Abstract
The E2A gene encodes the E47 and E12 basic helix-loop-helix (bHLH) transcription factors. T cell development in E2A-deficient mice is partially arrested before lineage commitment. Here we demonstrate that E47 expression becomes uniformly high at the point at which thymocytes begin to commit towards the T cell lineage. E47 protein levels remain high until the double positive developmental stage, at which point they drop to relatively moderate levels, and are further downregulated upon transition to the single positive stage. However, stimuli that mimic pre-T cell receptor (TCR) signaling in committed T cell precursors inhibit E47 DNA-binding activity and induce the bHLH inhibitor Id3 through a mitogen-activated protein kinase kinase–dependent pathway. Consistent with these observations, a deficiency in E2A proteins completely abrogates the developmental block observed in mice with defects in TCR rearrangement. Thus E2A proteins are necessary for both initiating T cell differentiation and inhibiting development in the absence of pre-TCR expression. Mechanistically, these data link pre-TCR mediated signaling and E2A downstream target genes into a common pathway.
Keywords: E2A, thymocyte, β selection, T cell commitment, Id
Introduction
The earliest T cell progenitors in the thymus are found within a minor population of lymphoid cells defined largely by the absence of expression of the CD4 and CD8 coreceptor molecules 1. The most immature of these double negative (DN) thymocytes are characterized by the expression of the CD44 and CD117 cell surface antigens; these cells still have multilineage developmental capacity. Commitment to the T cell lineage is largely associated with the induction of CD25 expression, followed by decreases in the levels of CD44 and CD117. Newly committed T cell precursors then initiate gene rearrangements at the TCR-γ, -δ, and -β loci. The generation of an in-frame TCR β gene allows for the expression of the pre-TCR complex, which contains the TCR β chain, the pre-Tα polypeptide, and the CD3 complex of signal transduction proteins. Signaling events dependent upon pre-TCR assembly result in a developmental transition referred to as β selection, characterized by the cessation of gene rearrangement, the initiation of several rounds of proliferation, and the differentiation into CD4+ CD8+ double positive (DP) thymocytes. DP cells, which comprise 75–90% of thymic cellularity, then exit the cell cycle and initiate TCR-α gene rearrangements. The expression of an αβ TCR allows DP cells to undergo affinity-based positive and negative selection. DP thymocytes that are positively selected downregulate either CD4 or CD8 and become mature single positive (SP) T cells 2.
E47 and E12 are basic helix-loop-helix (bHLH) transcription factors encoded by the E2A gene that bind DNA either as homodimers or as heterodimers with other bHLH proteins 3. E2A-encoded proteins are involved in the differentiation of a number of cell types and are particularly essential for lymphocyte development. In B-lineage cells the bHLH DNA binding complexes are composed of E47 homodimers whereas in thymocytes mainly heterodimers of E47 and a related bHLH protein, HEB have been detected. B cell development in E2A- and E47-deficient mice is completely arrested before Ig gene rearrangement 4 5 6. bHLH proteins have also been shown to be important at multiple stages of T cell development. Thymuses from E47-deficient mice are markedly hypocellular due to defects preceding T-lineage commitment and the initiation of TCR gene rearrangements 7. E47 has also been shown to be important for the selection process at the DP to SP transition 8 9. In addition, overexpression of E2A protein activity has been shown to induce either growth arrest or apoptosis, indicating that these proteins may regulate cell proliferation and survival 10 11 12.
E2A and HEB DNA binding activity is regulated by Id proteins, which are HLH proteins that lack DNA binding activity 13. There are four HLH proteins present in vertebrates, Id1–4, of which predominately Id2 and Id3 are expressed in lymphoid cells 3. Id3 is regulated by the Erk–mitogen-activated protein kinase (MAPK) pathway in DP thymocytes 9. Both positive and negative selection in Id3 null mutant thymocytes is severely perturbed 14. Additionally, Id3-deficient B-lineage cells display defects in the response to cytokines and mitogenic stimuli 15 16. Thus, the E2A proteins and their inhibitor Id3 play essential roles in lymphocyte maturation.
Here we have examined the role and regulation of E2A proteins in the development of DN thymocytes. Our observations indicate that E47 expression is activated in thymocytes at the point during which these cells begin to restrict their developmental potential towards the T-lineage. However, we show that E47 DNA binding activity is modulated upon signals that mimic β selection in DN thymocytes. We also demonstrate that pre-TCR signaling activates the expression of Id3, at least in part through an Erk–MAPK-dependent pathway. Furthermore, we demonstrate that E2A proteins inhibit the DN to DP transition in the absence of β selection. A null mutation in E47 completely abrogated the developmental block observed in both recombination activating gene (RAG)-deficient and scid mutant thymocytes, allowing survival and expansion to the DP stage and in some cases maturation to the mature CD8 SP stage as well. Interestingly, E47 heterozygosity could also lead to aberrant maturation in RAG-null mutant mice. These data strongly imply that the E2A proteins regulate genes required for both initiating T cell differentiation and inhibiting development at later stages in the absence of pre-TCR expression. Furthermore, our data suggest a model for how signaling from the pre-TCR complex can regulate E2A target genes.
Materials and Methods
Mice and Genotyping.
Either RAG-1−/− mice provided by Randall Johnson (University of California at San Diego) or RAG-2−/− mice obtained from Yang Xu (University of California at San Diego) were used for the anti-CD3ε injection experiments. 3–5-wk-old RAG-deficient littermates were injected intraperitoneally with 150 μg antibody against CD3ε (2C11 NA/LE; BD PharMingen). For the bone marrow transfer experiment, RAG-2−/− mice were irradiated with 950 rads from a Cesium source, and then injected intravenously with 7.5 × 106 bone marrow cells from an adult E47−/− RAG-1−/−mouse. Mice heterozygous for the E47 and RAG-1 null mutations in a mixed genetic background were obtained from Barbara Kee (University of California at San Diego) and interbred. scid mice in a C57BL/6 background were purchased from the Jackson ImmunoResearch Laboratories and interbred with mice carrying the E47-null mutation 6 in an FVB background. Mice were genotyped for the E47 and RAG-1 mutations by PCR amplification as described previously 6 17. Mice bred into a scid background were genotyped using a previously described strategy 18.
Preparation of Thymocyte Suspensions.
Thymuses were dissected from 3–5-wk-old RAG-deficient mice in HBSS. Thymocytes were released by pressing through 70 μm cell strainers (Falcon) and washed once in HBSS.
Flow Cytometric Analyses.
Thymocytes (1–10 × 105) were suspended in 0.1 ml FACS® buffer (PBS plus 0.1% FCS plus 0.02% sodium azide) plus the indicated antibodies on ice for 20 min. For analysis of DN thymocytes for expression of CD25 and CD44, cells were first incubated with biotinylated antibodies against CD4, CD8, CD3, CD11b, Gr-1, and TER-119, washed in 1 ml FACS® buffer, and stained anti-CD25-FITC and anti-CD4-PE together with strepavidin-cychrome or allophycocyanin. Cells were washed again and fixed in 0.5 ml PBS plus 1% paraformaldehyde. For analysis of BCL-2 expression cells were first stained with anti-CD8-PE and anti-CD4-tricolor, washed, and fixed in 1% paraformaldehyde, permeabilized with 0.1% saponin in PBS, incubated with anti–mouse BCL-2-FITC in PBS plus 0.3% saponin and 1% BSA for 30 min at 4°C, washed in PBS plus 0.1% saponin, and resuspended in FACS® buffer. For analysis of intracellular TCR-β or E47 expression cells were first stained for surface antigens, washed in FACS® buffer, and then fixed, permeabilized, and stained with anti–TCR-β-FITC or G127-32 (anti-E47) plus goat anti–mouse-FITC as described previously 19. Analysis was done using CELLQuest™ software on a FACScan™ or FACScalibur™ (Becton Dickinson). All antibodies were obtained from BD PharMingen except for anti-CD4-tricolor (Caltag).
Electrophoretic Mobility Shift Assay and Immunoblots.
Thymocyte suspensions were purified by centrifugation through Ficoll-Paque (Amersham Pharmacia Biotech) in order to remove dead cells and stroma. Cells were washed in HBSS, pelleted, and lysed at 4 × 107 cells per milliliter in a buffer containing 20 mM Hepes, pH 7.9, 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 10 mM NaPO4, 10 mM Na P2O7, 0.1 mM Na vanadate, protease inhibitors, and 1% NP-40. The insoluble fraction was then pelleted and removed. electrophoretic mobility shift assay (EMSA) was performed using 10–15 μg of whole cell extract as described in Bain et al. and references therein. Antibodies used for antibody interference analysis were G98-271 (mouse IgG1 specific only for human E2A), G127-32 (anti-E47), G127-34 (anti-E12), and G127-382 (anti-HEB, E12 and E2-2). Complexes interfered with by G127-382 preincubation were assumed to contain HEB, as these complexes did not react with anti-E12 (see Fig. 2 D), and E2-2 has weak EMSA activity (data not shown). For immunoblot analysis, 30 μg of whole cell extract was separated on 6% SDS-PAGE via electrophoresis and transferred to Immobilon (Millipore). The blots were stained with amido black to confirm that approximately equivalent amounts of protein were loaded, and then blocked in TBS plus 5% nonfat dry milk (Blotto). Anti-E47 was used at 1:250 in Blotto. Secondary goat anti–mouse conjugated to horseradish peroxidase was used at 1:104 and results were visualized with ECL+ (Amersham Pharmacia Biotech).
Figure 2.
