Abstract
Several reports have implicated reactive oxygen and nitrogen metabolites (RONS) in the initiation and/or progression of inflammatory bowel diseases (IBDs). We have investigated the role of three key RONS-metabolizing enzymes (inducible nitric oxide synthase [iNOS], superoxide dismutase [SOD], nicotinamide adenine dinucleotide phosphate [NADPH] oxidase) in a murine model of IBD. Mice genetically deficient (−/−) in either iNOS or the p47phox subunit of NADPH oxidase, transgenic (Tg) mice that overexpress SOD, and their respective wild-type (WT) littermates were fed dextran sulfate sodium (DSS) in drinking water for 7 days to induce colitis. In addition, the specific iNOS inhibitor 1400W was used in DSS-treated WT and p47phox−/− mice. WT mice responded to DSS feeding with progressive weight loss, bloody stools, elevated serum NOX and colonic mucosal injury with neutrophil infiltration. Both the onset and severity of colitis were significantly attenuated in iNOS−/− and 1400W-treated WT mice. While the responses to DSS did not differ between WT and p47phox−/− mice, enhanced protection was noted in 1400W-treated p47phox−/− mice. Interestingly, SODTg mice exhibited more severe colitis than their WT littermates. These findings reveal divergent roles for superoxide and iNOS-derived NO in intestinal inflammation.
Keywords: inflammatory bowel diseases, nitric oxide synthase, superoxide dismutase, NADPH oxidase, dextran sulfate sodium
Introduction
Reactive oxygen and nitrogen species (RONS)* have been implicated in the pathogenesis of a variety of acute and chronic inflammatory states, including ischemia-reperfusion injury (1), atherosclerosis (2), rheumatoid arthritis (3) and inflammatory bowel disease (IBD; e.g., Crohn's disease, ulcerative colitis; references 4 and 5). The two most extensively studied RONS, superoxide (O2 · −) and nitric oxide (NO·), are known to exert profound, and often opposing, effects in inflamed tissue. These actions of O· 2 − and NO· can be manifested as impaired endothelium-dependent vasodilation (6), activation of nuclear transcription factors, and the subsequent production of inflammatory cytokines (7), enhanced recruitment and activation of leukocytes (8), accelerated apoptosis (9), and parenchymal cell necrosis (10). Due to the opposing actions of O· 2 − and NO·, which may result from their ability to chemically react with and decompose each other, it is widely held that conditions which alter the balance between O· 2 − and NO· levels may promote inflammation. This concept has lead to extensive efforts to define the role of RONS in different experimental models of inflammation and has stimulated the search for agents that may alter the production and/or bioavailability of RONS.
The IBDs are chronic, idiopathic disorders primarily of the ileum and/or colon that are characterized by abdominal pain, severe diarrhea, rectal bleeding, and weight loss. Involved regions of the bowel often exhibit an intense infiltration of leukocytes (including granulocytes and lymphocytes), crypt cell hyperplasia, interstitial edema, and mucosal ulcerations. Accompanying the extensive gut inflammation and tissue injury is an enhanced production of reactive oxygen species such as superoxide, which appears to be largely generated by membrane-associated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in activated neutrophils, and a corresponding increase in the expression of the inducible isoform of NO synthase (iNOS).
Although several experimental strategies have been employed to address the importance of the enhanced production of superoxide and NO in the pathogenesis of IBD, inconsistent findings have left this issue largely unresolved. For example, some reports have described a beneficial effect of superoxide dismutase (SOD) treatment in experimental models of colitis (11–13), while SOD treatment in human IBD has shown limited benefit (14). Similarly, inhibitors of iNOS have yielded mixed results in various experimental models of IBD (15, 16) and conflicting results have been recently reported using mice that are genetically deficient in iNOS (17, 18). It has been proposed that the detrimental effects of enhanced NO production by iNOS are related to the generation of potent oxidants, such as peroxynitrite. However this concept is currently under debate (19). While the inconsistent findings with RONS-directed interventions may be attributed to a variety of factors, such as differences between experimental models, the specificity and cellular accessibility of agents used to manipulate RONS production and/or bioavailability remains an important concern. For example, the absence of protection in animals treated with SOD may reflect an inaccessibility of the enzyme to the intracellular compartment, rather than no role for superoxide in the injury process. Furthermore, the protection afforded by some inhibitors of iNOS in models of IBD models may reflect a nonspecific action of these agents on other enzymes or other cellular processes.
Recently, gene targeting technology has led to the production of mice that are either selectively deficient in, or overexpress, candidate mediator proteins, such as cell adhesion molecules, enzymes, or transcription factors. These mice are rapidly gaining acceptance as a tool for mechanistic studies of the inflammatory response that obviate some of the concerns (e.g., specificity) raised about previously employed interventional strategies. Indeed, the wide variety of mutant mice that have been produced for inflammation-related research makes this experimental strategy particularly promising for mechanistic investigations of the pathobiology of IBD. In the present study, gene-targeted animals were used to assess the contributions of three key RONS-related enzymes (iNOS, SOD, and NADPH oxidase) to the gut inflammation and tissue injury observed in a mouse model of dextran sulfate sodium (DSS)-induced colitis. The findings of this study reveal a significant role for both iNOS and SOD and a minor role for NADPH oxidase in DSS-induced colitis. The data also indicate divergent roles for superoxide and iNOS-derived NO, and suggest that NO derived products other than peroxynitrite may be important in the pathogenesis of DSS-induced colitis.
Materials and Methods
Mice
All experimental protocols were applied to either C57BL/6J-664 (wild-type [WT] control strain) mice (The Jackson Laboratory) or mutant mice developed on a C57BL/6 background strain, at 10–12 wk of age. All experiments were repeated at least once with 4–5 mice per group. The data displayed are the combined results of two series of experiments (n = 4 + 5 = 9/group), unless otherwise indicated. Some experiments were performed using transgenic (Tg) mice overexpressing human CuZn-SOD (TgN[SOD1]3Cje) at approximately three times the WT level as hemizygotes and five times the WT level as homozygotes (20). Breeder stocks for homozygous C57BL6-TgN(SOD1)3Cje from a backcross generation >9 were obtained from The Jackson Laboratory and used to breed the hemizygous positive Tg mice (SODTg) in our animal care facility. The SODTg mice were identified by qualitative demonstration of CuZn-SOD activity using nondenaturing gel electrophoresis followed by nitroblue tetrazolium staining. Some experiments were also performed using mice deficient in iNOS (C57BL/6-Nos2tm1Lau), which were purchased from The Jackson Laboratory after >10 generations of backcrossing. An additional series of experiments were performed using NADPH oxidase deficient (C57BL/6-p47phox−/−) mice developed by S. Holland, Laboratory of Host Defenses, National Institutes of Health (21). Breeder stocks for p47phox−/− mice were obtained from C. Ross (Kansas State University, Manhattan, KS). Heterozygote matings were used to produce the homozygous p47phox−/−. For the 1400W study (n = 5/group), WT-controls consisted of the p47phox+/+ littermates that resulted from breeding heterozygous p47phox+/− mice. The genetic identity of the p47phox mutants was determined by PCR analysis of DNA in tail clips obtained for each mouse. Since pilot experiments using the same colitis protocol (data not shown) showed no significant differences for all measured variables between C57BL/6J-664 mice and p47phox+/+ mice, C57BL/6J-664 mice were used as WT controls for the remainder of the study. All groups of animals were singly housed under specific pathogen-free (SPF) conditions in standard cages and were fed standard laboratory chow and water ad libidum until the desired age (10–12 wk) and/or weight (25–30 g). To investigate whether differences in environment could influence the susceptibility of iNOS−/− mice to DSS-mediated gut inflammation, additional groups of iNOS−/− mice and WT controls were housed for 2 wk under conventional (nSPF) conditions before induction of colitis (n = 5/group). All experimental procedures involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Committee of LSU Health Sciences Center and performed according to the criteria outlined by the National Institutes of Health.
DSS-induced Colitis
Colitis was induced by feeding mice 3% DSS (mol wt, 40,000; ICN Biomedicals) dissolved in drinking water (millipore water) for 7 d (22). In pilot experiments, WT mice were given 3 or 5% (wt/vol) DSS in drinking water for 7 d. Since the mortality rate of mice receiving 5% DSS was nearly one-third, 3% DSS treatment for 7 d (0% mortality) was chosen as an optimal dose for detailed analyses. In control mice, normal drinking water was replaced by millipore water.
