Abstract
In this study, we examined the immunogenic properties of the Leishmania infantum acidic ribosomal protein P0 (LiP0) in the BALB/c mouse model. The humoral and cellular responses induced by the administration of the LiP0 antigen, either as soluble recombinant LiP0 (rLiP0) or as a plasmid DNA formulation (pcDNA3-LiP0), were determined. Also, the immunological response associated with a prime-boost strategy, consisting of immunization with pcDNA3-LiP0 followed by a boost with rLiP0, was assayed. Immunization with rLiP0 induced a predominant Th2-like humoral response, but no anti-LiP0 antibodies were induced after immunization with pcDNA3-LiP0, whereas a strong humoral response consisting of a mixed immunoglobulin G2a (IgG2a)-IgG1 isotype profile was induced in mice immunized with the prime-boost regime. For all three immunization protocols, rLiP0-stimulated production of gamma interferon (IFN-γ) in both splenocytes and lymph node cells from immunized mice was observed. However, it was only when mice were immunized with pcDNA3-LiP0 that noticeable protection against L. major infection was achieved, as determined by both lesion development and parasite burden. Immunization of mice with LiP0-DNA primes both CD4+ and CD8+ T cells, which, with the L. major challenge, were boosted to produce significant levels of IL-12-dependent, antigen-specific IFN-γ. Taken together, these data indicate that genetic vaccination with LiP0 induces protective immunological effector mechanisms, yet the immunological response elicited by LiP0 is not sufficient to keep the infection from progressing.
Protozoa of the genus Leishmania are obligate intracellular parasites that infect cells of the mononuclear phagocyte lineage of their vertebrate hosts. These parasites are the etiological agents of leishmaniasis, a group of diseases characterized by a variety of clinical manifestations in humans, ranging from self-healing cutaneous ulcers to potentially fatal visceral infection (13). The development of the disease and the spread of the infection vary greatly from individual to individual, depending on the genetic background and the status of the immune system. The genetic predisposition for susceptibility or resistance is best illustrated by mouse Leishmania major infection (see reference 27 for a recent review). Most mouse genotypes control L. major infection; however, certain strains (such as BALB/c) develop progressive lesions and systemic disease. An interleukin-12 (IL-12)-driven gamma interferon (IFN-γ)-dominated Th1 response is associated with resistance to infection. In contrast, susceptible BALB/c mice show an IL-4-driven Th2 response.
Although there is evidence of acquired immunity and resistance to reinfection in natural Leishmania hosts, suggesting that a vaccine is feasible, there are no available vaccines against leishmaniasis (12). Several antigens have been used in experimental vaccination trials in murine leishmaniasis, achieving various levels of protection (references 18 and 27 and references therein). Genetic vaccination is a particularly appealing approach for generating protective responses against infectious diseases that require long-term cellular immunity, such as tuberculosis, malaria, or leishmaniasis (9, 16). In mouse models, DNA immunization diverts the immune response from Th2 to Th1 cell dominance. The usefulness of this approach is illustrated by considering, for example, the protective immunity generated by immunization with DNA encoding LACK (Leishmania homologue of receptor for activated C kinase). During L. major infection of susceptible mice, LACK antigen drives the early production of IL-4 from a specific population of CD4+ T cells, and IL-4 promotes the outgrowth of Th2 T cells and disease progression. Depletion of LACK-reactive T cells diminishes early IL-4 production, allowing the development of a protective Th1 response (14). Interestingly, immunization of susceptible BALB/c mice using LACK DNA induces protection against L. major (10). Control of disease progression in mice vaccinated with LACK DNA was associated with the enhancement of IL-12-dependent production of IFN-γ. Thus, immunization of mice with DNA-delivered LACK antigen promoted protection by redirecting the T-cell response away from the pathogenic IL-4 response toward a protective Th1 response. However, although LACK is highly conserved, the efficacy of this vaccine antigen seems to be restricted to the L. major-BALB/c infection model (20). Therefore, characterization of new Leishmania antigens and optimization of vaccination strategies are needed to develop potent and durable vaccines against the different forms of leishmaniasis and for different hosts. The ideal vaccine would be a pan-Leishmania vaccine including several molecules, preferably conserved among different species. In this regard, it is worthwhile to mention recent studies showing that cocktail and multicomponent DNA vaccines induce solid protection against both cutaneous and visceral leishmaniasis (3, 19, 21, 24).
In the present study, we examined the immunogenic properties of the Leishmania infantum acidic ribosomal protein P0 (LiP0). This protein was described as an immunodominant antigen recognized by sera from both patients and animals infected with Leishmania chagasi-L. infantum (30, 33). In addition, antibodies against the Plasmodium falciparum P0 phosphoprotein were detected extensively in malaria-immune persons, but not in malaria patients (5). Interestingly, antibodies against ribosomal P0 were found to protect mice against experimental infection with Plasmodium yoelii (6). In this study, we analyzed the induction of a protective immune response in BALB/c mice following several regimes of LiP0 delivery. Among them, genetic vaccination with plasmid DNA encoding LiP0 conferred partial protection against L. major infection in susceptible mice.
MATERIALS AND METHODS
Mice and parasites.
Female 6- to 8-week-old BALB/c mice were purchased from Harlan Interfauna Ibérica S.A. (Barcelona, Spain). L. major (WHOM/IR/-173) was a kind gift of R. M. Gonzalo (Centro Nacional de Biotecnología, Madrid, Spain). Promastigotes were cultured at 26°C in Schneider's medium (Gibco/BRL) supplemented with 20% heat-inactivated fetal calf serum (FCS). The parasites were kept in a virulent state by passage in BALB/c mice. L. major amastigotes were obtained from popliteal lymph nodes and cultured in Schneider's medium. For challenge, promastigotes were harvested at late stationary phase.
Plasmid constructs.