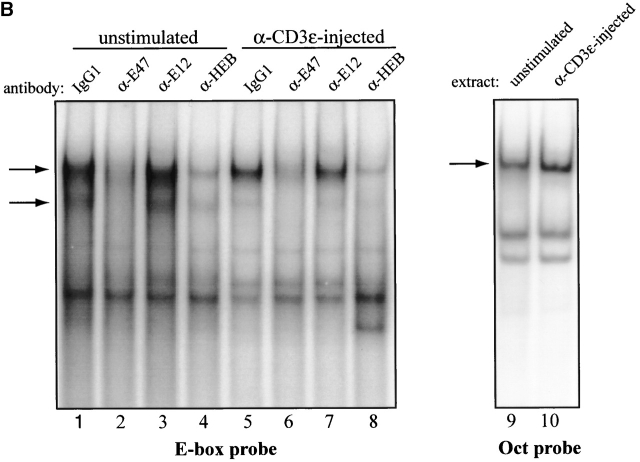
Regulation of E-box–binding activity during thymocyte β selection. RAG-1−/− thymocytes were harvested at indicated times after intraperitoneal anti-CD3ε injection. (A) Flow cytometric analysis of RAG-1−/− thymocytes at various time points after injection of anti-CD3ε. The number of cells per thymus for each time point is indicated above each column of dot plots. Top row of dot plots shows CD4 versus CD8 expression profile of live-gated cells. Bottom row shows CD44 versus CD25 profile of live-gated DN cells. (B) EMSA of thymic extracts binding to a μE5 oligonucleotide probe. (Left) Thymocyte whole cell extracts (15 μg) prepared from unstimulated RAG-2−/− mice (lanes 1–4) and RAG-2−/− mice injected with anti-CD3ε 24 h before extract preparation (lanes 5–8), were preincubated with 0.5 μg of irrelevant mouse IgG1 antibody (lanes 1 and 5), anti-E47 (lanes 2 and 6), anti-E12 (lanes 3 and 7), or anti-HEB (lanes 4 and 8), before performing EMSA using a μE5 probe. Arrows indicate specific complexes. (Right) The same extracts used in the left panel were tested for binding to an Oct probe at 10 μg per lane.
Northern Blot Analysis.
RNA was prepared using TRIzol (GIBCO BRL) from 106–107 thymocytes. DN and DP thymocyte subpopulations were obtained by a combination of complement lysis and cell sorting strategies. 10 μg RNA was separated by electrophoresis through a 1% agarose formaldehyde gel. The RNA was transferred to Nytran (Schleicher and Schuell) and cross-linked in a Stratalinker (Stratagene). Blots were probed with single stranded PCR-labeled DNA probes. The mouse Id2 probe from nucleotides 230–595 was isolated by reverse transcription PCR. The mouse Id3 probe was a 200-bp DNA fragment isolated by reverse transcription PCR with the following primers: for: 5′ CGCACTGTTTGCTGCTTTAGG 3′ and rev: 5′ GTAGCAGTGGTTC ATGTCGTC 3′.
Thymic Organ Culture.
Thymuses were harvested from neonatal RAG1−/− mice and cultured at the media interface on Costar Transwell inserts (Fisher Scientific), using IMDM (GIBCO BRL) plus 10% FCS. Penicillin/streptomycin/glutamate (GIBCO BRL), IL-7, and SCF. Organs were preincubated in the presence or absence of 30 μM PD98059 (Calbiochem) for 1 h, followed by 2 h of culture in 10 μg/ml α-CD3ε before harvest and RNA preparation.
Results
E47 Expression Is Activated shortly before Commitment to the T Cell Lineage.
Previous studies have suggested roles for E2A and HEB in the initiation of TCR gene rearrangements and the expression of the Rag and pre-Tα genes 20 21 22. These data imply that the expression of bHLH proteins may be required during the initial stage of T cell differentiation. Thus, we examined the expression of E47 in DN thymocytes by flow cytometric analysis of permeabilized cells stained with an antibody specific for E47. The expression of E47 was determined in four developmental stages of DN thymocyte development as defined by the expression of CD25 and CD44 23. The majority of CD44high CD25− (DN1) thymocytes, representing cells that have not committed to T cell development 24, expressed little or no E47 (Fig. 1 A). However, the expression of E47 was high within virtually all CD44high CD25+ (DN2) thymocytes, and was also uniformly high in the CD44low CD25+ (DN3) and CD44low CD25− (DN4) subsets (Fig. 1 A). Transition from the DN1 to the DN2 stage is closely correlated with a marked biasing of developmental potential towards the T cell lineage 24. Thus, when taken together with previous data demonstrating that developmental progression past the DN1 stage is defective in E2A-deficient thymocytes 7, these results suggest that the induction of E2A protein expression in DN thymocytes is necessary for efficient developmental progression towards the T-lineage. These data are also consistent with reports indicating that T cell commitment is blocked by Id3 overexpression in lymphocyte progenitors 25.
Figure 1.
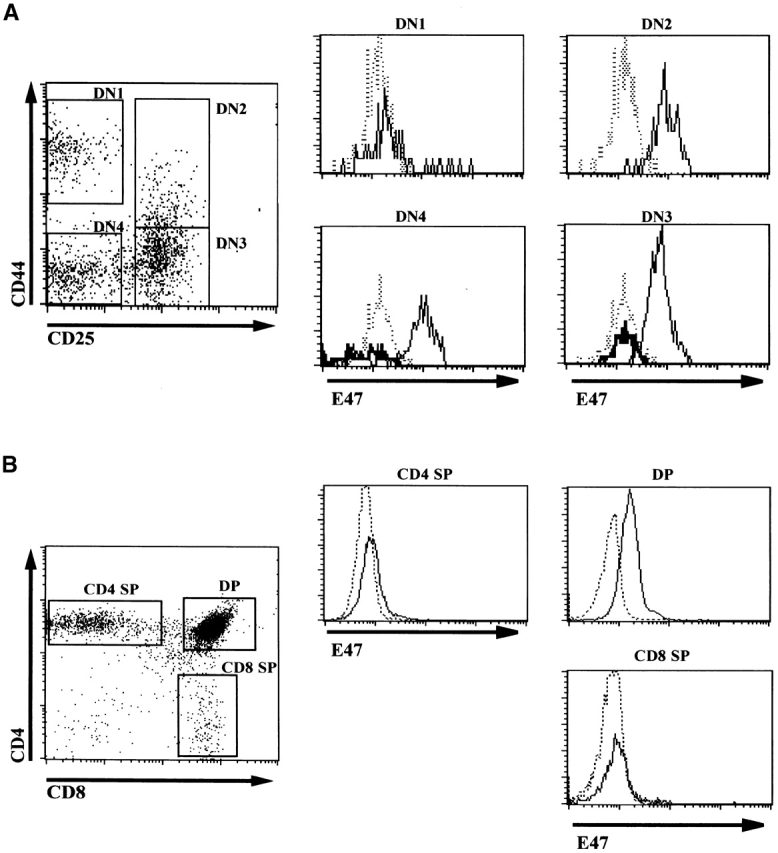
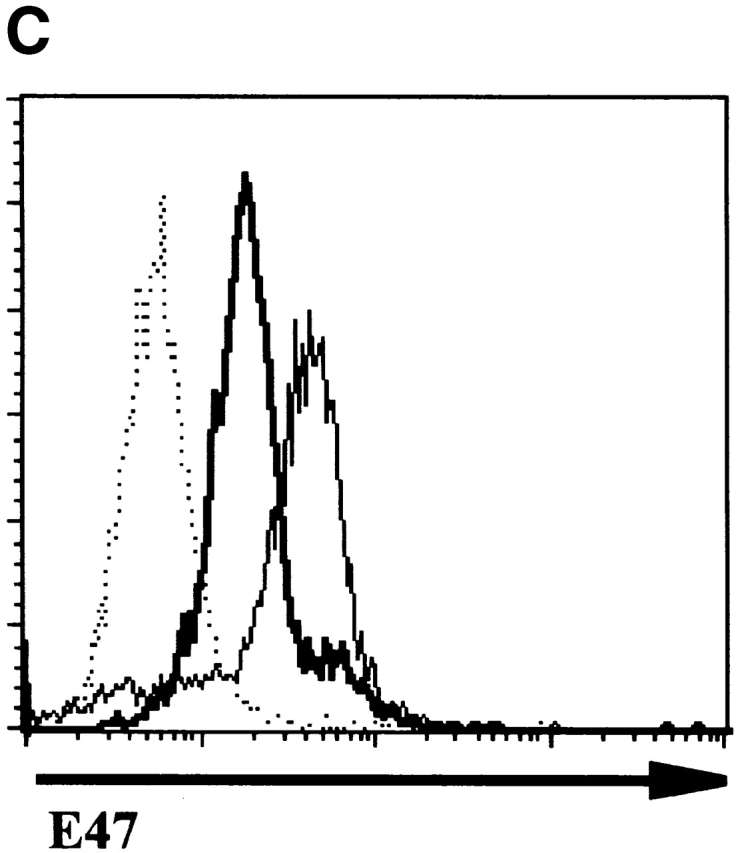
Expression of E47 in thymocyte subsets. The DN thymocytes from 5-wk-old E47 wild-type and −/− mice were analyzed for E47 expression by flow cytometry. (A) The dot plot depicts flow cytometric analysis of surface CD25 and CD44 expression in wild-type DN thymocytes, with gates drawn for the definition of the subpopulations analyzed for E47 expression. The histograms depict the staining of wild-type (solid line) cells within the DN subsets for E47, as compared with the staining of E47−/− DN1 thymocytes (dotted line). The DN3 and DN4 subset histograms also depict the staining of the appropriate subsets of E47−/− thymocytes (thick tracings). As expected, virtually no events were collected within the E47−/− DN2 gate. The high-level staining in a small percentage of wild-type DN1 cells was also observed when cells were stained with GAM-FITC but without anti-E47 (data not shown), therefore these cells are likely to be mature B cell contaminants, which are absent from E47-deficient mice (reference 6). The very dull “cells” in the DN4-gated populations most likely represent contaminating cellular debris, as their mean fluorescence is lower than the background fluorescence of viable cells, and the permeabilization method required for staining with the anti-E47 antibody decreases the discrimination between live cells and debris by flow cytometry. (B) The dot plot depicts analysis of CD8 and CD4 expression in wild-type thymocytes, with gates drawn for the definition of DP and SP populations. The histograms depict the staining of wild-type thymocytes (solid line) for E47, as compared with the staining of identically gated populations of E47−/− thymocytes (dotted line). (C) Histogram depicting the staining of wild-type (solid thick tracing), RAG-1−/− (solid thin line) and E47−/− thymocytes (dotted line). These experiments were repeated two or more times with similar results.