Treatment with a Specific iNOS-inhibitor
WT and p47phox−/− mice (n = 5/group) were anesthetized by isoflurane inhalation (FiO2 0.35 l/l isoflurane, IsoFlo®; Abbott Laboratories). Under sterile conditions miniosmotic pumps (alzet; ALZA Corp.) were implanted subcutaneously. They provided a continuous infusion (1 μl/h) of sterile saline alone (control group) or the specific iNOS inhibitor 1400W (Cayman Chemical) dissolved in sterile saline (treated group) and delivered at a rate of 10 mg/kg/h over the 7 d of DSS treatment (23, 24). 1400W has been shown to inhibit human eNOS and nNOS inefficiently, when compared with its inhibition of iNOS (23). The selectivity ratio of 1400W for human NOS isoenzymes was reported to be 62 for i-/eNOS and 4 for i/nNOS (25).
Assessment of Inflammation in DSS-treated Mice
Daily clinical assessment of DSS-treated animals included measurement of drinking volume and body weight, evaluation of stool consistency, and the presence of blood in the stools by a guaiac paper test (ColoScreen®; Helena Laboratories [26]). A previously validated clinical disease activity index (DAI; reference 27) ranging from 0 to 4 was calculated using the following parameters: stool consistency; presence or absence of fecal blood; and weight loss. Mice were killed at day 7, blood was collected by cardiac puncture, and spleens were weighed. Colons were removed; length and weight were measured after exclusion of the cecum and before histological and myeloperoxidase (MPO) activity evaluation.
Histology
Histological examination was performed on three samples of distal colon of each animal, which were fixed in Zamboni's solution before embedding in JB-4 (Polysciences) and staining with hematoxylin and eosin. All histological quantitation was performed in a blinded fashion using an established scoring system (28). In brief, three independent parameters were measured: severity of inflammation; depth of injury; and crypt damage. The score of each parameter was multiplied by a factor reflecting the percentage of tissue involvement and added to a sum. The maximum possible score is 40. In addition, the actual percentage of mucosal surface ulcerated was measured by computer-aided morphometric analysis.
Morphometric Analysis
In an attempt to quantify the extent of ulcerated mucosa, computerized morphometry was performed on affected sections of each colon of DSS-treated mice by using a METAMORPH® image acquisition and processing software system (Universal Imaging). Images of three cross-sections of the distal colonic wall were captured via an ECLIPSE E600 upright light microscope (Nikon) coupled to a SenSys CCD camera system (Roper Scientific Photometrics). The ulcerated mucosal surface was defined by loss of mucosal epithelium and expressed as a percentage of the total luminal perimeter for each section.
Serum TNF Measurements
On day 7 of the protocol, blood was collected from control and DSS-treated mice for determination of serum TNF-α levels by ELISA for murine TNF-α (detectable concentration <3.0 pg/ml; BioSource International). All cytokine determinations were performed in duplicate serial dilutions.
Tissue MPO Activity
Samples included tissue from mid to distal colon (adjacent to tissue used for histology) and were obtained either from control or DSS-treated animals, rinsed with cold PBS, blotted dry, and immediately frozen with liquid nitrogen. The samples were stored at −80°C until thawed for MPO activity determination using the O-dianisidine method described previously (29). The change in absorbance was read at 460 nm in a spectrophotometer (Shimadzu UV-1201 Series; Shimadzu Scientific Instruments). MPO activity was expressed as the amount of enzyme necessary to produce a change in absorbance of 1.0 per min per gram of wet weight of tissue.
Neutrophil Transmigration Assay
WT, iNOS−/−, and NADPH-oxidase−/− mice were injected (intraperitoneally with 25 mg of oyster glycogen suspended in 0.5 ml PBS 6 h before peritoneal lavage with 15 ml PBS containing 10 mmol/L D-glucose (D-PBS). Extravasated leukocytes from at least two mice per group were pooled, pelleted, and resuspended in 1 ml D-PBS for quantification before use in the superoxide and NOX production assays described below.
Measurement of Superoxide Production by PMNs
Superoxide production rates were determined as described previously (30) with minor modifications. Briefly, 106 extravasated neutrophils were incubated for 30 min at 37°C in the presence of 2 μg/ml PMA, 1 mg/ml cytochrome c, 30 μg/ml catalase, ±100 μg/ml SOD in D-glucose phosphate-buffered saline (D-PBS). The cells were then removed by centrifugation and the absorbance of reduced cytochrome c measured at 550 nm. The rate of superoxide production was calculated as follows: O2 − (nmol·min–1·106 cells–1) = (47.7 × [A–SOD − A+SOD])/30 min.
Measurement of NO Metabolites (NOX) Production by Cultured PMNs
NOX production by murine PMNs was measured by assaying the NO metabolites nitrite and nitrate in the supernatant of stimulated cells cultured in vitro. Briefly, PMN (106 cells) were suspended in 0.5 ml of phenol-free DMEM supplemented with 1 mM L-arg and 4 mM glutamine, and incubated at 37°C for 24 h in the presence of 10 U/ml recombinant murine IFN-γ (R&D Systems) and 1 μg/ml LPS (serotype O55:B5; Sigma-Aldrich). Cells were removed by centrifugation and the supernatant collected. NOX levels in collected supernatants were determined using the standard Griess reaction and the results expressed as μM total NOX.
Measurement of Serum NOX
Additional groups of WT, SODTg, and iNOS−/− mice plus the p47phox−/− mice with their WT controls from the 1400W study (n = 5/DSS-treated group, n = 3/H2O-treated group) were fasted 16 h before exsanguination. Blood was collected in microtainer serum separator tubes (Microtainer; Becton Dickinson). After clotting of blood on ice, samples were centrifuged and the supernatant serum collected. Serum samples were then passed through a size exclusion column (Ultrafree-MC 10,000 MW exclusion; Millipore Corporation) to remove free hemoglobin and the resulting ultrafiltrate assayed for total NOX using a modification of the standard Griess assay described previously (31).
Data Analysis
Statistical analyses were performed with StatView 4.5v software (Abacus Concepts) using a one-way ANOVA followed by Scheffé's (post hoc) test for parametric or the Kruskal-Wallis test for nonparametric data. All values are reported as means ± SEM. Statistical significance was set at P < 0.05.
Results
Neither a Genetic Deficiency of iNOS, p47phox (NADPH Oxidase) nor Overexpression of SOD Changed Gross Gastrointestinal Morphology or Function
None of the untreated, genetically engineered mice used in this study exhibited any of the clinical signs (diarrhea, rectal bleeding, weight loss) normally associated with spontaneous intestinal inflammation. Furthermore, gross examination of the gastrointestinal tract from untreated iNOS−/−, p47phox−/−, and SODTg mice did not reveal any evidence of inflammatory changes in the gut.
Serum TNF-α Was not Altered on Day 7 of Colitis
ELISA of serum samples (n = 8/group) revealed no significant change in TNF-α levels in any of the DSS-treated groups when compared with controls (values ranging from 0 to 127 pg/ml).
