Abstract
Certain peptide analogs that carry substitutions at residues other than the main major histocompatibility complex anchors and are surprisingly much more antigenic than wild-type peptide (heteroclitic analogs). To date, it was unknown how frequently wild-type epitopes could be modified to obtain heteroclitic activity. In this study, we analyzed a large panel of analogs of two different human histocompatibility leukocyte antigen (HLA)-A2.1–restricted epitopes and found that heteroclitic analogs were associated with higher magnitude responses and increased (up to 107-fold) sensitivity to antigen, and corresponded to conservative or semiconservative substitutions at odd-numbered positions in the middle of the peptide (positions 3, 5, or 7). These findings were validated by performing additional immunogenicity studies in murine and human systems with four additional epitopes. The biological relevance of heteroclitic analogs was underlined when predicted analogs of the p53.261 epitope was shown to induce cytotoxic T lymphocytes (CTLs) that recognize low concentrations of peptide (high avidity) in vivo and demonstrate in vitro antitumor recognition. Finally, in vitro immunization of human peripheral blood mononuclear cells with two heteroclitic analogs resulted in recruitment of more numerous CTLs which were associated with increased antigen sensitivity. In conclusion, heteroclitic analogs were identified in each of the six cases studied and structural features were defined which allow identification of such analogs. The strong CTL immunity elicited by heteroclitic epitopes suggest that they could be of significant value in vaccination against tolerant or weakly immunogenic tumor-associated and viral antigens.
Keywords: heteroclitic analogs, CTL, MHC, amino acid substitutions, vaccine
Introduction
Several studies suggest that CTLs play a central role in the eradication of infectious disease and cancer by the immune system 1 2. As CTLs recognize complexes of antigenic peptides and MHC, considerable effort is currently ongoing in developing epitope-based vaccines to stimulate CTL responses. Peptide analogs with increased binding affinity for their MHC restricting MHC molecule have been engineered by rational modification of the main MHC anchor residues. These “fixed anchor” analogs have been shown to be more antigenic and immunogenic in various experimental systems 3 4. Normally, single substitution analogs of an antigenic peptide at positions other than the main MHC anchor residues would be expected to have either no effect or a detrimental effect on T cell activation. Certain analogs, however, have unexpectedly increased potency and these analogs are defined as “heteroclitic analogs,” as heteroclitic, according to the Webster's dictionary 5, indicates “one that deviates from common norm.” These analogs may provide considerable benefit in vaccine development, as they induce stronger T cell responses than the native epitope 6 7, and have been shown to be associated with increased affinity of the epitope/MHC complex for the TCR molecule 8. Advantages associated with using such analogs in clinical applications are as follows: (a) heteroclitic analogs have been shown to break/overcome tolerance by reversing a state of T cell anergy and/or recruiting new T cell specificities 9 10 11; (b) significantly smaller amounts of heteroclitic analogs may be needed for treatment 12; and (c) peptide analogs have previously been demonstrated to induce immune deviation by modulating cytokine production 13 14 15, which may have implications in several disease states where generation of a specific subset of Th cell response is required 16 17 18.
For the aforementioned reasons, it would be extremely useful to predict amino acid substitutions that render heteroclitic activity to a given antigenic peptide. To date, however, there is no easy method for predicting such substitutions. Indeed, in previous studies 19 20 heteroclitic epitopes were fortuitously identified by eluting naturally occurring mutant peptides from melanoma cells or by systematically screening a large number of analogs and combinatorial libraries consisting of substitutions at almost every position in the epitope 6 7 21 22. Genetic approaches, such as screening of DNA expression libraries, have provided another method for generating CTL epitopes and analogs 23 24. However, this approach may be problematic given the potentially small quantities and complexity of epitopes generated.
In this study we examined whether heteroclitic analogs could be consistently identified for several different CTL epitopes, or only for relatively rare occurrences. Furthermore, we sought to understand the structural features associated with heteroclicity, thereby circumventing the need to employ time-consuming analogue screening methods for identification of heteroclitic analogs.
Materials and Methods
Peptide Synthesis and Generation of Peptide Analogs.
Peptides used in this study are shown in Table . All of the human CTL epitopes that were derived from tumor-associated and viral antigens have been described in previous studies where their immunogenicity in human and transgenic mouse systems was demonstrated 25 26.
Table 1.
Characterization of Heteroclitic Analogs Identified from Tumor and Viral Antigens
| Antigen | Sequence | Heteroclitic substitution | Type of substitution | Position of substitution | Th1 cytokines | Th2 cytokines | A0201 binding (IC50 nM) |
|---|---|---|---|---|---|---|---|
| CEA.691 | IMIGVLVGV | None (WT) | None | 1 | 10 | 54 | |
| CEA.691M3 | IMMGVLVGV | I→M | Conservative | 3 | 10−5 | 1 | 27 |
| CEA.691H5 | IMIGHLVGV | V→H | Semiconservative | 5 | 10−7 | 10−1 | 16 |
| MAGEA3.112 | KVAELVHFL | None (WT) | None | 1 | NS | 94 | |
| MAGEA3.112I5 | KVAEIVHFL | L→I | Conservative | 5 | 10−4 | NS | 66 |
| MAGEA3.112W7 | KVAELVWFL | H→W | Semiconservative | 7 | 10−7 | NS | 7 |
| MAGEA2.157 | YLQLVFGIEV | None (WT) | 1 | 10 | 40 | ||
| MAGEA2.157I5 | YLQLIFGIEV | V→I | Conservative | 5 | 10−4 | 10−2 | 476 |
| MAGEA2.157F5 | YLQLFFGIEV | V→F | Semiconservative | 5 | 10−2 | 10−2 | 212 |
| HBV Pol.455 | GLSRYVARL | None (WT) | 10 | 10 | 83 | ||
| HBV Pol.455P7 | GLSRYVPRL | A→P | Conservative | 7 | 10−2 | 10−2 | 267 |
| HIV Pol.476 | ILKEPVHGV | None (WT) | >10 | >10 | 369 | ||
| HIV Pol.476H3 | ILHEPVHGV | K→H | Conservative | 3 | 1 | 1 | 78 |
| HIV Pol.476L3 | ILLEPVHGV | K→L | Semiconservative | 3 | 10−1 | 1 | 63 |
WT, wild-type.
Large collections of peptides tested for heteroclitic activity were synthesized by Chiron Corp. as crude material. Further biological characterizations were performed with peptides synthesized and purified (≥ 95% purity) at Epimmune using described methods 27, and their identity was confirmed by mass spectral analysis before use.
Scheme for Selection of Single Amino Acid Substitutions.
The degree of similarity between amino acid pairs was quantified by averaging, for each amino acid pair, the rank coefficient scores for PAM250, hydrophobicity, and side chain volume (see below). Based on the average values of these composite rankings, shown in Fig. 1, similarity assignments between any given amino acid pair was derived so that a given amino acid substitution could be characterized as being a conservative, semiconservative, or nonconservative substitution.
Figure 1.
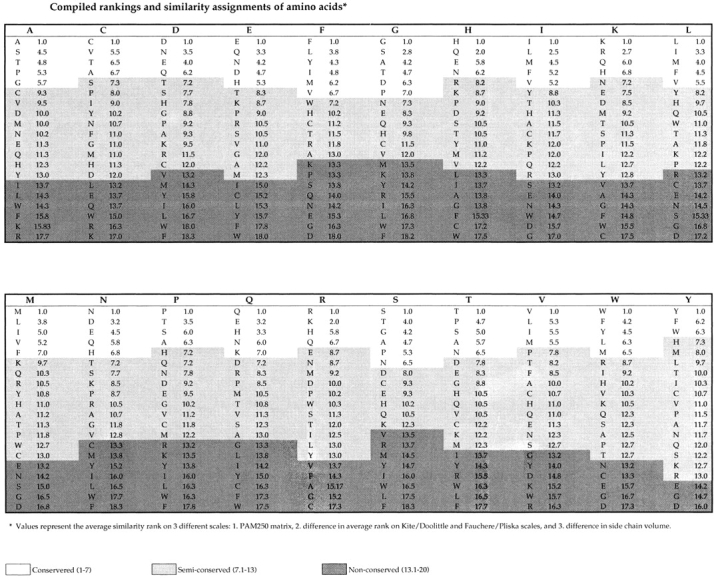
The Dayhoff PAM250 score (28, 29; http://prowl.rockefeller. edu/aainfo/pam250.html) is a commonly used protein alignment scoring matrix which measures the percentage of acceptable point mutations within a defined time frame. The frequencies of these mutations are different from what would be expected from the probability of random mutations, and presumably reflect a bias due to the degree of physical and chemical similarity of the amino acid pair involved in the substitution. To obtain a score of amino acid similarity that could be standardized with other measures of similarity, the PAM250 scores were converted to a rank value, where 1 indicates the highest probability of being an accepted mutation.
The most commonly used scales to represent the relative hydrophobicity of the 20 naturally occurring amino acids 30 are those developed on the basis of experimental data by Kyte and Doolittle 31, and by Fauchere and Pliska 32. The Kyte/Doolittle scale measures the H2O/organic solvent partition of individual amino acids. Because it considers the position of amino acids in folded proteins, it may most accurately reflect native hydrophobicity in the context of proteins. The Fauchere/Pliska scale measures the octanol/H2O partitioning of N-acetyl amino acid amides, and most accurately reflects hydrophobicity in the context of denatured proteins and/or small synthetic peptides. To obtain scores for hydrophobicity, each amino acid residue was ranked on both the Kyte/Doolittle and Fauchere/Pliska hydrophobicity scales. An average rank between the two scales was calculated and the average difference in hydrophobicity for each pair was calculated.
