at −80°C. We performed a total Ig ELISA on the supernatants, as described previously, to detect Ig-secreting clones. The fraction of Ig-negative wells from each plate was then plotted against the number of seeded cells per well. This gave us, according to the Poisson distribution, the limit for clonality. From wells containing clonal cultures, cDNA was prepared and analyzed with PCR for HPRT expression and J chain expression. PCR products were separated on agarose gels, vacuum blotted to nylonfilters (Zeta probe; Bio-Rad Laboratories), and then hybridized with [32P]-labeled probes for HPRT and J chain as indicated.
Results
Construction of the JDTA Mouse Strain.
To address directly whether a dichotomy of plasma cells could be distinguished based on J chain expression, we created a mouse strain where J chain–expressing cells were selectively depleted. We replaced the first exon in the mouse J chain locus with the coding sequence for the DTA. It has previously been shown in transgenic models that the tissue-specific expression of DTA can selectively deplete defined cell populations 44 45 46 or indeed whole organs 41 47. The strategy for gene targeting in the J chain locus and subsequent removal of the selection marker, enabling the exogenous gene to be expressed, is outlined in Fig. 1 A. A DNA construct was made where exon 1 of the J chain gene was replaced by the DTA gene. This was followed by the neor cassette flanked by loxP sequences, where the splice site of the J chain exon 1 was placed 3′ of the neor gene. Hence, no expression of DTA can occur until the neor cassette has been removed. The construct was electroporated into E14 ES cells and G418-resistant clones were screened by Southern blot analysis. Homologous recombinant clones were obtained at a frequency of 1/300 and mice derived from such an ES cell clone had a J chain−/− phenotype 25. To activate the DTA gene, the ES cell clone used to generate the J chain−/− mice was transiently transfected with a Cre expression plasmid to excise the neomycin cassette (excised clones were obtained at a frequency of 1/12). Fig. 1 A also shows a Southern blot analysis of KpnI-digested DNA from wt and heterozygous ES cells before (JDTAneo) and after the removal of the neomycin cassette (JDTA). No new integration of DNA into the genome could be detected after the transient transfection with Cre, as judged by Southern blot analysis and PCR analysis (data not shown). Subsequently, ES cells where the neor cassette had been removed were injected into blastocysts to generate chimeric mice, after which the JDTA mouse strain was established by backcrossing these chimeras to C57BL/6 mice. Litter sizes of the JDTA mice were normal and the offspring displayed a normal Mendelian distribution of wt, heterozygous, and homozygous mice.
To assess the expression of the inserted DTA gene, we performed RT-PCR analysis of RNA from splenocytes stimulated with LPS during 4 d (Fig. 1 B). Activation of J chain transcription could be detected in cells from both wt and JDTA heterozygous mice and transcription of the targeted JDTA locus was detected in both heterozygous and JDTA mice. Within the sensitivity of the PCR assay it appeared that JDTA heterozygous mice expressed higher levels of JDTA transcripts than did JDTA mice, which could be explained by that cell death occurs more rapidly in cells that are homozygous for the DTA toxin. Hence, we based the rest of the analysis in this paper on JDTA mice.
The Functional Capacity of the DTA Toxin Gene in the J Chain Locus of JDTA Mice.
A pertinent question to be addressed in order to interpret the data obtained in the JDTA model is the robustness of the model. To address this question we reasoned that fusion of splenocytes to SP2/0 plasmacytoma cells would result in hybridomas that have the secretory phenotype of this J chain–expressing fusion partner. To this end, we used B cells from wt, heterozygous JDTA, and JDTA mice stimulated with LPS for 48 h in order to increase fusion efficiency. These cell populations were fused to SP2/0 and plated at various SP2/0 cell concentrations in 96-well plates. After conventional selection procedures, the number of growing hybridomas were scored (Fig. 2). When plating 20,000 SP2/0 cells fused with wt B cells, hybridoma growth was detected in virtually 100% of the wells, indicating that most wells contained more than one growing clone according to Poisson statistics. Hybridoma growth was reduced by 40% at this SP2/0 cell density in the fusions with JDTA and JDTA heterozygous B cells. In the plates where 5, 000 or 1,250 SP2/0 cells had been plated, the number of recovered hybridomas from the fusion with B cells from JDTA mice was greatly reduced. Furthermore, at inputs of SP2/0 cells were limiting dilution conditions was established, 37% negative wells according to Poisson statistics, the numbers of recovered hybridomas from B cells from heterozygous JDTA mice were intermediary to that of JDTA B cells. This would be expected since the probability of incorporating one DTA-expressing allele in the hybridoma using heterozygous B cells should be only 50% of that when homozygous B cells are used. Thus, we conclude that the genetically manipulated JDTA locus is functional and has essentially a 100% phenotypic penetrance, given the proper intracellular environment.
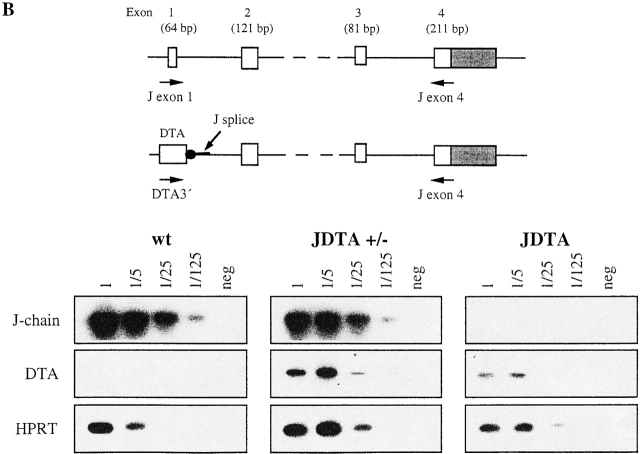
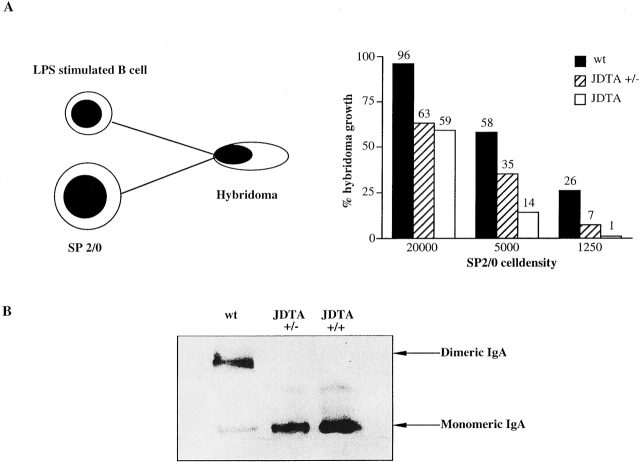
Figure 2.