Genetic Deficiency in iNOS Expression Attenuated Clinical Abnormalities Induced by DSS Colitis
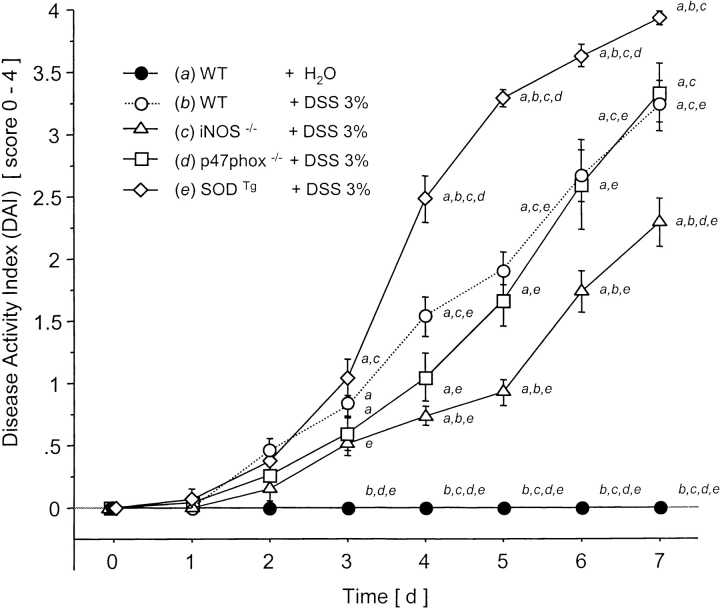
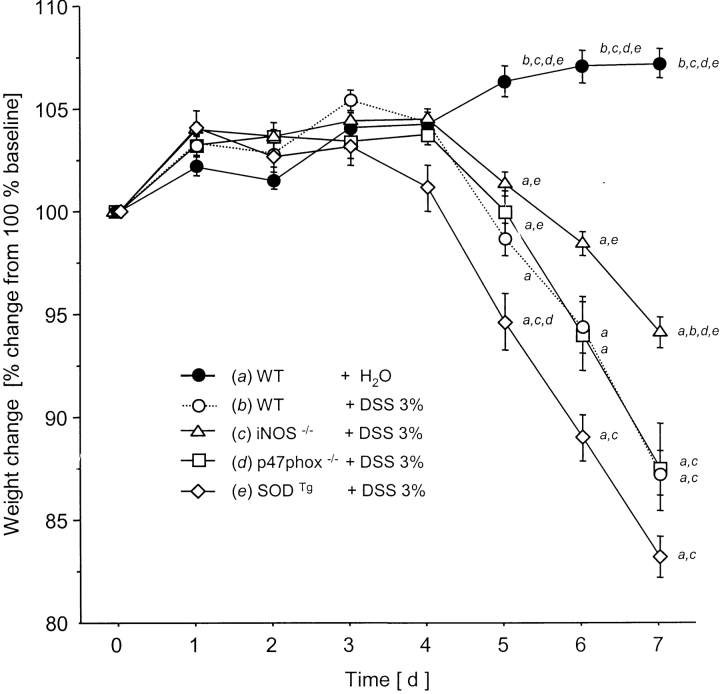
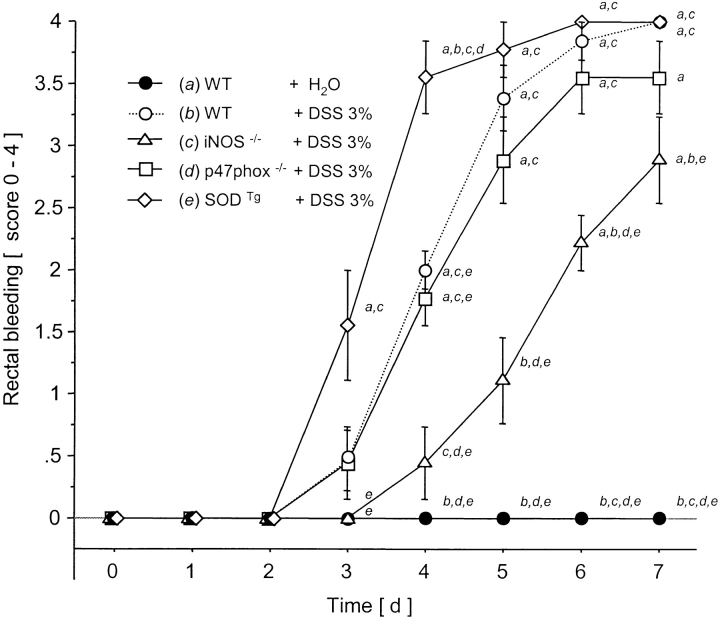
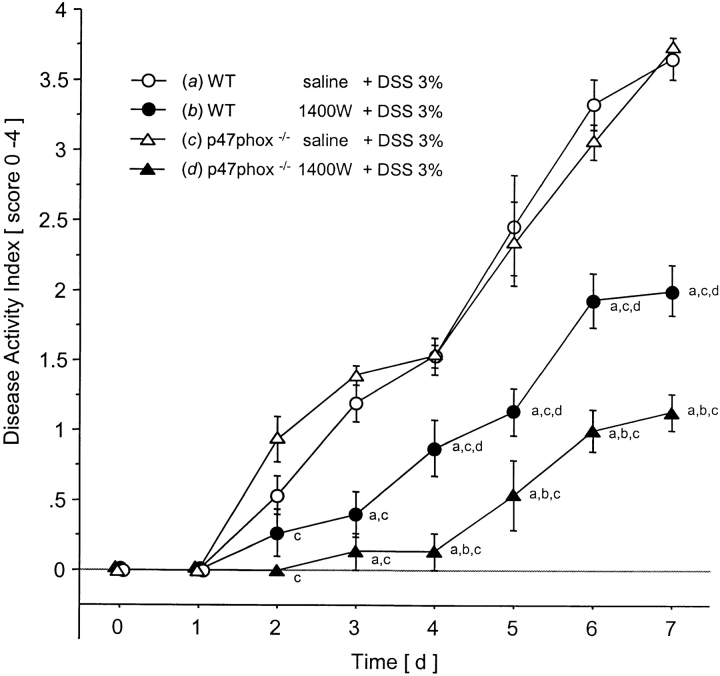
DSS treatment of WT mice for 7 d was associated with significant changes in body weight starting on day 5 (Fig. 1) and the appearance of fecal blood on day 4 (Fig. 2) . Only mild alterations were seen in stool consistency and no mortality was observed. Drinking volume was similar in all groups (data not shown). DAI scores in DSS-treated mice were lowest in the iNOS−/− mice compared with WT controls (significant from day 4 to day 7; Fig. 3) .
Figure 1.
Clinical score (DAI) over 7 d after water in WT or DSS oral treatment in WT, iNOS−/−, p47phox−/−, and SODTg mice (n = 9/group). a versus WT plus water; b versus WT plus DSS; c versus iNOS−/− plus DSS; d versus p47phox−/−; and e versus SODTg plus DSS. Mean ± SEM. P < 0.05.
Figure 2.
Body weight change from 100% baseline over 7 d after water in WT or DSS oral treatment in WT, iNOS−/−, p47phox−/−, and SODTg mice (n = 9/group). a versus WT plus water; b versus WT plus DSS; c versus iNOS−/− plus DSS; d versus p47phox−/−; and e versus SODTg plus DSS. Mean ± SEM. P < 0.05.
Figure 3.
Rectal bleeding score over 7 d after water in WT or DSS oral treatment in WT, iNOS−/−, p47phox−/−, and SODTg mice (n = 9/group). a versus WT plus water; b versus WT plus DSS; c versus iNOS−/− plus DSS; d versus p47phox−/−; and e versus SODTg plus DSS. Mean ± SEM. P < 0.05.
Tg Overexpression of SOD Exacerbated the Clinical Abnormalities Associated with DSS-induced Colitis
The clinical score was highest in the SODTg mice receiving DSS (Fig. 3) reaching statistical significance when compared with the WT mice from day 4 to 7.
Genetic Deficiency in p47phox Did not Influence Susceptibility to DSS-induced Colitis
Throughout the 7 d of DSS administration, the clinical score for p47phox−/− mice remained unchanged from WT mice (Fig. 3). These results are in contrast to the attenuated disease activity noted in mice lacking iNOS, and the more pronounced disease in SOD Tg mice.
iNOS Deficiency Attenuates, while SOD Overexpression Exaggerates, the Macroscopic Changes Associated with DSS Administration
DSS-treated WT mice exhibited the macroscopic changes, which are classically associated with this model of colitis. These changes included shortening of the colon and increases in weight of the colon and spleen (Table I). In DSS-treated iNOS − /− mice however, no increase in spleen weight was noted and colonic shortening and weight increase were both significantly attenuated. Compared with DSS-treated WT mice, SODTg mice showed a significant increase in spleen weight whereas colon length and weight remained unchanged. DSS-associated macroscopic changes in the colon were unaltered in p47phox− /− mice. Consistent with the observed clinical scores, SODTg mice exhibited the most aggressive injury, with an unchanged injury response in p47phox− /− and a blunted injury response in DSS-treated iNOS− /− mice.
Table I.
Inflammatory Changes in Colon and Spleen after DSS Administration
| Colon length | Colon weight/length ratio | Spleen weight | ||
|---|---|---|---|---|
| cm | mg/cm | % body weight | ||
| WT | plus water | 7.3 ± 0.2b&e b–e | 35.9 ±1.7b&e b–e | 0.31 ± 0.02b,e |
| WT | plus DSS | 4.8 ± 0.2a,c | 65.9 ± 3.5a,c | 0.54 ± 0.04a,c |
| iNOS−/− | plus DSS | 5.9 ± 0.2a,b,e | 51.3 ± 2.1a,b | 0.33 ± 0.02b,e |
| p47phox−/− | plus DSS | 5.3 ± 0.1a | 59.5 ± 4.8a | 0.39 ± 0.05e |
| SODTg | plus DSS | 4.7 ± 0.2a , c | 62.6 ± 2.2a | 0.65 ± 0.05a,c,d |
| WT (conv.) | plus DSS | 5.2 ± 0.3 | 58.0 ± 4.6 | 0.51 ± 0.06 |
| iNOS−/− | plus DSS | 6.4 ± 0.3f | 45.4 ± 4.2f | 0.30 ± 0.03f |
Colon and spleen changes after 7 d of oral treatment with either water in WT or DSS in WT, iNOS−/−, p47phox−/−, and SODTg mice (n = 9/group). In addition DSS-treated WT versus iNOS−/− mice under conventional housing conditions (n = 5/group). Mean ± SEM.