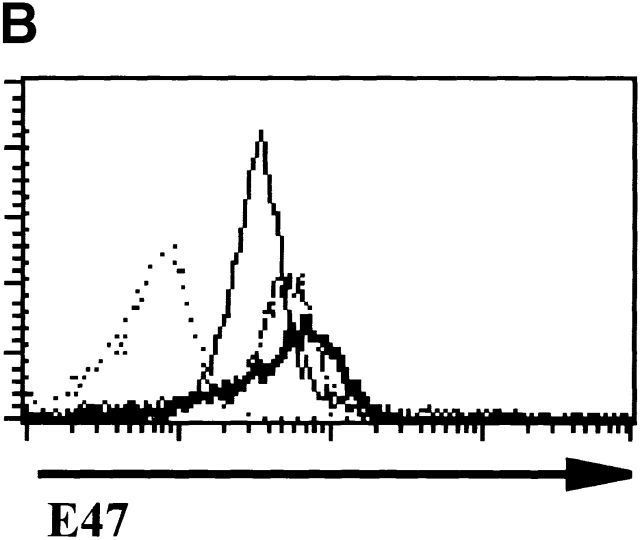
We also examined E47 expression in more mature thymocyte populations by flow cytometry. Immature SP cells, which represent a transitional stage from DN to DP, expressed levels similar to that of DN2, DN3, and DN4 thymocytes (reference 26, and data not shown). E47 was clearly expressed in DP thymocytes, although the majority of these cells expressed lower levels than that observed in the DN2, DN3, or DN4 subsets (Fig. 1 B). To confirm our assessment of the relative levels of E47 in DN and DP cells, we compared the expression of E47 in wild-type and RAG-deficient thymocytes. RAG-1 null thymocytes, which are predominately comprised of DN3 stage cells, expressed higher E47 levels than did the majority of wild-type thymocytes, which are largely DP (Fig. 1 C). E47 expression was downregulated further in mature CD4 and CD8 SP cells, to levels near the limit that we could detect by flow cytometry (Fig. 1 B). Thus, E47 protein levels in thymocytes become high at the point of T cell commitment, are lowered after the DN to the DP transition and barely detectable in SP cells.
E47 DNA Binding Activity Is Modulated upon pre-TCR-mediated Signaling.
E47 DNA binding has been shown to be modulated by TCR-mediated signaling in DP thymocytes 9. To examine whether bHLH DNA binding activity is also regulated during the DN to DP transition, RAG-deficient mice were injected with antibodies against CD3ε (anti-CD3ε). Thymocytes null for either RAG-1 or RAG-2 exhibit a developmental block at the DN3 stage due to the inability to rearrange the TCR genes 1. However, intraperitoneal injection of TCR-β–deficient mice with anti-CD3ε has been shown to induce developmental events associated with β selection, including progression from the DN3 to the DN4 and DP stages together with proliferation and expansion of the thymocyte pool 27 28. Thymocyte populations derived from RAG-deficient mice that were injected with anti-CD3 were harvested at various time points and analyzed by flow cytometry for developmental progression. Additionally, RNA and protein extracts were prepared from the remaining cells. Flow cytometric analysis confirmed that the thymocytes indeed progressed from DN3 to first DN4 and then the DP stage (Fig. 2 A).
Then, we analyzed the protein extracts for E-box DNA binding activity by EMSA using a canonical oligonucleotide probe. E-box binding activity decreased 2.5–5-fold within 12 h after anti-CD3ε injection (Fig. 2 B and data not shown). These data suggest that E-box–binding activity is regulated during the developmental transition induced by β selection. We characterized the effect of β-selection on the binding of individual E-proteins by determining the sensitivity of EMSA complexes from anti-CD3ε–treated and untreated RAG-deficient thymocyte extracts to interference by antibodies against specific E proteins. The E-box complexes in thymocyte extracts from RAG-deficient mice that were either untreated or injected with anti-CD3ε 24 h before preparation were primarily comprised of E47 and HEB (Fig. 2 B), which are also the principal components of E-box–binding complexes in wild-type thymocyte extracts 7. Although the overall level of E-box binding was decreased, the relative fractions of E-box complexes containing E47, HEB, or both proteins did not change significantly upon anti-CD3ε administration (Fig. 2 B). These data indicate that the developmental transition induced by β selection is associated with a general decrease in E-box DNA binding activity.
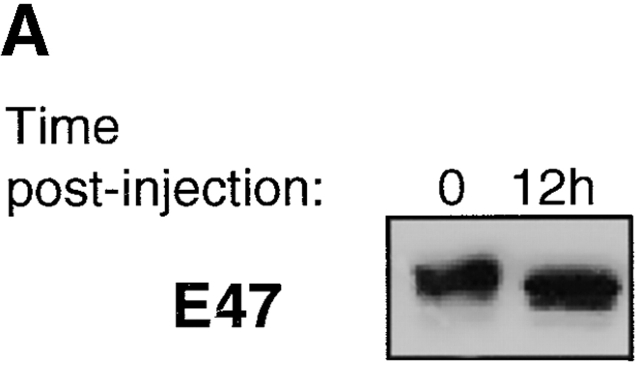
To determine the mechanism underlying the change in E-box–binding activity, we examined the levels of E47 and HEB proteins by immunoblotting. However, we found that the modulation of E-box binding could not be explained by changes in the overall levels of E47 (Fig. 3 A) or HEB (data not shown). We also determined the levels of E47 by flow cytometry in RAG-deficient DN4 and DP cells harvested 5 d after anti-CD3ε injection. Although the levels of E47 are lowered slightly after progression to the DP stage, this decrease comes well after the reduction in E-box binding (Fig. 3 B). These data are also consistent with the levels of E47 expression in wild-type thymocytes as detected by flow cytometry (Fig. 1).
Figure 3.
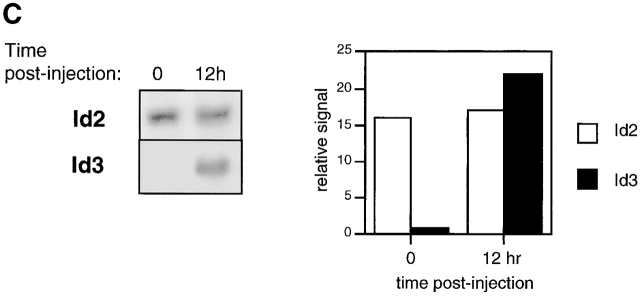
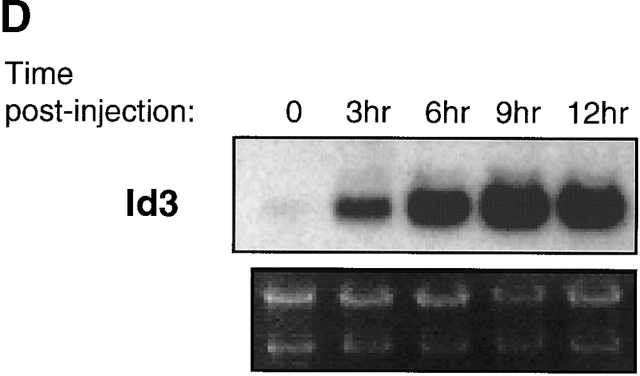
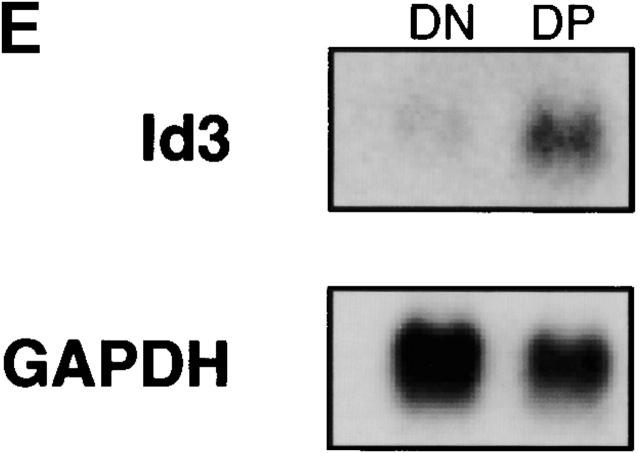
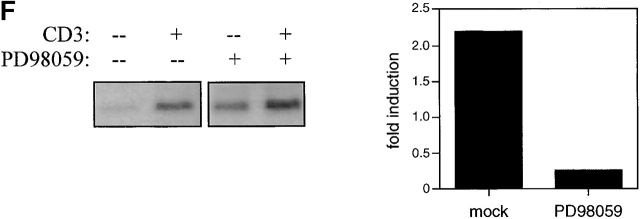
Regulation of E47, Id2, and Id3 during thymocyte β selection. (A) Immunoblot analysis of E47 protein levels in thymic whole cell extracts from RAG-1−/− mice prepared 0 or 12 h after anti-CD3ε injection. Protein loading was judged to be approximately equivalent by amido black staining (data not shown). (B) E47 levels as measured by flow cytometry in DP (solid thin line) and DN4 (solid thick line) thymocyte populations from RAG-deficient mice analyzed 5 d after anti-CD3ε injection, as compared with the staining of control RAG-deficient (stippled line) and E47−/− (dotted line) thymocytes. (C) Northern blot analysis of Id2 and Id3 transcripts in RNA from RAG-1−/− thymocytes prepared 0 or 12 h after anti-CD3ε injection. The bar graph depicts the relative signal strength of Id2 (white bar) and Id3 (black bar) as quantitated by phosphorimage analysis, normalizing to the signal after probing for GAPDH. (D) Northern blot analysis of Id3 expression in RAG-1−/− thymocytes harvested 0, 3, 6, 9, and 12 h after anti-CD3ε injection. The bottom panel depicts the staining of the transferred RNA samples with ethidium bromide. (E) Northern blot analysis of RNA from thymocyte populations from wild-type mice subdivided on the basis of CD4 and CD8 expression. (Top) Blot probed for Id3. (Bottom) The same blot probed for GAPDH. (F) Northern blot analysis of RAG-1−/− thymus organ cultures treated with or without anti-CD3ε for 2 h in the presence and absence of 30 μM PD98059. Bar graph depicts the normalized fold increase of Id3 RNA induced by treatment with anti-CD3ε in the presence and absence of PD98059.
