Abstract
Transgenic Balb/c mice expressing the transforming rat HER-2/neu oncogene develop early and multifocal mammary carcinomas. Within the first 5 months of life the tissue-specific expression of HER-2/neu causes a progression in all their 10 mammary glands from atypical hyperplasia to invasive carcinoma. It was previously observed that chronic administration of interleukin (IL)-12 increased tumor latency, but every mouse eventually succumbed to multiple carcinomas. A significant improvement in tumor prevention was sought by administering allogeneic mammary carcinoma cells expressing HER-2/neu combined with systemic IL-12. This treatment reduced tumor incidence by 90% and more than doubled mouse lifetime. For the maximum prevention p185neu antigen must be expressed by allogeneic cells. IL-12 treatment strongly increased the cell vaccine efficacy. The mammary glands of mice receiving the combined treatment displayed a markedly reduced epithelial cell proliferation, angiogenesis, and HER-2/neu expression, while the few hyperplastic foci were heavily infiltrated by granulocytes, macrophages, and CD8+ lymphocytes. Specific anti–HER-2/neu antibodies were produced and a nonpolarized activation of CD4+ and CD8+ cells secreting IL-4 and interferon (IFN)-γ were evident. A central role for IFN-γ in the preventive effect was proven by the lack of efficacy of vaccination in IFN-γ gene knockout HER-2/neu transgenic Balb/c mice. A possible requirement for IFN-γ is related to its effect on antibody production, in particular on IgG2a and IgG2b subclasses, that were not induced in IFN-γ knockout HER-2/neu mice. In conclusion, our data show that an allogeneic HER-2/neu–expressing cell vaccine combined with IL-12 systemic treatment can prevent the onset of genetically determined tumors.
Keywords: IL-12, allogeneic vaccine, HER-2/neu, mammary carcinoma, immunoprevention
Introduction
Immunological prevention of tumors is a concept based on the ability of immunity to arrest tumor progression in healthy individuals with a high risk of cancer. Successful results have been reported in mice treated with chemical carcinogen (1) and in transgenic mice that develop mammary (2) or prostate (3) carcinomas by using IL-12 (1, 2), cellular vaccines (4), DNA vaccines (5, 6), recombinant proteins (7), and adoptive immunotherapy (3).
While these studies provide significant proofs of concept, the protection afforded was markedly influenced by the model adopted. Balb/c female mice transgenic for the transforming rat HER-2/neu oncogene (Balb-neuT mice) display an early development of multifocal mammary carcinomas. In these mice, final tumor incidence was not affected but only significantly delayed by IL-12 systemic treatment, while a 50% reduction in tumor incidence was observed in FVB mice transgenic for rat HER-2/neu protooncogene (FVB-neuN), which develop fewer tumors with a longer latency period (2). Both cytokines and antigen-specific vaccines were effective, albeit through markedly different reaction mechanisms (1–7). It should be noted that in HER-2/neu transgenic mice the existence of immunological tolerance to HER-2/neu, similar to what is observed in patients with neu-expressing breast cancer, has been documented (6, 8).
This paper reports the efficacy of combined allogeneic tumor cell vaccination and IL-12 treatment in the prevention of mammary carcinomas in virgin Balb-neuT mice. Within the first 5 mo of age the tissue-specific expression of HER-2/neu causes a progression in all their ten mammary glands from atypical hyperplasia to an invasive carcinoma that closely resembles human neoplasia (9). The combination of cell vaccines and cytokine treatment we have chosen inhibited tumor onset in a model of rapid progression to malignant mammary carcinoma.
Materials and Methods
Mice
Balb-neuT female mice (H-2d) overexpressing the transforming activated rat HER-2/neu oncogene under control of the mouse mammary tumor virus promoter (2) were bred under specific pathogen-free conditions by Charles River Laboratories. IFN-γ gene knockout Balb/c mice from Jackson ImmunoResearch Laboratories were crossed with Balb-neuT, and IFN-γ knockout Balb-neuT mice (IFN-γ−/− Balb-neuT mice) selected by PCR analysis. The total number of backcrosses of Jackson IFN-γ knockout mice to Balb/c mice was 10. When spleen cells from IFN-γ knockout Balb-neuT mice were used as stimulator in mixed lymphocyte cultures, no significant activation of proliferative response of Balb-neuT responder T lymphocytes was ever found. Only individually tagged virgin females were used in experiments described here and were treated according to the European Community guidelines. Mammary glands were inspected weekly, and tumor masses measured with calipers in two perpendicular diameters. Progressively growing masses of >3 mm in mean diameter were regarded as tumors. Growth was monitored until all 10 mammary glands displayed a tumor or until a tumor exceeded a mean diameter of 1.5 cm, at which time mice were killed for humane reasons.