P < 0.05 vs. WT plus water.
P < 0.05 vs. WT plus DSS.
P < 0.05 vs. iNO−/− + DSS.
P < 0.05 vs. p47phox−/− plus DSS.
P < 0.05 vs. SODTg plus DSS.
P < 0.05 vs. WT (conv.) plus DSS.
Histologic Assessment Confirmed the Protection against DSS-induced Colitis by iNOS Deficiency and the Exaggerated Disease Response with SOD Overexpression
Blinded histologic injury scoring was quantified in the distal colon from WT, iNOS−/−, p47phox− /−, and SODTg mice (9/group) after 7 d of treatment with DSS 3%. Resulting colonic inflammation was mostly confined to the mucosa with loss of goblet cells, crypt damage, mucosal ulceration, and accompanying edema of the submucosa. Mucosal ulceration was significantly decreased (63.5%) in iNOS− /− mice (Table II). The severity of colitis was also significantly attenuated (36.5%) in iNOS− /− versus WT mice (Table II). Crypt damage in SODTg mice was significantly worse (1.6-fold) compared with WT mice. The total histologic score (Table II) was lowest in iNOS− /− (12.0 ± 1.5) and highest in SODTg mice (23.9 ± 1.3), each exhibiting a significant difference from WT mice (17.9 ± 0.7). Histologic findings in p47phox−/− mice were unchanged from their WT controls. These observations are consistent with the macroscopic findings (Table I) and further support the conclusion that susceptibility to DSS-induced colonic injury is decreased in iNOS− /−, increased in SODTg and unchanged in p47phox− /− mice.
Table II.
Histologic Alterations after DSS Administration
| Total histology score | Crypt damage | Severity of colitis | Ulceratedsurface | ||
|---|---|---|---|---|---|
| score | score | score | % | ||
| WT | plus DSS | 17.9 ± 0.7b,d | 6.7 ± 0.4b,d | 7.4 ± 0.3b | 20.3 ± 1.8b |
| iNOS−/− | plus DSS | 12.0 ± 1.5a,d | 4.4 ± 0.6a , d | 4.7 ± 0.7a,d | 7.4 ± 0.8a,c,d |
| p47phox−/− | plus DSS | 16.3 ± 1.4d | 6.9 ± 0.6d | 6.1 ± 0.7d | 15.9 ± 2.3b |
| SODTg | plus DSS | 23.9 ± 1.3a&c a–c | 10.4 ± 0.8a&c a–c | 9.1 ± 0.5b,c | 22.0 ± 2.0b |
| WT (conv.) | plus DSS | 18.8 ± 1.6 | 7.1 ± 0.5 | 7.8 ± 0.5 | 22.8 ± 3.5 |
| iNOS−/− (conv.) | plus DSS | 11.4 ± 2.0e | 3.9 ± 1.7e | 4.3 ± 0.9e | 6.2 ± 2.7e |
Blinded histologic assessment of colitis after oral treatment with DSS for 7 d in WT, iNOS−/−, p47phox−/−, and SODTg mice (n = 9/group). In addition DSS-treated WT versus iNOS−/− mice under conventional housing conditions (n = 5/group). Based on validated scoring system and morphometry described in Materials and Methods. Mean ± SEM.
P < 0.05 vs. WT.
P < 0.05 vs. iNOS−/−.
P < 0.05 vs. p47phox−/−.
P < 0.05 vs. SODTg.
P < 0.05 vs. WT (conv.).
Neutrophil Recruitment into the Colon during DSS Colitis Was Attenuated in the Absence of iNOS and Is Exaggerated by Overexpression of SOD
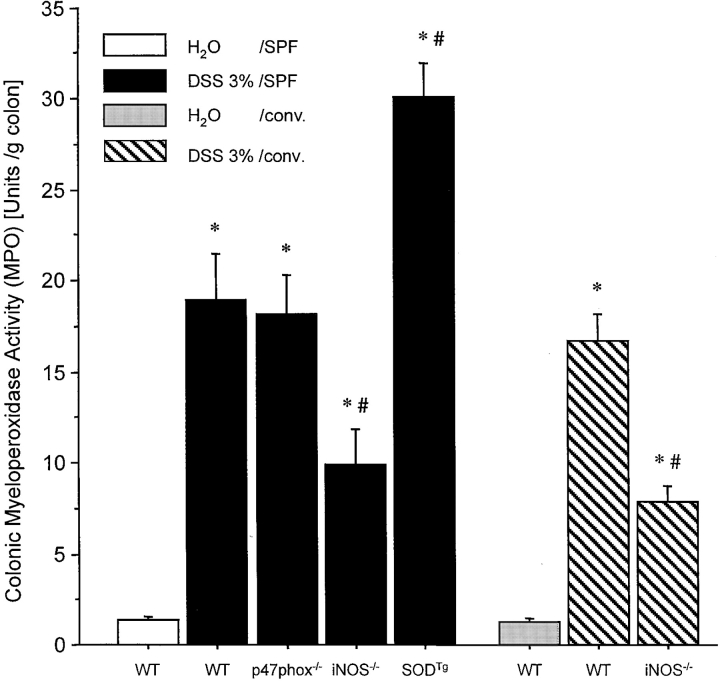
DSS administration was followed by a significant increase in MPO activity in all mice (Fig. 4) . MPO activity corresponded closely with the clinical and histological grading of inflammation in the different groups (Figs. 1–3 and Table I). Whereas WT and p47phox−/− mice showed approximately equivalent increases in MPO activity (14-fold and 13-fold, respectively), the increment in colonic MPO was significantly less pronounced (7.4-fold) in iNOS−/− mice, but more pronounced in SODTg mice (23-fold) after DSS administration (Fig. 4).
Figure 4.
MPO in colonic tissue of water-treated WT mice or after 7 d of treatment with DSS in WT, iNOS−/−, p47phox−/−, and SODTg mice that were housed under SPF conditions (n = 9/group) or WT and iNOS mice housed under conventional condition (n = 5/group). Tissue concentrations were measured by the O-dianisidine method. The change in absorbance was read at 460 nm in a spectrophotometer. MPO activity was expressed as the amount of enzyme necessary to produce a change in absorbance of 1.0 per min per gram of wet weight of tissue. Mean ± SEM. *P < 0.05 vs. water-treated WT. # P < 0.05 vs. DSS-treated WT.
Treatment of WT Mice with the Specific iNOS Inhibitor 1400W Attenuated DSS-induced Colitis
When compared with saline-treated controls the 1400W-treated WT mice lost significantly less weight (11.6 ± 1.8% versus 6.1 ± 0.9%, respectively), and exhibited minimal rectal bleeding that was detectable only by testing for fecal occult blood, whereas all controls exhibited gross rectal bleeding. Daily measurements of the DAI demonstrated the delayed and attenuated clinical course of colitis after blockade of iNOS by 1400W (Fig. 5) , which resulted in less tissue damage as summarized in Table III.
Figure 5.
Clinical score (DAI) during 7 d of DSS administration in WT and p47phox−/− mice that received concomitant 1400W (or saline in controls) using a subcutaneously implanted miniosmotic pump (n = 5/group). a versus WT saline plus DSS, b versus p47phox−/− 1400W plus DSS, c versus p47phox−/− saline plus DSS. Mean ± SEM. P < 0.05.
Table III.
Effects of a Specific iNOS Inhibitor on the Development of DSS Colitis
| Colonlength | Histology | MPOactivity | ||
|---|---|---|---|---|
| cm | score | U/g colon | ||
| WT | saline plus DSS | 5.1 ± 0.31 | 19.8 ± 1.7 | 21.1 ± 1.5 |
| WT | 1400W plus DSS | 6.6 ± 0.3a | 10.8 ± 1.4a | 10.6 ± 2.8a |
| p47phox−/− | saline plus DSS | 5.0 ± 0.1 | 19.2 ± 3.4 | 20.3 ± 2.5 |
| p47phox−/− | 1400W plus DSS | 7.9 ± 0.2b,c | 1.8 ± 0.8b,c | 5.2 ± 1.7c |
| WT | plus DSS | 4.8 ± 0.2 | 17.9 ± 0.7 | 19.0 ± 2.5 |
| iNOS−/− | plus DSS | 5.9 ± 0.2d | 12.0 ± 1.5d | 9.9 ± 2.0d |
Effects of treatment with 1400W on susceptibility to DSS-induced colitis in WT and p47phox−/− mice (n = 5/group). 1400W (or saline in controls) was administered over 7 d using a subcutaneously implanted miniosmotic pump parallel to feeding DSS in drinking water. To facilitate how the iNOS−/− compound compared in relation to 1400W-treated mice the result of DSS administration to iNOS−/− mice and their appropriate controls is shown as a subsection of this table.