Functional activity of the DTA transgene. (A) In vitro activation of the DTA gene by cell fusion. Percentage of hybridoma growth from wt, JDTA heterozygous (JDTA+/−), and JDTA day 2 LPS-stimulated splenic B cells fused with SP2/0 and plated at different SP2/0 cell densities. (B) Deletion of J chain–expressing cells in vivo. Serum IgA from wt, JDTA heterozygous, and JDTA mice were separated on a nondenaturating polyacrylamide gel and detected by immunoblotting.
To further establish that the DTA transgene was active and deleted J chain–expressing B cells in vivo we purified serum IgA from wt, heterozygous JDTA, and JDTA. After separation on nondenaturing polyacrylamide gels the expression of dimeric and monomeric IgA was analyzed (Fig. 2 B). While the expression of dimeric IgA was dominating in sera from wt mice, essentially no dimeric IgA was detected in sera from JDTA heterozygous mice indicating a complete deletion of J chain–expressing B cells. JDTA serum also contained only monomeric IgA as expected since both alleles of the J chain locus are inactivated in these animals. We conclude from these data that the presence of a DTA gene on one allele suffice for the elimination of J chain–expressing B cells in vivo.
JDTA Mice Have Perturbed Serum Ig Levels.
It has been claimed that all plasma cells in the mouse express J chain irrespective of which Ig isotype is expressed 30 31. However, upon analysis it was evident that JDTA mice still had significant levels of serum Ig (Fig. 3 A). The IgM and IgG serum levels of JDTA mice were found to be reduced six and eight times respectively when compared with wt controls, while the serum IgA level was upregulated 14-fold in JDTA mice.
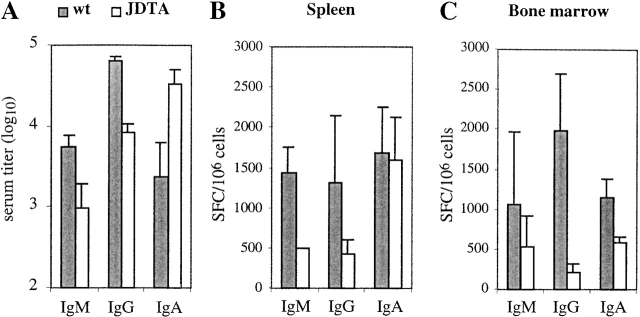
Figure 3.
Perturbed Ig production in the JDTA mice. (A) Serum IgM, IgG, and IgA levels in wt and JDTA mice at the age of 4 mo (n = 5). Number of Ig-secreting cells per 106 cells from wt and JDTA spleen (B) and bone marrow (C) as detected by ELISPOT assay (n = 3). Data shown from one out of three experiments giving similar results. SFC, spot-forming cell.
We also analyzed Ig secretion in bone marrow and spleen from JDTA mice at the single cell level using ELISPOT (Fig. 3 B and C) The number of IgM- and IgG-secreting cells per 106 lymphocytes in both organs were reduced in JDTA mice, as expected from the serum Ig levels. On the other hand, the number of IgA-secreting cells per 106 lymphocytes was reduced in bone marrow but normal in spleen when compared with wt controls. Taking into account the increase in spleen size in JDTA spleen (wt: 80 ± 24; JDTA: 114 ± 23, all numbers ×106 cells, n = 12), the total number of IgA-secreting cells per spleen was in fact increased in JDTA mice compared with wt while IgM- and IgG-secreting cells were reduced. More importantly, since there were significant numbers of Ig-secreting cells present in both spleen and bone marrow of JDTA mice, we conclude that there exist populations of Ig-secreting cells that have not activated the J chain locus. Finally, we also analyzed the various B cell populations in spleen and bone marrow using FACS® without finding significant alterations of a distinct B cell subset (data not shown).
J Chain-Negative, Secretory B Cells Are Readily Detected in wt Mice.
A key observation in this study is that Ig-secreting B cells either express the J chain locus or not. It could be argued that in the genetically manipulated mice, a strong selection pressure for rare populations of B cells was established that is not paralleled in normal animals. Therefore, we established a PCR analysis to detect the ratio between the expression of RNA for secretory IgM (μs) over the membrane form (μm) as an independent marker for secretory B cells. Fig. 4 A shows the outcome of this assay using a pre-B cell line, a B cell lymphoma cell line, and LPS-stimulated spleen cells. In the clonal cell lines the μm/μs ratio was close to 1, while in LPS-stimulated spleen RNA the μs RNA is dominating due to the presence of IgM-secreting plasma cells. It should be noted that J chain expression could easily be detected in both the pre-B and the B cell lymphoma cell line, correlating with the situation seen in cell lines representing early stages of human B cell development 32 33 34. Then we proceeded to analyze the correlation between dominant μs RNA expression and J chain expression in single, LPS-stimulated cells from the spleen of C57BL/6 mice. Fig. 4 B summarizes the results obtained from 38 single B cells. While 26 cells showed an abundance of μs expression, only 13 of these were positive for J chain mRNA (Fig. 4 C show examples of the raw data obtained). Thus, we conclude that also in wt mice B cells with a secretory phenotype that do not express the J chain locus can readily be detected and may constitute up to 50% of this cellular compartment.
Figure 4.