The L. infantum P0 coding sequence was PCR amplified, taking as a template the λEMBL-3 clone L27g-5, containing the L. infantum P0 gene cluster (32). The following oligonucleotides were used: sense, 5′-TCCATATGCCGTCTATCACCACTGC-3′ (positions 1 to 20 of the coding region); antisense, 5′-GGAATTCTTAGAAGAGACCG CCCATGC-3′ (reverse and complementary to positions 949 to 969 of the coding region). The underlined sequences are not present in the P0 gene. First, the amplification product was cloned in the SmaI site of the pBluescript plasmid (the resulting clone was named pBls-LiP0). The pBls-LiP0 insert was obtained by BamHI-EcoRV double digestion and subcloned in the corresponding sites of the eukaryotic expression plasmid pcDNA3 (Invitrogen, San Diego, Calif.) to produce clone pcDNA3-LiP0. Endotoxin-free plasmid DNA was isolated using the EndoFree Plasmid Giga kit (Qiagen, Hilden, Germany).
For expression of L. infantum P0 as a recombinant protein, the coding region was PCR amplified, taking as a template the pcDNA3-LiP0 clone with the following oligonucleotides as primers: sense, 5′-CGGGATCCATGTCCACCAAG TACCTCGC-3′ (positions 1 to 20 of the coding region); antisense, 5′-CCCAAGCTTA GAAGAGACCGCCCATGC-3′ (reverse and complementary to positions 949 to 969 of the coding region). The underlined sequences correspond to the BamHI and HindIII restriction sites, respectively, included for cloning purposes. Thus, the PCR product was double digested with BamHI and HindIII and was subsequently ligated into the pQE30 expression vector (Qiagen). The resulting clone was named pQE-LiP0.
Expression of pcDNA3-LiP0 in COS cells.
To confirm that the DNA construct was functional, COS7 cells were transfected with 20 μg of the pcDNA3-LiP0 plasmid using the Lipofectin reagent (Gibco/BRL) according to the manufacturer's protocol. Briefly, 3 × 106 cells were seeded on 100-mm-diameter plates in Dulbecco's modified Eagle's medium supplemented with 5% FCS until they reached 50 to 75% confluence. Seventy-two hours posttransfection, the cells were harvested, washed two times with ice-cold phosphate-buffered saline (PBS), and immediately lysed by the addition of Laemmli's buffer (15). The proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Amersham, Aylesbury, United Kingdom). The blots were probed with mouse antiserum raised against L. infantum LiP0 by immunization with the recombinant LiP0 protein.
Leishmanial antigens.
Recombinant LiP0 protein (rLiP0) was produced in pQE-LiP0-transformed Escherichia coli M15 (Qiagen) following standard procedures. After induction, the bacteria were harvested, lysed by sonication under denaturing conditions (8 M urea, 0.5 M NaCl, 20 mM Tris-HCl, pH 8.0), and loaded onto an Ni-nitrilotriacetic acid agarose column (Qiagen). The bound protein was gradually refolded on the affinity column as described previously (28). Afterwards, the bound protein was eluted with 0.3 M imidazol. The fractions containing the proteins, as determined by SDS-PAGE, were pooled and dialyzed against PBS. The recombinant protein was further passed through a polymyxin-agarose column (Sigma, St. Louis, Mo.) to eliminate endotoxins. The lipopolysaccharide content was measured by the Quantitative Chromogenic Limulus Amebocyte Assay QCL-1000 (BioWhittaker, Walkersville, Md.), showing that rLiP0 preparations were essentially free of endotoxin (<12 pg/μg of recombinant protein).
Total proteins of L. major were prepared from promastigotes by three cycles of freezing and thawing. After cell lysis, soluble proteins (soluble Leishmania antigen [SLA]) were separated from the insoluble fraction by centrifugation.
Immunizations and parasite challenge.
Immunization experiments were carried out in groups of 10 mice. Five micrograms of rLiP0 were injected twice intradermally (i.d.) at the base of the tail in a volume of 50 μl, while control mice received PBS alone. In DNA immunization experiments, mice were inoculated twice intramuscularly (i.m.) in both quadriceps with 100 μg of DNA (50 μg per leg) of either pcDNA3-LiP0 or pcDNA3 (controls) in a total volume of 100 μl of PBS. When “prime-boost” immunization was carried out, two inoculations of DNA and two inoculations of recombinant protein were administered. In all groups, the mice were inoculated at 2-week intervals. Also, 7 days after each inoculation, the mice were bled by orbital plexus puncture. Two weeks after the final inoculation, spleens and lymph nodes from five immunized mice per group were collected. Another five immunized mice per group were challenged with 5 × 104 stationary-phase promastigotes of L. major that were suspended in 50 μl of PBS and injected into the left hind footpad. The progress of infection was followed by measuring the footpad thickness with a metric caliper. The contralateral footpad of each animal injected with PBS represented the control value, and the swelling was calculated as follows: thickness of the left footpad − thickness of the right footpad. The animals were euthanized when the lesions became necrotic.
Determination of antibody titers and isotypes.
Specific antibody responses were measured by conventional enzyme-linked immunoadsorbent assay (ELISA). Briefly, standard ELISA plates were coated overnight at room temperature with 100 μl of rLiP0 (2 μg/ml in PBS) or SLA (2 μg/ml in PBS). A serial dilution of the sera was carried out in order to determine the titer, which is defined as the inverse of the highest serum dilution factor giving an absorbance of >0.2. The titers for immunoglobulin classes and immunoglobulin G (IgG) isotypes were determined using the following horseradish peroxidase-conjugated secondary antibodies (Nordic Immunological Laboratories, Tilburg, The Netherlands): goat anti-mouse IgG (1:1,000 and 1:2,000 dilutions), goat anti-mouse IgM (1:1,000 and 1:2,000 dilutions), goat anti-mouse IgG1 (1:1,000 and 1:2,000 dilutions), and goat anti-mouse IgG2a (1:500 and 1:1,000 dilutions). Ortophenylenediamine dihydrochloride (Dako A/S, Glostrup, Denmark) was used as a peroxidase substrate. After 15 min, the reaction was stopped by the addition of 100 μl of 1 M H2SO4, and the absorbance was read at 450 nm.