P < 0.05 vs. WT saline plus DSS.
P < 0.05 vs. WT 1400W plus DSS.
P < 0.05 vs. p47phox−/− saline plus DSS.
P < 0.05 vs. WT plus DSS.
Blockade of iNOS by 1400W in p47phox–deficient Mice Revealed an Additional Role for NADPH-Oxidase in DSS-induced Colitis
Saline-treated p47phox−/− mice and their saline-treated WT controls showed no differences in the onset and severity of DSS colitis and associated tissue damage (Fig. 5, Table III). However continuous treatment of p47phox−/− with the specific iNOS inhibitor 1400W resulted in protection that was not only significant when compared with the saline-treated p47phox−/− control mice, but was also superior to the protection achieved by treatment of WT mice with 1400W (P < 0.05). Indeed, 40% of 1400W-treated p47phox−/− mice did not exhibit rectal bleeding whereas all 1400W-treated WT mice were positive for fecal blood. Weight loss after 7 d of DSS administration was significantly reduced in 1400W-treated p47phox−/− mice (0.1 ± 0.6%) when compared with saline-treated p47phox−/− (12.4 ± 1.3%) and to 1400W-treated WT mice (6.1 ± 0.9%). Colonic shortening and histology confirmed the superior protective effect of combined pharmacologic iNOS blockade and p47phox deficiency (Table III).
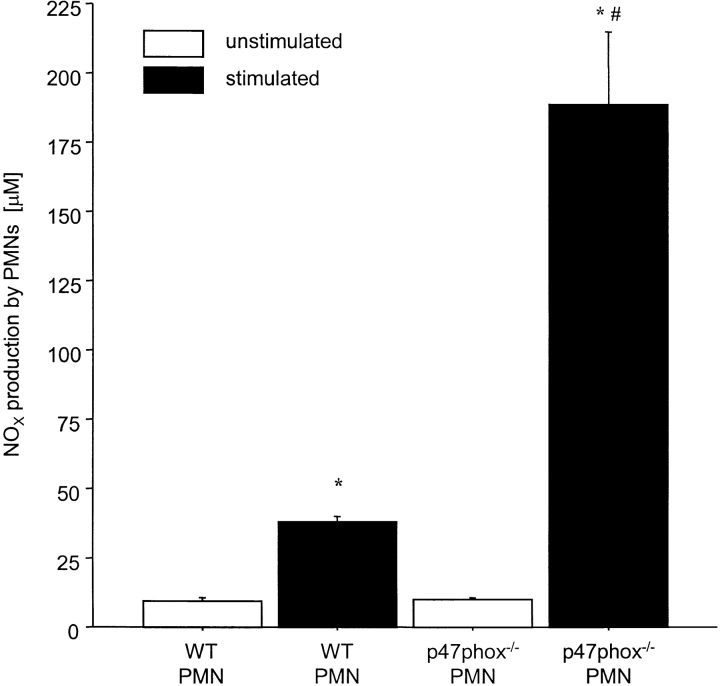
Stimulated p47phox−/− PMNs Generated More NOX Compared with WT (control) PMNs, Whereas O2 − Production by iNOS−/− PMNs Was not Significantly Different from WT (Control) PMNs
24 h after stimulation, PMNs from p47phox−/− mice produced almost five times more NOX than PMNs of their WT controls (188.6 ± 26.3 versus 37.8 ± 2.2 μM). NOX production by unstimulated PMNs did not differ between p47phox−/− and WT mice (Fig. 6) . The production of superoxide by PMNs was similar in iNOS−/− PMNs and WT (control) PMNs (1.4 ± 0.1 versus 1.6 ± 0.1 nmol.min−1.106 cells−1, respectively).
Figure 6.
Effect of stimulation (recombinant murine INF-γ 10 U/ml and LPS 1 μg/ml) of PMNs of WT and p47phox−/− mice on the production of NOX in vitro. NOX levels in the medium were assayed 24 h after incubation with the inflammatory mediators. PMNs of at least two mice per group were pooled and analyzed in triplicates. Concentrations were measured using a modification of the standard Griess assay and absorbances were read at 540 nm in a spectrophotometer. Results are expressed as μM NOX. Mean ± SEM. *P < 0.05 vs. unstimulated WT PMNs. # P < 0.05 vs. stimulated WT PMNs.
DSS Colitis Was Characterized by Elevated Serum NOX Levels that Were Dependent on iNOS Activity
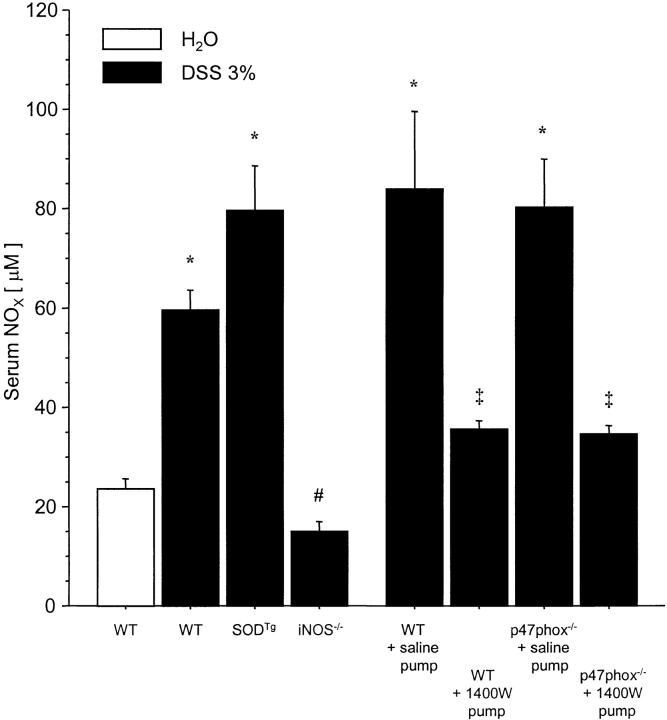
DSS administration in drinking water over 7 d resulted in a 250% increase of serum NOX in WT mice when compared with controls given millipore water. Serum levels of NOX after DSS were significantly lowered by either genetic deletion of iNOS (−75.1%) or blockade of iNOS using 1400W (up to −57.8%). Interestingly, serum NOX in DSS-treated SODTg was 33% higher compared with DSS-treated WT, however this change did not achieve statistical significance. No differences were found between WT and p47phox−/− mice after saline pump implantation and DSS administration. As expected, the lowest serum NOX levels were detected in DSS-treated iNOS−/− (Fig. 7) .
Figure 7.
NOX levels in serum of different mutant and WT mice that received DSS in drinking water over 7 d or millipore water (H2O) in controls (n = 5/DSS-treated group; n = 3/H2O-treated group). Serum concentrations were measured using a modification of the standard Griess assay and absorbances were read at 540 nm in a spectrophotometer. Results are expressed as μM NOX. Mean ± SEM. *P < 0.05 vs. H2O-treated WT. # P < 0.05 vs. DSS-treated WT. ‡ P < 0.05 vs. saline-pump.
Protection against DSS-induced Colitis by iNOS Deficiency Did not Depend on SPF Housing Conditions
The same histopathologic changes that were observed in DSS-treated mice housed under SPF conditions were also present in subsets of WT and iNOS−/− mice housed under conventional conditions before the experiment (Tables I and II). In addition, the attenuated clinical course of DSS colitis observed in iNOS−/− mice under SPF conditions was present under conventional conditions (data not shown).
Discussion
The objectives of this study were to assess the contribution of RONS-regulating enzymes in the acute phase of DSS-induced colitis by using gene-targeted mice that are genetically deficient in either iNOS or functional NADPH oxidase (p47phox), or which overexpress CuZn-SOD. The role of iNOS was further assessed using the specific iNOS inhibitor, 1400W. Our findings indicate that either the genetic absence or pharmacologic blockade of iNOS significantly attenuates the severity of colonic inflammation in DSS-induced colitis, a model that involves epithelial cell injury (27). On the other hand, mice that genetically overexpress CuZn-SOD exhibited exaggerated inflammatory and tissue injury responses to DSS treatment. The absence of functional NADPH oxidase, resulting from targeted disruption of p47phox, did not alter susceptibility to DSS-induced intestinal inflammation provided that iNOS remained functional. Using the specific iNOS inhibitor 1400W, we demonstrated that the combined blockade of iNOS (by 1400W) and NADPH oxidase (by genetic deletion of p47phox) was even more effective in protecting mice from DSS-colitis than either intervention alone. These results suggest that both iNOS and CuZn-SOD play an important role in the progression of DSS-induced colitis. NADPH oxidase per se may only play a minor role in the presence of functional iNOS since genetic blockade of this enzyme alone did not influence the course of colitis.