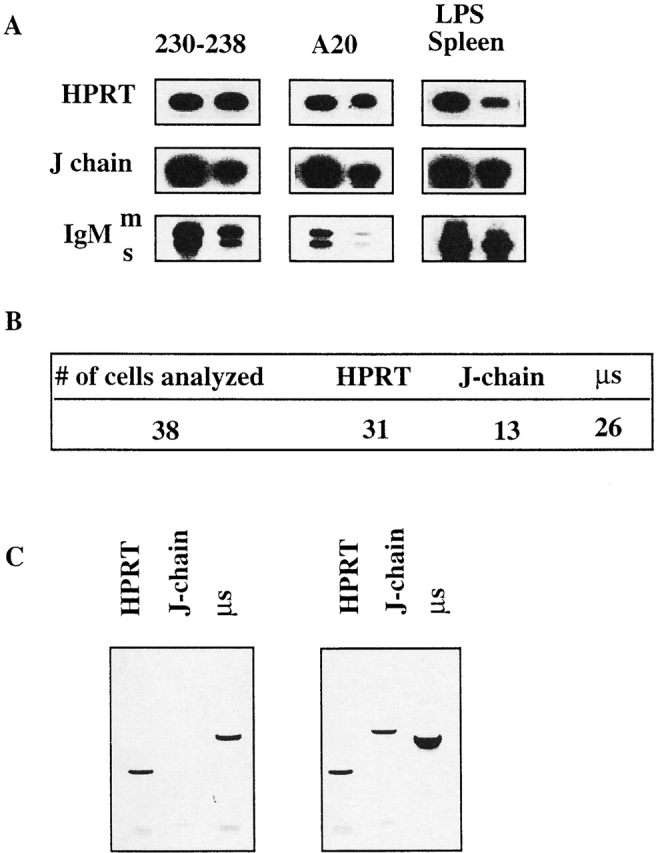
Single cell analysis of J chain expression in secretory B cells from wt mice. (A) RT-PCR analysis of J chain and secretory versus membrane bound IgM expression in two different murine cell lines and in LPS-stimulated total splenocytes representing different differential stages of B cell development. 230–238 represents a pre-B, A20 represents a mature B cell, and the LPS-stimulated total spleen represents the plasma cell stage. (B) Summary of single cell RT-PCR analysis of J chain and secretory IgM transcripts in LPS-stimulated splenic B cells. (C) Example of experimental data showing one example of a single cell expressing only secretory IgM and of a single cell expressing both J chain and secretory IgM. HPRT is used as a cDNA control.
Introduction of a Distinct Coding Region in the J Chain Locus Results in a Novel Phenotype.
As a control for the JDTA experiments we would also like to determine that targeting a distinct open reading frame into the J chain locus would result in a mouse strain that showed a distinct phenotype from JDTA and J−/− mice. Hence, we made a DNA construct based on the same principle as for constructing JDTA mice, but where, instead of the DTA gene, a cDNA for the c-myc oncogene was inserted (Fig. 5 A). ES cell clones were obtained at a frequency of 1/40 and one clone was used to generate JcMycneo mice, which had a J−/− phenotype (data not shown). To enable cMyc expression the JcMycneo mice were bred to mice constitutively expressing the Cre enzyme 42, enabling in vivo excision of the neomycin cassette. JcMyc mice were identified by Southern blot analysis on DNA from tail biopsies and backcrossed to C57BL/6 mice (Fig. 5 A). The expression of the cMyc gene from its position in the J chain locus was analyzed using a similar approach as for the DTA gene in JDTA mice above. As shown in Fig. 5 B, expression of the exogenous cMyc gene was readily detectable in both heterozygous JcMyc mice and JcMyc mice. Litter sizes of the JcMyc mice were normal and the offspring displayed a normal Mendelian distribution of wt, heterozygous, and homozygous mice. The cell numbers of the JcMyc spleen as well as the bone marrow were found to be normal when compared with wt littermates (data not shown). JcMyc spleen cell populations as well as bone marrow, thymus, and peritoneal cell populations were analyzed using FACS® and no significant differences when comparing to wt littermates was detected (data not shown).
With regard to the levels of serum Ig in JcMyc mice, both the total serum Ig (data not shown) and serum IgG levels in JcMyc mice were normal when compared with wt littermate controls (Fig. 5 C). The serum IgM levels of JcMyc mice were found to be equal to those observed in wt controls, in contrast to the situation in J−/− mice 25, while the serum IgA levels in the JcMyc mice was elevated about sevenfold compared with wt (Fig. 5 C). We also performed ELISPOT analysis on IgM-, IgA-, and IgG-secreting cells in bone marrow and spleen from JcMyc mice comparing them to wt littermates (Fig. 5 C). The number of IgM- and IgG- secreting cells per 106 lymphocytes in both spleen and bone marrow were normal, as expected from the serum Ig. The number of IgA-secreting cells in the JcMyc bone marrow was close to wt levels, while the number of IgA-secreting cells in the spleen was increased fivefold. We conclude from this experiment that the introduction of a distinct coding sequence into the J chain locus results in the generation of a mouse strain with a distinct phenotype monitored at the level of Ig secretion.
Mice Devoid of J Chain–expressing Cells Are Partially Immune Compromised.
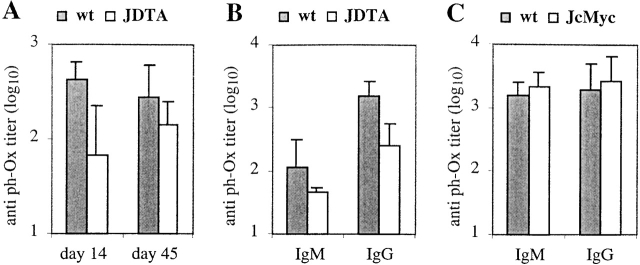
So far we had only investigated the levels of background Ig but we also wanted to analyze the humoral immune response in mice that can only produce J chain-negative plasma cells. To this end, we immunized JDTA mice with the T cell–dependent antigen 2-phenyl-5-oxazolone coupled to OVA (phOx-OVA). Serum was taken 14 and 45 d after primary immunization. As shown in Fig. 6 A, the levels of total phOx-specific antibodies were reduced about sixfold at day 14 of the primary response to this antigen, while only twofold at day 45 of the response. Fig. 6 B shows the levels of phOx-specific IgM and IgG antibodies at day 14, and both were reduced. We conclude from these data that JDTA mice are immune competent although immune compromised especially at early time points during the immune response.
Figure 6.
Humoral immune response in JDTA and JcMyc mice. (A) wt and JDTA mice were immunized with 100 μg of phOx-OVA intraperitoneally (n = 5) at the age of 4 mo. Sera were collected at day 14 and day 45 after immunization and total anti-phOx–specific antibodies were analyzed by ELISA. Data shown from one out of two experiments giving similar results. (B and C) wt, JDTA (B), and JcMyc (C) mice were immunized with 100 μg of phOx-OVA intraperitoneally (n = 5) at the age of 4 mo. Sera were collected at day 14 after immunization and IgM and IgG anti-phOx–specific antibodies were analyzed by ELISA. Data shown from one out of two experiments giving similar results.