Finally, for calculating amino acid side-chain volume, the partial volume in solution obtained by noting the increase in volume of water after adding either one molecule or 1 g of amino acid residue was considered 33 34. The absolute difference in the partial volume of each possible pairing of the 20 naturally occurring amino acids was calculated and ranked, where 1 indicated residues with the most similar volumes, and 20 the most dissimilar.
Measurement of Peptide Binding Affinity for the HLA-A2.1 Molecule.
Binding of test peptides to HLA-A2.1 was measured by determining the level of competition induced by the test peptide for binding of a radiolabeled standard peptide to purified HLA-A2.1 molecules. The percentage of MHC-bound radioactivity was determined by gel filtration and the concentration of test peptide that inhibited 50% of the binding of the labeled standard peptide (IC50%) was calculated 27 35. The standard peptide was the hepatitis B virus (HBV) Core.18 epitope (sequence FLPSDFFPSV).
Mice.
HLA-A2.1/Kbxs and HLA-A2.1/Kbxd transgenic mice were bred at Epimmune. These strains represent the F1 generation of a cross between an HLA-A2.1/Kb transgenic strain generated on the C57BL/6 background 36 and SJL or BALB/c mice (The Jackson Laboratory), respectively.
APC Lines.
The .221A2.1 cell line was generated by transfecting the HLA-A2.1 gene into the HLA-A, -B, -C-null mutant EBV-transformed human B-lymphoblastoid cell line 3A4–721.221 25. Jurkat cells and methylcholanthrene-induced sarcoma tumor cells (Meth A) were transfected with the HLA-A2.1 or A2.1/Kb molecule as described 26 36. The carcinoembryonic antigen (CEA)-expressing colon adenocarcinoma lines SW403 (A2+, CEA+) and HT29 (A2−, CEA+) were obtained from the American Tissue Type Collection. The tumor cell lines were treated with 100 IU/ml human IFN-γ (Genzyme) for 48 h at 37°C before using as APCs. All cells were grown in RPMI-1640 supplemented with antibiotics, sodium pyruvate, nonessential amino acids, and 10% FBS.
Primary In Vitro Induction of CTLs from PBMCs and Derivation of Human CTL Lines.
To generate peptide-specific CTL lines against the CEA.691 epitope and to examine the immunogenicity of CEA.691 analogs, PBMCs from normal subjects were stimulated repeatedly with peptide in vitro in 48-well plates 25. To check CTL activity after two rounds of stimulation, PBMCs from each culture (well) were cultured with .221-A2.1 APC in the presence or absence of peptide or, in some experiments, with tumor cells that endogenously express the wild-type epitope. Cultures were scored positive for CTL induction if the net IFN-γ production was >100 pg/well and IFN-γ production in the presence of peptide was at least twofold above background.
Murine CTL Lines.
CTL lines against epitopes HBV Pol.455 and HIV Pol.476 peptides were generated in HLA-A2.1/Kbxs transgenic mice by DNA immunization 26. Similarly, a CTL line against the MAGEA2.157 epitope was generated by immunizing HLA-A2.1/Kbxs mice subcutaneously at the tail base with 50 μg of peptide and 140 μg of the HBV Core.128 Th epitope (TPPAYRPPNAPIL), emulsified in IFA and restimulating primed splenocytes repeatedly in vitro with peptide 26.
Measurement of Murine and Human IFN-γ, IL-5, and IL-10 Production by CTLs.
An in situ capture ELISA was used for measuring IFN-γ release from mouse or human CTL 37. Anti–mouse or anti–human IFN-γ mAb for this assay were purchased from BD PharMingen. Murine and human IL-5 and IL-10 was measured in culture supernates using ELISA kits (R&D Systems).
Enzyme-linked Immunospot Assay for Measuring Ex Vivo Murine CTL Responses.
Enzyme-linked immunospot (ELISPOT) assays were performed according to standard protocols 38 39. Briefly, 4 × 105 splenic CD8+ cells isolated by magnetic beads (Miltenyi Biotec) and 5 × 104 Jurkat-A2.1/Kb cells pulsed with 10 μg/ml of peptide were cultured in flat-bottom 96-well nitrocellulose plates (Immobilon-P membrane; Millipore) which had been precoated with anti–IFN-γ mAb (BD PharMingen; 10 μg/ml). After 20 h incubation at 37°C, plates were washed with PBS/0.05% Tween and wells were incubated with a biotinylated anti–IFN-γ mAb (BD PharMingen; 2 μg/ml) for 4 h at 37°C. After additional washing, spots were developed by sequential incubation with Vectastain ABC peroxidase (Vector Laboratories) and 3-amino-9-ethyl carbazole (AEC) solution (Sigma-Aldrich) and counted by computer-assisted image analysis (ZEISS KS ELISPOT Reader). The net number of spots/106 CD8+ cells was calculated as follows: ([number of spots against relevant peptide] − [number of spots against irrelevant control peptide]) × 2.5. For human ELISPOT assays, 4 × 104 CTLs were cultured with 105 .221A2.1 cells pulsed with 10 μg/ml of peptide for 20 h. The antibodies used for this assay were the mouse anti–human IFN-γ clone1-D1K (Mabtech) and the biotinylated mouse anti–human IFN-γ clone 7-B6–1 (Mabtech). The protocol followed was identical to that described above.
Screening of Peptide Analogs for Heteroclitic Activity.
Prior to screening analogs, .221A2.1 tumor cells (105 cells/well) pulsed with varying doses of peptide were cultured with an equivalent number of murine or human CTLs. The level of IFN-γ released by CTLs was measured by the in situ capture ELISA assay after 24 h (murine) or 48 h (human) of incubation at 37°C. After determining a titration curve, a suboptimal peptide dose was selected for each CTL line where activity against wild-type peptide was barely detectable. This dose was typically in the range of 0.1–1 μg/ml. For screening of peptide analogs, .221A2.1 cells were pulsed with each analogue at the selected suboptimal dose and peptide-loaded APCs were cultured with CTLs as described above. Analogs inducing enhanced CTL responses relative to wild-type peptide were then selected for further characterization. These analogs were tested side-by-side with the wild-type epitope in a peptide dose titration under identical conditions described above. It should be noted that although murine CTL lines were generated in HLA-A2.1/Kbxs transgenic mice which express an HLA molecule with murine H-2Kb sequences in the third domain, all lines responded to peptide presented on APCs expressing the native HLA-A2.1 molecule.
In Vivo Immunogenicity of Predicted p53.261 Analogs in HLA-A2.1/Kbxd Transgenic Mice.
Immunogenicity for the murine p53.261 predicted analogs were tested in HLA-A2.1/Kbxd transgenic mice by co-immunizing mice with 50 μg of the p53.261 epitope (LLGRDSFEV) or its predicted analogs and 140 μg of the HBV Core.128 Th epitope in IFA. Splenocytes from analogue-primed animals were stimulated twice in vitro with either the respective immunizing analogue (to determine avidity and precursor frequency of CTLs responding to the analogue) or the wild-type peptide (to determine the cross-reactivity, avidity, and precursor frequency of CTLs that respond to wild-type antigen). Splenocytes from wild-type peptide-immunized animals were stimulated and tested against wild-type peptide. All short-term, bulk populations of CTL were tested for peptide specificity by the IFN-γ in situ ELISA 5 d after the second in vitro stimulation, using Jurkat-A2.1 as APC. Alternatively, CTL responses were performed on freshly isolated spleen cells from immunized animals using the ELISPOT assay.
Results
Identification of CEA.691 and MAGEA3.112 Analogs Associated with Increased IFN-γ Release.
To identify heteroclitic analogs, a total of 117 CEA.691 and 116 MAGEA3.112 analogs of the CEA.691 and MAGEA3.112 epitopes were generated by systematically replacing each residue with 17 different single amino acids. The residues Cys, Trp, and Met were in general avoided unless they corresponded to conservative changes. Substitutions were introduced at all positions in the peptide except at positions 2 and the COOH termini, the main MHC anchor positions for HLA-A2. These analogs were then tested in vitro for their antigenicity against polyclonal CTL lines specific for MAGEA3.112 and CEA.691 epitopes. It should be emphasized that polyclonal rather than clonal populations of T cells were used in the study. Our objective was to define patterns associated with heteroclicity that encompass multiple TCR families found in a polyclonal population, to avoid any potential bias introduced by examination of CTL clones expressing only a single TCR clonotype.
Preliminary dose titration experiments for each CTL line defined a suboptimal concentration of wild-type peptide (data not shown). This suboptimal concentration was then used for subsequent antigenicity assays of analogue peptides for each epitope, so that analogs associated with increased T cell stimulatory capacity could be identified. Results of such antigenicity analysis for the CEA.691 analogs are shown in Fig. 2 A. At the suboptimal 100 ng/ml dose the wild-type CEA.691 peptide induced only marginal IFN-γ production (<50 pg/well). By contrast, at the same dose, several CEA.691 analogs (M3, L4, P4, H5, L5, H6, T6, and I7) induced detectable levels of IFN-γ production in the 125 to 350 pg/well range. Similarly, for the MAGEA3.112-specific CTL line 100 ng/ml of wild-type peptide induced the release of 100 pg/ml of IFN-γ, whereas two analogs (I5 and W7) were associated with inducing IFN-γ levels of over 300 pg/well (Fig. 2 B).
Figure 2.