Lymphoproliferation assays.
Spleens and lymph nodes from BALB/c mice were removed aseptically. Lymph node cell (LNC) and spleen cell suspensions were prepared in complete RPMI medium (RPMI 1640 supplemented with 10% FCS, 2 mM glutamine, and 3 × 10−5 M 2-mercaptoethanol). Cells were plated at 2 × 106 per ml (final volume, 200 μl) in 96-well flat-bottom plates in the presence of various concentrations of rLiP0, 2 μg of lipopolysaccharide (Sigma)/ml, or 2 μg of concanavalin A (Sigma)/ml. The cell cultures were incubated for 72 h at 37°C in a humidified chamber containing 5% CO2. The cultures were pulsed with 1 μCi of [methyl-3H]thymidine (5 Ci/mmol; Amersham) for the final 16 h of culture. The pulsed cultures were harvested on filters using an automated multiple-sample harvester and dried. Incorporation of [methyl-3H]thymidine was determined by liquid scintillation counting.
Fluorescence-activated cell sorter analyses.
All reagents and conjugated monoclonal antibodies (MAbs) for fluorescence-activated cell sorter analyses were purchased from PharMingen (San Diego, Calif.). For analysis of the frequency of T-cell-producing IFN-γ by intracellular staining, pooled LNCs at 2 × 106/ml were cultured in flat-bottom 24-well plates and stimulated with rLiP0 (12 μg/ml) for 72 h at 37°C in 5% CO2. After incubation, the Golgi Stop reagent was added, and the cells were cultured for an additional 6 h. Afterwards, the cells were harvested, washed twice in PBS with 1% FCS, and stained with either phycoerythrin-conjugated rat anti-mouse CD4 MAb (GK1.5) or phycoerythrin-conjugated rat anti-mouse CD8 MAb (53-6.7) for 30 min on ice. The cells were then washed twice and fixed for 20 min in Cytofix/Cytoperm buffer. Next, the cells were washed twice in Cytowash buffer and incubated with fluorescein isothiocyanate-conjugated rat anti-mouse IFN-γ (XGM1.2) for 30 min at 4°C. Finally, the cells were washed twice and analyzed on a FACSCalibur flow cytometer. The specificity of the anti-cytokine MAb was confirmed by both negative staining of nonpermeabilized cells and a fluorescein isothiocyanate-conjugated isotype-matched control (R3-34).
Measurement of cytokines in culture supernatants.
Cytokine production was determined by commercial ELISA kits (Endogen, Woburn, Mass.). The release of IFN-γ and IL-4 was measured in the supernatants of splenocytes and LNC cultures (5 × 106 cells/ml) seeded in 24-well plates for 72 h at 37°C in the presence of rLiP0 (12 μg/ml) or concanavalin A (2 μg/ml). In parallel, splenocytes stimulated with rLiP0 were incubated in the presence of 10 μg of MAb against either mouse CD4 (GK 1.5), mouse IL-12 (C17.8), or mouse CD8 (53-6.7)/ml. Appropriate isotype-matched controls were also analyzed in the assay. The antibodies (no azide-low endotoxin) were purchased from PharMingen.
Evaluation of parasite burden.
The numbers of parasites in infected popliteal lymph nodes were quantified by limiting dilution (2). Briefly, each node was homogenized and serially diluted in a 96-well flat-bottom microtiter plate with Schneider's medium containing 20% FCS. The number of viable parasites per lymph node was determined from the highest dilution at which promastigotes could be grown after 7 days of incubation at 26°C.
Histological analysis.
For histopathological studies, specimens from the footpads of mice were fixed in formalin, embedded in paraffin, cut in 4-μm-thick sections, and stained with hematoxylin and eosin. Slide-mounted sections were investigated by light microscopy for granuloma formations and inflammatory-cell compounds in the dermis. Segments of epidermis were also examined for ulceration of the epithelium.
Statistical analysis.
Statistical analysis was performed by a Student's t test. Differences were considered significant when P was <0.05.
RESULTS
Immunogenicity of Leishmania LiP0 in BALB/c mice.
The immune responses to LiP0 were evaluated in BALB/c mice after administration of the protein either as soluble recombinant protein (rLiP0) or in a plasmid DNA format (pcDNA3-LiP0). Groups of 10 BALB/c mice were inoculated with either 5 μg of rLiP0 (i.d. route) or 100 μg of pcDNA3-LiP0 (i.m. route) on days 0 and 14. Soluble rLiP0, administered in PBS, elicited a substantial humoral response (Fig. 1) showing features of a conventional secondary response with a progressive switch from IgM to IgG antibodies (data not shown). A preponderance of IgG1 versus IgG2a was also patent (Fig. 1A), suggesting that rLiP0 elicits a Th2-type response. The relative production of IgG1 and IgG2a isotypes is used as a marker for the induction of Th2-type and Th1-type immune responses, respectively (7). In contrast, no antibodies against the LiP0 protein were detected in sera from mice inoculated with plasmid pcDNA3-LiP0. The possibility that the lack of humoral response in mice inoculated with pcDNA3-LiP0 was due to a deficient expression of this construct in mammalian cells must be excluded. As shown in Fig. 1B and C, COS cells transfected with pcDNA3-LiP0 produced high levels of Leishmania LiP0 that could be clearly detected by Western blotting. The experiment also showed that the protein produced by COS cells has the same size as the L. major P0 protein and that the elicited antibodies in rLiP0-inoculated mice are specific against the Leishmania protein.
FIG. 1.