To validate the finding in JDTA mice we also immunized JcMyc mice with phOx-OVA and analyzed serum anti-phOx antibodies at day 14 after immunization. The immune response in the JcMyc mice was quite distinct from that seen in JDTA mice. Hence, the level of phOx-specific IgM and IgG antibodies were similar, or even slightly increased, compared with wt levels (Fig. 6 C).
Next we analyzed if the JDTA mice were immune competent in the LP of the gut mucosa. After three oral immunizations with CT we analyzed the specific CT response in JDTA and wt mice (Table ). As the J−/− mice, the JDTA mice had undetectable levels of anti-CT IgA in gut lavage, which was expected since they cannot transport IgA across the mucosal surface due to the absence of J chain 24 27. When analyzing the anti-CT total Ig or anti-CT IgA contents in serum we found total Ig to be unaltered and anti-CT IgA near normal or slightly increased when compared with wt (Table ). Furthermore, detection of anti-CT–specific antibody-producing cells in the LP of immunized JDTA mice showed a 75% reduction in numbers of anti-CT IgA-secreting cells when compared with wt controls. Thus, the introduction of an exogenous gene into the J chain locus will qualitatively and quantitatively alter the immune response to a defined antigen.
Table 1.
Anti-CT Titers in Lavage and Serum, and Numbers of Anti-CT–specific Secreting Cells in LP from Immunized wt and JDTA Mice
| Genotye | Lavageanti-CT IgA | Serumanti-CT total Ig | Serumanti-CT IgA | Anti-CTIgA-secreting cells |
|---|---|---|---|---|
| Log10 titer | Log10 titer | Log10 titer | ||
| wt | 2.5 ± 0.2 | 4.7 ± 0.1 | 3.7 ± 0.4 | 16,000 ± 4,770 |
| JDTA | <1.0 | 4.5 ± 0.1 | 4.1 ± 0.2 | 3,792 ± 617 |
Commitment to J Chain Expression Is an Early Event during B Cell Differentiation.
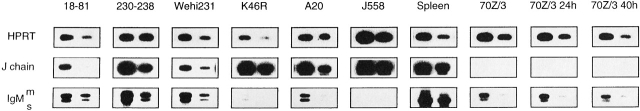
Our data indicates that once B cells are committed to secrete Ig at a high rate, they either express J chain or not. However, it cannot be excluded that J chain expression is a clonal property decided before the B lymphocyte reaches the terminal developmental stage of high Ig secretion. Evidence in favor of early commitment to Ig secretion can be found in the observation that some human cell lines and normal cells representing early stages of B cell development have been shown to express the J chain locus 32 33 34 35. We confirmed this finding using a panel of mouse B cell lines and RT-PCR (Fig. 7). The plasmacytoma J558 and total spleen cells expressed high levels of J chain RNA as expected. However, the B cell lymphomas WEHI231, A20, and K46R also expressed detectable levels of J chain RNA, as did the Abelson transformed pre-B cell lines 18–81 and 230–238. Interestingly, J chain transcripts were undetectable in the pre-B cell line 70Z/3 both before and after stimulation with LPS.
Figure 7.
RT-PCR analysis of J chain expression in mouse cell lines representing various stages of B cell differentiation. RT-PCR analysis was performed as described previously. The 70Z/3 cell line was analyzed both unstimulated but also after 24 and 40 h stimulation with LPS which triggers its differentiation to a surface Ig+ phenotype, as indicated.
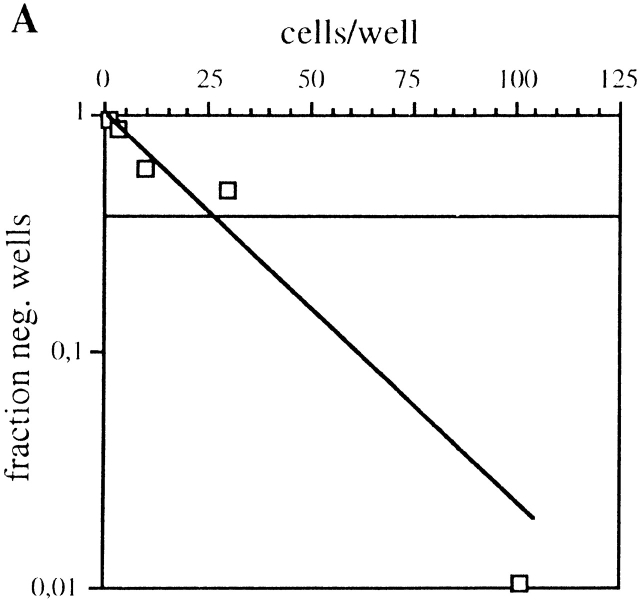
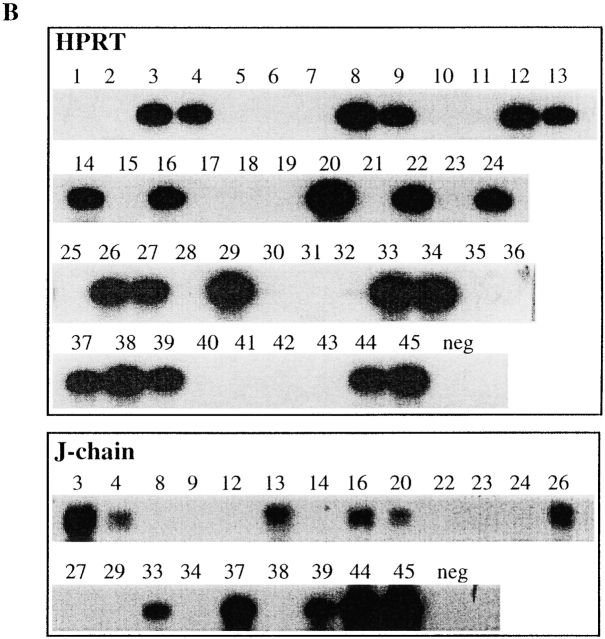
To expand this analysis to untransformed B cells we performed limiting dilution analysis of LPS-stimulated C57BL/6 splenic lymphocytes (Fig. 8). To reduce background in the subsequent PCR analysis the limiting dilution assay was performed in the absence of filler cells, and only 1 out of 25 cells plated gave rise to an Ig-positive well under our experimental conditions (Fig. 8 A). Individual wells that were Ig-positive and derived from a single precursor clone according to the Poisson distribution, were subsequently analyzed for HPRT and J chain mRNA expression using RT-PCR (Fig. 8 B). Under these experimental conditions, HPRT mRNA could be detected in ∼50% of the Ig+ wells but only between 20–50% of these HPRT-positive wells were also positive for J chain mRNA (Table ). No wells were found that were positive for J chain RNA and did not express HPRT mRNA. We conclude from this experiment that J chain expression is a clonal property of peripheral B lymphocytes already before they are committed to Ig secretion.