Cells
TT12 and N202.1E cell clones were derived from two independent mammary carcinomas of FVB-neuN no. 202 mice (H-2q), transgenic for the rat neu protooncogene (10). TT12 cells (referred to as Neu/H-2q) expressed high levels of p185neu; N202.1E cells (referred to as Neuneg/H-2q) lacked p185neu (11). TUBO cell clone (referred to as Neu/H-2d) was derived from a carcinoma of Balb-neuT mouse (12). Cells were cultured in Dulbecco's modified minimal essential medium supplemented with 20% FBS (Life Technologies) at 37°C in a humidified 5% CO2 atmosphere. In some experiments cells were treated with 40 μg/ml of mitomycin C (MitC; Sigma-Aldrich) to block cell proliferation. Surface expression of p185neu and class I H-2q molecules was assessed by flow cytometry as described previously (11) using monoclonal antibodies 7.16.4 (p185neu; Oncogene Research Products), KH114 (H-2Kq; BD PharMingen), 34-7-23S (H-2Dq; Cedarlane), and 28-14-8S (H-2D/Lq; BD PharMingen).
Vaccination and IL-12 Treatment
Starting at the sixth week of age Balb-neuT mice received successive 3-wk courses of four twice-weekly intraperitoneal vaccinations with 2 × 106 allogeneic Neu/H-2q mammary carcinoma cells in 0.4 ml of PBS, followed by five daily intraperitoneal administrations of recombinant mouse IL-12 (provided by S. Wolf, Genetics Institute, Andover, MA) in the third week. In the first course the five injections were of 50 ng of IL-12 in 0.2 ml of PBS supplemented with 0.01% mouse serum albumin (MSA; Sigma-Aldrich), the subsequent injections were of 100 ng. After 1 wk of rest, the course was repeated until mice were killed or were 1 y old. Control Balb-neuT mice received either mock vaccination or MSA administration. Their tumor progression mirrored that of untreated mice.
Morphologic and Immunohistochemical Analysis
Groups of three mice were killed at the indicated times. Tissue samples were processed as described previously (9) for histologic evaluation or for immunohistochemistry. The following antibodies were used: antidendritic cells (NLDC 145; Cedarlane); anti-CD4, anti-CD8a (both from Sera-Lab, Crawley Down); anti–Mac-1 (anti-CD11b/CD18), anti–Mac-3, and anti-Ia (all from Boehringer Mannheim); anti-PMN (clone RB6-8C5, provided by R.L. Coffman, Dynax); antiasialo GM1 (Wako Chemicals); anti–endothelial cells (mEC-13.324) and anti–endothelial leukocyte adhesion molecule E-selectin 1 (E-selectin), both provided by A. Vecchi, Istituto M. Negri, Milano, Italy; anti–intercellular adhesion molecule 1 (CD54), anti–vascular cell adhesion molecule 1 (BD PharMingen), and anti–monocyte chemotactic protein 1 (BD PharMingen); anti–macrophage inflammatory protein (MIP)-2 (Walter Occhiena Srl); anti-RANTES; anti–IFN-γ inducible protein (IP)*-10; antimonokine induced by IFN-γ (MIG; R&D Systems); anti–IL-1β (Genzyme), anti–TNF-α (ImmunoKontact), anti–IFN-γ (provided by S. Landolfo, University of Turin), and anti-inducible nitric oxide synthase (Transduction Laboratories); anti–vascular endothelial cell growth factor, anti–basic fibroblast growth factor (Santa Cruz Biotechnology, Inc.), anti-p185neu (C-18; Santa Cruz Biotechnology, Inc.), and anti–proliferating cell nuclear antigen (PCNA; Ylem). Quantitative studies of immunohistochemically stained sections were performed independently by three pathologists in a blind fashion. From mice with multiple tumors, one sample per tumor growth area and 10 randomly chosen fields in each sample were evaluated for each point determination. Positive cells were counted under a high-power (original magnification: ×630) microscope field (0.114 mm2 per field).
IFN-γ and IL-4 Production from Spleen Cells
Spleens were collected from treated and control mice, and single cell suspensions were prepared, washed in PBS, and resuspended in RPMI 1640 supplemented with 10% FBS. CD4+- and CD8+-enriched T cells were positively selected by paramagnetic beads conjugated with anti-CD4 and anti-CD8a monoclonal antibody, respectively (Miltenyi Biotec). Unseparated, CD4+ and CD8+ cells (5 × 105 cells per milliliter) were then restimulated with MitC-treated target cells (5 × 104 cells per milliliter) for 6 d at 37°C in RPMI 1640 with 10% FBS containing 10 U/ml recombinant mouse IL-2 (Peprotech). Supernatants were assayed for IFN-γ and IL-4 production by ELISA assays from Endogen.