Humoral response induced in BALB/c mice after rLiP0 inoculation. (A) The anti-rLiP0 reactivities of sera from 10 mice per group were individually assayed by ELISA 7 days after the first inoculation or 7 days after the second inoculation with rLiP0. The open bars correspond to mice inoculated with rLiP0 protein alone, whereas the hatched bars correspond to LiP0-DNA-primed mice boosted with rLiP0. Titers were determined for the IgG class and for IgG1 and IgG2a isotypes. Results are expressed as the mean ± standard deviation. None of the preimmune sera showed reactivity against the protein. (B) Coomassie blue-stained SDS-12% PAGE gel containing 1 μg of rLiP0 (lane 1), lysate from COS cells (lane 2), COS cells transfected with pcDNA3-LiP0 antigen (lane 3), and total proteins from L. major promastigotes (lane 4). (C) A gel equivalent to that in panel B was blotted and incubated with a pool of 10 sera from rLiP0-immunized mice at a final dilution of 1:200.
Since the prime-boost strategy has been shown to improve the immunogenicity of DNA inoculations (25), we decided to first inoculate an additional group of mice with pcDNA3-LiP0 and afterward to boost them with rLiP0. Thus, the mice were inoculated with plasmid DNA (100 μg by the i.m. route) on days 0 and 14, and subsequently, the mice received 5 μg of rLiP0 by the i.d. route on days 28 and 42. Indeed, mice primed with the plasmid DNA and boosted with rLiP0 developed higher titers of anti-LiP0 antibodies than did mice receiving only the soluble protein (Fig. 1A). After the first boost with LiP0, the anti-P0 IgG2a isotype was found to dominate over the IgG1 isotype. However, after the second boost, a mixed IgG1-IgG2a phenotype was observed. A likely interpretation of these observations is that immunization with the LiP0 gene primes a Th1-type response and that the posterior inoculation of the protein changed the response to a mixed Th1-Th2 pattern.
To investigate the cellular response elicited by immunization with LiP0 in each of the three formulations, splenocytes and LNCs were obtained 2 weeks after the last inoculation from five mice of each group. Suspensions of cells were stimulated in vitro with rLiP0, and the proliferative and cytokine levels were measured (Fig. 2). Lymphoproliferation above that of control mice was induced by rLiP0 in mice immunized with soluble rLiP0 and mainly in LiP0-DNA-primed and rLiP0-boosted mice; it was particularly evident in rLiP0-stimulated LNCs (Fig. 2A). It should be noted that rLiP0 has the capacity to induce proliferation of both spleen cells and LNCs from control mice. Preliminary data indicated that rLiP0 exerts a proliferative effect on B cells from naïve mice (data not shown).
FIG. 2.
Proliferation and cytokine production by LNCs and splenocytes from mice inoculated with LiP0 in different formulations. The immunization groups were as follows: rLiP0, mice inoculated with soluble rLiP0; LiP0-DNA, mice inoculated with pcDNA3-LiP0 DNA; LiP0-DNA + rLiP0, mice primed with pcDNA3-LiP0 DNA and boosted with rLiP0; Control, mice inoculated with PBS. LNCs (A) or splenocytes (B) at 4 × 105/well were incubated for 72 h in the presence of the indicated concentrations of rLiP0 and pulsed with 1 μCi of [methyl-3H]thymidine for the last 16 h. Cells from five mice per group were pooled. Each bar represents the mean ± standard deviation (SD) of triplicate wells. For IFN-γ determination, either LNCs (C) or splenocytes (D) (5 × 106) were cultured in the presence or absence of rLiP0 (12 μg/ml) for 72 h. Afterwards, the supernatants were harvested and assayed by ELISA for IFN-γ. The results are expressed as the mean ± SD. The experiment was repeated with similar results.
Concomitantly, cells from mice inoculated by the prime-boost regime secreted higher levels of IFN-γ than those from the other groups in response to in vitro stimulation with rLiP0 (Fig. 2C and D). For all immunization groups, the amounts of IFN-γ produced by both splenocytes and LNCs were larger than those induced in cells from naïve mice. In contrast, IL-4 was detected at low levels in the supernatants of both splenocyte and LNC cultures (data not shown). For most of the immunization groups, no increase in IL-4 production was observed after stimulation with rLiP0. However, cultured LNCs from LiP0-DNA-primed and rLiP0-boosted mice spontaneously secreted twofold more IL-4 than LNCs from the other groups.
Immunization with pcDNA3-LiP0 protects BALB/c mice against L. major infection.
In order to assess the protective capacity of LiP0, half of the mice included in each of the immunization groups (see above) were challenged with L. major promastigotes 14 days after the last inoculation. Contrary to our expectations, considering the high levels of IFN-γ produced by splenocytes and LNCs (Fig. 2), mice primed with LiP0-DNA and boosted with rLiP0 showed lesion development similar to that of the control groups (mice inoculated with either the empty vector pcDNA3 or PBS). Also, rLiP0-immunized mice were not protected against L. major infection. Instead, mice immunized with naked DNA containing the LiP0 gene experienced a delay in footpad swelling (Fig. 3A).
FIG.3.
Evaluation of protection against infection with L. major in BALB/c mice immunized with pcDNA3-LiP0. (A) Mice were immunized and boosted 2 weeks later with pcDNA3-LiP0, pcDNA3, or PBS. Also, a group was immunized two times with pcDNA3-LiP0 and boosted two times with rLiP0 (pcDNA3-LiP0 + rLiP0). Two weeks after the boost, the mice were challenged in the left hind footpad with 5 × 104 L. major promastigotes. Footpad swelling is given as the difference between the thicknesses of the infected and contralateral uninfected footpads. Weekly footpad measurements represent the average footpad scores ± standard deviations (SD). The data shown are representative of two different experiments with virtually the same results. The group marked with an asterisk showed significant reduction (P < 0.001) in lesion size compared to unvaccinated (PBS) and control-vaccinated (pcDNA3) mice. (B) Quantification of parasite loads at various times after infection. Cell suspensions were made from the popliteal lymph node of the infected leg, and the number of viable parasites was determined by limiting dilution. The results are expressed as means ± SD of the total number of parasites per popliteal lymph node (expressed as a decimal logarithm) from three mice. (*P < 0.05) (C to F) Histological studies showing the inflammatory responses in footpad lesions from mice vaccinated with either pcDNA3 (C and E) or pcDNA3-LiP0 (D and F) 3 (C and D) or 5 (E and F) weeks after L. major challenge. Preparations were stained with hematoxylin-eosin. The arrows point to areas of inflammation. The inset in each panel is an amplified image in which the mononuclear infiltrates with macrophages infected with L. major amastigotes are shown. Magnifications: panels, ×20; insets, ×400.
