Abstract
Removal of apoptotic cells is essential for maintenance of tissue homeostasis, organogenesis, remodeling, development, and maintenance of the immune system, protection against neoplasia, and resolution of inflammation. The mechanisms of this removal involve recognition of the apoptotic cell surface and initiation of phagocytic uptake into a variety of cell types. Here we provide evidence that C1q and mannose binding lectin (MBL), a member of the collectin family of proteins, bind to apoptotic cells and stimulate ingestion of these by ligation on the phagocyte surface of the multifunctional protein, calreticulin (also known as the cC1qR), which in turn is bound to the endocytic receptor protein CD91, also known as the α-2-macroglobulin receptor. Use of these proteins provides another example of apoptotic cell clearance mediated by pattern recognition molecules of the innate immune system. Ingestion of the apoptotic cells through calreticulin/CD91 stimulation is further shown to involve the process of macropinocytosis, implicated as a primitive and relatively nonselective uptake mechanism for C1q- and MBL-enhanced engulfment of whole, intact apoptotic cells, as well as cell debris and foreign organisms to which these molecules may bind.
Keywords: apoptosis, phagocytosis, macropinocytosis, C1q, MBL
Introduction
Collectins, also known as defense collagens, are a multifunctional family of proteins with C-type lectin domains and pattern recognition capability thought to be involved in the role of the innate immune system in protection from infectious organisms 1 2. Members of the family include mannose binding lectin (MBL), surfactant proteins A and D (SP-A and SP-D), and bovine conglutinin. C1q, a member of the first component of the classical complement pathway, is related to this family as it shares structural and functional homology with the collectins, although it does not exhibit lectin activity 3.
C1q deficiency is known to result in an increased risk for bacterial infections and autoimmune diseases such as systemic lupus erythematosus (SLE; references 4 5 6 7) and lupus nephritis 8. MBL deficiency is also associated with an increased susceptibility to infection and disease 9 10 11. For example, recent findings have shown a correlation between MBL deficiency and Pseudomonas infections in cystic fibrosis patients, suggesting that MBL is inherently involved in clearance of potential pathogens in the body 12.
C1q knockout mice exhibit a phenotype resembling some aspects of SLE. These mice develop autoantibodies and glomerulonephritis due to immune complex deposition, which may be exacerbated by the presence of multiple apoptotic bodies 13. Abnormal clearance of apoptotic cells with inappropriate levels of apoptotic nuclei have been suggested to contribute to SLE 14 and the knockout mice also show a defect in clearance of apoptotic cells in vivo 15. As C1q has been shown to recognize and bind to surface blebs on apoptotic keratinocytes 16, and apoptotic vascular endothelial cells 17, we questioned the potential role of C1q and the related protein MBL as mediators of apoptotic cell recognition and clearance and have begun to examine mechanisms of this engulfment.
Our studies demonstrate that C1q and MBL can bind to, and initiate uptake of, apoptotic cells into macrophages. In addition, evidence is provided to support a role for cell surface calreticulin (CRT) in (also known as the cC1qR) binding the collagenous tails of C1q and MBL attached to the apoptotic cell. This multifunctional protein does not have a transmembrane domain but appears to signal for apoptotic cell ingestion through association with CD91 (also known as the α2-macroglobulin (α2m) receptor or LDL receptor related–protein (LRP) on the macrophage cell surface. This is suggested to initiate engulfment of the apoptotic cells by a process leading to concurrent uptake of extracellular fluid and the formation of spacious phagosomes. It can also stimulate bystander engulfment of attached cells. Accordingly, uptake of apoptotic cells by these processes is suggested to occur by macropinocytosis.
Materials and Methods
Phagocytosis Assays.
Human monocytes were isolated on a Percoll gradient as described previously 18 and then washed twice in HBSS (Cellgro). They were resuspended in X-Vivo media (Biowhittaker) to a final concentration of 4 × 106 cells/ml and allowed to adhere in 48-well plates (0.5 ml/well) for 1 h at 37°C with 10% CO2. At this point, medium was changed to X-Vivo plus 10% heat-inactivated, pooled human serum. The cells were allowed to mature into macrophages over a 7–10-d period, with the medium changed at days 4 and 7. The macrophages were used after 7 d of culture.
Jurkat T cells were cultured in RPMI (Cellgro) plus 10% heat-killed fetal calf serum (Gemini) and penicillin/streptomycin plus l-glutamine (Sigma-Aldrich). Prior to phagocytosis, the cells were irradiated with UV (254 nm) for 10 min, then cultured at 37°C plus 5% CO2 for 3 h to induce apoptosis. The cells were washed with HBSS and resuspended in DMEM (Cellgro). The percentage apoptosis was routinely determined (usually 65–70%) by morphologic assessment of nuclear alterations after cytocentrifugation and staining with a modified Wright-Giemsa stain. Apoptosis was confirmed by propidium iodide staining for subdiploid DNA and by the ability to bind annexin V-FITC.
The human monocyte-derived macrophages (HMDMs) were treated with inhibitors half an hour before the addition of apoptotic cells. Antibodies to block uptake were added at a final concentration of 10 μg/ml and the cells incubated at 37°C in 10% CO2 for 30 min. The wells were washed with HBSS and apoptotic cells added for 1 h at 37°C. After removal of noningested apoptotic cells with PBS (Cellgro), the cells were then fixed and counted for uptake.
Uptake conditions and assessment have been described previously 19. The data are presented as a phagocytic index calculated as: (no. Møs with ingested apoptotic cells/total no. Møs) × (no. apoptotic cells per Mø/no. Møs with apoptotic cells).
Using this phagocytic index, data from experimental wells were compared with controls with no inhibitor or antibody added.
Inhibitors used included the following: anti-C1q, monoclonal from Quidel Corp. Sheep and goat polyclonal from ICN Biomedicals. Monoclonal anti-MBL was provided by Statens Serum Institut, Copenhagen, Denmark. Anti-C1qRp antibodies were provided by Andrea Tenner, University of California Irvine, CA. Monoclonal anti-CR1 (anti-CD35), anti-mannose receptor, anti-CD32 (BD PharMingen); monoclonal anti-CR3 (Dako); chicken polyclonal anti-CRT antibodies (Affinity BioReagents, Inc.); rabbit polyclonal anti-CRT (Upstate Biochemicals); rabbit polyclonal anti-cC1qR (from Dr. Ghebrehiwet); mouse anti–human CD91 (α chain) and anti-CD91 (β chain) monoclonal antibodies (American Diagnostica); (all antibodies anti–human). Control antibodies consisted of monoclonal and polyclonal anti-CD45 antibodies (BD PharMingen and The Binding Site, respectively); chicken IgY and human Ig, as well as FITC-conjugated goat anti–mouse IgG and Cy3-conjugated donkey anti–chicken IgY (Jackson ImmunoResearch Laboratories).
Purified human C1q was obtained from Quidel Corp. MBL was purified from acute-phase human plasma (obtained from Bonfils Blood Center, Denver, CO) as reported previously 20. C1q collagenous tails were prepared following a modified protocol from reference 21. Briefly, C1q was dialyzed overnight against 0.15 M NaCl, 0.1M NaAcetate, pH 4.5, and incubated for 4 h at 37°C with a 1:30 dilution of 10 mg/ml pepsin. After centrifugation for 10 min at 10,000 g, the supernatant was fractionated on an ACA34 column and tails eluted with Tris-buffered saline with 5 mM Ca2+. 1-ml aliquots were collected; tails were usually eluted around fraction 6. Human α-2-macroglobulin and bovine CRT were obtained from Sigma-Aldrich.
Surface Modulation Experiments.
Proteins were resuspended in DMEM to a concentration of 0.1 μg/ml and 100 μl added per well of a 48-well plate and kept at 4°C for 2 h. The plate was then warmed to 37°C, and 100 μl of a solution 5 × 106 HMDMs was plated on each well. These were obtained by maturing human monocytes as before but on 10-cm bacteriologic plates at 40 × 106 cells/well for 7 d. The plates were placed on ice with HBSS plus 10 mM EDTA for 15 min and cells harvested using cell lifters and washed twice with HBSS. These HMDMs were allowed to adhere on the proteins for 30 min at 37°C before adding apoptotic cells. Phagocytosis was allowed to occur for 1 h, and then cells were washed, fixed, and counted.
Flow Cytometry.
Cells were resuspended to 10 × 106 cells per ml in HBSS and 100 μl pipetted into each well of a 96-well, U-bottomed plate. The plates were centrifuged at 1,000 RPM for 2 min at 4°C. After two washes with Kreb's/Ringers phosphate and dextrose buffer (containing 0.9% NaCl, 1.15% KCl, 0.61% CaCl2, 3.82% MgSO4.7H2O, 0.2 M NaH2PO4, 0.2 M Na2HPO4, and dextrose; pH adjusted to 7.34; KRPD) plus 0.25% human serum albumin, the cells were resuspended in this buffer with FITC-conjugated protein (prepared using the FluoroTag™ FITC conjugation kit; Sigma-Aldrich), or with 5 μg primary antibody (rabbit anti-CRT or rabbit immunoglobulin; Jackson ImmunoResearch Laboratories) for 1 h on ice in the dark. Cells stained with antibody were then washed twice and resuspended in buffer and secondary antibody (1:200; FITC donkey anti–rabbit; Jackson ImmunoResearch Laboratories). These cells were incubated on ice, in the dark for 30 min, and then washed. After two washes, the cells were resuspended in 1 ml of buffer and analyzed on a FACScan™ (Becton Dickinson). The data were analyzed using PC Lysis software (Becton Dickinson).
Construction of Single Ligand Particles for Uptake (Ebab).
Human erythrocytes were obtained from normal donors and washed in 1× PBS, pH 8.0. 30 × 106 erythrocytes were resuspended in 800 μl PBS, pH 8.0, and incubated with EZ-Link Sulfo-NHS-LC-Biotin (Pierce Chemical Co.) according to manufacturer's instructions. These biotinylated erythrocytes (Eb) were washed twice, resuspended in 1× PBS, pH 8.0, and incubated with 120 μg streptavidin (Sigma-Aldrich) at room temperature for 30 min. Ebab were washed twice, resuspended in 1× PBS, pH 7.4, and incubated with 5–10 μg of biotinylated antibody or protein. Proper construction and equal ligand distribution of Ebab was confirmed by flow cytometry using the appropriate fluorochrome-conjugated antibody.
Ebab Phagocytosis Assays.
Serum-containing media was washed from cells and replaced with DMEM. Target Ebab were added to macrophages at a ratio of 15:1 and allowed to incubate at 37°C for 30 min. Unbound Ebab were washed away with HBSS. Lysis of uningested Ebab was performed by adding deionized H2O for 10 s, followed by immediate replacement with DMEM. Cells were fixed with 0.75% glutaraldehyde, stained with dianisidine and H2O2 (Sigma-Aldrich), and counterstained with eosin. 200 cells were scored using light microscopy to quantify binding (before distilled water lysis) and engulfment of Ebab (after lysis).
Fluorescence Microscopy.
Macrophages were plated onto coverslips in 24-well plates and allowed to mature over 7 d (106 cells/coverslip). The cells were washed twice with KRPD plus 0.25% heat stable antigen (HSA) buffer, and incubated in 0.5 ml buffer plus 1 μg human gamma globulin for 20 min on ice. The cells were washed twice, and then incubated in 0.5 ml of buffer plus 10 μg primary antibody (mouse anti–human CD45, BD PharMingen; chicken anti–human CRT, Affinity Bioreagents, Inc.; mouse anti–human CD32, BD PharMingen; mouse anti-CD91, American Diagnostica) for 1 h on ice. The cells were washed twice and incubated in 0.5 ml of buffer plus secondary antibody (FITC anti–mouse and Cy3 anti–chicken; Jackson ImmunoResearch Laboratories; 1:200) for 30 min on ice in the dark. The cells were then washed twice, and the coverslips removed from the wells and mounted onto slides and visualized by confocal microscopy.