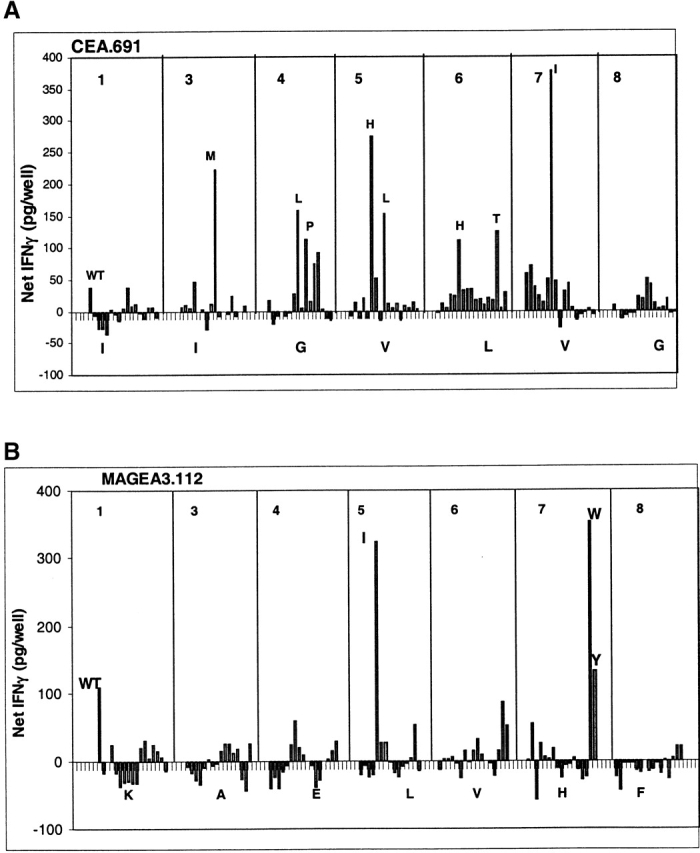
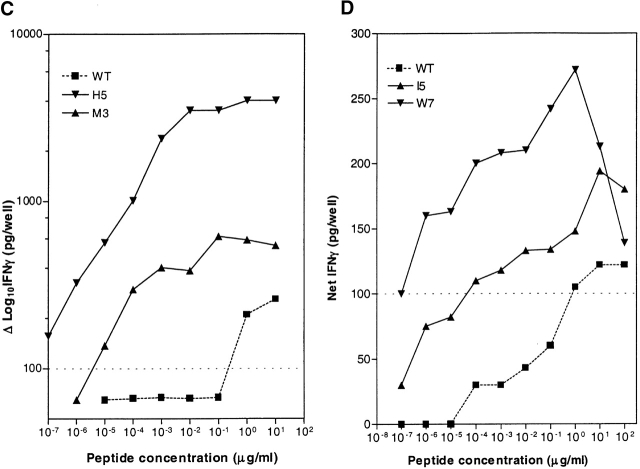
Antigenicity of CEA.691 or MAGEA3.112 analogs tested with CTLs specific for wild-type (WT) epitope. IFN-γ produced by CEA.691-specific CTLs (A) or MAGEA3.112-specific CTLs (B) was measured after stimulation with analogue peptides. The position number of the amino acid that was substituted appears at the top of each graph and the native residue corresponding that position is shown along the X-axis. Each bar represents the CTL response stimulated by each analogue. The amino acid substitution of analogs that were further characterized for heteroclitic activity in a dose titration is indicated next to the corresponding response and the CTL response against heteroclitic analogs of CEA.691 (C) and MAGEA3.112 (D) compared with WT is shown. Significant production of IFN-γ was considered to be levels >100 pg/well (dotted lines). Background IFN-γ release by CTL stimulated by unpulsed target cells ranged between 25–40 pg/well.
All analogs of both CEA.691 and MAGEA3.112 that stimulated IFN-γ above 100 pg/well were chosen for further characterization and a complete dose titration was performed to identify heteroclitic analogs. Herein, heteroclitic analogs are considered as those which stimulate significant IFN-γ release (>100 pg/well) at 10-fold or lower peptide concentrations than wild-type peptide. A 100 pg/well cutoff was selected, as it represented a level of IFN-γ that was threefold above background, based on analysis of various CTL lines in several independent experiments.
Dose titration analysis for the CEA.691 analogs resulted in the identification of two heteroclitic analogs, namely M3 and H5. As seen in Fig. 2 C, the wild-type CEA.691 peptide yielded a significant detectable IFN-γ signal in the 1 to 10 μg/ml dose range while the analogs M3 and H5 stimulated significant release with as little as 0.01 ng/ml and 0.1 pg/ml of peptide, respectively. The I7 analogue, which appeared to be heteroclitic in initial screening assays (Fig. 2 A), failed to demonstrate heteroclitic activity upon further testing.
Similar dose titration analysis for the MAGEA3.112 epitope resulted in the identification of two heteroclitic analogs, I5 and W7. As shown in Fig. 2 D, 1 μg/ml of wild-type peptide was required for significant IFN-γ release whereas ∼0.1 ng/ml of analogue I5 or 0.1 pg/ml of analogue W7 was required to stimulate an equivalent response. It should be pointed out that, in general, the heteroclitic analogs not only induced a dose response shift, but they also stimulated CTLs to produce higher levels of IFN-γ compared with wild-type peptide.
Distinct Structural Features Associated with Heteroclitic Substitutions.
The identification of four heteroclitic analogs described above suggested that certain properties might be associated with heteroclitic substitutions. Specifically, amino acid substitutions were conservative or semiconservative in nature, as defined by our scheme for assigning conservancy of single amino acid substitutions (see Materials and Methods). It should be noted that the similarities between amino acids according to this scheme are not always bidirectional. This may appear counterintuitive or even confusing at first, but it is a general feature of ranking of amino acid replacements which take into account similarities and replacement rates. For example, substituting an average size residue glutamine (Q) with either a small residue such as glycine (G) or a large one such as tryptophan (W) will rank as an extreme substitution in terms of size. By contrast, substituting the same small size residue (G) with an average size residue (Q) will rank only as average while the (W) substitution will rank as extreme in terms of size replacements. In addition, Dayhoff replacement frequencies tabulate substitutions which conserve biological function and these substitutions are also not always bidirectional. For example a tryptophan can be substituted by a positively charged (K) without drastic effect, while substituting a lysine (K) engaged in a salt bridge with tryptophan may lead to total loss of biological function.
Furthermore, substitutions tended to be at odd-numbered positions (position 3, 5, or 7) in the center of the peptide (Table ). To test whether conservative or semiconservative substitutions at odd-numbered positions in the middle of an epitope are indeed associated with heteroclicity, three additional A2.1-restricted epitopes, the MAGEA2.157 tumor epitope and two epitopes from viral antigens, HBV Pol.455 and HIV Pol.476, were analyzed. All three epitopes have been shown to be immunogenic for CTLs in earlier studies 25 26 40.
For this purpose a panel of 212 different analogs was synthesized. These analogs included five conservative and five nonconservative amino acid substitutions at predicted positions (3, 5, or 7) as well as at irrelevant positions (1, 4, 6, or 8). Due to the technical ease associated with generating and maintaining murine CTL lines, analogs were tested for heteroclicity using CTL lines generated in HLA-A2.1/Kbxs transgenic mice, following an experimental strategy similar to the one described above.
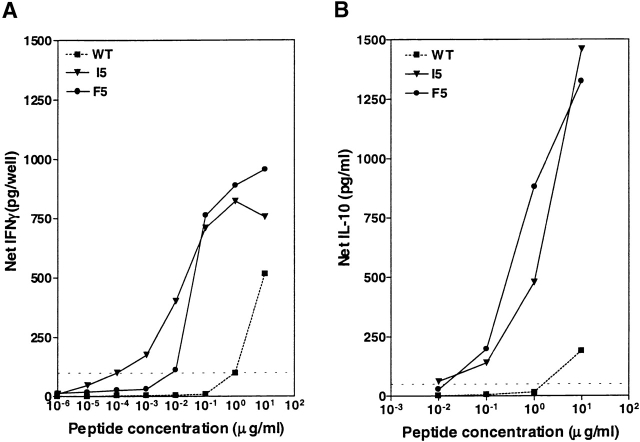
Antigenicity analysis of the MAGEA2.157 analogs is depicted in Fig. 3 A, where analogs inducing IFN-γ production in excess of 500 pg/well (specifically F1, H3, K3, E3, N3, I5, F5, T5, Y5, N7, and M8) were characterized further in a dose titration analysis. From a total of 77 different analogs tested two heteroclitic analogs were identified, F5 and I5, that stimulated IFN-γ responses at 100- to 10,000-fold lower doses than wild-type peptide (Table ). As before, both of these analogs had substitutions that were conservative or semiconservative in nature occurring at an odd-numbered position in the center of the peptide (position 5). Antigenicity analysis of analogs for two other epitopes, HBV Pol.455 and HIV Pol.476, are shown in Fig. 3B and Fig. C, respectively. Further characterization of the HBV Pol.455 analogs screened in Fig. 3 B resulted in the identification of one analogue of out of 66 initially tested that had a conservative substitution at position 7 (Table ). For the HIV Pol.476 epitope, out of 69 different analogs initially screened in Fig. 3 C, two analogs were identified as having heteroclitic activity (H3 and L3; Table ). Once again, both analogs carried either a conservative or semiconservative substitution at an odd-numbered position in the center of the peptide.
Figure 3.
