Abstract
Not all T cells specific for autoantigens are eliminated in the thymus, and therefore alternate mechanisms are required to prevent potentially autoreactive T cells from developing into effectors. Adoptive transfer of CD8+ T cells from influenza hemagglutinin-specific Clone 4 TCR transgenic mice into mice that express hemagluttinin in the pancreatic islets results in tolerance. This is preceded by activation of Clone 4 T cells that encounter antigen cross-presented in the draining lymph nodes of the pancreas. In this report we compare the phenotype, function, and costimulatory requirements of Clone 4 T cells activated by endogenous self-antigen, with Clone 4 T cells stimulated by influenza virus. The cells undergoing tolerance upregulate both CD69 and CD44, yet only partially downregulate CD62L, and do not express CD49d or CD25. Most importantly, they lack the ability to produce interferon-γ in response to antigen and show no cytolytic activity. Clone 4 T cells disappear after several cycles of division, apparently without leaving the site of initial activation. Surprisingly, despite the fact that such stimulation occurs through recognition of antigen that is cross-presented by a professional antigen-presenting cell, we find this activation is not dependent on costimulation through CD28. These data demonstrate that the recognition by naive CD8+ T cells of cross-presented self-antigen results in localized proliferation and deletion, without the production of effector cells.
Keywords: cytotoxic T lymphocytes, peripheral tolerance, T cell activation, B7, IFN-γ
Introduction
Negative selection in the thymus is arguably the major mechanism of T cell tolerance 1 2. However, as many peripherally expressed antigens are not present in the thymus at a level sufficient to eliminate all cognate T cells, there exist additional mechanisms in the periphery that prevent autoimmunity. One such mechanism involves peripheral deletion of CD8+ T cells activated through recognition of self-antigen that is picked up, processed, and presented by APCs 3. These APCs are presumed to be dendritic cells, as these have been shown to be unique in their ability to acquire antigen in the parenchyma, and present it to naive T cells in the draining LNs 4 5. Despite the fact the T cells are activated and undergo proliferation, this encounter results in tolerance rather than autoimmunity 3 6 7 8. The nature of the tolerizing signals is poorly understood, however, in all models in which this occurs, T cells recognize antigen that is presented in a noninflammatory environment.
Our laboratory has been studying such peripheral tolerance in InsHA mice that express the hemagglutinin (HA)protein from the influenza virus under the control of the rat insulin promoter 9. These mice are tolerant of HA that is expressed on the β cells of the pancreatic islets, such that the TCR repertoire is purged of T cells with high avidity for HA 9 10 11. We have demonstrated previously that HA-specific CD8+ T cells become tolerant after they leave the thymus 9 12. To study the mechanism of tolerance induction in InsHA mice, we produced Clone 4 TCR transgenic mice that express a relatively high affinity TCR specific for the major Kd-restricted HA epitope 12. This receptor was obtained from a B10.D2 animal that was immunized with influenza. Upon transfer of naive Clone 4 CD8+ T cells into InsHA recipients, the T cells become activated and proliferate in the draining LNs of the pancreas 8. The activated Clone 4 T cells did not cause diabetes and were either deleted or inactivated 8 13. To learn more about the basis for this apparent paradox, in which activation of CD8+ T cells is benign to the host and results in clonal elimination or inactivation, we have investigated the phenotype and functional capability of the Clone 4 CD8+ T cells that proliferate in the pancreatic LNs of InsHA mice.
Materials and Methods
Mice.
Balb/c mice were purchased from the breeding colony of The Scripps Research Institute. InsHA transgenic mice 9, homozygous for the HA gene, had been backcrossed with Balb/c at least eight generations. Clone 4 TCR transgenic mice 12 were also backcrossed with Balb/c mice for at least eight generations and were then crossed with Balb/c Thy1.1+/+ for two generations to achieve homozygosity for Thy1.1. Mice were propagated and maintained under specific pathogen-free conditions in The Scripps Research Institute's animal facility. All mice used in these studies were between 8 and 12 wk of age. Experimental procedures were performed according to the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Preparation of CFSE-labeled Clone 4 CD8+ T Cells.
CD8+ Thy1.1+ T cells from Clone 4 TCR were prepared as described previously 8 13 with certain modifications. Single cell suspensions were prepared from spleen and LNs of Clone 4 TCR transgenic mice and RBCs were lysed. Cells were then passed through a nylon wool column (Wako Chemicals) according to manufacturer's instructions. Nonadherent cells were incubated for 1 h at 4°C with anti-CD4 (clone RL172) mAb, in the form of tissue culture supernatant, at a concentration of 107 cells per milliliter. After centrifugation, Low-Tox rabbit complement (Accurate Chemical) was added to a final dilution of 10% in RPMI 1640 medium and cells were further incubated for 1 h at 37°C. Cells were finally washed with RPMI containing 10% FCS. 5 × 107 purified CD8+ T cells were incubated in 1 ml of 5 μM 5- and 6-carboxy-fluorescein succinimidyl ester (CFSE; Molecular Probes) in HBSS for 10 min at 37°C. Cells were then washed with ice-cold HBSS.
Adoptive Transfer, Immunization, and Ab Treatment.
Recipient InsHA or Balb/c mice were injected intravenously with 3 × 106 CFSE-labeled Clone 4 TCR CD8+ T cells in 200 μl of HBSS on day 0. Immediately after transfer, groups of mice were immunized intraperitoneally with 500 HA U of influenza virus A/PR/8/34 H1N1 that was grown in the allantoic cavity of 10-d-old hen's eggs. Mice immunized with a low dose of influenza virus received 12 HA U. Mice immunized with the Kd HA peptide (IYSTVASSL) received 100 μg of peptide in PBS intravenously.
In some experiments mice were treated with a combination of purified anti-B7.1 (clone 16-10A1; American Type Culture Collection[ATCC]) plus anti-B7.2 (clone GL1; ATCC) mAbs; 100 μg of each Ab per mouse per dose. Groups of mice treated with purified hamster IgG plus rat IgG (Jackson ImmunoResearch Laboratories) served as isotype controls. Abs were administered intraperitoneally in HBSS on days 0, 1, 2, 3, and in some cases on day 5.
Mice were monitored for diabetes by measuring blood glucose on days 4, 6, 8, and every 4 d thereafter. They were considered diabetic when glucose levels were >300 mg/dl.
Flow Cytometry.
4 or 8 d after receiving CFSE-labeled cells, pancreatic LNs and a mixture of other LNs including inguinal, axillary, cervical, and mandibular were excised and processed separately to obtain single cell suspensions. After counting, all the cells from the pancreatic LNs and an equivalent number of cells from the other LNs were stained with the indicated Abs. LNs from three mice receiving the same treatment were pooled together for staining.
All mAbs and secondary reagents were purchased from BD PharMingen. Donor Clone 4 T cells were detected and enumerated by virtue of their Thy1.1 expression. LN cells were incubated with anti-CD8α-PerCP and anti-Thy1.1-PE mAbs in HBSS, 0.1% BSA, 0.02% sodium azide for 30 min at 4°C. After washing, cells were analyzed with a FACSCalibur™ apparatus using CELLQuest™ software (Becton Dickinson). In analyzing each sample, 1.5 × 106 events were collected. The intensity of CFSE fluorescence was analyzed in the CD8+ Thy1.1+ subpopulation of lymphocytes.