Previous studies indicated that Id3 levels are activated upon TCR-mediated signaling in DP thymocytes, modulating the DNA binding activity of E47/HEB heterodimers 9. To examine whether RAG-deficient thymocytes treated with anti-CD3ε also activated Id RNA levels, we analyzed RNA derived from RAG-deficient thymocytes by Northern blot analysis. While Id2 RNA levels changed little throughout the time course we examined, Id3 RNA levels were elevated dramatically within 3 h after anti-CD3ε stimulation (Fig. 3c and Fig. d). These results suggest that the modulation of E box binding activity that occurs after CD3 cross-linking on DN thymocytes in vivo is caused, at least in part, by an induction of Id3 expression. To assess the regulation of Id3 during normal thymocyte development, we have examined the levels of Id3 RNA in purified thymocyte populations from wild-type mice defined on the basis of CD4 and CD8 expression. Id3 expression was found to be relatively high in DP thymocytes as compared with the DN fraction, which is primarily comprised of thymocytes that have not yet undergone β selection (Fig. 3 E). Taken together, these data suggest that Id3 RNA is induced in thymocytes after the expression of the pre-TCR complex.
The induction of Id3 through TCR cross-linking in DP thymocytes is blocked by the MEK inhibitor PD98059, indicating the involvement of the Ras-Raf-Erk signaling pathway in this process 9 29. We examined the role of Ras–Erk signaling in Id3 induction by anti-CD3ε in RAG-deficient thymocytes by treating cultured RAG-1−/− neonatal thymi with anti-CD3ε in the presence or absence of PD98059. Treatment with anti-CD3ε for 2 h was found to induce Id3 transcription in RAG-1−/− fetal thymi, and this induction was partially blocked by the presence of PD98059 (Fig. 3 F). These data demonstrate the similarity in the mechanisms of induction of Id3 upon CD3 cross-linking in DP and DN thymocytes.
E2A Protein Deficiency Promotes Developmental Progression in the Absence of pre-TCR-mediated Signaling.
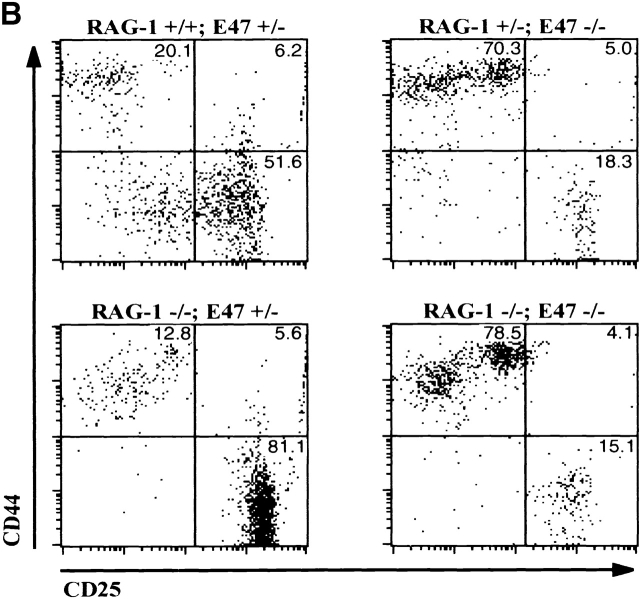
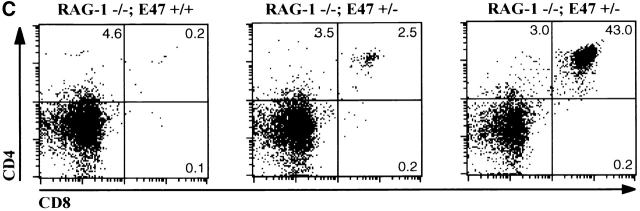
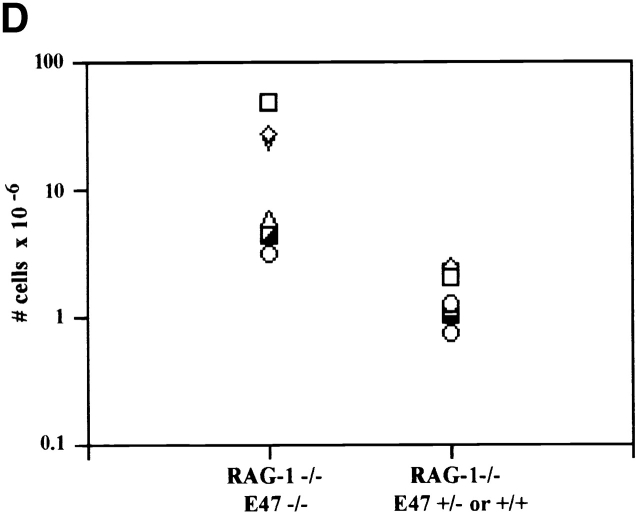
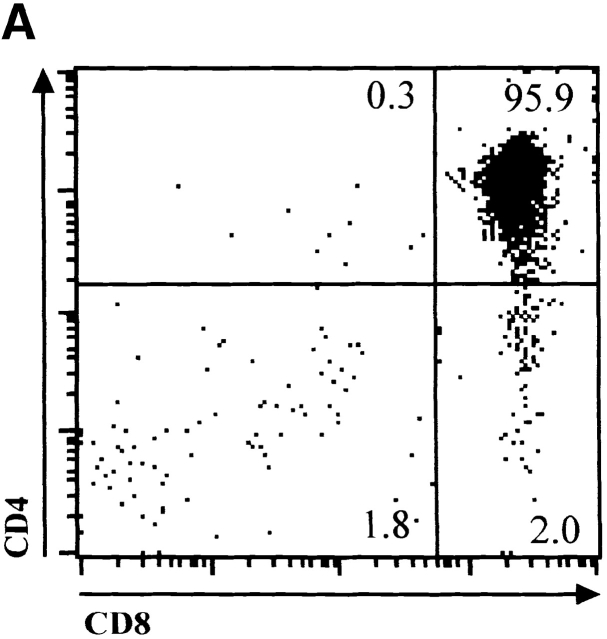
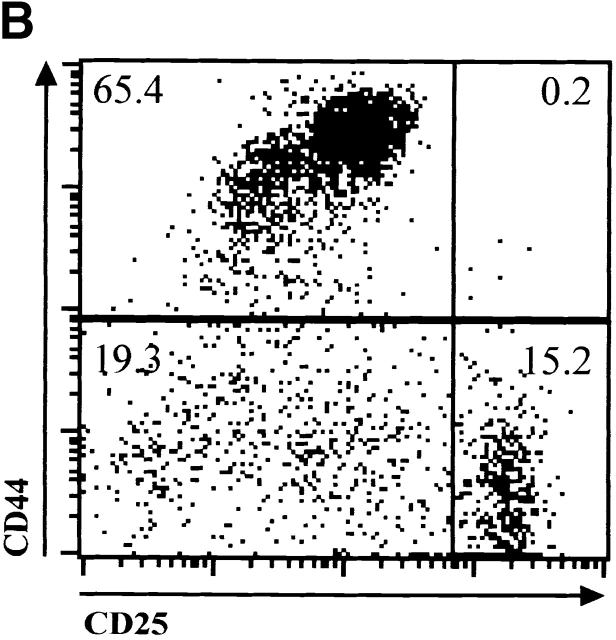
The observation that E47 DNA binding was inhibited upon cross-linking of the CD3 complex in DN thymocytes raised the possibility that E2A proteins play a role in blocking the DN to DP transition in the absence of β selection. Therefore, we generated mice deficient for both E47 and RAG-1 and analyzed thymocytes from these mice for the expression of CD4 and CD8 by flow cytometry. As demonstrated previously, E47-deficient mice showed significant decreases in the proportion of DP thymocytes compared with the wild-type control littermates (Fig. 4 A, reference 8). DP thymocytes were absent from RAG-1−/− E47+/+ mice, due to the block in TCR gene rearrangement (Fig. 4 A, reference 1). However, the overwhelming majority of the thymocytes from the doubly deficient mice expressed high levels of both CD4 and CD8 (Fig. 4 A). The DP thymocytes isolated from the RAG-1−/− E47−/− mice expressed high levels of CD95 and low levels of BCL-2, similar to that of wild-type DP thymocytes (Fig. 4e and Fig. f, and references 30 and 31). DP thymocytes from mice deficient in both RAG-1 and E47 were also similar in size and cell-cycle status to that of wild-type DP thymocytes (data not shown). Strikingly, we also observed significant numbers of DP thymocytes in 6 out of 11 mice analyzed with a RAG1−/− E47+/− genotype (Fig. 4 C and data not shown). Thus a wild-type gene dosage of E47 is required to ensure that all RAG-deficient thymocytes arrest at the β selection checkpoint.
Figure 4.