In order to determine more accurately the degree of protection against L. major infection induced by LiP0-DNA vaccination, we repeated the immunization and challenge of mice to monitor whether the course of disease as assessed by footpad swelling was correlated with the parasite burden. Thus, mice immunized with pcDNA3-LiP0 and the corresponding controls (immunized with pcDNA3) were sacrificed serially in weeks 3, 5, and 8 after L. major challenge. The parasite burden in the popliteal lymph node from the infected leg was determined by a limiting-dilution assay (Fig. 3B). Three weeks after challenge, the parasite burden was found to be significantly lower in mice vaccinated with pcDNA3-LiP0 than in controls (P < 0.05). Mice vaccinated with LiP0-DNA had a 99.1% reduction in the parasite burden 3 weeks after infection compared with mice vaccinated with control DNA. However, the number of parasites increased progressively in pcDNA3-LiP0-vaccinated mice, and in week 5 postinfection, even though the LiP0-DNA-vaccinated mice had a parasite burden 84.8% lower than that of control mice, the difference was not statistically significant.
Histological studies of footpads also corroborated the fact that immunization with pcDNA3-LiP0 induced a substantial delay in the development of pathological alterations caused by L. major infection. Thus, 3 weeks after challenge, the L. major-infected footpads of mice vaccinated with pcDNA3 showed a strong granulomatous inflammation in the deep dermis and numerous heavily parasite-infected macrophages (Fig. 3C). However, histological preparations of the footpads of mice vaccinated with pcDNA3-LiP0 after 3 weeks of L. major infection showed normal architecture; only a few inflammatory cells were observed, and infected macrophages were scarce (Fig. 3D). After 5 weeks of infection, the difference between pcDNA3- and pcDNA3-LiP0-immunized mice persisted. Although inflammation was observed in both groups, the granulomatous area was more extensive in the footpads of control mice (Fig. 3E) than in the footpads of pcDNA3-LiP0-immunized mice (Fig. 3F). Also, the number of infected macrophages was larger in control than LiP0-DNA immunized mice. These data demonstrate that immunization with pcDNA3-LiP0 induced a delay in the progression of the L. major infection.
Analysis of the immune response in mice vaccinated with pcDNA3-LiP0 and challenged with L. major.
Humoral and cellular responses against LiP0 antigen were analyzed in mice infected with L. major in order to determine the immunological parameters associated with the delay in lesion development observed in pcDNA3-LiP0-vaccinated mice. For this purpose, the spleen and draining lymph nodes were aseptically removed at various times after L. major infection. Splenocytes were stimulated in vitro with either rLiP0 or L. major SLA, and after 3 days of incubation, the presence of IFN-γ and IL-4 in the supernatants was assessed. As shown in Fig. 4A, splenocytes from mice vaccinated with pcDNA3-LiP0 made significantly more rLiP0-specific IFN-γ than splenocytes from the control mice. However, the production of IFN-γ by splenocytes decreased with time postinfection, suggesting that L. major infection exerts inhibitory effects on cells producing this cytokine. Interestingly, a transient increase in SLA-specific production of IFN-γ was observed 5 weeks postinfection in pcDNA3-LiP0-vaccinated mice. In contrast, IL-4 production was not stimulated by rLiP0 in any of the immunization groups, but remarkably, SLA-specific induction of IL-4 increased with the course of L. major infection and was higher in control mice than in mice vaccinated with pcDNA3-LiP0 (Fig. 4B).
FIG. 4.
Cytokine production in splenocytes from vaccinated mice during L. major infection. Splenocytes (5 × 106/ml) from either pcDNA3- or pcDNA3-LiP0-vaccinated mice (five per group) were cultured either without stimulus (none) or in the presence of rLiP0 (12 μg/ml) or SLA (24 μg/ml) for 72 h. The supernatants were harvested and assayed by ELISA for both IFN-γ (A) and IL-4 (B).