Jurkat cells (viable or apoptotic) were resuspended to 10 × 106 cells per ml in HBSS and 100 μl pipetted into each well of a 96-well, U-bottomed plate. The plates were centrifuged in plate spinners at 1,000 RPM for 2 min at 4°C. After two washes with Kreb's/Ringers phosphate and dextrose buffer (KRPD) plus 0.25% human serum albumin, the cells were resuspended in this buffer with 5 μg FITC-conjugated protein (prepared using FITC-conjugation kit; Sigma-Aldrich) for 1 h on ice in the dark. Cells were then washed twice, resuspended in buffer, mounted onto slides coated with GelMount (Biomeda), and visualized by confocal microscopy. A directly labeled FITC C1q or MBL (shown), unlabeled C1q or MBL with fluorochrome-conjugated polyclonal anti-C1q or monoclonal anti-MBL (not shown), or a biotinylated C1q or MBL and fluorochrome-conjugated streptavidin (not shown); all gave identical results.
Stimulated Macropinocytosis Experiments (Uptake of Lucifer Yellow).
HMDMs were incubated with 10 μg/ml anti-CRT anti-CD91 or, as control, anti-CD45 antibodies for 15 min at 37°C. In some experiments, appropriate cross-linking anti-immunoglobulin antibodies (5 μg/ml) were then added for an additional 10 min. Lucifer Yellow was then added to the medium (to a final concentration of 1.5 mg/ml) for 2 min as the cells were incubated at room temperature, in the dark, on a rocking surface. The cells were then washed and fixed in paraformaldehyde before being mounted onto coverslips and visualized via confocal microscopy.
Bystander Uptake Experiments.
RBCs coated with anti-CD36 were prepared as described above. These were added to HMDMs and allowed to adhere at 37°C for 15 min (although the Ebab-anti-CD36 cells became tethered to the macrophages, uptake did not occur). Antibodies to human CRT or CD91 present on the macrophage surface were added followed by buffer, or in some experiments, cross-linking anti–chicken immunoglobulin. Cross-linked mouse anti–human CD45 (anti-CD45) was used as a control. The cells were incubated for a further 30 min at 37°C. Cells were then washed, fixed, stained, and counted.
Concurrent Ingestion of Extracellular Fluid Marker.
Erythrocytes were prepared as described. Texas Red succinimidyl ester dye (Molecular Probes) was incubated with the erythrocytes for 25 min at 37°C. HMDMs, plated in wells containing sterile coverslips, were washed with HBSS, and incubated in X-vivo medium for 2 h before assay. Erythrocytes were washed and added to serum-starved macrophages along with Lucifer Yellow dye (Molecular Probes). Phagocytosis was allowed to occur for 15 min in the dark and at 37°C. Cells were washed, and incubated in PBS and DAPI (Calbiochem) stain for 10 min in the dark at room temperature. Cells were washed, fixed in paraformaldehyde, mounted onto slides, and visualized by confocal microscopy.
Statistical analysis was performed by the one-way analysis of variance (Anova) and Dunnett's comparison of means using JMP 3.2.6 software (SAS Institute). * denotes statistical significance. A value is deemed significant if P ≤ 0.05.
Results
C1q and MBL Recognize Apoptotic Cells.
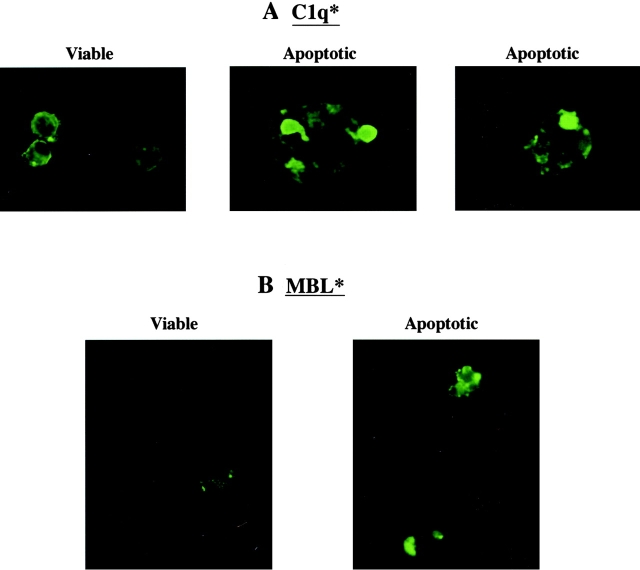
C1q has been reported to bind to surface blebs on apoptotic human keratinocytes 16 and apoptotic vascular endothelial cells 17. We show here that C1q also attaches to blebs on apoptotic Jurkat T cells (Fig. 1 A). While binding was also seen with nonapoptotic cells, the distribution in this circumstance was uniform over the cell surface without the high-density localization apparent on the apoptotic cells. This high local concentration of C1q binding may be important in interaction with the phagocyte receptor. On the other hand, MBL bound preferentially to apoptotic Jurkat T cells compared with fresh control cells (Fig. 1 B).
Figure 1.
C1q and MBL bind to the surface of apoptotic cells. Both Jurkat T cells and human neutrophils (not shown) bound C1q in their fresh and apoptotic states (A). C1q exhibited diffuse surface binding to the viable cells, while it bound in a localized pattern to apparent blebs on the surface of apoptotic cells. MBL showed little or no binding to fresh cells and robust, localized binding to the surface of apoptotic cells (B).
The experiments herein were performed using apoptotic Jurkat T cells (shown) or apoptotic human neutrophils (not shown), and comparable results were obtained with both cell types. Specific ligands on the apoptotic cells for these two proteins have not been identified, but pretreatment with mannosidase or inclusion of high concentrations of mannose (20 mM) in the medium both reduced apoptotic cell uptake and attachment of MBL to the apoptotic cells (data not shown) and support the concept that MBL is binding by virtue of its globular head groups. As isolated C1q collagenous tails did not bind to the apoptotic Jurkat T cells (data not shown), it is suggested that for C1q, too, it is the globular heads that recognize the apoptotic cell surface; however, the ligand to which it binds is not known.
Apoptotic Cell Uptake Mediated by C1q or MBL.
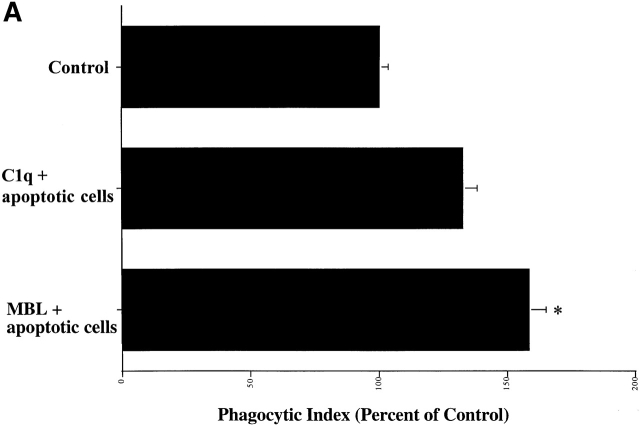
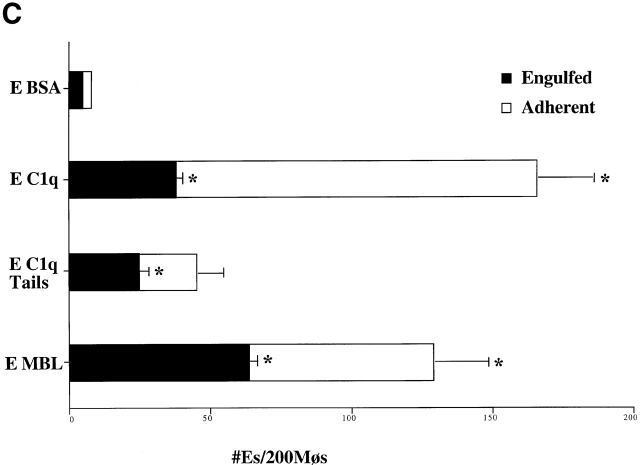
Deliberate preincubation of either C1q or MBL with the target apoptotic cells enhanced uptake of these cells by HMDMs (Fig. 2 A). While the observation supports the concept of apoptotic cell “opsonization” by the two collectin family members, there was significant uptake even in the absence of added C1q or MBL. In part this may be explained by the ability of the macrophages to synthesize their own C1q which seems to contribute to the uptake. They may also be able to synthesize MBL (preliminary data not shown). Thus, even in the absence of added C1q or MBL, antibodies to each of these proteins were able to partially reduce uptake of apoptotic Jurkat T cells (Fig. 2 B). Second, C1q and MBL-mediated uptake is not the only process involved. A number of other ligands and receptors may be active 19 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46, including a receptor for phosphatidylserine 47. To obviate this difficulty, we have recently developed a system to examine single ligands in order to address receptor usage and mechanisms of ingestion. A sandwich is created on human erythrocytes with biotin, streptavidin, and then a final coating of the biotinylated protein of interest, whether antibody or natural ligand (unpublished data). The erythrocytes neither bind nor are ingested without appropriate ligands. Moreover, hypotonic lysis can be used to distinguish bound from ingested cells 48. Erythrocytes coated with biotinylated BSA (Sigma-Aldrich) were used as a negative control; these cells neither bind to nor are taken up by macrophages. As depicted in Fig. 2 C, erythrocytes (coated with C1q, C1q collagenous tails, or with MBL) bound to, and were ingested by, human macrophages.
Figure 2.
C1q and MBL facilitate particle clearance. (A) Pretreatment of apoptotic cells with C1q or MBL: apoptotic cells (5 × 106) were incubated with 50 μg of purified C1q or MBL. These cells were tumbled for 30 min at 37°C and washed with HBSS before addition to macrophages for a phagocytosis assay (as described in Materials and Methods). n = 4 ± SEM (control mean phagocytic index = 44.2 ± 6.6, P < 0.05). (B) Antibody against human C1q and against human MBL inhibits uptake of apoptotic cells into HMDMs. HMDMs were treated with 10 μg antibody (polyclonal goat anti–human C1q, goat control antibody, mouse monoclonal anti-CD45 antibody as control, and monoclonal mouse anti–human MBL, shown) for 30 min at 37°C. Media was then aspirated, and apoptotic cells were added for 1 h for a phagocytosis assay. n = 4 ± SEM (control mean phagocytic index = 37.0 ± 6.1, *P < 0.005). (C) C1q, C1q Tails, or MBL Ebab are ingested by HMDMs. Single ligand coated particles coated with control protein, MBL, C1q, or the collagenous tail fragment of C1q were fed to HMDMs for 20 min at 37°C. The cells were then washed, fixed, and counted (as described in the Materials and Methods section). n = 3 ± SEM. P < 0.005 Engulfed; P < 0.003 Adherent.