The data derived from iNOS−/− mice and 1400W-treated WT mice exposed to DSS suggest that iNOS-derived NO either directly or indirectly contributes to the initiation and/or progression of inflammation and tissue injury. This contention is supported by previous reports that demonstrate increased iNOS activity and NO production by epithelial cells and leukocytes harvested from colonic specimens derived from humans or rodents with colitis (17, 32, 33). Furthermore, the fact that a similar protection from DSS colitis was achieved by treatment of WT mice with either 1400W or genetic disruption of iNOS supports a proinflammatory role for iNOS. Our findings are consistent with several studies showing attenuated colonic injury in different models of IBD treated with iNOS inhibitors given in the drinking water (15, 34, 35). However, our results in iNOS−/− mice contrast with recently published reports that employed these same mutants in the trinitrobenzene sulphonic acid (TNBS/ETOH; references 17 and 18) and acetic acid (AA; reference 33) models of colonic injury and inflammation. The results of the TNBS/ETOH studies were inconsistent since iNOS-deficient mice were shown to exhibit an exaggerated course of the acute phase of TNBS/ETOH colitis in one study (17) and an attenuated course in the other study (18). A notable difference between these studies was the housing environments of the mice. The iNOS−/− mice showing protection were housed under SPF conditions (18) whereas the others were conventionally housed (17). The greater sensitivity of iNOS−/− to a number of pathogens was proposed as a possible explanation for the increased susceptibility to TNBS/ETOH-induced colitis after conventional housing.
SPF-housed mice harbor indigenous microflora (∼500 species) as do conventional mice. Since SPF mice are kept in a clean controlled environment, they do not acquire additional opportunistic pathogens, such as Salmonella or Pseudomonas that could alter the experimental results. Therefore, “clean” SPF-housed mice may be more relevant to human disease than “dirty” conventionally housed mice. In our experimental model, conventional or SPF conditions did not influence the outcome of colitis in iNOS−/− mice. The study using iNOS−/− in AA-induced colitis showed a reduced ability to repair colitis-associated tissue injury (38), when compared with WT mice.
There are fundamental differences between the DSS model of colitis and both the TNBS/ETOH and AA models that may account for the differential responses of iNOS-deficient mice. The use of AA and TNBS/ETOH to induce epithelial injury represents an acute and massive insult that occurs within 30 min after intrarectal administration (36). The protective effect observed in TNBS/ETOH-treated iNOS−/− mice was limited only to the first 24 h and absent at 72 h after the inflammatory stimulus (17). In the DSS model used in our study, colitis develops gradually in response to continuous administration (7 d) of an inflammatory stimulus. Attenuation of clinical symptoms in DSS-treated iNOS−/− mice was not statistically significant until day 4 and MPO activity was not measured until day 7 of inflammation, since neutrophils do not accumulate to a significant degree at an earlier stage. Different pathophysiological mechanisms may be involved in tissue repair after a single insult when compared with a continuous insult. Therefore, we propose that part of the controversy regarding whether iNOS−/− mice are more or less susceptible to colonic inflammation may relate to differences in the pathogenic mechanisms that underlie TNBS/ETOH-, AA-, and DSS-induced colitis.
An enhanced iNOS-dependent production of NO may modify inflammation by one or more mechanisms, thereby leading either to an improvement or exacerbation of inflammation. iNOS−/− mice may be protected against DSS-induced colitis because of decreased production of the highly cytotoxic oxidant ONOO−, which results from the interaction between NO and O· 2 − (37). This mechanism however seems unlikely in view of our results in SODTg mice and p47phox−/−, where a reduction in O· 2 − production (p47phox−/−) or accumulation (CuZn-SODTg) would be expected to decrease ONOO− formation and thus decrease colonic injury and inflammation. Indeed, the unchanged susceptibility of p47pox−/− mice to DSS treatment and the additional protection afforded by p47phox deletion that was only detectable after iNOS blockade (using 1400W) support the theory that phagocyte-derived reactive oxygen metabolites play little or no role in the presence of iNOS in DSS colitis (38).
The observation that the combination of iNOS blockade (by 1400W) and nonfunctional NADPH oxidase (by genetic deletion of p47phox) resulted in additional protection may be explained by the scavenging properties of NO. It is known for example that NO can directly inhibit NADPH oxidase and thereby limit superoxide generation (39). Suppression of NO production (induced by 1400W treatment) and the resultant enhancement of NADPH oxidase function may therefore account for the more severe inflammation noted in 1400W-treated WT mice, compared with 1400W-treated p47phox−/− mice. P47phox-independent production of reactive oxygen intermediates must also be considered as a reason why p47phox−/− mice did not show protection when compared with their WT controls. However, the additional protection that can be achieved by p47phox deletion in 1400W-treated mice does not support this possibility. It would be interesting to determine whether similar results could be achieved in mice that are doubly deficient in both enzymes (p47phox−/−/iNOS−/−). Unfortunately, p47phox−/−/iNOS−/− mice form massive abscesses containing mostly enteric bacteria, even when reared under SPF conditions with antibiotics whereas neither parental strain shows such infections (40). Thus, p47phox compensates for a deficiency in iNOS and vice verse thereby providing resistance to indigenous bacteria.
We were able to demonstrate that iNOS-deficient PMNs can produce superoxide and that p47phox-deficient PMNs can generate NO. Therefore, it is also possible that p47phox−/− mice can compensate for the loss of phagocytic activity from reactive oxygen species by generating larger amounts of reactive nitrogen species. Our finding that p47phox−/− PMNs generated significantly more NOX when compared with WT control PMNs 24 h after LPS/IFN-γ stimulation further supports this hypothesis. The fact that iNOS deficiency only results in partial protection, while combined blockade of iNOS and NADPH oxidase offers nearly complete protection suggests that compensatory mechanisms may exist that allow NO and superoxide to replace each other's proinflammatory activity. Similar compensatory responses have been described in studies that examine the role of RONS in microbicidal processes (40).
Another interesting finding in the present study is that neutrophils from phox47−/− animals produced more NO than their wt counterparts, but total animal NO production did not differ between the groups (Figs. 6 and 7). Our data does not allow for resolution of this issue, however, it should be noted that the method for stimulating NO production differed for the two measurements (in vitro LPS/IFN-γ versus in vivo DSS). Furthermore, there are sources of iNOS-derived NO other than PMNs present in vivo (i.e., macrophages), which may contribute to serum NO, whereas the in vitro experiments specifically addressed PMN-derived NO production.
Another possible mechanism by which iNOS-derived NO could mediate colonic inflammation and injury is through the production of nitroxyl anions (NO−, reference 41). NO− is a potent cytotoxic agent (42–44) that has been reported to be up to 1,000× more toxic than NO· (42). The absence of NO− formation in iNOS−/− mice or after 1400W treatment could protect these mice against the relatively severe form of DSS colitis that is seen in WT control mice.
An altered response of the gut microvasculature to DSS-induced injury in the absence of iNOS could also explain our findings. This is supported by recently published data showing that microvascular reactivity and permeability of the mesenteric vascular bed were altered to a lesser degree in iNOS−/− mice after topical or systemic challenge with bacterial endotoxin, compared with the responses observed in their wt littermates (6). Also the residual mucosal injury observed in iNOS−/− mice suggests other significant mechanisms that are NO independent.
The exaggerated DSS-induced inflammation that we observed in SODTg mice was unexpected since SOD is known to protect tissues against inflammation-mediated injury. The antiinflammatory properties of SOD, which relate to the enzyme's ability to eliminate superoxide, have been demonstrated in a variety of experimental models (11, 45). Furthermore, SOD treatment has proven to be somewhat effective in the treatment of patients with Crohn's disease (14). This enzyme catalyzes the dismutation of O· 2 − to H2O2 and molecular oxygen. Virtually all eukaryotic cells contain SOD in one or more isoforms, CuZnSOD in the cytoplasm and MnSOD in mitochondria. The SOD-catalyzed reaction is 10,000 times faster than the spontaneous dismutation of O· 2 −, thus O· 2 − levels are usually kept very low (46). The H2O2 generated by SOD is also removed enzymatically by catalase, which is particularly efficient at high concentrations of H2O2 and catalyses the detoxication of H2O2 to water and molecular oxygen. Although some investigators have suggested that SOD overexpression may produce enhanced amounts of H2O2 and thus may promote rather than attenuate inflammation (47). This appears highly unlikely since it is well appreciated that the amount of H2O2 generated by SOD-catalyzed dismutation will be identical to the amount produced by the spontaneous dismutation of O2 − (48). However, a more tenable explanation for the results obtained with SODTg mice is that overexpression of SOD may enhance NF-κB activation (49). Although NF-κB may be indispensable as an inducer of many immediate-early inflammatory and immune genes, it is probably harmful or even fatal in diseases and syndromes that involve an aberrant expression of inflammatory cytokines, such as colitis. This hypothesis is further supported by a recently published study showing increased colonic NF-κB DNA-binding activity in DSS-induced colitis. Furthermore suppression of NF-κB by gliotoxin protected mice from DSS-colitis, which underscores the essential role of NF-κB in DSS-induced colitis (50). In addition, iNOS expression in experimental colitis can be down regulated by blockade of NF-κB activity using proteasome inhibitors (51).