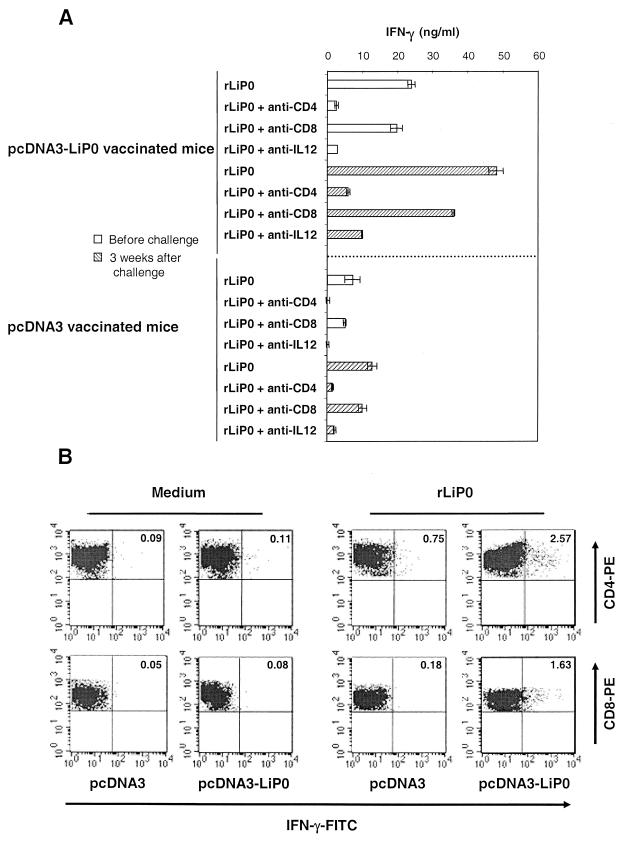
As shown in Fig. 5A, the LiP0-specific production of IFN-γ in splenocyte cultures from pcDNA3-LiP0-vaccinated mice increased after L. major challenge, suggesting that infection induces a boost in LiP0-primed cells. The contributions of CD4+ and CD8+ T cells to the production of IFN-γ and the dependence on IL-12 were also analyzed before and after challenge. In both situations, before and after challenge, the rLiP0-specific production of IFN-γ by splenocytes from pcDNA3-LiP0-vaccinated mice was found to be IL-12 dependent and largely inhibited by anti-CD4 antibodies (Fig. 5A). Interestingly, it was observed that the contribution of CD8+ cells to the specific production of IFN-γ by splenocytes from pcDNA3-LiP0-vaccinated mice increased after challenge, suggesting that L. major infection activated the rLiP0-primed CD8+ T cells. These findings could be an indication that LiP0-specific CD8+ T cells are responsible, in part, for the protection of mice vaccinated with pcDNA3-LiP0. As a confirmatory experiment, the frequencies of LiP0-specific CD4+ and CD8+ IFN-γ-producing T cells in LNCs from both pcDNA3- and pcDNA3-LiP0-vaccinated mice were determined 3 weeks after L. major infection (Fig. 5B). In agreement with the data on IFN-γ production in culture supernatants (Fig. 5A), the percentages of both CD4+ and CD8+ T cells producing LiP0-specific IFN-γ were larger in LiP0-DNA-vaccinated mice than in control mice. In fact, the frequency of CD8+ IFN-γ-producing T cells increased only after L. major challenge in mice vaccinated with pcDNA3-LiP0.
FIG.5.
rLiP0-specific production of IFN-γ in vaccinated mice after infection with L. major. (A) Two weeks after the second immunization with either pcDNA3-LiP0 or pcDNA3 (control) and 3 weeks after L. major challenge of both immunization groups, splenocytes were pooled and stimulated with rLiP0 alone or with rLiP0 in the presence of either anti-IL-12, anti-CD8, or anti-CD4 MAb. The specific production of IFN-γ was determined in the supernatants after 72 h of incubation. The results are expressed as means ± standard deviations. (B) Frequencies of rLiP0-specific LNCs producing IFN-γ from control (pcDNA3) and vaccinated (pcDNA3-LiP0) mice. The frequencies of CD4+ and CD8+ T cells were assessed in pooled LNCs derived from mice 3 weeks postinfection. LNCs (2 × 106/ml) were stimulated with rLiP0 (12 μg/ml) for 72 h. Afterwards, Golgi Stop solution was added, and the cultures were incubated for six additional hours. Finally, the cells were harvested and processed for intracellular IFN-γ and the presence of either CD4+ or CD8+ surface marker. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
DISCUSSION
Leishmanial mechanisms of virulence have been proposed to involve two different groups of parasite molecules (4). One group consists of surface and secretory products that are necessary for the establishment of infection as a prerequisite for virulence but that themselves do not cause disease. The second group of parasite molecules consists of highly conserved, intracellular molecules referred to as “pathoantigens.” Immune response against these antigens is thought to result in immunopathology manifested as clinical symptoms, namely, the virulence phenotype. As a conclusion, the model suggests that different parasite determinants may be targeted by different strategies to achieve more effective control of leishmaniasis. In fact, there are several examples of Leishmania pathoantigens that have been shown to confer protective immunity on mice infected with L. major: LACK (10), LeIF (31), cysteine proteinases (24), LmSTI1, and TSA (3).
In this study, we have analyzed the immunogenic properties of Leishmania LiP0, a ribosomal protein that possesses all of the features needed to be considered a pathoantigen (4, 26). Thus, BALB/c mice were immunized with this antigen administered either as a recombinant protein or as a DNA vaccine. Also, a combined strategy of priming with LiP0-DNA and boosting with rLiP0 was employed. A substantial humoral response was induced after immunization with rLiP0, whereas anti-LiP0 antibodies were not observed in sera from pcDNA3-LiP0-vaccinated mice. However, both immunization forms activate the cellular immune response to similar extents, as determined by lymphoproliferation assays and LiP0-specific production of IFN-γ. Interestingly, the prime-boost strategy elicited humoral and cellular responses fivefold higher than those observed after immunization with rLiP0 (Fig. 1 and 2). Also, the prime-boost strategy induced an isotype profile of anti-P0 antibodies different from that observed after immunization with protein alone. While immunization with rLiP0 generated mainly antibodies of the IgG1 isotype, priming with LiP0-DNA and boosting with the rLiP0 protein elicited similar levels of IgG1 and IgG2a isotypes. In summary, it can be concluded that DNA vaccination with the LiP0 gene induced a pure Th1-type response consisting of antigen-specific production of IFN-γ without induction of antigen-specific antibodies. This finding was expected, since it has been demonstrated that DNA vaccines have the ability to preferentially generate Th1 responses in mouse models (9). On the other hand, immunization with LiP0-DNA followed by an rLiP0 boost induced a mixed Th1-Th2 response, as deduced from the presence in the sera from vaccinated mice of similar levels of IgG1 and IgG2a antibodies against Leishmania P0. Finally, immunization with rLiP0 seems to induce a predominant Th2 response, since the anti-P0 antibodies elicited were mainly of the IgG1 isotype.