Antibody Response
Sera collected from tumor-free mice at 13 wk of age were analyzed by flow cytometry, Western blot analysis, and complement-mediated cytotoxicity as described previously (11) using various p185neu-positive and -negative cells. Ig subclasses analysis was carried out by an indirect immunofluorescence procedure. Secondary fluorescein-conjugated monoclonal antibodies directed against Ig subclasses were as follows: anti–mouse IgG1 clone A85-1; anti–mouse IgG2a clone R19-15; anti–mouse IgG2b clone R12-3; anti–mouse IgG3 clone R40-82; anti–mouse IgM clone R6-60.2; anti–mouse IgA clone C10-3; and anti–mouse IgE clone R35-72. All of them were purchased from BD PharMingen.
Expression of Angiogenic and Antiangiogenic Factors
Neu/H-2d mammary carcinoma cells were cultured for 48 h in the presence and absence of 100 U/ml of mouse IFN-γ (given by G.R. Adolf, Ernst-Boehringer Institute, Wien, Austria). Total RNA was extracted, retrotranscribed, and semiquantitative reverse transcriptase polymerase chain reaction was performed as described previously (13) with the following primers: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), purchased from CLONTECH; matrix metalloproteinase (MMP-9; reference 14); MIG (forward 5′-TCC GCT GTT CTT TTC CTT TTG G-3′, reverse 5′-TTG AAC GAC GAC GAC TTT GGG G-3′); IP-10 (forward 5′-CTC CCC ATC AGC ACC ATG AAC-3′, reverse 5′-GTG GCT TCT CTC CAG TTA AGG A-3′).
Results
Prevention of Mammary Carcinogenesis
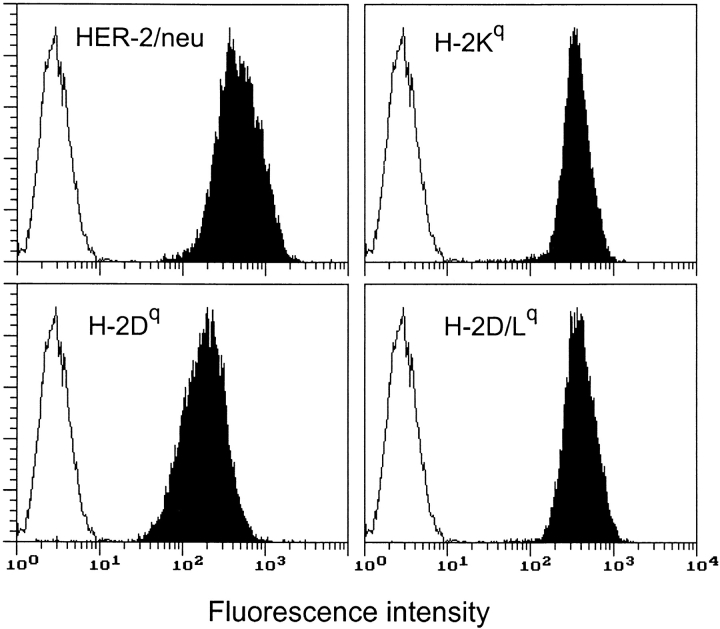
Vaccination with allogeneic mammary carcinoma cells (Neu/H-2q) expressing high surface levels of both p185neu and H-2q class I molecules (Fig. 1) followed by intraperitoneal administration of IL-12 was used to halt mammary carcinogenesis in Balb-neuT mice. IL-12 was chosen because of its ability to significantly delay tumor onset (1, 2). Beginning when the mice were 6 wk old, they received Neu/H-2q cells twice-weekly in the first and second week, followed by five daily administrations of IL-12 in the third week. After 1 wk of rest this course was repeated until mice were killed or reached the age of 1 y.
Figure 1.
Cytofluorometric analysis of HER-2/neu and MHC class I glycoprotein expression in Neu/H-2q cells. Open profiles represent cells stained with secondary antibody alone; solid profiles represent cells incubated with the indicated antibodies. In each panel, the ordinate represents the number of cells. Data from an experiment representative of at least three similar experiments are shown.