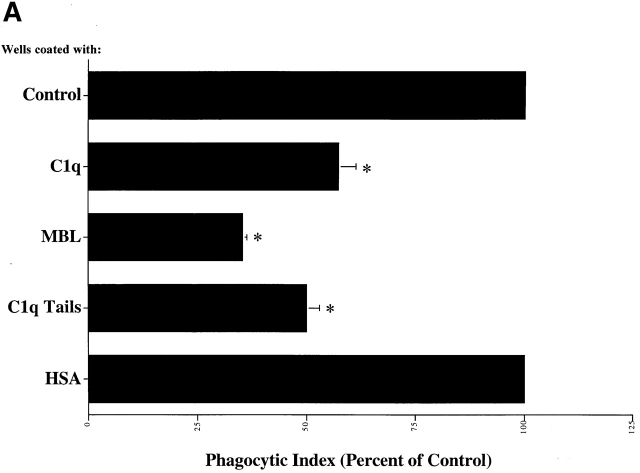
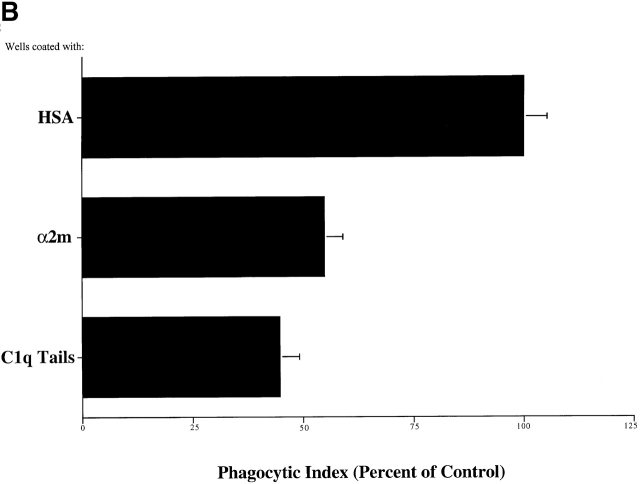
Phagocyte “Receptors” for the Collagenous Tails of C1q and MBL — The Role of CRT.
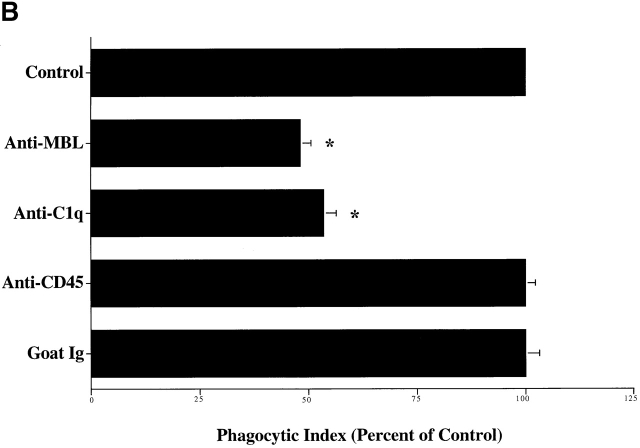
Macrophages plated on surfaces coated with purified C1q, the collagenous “tail” domain of C1q, or MBL before the addition of apoptotic targets, exhibited a decreased uptake of apoptotic Jurkat T cells, in contrast to cells plated on control proteins (Fig. 3 A). Modulation of surface receptors for C1q and MBL on the cell surface is suggested. The ability of C1q tails to modulate uptake in this system, or to induce uptake when attached to erythrocytes (Fig. 2 C), supports a model in which the C1q and MBL bind to apoptotic cells via their globular head groups and initiate uptake by interacting with phagocyte receptors for their structurally homologous collagenous tail regions.
Figure 3.
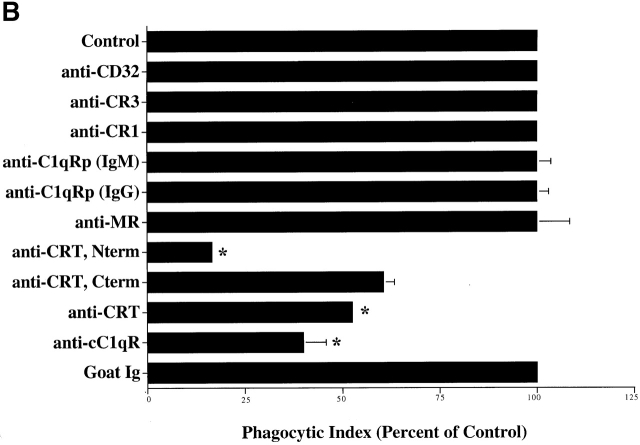
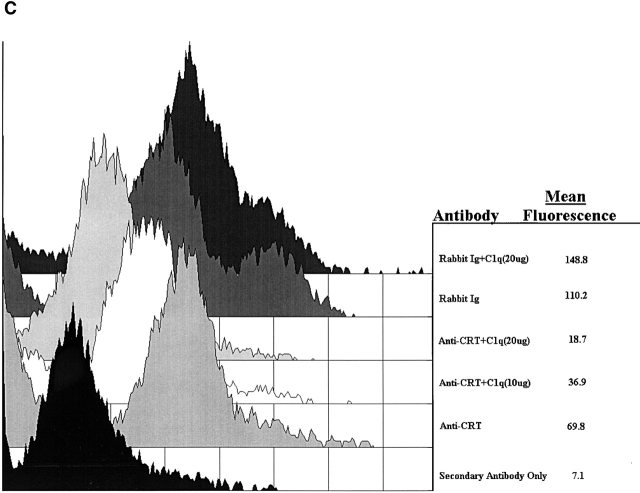
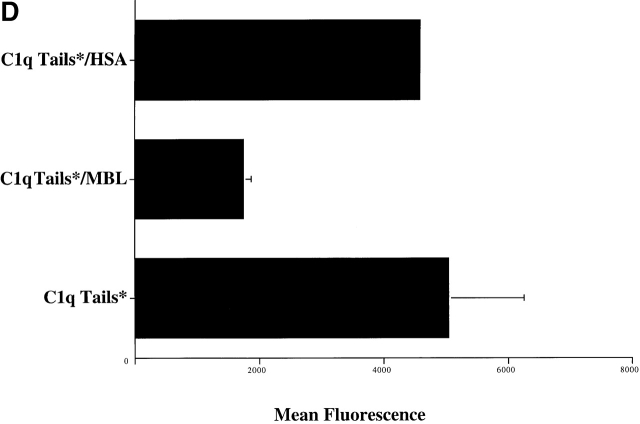
C1q and MBL facilitate clearance of apoptotic cells via cell-surface CRT. (A) Modulation of membrane receptor(s) by surface-bound C1q, C1q tails, or MBL. Macrophages were plated onto wells coated with control protein (HSA), MBL, C1q, or C1q tails. Apoptotic cells were then added for a phagocytosis assay. n = 7 ± SEM (control mean phagocytic index = 38.5 ± 4.4, P < 0.01). (B) Anti-CRT antibodies inhibit uptake of apoptotic cells by HMDMs. Other anti-receptor antibodies had no effect. anti-Nterm, anti-CRT, NH2 terminus; anti-Cterm, anti-CRT, COOH terminus; anti-MR, anti-mannose receptor; anti-CR3, anti-complement receptor 3; anti-CR1, anti-complement receptor 1. n = 4 ± SEM (control mean phagocytic index = 40.0 ± 4.3, P < 0.05). (C) Binding of anti-CRT antibody to the surface of HMDMs. Several polyclonal anti–human CRT antibodies were found to bind to the surface of HMDMs (see Materials and Methods) in a similar fashion. Binding of a rabbit anti–human polyclonal anti-CRT antibody was blocked by C1q. C1q did not inhibit binding of irrelevant antibody HMDM surfaces (inset). n = 3, representative experiment shown. (D) MBL and C1q bind to same receptor. C1q tails were FITC-conjugated (see Materials and Methods) and bound to HMDM surfaces. Unlabeled, whole MBL decreased this binding when incubated with the HMDMs along with the FITC-labeled tails, n = 3.
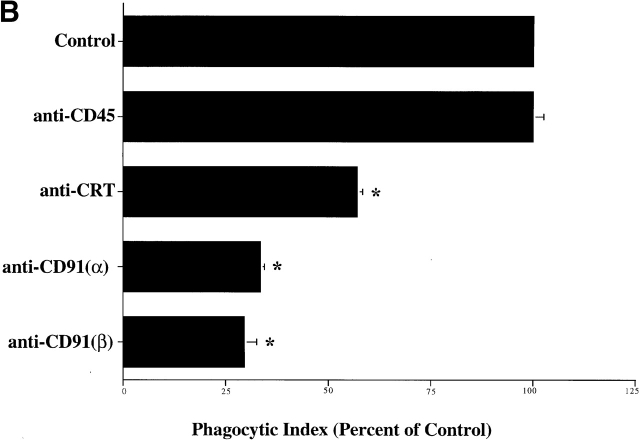
A number of C1q and collectin receptors have been proposed (1, 49, 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91). Accordingly, antibodies to many of these candidate molecules were examined for the ability to block uptake of apoptotic cells. Only antibodies to the cC1q receptor (for “collagenous tail C1q receptor”; reference 65), and CRT, molecules now known to be identical 49, were able to inhibit uptake (Fig. 3 B). Uptake of erythrocytes coated with MBL or C1q tails was also blocked by a variety of different antibodies directed against CRT, particularly those raised against the N domain of the molecule. As shown in Fig. 2 C, C1q-coated erythrocytes were also taken up by the macrophages; however, inhibition of uptake with anti-CRT was not performed here, for fear that the globular heads of intact C1q would bind antibody nonspecifically, giving a false positive result.
Despite its known role as a chaperone and calcium-binding protein of the endoplasmic reticulum, CRT has been demonstrated on the surface of a large number of cell types 55 92 93 94 95 96 and to bind the collagenous tails of members of the collectin family, including C1q 2 97. As shown in Fig. 3 C, rabbit anti-CRT antibody readily detected this molecule on the surface of HMDMs and this binding was blocked by pretreatment of the cells with C1q or C1q collagen tails (not shown). Antibody to human CD45, used as a positive control for binding, was not prevented from binding to the macrophage cell surface by C1q tails.
Finally, experiments were performed to support the suggestion that MBL and C1q interact with the same receptor. As shown in Fig. 3 D, MBL was able to inhibit the binding of FITC-tagged C1q tails to HMDM surfaces (10 μg/ml protein used). Irrelevant protein (HSA) had no effect on C1q tail binding. Conversely, C1q inhibited binding of FITC-tagged MBL to HMDM surfaces (not shown).
A Role for CD91 in Collectin–CRT Signaling for Ingestion.
While CRT is present on the phagocyte surface, it is not clear how it gets there. As a protein with no transmembrane domain, it also presumably requires a partner to transduce the signals to initiate engulfment. Recent evidence has been presented by Basu et al. demonstrating the binding of CRT, as well as various heat shock proteins, to CD91 on the surface of macrophages (among many other cell types 98). This protein, better known as the α-2-macroglobulin receptor or LRP, can signal for the endocytosis of adherent proteins. Accordingly it was examined as a candidate signaling partner for CRT in the C1q and MBL-mediated uptake of apoptotic cells.