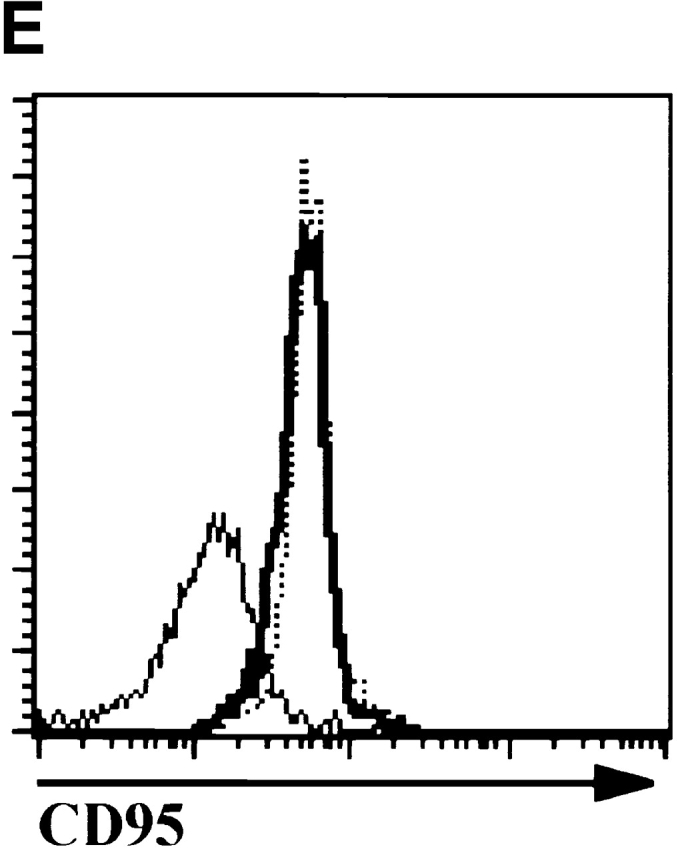
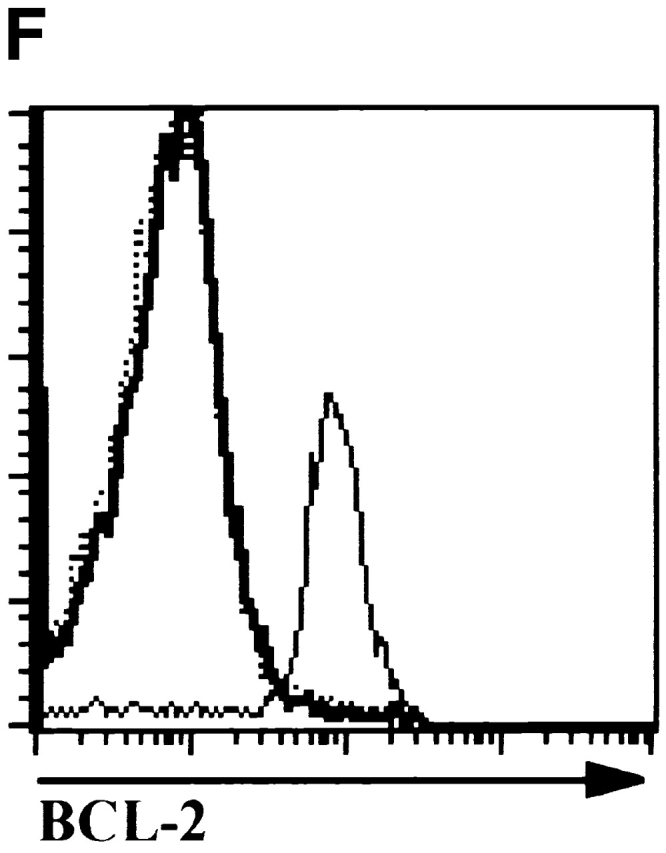
E47 deficiency promotes the aberrant development of RAG-1 null thymocytes. (A) Dot plots depicting surface CD4 and CD8 expression, as determined by flow cytometry, on thymocytes from 1-mo-old mice with RAG-1+/− E47+/−, RAG-1+/− E47−/−, RAG-1−/− E47−/−, and RAG-1−/− E4−/− genotypes. The RAG-1+/− E47−/− mouse used for this experiment was from a separate litter and analyzed separately from the other three mice. Numbers indicate the percentage of viable cells found in each quadrant. (B) Flow cytometric analysis of surface CD44 and CD25 expression on CD3−CD4−CD8− thymocytes from a 3.5-wk-old RAG-1−/− E47−/− mouse and three littermates. Numbers indicate the percentage of viable DN cells found in each quadrant. (C) Dot plots depicting surface CD4 and CD8 expression on thymocytes from 1-mo-old RAG-1−/− littermates that were either wild-type or heterozygous for E47, illustrating the appearance of DP thymocytes in some RAG-1−/− E47+/− mice. The percentage of DP thymocytes observed in RAG-1−/− E47+/− mice with significant numbers of DP thymocytes has ranged from ∼2–85% (data not shown). (D) Scatter plot indicating the number of thymocytes obtained from six RAG-1−/− E47−/− mice as compared with RAG-1−/− E47 wt or +/− littermates. Thymus cellularity in E47−/− mice typically ranges from 1.5–5 × 107 cells (data not shown). (E) Histograms of surface CD95 (Fas antigen) expression in selected thymocyte populations as measured by flow cytometry. (Thick line) DP thymocytes from a 4-wk-old E47−/− RAG-1−/− mouse. (Thin line) DN thymocytes from a RAG-1−/− E47+/− littermate. (Dotted line) DP thymocytes from a RAG-1−/− E47+/− littermate. (F) Histograms of BCL-2 expression levels in selected thymocyte populations as measured by flow cytometry. (Thick line) DP thymocytes from a 1-mo-old E47−/− RAG-1−/− mouse. (Thin line) DN thymocytes from a RAG-1−/− E47 wild-type littermate. (Dotted line) DP thymocytes from a RAG-1+/− E47+/+ littermate. All data presented are representative of analyses of at least three individual RAG-1−/− E47−/− mice and littermate controls.
Upon β selection thymocytes undergo 6–7 rounds of mitosis that result in an ∼100-fold expansion as the cells differentiate to the DP stage 32. Thus even a weak disruption of the β selection checkpoint can result in a thymus that consists primarily of DP cells. To assess directly the effect of E2A deficiency on the β selection checkpoint, we analyzed the DN thymocytes in RAG-1−/− E47+/− mice. We found that the accumulation of cells in the DN3 subset that is normally observed in RAG-1 null mice was not seen in RAG-1−/− E47−/− thymocytes (Fig. 4 B). These data show that a deficiency in E2A protein results in complete abrogation of the developmental block normally exhibited by thymocytes incapable of antigen receptor rearrangement.
E2A Protein Deficiency Allows Thymocyte Expansion in the Absence of pre-TCR-mediated Signaling.
Thymuses from E47-deficient mice are hypocellular as compared with wild-type littermate controls, due to a partial block at the DN1 stage that precedes TCR gene rearrangement 7. Thus, if a deficiency in E47 had no effect on the block in β selection–driven proliferation observed in a RAG-1-null background, then RAG-1−/− E47−/− mice would have fewer thymocytes than littermates deficient for RAG-1 but not E47. However, mice that were null for both RAG-1 and E47 consistently exhibited increases in total thymocyte numbers relative to RAG-1−/− E47+/+ or +/− littermates (Fig. 4 D). Although the degree of this increase in cellularity was variable, it should be noted that the numbers of thymocytes recovered from RAG-1 E47 doubly null mice were often well within the range recovered from mice deficient only for E47 (reference 8 and data not shown). RAG-1−/− E47+/− thymuses, either with or without DP cells, in most cases did not exhibit significant increases in cellularity relative to that observed in RAG-1–null E47+/+ mice (data not shown). These data indicate that E2A proteins are required for inhibiting both the differentiation and proliferation of RAG-1 null thymocytes.
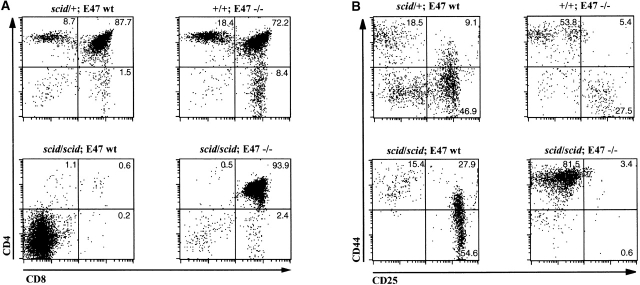
E2A Protein Deficiency Promotes the Developmental Progression of Thymocytes in scid Mice through a Mechanism Distinct from p53.
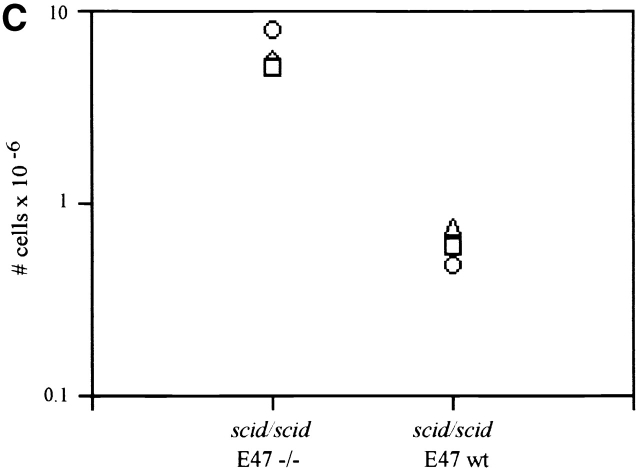
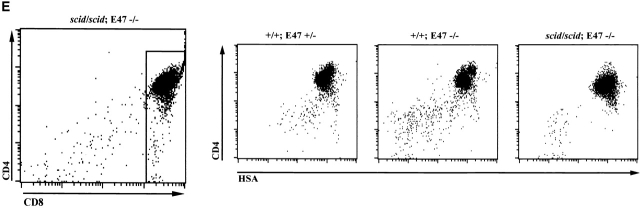
We also examined the effect of E2A deficiency on the T cell developmental block in mice homozygous for the scid mutation. The scid defect is caused by a mutation that results in a severe reduction in DNA-dependent protein kinase (DNA–PK) activity 33. Because DNA-PK activity is required for the efficient joining of broken DNA ends generated by RAG-mediated cleavage, scid thymocytes are unable to complete TCR gene rearrangement, and so are arrested at β selection 23. However, the overwhelming majority of the thymocytes from the scid E47−/− mice, like those from E47 and RAG-1–deficient mice, expressed high levels of both CD4 and CD8 (Fig. 5 A). Analysis of the DN thymocytes confirmed that the developmental block at the DN3 stage in scid mice is relieved by a deficiency in E47 (Fig. 5 B). Cellular expansion was also significantly rescued in scid mice that lacked E47 expression, as compared with scid E47 wild-type littermates (Fig. 5 C).
Figure 5.