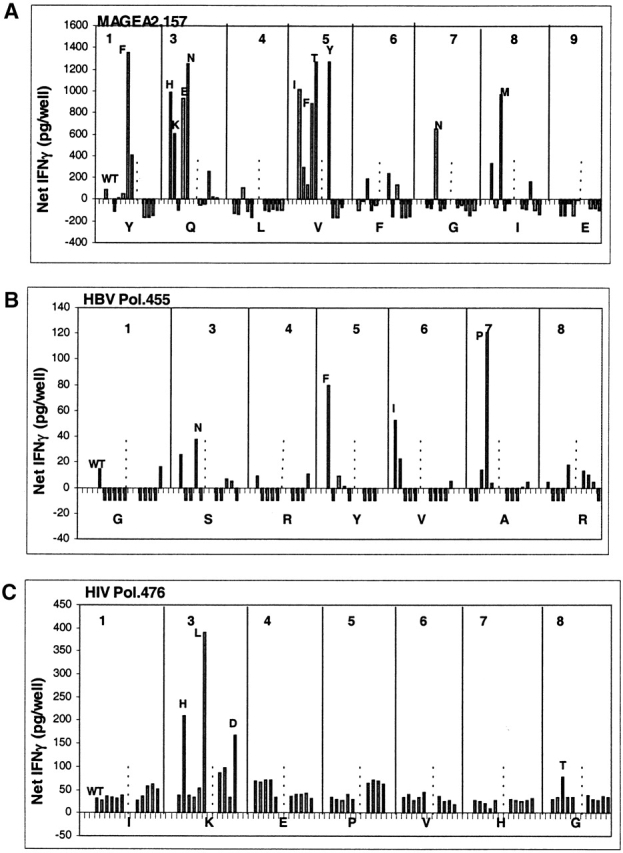
Antigenicity of analogs from three additional epitopes of tumor and viral antigens. IFN-γ production by CTLs specific for MAGEA2.157 (A), HBV Pol.455 (B), and HIV Pol.476 (C) in response to analogue-pulsed or wild-type (WT)-pulsed .221A2.1 cells is shown. The dotted line at each position demarcates the groups of five analogs with conservative/semiconservative substitutions (left of the line) and nonconservative substitutions (right of the line). Background IFN-γ responses were <25 pg/well.
In summary, analysis of data obtained from this second study, consisting of 212 analogs for three additional epitopes of tumor and viral origin were consistent with our previous analysis of the MAGEA3.112 and CEA.691 epitopes. These data validated our earlier patterns identified for heteroclitic substitutions inasmuch as all five of the heteroclitic analogs identified in the second study had substitutions that were of conservative or semiconservative nature, and in all cases substitutions occurred at odd-numbered positions (3, 5, or 7) in the middle of the peptide.
Lymphokine Profile Induced by Heteroclitic Analogs.
Heteroclitic analogs have been shown previously to differentially activate cytokine production from T cells such that some analogs specifically activate Th1 cytokine production while others preferentially activate production of Th2 cytokines 13 14. To investigate the cytokine pattern associated with the heteroclitic analogs, the production of the Th2 cytokines IL-5 and/or IL-10 from CTL lines was compared with the production of a Th1 cytokine IFN-γ. Representative data is shown in Fig. 4. The F5 and I5 analogs of MAGEA2.157 induced significant levels of IFN-γ production at 100-fold or 10,000-fold lower concentrations than wild-type peptide, respectively (Fig. 4 A). Moreover, the same analogs also induced significant IL-10 production at a 100-fold lower peptide concentration than wild-type peptide (Fig. 4 B).
Figure 4.
Lymphokine profile induced by MAGEA2. 157 analogs. IFN-γ (A) and IL-10 (B) produced by 105 MAGEA2.157-specific CTLs in response to 105 .221A2.1 targets pulsed with analogs I5 or F5, or wild-type (WT) peptide was measured over several different doses. Dotted lines indicate significant levels of IFN-γ (100 pg/well) or IL-10 (50 pg/ml). Background responses were 30 pg/well for IFN-γ and <10 pg/well for IL-10.
HLA-A2.1 Binding Affinity of Heteroclitic Analogs.
In the course of the studies described above the MHC contact residues were not intentionally substituted. However, we wished to verify that the enhanced recognition by CTL lines observed was not due to a fortuitous increase in MHC binding capacity of the analogue epitope. The MHC binding affinity of all heteroclitic analogs was therefore measured and compared with their unmodified wild-type counterparts. As summarized in Table , three analogs bound to HLA-A2.1 with fourfold or higher affinity than wild-type peptide (MAGEA3.112W7, HIV Pol.476H3, and HIV Pol.476L3) and two analogs bound with lower affinity (MAGEA2.157I5, MAGEA2.157F5). The four remaining heteroclitic analogs, MAGEA3.112I5, CEA.691M3, CEA.691H5, and HBV Pol.455P7, were associated with little or no change in HLA-A2.1 binding capacity. This lack of correlation between changes in HLA binding and heteroclicity suggests that heteroclicity is not mediated by an increase in MHC binding affinity, unlike the increased T cell responses resulting from substitutions of MHC anchor residues 41 42. Our studies, however, do not exclude the possibility that alterations in MHC binding affinity might indirectly affect T cell recognition through the introduction of subtle structural modifications in the peptide regions recognized by the TCR. Minor structural modifications, not unlike what we are proposing for heteroclitic analogs, have been previously reported to affect TCR recognition and generate antagonist or agonist peptides 43 44.
Prediction and Immunogenicity of Analogs for the Murine p53.261 Epitope.
To further validate the heteroclitic substitution rules, we performed additional studies where heteroclitic analogs were predicted according to the structural features described above and then tested for their immunogenicity in vivo. For these studies, the HLA-A2.1–restricted murine p53.261 epitope was selected, as CTL responses against this epitope have been shown to be partially tolerized in HLA-A2.1/Kb transgenic mice 45 46, thus allowing the opportunity to analyze the capacity of predicted heteroclitic analogs to increase CTL responses against an epitope that is weakly immunogenic in vivo.
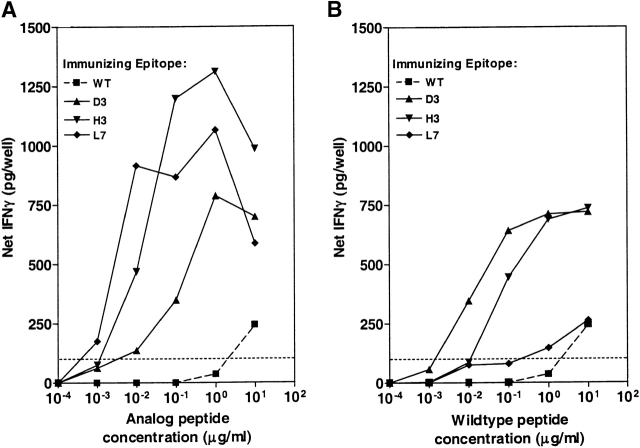
A panel of nine analogs of the p53.261 epitope consisting of three conservative or semiconservative substitutions at positions 3, 5, and 7 of the nonamer peptide was tested for immunogenicity in HLA-A2.1/Kbxd transgenic mice. Immunization of mice with each of the nine analogs resulted in identification of three analogs (D3, H3, L7) that gave CTL responses characterized by IFN-γ production of 100 pg/well at lower peptide concentrations compared with CTLs induced with wild-type peptide (Fig. 5 A). These results indicate that a significant percentage (33%) of the predicted analogs induce CTLs of a higher avidity than those induced by wild-type peptide itself.
Figure 5.
Reactivity of CTLs lines from p53.261 analogue-immunized mice against analogue and wild-type (WT) peptide. Spleen cells from mice immunized with either WT or analogs were stimulated in vitro with the immunizing peptide (A) or with wild-type peptide (B). IFN-γ release by these CTLs after two rounds of in vitro stimulation was then measured over a dose range against targets pulsed with the immunizing peptide (A) or with wild-type peptide (B). IFN-γ release at 100 pg/well is shown as a dotted line. Background IFN-γ levels were <50 pg/well.
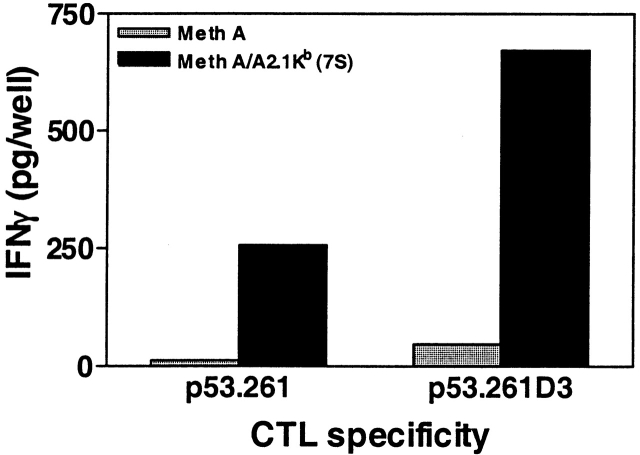
The cross-reactivity of CTLs primed with these heteroclitic analogs with wild-type peptide is shown in Fig. 5 B. CTLs obtained from animals immunized and restimulated with wild-type peptide induced 100 pg/well IFN-γ at peptide doses between 1–10 μg/ml. In contrast, CTLs obtained from animals immunized with analogs L7, H3, and D3 that were stimulated and tested in vitro with wild-type peptide required lower doses of wild-type peptide to induce 100 pg/well of IFN-γ in the range of 0.2 to 0.001 μg/ml (Fig. 5 B). Thus, in three out of nine cases the predicted heteroclitic analogs induced CTL in vivo which were 10- to a 1,000-fold more active/potent against a weakly immunogenic wild-type peptide compared with wild-type–induced CTL. Although the heteroclitic properties of the L7 analogue appear marginal in Fig. 5 B, data from precursor frequency analysis, described below, confirmed its heteroclicity. The biological relevance of the analogue-induced CTL responses was underlined when a p53+ Meth A–transformed tumor cell clone, transfected with HLA-A2.1/Kb, was used as APC. Significantly greater production of IFN-γ was observed when analogue-induced CTL were stimulated with the endogenously-processed wild-type epitope expressed by the HLA-A2.1/Kb+ Meth A tumor clone compared with CTLs generated by the wild-type peptide (Fig. 6).