Another explanation for the exaggerated inflammatory responses in CuZn-SODTg mice relates to the ability of SOD to chemically reduce NO to yield the cytotoxic and proinflammatory NO− (52). Since iNOS−/− mice were protected against DSS colitis, we postulated that NO derived from inducible NOS may be an important mediator of inflammation. Therefore, one might expect that toxic amounts of NO·-derived nitroxyl could accumulate in the colon of DSS-treated SODTg mice and the nitroxyl may be responsible for the exacerbated colonic injury observed in these animals. In this context, our results in SODTg mice provide additional support for the hypothesis that NO plays an important role in the progression of DSS-related tissue injury.
There is also evidence that overexpression of CuZn-SOD can impair the microbicidal and fungicidal activity of macrophages (53). This impaired macrophage function was associated with decreased NF-κB activation and reduced NO production. In the previously mentioned study, it was also shown that activated macrophages from Cu/Zn-SOD Tg mice produced significantly more H2O2 than their wt counterparts (54). This may explain why high concentrations of SOD are toxic in a model of cardiac ischemia-reperfusion injury, a response that is prevented by catalase (55). In view of these findings, one might expect overexpression of Cu/Zn-SOD to decrease the host's ability to defend against invading bacteria in the gut. Mucosal CuZn-SOD activity appears to be reduced in humans with Crohn's disease and unchanged in ulcerative colitis (56).
MPO activity has been shown by others to be proportional to the number of neutrophils in the inflamed tissue (57). In this study, it was demonstrated that MPO activity is strongly associated with the colonic tissue injury resulting from DSS administration in the different study groups, suggesting that RONS may be important in the signaling process for neutrophil recruitment. There is also good evidence that leukocytes and possibly neutrophils contribute significantly to the DSS-associated tissue injury since others have demonstrated that intercellular adhesion molecule 1–deficient mice exhibit an attenuated injury response in the same model of murine colitis (58). However, it remains unclear, whether neutrophils act as direct mediators of DSS-injury or their recruitment is secondary to DSS-induced damage.
Acknowledgments
This study was supported by grants from the National Institutes of Health P01DK43785 (to D.N. Granger), R01DK47663 (to M.B. Grisham), R15HL64595 (to C.R. Ross), and the Center of Excellence in Arthritis and Rheumatism at LSU Health Sciences Center at Shreveport.
Footnotes
Abbreviations used in this paper: AA, acetic acid; DAI, disease activity index; DSS, dextran sulfate sodium; IBD, inflammatory bowel disease; iNOS, inducible NO synthase; MPO, myeloperoxidase; NO, nitric oxide; NADP, nicotinamide adenine dinucleotide phosphate; RONS, reactive oxygen and nitrogen species; SPF, specific pathogen-free; SOD, superoxide dismutase; Tg, transgenic; WT, wild-type.
References
- 1.Carden, D.L., and D.N. Granger. 2000. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 190:255–266. [DOI] [PubMed] [Google Scholar]
- 2.Patel, R.P., D. Moellering, J. Murphy-Ullrich, H. Jo, J.S. Beckman, and V.M. Darley-Usmar. 2000. Cell signaling by reactive nitrogen and oxygen species in atherosclerosis. Free Radic. Biol. Med. 28:1780–1794. [DOI] [PubMed] [Google Scholar]
- 3.Bauerova, K., and A. Bezek. 1999. Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis. Gen. Physiol. Biophys. 18:15–20. [PubMed] [Google Scholar]
- 4.Babbs, C.F. 1992. Oxygen radicals in ulcerative colitis. Free Radic. Biol. Med. 13:169–181. [DOI] [PubMed] [Google Scholar]
- 5.Grisham, M.B. 1994. Oxidants and free radicals in inflammatory bowel disease. Lancet. 344:859–861. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki, Y., E.A. Deitch, S. Mishima, W.N. Duran, and D.Z. Xu. 2000. Endotoxin-induced mesenteric microvascular changes involve iNOS-derived nitric oxide: results from a study using iNOS knock out mice. Shock. 13:397–403. [DOI] [PubMed] [Google Scholar]
- 7.Flohe, L., R. Brigelius-Flohe, C. Saliou, M.G. Traber, and L. Packer. 1997. Redox regulation of NF-κB activation. Free Radic. Biol. Med. 22:1115–1126. [DOI] [PubMed] [Google Scholar]
- 8.Kubes, P., M. Suzuki, and D.N. Granger. 1991. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA. 88:4651–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai, Q., H. Ji, Z. Zheng, X. Yu, L. Sun, and X. Liu. 2000. Copper induces apoptosis in BA/F3β cells: Bax, reactive oxygen species, and NFκB are involved. J. Cell Physiol. 184:161–170. [DOI] [PubMed] [Google Scholar]
- 10.McKenzie, S.J., M.S. Baker, G.D. Buffinton, and W.F. Doe. 1996. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J. Clin. Invest. 98:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keshavarzian, A., G. Morgan, S. Sedghi, J.H. Gordon, and M. Doria. 1990. Role of reactive oxygen metabolites in experimental colitis. Gut. 31:786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller, M.J., H. McNeill, K.M. Mullane, S.J. Caravella, and D.A. Clark. 1988. SOD prevents damage and attenuates eicosanoid release in a rabbit model of necrotizing enterocolitis. Am. J. Physiol. 255:G556–G565. [DOI] [PubMed] [Google Scholar]
- 13.Clark, D.A., D.M. Fornabaio, H. McNeill, K.M. Mullane, S.J. Caravella, and M.J. Miller. 1988. Contribution of oxygen-derived free radicals to experimental necrotizing enterocolitis. Am. J. Pathol. 130:537–542. [PMC free article] [PubMed] [Google Scholar]
- 14.Emerit, J., S. Pelletier, J. Likforman, C. Pasquier, and A. Thuillier. 1991. Phase II trial of copper zinc superoxide dismutase (CuZn SOD) in the treatment of Crohn's disease. Free Radic. Res. Commun. 12:563–569. [DOI] [PubMed] [Google Scholar]
- 15.Rachmilewitz, D., F. Karmeli, E. Okon, and M. Bursztyn. 1995. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 37:247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida, Y., A. Iwai, K. Itoh, M. Tanaka, S. Kato, R. Hokari, T. Miyahara, H. Koyama, S. Miura, and M. Kobayashi. 2000. Role of inducible nitric oxide synthase in dextran sulfate sodium-induced colitis. Aliment. Pharmacol. Ther. 14:26–32. [DOI] [PubMed] [Google Scholar]
- 17.McCafferty, D.M., M. Miampamba, E. Sihota, K.A. Sharkey, and P. Kubes. 1999. Role of inducible nitric oxide synthase in trinitrobenzene sulphonic acid induced colitis in mice. Gut. 45:864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zingarelli, B., C. Szabo, and A.L. Salzman. 1999. Reduced oxidative and nitrosative damage in murine experimental colitis in the absence of inducible nitric oxide synthase. Gut. 45:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubes, P., and D.M. McCafferty. 2000. Nitric oxide and intestinal inflammation. Am. J. Med. 109:150–158. [DOI] [PubMed] [Google Scholar]
- 20.Epstein, C.J., K.B. Avraham, M. Lovett, S. Smith, O. Elroy-Stein, G. Rotman, C. Bry, and Y. Groner. 1987. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc. Natl. Acad. Sci. USA. 84:8044–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson, S.H., J.I. Gallin, and S.M. Holland. 1995. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 182:751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okayasu, I., S. Hatakeyama, M. Yamada, T. Ohkusa, Y. Inagaki, and R. Nakaya. 1990. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 98:694–702. [DOI] [PubMed] [Google Scholar]
- 23.Garvey, E.P., J.A. Oplinger, E.S. Furfine, R.J. Kiff, F. Laszlo, B.J. Whittle, and R.G. Knowles. 1997. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J. Biol. Chem. 272:4959–4963. [DOI] [PubMed] [Google Scholar]
- 24.Thomsen, L.L., J.M. Scott, P. Topley, R.G. Knowles, A.J. Keerie, and A.J. Frend. 1997. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res. 57:3300–3304. [PubMed] [Google Scholar]
- 25.Boer, R., W.R. Ulrich, T. Klein, B. Mirau, S. Haas, and I. Baur. 2000. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol. Pharmacol. 58:1026–1034. [PubMed] [Google Scholar]
- 26.Ebaugh, F.G. 1959. Quantitative measurement of gastrointestinal blood loss. J. Lab. Clin. Med. 53:77–82. [PubMed] [Google Scholar]
- 27.Cooper, H.S., S.N.S. Murthy, R.S. Shah, and D.J. Sedergran. 1993. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 69:238–249. [PubMed] [Google Scholar]
- 28.Dieleman, L.A., M.J. Palmen, H. Akol, E. Bloemena, A.S. Pena, S.G. Meuwissen, and E.P. Van Rees. 1998. Chronic experimental colitis induced by dextran sulfate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 114:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer, P., J.M. Russel, and D.N. Granger. 1999. Role of endotoxin in intestinal reperfusion-induced expression of E-selectin. Am. J. Physiol. 276:G479–G484. [DOI] [PubMed] [Google Scholar]
- 31.30. Colowick, S.P., and N.O. Kaplan. 1984. Methods in Enzymology. Harcourt, Orlando. pp. 358–365.