For the phenotypic characterization of dividing Clone 4 CD8+ T cells, pancreatic LN cells were previously incubated with either biotin-labeled anti-CD25, anti-CD44, anti-CD69, anti-CD49d, or anti-CD62L mAbs for 30 min at 4°C, washed, and incubated again with a mixture of streptavidin-APC, anti-CD8α–PerCP, and anti-Thy1.1–PE mAbs for 30 min at 4°C.
Biotin-labeled annexin V was used for the detection of apoptotic Clone 4 cells. In this case incubation times were reduced to 15 min and performed at room temperature in annexin V binding buffer (BD PharMingen).
To assess production of IFN-γ in response to antigen, LN cells were incubated in RPMI 1640 10% FCS with 1 μg/ml of the Kd HA peptide and 1 μl/ml of Brefeldin A containing Golgi-Plug solution (BD PharMingen) for 6 h at 37°C. An irrelevant peptide was used instead of the Kd HA peptide as a negative control. After washing cells were stained to detect cell surface CD8 and Thy1.1 as described previously. Cells were then permeabilized and stained to detect intracellular IFN-γ with anti-IFN-γ-APC mAb using the Cytofix/Cytoperm Plus kit (BD PharMingen) according to manufacturer's instructions.
In Vivo Cytotoxicity Assay.
Mice were injected with 3 × 106 nonlabeled, purified Clone 4 CD8+ T cells. Syngenic spleen cells were labeled by incubation for 15 min at 37°C with either 5 μM CFSE in HBSS (CFSEhigh cells) or 0.5 μM CFSE in HBSS (CFSElow cells) and washed twice with HBSS. CFSEhigh cells were pulsed with Kd HA peptide at 1 μg/ml for 1 h at 37°C. CFSElow cells were not pulsed and served as an internal control. On day 4 mice were injected intravenously with a mixture of 2.5 × 106 CFSEhigh peptide-pulsed cells plus 2.5 × 106 CFSElow nonpulsed cells. Pancreatic LNs were excised 10 h later and single cell suspensions were analyzed for detection and quantification of CFSE-labeled cells.
Results
The Fate of Clone 4 CD8+ T Cells in InsHA Mice.
We have previously demonstrated that CSFE-labeled Clone 4 CD8+ T cells adoptively transferred into InsHA mice become activated and proliferate in the pancreatic LNs, but not elsewhere in the host 8. This activation was a prerequisite for tolerance induction of the Clone 4 cells. In this model, the rate of T cell activation, and consequently, the rate of tolerance induction is limited by the amount of antigen made available for stimulation through cross-presentation 13. To more carefully examine the fate of Clone 4 cells as they undergo tolerance in InsHA mice, we have extended those studies by tracking the T cells at later time points after adoptive transfer (Fig. 1). We have also compared the consequence of activation of Clone 4 cells by endogenous HA, which results in tolerance, with activation by influenza virus, which results in insulitis and diabetes in InsHA mice 13.
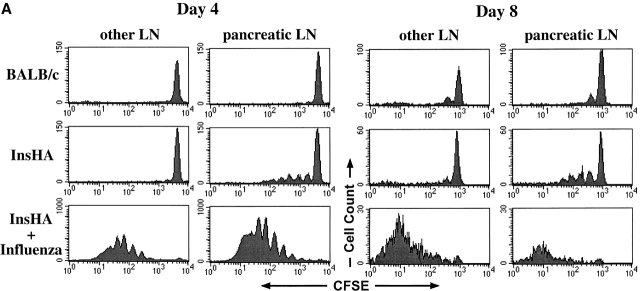
Figure 1.
Clone 4 CD8+ T cells proliferate in the pancreatic LNs of InsHA mice but do not accumulate over time. 3 × 106 CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into either Balb/c or InsHA hosts. Injected InsHA mice were split into two groups, one was immunized with influenza virus at the time of transfer and the other was left untreated. Mice were killed on days 4 or 8 after transfer and cells from pooled LNs were analyzed by FACS®. (A) Histograms represent the amount of CFSE label gating on CD8+ Thy1.1+ lymphocytes. This experiment has been repeated five times for InsHA mice and three times for influenza immunized InsHA and Balb/c mice. (B) Total numbers of CD8+ Thy1.1+ cells in the pancreatic LNs of host mice. Data represent the mean of all independent experiments performed. Only negative standard deviation is depicted to achieve greater sensitivity in the graph.
Clone 4 CD8+ T cells do not proliferate when transferred into syngenic Balb/c hosts that lack expression of HA (Fig. 1 A). However, by day 8 after transfer some cells were detected that had undergone a single cycle of division (Fig. 1 A). This is most likely due to homeostatic proliferation. The total number of Clone 4 cells present in the pancreatic LNs of Balb/c recipients is similar at 4 or 8 d after transfer (Fig. 1 B). In InsHA recipients, proliferation driven by cross-presentation of endogenous antigen is detectable in the pancreatic LNs but not in other LNs at days 4 and 8. The number of division cycles and the numbers of proliferating cells within each peak are similar on days 4 or 8. However, the peak corresponding to undivided cells is reduced by half during this time period (note change in scale), indicating a gradual consumption of the naive cells (Fig. 1 A). There was no evidence for increased numbers of activated cells. Also, by counting the total numbers of Clone 4 cells in the pancreatic LNs we could not detect accumulation over time (Fig. 1 B), despite evidence of proliferation. Indeed, a slight but significant decrease in total number of Clone 4 cells was evident between days 4 and 8. Of interest, the proliferating cells did not migrate from the pancreatic LNs to appear at different locations in the lymphoid system (Fig. 1 A). Taken together, these data indicate that only a small proportion of the T cells are stimulated at any given point in time, and that the activated cells do not accumulate within the lymphoid tissue.
This observation raised the question of what happens to the cells that divided previously. One possible explanation is that these activated HA-specific T cells had migrated to the pancreas where there is expression of cognate antigen. However, immunohistochemical examination of the pancreas indicated no such infiltration (data not shown). An alternative possibility, and one that is consistent with the fact the cells become tolerized in InsHA mice 8 13, is that the cells are both proliferating and dying within the pancreatic LNs. To determine if this was the case, annexin V staining was performed to detect apoptotic cells in the pancreatic LNs of InsHA mice (Fig. 2). On day 4, an average of 7% of all Clone 4 CD8+ T cells were annexin V positive, indicating an early stage in apoptosis, while <1% of the Clone 4 cells in Balb/c hosts were found to be positive. Taken together, these data suggest that the Clone 4 CD8+ T cells slowly become activated and die within the lymphoid tissue of InsHA mice.
Figure 2.