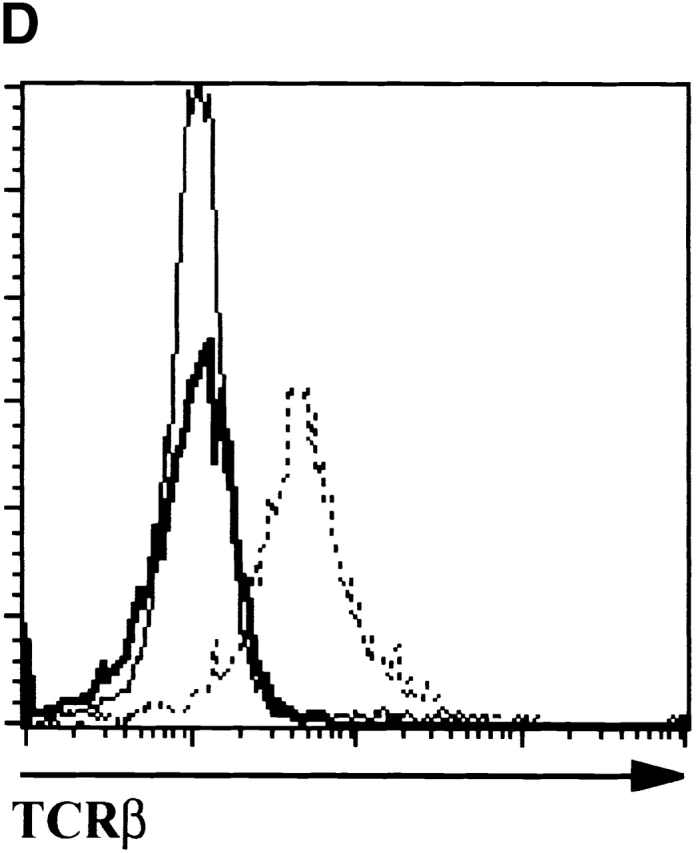
Analyses of thymocytes from mice homozygous for the scid and E47-null mutations. (A) Surface CD4 and CD8 expression, as determined by flow cytometry, on thymocytes from a 5-wk-old scid/scid E47−/− mouse and three littermates. Numbers indicate the percentage of viable cells found in each quadrant. (B) Flow cytometric analysis of surface CD44 and CD25 expression on DN thymocytes from a 5-wk-old scid/scid E47−/− mouse and three littermates. Numbers indicate the percentage of viable, DN cells found in each quadrant. (C) Scatter plot indicating the number of thymocytes obtained from three scid/scid E47−/− mice as compared with scid/scid E47 wild-type littermates. (D) Expression of TCR β chain in selected populations of fixed and permeabilized thymocytes as determined by flow cytometry. Thick line: DP thymocytes from a 5-wk-old scid/scid E47−/− mouse. (Dashed line) DP thymocytes from a+/− E47+/− littermate. (Thin line) DN thymocytes from a 6-wk-old RAG-1−/− E47 wild-type mouse. (E) CD4 and HSA expression on the CD8high thymocytes from a 5-wk-old scid/scid E47−/− mouse and littermates. Plot on left depicts CD4 and CD8 expression in scid/scid E47−/− thymocytes, with gate drawn to define CD8high cells. The other plots depict the expression of CD4 and HSA in the CD8high population of scid/scid E47−/− and littermate control thymocytes. Note that the CD8high CD4− (CD8 SP) scid/scid E47−/− thymocytes are also HSAlow. Similar data were obtained from RAG-1−/− E47−/− mice; however in some RAG-1−/− E47−/− and scid/scid E47−/− mice we did not detect CD8 SP HSAlow thymocytes (data not shown). All other data presented in Fig. 5 are representative of analyses of three individual scid/scid E47−/− mice and littermate controls.
Thymuses from mice deficient for p53 in a scid background also contain some DP thymocytes 34 35 36. A large fraction of the DP thymocytes isolated from scid p53−/− mice expressed the TCR β chain 35. These observations led to the conclusion that a deficiency in p53 allows some scid thymocytes to repair RAG-mediated DNA breaks through alternative repair pathways and undergo β selection-driven expansion and differentiation. To determine whether similar phenomena occur in scid E47−/− mice, cells were made permeable and analyzed for the presence of TCR β chain by flow cytometry. We found that DP thymocytes from mice homozygous for the E47 and scid mutations did not show detectable levels of β chain expression (Fig. 5 D). Thus, a deficit in E2A proteins allows thymocyte expansion and differentiation in mice with defects in TCR rearrangement through a pathway distinct from those regulated by p53.
E2A Protein Deficiency Can Allow for Differentiation of RAG-1 or scid Mutant Thymocytes to the CD8 SP Stage.
We have also observed in some mice deficient for E47 and either RAG-1 or DNA-PK a thymocyte population that expresses high levels of CD8 but low levels of CD4 (Fig. 4 A and 5 A). This phenotype could be representative of either an immature SP population that is in transition from the DN4 to the DP stage, or cells that have differentiated from the DP to the mature CD8 SP stage. To determine if these cells represented mature CD8 SP cells, we analyzed thymocytes from E47-null mice deficient for either RAG or DNA–PK activity for the expression of the heat stable antigen (HSA). HSA is expressed at high levels in immature SP and DP thymocytes but is downregulated upon transition to the SP stage 26. Interestingly, the CD8hi CD4lo cells present in E47−/− RAG-1−/− and E47−/− scid mice were often predominately HSAlo (Fig. 5 E). However, we note that CD8hi CD4lo HSAlo thymocytes were absent from some of the E47−/− RAG-1 or scid mutant mice examined. We have not detected CD4 SP thymocytes with a mature phenotype (Fig. 4 and Fig. 5 A, and data not shown). These data suggest that thymocytes lacking the expression of a TCR complex in the absence of E47 can progress to the mature CD8 but not the CD4 SP stage. However, the development of CD8 SP cells in an E47- and TCR-deficient background is likely influenced by other genetic or environmental factors.
E2A Protein Deficiency Promotes the Thymic Developmental Progression of RAG-deficient Bone Marrow Cells Transferred into an Irradiated E2A+/+ Host.
The observation that E47 DNA binding activity in RAG-deficient thymoyctes was inhibited by cross-linking of the CD3 complex strongly suggested that the effects of E2A protein deficiency in RAG-null mice are due to a deficit of E2A activity in the developing thymocytes. However, it remained formally possible that defects in E2A-deficient thymic stromal cells contributed to the phenotype of RAG-1−/− E47−/− thymocytes. Thus we reconstituted a lethally irradiated, RAG-2−/− mouse with bone marrow from an E47−/− RAG-1−/− mouse, and then analyzed the thymocytes 6 wk after transfer. The thymocytes obtained from this chimeric mouse showed a surface phenotype that was indistinguishable from that of the E47−/− RAG-1−/− and E47−/− scid mice we have analyzed (Fig. 4 Fig. 5 Fig. 6). Thus defects in radioresistant thymic epithelial cells do not contribute to the aberrant developmental progression observed in thymocytes deficient for both E2A and RAG activity.
Figure 6.
Surface staining of thymocytes from a RAG-2−/− mouse that was lethally irradiated and reconstituted with E47−/− RAG-1−/− bone marrow 6 wk before analysis. A RAG-deficient host was used because the donor bone marrow was from a mixed genetic background. (A) Surface CD8 and CD4 expression. (B) Surface CD44 and CD25 expression on DN thymocytes. The DN3 cells were found to be ∼3% positive when stained with anti-E47, indicating that the vast majority of the DN thymocytes were donor derived (data not shown). 4.2 × 106 cells were recovered from this thymus, which is within the range obtained from irradiated hosts reconstituted with E2A-deficient bone marrow (data not shown).
Discussion
The data presented here demonstrate that E2A proteins play important roles in at least two distinct stages of early T cell development. Flow cytometric analysis reveals that thymocytes start to express uniformly high levels of E47 at the point at which their developmental potential begins to be narrowed towards a T cell fate (Fig. 1). These data are consistent with observations of the phenotype of E2A-deficient thymocytes, which display a developmental arrest just before the stage at which E47 is normally induced. However, while E47 expression remains high during subsequent stages of DN thymocyte maturation, our data reveal that E47 DNA binding activity is inhibited by signaling through the pre-TCR complex (Fig. 1 Fig. 2 Fig. 3). Furthermore, we find that E2A proteins are required to prevent the developmental progression of DN thymocytes in the absence of TCR-β rearrangement, and hence pre-TCR expression (Fig. 4 and Fig. 5). The combination of the genetic and biochemical evidence strongly imply that E2A proteins act to block thymocyte maturation in the absence of pre-TCR expression, and that pre-TCR signaling acts to promote development in part by modulating E2A activity. As expected, we did not observe any effect of rearrangement defects on the B cell phenotype of E2A-deficient mice, as B cell development in E47−/− mice is completely blocked before Ig rearrangement (data not shown, and reference 6). Taken together, the studies described here provide a mechanism linking pre-TCR signaling and E2A to developmental progression and cellular expansion.
E2A Proteins Are Negatively Regulated during the Differentiation of both DN and DP Thymocytes.
Many of the signaling molecules that are necessary for β selection have also been shown to play roles in the transition of thymocytes from the DP to the SP stage. This is not surprising, given that both the pre-TCR and the αβ TCR utilize the CD3 antigen complex for the initiation of signal transduction upon receptor binding 37. Recent reports have demonstrated that E2A functions to attenuate positive selection, and that E2A activity is inhibited by TCR cross-linking in DP thymocytes 8. Taken together with the results reported here, these data suggest that the signaling pathways activated by both the TCR and pre-TCR negatively regulate E2A protein activity, and that modulation of E2A is critical to multiple stages of T cell development.
The bHLH inhibitor Id3 has been shown to be upregulated by TCR cross-linking in DP thymocytes through a pathway dependent upon the Erk–MAPK cascade 9. We have also observed that Id3 RNA is dramatically upregulated in RAG-1−/− thymocytes stimulated by anti-CD3ε (Fig. 3c and Fig. d), and that this induction of Id3 is also sensitive to inhibitors of the Ras-Erk–MAPK signaling pathway (Fig. 3 E). Thus it is likely that similar signaling pathways are involved in the inhibition of E47 activity upon cross-linking antigen receptor complexes in DN and DP thymocytes.
The presence of CD8 SP thymocytes with a mature phenotype in several E47−/− mice with blocks in TCR β chain rearrangement provides additional evidence demonstrating that E2A proteins are important negative regulators of the positive selection of DP thymocytes (Fig. 5 E). The consistent absence of mature CD4 SP thymocytes from E47−/− RAG-1−/− or E47−/− scid mice defines a distinction between the signaling events required for the generation of CD8 and CD4 SP cells. Thus while a decrease in E2A activity may be sufficient for the transition of thymocytes from the DP to the CD8 SP stage, additional events appear to be necessary for the positive selection of CD4 SP thymocytes. Our observations are also consistent with data demonstrating that the absence of E47 results in a greater enhancement of selection to the CD8 lineage than to CD4 8. Furthermore, it has been shown that E2A deficiency allows the development of some CD8 SP T cells in a β2 microglobulin–null background, in which positive selection to the CD8 lineage is normally blocked 8.