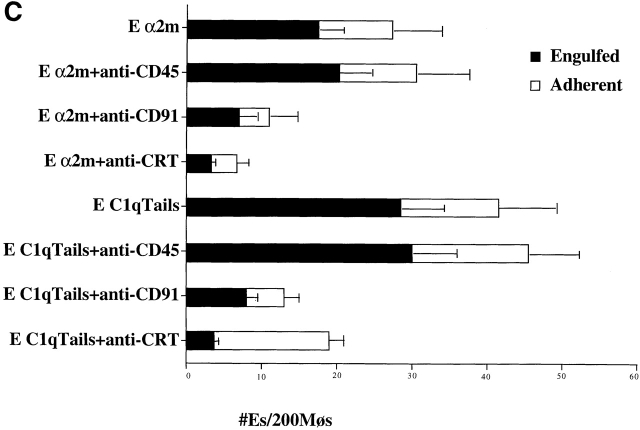
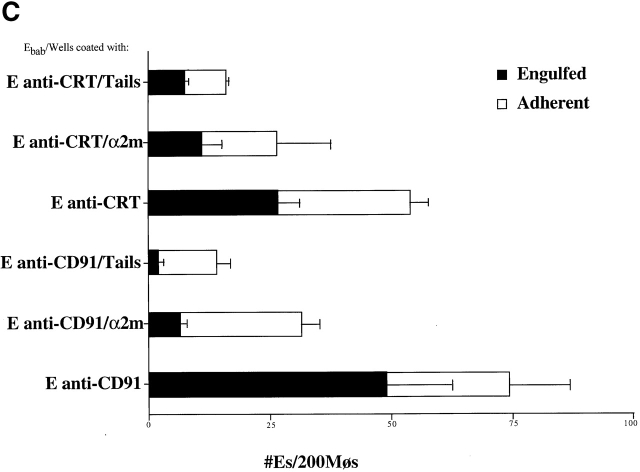
First, CRT and CD91 were seen to colocalize on the surface HMDM cell membrane (Fig. 4 A). Second, antibodies to CD91 were found to block the uptake of apoptotic Jurkat T cells by HMDMs (Fig. 4 B), as well as of erythrocytes carrying C1q tails, a ligand for CRT, or α2m, a ligand for CD91 (Fig. 4 C). The ability of anti-CRT (CRT) pretreatment to reduce uptake of α2m Ebab suggests preassociation of CRT with CD91 so that antibody can modulate/block the CD91/CRT complex and prevent α2m Ebab binding or engulfment.
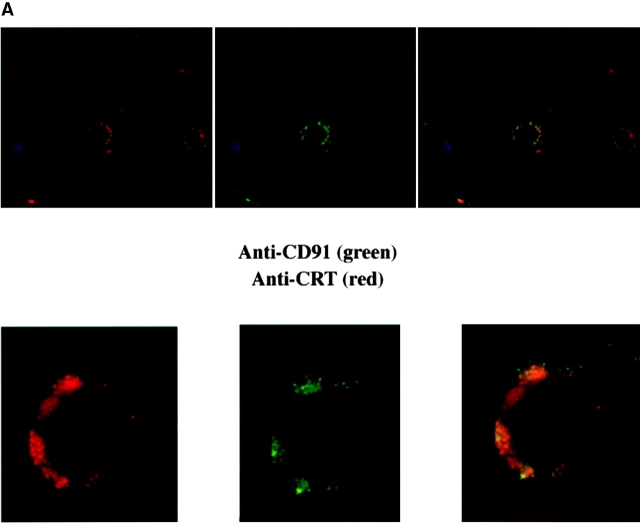
Figure 4.
CD91 is involved in C1q- and MBL-mediated uptake via CRT. (A) CRT colocalizes with CD91 on the HMDM cell surface. HMDMs were stained with chicken anti–human CRT (anti–N-terminus, shown) and mouse anti–human CD91α and β (see Materials and Methods). Antibody staining was detected with FITC anti–mouse and Cy3 anti–chicken. n = 3, representative experiment shown. (B) Uptake of apoptotic cells is inhibited by antibody to CD91. HMDMs were preincubated with anti-CD91 antibody for 30 min at 37°C. Media was then aspirated and apoptotic cells were added for 1 h for a phagocytosis assay (see Materials and Methods). anti-CRT, chicken anti-CRT, NH2 terminus; anti-CD91α, anti-CD91, α chain; anti-CD91β, anti-CD91, β chain. n = 3 ± SEM (control mean phagocytic index = 20.3 ± 1.5, P < 0.002). (C) C1q tail Ebab and α2m Ebab are taken up into HMDMs; this uptake is inhibited by anti-CD91 and by anti-CRT. Ebab coated with C1q tails, α2m, or BSA (not shown) were fed to HMDMs for 20 min at 37°C (see Materials and Methods). n = 4. P < 0.1 Engulfed; P < 0.1 Adherent.
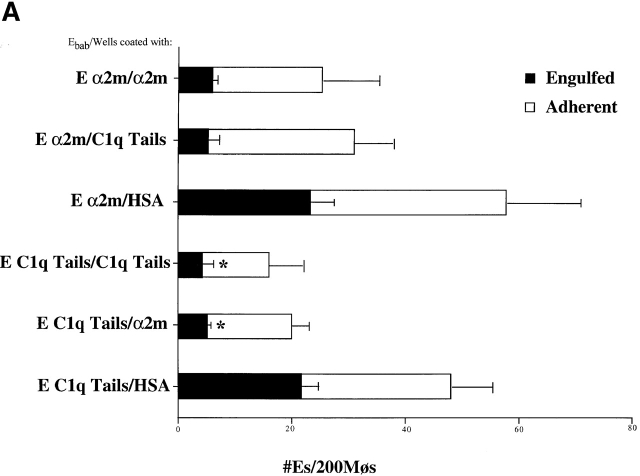
In Fig. 5 are depicted receptor modulation experiments to support the triple involvement of C1q or MBL, CRT, and CD91. Plating the macrophages on wells precoated with ligand to CD91, α2m, led to impairment of their ability to recognize and engulf erythrocytes coated with C1q tails or α2m (Fig. 5 A); engulfment of anti-CD32 coated Ebab was unaffected (not shown). These macrophages also showed reduced uptake of apoptotic cells (Fig. 5 B). Plating the macrophages on C1q tails had the same effect (Fig. 5a and Fig. b) and, by reducing binding of erythrocytes coated with α2m, supports the suggestion that these two molecules are associated on the membrane, are similarly modulated, and are both required for uptake of C1q- or MBL-bound cells. Fig. 5 C further supports this hypothesis, demonstrating an inhibition of clearance of anti-CD91 or anti-CRT coated Ebab by macrophages plated onto wells coated with α2m or C1q tails.
Figure 5.
CD91 and CRT facilitate particle clearance. (A) α2m and C1q Tails modulate the uptake of tail Ebab and of α2m Ebab. HMDMs were plated onto wells coated with either HSA, α2m, or C1q tails (see Materials and Methods). These macrophages were then fed erythrocytes coated with C1q tails, α2m, or BSA (not shown) (α2m/HSA, for example, stands for α2m Ebab fed to macrophages plated onto wells coated with HSA). n = 3 ± SEM. P < 0.02 Engulfed. (B) α2m and C1q tails modulate the uptake of apoptotic cells. HMDMs were plated onto wells coated with HSA, α2m, or C1q tails. Apoptotic cells were fed to the macrophages for 1 h at 37°C for a phagocytosis assay. n = 3 ± SEM (control mean phagocytic index= 21.3 ± 5.3, P < 0.34). (C) α2m or C1q tails modulate the uptake of anti-CD91 or anti-CRT Ebab. HMDMs were plated onto wells coated with HSA, α2m, or C1q tails. The macrophages were then fed erythrocytes coated with BSA, anti-CD32 (neither shown), anti-CD91, or anti-CRT, NH2 terminus (here, anti-CRT). These Ebab were incubated with the HMDMs for 20 min. n = 3 ± SEM. P < 0.11 Engulfed; P < 0.06 Adherent.
Mevorach et al. 46 have reported the opsonization by serum of apoptotic cells and resultant increase in phagocytosis of these cells by macrophages. Complement components present in the serum have been implicated as the opsonizing agents. In vivo, C1q and MBL present in the plasma or inflammatory exudate likely represent the major source for possible apoptotic cell opsonization. Accordingly, preincubation of the apoptotic cells with serum enhanced uptake and, as expected, this was blocked by anti-CRT and by anti-CD91 antibodies (data not shown).
C1q- or MBL-mediated Engulfment Occurs via CD91-induced Macropinocytosis.
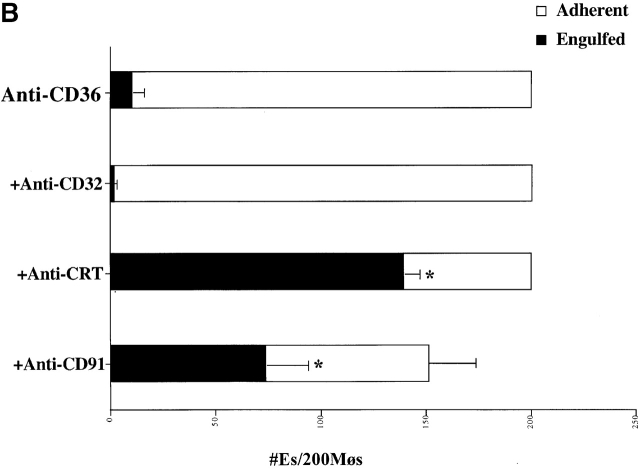
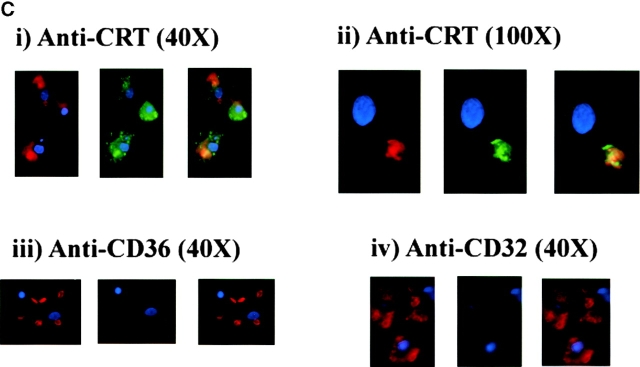
In exploring the mechanisms of apoptotic cell uptake through the PS receptor we have recently provided evidence that engulfment appeared to occur by a process of “stimulated phagocytosis” or macropinocytosis (unpublished data). Importantly, surface CRT or CD91 ligation also appears to initiate macropinocytosis. Thus, either anti-CRT or anti-CD91 was shown capable of inducing uptake of the water-soluble dye Lucifer Yellow into large macropinosomes within the macrophages (Fig. 6 A). Antibodies to unrelated surface molecules did not have this property (some background pinocytosis was seen in stimulated as well as control macrophages), although the growth factor M-CSF was effective (unpublished data). To confirm the involvement of a stimulated macropinocytosis mechanism, ligation of CRT or CD91 was used to initiate bystander uptake of previously attached erythrocytes (Ebab). Ebab were coated with antibodies to another putative apoptotic cell recognition molecule, the B type scavenger receptor, CD36 19 27. The anti-CD36 Ebab became tethered to the macrophage surface but were not internalized. The addition of soluble anti-CRT or anti-CD91 antibodies, but not anti-CD32 antibody initiated internalization of the tethered erythrocytes (Fig. 6 B). Crosslinking of these antibodies was even more effective. Finally, erythrocytes were prelabeled with an intracellular red dye (Texas red succinimidyl ester) and then coated with antibodies or collectin ligands for uptake. Ingestion by the macrophages in the presence of Lucifer Yellow led to phagosomes with coingested dye (Fig. 6 C). This implies a spacious phagosome with significant concurrent ingestion of extracellular fluid, an effect more consistent with macropinocytosis 99 100 101 102 than with the “zipper” mechanism used during uptake via the FcR 103 104. A similar effect was seen with whole apoptotic cells but in this case could not definitively be attributed to effects mediated by C1q or MBL or through CD91 since other processes (e.g., the phosphatidylserine receptor) may also be operative. The data support the hypothesis that macropinocytosis is the primary mechanism for uptake of apoptotic cells.