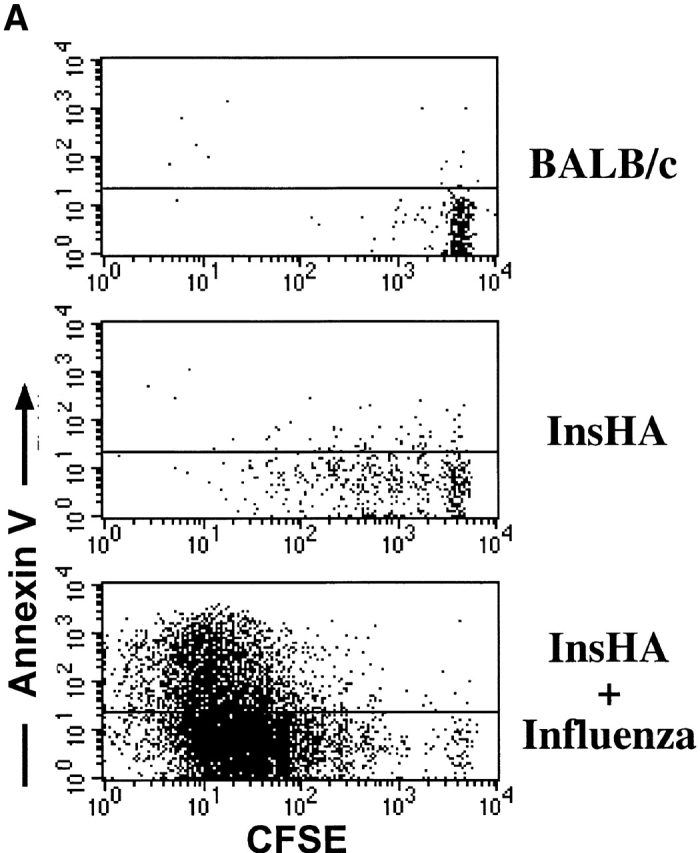
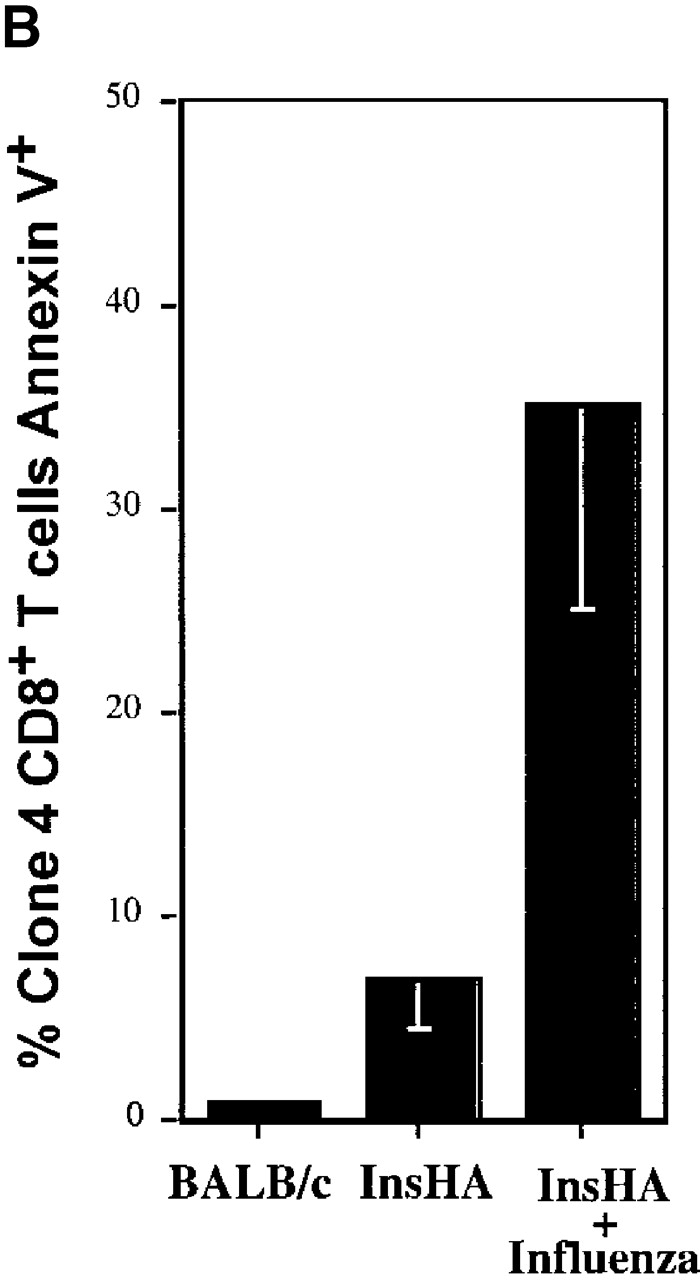
Detection of apoptotic Clone 4 CD8+ T cells. CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into the indicated hosts as in Fig. 1. On day 4 after transfer mice were killed and cells from pooled pancreatic LNs were analyzed by FACS® to detect apoptotic cells through annexin V binding. (A) Plots represent the amount of CFSE label versus annexin V binding–intensity gating on lymphocytes CD8+ Thy1.1+. One representative experiment out three independent experiments is shown. (B) Percentage of Clone 4 Thy1.1+ CD8+ T cells that are annexin V+ in the pancreatic LNs of recipient mice. Data represent the mean of three independent experiments performed.
In parallel experiments, we examined the fate of the Clone 4 cells in InsHA mice that were infected with influenza. On day 4 after the InsHA mice received both Clone 4 CD8+ T cells and influenza, there was vigorous activation and proliferation, with a clear accumulation of cells in the pancreatic LNs as well as other LNs. By day 8, the numbers of Clone 4 cells had dramatically declined in the lymphoid tissue (Fig. 1 A, note change in scale from days 4–8). This was clearly reflected in the decrease in the total number of Clone 4 CD8+ T cells recovered from the pancreatic LNs (Fig. 1 B). Of interest, when stained with annexin V on day 4, there was evidence that many of the virus-stimulated T cells, an average of ∼40%, were undergoing apoptosis beginning after 4 cycles of division. Presumably this represents activation-induced cell death (Fig. 2). These mice developed diabetes by days 6–8, due to the migration of activated Clone 4 CD8+ T cells to the pancreatic islets (data not shown).
Clone 4 CD8+ T Cells Responding to Endogenous Antigen Display a Distinctive Phenotype.
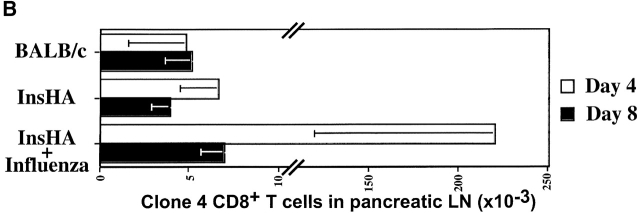
In an effort to understand the basis for the different fate of the Clone 4 cells, depending upon whether they were activated through encounter with self-antigen or virus infection, we analyzed the Clone 4 CD8+ T cells, obtained at day 4 from the pancreatic LNs of InsHA mice, for their expression of key activation markers (Fig. 3). Naive CD8+ T cells, like the Clone 4 cells transferred into Balb/c hosts, are characterized by a CD25−, CD69low, CD44low, CD62Lhigh, CD49dlow phenotype (Fig. 3; reference 14). In contrast, T cells activated by influenza virus proliferated vigorously and the expression pattern of these molecules switched to that characteristic of effector CD8+ T cells. They became CD25+, CD69high, CD44high, CD62Llow, CD49dhigh (Fig. 3). Some of these changes required that cells first undergo several rounds of division before they achieved the full activation phenotype. For example, maximal levels of expression of VLA-4 (CD49d) and IL-2Rα (CD25) did not occur until division cycle 5 or 6 (Fig. 3 B). However, the cells exhibited a gradual increase in expression that could be seen in earlier divisions.