Figure 6.
CTL generated by p53.261 analog respond to endogenously-processed p53.261 wild-type epitope. CTL (105/well) from mice immunized with wild-type epitope or the D3 analogue were cultured with a p53+ Meth A tumor cell clone 7S which had been transfected with HLA-A2.1/Kb or the untransfected parental cell line (2.5 × 104/well), and IFN-γ release was measured by the in situ ELISA.
Precursor Frequency Analysis Using the ELISPOT Assay.
To determine the frequencies of wild-type–reactive CTLs, splenic CD8+ cells were isolated from mice immunized with heteroclitic analogs or wild-type peptide and tested for reactivity against the wild-type peptide using an ELISPOT assay without any prior CTL expansion in vitro. As shown in Fig. 7, immunization with the heteroclitic p53.261 analogs D3, H3, and L7 induced a significantly higher level of CTL effectors responding to the wild-type epitope (CTL frequency of ∼1/15,000, or 60–75 spots/106 cells), compared with immunization with wild-type epitope (frequency of 1/67,000 or 15 spots/106 cells).
Figure 7.
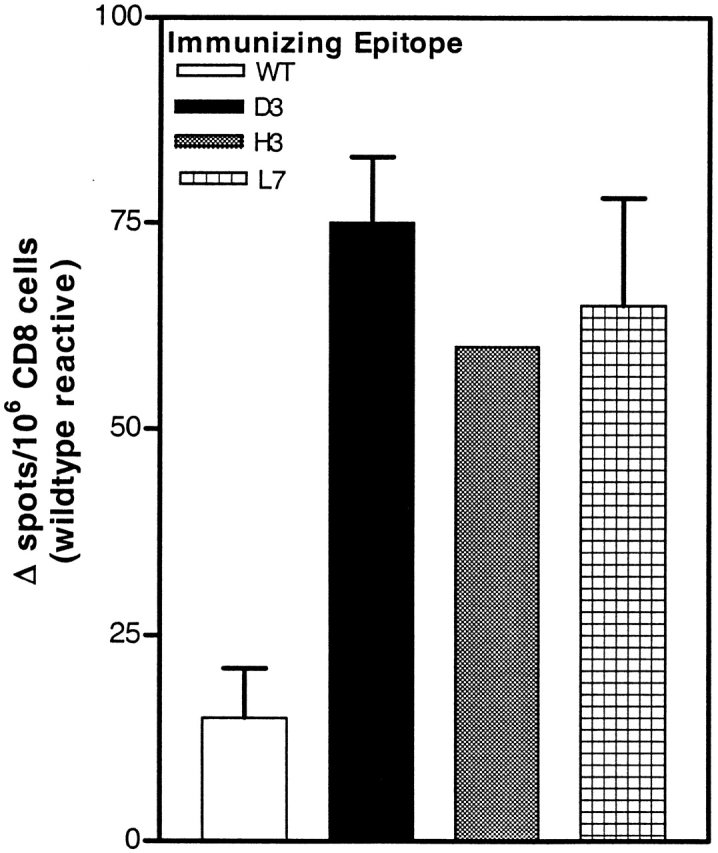
Precursor frequencies of wild-type (WT)-reactive cells in mice immunized with heteroclitic analogs. CD8+ cells isolated from mice immunized with either wild-type peptide or the D3, H3, and L7 analogs were analyzed for their ability to release IFN-γ when stimulated with wild-type peptide for 24 h in an ELISPOT assay. Background CTL responses when stimulated with target cells in the absence of peptide were between 3–5 spots/4 × 105 CTLs.
Heteroclitic Analogs Induce Human CTLs Capable of Recognizing Tumor Cells In Vitro.
To further underline the physiologic relevance of our observations, we examined whether heteroclitic analogs of the CEA.691 epitopes could induce human CTLs in a primary in vitro induction system. Fresh naive human PBMC from normal donors were stimulated repetitively in vitro with either the wild-type CEA.691 epitope or the M3 and H5 analogs. A higher number of CTL-positive cultures was detected when PBMCs were stimulated with two of the heteroclitic CEA analogs (Fig. 8b and Fig. c) compared with the wild-type epitope (Fig. 8 A; 11 positive/48 total wells for the M3 analogue and 10/48 for the H5 analogue versus 2/48 for wild-type). More importantly, a higher frequency of analogue-induced CTL cultures recognized the naturally processed form of the wild-type epitope (9 positive/48 total wells for both analogs, versus 2/48 for wild-type immunogen; P value < 0.02). Only CTLs from cultures that displayed peptide specificity also recognized endogenous epitope-expressing tumor cells indicating that the latter reactivity was epitope-specific.
Figure 8.
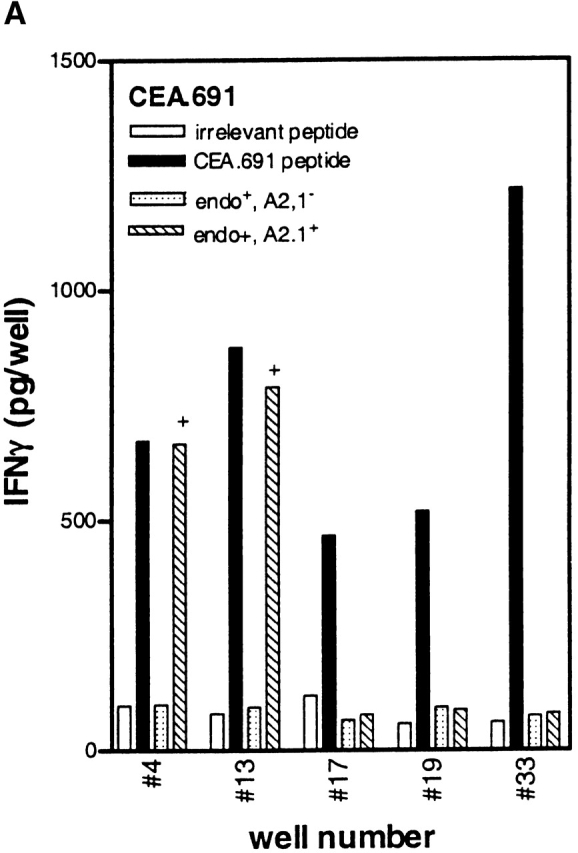
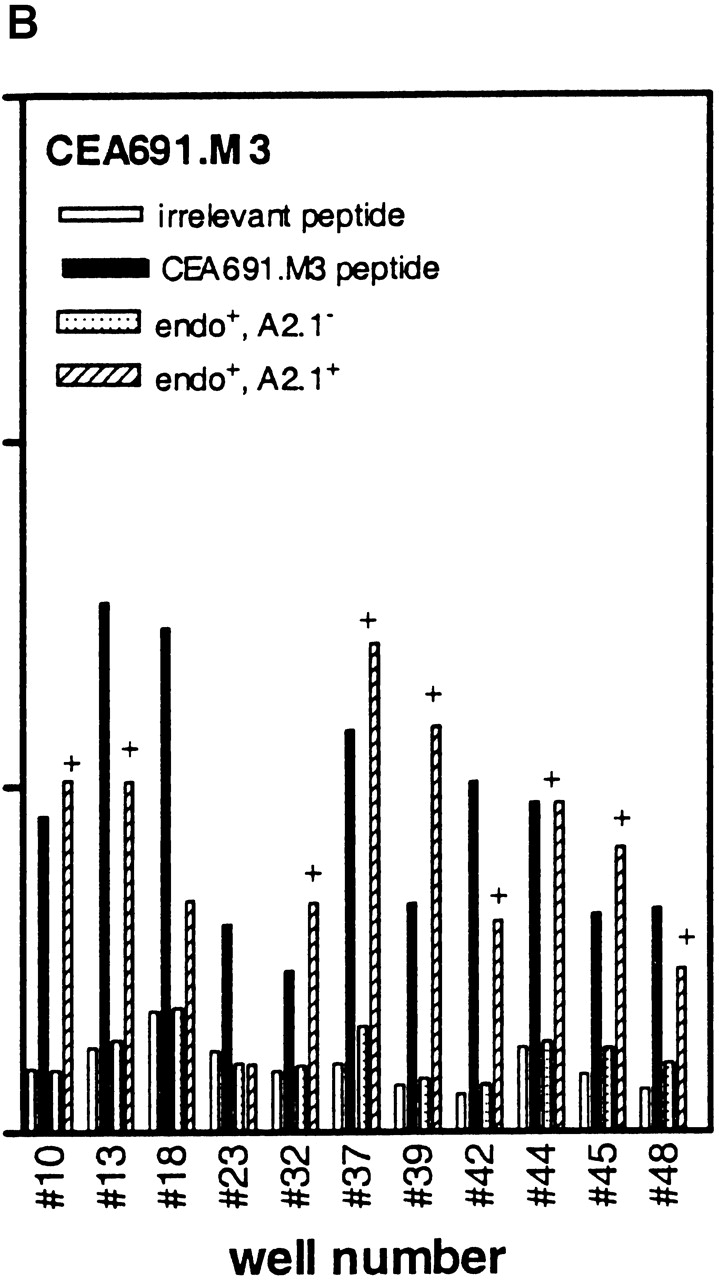
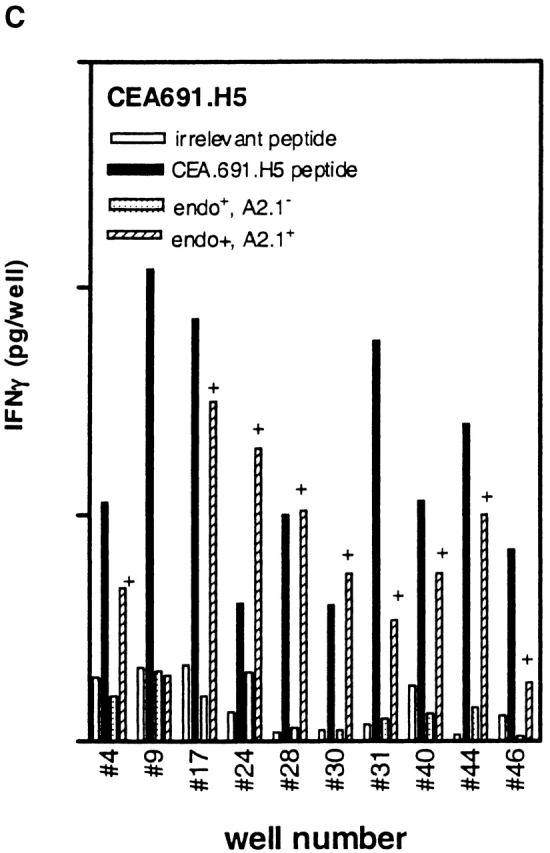
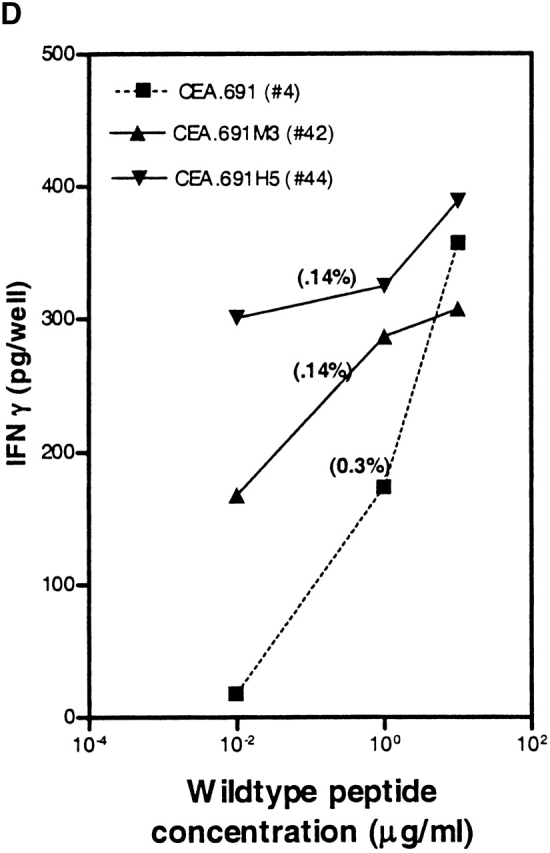
Higher frequencies of CTLs recognizing endogenous antigen are induced with CEA.691 analogs M3 and H5 than with wild-type epitope. IFN-γ production by CTLs induced after repetitive in vitro stimulation with either wild-type peptide (A), analogue M3 (B), or analogue H5 (C) was measured. PBMCs were stimulated in a 48-well plate with each epitope and tested against .221A2.1 cells pulsed with 10 μg/ml of the corresponding inducing peptide or an irrelevant MAGEA3.112 peptide. CTLs were also tested against the HLA-A2.1+ tumor cell line SW403 which express the endogenous CEA.691 epitope, and an allogeneic endogenous epitope-positive tumor cell line, HT29. Only responses from wells with peptide-specific CTLs are shown and the number assigned to that well is indicated along the X-axis. Corresponding responses showing positive endogenous epitope recognition is denoted by “+.” Representative responses of three CTL-positive wells against WT epitope, after an additional restimulation with adherent monocytes pulsed with the immunizing peptide, are shown in D. The well numbers correspond to those tested in A, B, and C. The percentage of wild-type reactive cells in each of these three representative wells (Fig. 8 D) as determined by an ELISPOT assay are indicated in parentheses above the dose titration curve of each corresponding CTL line. Background responses of each CTL line was <10 pg/well.