- 31.Grisham, M.B., G.G. Johnson, and J.R. Lancaster, Jr. 1996. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 268:237–246. [DOI] [PubMed] [Google Scholar]
- 32.Kolios, G., N. Rooney, C.T. Murphy, D.A. Robertson, and J. Westwick. 1998. Expression of inducible nitric oxide synthase activity in human colon epithelial cells: modulation by T lymphocyte derived cytokines. Gut. 43:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCafferty, D.M., J.S. Mudgett, M.G. Swain, and P. Kubes. 1997. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 112:1022–1027. [DOI] [PubMed] [Google Scholar]
- 34.Grisham, M.B., R.D. Specian, and T.E. Zimmerman. 1994. Effects of nitric oxide synthase inhibition on the pathophysiology observed in a model of chronic granulomatous colitis. J. Pharm. Exp. Ther. 271:1114–1121. [PubMed] [Google Scholar]
- 35.Hogaboam, C.M., K. Jacobson, S.M. Collins, and M.G. Blennerhassett. 1995. The selective beneficial effects of nitric oxide inhibition in experimental colitis. Am. J. Physiol. 268:G673–G684. [DOI] [PubMed] [Google Scholar]
- 36.Yamada, Y., S. Marshall, R.D. Specian, and M.B. Grisham. 1992. A comparative analysis of two models of colitis in rats. Gastroenterology. 102:1524–1534. [DOI] [PubMed] [Google Scholar]
- 37.Ischiropoulos, H., L. Zhu, and J.S. Beckman. 1992. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 298:446–451. [DOI] [PubMed] [Google Scholar]
- 38.Blackburn, A.C., W.F. Doe, and G.D. Buffinton. 1998. Salicylate hydroxylation as an indicator of hydroxyl radical generation in dextran sulfate-induced colitis. Free Radic. Biol. Med. 25:305–313. [DOI] [PubMed] [Google Scholar]
- 39.Clancy, R.M., J. Leszczynska-Piziak, and S.B. Abramson. 1992. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J. Clin. Invest. 90:1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiloh, M.U., J.D. MacMicking, S. Nicholson, J.E. Brause, S. Potter, M. Marino, F. Fang, M. Dinauer, and C. Nathan. 1999. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 10:29–38. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt, H.H., H. Hofmann, U. Schindler, Z.S. Shutenko, D.D. Cunningham, and M. Feelisch. 1996. No NO from NO synthase. Proc. Natl. Acad. Sci. USA. 93:14492–14497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Espey, M.G., K.M. Miranda, M. Feelisch, J. Fukuto, M.B. Grisham, M.P. Vitek, and D.A. Wink. 2000. Mechanisms of cell death governed by the balance between nitrosative and oxidative stress. Ann. NY Acad. Sci. 899:209–221. [DOI] [PubMed] [Google Scholar]
- 43.Ma, X.L., F. Gao, G.L. Liu, B.L. Lopez, T.A. Christopher, J.M. Fukuto, D.A. Wink, and M. Feelisch. 1999. Opposite effects of nitric oxide and nitroxyl on postischemic myocardial injury. Proc. Natl. Acad. Sci. USA. 96:14617–14622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wink, D.A., M. Feelisch, J. Fukuto, D. Chistodoulou, D. Jourd'Heuil, M.B. Grisham, Y. Vodovotz, J.A. Cook, M. Krishna, W.G. DeGraff, et al. 1998. The cytotoxicity of nitroxyl: possible implications for the pathophysiological role of NO. Arch. Biochem. Biophys. 351:66–74. [DOI] [PubMed] [Google Scholar]
- 45.Perry, M.A., S. Wadhwa, D.A. Parks, W. Pickard, and D.N. Granger. 1986. Role of oxygen radicals in ischemia-induced lesions in the cat stomach. Gastroenterology. 90:362–367. [DOI] [PubMed] [Google Scholar]
- 46.Halliwell, B., and J.M.C. Gutteridge. 1999. Free Radicals in Biology and Medicine. 3rd ed. Oxford University Press, New York. 936 pp.
- 47.Wenk, J., P. Brenneisen, M. Wlaschek, A. Poswig, K. Briviba, T.D. Oberley, and K. Scharffetter-Kochanek. 1999. Stable overexpression of manganese superoxide dismutase in mitochondria identifies hydrogen peroxide as a major oxidant in the AP-1-mediated induction of matrix-degrading metalloprotease-1. J. Biol. Chem. 274:25869–25876. [DOI] [PubMed] [Google Scholar]
- 48.Teixeira, H.D., R.I. Schumacher, and R. Meneghini. 1998. Lower intracellular hydrogen peroxide levels in cells overexpressing CuZn-superoxide dismutase. Proc. Natl. Acad. Sci. USA. 95:7872–7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt, K.N., P. Amstad, P. Cerutti, and P.A. Baeuerle. 1995. The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-κB. Chem. Biol. 2:13–22. [DOI] [PubMed] [Google Scholar]
- 50.Herfarth, H., K. Brand, H.C. Rath, G. Rogler, J. Schölmerich, and W. Falk. 2000. Nuclear factor-κB activity and intestinal inflammation in dextran sulfate sodium (DSS)-induced colitis in mice is suppressed by gliotoxin. Clin. Exp. Immunol. 120:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conner, E.M., S. Brand, J.M. Davis, F.S. Laroux, V.J. Palombella, J.W. Fuseler, D.Y. Kang, R.E. Wolf, and M.B. Grisham. 1997. Proteasome inhibition attenuates nitric oxide synthase expression, VCAM-1 transcription and the development of chronic colitis. J. Pharmacol. Exp. Ther. 282:1615–1622. [PubMed] [Google Scholar]
- 52.Murphy, M.E., and H. Sies. 1991. Reversible conversion of nitroxyl anion to nitric oxide by superoxide dismutase. Proc. Natl. Acad. Sci. USA. 88:10860–10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirochnitchenko, O., and M. Inouye. 1996. Effect of overexpression of human CuZn superoxide dismutase in transgenic mice on macrophage functions. J. Immunol. 156:1578–1586. [PubMed] [Google Scholar]
- 54.Koningsberger, J.C., B.S. van Asbeck, E. van Faassen, L.J. Wiegman, J. van Hattum, G.P. van Berge Henegouwen, and J.J. Marx. 1994. Copper, zinc-superoxide dismutase and hydrogen peroxide: a hydroxyl radical generating system. Clin. Chim. Acta. 230:51–61. [DOI] [PubMed] [Google Scholar]
- 55.Mao, G.D., P.D. Thomas, G.D. Lopaschuk, and M.J. Poznansky. 1993. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the Fenton reaction in SOD toxicity. J. Biol. Chem. 268:416–420. [PubMed] [Google Scholar]
- 56.Lih-Brody, L., S.R. Powell, K.P. Collier, G.M. Reddy, R. Cerchia, E. Kahn, G.S. Weissman, S. Katz, R.A. Floyd, M.J. McKinley, et al. 1996. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 41:2078–2086. [DOI] [PubMed] [Google Scholar]
- 57.Krawisz, J.E., P. Sharon, and W.F. Stenson. 1984. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 87:1344–1350. [PubMed] [Google Scholar]
- 58.Bendjelloul, F., P. Maly, V. Mandys, M. Jirkovska, L. Prokesova, L. Tuckova, and H. Tlaskalova-Hogenova. 2000. Intercellular adhesion molecule-1 (ICAM-1) deficiency protects mice against severe forms of experimentally induced colitis. Clin. Exp. Immunol. 119:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]