Figure 3.
Phenotypic characterization of proliferating Clone 4 CD8+ T cells. CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into different hosts as in Fig. 1. On day 4 after transfer mice were killed and cells from pooled pancreatic LNs were analyzed by FACS® to detect expression of key activation surface markers. (A) Plots represent the amount of CFSE label versus the intensity of expression of five different activation markers gating on lymphocytes CD8+ Thy1.1+. One out of two independent experiments with similar results is shown. (B) Representation of the geometrical mean fluorescence intensity (GMFI) of the different activation markers from A examined as a function of the division cycles. GMFI was selected for this analysis due to the small numbers of cells involved in the analysis of each cycle of division, particularly in the case of Clone 4 T cells activated by endogenous expression of HA.
In contrast, Clone 4 CD8+ T cells that were activated to proliferate in response to self-antigen in the InsHA mice demonstrated a different phenotype. Although an early activation marker, CD44, became efficiently upregulated soon after the first round of division, there was no upregulation of CD25 or CD49d, and less downregulation of CD62L. Also, even though the expression pattern of CD69 was similar to that of Clone 4 cells taken from influenza infected mice, the maximum levels reached by each cell population was clearly different (Fig. 3 B). Thus, clear differences in cell surface expression of activation markers were exhibited by the Clone 4 cells depending upon whether they were stimulated with endogenous antigen or virus.
Clone 4 CD8+ T Cells Proliferating in Response to Endogenous Antigen Do Not Develop Effector Function.
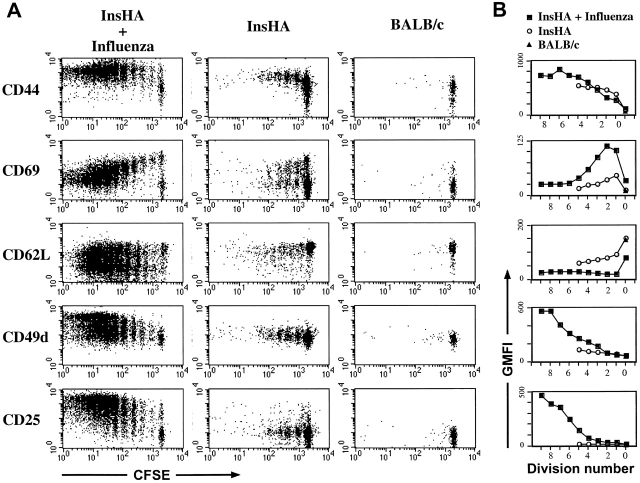
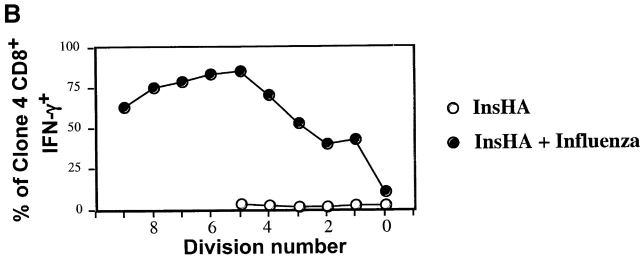
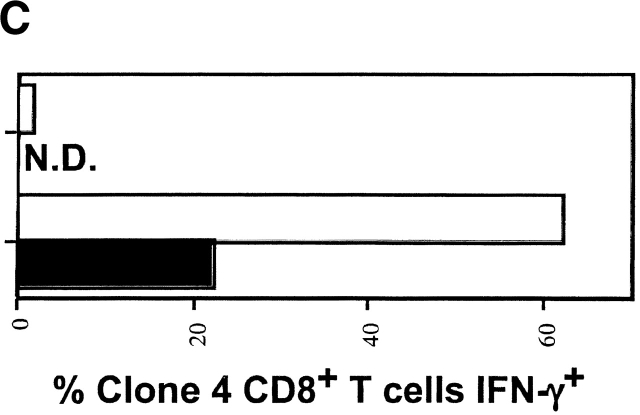
The observation that Clone 4 CD8+ T cells stimulated by endogenous self-antigen did not express a “classical” effector CTL phenotype led us to question whether the activated cells demonstrated effector function. Production of IFN-γ in response to antigen represents an early effector function. Indeed, a total of >60% of the Clone 4 cells obtained from influenza-infected mice exhibited the ability to produce IFN-γ at day 4 after transfer (Fig. 4). This cytokine was clearly detected even in cells that had divided only once or twice (Fig. 4). In contrast, the Clone 4 T cells taken from the pancreatic LNs of InsHA mice did not produce IFN-γ at day 4 after transfer (Fig. 4). Due to the limiting amount of cross-presented antigen few of the Clone 4 T cells transferred into InsHA are activated and proliferate at any given time. This is an ongoing process that continues until all naive Clone 4 cells are tolerized 13. Therefore, the cells that have experienced fewer rounds of division are likely to be derived from naive cells that were more recently activated. Accordingly, these data argues against the possibility that cells undergoing tolerance may produce IFN-γ at an early stage in division. In addition, examination of the Clone 4 cells taken from InsHA mice at day 2 after transfer, a time point when they have only divided one or two times, also showed no production of IFN-γ (data not shown).
Figure 4.
IFN-γ production by proliferating Clone 4 CD8+ T cells. 3 × 106 CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into InsHA recipients that were either immunized with influenza virus or left untreated. On day 4 after transfer cells from pooled pancreatic LNs were incubated with Kd HA peptide (stimulated) or an irrelevant peptide (nonstimulated) for 6 h at 37°C. Then, cells were analyzed by FACS® to detect accumulation of intracellular IFN-γ. (A) Plots represent the amount of CFSE label versus the intensity of IFN-γ produced gating on lymphocytes CD8+ Thy1.1+. Data correspond to one out of two independent experiments with similar results. (B) Representation of the percentage of CD8+ Thy1.1+ T cells that are IFN-γ+ from A as a function of the division cycle.