In addition, our data indicate that the levels of E2A proteins are also regulated during thymocyte development. E47 levels become relatively high at the DN2 stage, then are lowered in DP cells and barely detectable in SP thymocytes. E2A RNA levels are about the same in the DN, DP, and SP populations, suggesting that E47 expression in thymocytes is likely to be posttranscriptionally regulated (unpublished data). The specific mechanisms by which E47 protein levels are regulated remain to be elucidated. Thus our data indicate that E2A activity is regulated during thymocyte development through mechanisms that affect the levels of E2A protein and Id3.
Deficiencies in E47 and p53 Allow Thymocyte Progression through Distinct Mechanisms.
The p53 tumor suppressor is another transcription factor that has a role in preventing maturation in the absence of pre-TCR expression 34 35 36 38 39. However, although both E2A and p53 have been shown to be required for the suppression of the DN to DP transition in scid thymocytes, there are sharp differences in the way they mediate this block. p53 is known to be activated by double strand DNA breaks, and has been shown to be expressed at elevated levels in scid thymocytes, which contain RAG-mediated strand breaks that cannot be efficiently repaired 35. The DP thymocytes that develop in mice deficient for both p53 and DNA–PK have been reported to express the TCR β chain 35. These data support a model in which a deficiency in p53 allows some scid thymocytes to complete in frame VDJβ rearrangement and thus pass the β selection checkpoint. In contrast, DP thymocytes that are homozygous for the E47 and scid mutations do not express the β chain (Fig. 5 D). We should add that E2A deficiency by itself does not significantly affect the expression levels of either surface or intracellular β chain (data not shown). Thus the rescue of development in scid mutant mice to the DP stage by E47 deficiency is not likely to occur by a delay in apoptosis that allows β rearrangement, as is the case in a p53-deficient background.
Role of E2A Proteins during Thymocyte Development.
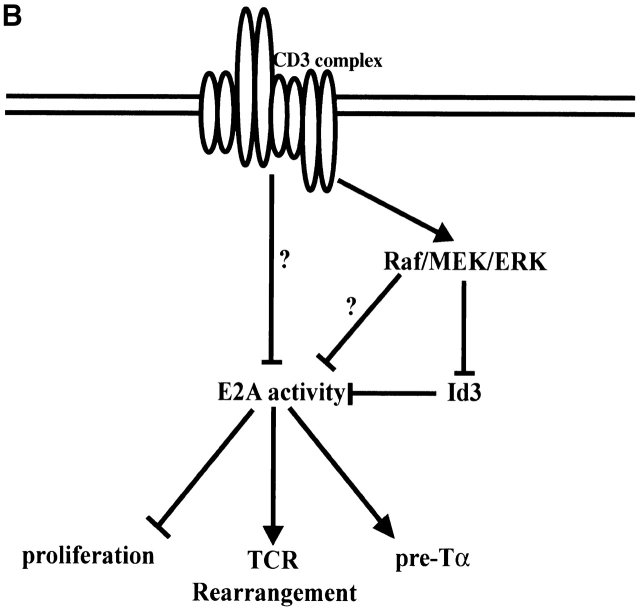
The data presented above, together with other recent studies of the roles of E-proteins at several stages of T cell development, suggest a model for how modulation of E2A protein activity acts to promote thymocyte differentiation (Fig. 7 A). The observation that the developmental arrest in E2A-deficient mice occurs just before the appearance of the earliest thymocyte subset to express uniformly high levels of E47 strongly implies that E2A expression in thymocytes is important for the initial stages of T cell differentiation 7. There have been a number of studies implicating E2A proteins in the regulation of genes required for early T cell development (Fig. 7 B). Id3 overexpression has been shown to inhibit pre-Tα transcription in committed T cell progenitors 22. The proteins encoded by E2A and HEB positively regulate the RAG-1 and RAG-2 genes 21. In addition, we have recently demonstrated that, using a RAG–GFP transgene, proper RAG expression in DN thymocytes requires the presence of the E2A proteins (unpublished data). Furthermore, bHLH proteins have also been shown to be required to promote TCR V(D)Jβ rearrangement in thymocytes 20. Thus E2A proteins have an important role in initiating the T cell developmental program.
Figure 7.
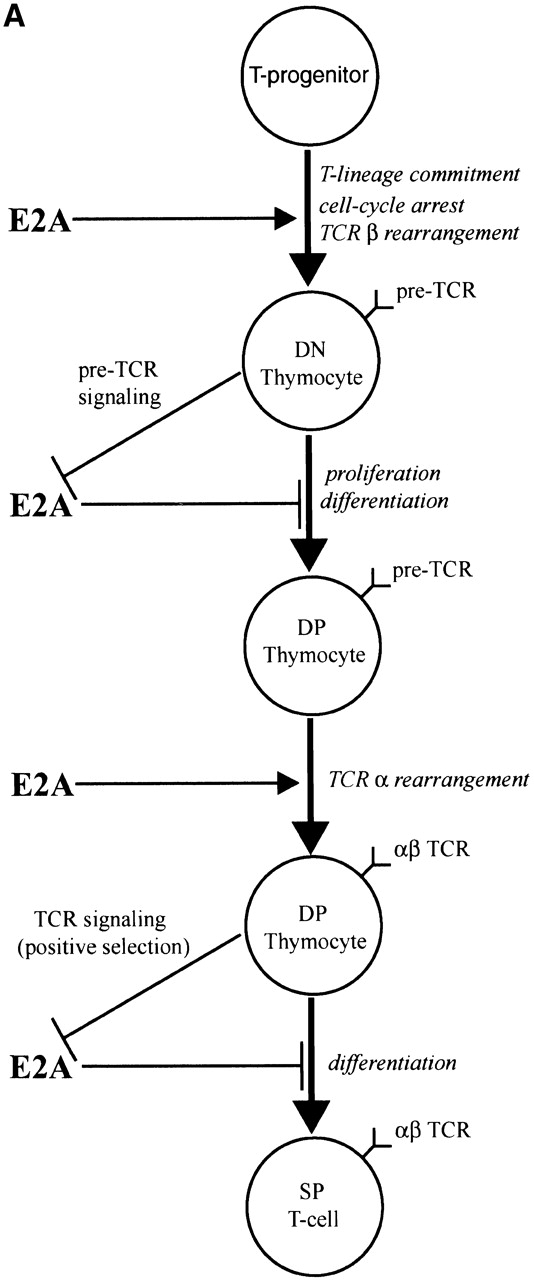
(A) Model outlining the role and regulation of E2A during T cell development. (B) Model depicting mechanisms by which TCR signaling inhibits E2A activity and defined functions of E2A that may act to promote normal thymocyte development.
E47 DNA binding activity is then lowered at subsequent stages of thymocyte development by signaling initiated through the CD3 complex. The downregulation of E2A activity is dependent upon signaling through the pre-TCR complex in DN thymocytes and appears necessary for the β selection developmental transition (Fig. 2, Fig. 4, and Fig. 5). It is conceivable that this modulation of E2A DNA binding affects RAG gene expression and inhibits accessibility to the TCR-β locus. The roles of E2A proteins in TCR rearrangement and RAG gene expression also imply that the reduction of E-box–binding activity upon β selection may contribute to allelic exclusion as well.
Our data also indicate that a deficit of E2A promotes cellular expansion, which suggests that E2A proteins function to block the proliferation of DN thymocytes in the absence of β selection. E2A activity has previously been shown to induce cell-cycle arrest in fibroblasts and lymphoid cells 10 11. We have also observed that E2A proteins can induce G1 arrest in some lymphoma lines (unpublished data). These findings are consistent with our observation that E2A proteins are required to prevent the proliferative expansion of thymocytes in the absence of pre-TCR expression and suggest that the downregulation of E2A activity by pre-TCR signaling could help promote cell-cycle progression in DN3 thymocytes. It is also possible that E2A proteins may contribute to the induction of apoptosis in DN thymocytes that have failed β selection, as E2A protein activity can also induce apoptosis in some cell lines 11 12.
E2A Proteins and Lymphomagenesis.
Genetic alterations that allow for development to the DP stage in the absence of β selection are frequently oncogenic. Thus deficiencies in E47, p53, or Ikaros, as well as overexpression of Lck or Pim-1 result in both the generation of DP cells in RAG-null or scid mice and in a high susceptibility to thymic lymphoma 7 36 40 41 42 43 44 45 46. Since the β selection developmental transition is marked by rapid proliferation, it is possible that factors that block aberrant progression of DN thymocytes also inhibit the growth of transformed T lymphocytes. It will thus be interesting to explore the relationship of the roles of E2A proteins in normal thymocyte development and lymphoma suppression.
Acknowledgments
We thank Barbara Kee, Yang Xu, and Randall Johnson for providing us with mice and Yang Xu for providing a protocol for genotyping the scid mutation.
Footnotes
I. Engel and C. Johns contributed equally to this paper.
Abbreviations used in this paper: bHLH, basic helix-loop-helix; DN, double negative; DP, double positive; HSA, heat stable antigen; MAPK, mitogen-activated protein kinase; RAG, recombination activating gene; SP, single positive.