Figure 6.
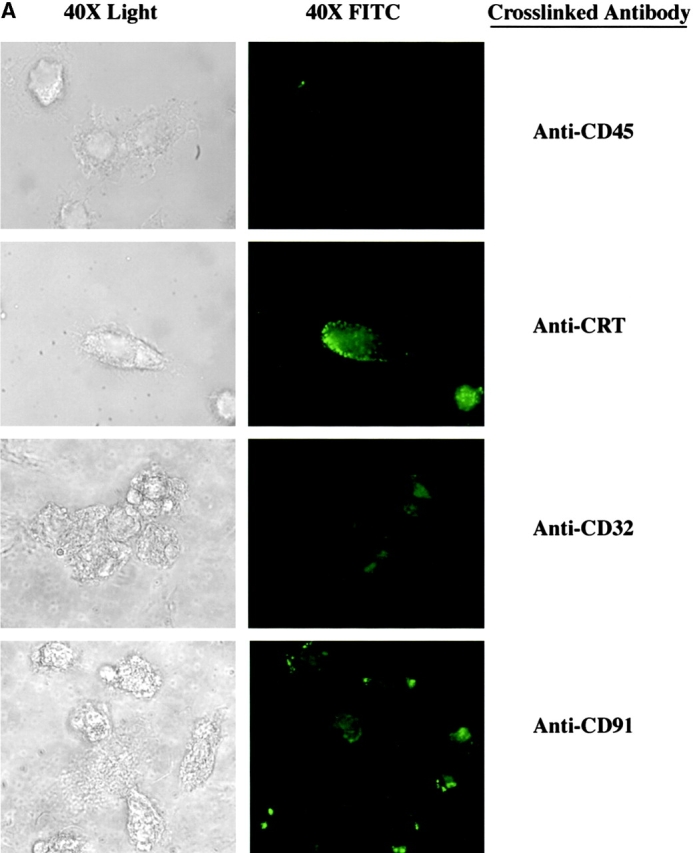
Uptake through CRT/CD91 occurs through a mechanism of macropinocytosis. (A) Stimulation of CRT and of CD91, but not of CD45 or CD32, initiate macropinocytosis. Macrophages were incubated in the presence of Lucifer Yellow. Anti-CD45 (above right) or anti-CRT, NH2 terminus (anti-CRT) (bottom right) were added to the macrophages (5 μg/ml) and allowed to interact for 15 min at room temperature. Cross-linking antibodies were then added (2.5 μg/ml), and the macrophages were incubated with Lucifer Yellow in the dark at 37°C for 5 min. Anti-CRT and anti-CD91 antibodies stimulated macropinocytosis and subsequent uptake of Lucifer Yellow dye, whereas anti-CD45 did not. (B) Bystander uptake of adherent particles by HMDMs occurs via CD91- and CRT-stimulated macropinocytosis. Chicken anti–human CRT (N-terminus) or mouse anti–human CD91 were used to stimulate HMDMs with anti-CD36–coated particles adherent to their surfaces. Cross-linking antibodies were then added to enhance the effect and phagocytosis was allowed to occur for 20 min. Each bar represents the total number of adherent cells plus engulfed cells. n = 5 ± SEM. P < 0.0001 Engulfed. (C) Engulfment of erythrocytes (E) coated with anti-CRT antibody involves formation of spacious phagosomes and concomitant uptake of Lucifer Yellow. RBCs labeled with anti-CRT, NH2 terminus (anti-CRT) (i and ii), anti-CD36 (iii), or anti-CD32 (iv) were stained with Texas red and added to HMDMs in the presence of Lucifer Yellow. Anti-CD36 RBCs (iii) were not taken up into the macrophages, anti-CD32 RBCs (iv) were ingested by macrophages, but with no accompanying uptake of Lucifer Yellow. In contrast, anti-CRT RBCs (i and ii) were taken up into HMDMs along with Lucifer Yellow, which colocalized in the same phagosome. HMDM nuclei stained with DAPI (blue).
Discussion
Collectins are proteins of the innate immune system involved in pattern recognition and opsonization of foreign particles. Recent evidence suggests a role for other members of the collectin family, specifically SP-A, in the clearance of apoptotic cells 105.
Here, we propose that a member of this family, MBL, and a close relative, C1q, bind to apoptotic cells and initiate their uptake into macrophages. MBL was found to bind primarily to apoptotic cells and showed little evidence of interaction with viable cells. C1q has been reported to bind to the surface of a number of different cell types 55 106 107 108 109 110 111 112 and here was shown to attach to both viable and apoptotic Jurkat T cells. Despite this binding, only apoptotic cells are engulfed by macrophages. However, a careful examination of the of C1q binding revealed a clustered distribution on apoptotic cells. This was similar to C1q binding shown previously on apoptotic keratinocytes 16 or vascular endothelial cells 17. On viable Jurkat T cells, C1q binding was diffuse. Reexamination of MBL attachment suggested a similar distribution on apoptotic cells and implicates significantly altered, but to date uncharacterized, surface structures on the membrane blebs of apoptotic cells.
The multifunctional properties and binding sites on MBL and C1q make the orientation of their attachment to the apoptotic cells difficult to clearly identify. The data presented herein suggest that for productive initiation of apoptotic cell engulfment, C1q and MBL bind to the apoptotic cells by their globular domains and to the phagocyte through their collagen-like tails.
The globular head regions of the collectins are known to be involved in pattern recognition and binding and it is suggested that they interact with the ligands on the surface of the apoptotic cells. A report of increased mannose expression on apoptotic hepatocytes and lymphocytes is noteworthy in this regard 23 113 114 115. It can be inferred from the data that after binding and/or aggregation of the collectins on the apoptotic cell surface, their collagenous tails interact with receptor on the surface of the phagocyte. It seems likely that this interaction is low affinity and requires significant aggregation to mediate signaling and uptake.
Complement components have been shown to bind to and opsonize apoptotic cells 46 116 117. Preincubation of the apoptotic cells with C1q or MBL resulted in some increased uptake, suggesting opsonization of the target cells (viable cells, regardless of pretreatment, were not engulfed by macrophages). However, significant ingestion was seen even in the absence of prior opsonization and this uptake was markedly reduced in the presence of anti-C1q or anti-MBL. The data suggest involvement of the two collectin family members even in the absence of added protein and imply production by the macrophages. Macrophages are known to synthesize and secrete C1q 3 and preliminary experiments suggest they may produce MBL as well (data not shown). In vivo, these two proteins will likely be supplied from plasma, and addition of serum to the macrophage uptake system did increase the ingestion of apoptotic, but not viable, cells (data not shown). On the other hand, not all of the uptake is expected to occur via these C1q or MBL processes and, as with most attempts to identify apoptotic uptake receptors by ligand blockade 19 118 119, inhibition was never complete.
Preincubation of the phagocytes with the C1q or MBL before addition of apoptotic targets resulted in a reduced phagocytic index. Plating macrophages onto wells coated with C1q or MBL before adding target apoptotic cells yielded the same result, suggested to reflect modulation of the phagocytic receptors. The ability of isolated C1q tails to similarly modulate the functional receptor further supports the concept that it is this portion of the protein that is primarily recognized by the phagocytes.
The redundancy of recognition and uptake mechanisms driven by multiple ligands and receptors makes detailed analysis of any one of these particularly complex. To simplify the examination of C1q- or MBL-induced uptake, a single ligand-coated particle was used. Human erythrocytes were coated with biotin (Eb), and a sandwich was created with avidin and the biotinylated ligand of choice (Ebab-X). These Ebab cells have a number of advantages, including the ability to distinguish binding to the phagocyte surface from engulfment as well as complete absence of binding and engulfment with control erythrocytes. This system demonstrated that Ebab coated with C1q, MBL, or purified C1q collagenous tails both bound to, and were directly taken up into, macrophages.
Several receptors have been postulated to play a role in C1q- or collectin-mediated uptake of particles by macrophages or other cell types. These include CR1, CR3, C1qRp, and cC1qR 50 59 60 61 65 69 97. Of these, only antibodies to the cC1qR, or CRT, were found to be effective in inhibiting phagocytosis of apoptotic cells by HMDMs. CRT has been shown by several groups to bind to the collagenous tail regions of MBL, SP-A, conglutinin, and C1q 1 74 or SPD (unpublished data). In fact, all anti-CRT antibodies tested were effective in inhibiting the apoptotic cell engulfment, including polyclonal antibodies produced against the N-domain of the protein, which contains the region shown to bind C1q collagenous tails 78 120. The efficacy of antibody against the C-domain may result from blockade of collectin or C1q tail binding or perhaps as a consequence of receptor modulation. Antibodies to a wide variety of alternative receptors or binding sites were inactive. Ingestion of Ebab coated with the purified tail region of C1q, which shares functional homology with the collagenous tail region of MBL and other collectins, was also inhibited by antibody against CRT.
Although CRT serves a number of critical functions within the endoplasmic reticulum 78 93, it has also been shown to be expressed on the surface of many cell types, including human macrophages 49 84 86 96 121 122. Although cultured HMDMs display much phenotypic heterogeneity 123, a large portion of the population tested demonstrated surface expression of CRT. However, the mode of access to the cell surface and the mechanism of retention at this site is not at all clear — it may be transported from the endoplasmic reticulum or bind to the cell surface from the extracellular milieu 98 124. However, either route could readily allow for attachment of C1q or MBL collagenous tails. On the other hand, as CRT is not a transmembrane protein, actual signaling for uptake of apoptotic cells must be presumed to involve some other membrane structure recruited for the process.
A recent report has suggested that CRT may bind to the multifunctional receptor, CD91 98; we show colocalization of CRT with CD91 on the HMDM cell surface (Fig. 4 A). This molecule, also known as the α2m receptor or LRP, is a type 1, 600-kD transmembrane protein made up of an α and a β chain which are noncovalently, but tightly attached. Importantly, the receptor has a 100 amino acid cytoplasm tail that contains two NPXY endocytosis signal sequences 125. It shares sequence homology in the cytoplasmic tail with other receptors used for endocytosis, including the newly cloned ced-1 gene from Caenorhabiditis elegans known to play a role in the phagocytosis of apoptotic cells in these organisms 126 127. CD91 is found on the surface of HMDMs (among many other cell types) and appeared to be a prime candidate for signaling for apoptotic cell uptake into these cells. Accordingly, antibodies to CD91 were found effective in inhibiting uptake of apoptotic cells, or Ebab coated with MBL, C1q, or C1q collagenous tails in exactly the same fashion as shown for anti-CRT antibodies.
The hypothesis, then, is that CRT acts to bind the collagenous tails of C1q and MBL and then signals for uptake due to its interaction with CD91. To support this likelihood, a number of different experiments were performed. Importantly, CD91 could be modulated from the upper surface of macrophages by plating the cells onto wells coated with a ligand for this molecule, α2m. Not only were the upper macrophage surfaces depleted of CD91 (shown by reduction in attachment of Ebab-anti-CD91) but also in CRT (shown by reduction of attachment of anti-CRT Ebab). Plating macrophages onto wells coated with control proteins did not modulate these receptors. Macrophages plated on either α2M or C1q tails showed diminished binding or uptake of Ebab coated with MBL, C1q, C1q tails, or α2m (Ebab-α2m were also shown to be bound and ingested by the macrophages). These macrophages were still able to bind and ingest Ebab coated with anti-Fc receptor antibody (anti-CD32 Ebab). Finally, modulation of the macrophage receptors by plating the macrophages onto wells coated with either α2m or C1q tails blocked uptake of apoptotic cells. It seems reasonable to suggest that this demonstration of a role for CD91 in collectin family mediated ingestion of apoptotic cells may be extended to systems in which these innate immune system, pattern recognition molecules participate in recognition and removal of foreign organisms and cell debris.