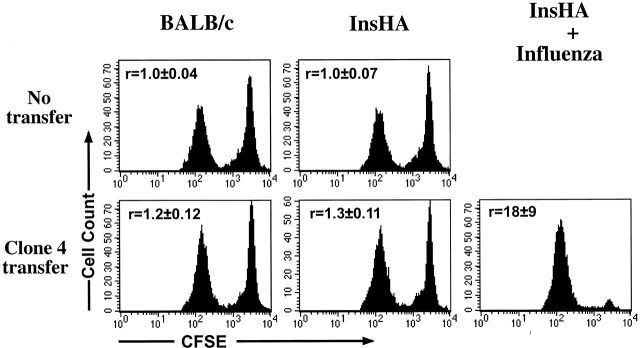
Next, we compared the ability of the Clone 4 T activated by either endogenous antigen or influenza virus, to lyse target cells that were pulsed with the HA peptide (Fig. 5). Peptide pulsed and nonpulsed spleen cells that were differentially labeled with different concentrations of CFSE were injected into InsHA or Balb/c mice that had received Clone 4 T cells 4 d earlier. After 10 h, cells from the pancreatic LNs were examined for evidence of lysis of targets pulsed with the HA peptide antigen. In Balb/c mice which received Clone 4 cells, a slight reduction was observed in the peak of CFSEhigh peptide pulsed targets, relative to the internal control of CFSElow cells that had not been pulsed with peptide (ratio CFSElow/CFSEhigh = 1.2 ± 0.1; Fig. 5). Essentially identical results were found when Clone 4 cells were injected into InsHA mice (ratio CFSElow/CFSEhigh = 1.3 ± 0.1; Fig. 5). This small reduction observed in the CFSEhigh peptide pulsed cells may be attributable to activation of naive, undivided Clone 4 cells by the CFSE targets, as it was not observed in mice that had not received Clone 4 cells (ratio = 1.0 in both Balb/c and InsHA). In contrast, cytolytic activity was clearly evident in recipients that had been infected on day 0 with influenza (ratio CFSElow/CFSEhigh = 18 ± 9; Fig. 5). These data indicate that activation of Clone 4 cells by endogenous HA alone did not result in cytolytic activity.
Figure 5.
In vivo cytolytic activity of proliferating Clone 4 CD8+ T cells. 3 × 106 purified Clone 4 CD8+ T cells were injected into either Balb/c or InsHA hosts. Injected InsHA mice were split into two groups, one was immunized with influenza virus at the time of transfer and the other was left untreated. 4 d after transfer mice were injected with a mixture of 2.5 × 106 CFSEhigh-labeled, Kd HA peptide pulsed syngenic spleen cells, and 2.5 × 106 CFSElow-labeled, nonpulsed cells, as targets. Groups of mice that did not receive Clone 4 cells were used as controls and were also given CFSE-labeled target cells. 10 h later lymphocytes from the pancreatic LNs of four individual mice per group (n = 4) were examined by FACS® to detect and quantify CFSE-labeled cells. Histograms represent the amount of CFSE label of one representative mouse per group. The mean ± SD of the ratio of the number of CFSElow/CFSEhigh cells (r) for all mice in each group is indicated. Data represent one out of two experiments with similar results.
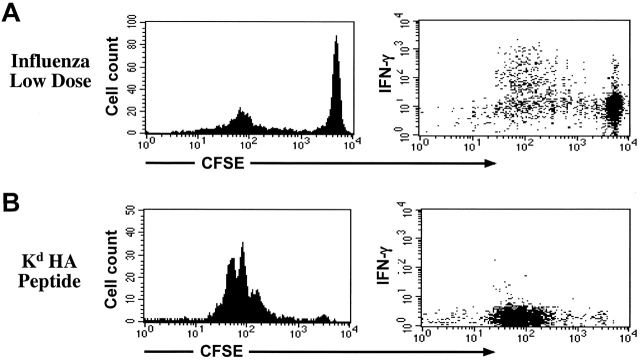
In the experiments described above, influenza virus–immunized mice received a high dose of virus (500 HA U). In this situation, the amount of antigen available to Clone 4 cells is much higher than when the only source of Kd HA peptide is the pancreatic HA protein. This is readily appreciated by comparing the number of cells that remained undivided in both situations (Fig. 1 A). Such a difference in antigen load could be responsible for the differential potential of Clone 4 cells of gaining effector function. To address this issue we decreased the amount of virus used for immunization to achieve conditions in which only a small percentage of the transferred cells divided (Fig. 6 A). Balb/c mice were used in these experiments to avoid interference with the endogenous antigen. Clone 4 cells stimulated by this “low dose” of virus (12 HA U), however, divided more that seven times and were able to efficiently secrete IFN-γ (Fig. 6 A). As another approach, we looked at IFN-γ production by Clone 4 T cells in a situation in which tolerance occurs after infusion of a high dose of peptide antigen. Systemic injection of 100 μg of soluble Kd HA peptide after adoptive transfer of Clone 4 CD8+ T cells induces a dramatic expansion of these cells followed by a rapid deletion without development of diabetes in InsHA mice (unpublished data). Clone 4 cells proliferating in Balb/c mice in response to soluble peptide did not produce IFN-γ (Fig. 6 B). Taking together, our results indicate that the development of effector function is not related to the antigen dose but rather to the context in which antigen is recognized.
Figure 6.
IFN-γ production by proliferating Clone 4 CD8+ T cells is not dependent on the antigen dose. 3 × 106 CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into Balb/c recipients. Cells recovered from the pancreatic LNs were analyzed for their ability to produce IFN-γ as described in Fig. 4. (A) Mice were immunized with 12 HA U of influenza virus at the time of cell transfer and killed on day 4. (B) Mice were immunized with 100 μg of Kd HA peptide intravenously at the time of cell transfer and killed on day 3.
Proliferation of Clone 4 CD8+ T Cells in the Pancreatic LNs Occurs Independent of Costimulation by B7.1+7.2.