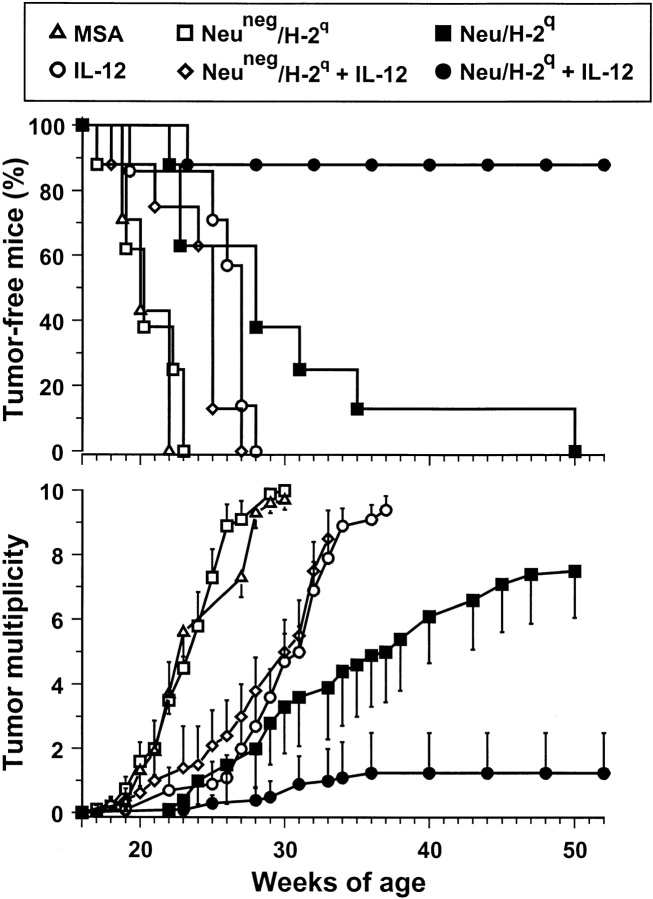
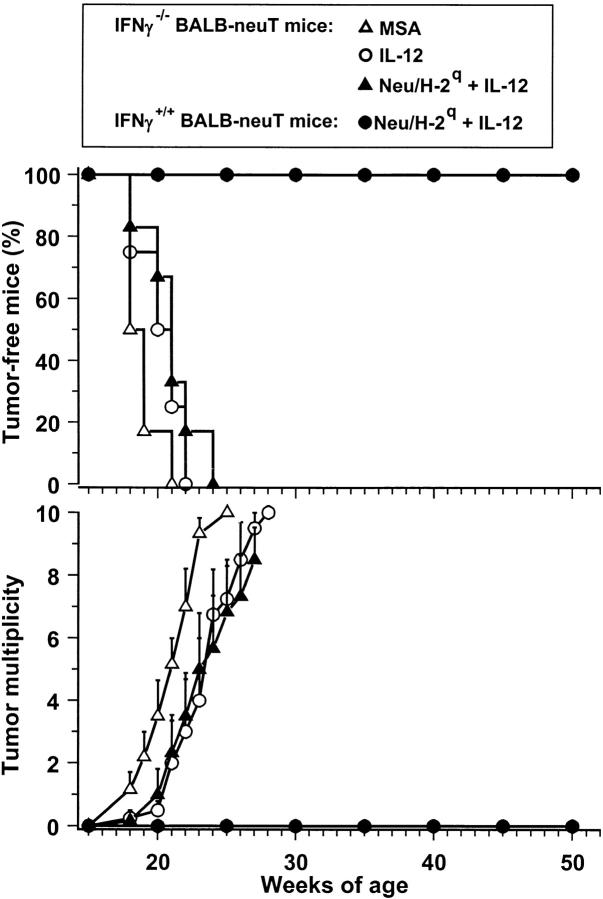
This combined treatment effectively inhibited the development of mammary carcinomas (Fig. 2) . All control mice developed their first mammary tumor within 22 wk, when all mice treated with Neu/H-2q cells plus IL-12 were completely tumor free. Within 30 wk all control mice developed tumors in all 10 mammary glands. At 52 wk (end of the study) 88% of mice treated with Neu/H-2q cells plus IL-12 were still completely tumor free; their lifetime was more than doubled.
Figure 2.
Inhibition of mammary carcinogenesis in Balb-neuT female mice. Groups of 7–8 mice received the indicated treatments. Tumor-free survival curve of mice treated with Neu/H-2q cells plus IL-12 is significantly different from all other curves (P < 0.005 at least by the Mantel-Haenszel test); tumor-free survival curves of mice treated with IL-12 or Neu/H-2q cells are significantly different (P < 0.05 at least) from MSA controls. Tumor multiplicity is calculated as the cumulative number of incident tumors/total number of mice and is shown as mean ± SEM.
Weight of the Treatment Components
IL-12 alone produced a significant delay in tumor latency and a significant reduction in tumor multiplicity, but did not affect tumor incidence (Fig. 2), in agreement with our previous findings (2). Vaccination with Neu/H-2q cells alone delayed tumor onset, but did not result in tumor-free mice (Fig. 2), thus indicating that IL-12 markedly contributes to the efficacy of the combined treatment. Vaccination with the Neuneg/H-2q cells alone was ineffective and its combination with IL-12 resulted in a protection similar to that afforded by IL-12 alone (Fig. 2). Therefore, p185neu recognition appears to play a central role in halting HER-2/neu carcinogenesis.