We have recently provided evidence that uptake of apoptotic cells through the phosphatidylserine receptor is mediated by a process of macropinocytosis (unpublished data). Data presented herein support the ability of CRT/CD91 ligation by C1q or MBL to induce macropinocytosis and that this is the mechanism by which these collectin family members initiate uptake of apoptotic cells. Thus anti-CRT and anti-CD91 antibodies were shown to directly stimulate macropinocytosis of the water soluble dye Lucifer Yellow. Inclusion of this dye during uptake of apoptotic cells resulted in simultaneous ingestion of apoptotic cell and dye (data not shown). Similarly in these studies, Ebab coated with anti-CRT were shown to be ingested in a fashion that was accompanied by Lucifer Yellow uptake into the same compartment. This could be contrasted with uptake of Ebab via the Fc receptor. Finally, CRT and CD91 were shown capable of mediating bystander uptake of attached cells into macrophages. Erythrocytes coated with an anti-CD36 antibody were found to attach to the macrophage surface without subsequent ingestion (Fig. 6 C). Antibody ligation of either surface CRT or CD91 in this system was shown to drive engulfment of the attached E (Fig. 6 B). The growth factor M-CSF had the same effect on attached erythrocytes (not shown). Antibodies to other surface molecules, including anti-FcR, had no effect on uptake. In these systems involving stimulation of CRT and CD91 with antibody, crosslinking the primary antibody led to an enhanced effect. The multivalency of C1q and the collectins, as well as their localized binding to the apoptotic cell surface, is in keeping with this concept. Similarly, CD91 mediates endocytosis of α2m/proteinase complexes most effectively when crosslinked (Fig. 6 A).
The ability of CRT and CD91 ligation to initiate macropinocytosis, therefore, suggests a mechanism for uptake of apoptotic cells. It may also implicate macropinocytosis, as well as CD91 involvement as an explanation for the known ability of collectins to enhance phagocytosis of foreign particles and organisms 1 52 110. We also suspect their similar participation in recognition and uptake of cell debris during removal of damaged and necrotic cells. C1q avidly binds via its globular head groups to free mitochondria 128 129. Pattern recognition by collectin family members of the innate immune system could, by engaging CRT and then CD91, provide significantly enhanced signaling potential, not only quantitatively, but also, because of the broad specificity of their globular head recognition domains, to involve a very wide variety of ligands. Finally, CD91 engagement has been shown to initiate maturation of immature, dendritic cells 124 and enhancement of antigen presentation 130.
C1q and MBL bind to and facilitate the ingestion of apoptotic cells by human macrophages. The structurally and functionally similar collagenous tails of C1q and MBL bind to CRT, which, in turn is bound to CD91 on the macrophage cell surface. Engagement of this receptor initiates a process of macropinocytosis and eventual engulfment of the apoptotic cell. This mechanism, which involves pattern recognition molecules of the innate immune system, a multifunctional cellular protein, and an evolutionarily conserved clearance receptor, may be an ancient method the body has evolved for ridding itself of a potentially harmful source of self-antigen.
Acknowledgments
This work was supported by National Institutes of Health grants GM 48211 and GM 61031.
Footnotes
Abbreviations used in this paper: CRT, calreticulin; HMDM, human monocyte-derived macrophage; HSA, heat stable antigen; LRP, LDL receptor–related protein; MBL, mannose binding lectin; SLE, systemic lupus erythematosus.
References
- Holmskov U., Malhotra R., Sim R.B., Jensenius J.C. Collectinscollagenous C-type lectins of the innate immune defense system. Immunol. Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Hansen S., Holmskov U. Structural aspects of collectins and receptors for collectins. Immunobiology. 1998;199:165–189. doi: 10.1016/S0171-2985(98)80025-9. [DOI] [PubMed] [Google Scholar]
- Loos M., Martin H., Petry F. The biosynthesis of C1q, the collagen-like and Fc-recognizing molecule of the complement system. Behring Inst. Mitt. 1989;84:32–41. [PubMed] [Google Scholar]
- Bowness P., Davies K.A., Norsworthy P.J., Athanassiou P., Taylor-Wiedeman J., Borysiewicz L.K., Meyer P.A., Walport M.J. Hereditary C1q deficiency and systemic lupus erythematosus. QJM. 1994;87:455–464. [PubMed] [Google Scholar]
- Carroll M.C. The lupus paradox. Nat. Gen. 1998;19:3–4. doi: 10.1038/ng0598-3. [DOI] [PubMed] [Google Scholar]
- Petry F. Molecular basis of hereditary C1q deficiency. Immunobiology. 1998;199:286–294. doi: 10.1016/S0171-2985(98)80033-8. [DOI] [PubMed] [Google Scholar]
- Stone N.M., Williams A., Wilkinson J.D., Bird G. Systemic lupus erythematosus with C1q deficiency. Br. J. Dermatol. 2000;142:521–524. doi: 10.1046/j.1365-2133.2000.03369.x. [DOI] [PubMed] [Google Scholar]
- Tsao B.P. Genetic susceptibility to lupus nephritis. Lupus. 1998;7:585–590. doi: 10.1191/096120398678920695. [DOI] [PubMed] [Google Scholar]
- Sullivan K.E., Wooten C., Goldman D., Petri M. Mannose-binding protein genetic polymorphisms in black patients with systemic lupus erythematosus. Arthritis Rheum. 1996;12:2046–2051. doi: 10.1002/art.1780391214. [DOI] [PubMed] [Google Scholar]
- Thomas H.C., Foster G.R., Sumiya M., McIntosh D., Jack D.L., Turner M.W., Summerfield J.A. Mutation of gene of mannose-binding protein associated with chronic hepatitis B viral infection. Lancet. 1996;348:1417–1419. doi: 10.1016/s0140-6736(96)05409-8. [DOI] [PubMed] [Google Scholar]
- Davies E.J., Snowden N., Hillarby M.C., Carthy D., Grennan D.M., Thomson W., Ollier W.E. Mannose-binding protein gene polymorphism in systemic lupus erythematosus. Arthritis Rheum. 1995;38:110–114. doi: 10.1002/art.1780380117. [DOI] [PubMed] [Google Scholar]
- Gabolde M., Guilloud-Bataille M., Feingold J., Besmond C. Association of variant alleles of mannose binding lectin with severity of pulmonary disease in cystic fibrosiscohort study. BMJ. 1999;319:1166–1167. doi: 10.1136/bmj.319.7218.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto M., Dell'Agnola C., Bygrave A.E., Thompson E.M., Cook H.T., Petry F., Loos M., Pandolfi P.P., Walport M.J. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Mevorach D., Zhou J.L., Song X., Elkon K.B. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J. Exp. Med. 1998;188:387–392. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P.R., Carugati A., Fadok V.A., Cook H.T., Andrews M., Carroll M.C., Savill J.S., Henson P.M., Botto M., Walport M.J. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J. Exp. Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb L.C., Ahearn J.M. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytescomplement deficiency and systemic lupus erythematosus revisited. J. Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- Navratil J.S., Watkins S.C., Wisnieski J.J., Ahearn J.M. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J. Immunol. 2001;166:3231–3239. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- Haslett C., Guthrie L.A., Kopaniak M.M., Johnston R.B., Jr., Henson P.M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am. J. Pathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- Fadok V.A., Warner M.L., Bratton D.L., Henson P.M. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3) J. Immunol. 1998;161:6250–6257. [PubMed] [Google Scholar]
- Ezekowitz R.A., Kuhlman M., Groopman J.E., Byrn R.A. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J. Exp. Med. 1989;169:185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K.B. Isolation, by partial pepsin digestion, of the three collagen-like regions present in subcomponent Clq of the first component of human complement. Biochem. J. 1976;155:5–17. doi: 10.1042/bj1550005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall E., Wyllie A.H., Morris R.G. Macrophage recognition of cells undergoing programmed cell death (apoptosis) Immunology. 1985;56:351–358. [PMC free article] [PubMed] [Google Scholar]
- Dini L., Lentini A., Diez G.D., Rocha M., Falasca L., Serafino L., Vidal-Vanaclocha F. Phagocytosis of apoptotic bodies by liver endothelial cells. J. Cell Sci. 1995;108:967–973. doi: 10.1242/jcs.108.3.967. [DOI] [PubMed] [Google Scholar]
- Fadok V.A., Savill J.S., Haslett C., Bratton D.L., Doherty D.E., Campbell P.A., Henson P.M. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 1992;149:4029–4035. [PubMed] [Google Scholar]
- Fadok V.A., Henson P.M. Apoptosisgetting rid of the bodies. Curr. Biol. 1998;8:R693–R695. doi: 10.1016/s0960-9822(98)70438-5. [DOI] [PubMed] [Google Scholar]
- Savill J., Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- Savill J. Recognition and phagocytosis of cells undergoing apoptosis. Br. Med. Bull. 1997;53:491–508. doi: 10.1093/oxfordjournals.bmb.a011626. [DOI] [PubMed] [Google Scholar]
- Gregory C.D., Devitt A., Moffatt O. Roles of ICAM-3 and CD14 in the recognition and phagocytosis of apoptotic cells by macrophages. Biochem. Soc. Trans. 1998;26:644–649. doi: 10.1042/bst0260644. [DOI] [PubMed] [Google Scholar]
- Moffatt O.D., Devitt A., Bell E.D., Simmons D.L., Gregory C.D. Macrophage recognition of ICAM-3 on apoptotic leukocytes. J. Immunol. 1999;162:6800–6810. [PubMed] [Google Scholar]
- Devitt A., Moffatt O.D., Raykundalia C., Capra J.D., Simmons D.L., Gregory C.D. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- Gregory C.D. CD14-dependent clearance of apoptotic cellsrelevance to the immune system. Curr. Opin. Immunol. 2000;12:27–34. doi: 10.1016/s0952-7915(99)00047-3. [DOI] [PubMed] [Google Scholar]
- Gregory C.D. Non-inflammatory/anti-inflammatory CD14 responsesCD14 in apoptosis. Chem. Immunol. 2000;74:122–140. [PubMed] [Google Scholar]
- Ramprasad M.P., Fischer W., Witztum J.L., Sambrano G.R., Quehenberger O., Steinberg D. The 94- to 97-kDa mouse macrophage membrane protein that recognizes oxidized low density lipoprotein and phosphatidylserine-rich liposomes is identical to macrosialin, the mouse homologue of human CD68. Proc. Natl. Acad. Sci. USA. 1995;92:9580–9584. doi: 10.1073/pnas.92.21.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth R., Platt N., Keshav S., Hughes D., Darley E., Suzuki H., Kurihara Y., Kodama T., Gordon S. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J. Exp. Med. 1997;186:1431–1439. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt N., Suzuki H., Kodama T., Gordon S. Apoptotic thymocyte clearance in scavenger receptor class A-deficient mice is apparently normal. J. Immunol. 2000;164:4861–4867. doi: 10.4049/jimmunol.164.9.4861. [DOI] [PubMed] [Google Scholar]