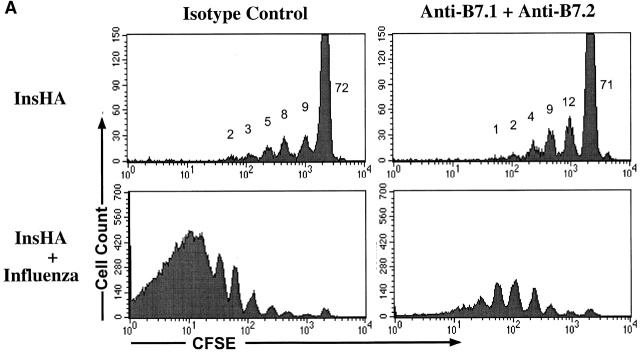
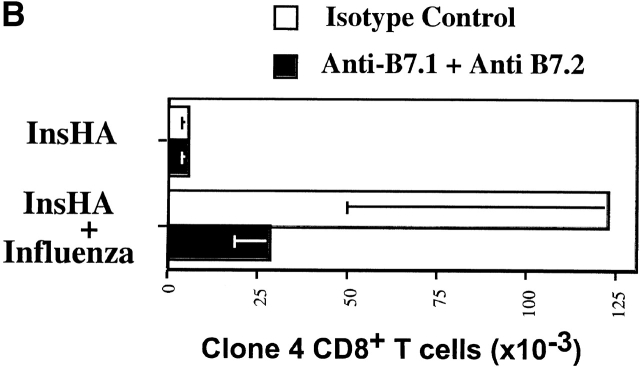
The important role of costimulation through CD28 in the development of a CD8+ T cell response is well established (for a review, see references 15 16 17). This occurs when T cells are stimulated by APCs that express B7.1/7.2 molecules. However, the absence or presence of this type of costimulation in the induction of T cell tolerance in vivo is still controversial and varies enormously depending on the system under examination 18 19. The role of costimulation in tolerance resulting from cross-presentation of self-antigen has not been addressed previously. To determine whether such costimulation was required for activation of Clone 4 T cells in response to endogenous self-antigen, we examined the effect of anti-B7 blocking mAbs. Anti-B7.1 mAb 16-10A1 and anti-B7.2 mAb GL1 have been used successfully to block CD28/B7 interactions 20. In vitro, a combination of both Abs was able to block the development of Clone 4 cells into effector CTLs and substantially reduced their proliferative potential in response to influenza virus–infected cells (data not shown). Individually, these Abs blocked neither proliferation nor CTL function. Surprisingly, when administered to InsHA mice, the amount of activation and proliferation of Clone 4 T cells in response to endogenous HA was found to be similar in the presence and absence of anti-B7 mAbs (Fig. 7 A). The number of division cycles observed and the percentage of cells within each cycle were almost identical. Also, the total numbers of Clone 4 cells in the pancreatic LNs were identical in both situations (Fig. 7 B). In contrast, these reagents had a profound effect on expansion of Clone 4 CD8+ T cells stimulated by influenza virus (Fig. 7a and Fig. b). A fourfold lower number of cells were recovered from the pancreatic LNs of virus-infected mice that received these Abs, and the CD8 cells that were recovered expressed threefold less IFN-γ (Fig. 7 C) and did not induce diabetes.
Figure 7.
Proliferation of Clone 4 cells in the pancreatic LNs of InsHA mice is B7 co-stimulation independent. 3 × 106 CFSE-labeled, purified, Thy1.1+ Clone 4 CD8+ T cells were injected into InsHA recipients that were either immunized with influenza virus or not. Half of the mice from the two previous groups were treated anti-B7.1 plus anti-B7.2 mAbs and the rest of the mice were treated with rat plus hamster IgG as isotype controls. On day 4 after transfer, cells from the pancreatic LNs were analyzed by FACS®. (A) Histograms represent the amount of CFSE label gating on lymphocytes CD8+ Thy1.1+. Percentages of cells within each division cycle are indicated in the top histograms. Data correspond to one out of three independent experiments with similar results. (B) Total numbers of CD8+ Thy1.1+ T cells in the pancreatic LNs of recipient mice. Data represents the mean of all experiments performed. (C) Cells from the pancreatic LNs were processed as described in Fig. 4 to detect IFN-γ production. The percentage of lymphocytes CD8+ Thy1.1+ that are IFN-γ+ is represented.
Discussion
Cross-presentation by APCs of exogenous antigen leads to the activation of naive, antigen-specific T cells in the periphery. In the presence of inflammatory agents, such as pathogens, activation results in the development of a vigorous T cell response. However, in the case of cross-presentation of self-antigens, activation results in tolerance induction (for a review, see reference 21). The current study represents the first detailed analysis of the phenotypic characteristics, functional capabilities, and costimulation requirements of T cells that are tolerized specifically by cross-presentation of self-antigen. Although others have previously studied some of these characteristics in T cells undergoing in vivo peripheral tolerance in response to exogenously provided or ubiquitously expressed antigens 22 23 24 25 in each of these situations, multiple cell types are involved in antigen presentation. The current study has examined tolerance as it occurs to an antigen expressed in the pancreatic islets via cross-presentation of antigen by a professional APCs. We have contrasted the phenotype and effector function of naive Clone 4 TCR cells that are activated in vivo either by cross-presentation of an endogenously expressed antigen, or by infection with influenza virus. The signals delivered in the former situation induce a partial activation program in which the HA-specific CD8+ T cells acquire a limited proliferation potential and display a distinctive phenotype, CD44high, CD69intermediate, CD25−, CD62Lintermediate, CD49d low. Moreover, they do not develop a key early effector function, the production of IFN-γ, and do not lyse HA-pulsed targets in vivo.
A number of studies have concluded that there is a strong link between the number of cell divisions by activated T cells, and the expression of activation markers and cytokines 26 27 28. Although there is a marked difference in the proliferative potential of Clone 4 cells that are stimulated by cross-presentation of self-antigen as compared with influenza infection, some of the differences observed in phenotype and function appear to be related to the quality of the signals received rather than the fact that they undergo fewer division cycles. Clone 4 cells obtained from virus infected mice demonstrate downregulation of CD62L after a single division cycle. Upregulation of CD25 and CD49d is noticeable after the second division, as is the ability to produce IFN-γ. In contrast, although we can detect cells that have undergone up to five division cycles in response to endogenous self-antigen, in the case of expression of CD25, CD49d, and IFN-γ, these activation and effector markers were not evident. It has also been proposed that gain of effector function and activation markers in T cells correlates with proliferative capacity and is independent of the signals inducing proliferation in vivo 27. In this previous study it was observed that CD4+ T cells which underwent division as a consequence of stimulation through TCR alone (in the absence of costimulation) expressed the same phenotype as cells that received stimulation through both TCR and CD28, as long as they underwent comparable numbers of divisions. The differences between the previous findings and the current study is unclear, but may be related to the fact that a high amount of peptide antigen was used to prime the OVA-specific CD4+ T cells in vivo.
The inability of the Clone 4 cells, undergoing activation in response to cross-presented self-antigen, to fully downregulate CD62L and upregulate CD49d is consistent with the finding that the cells undergoing tolerance are unable to leave the lymph and enter the pancreas 29 30 31. Despite their proliferation, Clone 4 cells did not accumulate in the pancreatic LNs and could not be detected in other LNs or at the site of expression of antigen in the pancreas. Evidence was found that some cells in the pancreatic LNs were undergoing apoptosis. Most likely the efficient clearance of small numbers of apoptotic cells by phagocytic cells prevented the detection of greater number of apoptotic cells 32. Taken together with our previous results, which demonstrated that tolerance induction occurs under this activation condition 8 13, we conclude the cells are being deleted from the repertoire.
It is likely that lack of expression of IL-2Rα, which may be a direct consequence of the absence of B7 costimulation 33, could prevent Clone 4 cells from continued proliferation and survival. Also, CD28/B7 ligation has been shown to enhance expression of Bcl-xL in T cells, which prevents death induced by Fas, TCR cross-linking, or withdrawal of IL-2 34. Since Clone 4 cells activated by endogenous antigen do not receive a costimulatory signal trough CD28, they would not upregulate expression of this antiapoptotic molecule. Furthermore, signaling through CD28 may be required to express additional costimulatory molecules that promote viability and proliferation, as has recently been described for expression of ICOS 35. Taken together, these results suggest that the Clone 4 T cells undergo “death by neglect” also, referred to as passive cell death, as opposed to AICD 36. The molecules involved in signaling apoptosis in the InsHA mice is currently under investigation. However, preliminary experiments suggest Fas is not involved in tolerance in InsHA mice (unpublished data). This is consistent with the reports that Fas plays no role in passive cell death 36. Interestingly, passive death has also been shown to be involved as a mechanism of peripheral tolerance in deletion of CD8+ T cells specific for a neoantigen expressed in the liver 37. Similarly, those cells are activated by antigen in the absence of costimulation. On the other hand, several other studies had reported a role for Fas in the peripheral deletion of T cells 38 39 40. However, in these studies there is extensive antigen stimulation and therefore, deletion in these other models may share features of AICD.