The immune response observed correlated well with the protection experiments. Thus, it was only when Leishmania P0 was injected in the DNA formulation that protection against L. major challenge was achieved. In contrast, vaccination either with the rLiP0 protein or following a prime-boost strategy (immunization with LiP0-DNA followed by an rLiP0 boost) did not result in protection upon challenge with live parasites. It is now well accepted that the susceptibility to infection that characterizes mice of the BALB/c strain results from the development of a Th2 antibody-mediated immune response (reviewed in reference 17). Therefore, the lack of protection against L. major infection in rLiP0-vaccinated mice can be explained by the induction by the soluble protein of high titers of specific IgG1 antibodies. However, we have not found an easy explanation for the lack of protection showed by the mice immunized following a prime-boost strategy, taking into account the facts that splenocytes and LNCs from these mice, when stimulated in vitro with rLiP0, produced high levels of IFN-γ and that this vaccination procedure stimulated a humoral response consisting of high titers of both IgG1 and IgG2a isotypes. In a recent work, Rafati et al. (24) described a protective vaccine against murine cutaneous leishmaniasis consisting of the injection of DNA encoding the cysteine proteinases CPa and CPb of L. major followed by a boost with the recombinant proteins. Analysis of the immune response showed that protected mice developed a specific Th1 immune response, which was associated with an increase in IFN-γ production and substantially higher levels of CP-specific IgG2a antibodies than of IgG1-isotype antibodies. Therefore, based on the differences in IgG isotypes, we speculate that the lack of protection against infection after vaccination with LiP0 following the prime-boost regime could be related to the stimulation of a consistent anti-P0 IgG1 antibody response. In addition, it was observed that cultures of LNCs from mice primed with pcDNA3-LiP0 and boosted with rLiP0 spontaneously secrete twofold more IL-4 than either pcDNA3-LiP0- or rLiP0-vaccinated mice (data not shown), suggesting that the prime-boost regime also induces CD4+ Th2 cells. Sjölander and coworkers (29) have indeed also suggested that a Th1 response is sufficient to protect against cutaneous leishmaniasis but that the induction of a simultaneous Th2 response abrogates the Th1 function. These authors found that immunization of mice with parasite Ag-2 antigen in immune-response-stimulating complexes generated an immune response with mixed Th1 and Th2 properties that was not protective despite the activation of large numbers of CD4+ T cells secreting IFN-γ.
DNA vaccination of BALB/c mice with LiP0 generated an initial significant reduction in lesion size after challenge; however, the mice ultimately developed nonhealing lesions. The delay in the onset of lesion growth in LiP0-DNA-vaccinated mice was accompanied by a substantial decrease in the parasite load and a more controlled inflammatory response (Fig. 3). Also, the immunological analyses performed after L. major infection indicated that protective immunological effector mechanisms were activated at challenge but that they were not sufficient to keep the infection from progressing. Thus, we have demonstrated that after L. major infection, LiP0-DNA-vaccinated mice developed an initial IgG2a antibody response against LiP0 that changed to a mixed IgG1-IgG2a profile in week 8 postinfection (data not shown). In addition, splenocytes from LiP0-DNA-vaccinated mice secreted smaller amounts of IL-4 after in vitro stimulation with SLA than control mice (Fig. 4B). Also, the ability of splenocytes from pcDNA3-LiP0-vaccinated mice to produce rLiP0-specific IFN-γ significantly increased after L. major challenge (Fig. 5A). In these studies, it was found that LiP0-DNA vaccination induces cells producing IFN-γ from both CD4+ and CD8+ T cells in mice infected 2 weeks after vaccination, whereas IFN-γ derives mainly from CD4+ T cells in mice vaccinated with control DNA (pcDNA3). This finding was further corroborated by the analysis of frequencies of LiP0-specific CD4+ and CD8+ IFN-γ-producing T cells, as determined using intracellular cytokine staining (Fig. 5B). These data demonstrate that vaccination with pcDNA3-LiP0 primes CD8+ T cells that have the ability to produce LiP0-specific IFN-γ in response to L. major infection. Several studies have shown that CD8+ T cells have an important role in mediating immunity against L. major (1, 11, 22). Altogether, our data indicate that genetic immunization with Leishmania P0 antigen induces a protective immunological status in mice but that its strength is not enough to achieve total protection of mice from a high challenge dose of a highly virulent strain like that used in this study (23). In summary, the evidence presented here shows that LiP0 is a potential vaccine candidate when administered in a DNA formulation. We think that inclusion of this antigen as a component of a multiantigen vaccine, a strategy that has been demonstrated to be effective against murine cutaneous leishmaniasis (3, 8, 24), will contribute to the development of an effective vaccine against Leishmania.
Acknowledgments
This work was supported by grant BIO2002-04049-C02-C1 from The Spanish Ministerio de Ciencia y Tecnología. An institutional grant and direct support for the project from Fundación Ramón Areces are acknowledged. E.F. was supported by the Ramón y Cajal Program.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Belkaid, Y., E. Von Stebut, S. Mendez, R. Lira, E. Caler, S. Bertholet, M. C. Udey, and D. Sacks. 2002. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J. Immunol. 168:3992-4000. [DOI] [PubMed] [Google Scholar]