- Platt N., Suzuki H., Kurihara Y., Kodama T., Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc. Natl. Acad. Sci. USA. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi A., Nakanishi Y. Elimination of unwanted cells by phagocytes. Seikagaku. 1999;71:282–286. [PubMed] [Google Scholar]
- Yamada Y., Doi T., Hamakubo T., Kodama T. Scavenger receptor family proteinsroles for atherosclerosis, host defence and disorders of the central nervous system. Cell. Mol. Life Sci. 1998;54:628–640. doi: 10.1007/s000180050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka K., Sawamura T., Kikuta K., Itokawa S., Kume N., Kita T., Masaki T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc. Natl. Acad. Sci. USA. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto Y., Ohashi K., Mizuno K., Nakano T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J. Biochem. 2000;127:411–417. doi: 10.1093/oxfordjournals.jbchem.a022622. [DOI] [PubMed] [Google Scholar]
- D'Cruz P.M., Yasumura D., Weir J., Matthes M.T., Abderrahim H., LaVail M.M., Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- Nandrot E., Dufour E.M., Provost A.C., Pequignot M.O., Bonnel S., Gogat K., Marchant D., Rouillac C., Sepulchre de Conde B., Bihoreau M.T. Homozygous deletion in the coding sequence of the c-mer gene in RCS rats unravels general mechanisms of physiological cell adhesion and apoptosis. Neurobiol. Dis. 2000;7:586–599. doi: 10.1006/nbdi.2000.0328. [DOI] [PubMed] [Google Scholar]
- Bondanza A., Sabbadini M.G., Pellegatta F., Zimmermann V.S., Tincani A., Balestrieri G., Manfredi A.A., Rovere P. Anti-beta2 glycoprotein I antibodies prevent the De-activation of platelets and sustain their phagocytic clearance. J. Autoimmun. 2000;15:469–477. doi: 10.1006/jaut.2000.0449. [DOI] [PubMed] [Google Scholar]
- Chan A., Magnus T., Gold R. Phagocytosis of apoptotic inflammatory cells by microglia and modulation by different cytokinesmechanism for removal of apoptotic cells in the inflamed nervous system. Glia. 2001;33:87–95. doi: 10.1002/1098-1136(20010101)33:1<87::aid-glia1008>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Rovere P., Sabbadini M.G., Vallinoto C., Fascio U., Recigno M., Crosti M., Ricciardi-Castagnoli P., Balestrieri G., Tincani A., Manfredi A.A. Dendritic cell presentation of antigens from apoptotic cells in a proinflammatory contextrole of opsonizing anti-beta2-glycoprotein I antibodies. Arthritis Rheum. 1999;42:1412–1420. doi: 10.1002/1529-0131(199907)42:7<1412::AID-ANR15>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Mevorach D., Mascarenhas J.O., Gershov D., Elkon K.B. Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V.A., Bratton D.L., Rose D.M., Pearson A., Ezekewitz R.A., Henson P.M. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- Gigli I., Nelson R.A., Jr. Complement dependent immune phagocytosis. I. Requirements for C'1, C'4, C'2, C'3. Exp. Cell Res. 1968;51:45–67. doi: 10.1016/0014-4827(68)90158-4. [DOI] [PubMed] [Google Scholar]
- Stuart G.R., Lynch N.J., Day A.J., Schwaeble W.J., Sim R.B. The C1q and collectin binding site within C1q receptor (cell surface calreticulin) Immunopharmacology. 1997;38:73–80. doi: 10.1016/s0162-3109(97)00076-3. [DOI] [PubMed] [Google Scholar]
- Eggleton P., Reid K.B., Tenner A.J. C1q—how many functions? How many receptors? Trends Cell Biol. 1998;8:428–431. doi: 10.1016/s0962-8924(98)01373-7. [DOI] [PubMed] [Google Scholar]
- Tenner A.J. C1q receptorsregulating specific functions of phagocytic cells. Immunobiology. 1998;199:250–264. doi: 10.1016/S0171-2985(98)80031-4. [DOI] [PubMed] [Google Scholar]
- Nepomuceno R.R., Tenner A.J. C1qRP, the C1q receptor that enhances phagocytosis, is detected specifically in human cells of myeloid lineage, endothelial cells, and platelets. J. Immunol. 1998;160:1929–1935. [PubMed] [Google Scholar]
- Braun L., Ghebrehiwet B., Cossart P. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes . EMBO J. 2000;19:1458–1466. doi: 10.1093/emboj/19.7.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson R., Bronson S., Oula L., Zhang W., Ghebrehiwet B. Detection of complement C1q receptors on human spermatozoa. J. Reprod. Immunol. 1998;38:1–14. doi: 10.1016/s0165-0378(98)00006-0. [DOI] [PubMed] [Google Scholar]
- Bordin S., Ghebrehiwet B., Page R.C. Participation of C1q and its receptor in adherence of human diploid fibroblast. J. Immunol. 1990;145:2520–2526. [PubMed] [Google Scholar]
- Bordin S., Smith M., Ghebrehiwet B., Oda D., Page R.C. Smooth muscle and epithelial cells express specific binding sites for the C1q component of complement. Clin. Immunol. Immunopathol. 1992;63:51–57. doi: 10.1016/0090-1229(92)90093-4. [DOI] [PubMed] [Google Scholar]
- Crouch E., Hartshorn K., Ofek I. Collectins and pulmonary innate immunity. Immunol. Rev. 2000;173:52–65. doi: 10.1034/j.1600-065x.2000.917311.x. [DOI] [PubMed] [Google Scholar]
- Dedio J., Jahnen-Dechent W., Bachmann M., Muller-Esterl W. The multiligand-binding protein gC1qR, a putative C1q receptor, is a mitochondrial protein. J. Immunol. 1998;160:3534–3542. [PubMed] [Google Scholar]
- Erdei A., Reid K.B. The C1q receptor. Mol. Immunol. 1988;25:1067–1073. doi: 10.1016/0161-5890(88)90139-3. [DOI] [PubMed] [Google Scholar]
- Erdei A., Reid K.B. Characterization of the human C1q receptor. Behring Inst. Mitt. 1989;84:216–219. [PubMed] [Google Scholar]
- Erdei A. C1q receptor on murine cells. J. Immunol. 1990;145:1754–1760. [PubMed] [Google Scholar]
- Eggleton P., Ghebrehiwet B., Sastry K.N., Coburn J.P., Zaner K.S., Reid K.B., Tauber A.I. Identification of a gC1q-binding protein (gC1q-R) on the surface of human neutrophils. Subcellular localization and binding properties in comparison with the cC1q-R. J. Clin. Invest. 1995;95:1569–1578. doi: 10.1172/JCI117830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusi F., Bronson R.A., Hong Y., Ghebrehiwet B. Complement component C1q and its receptor are involved in the interaction of human sperm with zona-free hamster eggs. Mol. Reprod. Dev. 1991;29:180–188. doi: 10.1002/mrd.1080290214. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B., Lu P.D., Zhang W., Keilbaugh S.A., Leigh L.E., Eggleton P., Reid K.B., Peerschke E.I. Evidence that the two C1q binding membrane proteins, gC1q-R and cC1q-R, associate to form a complex. J. Immunol. 1997;159:1429–1436. [PubMed] [Google Scholar]
- Ghebrehiwet B. C1q receptor. Methods Enzymol. 1987;150:558–578. doi: 10.1016/0076-6879(87)50108-2. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B., Peerschke E.I. Structure and function of gC1q-Ra multiligand binding cellular protein. Immunobiology. 1998;199:225–238. doi: 10.1016/S0171-2985(98)80029-6. [DOI] [PubMed] [Google Scholar]
- Kaul M., Loos M. Collagen-like complement component C1q is a membrane protein of human monocyte-derived macrophages that mediates endocytosis. J. Immunol. 1995;155:5795–5802. [PubMed] [Google Scholar]
- Jack R.M., Lowenstein B.A., Nicholson-Weller A. Regulation of C1q receptor expression on human polymorphonuclear leukocytes. J. Immunol. 1994;153:262–269. [PubMed] [Google Scholar]
- Klickstein L.B., Barbashov S.F., Liu T., Jack R.M., Nicholson-Weller A. Complement receptor type 1 (CR1, CD35) is a receptor for C1q. Immunity. 1997;7:345–355. doi: 10.1016/s1074-7613(00)80356-8. [DOI] [PubMed] [Google Scholar]
- Lim B.L., Reid K.B., Ghebrehiwet B., Peerschke E.I., Leigh L.A., Preissner K.T. The binding protein for globular heads of complement C1q, gC1qR. Functional expression and characterization as a novel vitronectin binding factor. J. Biol. Chem. 1996;271:26739–26744. doi: 10.1074/jbc.271.43.26739. [DOI] [PubMed] [Google Scholar]
- Lim B.L., White R.A., Hummel G.S., Schwaeble W., Lynch N.J., Peerschke E.I., Reid K.B., Ghebrehiwet B. Characterization of the murine gene of gC1qBP, a novel cell protein that binds the globular heads of C1q, vitronectin, high molecular weight kininogen and factor XII. Gene. 1998;209:229–237. doi: 10.1016/s0378-1119(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Lurton J., Soto H., Narayanan A.S., Raghu G. Regulation of human lung fibroblast C1q-receptors by transforming growth factor-beta and tumor necrosis factor-alpha. Exp. Lung Res. 1999;25:151–164. doi: 10.1080/019021499270367. [DOI] [PubMed] [Google Scholar]
- Lynch N.J., Reid K.B., van den Berg R.H., Daha M.R., Leigh L.A., Ghebrehiwet B., Lim W.B., Schwaeble W.J. Characterisation of the rat and mouse homologues of gC1qBP, a 33 kDa glycoprotein that binds to the globular ‘heads’ of C1q. FEBS Lett. 1997;418:111–114. doi: 10.1016/s0014-5793(97)01348-3. [DOI] [PubMed] [Google Scholar]
- Malhotra R., Thail S., Reid K.B.M., Sim R.B. Human leukocyte C1q receptor binds other soluble proteins with collagen domains. J. Exp. Med. 1990;172:955–959. doi: 10.1084/jem.172.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R., Willis A.C., Jensenius J.-C., Jackson J., Sim R.B. Structure and homology of the human C1q receptor. Immunology. 1993;78:341–348. [PMC free article] [PubMed] [Google Scholar]
- Malhotra R., Laursen S.B., Willis A.C., Sim R.B. Localization of the receptor-binding site in the collectin family of proteins. Biochem. J. 1993;293:15–19. doi: 10.1042/bj2930015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R., Lu J., Holmskov U., Sim R.B. Collectins, collectin receptors and the lectin pathway of complement activation Clin. Exp. Immunol. 97Suppl 21994. 4 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M., Corbett E.F., Mesaeli N., Nakamura K., Opas M. Calreticulinone protein, one gene, many functions. Biochem. J. 1999;344:281–292. [PMC free article] [PubMed] [Google Scholar]
- Narayanan A.S., Lurton J., Raghu G. Distribution of receptors to collagen and globular domains of C1q in human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 1997;17:84–90. doi: 10.1165/ajrcmb.17.1.2732. [DOI] [PubMed] [Google Scholar]
- Nepomuceno R.P., Henscen-Edman A.H., Burgess W.H., Tenner A.J. cDNA cloning and primary structure analysis of C1qRp, the human C1q/MBP/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity. 1997;6:119–129. doi: 10.1016/s1074-7613(00)80419-7. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Ghebrehiwet B., Peerschke E.I. Staphylococcus aureus protein A recognizes platelet gC1qR/p33a novel mechanism for staphylococcal interactions with platelets. Infect. Immun. 2000;68:2061–2068. doi: 10.1128/iai.68.4.2061-2068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerschke E.I., Ghebrehiwet B. Platelet C1q receptor interactions with collagen- and C1q-coated surfaces. J. Immunol. 1990;145:2984–2988. [PubMed] [Google Scholar]