References
- Fehling H.J., Boehmer H.V. Early αβ T cell development in the thymus of normal and genetically altered mice. Curr. Opin. Immunol. 1997;9:263–275. doi: 10.1016/s0952-7915(97)80146-x. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Massari M.E., Murre C. Helix-loop-helix proteinsregulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G., Maandag E., Izon D., Amsen D., Kruisbeek A., Weintraub B., Krop I., Schlissel M., Feeney A., van Roon M. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Soriano P., Weintraub H. The helix-loop-helix gene E2A is required for B cell formation. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Bain G., Maandag E.C.R., Riele H., Feeney A.J., Sheehy A., Schlissel M., Shinton S.A., Hardy R.R., Murre C. Both E12 and E47 allow commitment to the B cell lineage. Immunity. 1997;6:145–154. doi: 10.1016/s1074-7613(00)80421-5. [DOI] [PubMed] [Google Scholar]
- Bain G., Engel I., Maandag E.C.R., Riele H.P.J.T., Voland J.R., Sharp L.L., Chun J., Huey B., Pinkel D., Murre C. E2A deficiency leads to abnormalities in αβ T-cell development and to rapid development of T-cell lymphomas. Mol. Cell. Biol. 1997;17:4782–4791. doi: 10.1128/mcb.17.8.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G., Quong M.W., Soloff R.S., Hedrick S.M., Murre C. Thymocyte maturation is regulated by the activity of the helix-loop-helix protein, E47. J. Exp. Med. 1999;190:1605–1616. doi: 10.1084/jem.190.11.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G., Cravatt C.B., Loomans C., Alberola-Ila J., Hedrick S.M., Murre C. Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat. Immunol. 2001;2:165–171. doi: 10.1038/84273. [DOI] [PubMed] [Google Scholar]
- Peverali F., Ramqvist T., Saffrich R., Pepperkok R., Barone M., Philipson L. Regulation of G1 progression by E2A and Id helix-loop-helix proteins. EMBO J. 1994;13:4291–4301. doi: 10.1002/j.1460-2075.1994.tb06749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.T., Nolan G.P., Sun X.-H. Growth inhibition and apoptosis due to restoration of E2A activity in T cell acute lymphoblastic leukemia cells. J. Exp. Med. 1999;189:501–508. doi: 10.1084/jem.189.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel I., Murre C. Ectopic expression of E47 or E12 promotes the death of E2A-deficient lymphomas. Proc. Natl. Acad. Sci. USA. 1999;96:996–1001. doi: 10.1073/pnas.96.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R., Davis R., Lockshon D., Turner D., Weintraub H. The protein Ida negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Rivera R.R., Johns C.P., Quan J., Johnson R.S., Murre C. Thymocyte selection is regulated by the helix-loop-helix inhibitor protein, Id3. Immunity. 2000;12:17–26. doi: 10.1016/s1074-7613(00)80155-7. [DOI] [PubMed] [Google Scholar]
- Pan L., Sato S., Frederick J.P., Sun X.H., Zhuang Y. Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol. Cell. Biol. 1999;19:5969–5980. doi: 10.1128/mcb.19.9.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee B.L., Rivera R.R., Murre C. Id3 inhibits B lymphocyte progenitor growth and survival in response to TGF-β. Nat. Immunol. 2001;2:242–247. doi: 10.1038/85303. [DOI] [PubMed] [Google Scholar]
- Liao M.J., Zhang X.X., Hill R., Gago J.J., Qumsiyeh M.B., Nichols W., VanDyke T. No requirement for V(D)J recombination in p53-deficient thymic lymphoma. Mol. Cell. Biol. 1998;18:3495–3501. doi: 10.1128/mcb.18.6.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt T., Gell D., Fox M., Taccioli G.E., Lehmann A.R., Jackson S.P., Jeggo P.A. Identification of a nonsense mutation in the carboxyl-terminal region of dna-dependent protein kinase catalytic subunit in the scid mouse. Proc. Natl. Acad. Sci. USA. 1996;93:10285–10290. doi: 10.1073/pnas.93.19.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid I., Uittenbogaart C.H., Giorgi J.V. A gentle fixation and permeabilization method for combined cell surface and intracellular staining with improved precision in DNA quantification. Cytometry. 1991;12:279–285. doi: 10.1002/cyto.990120312. [DOI] [PubMed] [Google Scholar]
- Barndt R.J., Dai M., Zhuang Y. Functions of E2A/HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB. Mol. Cell. Biol. 2000;20:6677–6685. doi: 10.1128/mcb.20.18.6677-6685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel M., Voronova A., Baltimore D. Helix loop helix transcription factor-E47 activates germ-line immunoglobulin heavy-chain gene transcription and rearrangement in a pre-T-cell line. Genes Dev. 1991;5:1367–1376. doi: 10.1101/gad.5.8.1367. [DOI] [PubMed] [Google Scholar]
- Blom B., Heemskerk M.H.M., Verschuren M.C.M., Dongen J.J.M.V., Stegmann A.P.A., Bakker A.Q., Couwenberg F., Res P.C.M., Spits H. Disruption of αβ but not γδ T cell development by overexpression of the helix-loop-helix protein Id3 in committed T cell progenitors. EMBO J. 1999;18:2793–2802. doi: 10.1093/emboj/18.10.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D.I., Zlotnik A. Control points in early T-cell development. Immunol. Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- Shortman K., Wu L. Early T lymphocyte progenitors. Annu. Rev. Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- Heemskerk M.H.M., Blom B., Nolan G., Stegmann A.P.A., Bakker A.Q., Weijer K., Res P.C.M., Spits H. Inhibition of T cell and promotion of natural killer cell development by the dominant negative helix loop helix factor Id3. J. Exp. Med. 1997;186:1597–1602. doi: 10.1084/jem.186.9.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe I.N., Bevan M.J. Expression and functional significance of the J11d marker on mouse thymocytes. J. Immunol. 1987;138:2013–2018. [PubMed] [Google Scholar]
- Levelt C.N., Mombaerts P., Iglesias A., Tonegawa S., Eichmann K. Restoration of early thymocyte differentiation in T-cell receptor β-chain-deficient mutant mice by transmembrane signaling through CD3 epsilon. Proc. Natl. Acad. Sci. USA. 1993;90:11401–11405. doi: 10.1073/pnas.90.23.11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y., Alt F.W. CD3ε-mediated signals rescue the development of CD4+CD8+ thymocytes in RAG-2−/− mice in the absence of TCRβ chain expression. Int. Immunol. 1994;6:995–1001. doi: 10.1093/intimm/6.7.995. [DOI] [PubMed] [Google Scholar]
- Alessi D.R., Cuenda A., Cohen P., Dudley D.T., Saltiel A.R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Ishii A., Kobayashi Y., Yamasaki Y., Yonehara S. Expression and function of mouse Fas antigen on immature and mature T cells. J. Immunol. 1995;154:4395–4403. [PubMed] [Google Scholar]
- Veis D.J., Sentman C.L., Bach E.A., Korsmeyer S.J. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T-lymphocytes. J. Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- Penit C., Lucas B., Vasseur F. Cell expansion and growth arrest phases during the transition from precursor CD4−8− to immature CD4+8+ thymocytes in normal and genetically modified mice. J. Immunol. 1995;154:5103–5113. [PubMed] [Google Scholar]
- Danska J.S., Guidos C.J. Essential and perilousV(D)J recombination and DNA damage checkpoints in lymphocyte precursors. Sem. Immunol. 1997;9:199–206. doi: 10.1006/smim.1997.0072. [DOI] [PubMed] [Google Scholar]
- Nacht M., Strasser A., Chan Y.R., Harris A.W., Schlissel M., Bronson R.T., Jacks T. Mutations in the p53 and SCID genes cooperate in tumorigenesis. Genes Dev. 1996;10:2055–2066. doi: 10.1101/gad.10.16.2055. [DOI] [PubMed] [Google Scholar]
- Guidos C.J., Williams C.J., Grandal I., Knowles G., Huang M.T.F., Danska J.S. V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphocyte precursors. Genes Dev. 1996;10:2038–2054. doi: 10.1101/gad.10.16.2038. [DOI] [PubMed] [Google Scholar]
- Bogue M.A., Zhu C., Aguilar-Cordova E., Donehower L.A., Roth D.B. p53 is required for both radiation-induced differentiation and rescue of V(D)J rearrangement in scid mouse thymocytes. Genes Dev. 1996;10:553–565. doi: 10.1101/gad.10.5.553. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Fehling H.J. Structure and function of the pre-T cell receptor. Annu. Rev. Immunol. 1997;15:433–452. doi: 10.1146/annurev.immunol.15.1.433. [DOI] [PubMed] [Google Scholar]
- Haks M.C., Krimpenfort P., Brakel J.H.N.V.D., Kruisbeek A.M. Pre-TCR signaling and inactivation of p53 induces crucial cell survival pathways in pre-T cells. Immunity. 1999;11:91–101. doi: 10.1016/s1074-7613(00)80084-9. [DOI] [PubMed] [Google Scholar]
- Jiang D., Lenardo M.J., Zuniga-Pflucker J.C. p53 prevents maturation to the CD4+CD8+ stage of thymocyte differentiation in the absence of T cell receptor rearrangement. J. Exp. Med. 1996;183:1923–1928. doi: 10.1084/jem.183.4.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Young A.Z., Soares V.C., Kelley R., Benezra R., Zhuang Y. High incidence of T-cell tumors in E2A-null mice and E2A/Id1 double-knockout mice. Mol. Cell. Biol. 1997;17:7317–7327. doi: 10.1128/mcb.17.12.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Remington L., Williams B.O., Schmitt E.M., Halachmi S., Bronson R.T., Weinberg R.A. Tumor spectrum analysis in P53-mutant mice. Curr. Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Winandy S., Wu L., Wang J.-H., Georgopoulos K. Pre-T cell receptor (TCR) and TCR-controlled checkpoints in T cell differentiation are set by Ikaros. J. Exp. Med. 1999;190:1039–1048. doi: 10.1084/jem.190.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham K.M., Levin S.D., Marth J.D., Forbush K.A., Perlmutter R.M. Thymic tumorigenesis induced by overexpression of p56lck. Proc. Natl. Acad. Sci. USA. 1991;88:3977–3981. doi: 10.1073/pnas.88.9.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Anderson S.J., Perlmutter R.M., Mak T.W., Tonegawa S. An activated lck transgene promotes thymocyte development in RAG-1 mutant mice. Immunity. 1994;1:261–267. doi: 10.1016/1074-7613(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Schmidt T., Karsunky H., Rodel B., Zevnik B., Elsasser H., Moroy T. Evidence implicating Gfi-1 and Pim-1 in pre-T-cell differentiation steps associated with β-selection. EMBO J. 1998;17:5349–5359. doi: 10.1093/emboj/17.18.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lohuizen M., Verbeek S., Krimpenfort P., Domen J., Saris C., Radaszkiewics T., Berns A. Predisposition to lymphomagenesis in pim-1 transgenic micecooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989;56:673–682. doi: 10.1016/0092-8674(89)90589-8. [DOI] [PubMed] [Google Scholar]