- 2.Buffet, P. A., A. Sulahian, Y. J. Garin, N. Nassar, and F. Derouin,. 1995. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob. Agents Chemother. 39:2167-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos-Neto, A., J. R. Webb, K. Greeson, R. N. Coler, Y. A. W. Skeiky, and S. G. Reed. 2002. Vaccination with plasmid DNA encoding TSA/LmSTI1 leishmanial fusion proteins confers protection against Leishmania major infection in susceptible BALB/c mice. Infect. Immun. 70:2828-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, K. P., S. G. Reed, B. S. McGwire, and L. Soong. 2003. Leishmania model for microbial virulence: the relevance of parasite multiplication and pathoantigenicity. Acta Trop. 85:375-390. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee, S., S. Singh, R. Sohoni, V. Kattige, C. Deshpande, S. Chiplunkar, N. Kumar, and S. Sharma. 2000. Characterization of domains of the phosphoriboprotein P0 of Plasmodium falciparum. Mol. Biochem. Parasitol. 107:143-154. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee, S., S. Singh, R. Sohoni, N. J. Singh, A. Vaidya, C. Long, and S. Sharma. 2000. Antibodies against ribosomal phosphoprotein P0 of Plasmodium falciparum protect mice against challenge with Plasmodium yoelii. Infect. Immun. 68:4312-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman, R. L., D. A. Lehman, and P. Rothman. 1993. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 54:229-270. [DOI] [PubMed] [Google Scholar]
- 8.Coler, R. N., Y. A. W. Skeiky, K. Bernards, K. Greeson, D. Carter, C. D. Cornellison, F. Modabber, A. Campos-Neto, and S. G. Reed. 2002. Immunization with a polyprotein vaccine consisting of the T-cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infect. Immun. 70:4215-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 10.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 186:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurunathan, S., L. Stobie, C. Prussin, D. L. Sacks, N. Glaichenhaus, D. J. Fowell, R. M. Locksley, J. T. Chang, W. Chang-You, and R. A. Seder. 2000. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells. J. Immunol. 165:915-924. [DOI] [PubMed] [Google Scholar]
- 12.Handman, E. 2001. Leishmaniasis: current status of vaccine development. Clin. Microbiol. Rev. 14:229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 14.Julia, V., M. Rassoulzadegan, and N. Glaichenhaus. 1996. Resistance to Leishmania major induced by tolerance to a single antigen. Science 274:421-423. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structured proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Lai, W. C., and M. Bennett. 1998. DNA vaccines. Crit. Rev. Immunol. 18:449-484. [DOI] [PubMed] [Google Scholar]
- 17.Louis, J., H. Himmelrich, C. Parra-Lopez, F. Tacchini-Cottier, and P. Launois. 1998. Regulation of protective immunity against Leishmania major in mice. Curr. Opin. Immunol. 10:459-464. [DOI] [PubMed] [Google Scholar]
- 18.Melby, P. C. 2002. Vaccination against cutaneous leishmaniasis: current status. Am. J. Clin. Dermatol. 3:557-570. [DOI] [PubMed] [Google Scholar]
- 19.Melby, P. C., G. B. Ogden, H. A. Flores, W. Zhao, C. Geldmacher, N. M. Biediger, S. K. Ahuja, J. Uranga, and M. Melendez. 2000. Identification of vaccine candidates for experimental visceral leishmaniasis by immunization with sequential fractions of a cDNA expression library. Infect. Immun. 68:5595-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melby, P. C., J. Yang, W. Zhao, L. E. Perez, and J. Cheng. 2001. Leishmania donovani p36(LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis. Infect. Immun. 69:4719-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendez, S., Y. Belkaid, R. A. Seder, and D. Sacks. 2002. Optimization of DNA vaccination against cutaneous leishmaniasis. Vaccine 20:3702-3708. [DOI] [PubMed] [Google Scholar]
- 22.Mendez, S., S. Gurunathan, S. Kamhawi, Y. Belkaid, M. A. Moga, Y. A. W. Skeiky, A. Campos-Neto, S. Reed, R. A. Seder, and D. Sacks. 2001. The potency and durability of DNA- and protein-based vaccines against Leishmania major evaluated using low-dose, intradermal challenge. J. Immunol. 166:5122-5128. [DOI] [PubMed] [Google Scholar]
- 23.Menon, J. N., and P. A. Bretscher. 1996. Characterization of the immunological memory state generated in mice susceptible to Leishmania major following exposure to low doses of L. major and resulting in resistance to a normally pathogenic challenge. Eur. J. Immunol. 26:243-249. [DOI] [PubMed] [Google Scholar]
- 24.Rafati, S., A.-H. Salmanian, T. Taheri, M. Vafa, and N. Fasel. 2001. A protective cocktail vaccine against murine cutaneous leishmaniasis with DNA encoding cysteine proteinases of Leishmania major. Vaccine 19:3369-3375. [DOI] [PubMed] [Google Scholar]
- 25.Ramshaw, I. A., and A. J. Ramsay. 2000. The prime-boost strategy: exciting prospects for improved vaccination. Immunol. Today 21:163-165. [DOI] [PubMed] [Google Scholar]
- 26.Requena, J. M., C. Alonso, and M. Soto. 2000. Evolutionarily conserved proteins as prominent immunogens during Leishmania infections. Parasitol. Today 16:246-250. [DOI] [PubMed] [Google Scholar]
- 27.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 28.Shi, P. Y., N. Maizels, and A. M. Weiner. 1997. Recovery of soluble, active recombinant protein from inclusion bodies. BioTechniques 23:1036-1038. [DOI] [PubMed] [Google Scholar]
- 29.Sjölander, A., T. M. Baldwin, J. M. Curtis, and E. Handman. 1998. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response are required for a generation of immunity to leishmaniasis. J. Immunol. 160:3949-3957. [PubMed] [Google Scholar]
- 30.Skeiky, Y. A. W., D. R. Benson, M. Elwasila, R. Badaro, J. M. Burns, Jr., and S. G. Reed. 1994. Antigens shared by Leishmania species and Trypanosoma cruzi: immunological comparison of the acidic ribosomal P0 proteins. Infect. Immun. 62:1643-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skeiky, Y. A. W., M. Kennedy, D. Kaufman, M. M. Borges, J. A. Guderian, J. K. Scholler, P. J. Ovendale, K. S. Picha, P. J. Morrissey, K. H. Grabstein, A. Campos-Neto, and S. G. Reed. 1998. LeIF: a recombinant Leishmania protein that induces an IL-12-mediated Th1 cytokine profile. J. Immunol. 161:6171-6179. [PubMed] [Google Scholar]
- 32.Soto, M., J. M. Requena, and C. Alonso. 1993. Isolation, characterization and analysis of the expression of the Leishmania ribosomal PO protein genes. Mol. Biochem. Parasitol. 61:265-274. [DOI] [PubMed] [Google Scholar]
- 33.Soto, M., J. M. Requena, L. Quijada, F. Guzman, M. E. Patarroyo, and C. Alonso. 1995. Identification of the Leishmania infantum P0 ribosomal protein epitope in canine visceral leishmaniasis. Immunol. Lett. 48:23-28. [DOI] [PubMed] [Google Scholar]