Figure 8.
Limiting dilution analysis on LPS stimulated wt C57BL/6 splenic lymphocytes at day 7. (A) Fraction of Ig-negative wells plotted against number of cells seeded per well. The intercept between the 0.37 fraction of negative wells and the linear distribution of the samples gives the limit of clonality, according to the Poisson distribution. One representative plot out of three experiments is shown. (B) Expression analysis by RT-PCR for HPRT and J chain on Ig-positive wells.
Table 2.
| Frequency J+ | Percentage J+ | |
|---|---|---|
| Exp. 1 | 5/24 | 21 |
| Exp. 2 | 11/21 | 52 |
| Exp. 3 | 3/15 | 20 |
One representative experiment out of three is shown. The frequency of J chain–expressing clones is from three independent experiments. Exp., experiment.
Discussion
The goal with the current investigation was to directly address the heterogeneity of plasma cell populations in mice using J chain expression as a marker. Hence, while it has been claimed, and widely accepted, that all plasma cells in the mouse express the J chain locus 30 31 detailed studies have been hampered by the absence of robust serological reagents for detection of J chain protein. In humans such reagents exist and have been used to correlate J chain expression and plasma cell differentiation. Thus, it has been shown in human systems that there was a positive correlation between the production of polymeric IgA and the presence of cytoplasmic J chain; tissues that produced predominantly monomeric IgA generally displayed fewer cells containing cytoplasmic J chain 37 40. Furthermore, tissues associated with mucosal surfaces provide a greater proportion of polymeric IgA than other lymphoid tissues, whereas bone marrow, spleen, and lymph nodes produce mostly monomeric IgA 37 39 40. In addition, it has been shown that 50–70% of the IgG-secreting cells in human glandular tissue or in activated germinal centers are J chain-positive 39 48. In contrast, only a small percentage of the IgG- secreting cells in extra follicular sites or in chronic foci of inflammation contain detectable J chain 39 48. In studies of IgA M components it was noted that the most common ratio of J chain over IgA was 0, rather than the value of 0.5 expected from dimeric IgA 49; a finding that also would implicate that myelomas may arise in J chain-negative and in J chain-positive plasma cells.
We show that mice where J chain–expressing B cells are deleted still have serum Ig and can mount specific humoral immune responses to T cell–dependent antigens. Although clearly immune compromised the phenotype of JDTA mice was not dramatic. We would explain this by considering the complexity of the regulatory circuits involving serum Ig 5 50. On the other hand, we are well aware of that our experimental approach is highly dependent on the reliable expression of DTA and subsequent deletion of the proper cells. To verify this we detected DTA mRNA expression in LPS-stimulated cells from JDTA mice originating from the genetically altered J chain locus. Furthermore, the functional deletion properties of the DTA-containing J chain locus was verified by the selective deletion of hybridomas generated from JDTA mice which was gene-dosage dependent, and the finding that in serum from mice heterozygous for the DTA transgene no dimeric IgA could be detected. As a further verification we integrated a c-myc cDNA sequence into the J chain locus and generated a separate mouse strain. These mice had a subtle but easily detectable phenotype distinct from JDTA mice. All in all, we consider that our experimental system has been properly validated and can be used as a reliable base for our conclusions.
One major conclusion from this study is that plasma cells can be separated into two subsets based on J chain expression. This finding also has bearing on the generation of serum IgA. While being mostly associated with mucosal immune responses, IgA is also a common serum Ig isotype and the bone marrow of humans contain high numbers of IgA plasma cells 37 40 51. In addition, the bulk of serum IgA in humans is monomeric 37 51, while there seems to be a 1:1 relationship between monomeric and dimeric IgA in mouse serum 52. One model to explain this distribution would be that IgA-producing plasma cells are unable to efficiently assemble dimers and hence the same cell that produce dimeric IgA would also secrete a significant amount of monomeric IgA. Since only J chain–containing, dimeric IgA would be transported over the mucosal lining 24 53, the monomeric IgA would as a consequence accumulate in the serum. The experiments presented herein excludes that this model is exclusive 40, and conclusively show that the production of monomeric and dimeric IgA can be clonally distributed. Especially compelling in this context was the finding that serum IgA levels were even elevated in JDTA mice, while the gut immune response was compromised. That some IgA-secreting cells in normal mice produce monomeric and dimeric IgA carrying the same paratope cannot be formally excluded by our studies. However, it should be remembered that in humans the α2 heavy chain gene seems to be used preferentially in dimeric IgA, while the α1 heavy chain gene dominates in monomeric serum IgA (51, 54). This finding would support the notion of a clonal separation of the production of these two IgA forms that in turn seems to correlate with J chain expression.
The elevation of serum IgA observed in J−/− mice, JDTA mice, and in JcMyc mice merits discussion (for a summary of the phenotypes see Table ). The conclusion drawn from the situation in J−/− mice, that the high serum IgA levels was a consequence of impaired polyIgR-mediated transport of IgA in the absence of J chain 24 can now be extended. Part of this phenomenon could also be due to a compensatory mechanism for the reduced serum IgM levels in these mice. The same population of B cells/plasma cells seems to be responsible for the upkeep of both serum IgM and serum IgA levels 55 56, and it can be envisioned that the low IgM levels would trigger an increased IgA production from the same subset via unknown homeostatic mechanisms 5 50. In addition, it has been shown that the IgM detected by ELISA in these mice is of heterogeneous stoichiometry 25. This observation, taken together with the accentuated IgA production seen in JcMyc mice where the IgM level is normal, would indicate that what is regulated is the level of pentameric (or potentially hexameric) IgM. These interesting questions with regard to regulation of serum Ig homeostasis can now be further explored using the experimental models described herein. With regard to the other Ig isotypes, the reduced IgM level in JDTA mice was similar to that observed in J−/− mice 25, while serum IgM levels were at wt levels in JcMyc mice. This would indicate that the expression of c-myc from its position in the J chain locus positively selects these plasma cells and compensates for the reduced IgM levels seen in J−/− mice. In addition, IgG levels were also reduced in JDTA mice, indicating that a significant amount of serum IgG in normal mice is generated from the J chain–expressing B cell linage. Hence, the notion that commitment to J chain expression is independent of Ig heavy chain isotype expression was confirmed 30 57.