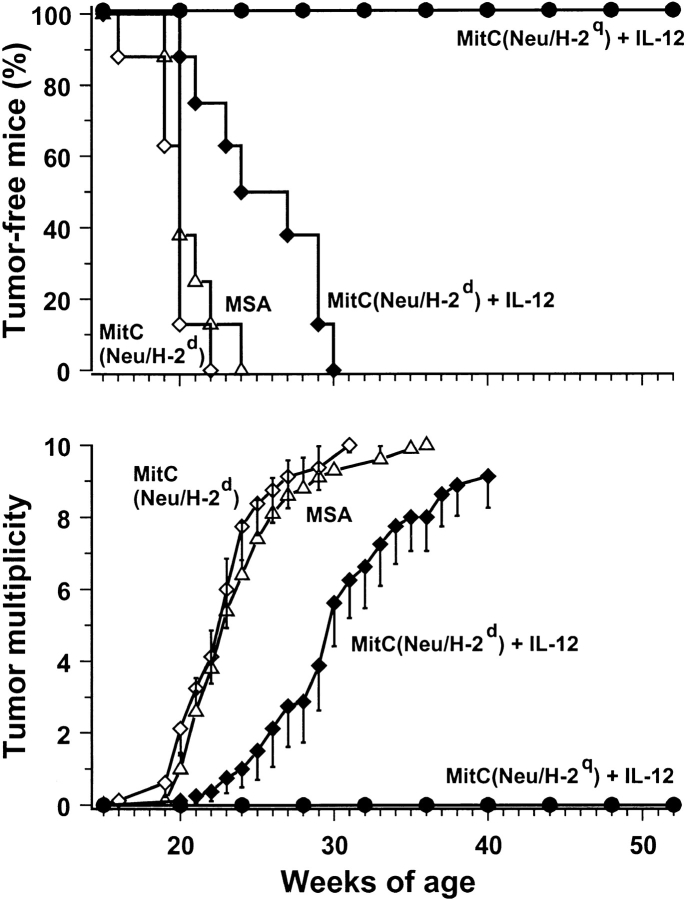
As the block of cell proliferation by MitC may reduce the immunogenicity of a vaccine (15), while vaccination with replicating cells poses practical issues, the need for live and replicating cells was also evaluated. The combination of MitC-treated Neu/H-2q cells with IL-12 resulted in a block of carcinogenesis and all mice were tumor free at 1 yr (Fig. 3) . Vaccination with MitC-treated cells allowed us to evaluate also the efficacy of syngeneic Neu/H-2d cells, which otherwise would be tumorigenic; no protection was obtained with such cells alone, whereas the delay in tumor occurrence induced by Neu/H-2d cells plus IL-12 was not longer than that elicited by IL-12 alone (see Fig. 2 for comparison). These data point to a requirement for both allogeneic MHC molecules and p185neu recognition for an effective protection.
Figure 3.
Inhibition of mammary carcinogenesis in Balb-neuT mice with MitC-treated allogeneic Neu/H-2q cells plus IL-12. Groups of eight mice received the indicated treatments. Tumor-free survival curves are significantly different (P < 0.0005 by the Mantel-Haenszel test). Tumor multiplicity is calculated as the cumulative number of incident tumors/total number of mice and is shown as mean ± SEM.
Pathological Evolution of the Mammary Gland
By the third week of age foci of atypical hyperplasia were evident in the numerous side buds (9, 16) of control mice and subsequently extended to all 10 mammary glands. Foci of carcinoma in situ first apparent around the fifteenth week (Fig. 4 A) evolved to invasive lobular carcinomas by the twentieth. 10 wk later invasive lobular carcinomas were present in all the glands.
Figure 4.
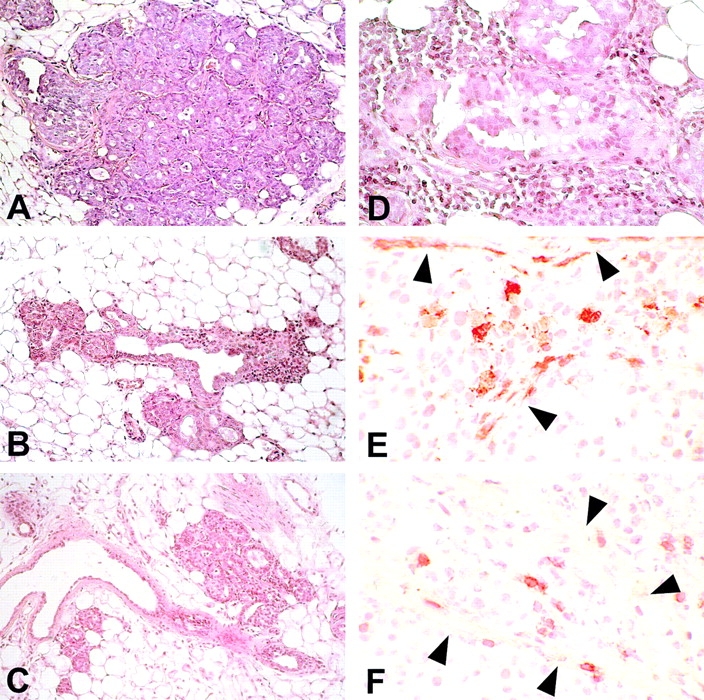
Morphologic and immunohistochemical analysis of mammary glands. Histology shows that at week 15 the mammary tissue of control mice (A) contains numerous foci of atypical hyperplasia with areas of lobular carcinoma in situ; the hyperplastic and neoplastic epithelial cells occupy and fill out all the ductal-lobular structures. The hyperplastic foci found in mice receiving IL-12 (B) or Neu/H-2q cells plus IL-12 (C) are less numerous and prosperous than in control mice and surrounded by an evident reactive infiltrate. At 27 wk in mice treated with Neu/H-2q cells plus IL-12 the few surviving foci of hyperplasia (D) are surrounded by an evident infiltrate composed of reactive cells that sometimes are found within epithelial cells after crossing or damaging the basal membrane. Immunohistochemistry reveals that granulocytes (E) and CD8+ lymphocytes (F) are present not only in the stroma but also inside the lobular structure after crossing the damaged basal membrane (arrowheads). A–C, original magnification: ×200; D, original magnification: ×400; E and F, original magnification: ×630.