Avidity analysis of peptide-reactive CTL cultures from the above experiment was performed by restimulating a small number of cultures one additional time in vitro. When tested against 221A2.1 cells pulsed with different concentrations of wild-type peptide, CTLs induced with the CEA analogs H5 and M3 demonstrated higher avidity than those induced with wild-type peptide (Fig. 8 D), in keeping with our results with the murine p53.261 epitope. Moreover, we determined the number of wild-type reactive cells using an ELISPOT assay for these indicated wells to substantiate the findings that the higher avidity of these cultures is not a result of greater number of wild-type specific CTLs present in cultures induced in vitro with analogue peptides. As indicated in the Fig. 8 D, the number of wild-type reactive cells in well #4 (induced with wild-type peptide) was 3,060/106 (0.3%) whereas in case of well #42 (induced with analogue M3) and well #44 (induced with analogue H5) the number of wild-type reactive cells were 1,420/106 (0.14%) and 1,360/106 (0.14%), respectively. This indicates that the CTL cultures induced by analogs did indeed recognize lower concentrations of wild-type peptide (higher avidity) and the effect was not do to greater numbers of wild-type reactive cells present in the analogue induced cultures. In summary, our results demonstrate that heteroclitic analogs can induce high avidity, physiologically relevant human CTLs that recognize endogenously generated wild-type peptide presented by tumor cells and that the analogs identified by the heteroclitic substitution patterns are relevant in both human and transgenic mouse systems.
Discussion
In this study, we report the identification of heteroclitic analogs of five different HLA-A2.1–restricted CTL epitopes of cancer and viral origin, that were characterized as CTL epitopes in our previous studies and from other studies reported in the literature 25 26 40. In initial experiments for this study, the antigenicity of 233 analogs of the CEA.691 and MAGEA3.112 CTL epitopes was investigated to determine the commonality of heteroclitic analogs and to identify patterns of heteroclitic substitutions. The nature of the four heteroclitic analogs identified suggested that heteroclitic substitutions tended to involve conservative/semiconservative substitutions at odd-numbered positions in the center of the peptide. This hypothesis was tested in a subsequent study involving three additional well-characterized epitopes, MAGEA2.157, HIV Pol.476, and HBV Pol.455. All of the heteroclitic analogs identified in this study followed the previously observed pattern of conservative or semiconservative substitutions at odd-numbered positions.
To more closely mimic the clinical application of heteroclitic analogs in cancer immunotherapy, the murine epitope p53.261, for which a partial state of T cell tolerance has been reported previously in HLA-A2.1/Kb transgenic mice, was selected 45 46. Out of a panel of nine predicted analogs, three p53.261 analogs induced CTLs which responded vigorously against the native p53.261 epitope. Finally, the relevance of these findings for human CTLs was addressed by demonstrating that heteroclitic analogs of the CEA.691 epitope are immunogenic for human T cells in vitro. The CTLs induced by primary in vitro induction with analogs were able to recognize the naturally processed wild-type epitope expressed on tumor cell lines. These studies therefore suggest that heteroclitic analogs are significantly better at inducing immune responses than wild-type antigen and could offer substantial improvements in the design of epitope-based vaccines.
Our studies demonstrate that heteroclicity is a fairly common phenomenon as heteroclitic analogs were identified for all six epitopes studied. In addition, we have shown that it is possible to detect heteroclitic analogs using bulk T cell populations, not unlike studies from other laboratories that have used more clonal T cell populations. In this study we intended to avoid the limitation of focusing heteroclitic peptide identification to epitopes recognized by single CTL clones and for this reason bulk CTL lines were used. However, in future studies it will be interesting to formally prove that the selected peptide analogs do have heteroclitic activity for a broad range of CTL clones directed against the same nominal peptide antigen.