Table 3.
Summary of Mouse Strains with Disrupted J Chain Locus
| JDTA | JcMyc | J−/− | |
|---|---|---|---|
| Serum Ig | − | +/− | +/− |
| Serum IgM | − | +/− | − |
| Serum IgG | − | +/− | +/− |
| Serum IgA | ++ | ++ | + |
| LP SFC | − | ND | +/− |
| BM SFC | − | +/− | − |
| Spleen SFC | − | + | − |
| Systemic IR | − | + | +/− |
| Gut | − | ND | − |
+, increased; −, decreased; +/−; unchanged; IR, immune response; and SFC, spot-forming cell.
The humoral immune response (IgM and IgG) seen in JDTA mice after immunization with a T cell–dependent antigen was significantly reduced at day 14 after immunization. However, at day 45 a significant level of serum antibodies specific for the antigen could be detected. In separate studies we also immunized the JDTA mice with a T cell–independent antigen and obtained similar results (data not shown). As a comparison, the IgM and IgG immune response seen in the JcMyc mice was similar to wt controls at day 14. From these results one may speculate that J chain–expressing plasma cells could be more prominent in Ig production during the early phases of the immune response (day 14), while the long-lasting Ig production seen at later stages might be more influenced by J chain-negative plasma cells 11 12 15.
From the same line of reasoning it would also follow that long-term antibody secretion, and hence immunity dependent on persisting antibody on mucosal surfaces, would be hard to achieve in the gut where the effector plasma cells by necessity must be J chain producing 24. Supporting this is the finding in JDTA mice immunized orally with CT where the serum anti-CT IgA levels were nearly normal, while the number of anti-CT IgA-secreting cells in the LP of the intestine was reduced. Only ∼25% of the anti-CT IgA-secreting cells were left in the LP of JDTA mice. In contrast, the same experiment made in J−/− mice showed that the number of anti-CT IgA-secreting cells in the LP was normal (27, Table III).
The last question asked relates to the timing of commitment to J chain expression. It has been claimed that J chain transcription was an exclusive marker for plasma cell differentiation 30 31, but in later studies J chain RNA has been detected in early B cells in humans 35. We also show here that this is true using transformed mouse B cell lines. To confirm our conclusion concerning the presence of J chain–expressing and –nonexpressing plasma cells we performed single cell PCR analysis of LPS-stimulated B cells from normal C57BL/6 mice. We here used the shift of IgM transcript to the secretory form as an independent control for commitment to secretion. From this experiment it was evident that among IgM-expressing B cells there is a significant fraction that does not express the J chain locus. It should also be noted that monomeric IgM, in contrast to IgA, is never detected in serum of healthy individuals due to regulation during the assembly process, again hinting towards a critical function for pentameric/hexameric IgM 58. Thus, J chain-negative plasma cells could be generated in normal individuals without the presence of IgM monomers in serum. Whether the hexameric IgM observed in serum 59 60 is produced by J chain-negative plasma cells remains an open question.
We further extended our analysis to the clonal level of splenic B cells using a limiting dilution approach. Using this analysis we could show that J chain expression was a clonal property of single splenic B cells, indicating that the commitment to J chain expression is already established in LPS-reactive, splenic B cells. This somewhat surprising result is compatible with earlier observations where J chain RNA expression has been detected in human pre-B cells 35. It also brings into question the mechanism for activation/silencing of the J chain locus and indicates that this might be an early event during B cell differentiation. It should here be remembered that the J chain promoter region resembles Ig light chain promoters 61, and that a similar molecular machinery as that used for allelic exclusion of the Ig loci might also operate on the J chain locus.
In summary, we have shown that two different B cell lineages can be defined with regard to their ability to express J chain. The functional differences between these two B cell populations remains to be analyzed in detail but one may speculate that longevity, response to different signals, and homing properties might differ. The rational for the existence of these two B cell lineages is unclear. It is tempting to speculate that J chain–expressing, Ig-secreting cells were imperative early in evolution when local immune responses to protect the host against microbes in the digestive tract was the dominant feature of the immune system. Indeed, J chain expression has been detected in evolution before the presence of Ig 36, and it can be speculated that Ig was not the first molecule to be transported by the J chain/polyIg receptor system. As evolution generated more refined, multicellular organisms systemic immunity and serum Ig started to play a role and it became pertinent that not all Ig produced was transported out onto mucosal surfaces. A stochastic mechanism that generates a certain fraction of Ig-producing cells unable to produce J chain would be a mechanism to ensure this balance.
Acknowledgments
We thank Eva Miller for her expert technical assistance, Dr. Reinhard Fassler for help with blastocyst injections, and Drs. F. Ivars and W. Agace for critical reading of the manuscript.
This study was supported by the Swedish Cancer Society, the Swedish Medical Research Council, and The Swedish Strategic Foundation.
Footnotes
Abbreviations used in this paper: CT, Cholera toxin; DTA, Diphtheria toxin A chain; ELISPOT, enzyme-linked immunospot; ES, embryonic stem; HRP, horseradish peroxidase; J chain, Joining chain; LP, lamina propria; RT, reverse transcription; wt, wild-type.