Treatment with IL-12 alone slowed down this progression. The atypical foci grew much more slowly and the onset of in situ and invasive carcinomas was delayed by 5–6 wk. The lesions were invaded by reactive cells (Fig. 4 B), whereas a similar infiltrate was barely visible in the controls. At the fifteenth week of age the side buds and the terminal ductal end buds (TEBs) of mice treated with Neu/H-2q cells plus IL-12 were carpeted with a single layer of epithelial cells and surrounded by reactive cells. The few small hyperplastic foci were the scene of intense inflammation (Fig. 4 C). After the fifth course (at 27 wk), the mammary tissue was mainly composed of small ducts with few TEBs. Remaining side buds and TEBs were slightly hyperplastic and surrounded by reactive cells. These were sometimes found inside the lobular structures among the epithelial cells (Fig. 4 D–F).
In mice treated with Neu/H-2q cells plus IL-12, attenuated inflammation was present at 35, 40, and 45 wk of age after the eighth or tenth course. In very few mice only invasive lobular carcinomas developing from hyperplastic foci were microscopically detectable. Their lobules were less compact than in the controls, while the neoplastic epithelial cells were poorly coherent and frequently disaggregated, and gave rise to fissures. The stroma inside and around the tumor was more abundant and occupied by a distinct inflammatory infiltrate. Reactive cells were numerous in the stroma of both the hyperplastic and the tumor lesions, and occasionally intermingled with the neoplastic cells after crossing or damaging the basal membrane.
Immunohistochemical examination after the second course (fifteenth week of age) revealed a reduction of hyperplastic foci and TEBs containing epithelial cells expressing p185neu. At 27 wk, the mammary tissue of mice treated with Neu/H-2q cells plus IL-12 was only constituted by small ducts lined by a single layer of epithelial cells without or with a slight p185neu expression confined to the cytoplasm (Fig. 5 A, C, and E) associated with a poor positivity to PCNA (Fig. 5 B, D, and F). By contrast, a marked cytoplasmic and membrane p185neu expression, similar to hyperplastic and neoplastic lesions from untreated and IL-12–treated mice, was displayed by the few carcinomas detected in mice treated with IL-12 and cell vaccine.
Figure 5.
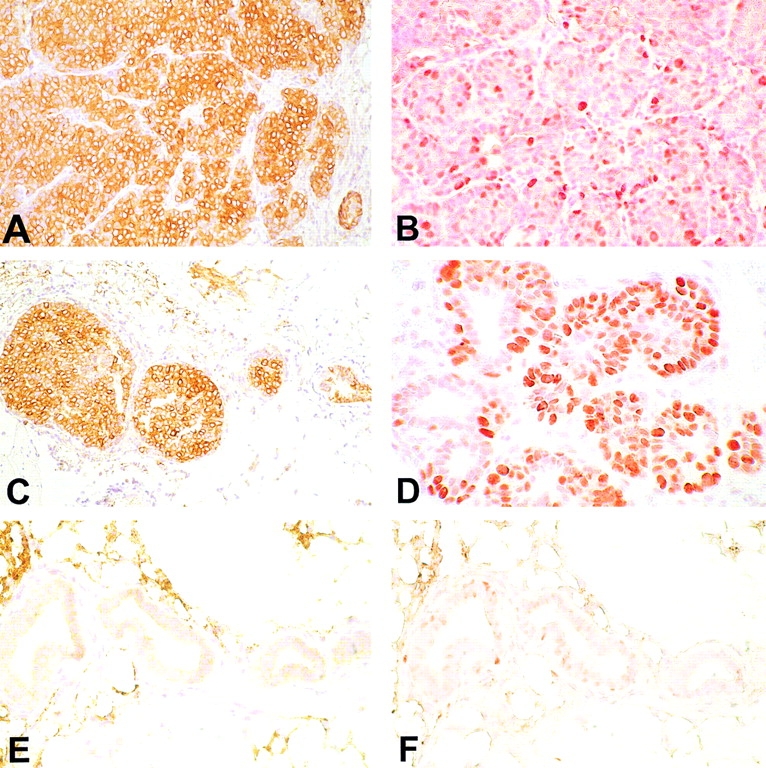
Mammary tissue from untreated 27-wk-old mice (A and B), mice receiving IL-12 (C and D), or Neu/H-2q cells plus IL-12 (E and F). Immunohistochemistry with anti-p185neu antibody reveals that all the neoplastic cells express the p185neu in the cytoplasm and on their membrane (A). A similar p185neu expression pattern is evident in the mammary epithelial cells of the carcinoma in situ present in the IL-12–treated mouse (C). The cytoplasmic and membrane expression of the p185neu is associated with a marked positivity of PCNA (B and D). The mammary glands of mice treated with Neu/H-2q cells plus IL-12 are mainly composed of ductules lined by a single layer of epithelial cells without or with a slight p185neu expression mainly confined in the epithelial cell cytoplasm (E); the epithelial proliferation rate was low as assessed by PCNA positivity (F). A–F, original magnification: ×400.