Finally, we have demonstrated that heteroclicity (at least in the HLA-A2.1 system) is associated with discrete structural features which allow rational prediction of heteroclicity. Our method involved developing a composite value based on a protein alignment scoring matrix, two hydrophobicity rankings, and a measurement of side chain volume derived from the partial specific volume of each amino acid. The ranking produced some unexpected similarities. For example, substitutions like A-P, G-D, H-E, G-P, and S-P might be somewhat unanticipated as conservative shifts. However, based on our results, development of an objective ranking scheme appears to constitute a much improved approach to identifying altered peptide ligands with heteroclitic activity. The first feature associated with heteroclitic effects for HLA-A2.1 epitopes was the occurrence of conservative or semiconservative substitutions at odd-numbered positions (3, 5, or 7) in the middle of the peptide. While heteroclicity is ultimately dependent on the orientation of specific side chains of the analogue in the structure of the trimeric complex (TCR-MHC-peptide), insertion of substitutions at odd-numbered positions can provide an empirical guide for the identification of heteroclitic analogs that enhance T cell activation. In these positions we frequently observed either a positive or a negative alteration in MHC binding, and some of these positions, particularly 3, 7, and in some cases position 5 have been shown to be secondary anchor positions for binding to the HLA-A2.1 molecule 27 47 48. Moreover, these positions have less frequently been identified as TCR contact positions by crystallization studies of HLA-A2.1 with viral peptides, as described by Madden et al. 48. Alteration of such secondary anchor positions which translate into T cell recognition differences have been reported 43 44, however, in these previous studies T cell recognition differences were associated with changes in MHC binding and no specific rules were defined for the kinds of amino acid substitutions involved in obtaining higher activity analogs. It should be emphasized, however, that the enhanced T cell recognition of analogs identified in this study is not likely due to increases in MHC binding affinity since higher activity was associated with analogs with invariant or even decreased MHC binding affinity. Increased binding is likely to play an important role in the case of analogs where primary anchor positions have been optimized; however, in this study we purposely did not substitute primary MHC anchor residues. A recent study by Slansky 8 and colleagues has shed some light on the potential mechanism of heteroclicity. In their studies heteroclicity of the AH1 analogue of a H-2Ld–restricted gp70 tumor epitope was associated with an enhancement in the TCR binding affinity for the peptide-MHC complex compared with the cognate wild-type epitope and not to an increase in binding to the MHC molecule. When examined in more detail, the enhanced TCR affinity of the AH1 analogue was not associated with changes in the main TCR contact residue or the main MHC contact residues at positions 2 and 9, suggesting that, similar to our findings, subtle changes in peptide structure may be contributing to heteroclicity. Some previous studies implied that modulation of T cell responses by heteroclitic analogs directly involve main TCR contact residues 6 7 49 but this finding is not corroborated by the current systematic analysis. In conclusion, our study suggests that heteroclitic analogs are most likely generated by subtle alterations in conformation rather than by gross alterations of TCR or MHC binding capacity.
It is of interest to compare our results with other published studies that have identified heteroclitic analogs for CTL. A heteroclitic analogue of a MART1/Melan A epitope has been shown to generate tumor-specific CTLs in vitro from PBMC of melanoma patients more efficiently than the wild-type peptide itself 12. Similarly, Schlom and colleagues 6 reported similar findings with a heteroclitic analogue of the CEA-derived CAP1 epitope although a side-by-side precursor frequency analysis or a TCR avidity analysis against wild-type peptide was not performed. More recently, Slansky 8 identified that a conservative substitution of valine to alanine at an odd-numbered position (position 5) in the wild-type gp70 epitope was associated with heteroclitic activity. Our current study extend their findings in several areas. First, although Slansky et al. reported that in vivo immunization with the AH1 analogue resulted in increases in CTL precursor frequency, their analysis was performed with in vitro stimulated cells where a potential for skewing the immune response exists. Herein, we have demonstrated that not only do heteroclitic analogs of p53.261 induce CTLs with higher avidity following in vivo immunization, they also induce a higher precursor frequency than wild-type peptide when measured without any in vitro manipulation of effector cells. Second, in studies reported herein we have defined some predictions for heteroclitic substitutions that is applicable for a wider array of HLA-A2.1-restricted epitopes and which have important implications in designing multi-epitope CTL vaccines for humans.
It should be noted that exceptions to the substitution patterns we describe in this study have been reported for heteroclitic analogs identified from other laboratories. For example, substitutions at position 1 for a MART1 epitope 12 and at position 6 for a CEA epitope 6 have resulted in heteroclitic activity. Thus, our predictions should not be taken as absolute rules but rather as a very efficient “shortcut” for predicting heteroclitic analogs. In this regard, current studies are ongoing to determine whether the substitution patterns identified for HLA-A2.1 are applicable for other HLA haplotypes.
Interestingly, the heteroclitic analogs identified in this study did not differentially regulate the production of Th1 or Th2 cytokines, as have been reported for other heteroclitic analogs 14. Our data suggests that heteroclitic analogs increase the production of both Th1 and Th2 cytokines by CTLs in terms of both a greater magnitude of cytokine production and more efficient stimulation at lower peptide doses. In fact, such overall stimulation by peptide analogs has been recently reported by some groups 49 50. One possible explanation for the increases in both Th1 as well as Th2 cytokines from CTLs stimulated with heteroclitic analogs reported herein may be that CTL lines used in this study were predominantly of the Th0 phenotype whereas other studies showing differential cytokine responses may have used T cell clones or lines that were terminally differentiated.
Our findings have implications from the standpoint of vaccine development and clinical applications. First, through the application of our heteroclitic substitution rules, we increased the efficiency of detecting heteroclitic analogs from 2% (four identified from screening of 233 CEA.691 and MAGEA3.112 analogs) to 33% (three identified by screening of nine predicted p53.261 analogs). As a consequence, the labor intensive process of screening analogs for heteroclicity should be dramatically simplified thus making it more cost effective and amenable to high throughput. Second, we demonstrate that heteroclitic analogs are effective in raising polyclonal populations of specific T cells after in vivo immunization which may be more efficacious in resolving disease states in a clinical setting. Finally, the ability to generate high precursor frequencies of CTL possessing strong cross-reactive avidity against wild-type epitope may be important in instances where effective CTL responses against epitopes, normally tolerant to the immune system, are required.
It will be of interest, in future studies to examine to which degree this approach is applicable to other HLA-A and HLA-B alleles. As HLA-A2 is expressed in ∼50% of the population and several different laboratories have already identified HLA-A2 restricted immunodominant epitopes of tumor antigens, our approach has immediate potential for increasing the activity of these HLA-A2 epitopes. While this approach may involve precise identification of HLA types and peptide epitopes, most of these procedures have now been simplified and automated. Thus, we believe that our approach has great potential in the field of immunotherapy of cancer and infectious disease in general.
Acknowledgments
We would like to thank Ajesh Maewal and Anthony Chiem for synthesis of peptides, and to Mingsheng Qin for technical assistance. We are also grateful to Robin Delp for secretarial assistance.
Supported in part by funds provided through National Institutes of Health-National Institute of Allergy and Infectious Diseases contract NO1-AI-95362.
Footnotes
Abbreviations used in this paper: CEA, carcinoembryonic antigen; HBV, hepatitis B virus; ELISPOT, enzyme-linked immunospot.
References
- Kagi D., Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr. Opin. Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- Greenberg P.D. Adoptive T cell therapy of tumorsmechanisms operative in the recognition and elimination of tumor cells. Adv. Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- Valmori D., Fonteneau J.F., Lizana C.M., Gervois N., Lienard D., Rimoldi D., Jongeneel V., Jotereau F., Cerottini J.C., Romero P. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J. Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- Bakker A.B., van der Burg S.H., Huijbens R.J., Drijfhout J.W., Melief C.J., Adema G.J., Figdor C.G. Analogues of CTL epitopes with improved MHC class I binding capacity elicit anti-melanoma CTL recognizing the wildtype epitope. Int. J. Cancer. 1997;70:302–308. doi: 10.1002/(sici)1097-0215(19970127)70:3<302::aid-ijc10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Webster's Ninth New Collegiate Dictionary. 1990. Merriam Webster Inc., Springfield, MA.