- Peerschke E.I., Ghebrehiwet B. Modulation of platelet responses to collagen by Clq receptors. J. Immunol. 1990;144:221–225. [PubMed] [Google Scholar]
- Peerschke E.I., Malhotra R., Ghebrehiwet B., Reid K.B., Willis A.C., Sim R.B. Isolation of a human endothelial cell C1q receptor (C1qR) J. Leukoc. Biol. 1993;53:179–184. doi: 10.1002/jlb.53.2.179. [DOI] [PubMed] [Google Scholar]
- Peerschke E.I., Reid K.B., Ghebrehiwet B. Identification of a novel 33-kDa C1q-binding site on human blood platelets. J. Immunol. 1994;152:5896–5901. [PubMed] [Google Scholar]
- Peerschke E.I., Smyth S.S., Teng E.I., Dalzell M., Ghebrehiwet B. Human umbilical vein endothelial cells possess binding sites for the globular domain of C1q. J. Immunol. 1996;157:4154–4158. [PubMed] [Google Scholar]
- Peterson K.L., Zhang W., Lu P.D., Keilbaugh S.A., Peerschke E.I., Ghebrehiwet B. The C1q-binding cell membrane proteins cC1q-R and gC1q-R are released from activated cellssubcellular distribution and immunochemical characterization. Clin. Immunol. Immunopathol. 1997;84:17–26. doi: 10.1006/clin.1997.4374. [DOI] [PubMed] [Google Scholar]
- Sim R.B., Moestrup S.K., Stuart G.R., Lynch N.J., Lu J., Schwaeble W.J., Malhotra R. Interaction of C1q and the collectins with the potential receptors calreticulin (cC1qR/collectin receptor) and megalin. Immunobiology. 1998;199:208–224. doi: 10.1016/s0171-2985(98)80028-4. [DOI] [PubMed] [Google Scholar]
- Tenner A.J., Robinson S.L., Ezekowitz R.A. Mannose binding protein (MBP) enhances mononuclear phagocyte function via a receptor that contains the 126,000 Mr component of the C1q receptor. Immunity. 1995;3:485–493. doi: 10.1016/1074-7613(95)90177-9. [DOI] [PubMed] [Google Scholar]
- Van Den Berg R.H., Siegert C.E.H., Faber-Krol M.C., Huizinga T.W.J., Van Es L.A., Daha M.R. Anti-C1q receptor/calreticulin autoantibodies in patients with systemic lupus erythematosus (SLE) Clin. Exp. Immunol. 1998;111:359–364. doi: 10.1046/j.1365-2249.1998.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevorach D. Opsonization of apoptotic cells. Implications for uptake and autoimmunity. Ann. NY Acad. Sci. 2000;926:226–235. doi: 10.1111/j.1749-6632.2000.tb05615.x. [DOI] [PubMed] [Google Scholar]
- Coppolino M.G., Woodside M.J., Demaurex N., Grinstein S., St-Arnaud R., Dedhar S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997;386:843–847. doi: 10.1038/386843a0. [DOI] [PubMed] [Google Scholar]
- Coppolino M.G., Dedhar S. Calreticulin. Int. J. Biochem. Cell Biol. 1998;30:553–558. doi: 10.1016/s1357-2725(97)00153-2. [DOI] [PubMed] [Google Scholar]
- Coppolino M.G., Dedhar S. Ligand-specific, transient interaction between integrins and calreticulin during cell adhesion to extracellular matrix proteins is dependent upon phosphorylation/dephosphorylation events. Biochem. J. 1999;340:41–50. [PMC free article] [PubMed] [Google Scholar]
- Coppolino M.G., Dedhar S. Bi-directional signal transduction by integrin receptors. Int. J. Biochem. Cell Biol. 2000;32:171–188. doi: 10.1016/s1357-2725(99)00043-6. [DOI] [PubMed] [Google Scholar]
- Arosa F.A., de Jesus O., Porto G., Carmo A.M., de Sousa M. Calreticulin is expressed on the cell surface of activated human peripheral blood T lymphocytes in association with major histocompatibility complex class I molecules. J. Biol. Chem. 1999;274:16917–16922. doi: 10.1074/jbc.274.24.16917. [DOI] [PubMed] [Google Scholar]
- Eggleton P., Tenner A.J., Reid K.B. C1q receptors. Clin. Exp. Immunol. 2000;120:406–412. doi: 10.1046/j.1365-2249.2000.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Binder R.J., Ramalingam T., Srivastava P.K. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- Racoosin E.L., Swanson J.A. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J. Cell Biol. 1993;121:1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racoosin E.L., Swanson J.A. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J. Cell Sci. 1992;102:867–880. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- Racoosin E.L., Swanson J.A. Macrophage colony-stimulating factor (rM-CSF) stimulates pinocytosis in bone marrow–derived macrophages. J. Exp. Med. 1989;170:1635–1648. doi: 10.1084/jem.170.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett L.J., Prescott A.R., Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J. Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F.M., Griffin J.A., Leider J.E., Silverstein S.C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J. Exp. Med. 1975;142:1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F.M., Griffin J.A., Silverstein S.C. Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow–derived lymphocytes. J. Exp. Med. 1976;144:788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagat T.L., Wofford J.A., Wright J.R. Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J. Immunol. 2001;166:2727–2733. doi: 10.4049/jimmunol.166.4.2727. [DOI] [PubMed] [Google Scholar]
- Bradley A.J., Brooks D.E., Norris-Jones R., Devine D.V. C1q binding to liposomes is surface charge dependent and is inhibited by peptides consisting of residues 14-26 of the human C1qA chain in a sequence independent manner. Biochim. Biophys. Acta. 1999;1418:19–30. doi: 10.1016/s0005-2736(99)00013-9. [DOI] [PubMed] [Google Scholar]
- Chen A., Gaddipati S., Hong Y., Volkman D.J., Peerschke E.I., Ghebrehiwet B. Human T cells express specific binding sites for C1q. Role in T cell activation and proliferation. J. Immunol. 1994;153:1430–1440. [PubMed] [Google Scholar]
- Ghebrehiwet B., Habicht G.S., Beck G. Interaction of C1q with its receptor on cultured cell lines induces an anti-proliferative response. Clin. Immunol. Immunopathol. 1990;54:148–160. doi: 10.1016/0090-1229(90)90014-h. [DOI] [PubMed] [Google Scholar]
- Goodman E.B., Tenner A.J. Signal transduction mechanisms of C1q-mediated superoxide production. Evidence for the involvement of temporally distinct staurosporine-insensitive and sensitive pathways. J. Immunol. 1992;148:3920–3928. [PubMed] [Google Scholar]
- Guan E.N., Burgess W.H., Robinson S.L., Goodman E.B., McTigue K.J., Tenner A.J. Phagocytic cell molecules that bind the collagen-like region of C1q. Involvement in the C1q-mediated enhancement of phagocytosis. J. Biol. Chem. 1991;266:20345–20355. [PubMed] [Google Scholar]
- Kovacs H., Campbell I.D., Strong P., Johnson S., Ward F.J., Reid K.B., Eggleton P. Evidence that C1q binds specifically to CH2-like immunoglobulin gamma motifs present in the autoantigen calreticulin and interferes with complement activation. Biochemistry. 1998;37:17865–17874. doi: 10.1021/bi973197p. [DOI] [PubMed] [Google Scholar]
- Tan X., Wong S.T., Ghebrehiwet B., Storm D.R., Bordin S. Complement C1q inhibits cellular spreading and stimulates adenylyl cyclase activity of fibroblasts. Clin. Immunol. Immunopathol. 1998;87:193–204. doi: 10.1006/clin.1997.4485. [DOI] [PubMed] [Google Scholar]
- Dini L. Endothelial liver cell recognition of apoptotic peripheral blood lymphocytes. Biochem. Soc. Trans. 1998;26:635–639. doi: 10.1042/bst0260635. [DOI] [PubMed] [Google Scholar]
- Dini L. Recognizing deathliver phagocytosis of apoptotic cells. Eur. J. Histochem. 2000;44:217–227. [PubMed] [Google Scholar]
- Ruzittu M., Carla E.C., Montinari M.R., Maietta G., Dini L. Modulation of cell surface expression of liver carbohydrate receptors during in vivo induction of apoptosis with lead nitrate. Cell Tissue Res. 1999;298:105–112. doi: 10.1007/s004419900059. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Kaji K., Nagasawa S. Activation of the alternative pathway of human complement by apoptotic human umbilical vein endothelial cells. J. Biochem. 1994;116:794–800. doi: 10.1093/oxfordjournals.jbchem.a124598. [DOI] [PubMed] [Google Scholar]
- Gershov D., Kim S., Brot N., Elkon K.B. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune responseimplications for systemic autoimmunity. J. Exp. Med. 2000;192:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V.A., Bratton D.L., Frasch S.C., Warner M.L., Henson P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998;5:551–562. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- Savill J.S., Henson P.M., Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel “charge-sensitive” recognition mechanism. J. Clin. Invest. 1989;84:1518–1527. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G.R., Lynch N.J., Lu J., Geick A., Moffatt B.E., Sim R.B., Schwaeble W.J. Localisation of the C1q binding site within C1q receptor/calreticulin. FEBS Lett. 1996;397:245–249. doi: 10.1016/s0014-5793(96)01156-8. [DOI] [PubMed] [Google Scholar]
- Peerschke E.I.B., Reid K.B.M., Ghebrehiwet B. Identification of a novel 33-kDa binding site on human platelets. J. Immunol. 1994;152:5896–5901. [PubMed] [Google Scholar]
- Sengelov H. Complement receptors in neutrophils. Crit. Rev. Immunol. 1995;15:107–131. [PubMed] [Google Scholar]
- Dougherty G.J., McBride W.H. Macrophage heterogeneity. J. Clin. Lab. Immunol. 1984;14:1–11. [PubMed] [Google Scholar]
- Basu S., Binder R.J., Suto R., Anderson K.M., Srivastava P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappaB pathway. Int. Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Nykjaer A., Petersen C.M., Jorgensen K.E., Nielsen M., Andreasen P.A., Christensen E.I., Lookene A., Olivecrona G., Moestrup S.K. The multiligand alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein (alpha 2MR/LRP). Binding and endocytosis of fluid phase and membrane-associated ligands. Ann. NY Acad. Sci. 1994;737:20–38. doi: 10.1111/j.1749-6632.1994.tb44299.x. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Hartwieg E., Horvitz H.R. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans . Cell. 2001;104:43–56. doi: 10.1016/s0092-8674(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Ellis R.E., Jacobson D.M., Horvitz H.R. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans . Genetics. 1991;129:79–94. doi: 10.1093/genetics/129.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W.P., Massoni R.J., Niemann M.A., Giclas P.C. Absence of induction of IL-1 production in human monocytes by complement fragments. J. Immunol. 1989;142:173–178. [PubMed] [Google Scholar]
- Pinckard R.N., Olson M.S., Giclas P.C., Terry R., Boyer J.T., O'Rourke R.A. Consumption of classical complement components by heart subcellular membranes in vitro and in patients after acute myocardial infarction. J. Clin. Invest. 1975;56:740–750. doi: 10.1172/JCI108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder R.J., Han D.K., Srivastava P.K. CD91a receptor for heat shock protein gp96. Nat. Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]