A conspicuous macrophage, neutrophil, and CD8+ lymphocyte infiltrate was present in the hyperplastic foci and in the tumor stroma of mice treated with Neu/H-2q cells plus IL-12 (Table I) . The number of dendritic and NK cells also increased, though it was not higher than in mice treated with IL-12 only. Recruitment of reactive cells was accompanied by overexpression of endothelial adhesion molecules in the small vessels.
Table I.
Reactive Cell Content, Expression of Endothelial Adhesion Molecules, and Production of Cytokines, Angiogenic Factors, and Mediators in Tumors Growing in Balb-neuT Mice
| MSA | IL-12 | Neu/H-2q cellsplus IL-12 | |
|---|---|---|---|
| Reactive cells a | |||
| Dendritic cells | 1.4 ± 0.7 | 11.0 ± 3.2b | 9.3 ± 2.8b |
| Macrophages | 3.1 ± 1.3 | 4.2 ± 1.8 | 22.1 ± 4.2c |
| PMN | 4.5 ± 2.0 | 4.6 ± 2.3 | 23.3 ± 5.2c |
| CD8+ lymphocytes | 1.3 ± 0.9 | 6.7 ± 2.4b | 12.7 ± 3.7c |
| CD4+ lymphocytes | 0.0 | 1.3 ± 0.4b | 2.0 ± 0.6 |
| NK cells | 5.1 ± 1.9 | 14.6 ± 3.3b | 11.2 ± 3.5b |
| Microvessel count | 14.4 ± 2.2 | 9.0 ± 1.8b | 6.1 ± 1.7b |
| Endothelial adhesion molecules d | |||
| ELAM-1 | – | – | + |
| ICAM-1 | + | + | ++ |
| VCAM-1 | – | – | ± |
| Cytokines andmediators d | |||
| IL-1β | – | + | ++ |
| TNF-α | ± | ± | + |
| IFN-γ | – | ± | + |
| MCP-1 | – | + | ++ |
| MIP-2 | ± | ± | + |
| RANTES | – | – | ± |
| IP-10 | + | ++ | ++ |
| MIG | + | ++ | ++ |
| iNOS | – | + | + |
| Angiogenic factors d | |||
| VEGF | ± | ± | ± |
| bFGF | + | + | + |
Cell counts performed in ten randomly chosen high-power fields per sample. Results are mean ± SD of positive cell per field.
Significantly different (P < 0.001) from corresponding values in MSA control mice.
Significantly different (P < 0.001) from corresponding values in MSA control and IL-12–treated mice.
The expression of adhesion molecules, cytokines, mediators, and angiogenic factors was defined as absent (-), scarcely (±), moderately (+), and strongly (++) present on cryostat sections decorated with the antibody.
bFGF, basic fibroblast growth factor; ELAM, endothelial leukocyte adhesion molecule E-selectin; ICAM, intercellular adhesion molecule; iNOS, inducible nitric oxide synthase; MCP, monocyte chemotactic protein; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial cell growth factor.
Significantly fewer blood vessels were found in mammary glands of mice treated with Neu/H-2q cells plus IL-12 and of those receiving IL-12 only than in the controls. By contrast, proinflammatory cytokines and chemokines were more evident in mice treated with Neu/H-2q cells plus IL-12 than in mice receiving IL-12 only and were almost absent in mammary glands from untreated controls.