- Zaremba S., Barzaga E., Zhu M., Soares N., Tsang K.Y., Schlom J. Identification of an enhancer agonist cytotoxic T lymphocyte peptide from human carcinoembryonic antigen. Cancer Res. 1997;57:4570–4577. [PubMed] [Google Scholar]
- Loftus D.J., Squarcina P., Nielsen M.B., Geisler C., Castelli C., Odum N., Apella E., Parmiani G., Rivoltini L. Peptides derived from self-proteins as partial agonists and antagonists of human CD8+ T cell clones reactive to Melanoma/Melanocyte epitope MART-1. Cancer Res. 1998;58:2433–2439. [PubMed] [Google Scholar]
- Slansky E.J., Rattis M.F., Boyd F.L., Fahmy T., Jaffee M.E., Schneck P.J., Margulies H.D., Pardoll M.D. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 2000;13:529–538. doi: 10.1016/s1074-7613(00)00052-2. [DOI] [PubMed] [Google Scholar]
- Zugel U., Wang R., Shih G., Sette A., Alexander J., Grey H.M. Termination of peripheral tolerance to a T cell epitope by heteroclitic antigen analogues. J. Immunol. 1998;161:1705–1709. [PubMed] [Google Scholar]
- Wang R., Wang-Zhu Y., Gabaglia C.R., Kimachi K., Grey H.M. The stimulation of low-affinity non-tolerized clones by heteroclitic antigen analogues causes the breaking of tolerance established to an immunodominant T cell epitope. J. Exp. Med. 1999;190:983–994. doi: 10.1084/jem.190.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men Y., Miconnet I., Valmori D., Rimoldi D., Cerottini J.C., Romero P. Assessment of immunogenicity of human Melan-A peptide analogues in HLA-A*0201/Kb transgenic mice. J. Immunol. 1999;162:3566–3573. [PubMed] [Google Scholar]
- Rivoltini L., Squarcina P., Loftus D.J., Castelli C., Tarsini P., Mazzocchi A., Rini F., Viggiano V., Belli F., Parmiani G. A superagonist variant of peptide MART1/Melan A elicits anti-melanoma CD8+ T cells with enhanced functional characteristicsimplication for more effective immunotherapy. Cancer Res. 1999;59:301–306. [PubMed] [Google Scholar]
- Tao X., Grant C., Constant S., Bottomly K. Induction of IL-4 producing CD4+ T cells by antigenic peptides altered for TCR binding. J. Immunol. 1997;158:4237–4244. [PubMed] [Google Scholar]
- Salazar E., Zaremba S., Allen P.A., Tsang K.Y., Schlom J.A. Agonist peptide from a cytotoxic T-lymphocyte epitope of human carcinoembryonic antigen stimulates production of Tc1-type cytokines and increases tyrosine phosphorylation more efficiently than cognate peptide. Int. J. Cancer. 2000;85:829–838. doi: 10.1002/(sici)1097-0215(20000315)85:6<829::aid-ijc16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Nicholson L.B., Anderson A.C., Kuchroo V.K. Tuning T cell activation threshold and effector function with cross-reactive peptide ligands. Int. Immunol. 2000;12:205–213. doi: 10.1093/intimm/12.2.205. [DOI] [PubMed] [Google Scholar]
- Zitvogel L., Mayordomo J.L., Tjandrawan T., DeLeo A.B., Clarke M.R., Lotze M.T., Storkus W.J. Therapy of murine tumors with tumor peptide-pulsed dendritic cellsdependence on T cell, B7 costimulation and T helper cell 1-associated cytokines. J. Exp. Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celluzzi C.M., Mayordomo J.I., Storkus W.J., Lotze M.T., Falo L.D., Jr. Peptide pulsed dendritic cells induce antigen specific CTL-mediated protective tumor immunity. J. Exp. Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Lymphokine production by human T cells in disease states. Annu. Rev. Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- Selby M., Erickson A., Dong C., Cooper S., Parham P., Houghton M., Walker C.M. Hepatitis C virus envelope glycoprotein E1 originates in the endoplasmic reticulum and requires cytoplasmic processing for presentation by class I MHC molecules. J. Immunol. 1999;162:669–676. [PubMed] [Google Scholar]
- Skipper J.C., Hendrickson R.C., Gulden P.H., Brichard V., Van Pel A., Chen Y., Shabanowitz J., Wolfel T., Slingluff C.L., Jr., Boon T. An HLA-A2 tyrosinase antigen on melanoma cells results from post-translational modification and suggests a novel pathway for processing of membrane proteins. J. Exp. Med. 1996;183:527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake J., Johnston J.V., Hellstrom K.E., Marquardt H., Chen L. Use of combinatorial peptide libraries to construct functional mimics of tumor epitopes recognized by MHC class I–restricted cytolytic T lymphocytes. J. Exp. Med. 1996;18:121–130. doi: 10.1084/jem.184.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinilla C., Martin R., Gran B., Appel J.R., Boggiano C., Wilson D.B., Houghten R.A. Exploring immunological specificity using synthetic peptide combinatorial libraries. Curr. Opin. Immunol. 1999;11:193–202. doi: 10.1016/s0952-7915(99)80033-8. [DOI] [PubMed] [Google Scholar]
- Boon T., Cerottini J.C., Van den Eynde B., Van der Bruggen P., Van Pel A. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- Gavin M.A., Dere B., Grandea A.G., Hogquist K.A., Bevan M.J. Major histocompatibility complex class I allele-specific peptide librariesIdentification of peptides that mimic an H-Y T cell epitope. Eur. J. Immunol. 1994;24:2124–2133. doi: 10.1002/eji.1830240929. [DOI] [PubMed] [Google Scholar]
- Kawashima I., Tsai V., Southwood S., Takesako K., Celis E., Sette A. The multi-epitope approach for immunotherapy of canceridentification of several CTL epitopes from various tumor-associated antigens expressed on solid epithelial tumors. Hum. Immunol. 1998;59:1–14. doi: 10.1016/s0198-8859(97)00255-3. [DOI] [PubMed] [Google Scholar]
- Ishioka G.Y., Fikes J., Hermanson G., Livingston B., Crimi C., Qin M., del Guercio M.F., Dahlberg C., Alexander J., Chesnut R.W., Sette A. Utilization of MHC class I transgenic mice for development of minigene DNA vaccines encoding multiple HLA-restricted CTL epitopes. J. Immunol. 1999;162:3915–3925. [PubMed] [Google Scholar]
- Ruppert J., Sidney J., Celis E., Kubo R.T., Grey H.M., Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993;74:929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- Dayhoff M.O., Schwartz R.M., Orcutt B.C. A model for evolutionary change. In: Dayhoff M.O., editor. Atlas of Protein Sequence and Structure, Vol. 5, Suppl. 3. National Biomedical Research Foundation; Washington, DC: 1998. pp. 345–359. [Google Scholar]
- Creighton T.E. ProteinsStructures and Molecular Properties 2nd ed 1993. W.H. Freeman and Company, ; New York: pp. 570 pp [Google Scholar]
- Cornette J.L., Cease K.B., Margalit H., Spouge J.L., Berzofsky J.A., DeLisi C. Hydrophobicity scales and computational techniques for detecting amphipathic structures in proteins. J. Mol. Biol. 1987;195:659–685. doi: 10.1016/0022-2836(87)90189-6. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Fauchere J., Pliska V. Hydrophobic parameters pi of amino-acid side chains from the partitioning of N-acetyl-amino-acid amides. Eur. J. Med. Chem. 1983;18:369–383. [Google Scholar]
- Zamyatnin A.A. Amino acid, peptide, and protein volume in solution. Annu. Rev. Biophys. Bioeng. 1984;13:145–165. doi: 10.1146/annurev.bb.13.060184.001045. [DOI] [PubMed] [Google Scholar]
- Zamyatnin A.A. Protein volume in solution. Prog. Biophys. Mol. Biol. 1972;24:107–123. doi: 10.1016/0079-6107(72)90005-3. [DOI] [PubMed] [Google Scholar]
- Sette A., Sidney J., del Guercio M.F., Southwood S., Ruppert J., Dahlberg C., Grey H.M., Kubo R.T. Peptide binding to the most frequent HLA-A class I alleles measured by quantitative molecular binding assays. Mol. Immunol. 1994;31:813–822. doi: 10.1016/0161-5890(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Vitiello A., Marchesini D., Furze J., Sherman L.A., Chesnut R.W. Analysis of the HLA-restricted influenza specific cytotoxic T lymphocyte responses in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J. Exp. Med. 1991;173:1007–1015. doi: 10.1084/jem.173.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney D., Skvoretz R., Qin M., Ishioka G., Sette A. Characterization of an in situ IFN-γ ELISA assay which is able to detect specific peptide responses from freshly isolated splenocytes induced by DNA minigene immunization. J. Immunol. Methods. 2000;237:105–107. doi: 10.1016/s0022-1759(00)00138-1. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K., Altman J.D., Suresh M., Sourdive D.J., Zajac A.J., Miller J.D., Slansky J., Ahmed R. Counting antigen-specific CD8 T cellsa re-evaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Lewis J.J., Janetzki S., Schaed S., Panageas K.S., Wang S., Williams L., Meyers L., Butterworth L., Livingston P.O., Chapman P.B., Houghton A.N. Evaluation of CD8+ T-cell frequencies by the ELISPOT assay in healthy individuals and in patients with metastatic melanoma immunized with tyrosinase peptide. Int. J. Cancer. 2000;87:391–398. doi: 10.1002/1097-0215(20000801)87:3<391::aid-ijc13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Visseren M.J., van der Burg S.H., van der Voort E.I., Brandt R.M., Schrier P.I., van der Bruggen P., Boon T., Melief C.J., Kast W.M. Identification of HLA-A*0201 restricted CTL epitopes encoded by the tumor specific MAGE-2 gene product. Int. J. Cancer. 1997;73:125–130. doi: 10.1002/(sici)1097-0215(19970926)73:1<125::aid-ijc19>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Dyall R., Bowne W.B., Weber L.W., LeMaoult J., Szabo P., Moroi Y., Piskun G., Lewis J.J., Houghton A.N., Nikolic-Zugic J. Heteroclitic immunization induces tumor immunity. J. Exp. Med. 1998;188:1553–1561. doi: 10.1084/jem.188.9.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst M.R., Salgaller M.R., Southwood S., Robbins P.F., Sette A., Rosenberg S.A., Kawakami Y. Improved induction of melanoma reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A201 binding residues. J. Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- Valmori D., Gervois N., Rimoldi D., Fonteneau J.F., Bonelo A., Lienard D., Rivoltini L., Jotereau F., Cerottini J.C., Romero P. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J. Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- Davis M.M., Boniface J.J., Reich Z., Lyons D., Hampl J., Arden B., Chien Y. Ligand recognition by alpha beta T cell receptors. Annu. Rev. Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- Theobald M., Biggs J., Dittmer D., Levine A.J., Sherman L.A. Targeting p53 as a general tumor antigen. Proc. Natl. Acad. Sci. USA. 1995;92:11993–11997. doi: 10.1073/pnas.92.26.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald M., Biggs J., Hernandez J., Lustgarten J., Labadie C., Sherman L.A. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J. Exp. Med. 1997;185:833–841. doi: 10.1084/jem.185.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden D.R. The three-dimensional structure of peptide-MHC complexes. Annu. Rev. Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- Madden D.R., Garboczi D.N., Wiley D.C. The antigenic identity of peptide-MHC complexesa comparison of the conformations of five viral peptides presented by HLA-A2. Cell. 1993;75:693–708. doi: 10.1016/0092-8674(93)90490-h. [DOI] [PubMed] [Google Scholar]
- Dressel A., Chin J.L., Sette A., Gausling R., Hollsberg P., Hafler D.A. Autoantigen recognition by human CD8 T cell clones. J. Immunol. 1997;159:4943–4951. [PubMed] [Google Scholar]
- Grakoui A., Donermeyer D.L., Kanagawa O., Murphy K.M., Allen P.M. TCR-independent pathways mediate the effects of antigen dose and altered peptide ligands on Th cell polarization. J. Immunol. 1999;162:1923–1930. [PubMed] [Google Scholar]