References
- Avrameas S. Natural autoantibodiesfrom “horror autotoxicus” to “gnothi seauton”. Immunol. Today. 1991;5:154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- Boes M., Prodeus A.P., Schmidt T., Carroll M.C., Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 1998;12:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Kazatchkine M.D., Avrameas S. Natural autoantibodies. Curr. Opin. Immunol. 1995;6:812–818. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Varela F., Andersson A., Dietrich G., Sundblad A., Holmberg D., Kazatchkine M., Coutinho A. Population dynamics of natural antibodies in normal and autoimmune individuals. Proc. Natl. Acad. Sci. USA. 1991;13:5917–5921. doi: 10.1073/pnas.88.13.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agenes F., Freitas A.A. Transfer of small resting B cells into immunodeficient hosts results in the selection of a self-renewing activated B cell population. J. Exp. Med. 1999;189:319–330. doi: 10.1084/jem.189.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C. The development of B cells and the B-cell repertoire in the microenvironment of the germinal center. Immunol. Rev. 1992;126:5–19. doi: 10.1111/j.1600-065x.1992.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Nossal G.J. The molecular and cellular basis of affinity maturation in the antibody response. Cell. 1992;68:1–2. doi: 10.1016/0092-8674(92)90198-l. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R.M., Bachmann M.F., Kundig T.M., Oehen S., Pirchet H., Hengartner H. On immunological memory. Annu. Rev. Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- Hibi T., Dosch H.M. Limiting dilution analysis of the B cell compartment in human bone marrow. Eur. J. Immunol. 1986;16:139–145. doi: 10.1002/eji.1830160206. [DOI] [PubMed] [Google Scholar]
- Benner R., Hijmans W., Haaijman J.J. The bone marrowthe major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin. Exp. Immunol. 1981;46:1–8. [PMC free article] [PubMed] [Google Scholar]
- Ho F., Lortan J.E., MacLennan I.C., Khan M. Distinct short-lived and long-lived antibody-producing cell populations. Eur. J. Immunol. 1986;16:1297–1301. doi: 10.1002/eji.1830161018. [DOI] [PubMed] [Google Scholar]
- Manz R.A., Thiel A., Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- MacLennan I.C., Liu Y.J., Johnson G.D. Maturation and dispersal of B-cell clones during T cell-dependent antibody responses. Immunol. Rev. 1992;126:143–161. doi: 10.1111/j.1600-065x.1992.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Tew J.G., Kosco M.H., Burton G.F., Szakal A.K. Follicular dendritic cells as accessory cells. Immunol. Rev. 1990;117:185–211. doi: 10.1111/j.1600-065x.1990.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Slifka M.K., Antia R., Whitmire J.K., Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- Slifka M.K., Matloubian M., Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 1995;69:1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka M.K., Ahmed R. Long-term antibody production is sustained by antibody-secreting cells in the bone marrow following acute viral infection. Ann. NY. Acad. Sci. 1996;797:166–176. doi: 10.1111/j.1749-6632.1996.tb52958.x. [DOI] [PubMed] [Google Scholar]
- Randall T.D., Brewer J.W., Corley R.B. Direct evidence that J chain regulates the polymeric structure of IgM in antibody-secreting B cells. J. Biol. Chem. 1992;267:18002–18007. [PubMed] [Google Scholar]
- Mestecky J., McGhee J.R. Immunoglobulin A (IgA)molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv. Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Cattaneo A., Neuberger M.S. Polymeric immunoglobulin M is secreted by transfectants of non-lymphoid cells in the absence of immunoglobulin J chain. EMBO J. 1987;6:2753–2758. doi: 10.1002/j.1460-2075.1987.tb02569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Role of J chain and secretory component in receptor-mediated glandular and hepatic transport of immunoglobulins in man. Scand. J. Immunol. 1985;22:111–146. doi: 10.1111/j.1365-3083.1985.tb01866.x. [DOI] [PubMed] [Google Scholar]
- Jerry L.M., Kunkel H.G., Adams L. Stabilization of dissociable IgA2 proteins by secretory component. J. Immunol. 1972;109:275–283. [PubMed] [Google Scholar]
- Orlans E., Peppard J., Fry J.F., Hinton R.H., Mullock B.M. Secretory component as the receptor for polymeric IgA on rat hepatocytes. J. Exp. Med. 1979;150:1577–1581. doi: 10.1084/jem.150.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson B.A., Conner D.A., Ladd D.J., Kendall D., Casanova J.E., Corthesy B., Max E.E., Neutra M.R., Seidman C.E., Seidman J.G. Altered hepatic transport of immunoglobulin A in mice lacking the J chain. J. Exp. Med. 1995;182:1905–1911. doi: 10.1084/jem.182.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsson L., Andersson K., Sigvardsson M., Lycke N., Leanderson T. Mice with an inactivated joining chain locus have perturbed IgM secretion. Eur. J. Immunol. 1998;28:2355–2365. doi: 10.1002/(SICI)1521-4141(199808)28:08<2355::AID-IMMU2355>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Hendrickson B.A., Rindisbacher L., Corthesy B., Kendall D., Waltz D.A., Neutra M.R., Seidman J.G. Lack of association of secretory component with IgA in J chain-deficient mice. J. Immunol. 1996;157:750–754. [PubMed] [Google Scholar]
- Lycke N., Erlandsson L., Ekman L., Schon K., Leanderson T. Lack of J chain inhibits the transport of gut IgA and abrogates the development of intestinal antitoxic protection. J. Immunol. 1999;163:913–919. [PubMed] [Google Scholar]
- Shimada S., Kawaguchi-Miyashita M., Kushiro A., Sato T., Nanno M., Sako T., Matsuoka Y., Sudo K., Tagawa Y., Iwakura Y., Ohwaki M. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J. Immunol. 1999;163:5367–5373. [PubMed] [Google Scholar]
- Johansen F.E., Pekna M., Norderhaug I.N., Haneberg B., Hietala M.A., Krajci P., Betsholtz C., Brandtzaeg P. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 1999;190:915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland M.E. The coming of age of the immunoglobulin J chain. Annu. Rev. Immunol. 1985;3:425–453. doi: 10.1146/annurev.iy.03.040185.002233. [DOI] [PubMed] [Google Scholar]
- Mather E.L., Alt F.W., Bothwell A.L., Baltimore D., Koshland M.E. Expression of J chain RNA in cell lines representing different stages of B lymphocyte differentiation. Cell. 1981;23:369–378. doi: 10.1016/0092-8674(81)90132-x. [DOI] [PubMed] [Google Scholar]