It has recently been shown that DCs induce CD8+ T cells proliferation by cross-presentation of endogenous self-antigens in vivo 41. Furthermore, there is evidence that it is the CD11c+ CD8+ DC subset the one responsible for activation of CTLs through cross-presentation of antigen 42. It is not yet known which type of APCs is responsible for tolerance as it occurs in InsHA mice. Future studies will attempt to determine the precise phenotype of the APCs involved in such tolerance. At first glance, the finding that the mechanism by which Clone 4 T cells are tolerized involves a lack of costimulation would seem to counterindicate a role for DCs, as it would be expected that any DCs that acquired HA from the islets and presented it in the pancreatic LNs should be a mature DCs and therefore express high levels of costimulatory molecules 43. However, several theories have been proposed to explain tolerance induction by DCs. They evoke the existence of a different subclass of DCs which is involved in tolerance 44 45 or a different state of activation for DCs responsible for priming versus tolerance induction 46. Bacterial products, viral infection, inflammatory cytokines, necrotic cells, CD4+ T cells activated through CD40 or TNF-related activation-induced cytokine expression (TRANCE)-receptor (TRANCE-R) ligation, and activated CD8+ T cells 46 47 48 have been shown to induce maturation or further activate mature DCs and increase expression of costimulatory molecules. A third model that could integrate the previous two has been proposed recently 49. Short lived, immature DCs may transport antigen to the lymph 50, where resident lymphoid-tolerizing DCs could phagocyte them and present antigen to T cells. Our data cannot discriminate among these different hypothesis, but does indicate that regardless of the lineage or maturation status of the tolerizing DCs, it is likely they are not activated and therefore do not express high levels of costimulatory molecules.
Of interest, the Clone 4 T cells activated to proliferate by endogenous self-antigen exhibit many characteristics that are similar to CD8+ T cells undergoing homeostatic proliferation in lymphopenic hosts 51 52. In both cases, activation is independent of CD28 52. The cells undergoing homeostatic division do not upregulate CD25 or CD49d, and are unable to efficiently downregulate CD62L. Also, within the first few weeks they did not develop effector activity. However, as opposed to our results with Clone 4 cells, CD8+ T cells expanding in lymphopenic hosts are able to persist for a long period of time 28 52, whereas the Clone 4 cells undergoing proliferation in response to endogenous antigen are deleted. It has been demonstrated that homeostatic division occurs in response to low affinity recognition of self-MHC–peptide complexes 52. It is interesting to speculate that the seminal difference between homeostatic division and the type of peripheral tolerance observed in this study, may be the affinity of the TCR for self-antigen. This draws obvious parallels to positive versus negative selection in the thymus.
Numerous studies have examined peripheral induction of tolerance in vivo 53. However, it is unclear whether the same mechanistic principles apply in the different models. In many cases, the nature of the APC is undefined. It has been shown that B7 costimulation is required during peptide-induced anergy in CD4+ T cells and tumor-induced anergy in CD8+ T cells 19 54. In this last report, CD8+ T cells displayed an activated phenotype and effector function, yet they remained in an unresponsive state. Here we have shown that Clone 4 CD8+ T cells do not utilize costimulation through CD28 when stimulated by cross-presentation of endogenous self-antigen. In this condition, proliferating Clone 4 cells do not express CD25 and presumably they do not secrete IL-2. This is reminiscent of an anergic phenotype; however, these cells are soon deleted, whereas anergic cells can persist in vivo for long periods of time 55. Deletion of mature CD8+ T cells as a consequence of self-antigen recognition in the absence of costimulation has been reported 56. In this case, T cells encounter antigen expressed on hepatocytes that lack expression of B7 molecules. What is surprising in our results is the fact that Clone 4 cells encounter antigen on professional APCs, yet no costimulation is provided.
Here we have characterized a novel activation phenotype in CD8+ T cells, which occurs after encounter with endogenous antigen. The fate of these cells is deletion. It was demonstrated previously that InsHA mice are tolerant of the HA antigen in both the CD8 and CD4 compartments 9. Thus, it is likely that Clone 4 cells are devoid of cognate CD4+ help in InsHA mice. We are currently evaluating the ability of HA-specific CD4+ T cell help to prevent such tolerance.
Acknowledgments
This work was supported by grants DK50824 and CA57855 from the National Institutes of Health.
Footnotes
Abbreviations used in this paper: CFSE, 5- and 6-carboxy-fluorescein succinimidyl ester; HA, hemagglutinin.
References
- Kappler J.W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Bluthmann H., Staerz U.D., Steinmetz M., Boehmer H.V. Tolerance in T cell receptor transgenic mice involves deletion of non mature CD4+8− thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Kurts C., Kosaka H., Carbone F.R., Miller J.F.A.P., Heath W.R. Class I-restricted cross-presentation of exogenous self antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Cella M., Danieli C., Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartmentdownregulation by cytokines and bacterial products. J. Exp. Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Forster I., Lieberam I. Peripheral tolerance of CD4+ T cells following local activation in adolescent mice. Eur. J. Immunol. 1996;26:3194–3202. doi: 10.1002/eji.1830261253. [DOI] [PubMed] [Google Scholar]
- Adler A.J., Marsh D.W., Yochum G.S., Guzzo J.L., Nigam A., Nelson W.G., Pardoll D.M. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J. Exp. Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.J., Kurts C., Kreuwel H.T.C., Holst K.L., Heath W.R., Sherman L.A. Ontogeny of T cell tolerance to peripherally-expressed antigens. Proc. Natl. Acad. Sci. USA. 1999;95:11377–11382. doi: 10.1073/pnas.96.7.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo D., Freedman J., Hesse J., Palmiter R.D., Brinster R.L., Sherman L.A. Peripheral tolerance to an islet cell specific hemagglutinin transgene affects both CD4+ and CD8+ T cells. Eur. J. Immunol. 1992;22:1013–1022. doi: 10.1002/eji.1830220421. [DOI] [PubMed] [Google Scholar]
- Morgan D.J., Kreuwel H.T.C., Fleck S., Levitsky H.I., Pardoll D.M., Sherman L.A. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J. Immunol. 1998;160:643–651. [PubMed] [Google Scholar]
- Nugent C.T., Morgan D.J., Biggs J.A., Ko A., Pilip I.M., Pamer E.G., Sherman L.A. Characterization of CD8+ T lymphocytes that persist after peripheral tolerance to a self antigen expressed in the pancreas. J. Immunol. 2000;164:191–200. doi: 10.4049/jimmunol.164.1.191. [DOI] [PubMed] [Google Scholar]
- Morgan D.J., Liblau R., Scott B., Fleck S., McDevitt H.O., Sarvetnick N., Lo D., Sherman L.A. CD8+ cell-mediated spontaneous diabetes in neonatal mice. J. Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- Morgan D.J., Kreuwel H.T.C., Sherman L.A. Antigen concentration and precursor frequency determine the rate of CD8+ T cell tolerance to peripherally expressed antigens. J. Immunol. 1999;163:723–727. [PubMed] [Google Scholar]
- Linton P.J., Haynes L., Tsui L., Zhang X., Swain S. From naive to effector—alterations with aging. Immunol. Rev. 1997;160:9–18. doi: 10.1111/j.1600-065x.1997.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Allison J.P. CD28-B7 interactions in T-cell activation. Curr. Opin. Immunol. 1994;6:414–419. doi: 10.1016/0952-7915(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Lenschow D.J., Walunas T.L., Bluestone J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- McAdam A.J., Schweitzer A.N., Sharpe A.H. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol. Rev. 1998;165:231–247. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., McKall-Faienza K., Schmits R., Bouchard D., Beach J., Speiser D.E., Mak T.W., Ohashi P.S. Distinct roles for LFA-1 and CD28 during activation of naive T cellsadhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- Perez V.L., Van Parijs L., Biuckians A., Zheng X.X., Strom T.B., Abbas A.K. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- Kearney E.R., Walunas T.L., Karr R.W., Morton P.A., Loh D.Y., Bluestone J.A., Jenkins M.K. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J. Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- Heath W.R., Carbone F.R. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- Ehl S., Hornbach J., Aichele P., Rulicke T., Odermatt B., Hengartner H., Zinkernagel R., Pircher H. Viral and bacterial infections interfere with peripheral tolerance induction and activate CD8+ T cells to cause immunopathology. J. Exp. Med. 1998;187:763–774. doi: 10.1084/jem.187.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cellsclonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Rocha B., von Boehmer B.H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Vella A.T., McCormack J.E., Linsley P.S., Kappler J.W., Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2:261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Oehen S., Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTLcorrelation of effector function with phenotype and cell division. J. Immunol. 1998;161:5338–5346. [PubMed] [Google Scholar]
- Gudmundsdottir H., Wells A.D., Turka L.A. Dynamics and requirements of T cell clonal expansion in vivo at the single-cell leveleffector function is linked to proliferative capacity. J. Immunol. 1999;162:5212–5223. [PubMed] [Google Scholar]
- Murali-Krishna K., Ahmed R. Cutting edgenaive T cells masquerading as memory cells. J. Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- Gallatin W.M., Weissman I.L., Butcher E.C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Baron J.L., Madri J.A., Ruddle N.H., Hashim G., Janeway C.A., Jr. Surface expression of α 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J. Exp. Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C.R. Homing of naive, memory and effector lymphocytes. Curr. Opin. Immunol. 1993;5:423–427. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- Savill J., Fadok V., Henson P., Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol. Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Cerdan C., Martin Y., Courcoul M., Brailly H., Mawas C., Birg F., Olive D. Prolonged IL-2 receptor α/CD25 expression after T cell activation via the adhesion molecules CD2 and CD28. Demonstration of combined transcriptional and post-transcriptional regulation. J. Immunol. 1992;149:2255–2261. [PubMed] [Google Scholar]
- Boise L.H., Minn A.J., Noel P.J., June C.H., Accavitti M.A., Lindsten T., Thompson C.B. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- McAdam A.J., Chang T.T., Lumelsky A.E., Greenfield E.A., Boussiotis V.A., Duke-Cohan J.S., Chernova T., Malenkovich N., Jabs C., Kuchroo V.K. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J. Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- Lenardo M., Chan K.M., Hornung F., McFarland H., Siegel R., Wang J., Zheng L. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- Bertolino P., Trescol-Biemont M.C., Thomas J., de St. Groth B.F., Pihlgren M., Marvel J., Rabourdin-Combe C. Death by neglect as a deletional mechanism of peripheral tolerance. Int. Immunol. 1999;11:1225–1238. doi: 10.1093/intimm/11.8.1225. [DOI] [PubMed] [Google Scholar]
- Singer G.G., Abbas A.K. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Sytwu H.K., Liblau R.S., McDevitt H.O. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- Kurts C., Heath W.R., Kosada H., Miller J.F., Carbone F.R. The peripheral deletion of autoreactive CD8+ T cells induced by cross-presentation of self-antigens involves signaling through CD95 (Fas, Apo-1) J. Exp. Med. 1998;188:415–420. doi: 10.1084/jem.188.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Cannarile M., Klebba I., Brocker T. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J. Immunol. 2001;166:1439–1442. doi: 10.4049/jimmunol.166.3.1439. [DOI] [PubMed] [Google Scholar]
- den Haan J.M.M., Lehar S.M., Bevan M.J. CD8+ but not CD8-dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Witmer-Pack M., Inaba M., Hathcock K.S., Sakuta H., Azuma M., Yagita H., Okumura K., Linsley P.S., Ikehara S. The tissue distribution of the B7-2 costimulator in miceabundant expression on dendritic cells in situ and during maturation in vitro. J. Exp. Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suss G., Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J. Exp. Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronin V., Winkel K., Suss G., Kelso A., Heath W., Kirberg J., von Boehmer H., Shortman K. A subclass of dendritic cells regulates the response of naive CD8 T cells by limiting their IL-2 production. J. Immunol. 1996;157:3819–3827. [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J. Exp. Med. 1999;189:611–614. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedl C., Kopf M., Bachmann M.F. CD8+ T cells mediate CD40-independent maturation of dendritic cells in vivo. J. Exp. Med. 1999;189:1875–1884. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter B., Albert M.L., Francisco L., Larsson M., Somersan S., Bhardwaj N. Consequences of cell deathexposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R.M., Turley S., Mellman I., Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F.P., Platt N., Wykes M., Major J.R., Powell T.J., Jenkins C.D., MacPherson G.G. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B.K., Rao V.P., Ge Q., Eisen H.N., Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath A.W., Bevan M.J. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B. T lymphocyte tolerancefrom thymic deletion to peripheral control mechanisms. Adv. Immunol. 1999;71:229–265. doi: 10.1016/s0065-2776(08)60404-6. [DOI] [PubMed] [Google Scholar]
- Deeths M.J., Kedl R.M., Mescher M.F. CD8+ T cells become nonresponsive (anergic) following activation in the presence of co-stimulation. J. Immunol. 1999;163:102–110. [PubMed] [Google Scholar]
- Pape K.A., Merica R., Mondino A., Khoruts A., Jenkins M.K. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J. Immunol. 1998;160:4719–4729. [PubMed] [Google Scholar]
- Bertolino P., Heath W.R., Hardy C.L., Morahan G., Miller J.F.A.P. Peripheral deletion of autoreactive CD8+ T cells in transgenic mice expressing H-2Kb in the liver. Eur. J. Immunol. 1995;25:1932–1942. doi: 10.1002/eji.1830250721. [DOI] [PubMed] [Google Scholar]