- McCune J.M., Fu S.M., Kunkel H.G. J chain biosynthesis in pre-B cells and other possible precursor B cells. J. Exp. Med. 1981;154:138–145. doi: 10.1084/jem.154.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E.E., Korsmeyer S.J. Human J chain gene. Structure and expression in B lymphoid cells. J. Exp. Med. 1985;161:832–849. doi: 10.1084/jem.161.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu I., Moldoveanu Z., Cooper M.D., Mestecky J. Ultrastructural studies of human lymphoid cells. Mu and J chain expression as a function of B cell differentiation. J. Exp. Med. 1983;158:1993–2006. doi: 10.1084/jem.158.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand F.E., III, Billips L.G., Gartland G.L., Kubagawa H., Schroeder H.W., Jr. The J chain gene is transcribed during B and T lymphopoiesis in humans. J. Immunol. 1996;156:4240–4244. [PubMed] [Google Scholar]
- Takahashi T., Iwase T., Takenouchi N., Saito M., Kobayashi K., Moldoveanu Z., Mestecky J., Moro I. The joining (J) chain is present in invertebrates that do not express immunoglobulins. Proc. Natl. Acad. Sci. USA. 1996;93:1886–1891. doi: 10.1073/pnas.93.5.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutteh W.H., Prince S.J., Mestecky J. Tissue origins of human polymeric and monomeric IgA. J. Immunol. 1982;128:990–995. [PubMed] [Google Scholar]
- Haber P.L., Mestecky J. J-chain expression in human cells producing IgG subclasses. Cell. Immunol. 1985;91:515–519. doi: 10.1016/0008-8749(85)90249-7. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Immunohistochemical characterization of intracellular J-chain and binding site for secretory component (SC) in human immunoglobulin (Ig)-producing cells. Mol. Immunol. 1983;20:941–966. doi: 10.1016/0161-5890(83)90036-6. [DOI] [PubMed] [Google Scholar]
- Tarkowski A., Moldoveanu Z., Koopman W.J., Radl J., Haaijman J.J., Mestecky J. Cellular origins of human polymeric and monomeric IgAenumeration of single cells secreting polymeric IgA1 and IgA2 in peripheral blood, bone marrow, spleen, gingiva and synovial tissue. Clin. Exp. Immunol. 1991;85:341–348. doi: 10.1111/j.1365-2249.1991.tb05730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R.D., Behringer R.R., Quaife C.J., Maxwell F., Maxwell I.H., Brinster R.L. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987;50:435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- Schwenk F., Baron U., Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucl. Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C.O., Ealding W., Lefkowitz J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J. Immunol. Methods. 1984;67:101–108. doi: 10.1016/0022-1759(84)90089-9. [DOI] [PubMed] [Google Scholar]
- Maxwell I.H., Glode L.M., Maxwell F. Expression of diphtheria toxin A-chain in mature B-cellsa potential approach to therapy of B-lymphoid malignancy. Leuk. Lymphoma. 1992;7:457–462. doi: 10.3109/10428199209049802. [DOI] [PubMed] [Google Scholar]
- Maxwell I.H., Maxwell F., Glode L.M. Regulated expression of a diphtheria toxin A-chain gene transfected into human cellspossible strategy for inducing cancer cell suicide. Cancer Res. 1986;46:4660–4664. [PubMed] [Google Scholar]
- Ross S.R., Graves R.A., Spiegelman B.M. Targeted expression of a toxin gene to adipose tissuetransgenic mice resistant to obesity. Genes Dev. 1993;7:1318–1324. doi: 10.1101/gad.7.7b.1318. [DOI] [PubMed] [Google Scholar]
- Breitman M.L., Clapoff S., Rossant J., Tsui L.C., Glode L.M., Maxwell I.H., Bernstein A. Genetic ablationtargeted expression of a toxin gene causes microphthalmia in transgenic mice. Science. 1987;238:1563–1655. doi: 10.1126/science.3685993. [DOI] [PubMed] [Google Scholar]
- Korsrud F.R., Brandtzaeg P. Immunohistochemical evaluation of J-chain expression by intra- and extra-follicular immunoglobulin-producing human tonsillar cells. Scand. J. Immunol. 1981;13:271–280. doi: 10.1111/j.1365-3083.1981.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Grubb A.O. Quantitation of J chain in human biological fluids by a simple immunochemical procedure. Acta. Med. Scand. 1978;204:453–465. doi: 10.1111/j.0954-6820.1978.tb08473.x. [DOI] [PubMed] [Google Scholar]
- Agenes F., Rosado M.M., Freitas A.A. Independent homeostatic regulation of B cell compartments. Eur. J. Immunol. 1997;27:1801–1807. doi: 10.1002/eji.1830270731. [DOI] [PubMed] [Google Scholar]
- Mestecky J. Immunobiology of IgA. Am. J. Kidney Dis. 1988;12:378–383. doi: 10.1016/s0272-6386(88)80029-5. [DOI] [PubMed] [Google Scholar]
- Delacroix D.L., Malburny G.N., Vaerman J.P. Hepatobiliary transport of plasma IgA in the mousecontribution to clearance of intravascular IgA. Eur. J. Immunol. 1985;15:893–899. doi: 10.1002/eji.1830150906. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Transport models for secretory IgA and secretory IgM. Clin. Exp. Immunol. 1981;44:221–232. [PMC free article] [PubMed] [Google Scholar]
- Russell M.W., Kilian M. Biological activities of IgA Ogra P.L., Strober W., Mestecky J., McGhee J.R., Lamm M.E., Bienenstock J. Mucosal Immunology 1999. 225 235 Academic Press, Inc; San Diego, CA: pp [Google Scholar]
- Herzenberg L.A. B-1 cellsthe lineage question revisited. Immunol. Rev. 2000;175:9–22. [PubMed] [Google Scholar]
- Kroese F.G., Butcher E.C., Stall A.M., Lalor P.A., Adams S., Herzenberg L.A. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int. Immunol. 1989;1:75–84. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Winchester R.J., Hoffman T., Kunkel H.G. Parallel synthesis of immunoglobulins and J chain in pokeweed mitogen-stimulated normal cells and in lymphoblastoid cell lines. J. Exp. Med. 1977;145:760–765. doi: 10.1084/jem.145.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Neuberger M., Alberini C., Bet P., Fra A., Valetti C., Williams G., Milstein C. Developmental regulation of IgM secretionthe role of the carboxy-terminal cysteine. Cell. 1990;60:781–790. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Randall T.D., King L.B., Corley R.B. The biological effects of IgM hexamer formation. Eur. J. Immunol. 1990;20:1971–1979. doi: 10.1002/eji.1830200915. [DOI] [PubMed] [Google Scholar]
- Parkhouse R.M., Askonas B.A., Dourmashkin R.R. Electron microscopic studies of mouse immunoglobulin M; structure and reconstitution following reduction. Immunology. 1970;18:575–584. [PMC free article] [PubMed] [Google Scholar]
- Sigvardsson M., Olsson L., Hogbom E., Leanderson T. Characterization of the joining chain (J-chain) promoter. Scand. J. Immunol. 1993;38:411–416. doi: 10.1111/j.1365-3083.1993.tb02581.x. [DOI] [PubMed] [Google Scholar]