Cell-mediated Reactivity
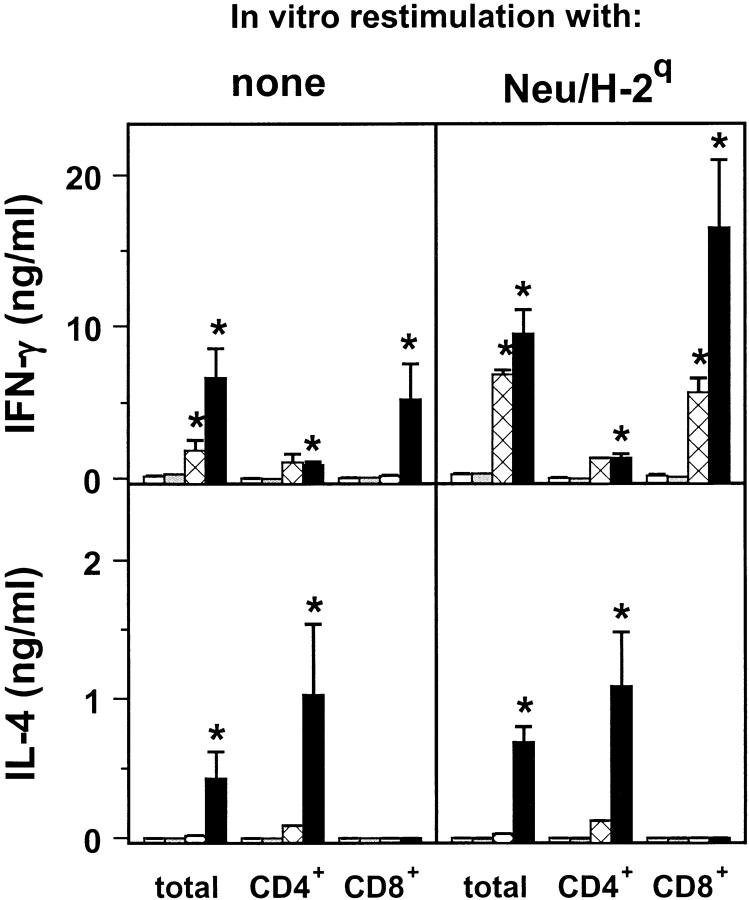
At the fifteenth week of age, after the second course, groups of 3–5 control and treated mice were killed to evaluate spleen cell reactivity. Both total spleen cell yield and the absolute number of CD4+ cells were significantly higher in mice treated with Neu/H-2q cells plus IL-12. However, CTL assays against HER-2/neu-positive or -negative targets showed only ∼10% of specific lysis of HER-2/neu-positive targets by spleen cells from Balb-neuT mice vaccinated with Neu/H-2q cells plus IL-12. These spleen cells did not display any protective activity when they were admixed with a tumorigenic dose of Neu/H-2d syngeneic mammary tumor cells in a Winn-type neutralization assay in Balb-neuT mice (data not shown). On the other hand, an increased spontaneous proliferation was displayed in vitro by the CD4+ lymphocyte subpopulation. It was further increased in an antigen-specific manner by the addition of mitomycin-blocked syngeneic Neu/H-2d cells. Moreover, total spleen cells and CD8+ lymphocytes of mice repeatedly treated with Neu/H-2q cells plus IL-12 spontaneously released more IFN-γ than leukocytes from control or IL-12–treated mice (Fig. 6) . Large amounts of IFN-γ were released by total and CD8+ spleen cells after restimulation by Neu/H-2q cells. IL-4 was secreted spontaneously or after restimulation by both total spleen cells and CD4+ lymphocytes (but not by CD8+ lymphocytes) from mice treated with Neu/H-2q cells plus IL-12. This cytokine release pattern was triggered by the recognition of both H-2q allogeneic MHC glycoproteins and p185neu since IFN-γ and IL-4 were produced not only in response to p185neu plus alloantigens (Neu/H-2q) but also by the presentation of p185neu on syngeneic cells (Neu/H-2d) as well as by alloantigens alone (Neuneg/H-2q) (Table II).
Figure 6.
Release of cytokines by spleen cells restimulated or not in vitro with Neu/H-2q cells. Spleen cells from untreated control mice (white bar); from mice treated with IL-12 (gray bar); from mice treated with Neu/H-2q cells (cross-hatched bar); and from mice treated with Neu/H-2q cells plus IL-12 (black bar). Asterisks denote a significant difference (P < 0.05 at least by Student's t test) with untreated controls. Mean ± SEM of groups of 3–5 Balb-neuT mice.
Table II.
In Vitro Production of Cytokines in Spleen Cells from Control and Vaccinated Balb-neuT mice after Restimulation with HER-2/neu-positive (Neu/H-2q) and -negative (Neuneg/H-2q) Allogeneic Cells or with HER-2/neu-positive Syngeneic Cells (Neu/H-2d)
| Released cytokine (ng/ml)
|
|||
|---|---|---|---|
| Vaccinated micea | In vitro stimulation | IFN-γ | IL-4 |
| MSA | None | 0.12 ± 0.07 | 0.00 ± 0.00 |
| Neu/H-2q | 0.27 ± 0.07 | 0.00 ± 0.00 | |
| Neuneg/H-2q | 0.40 ± 0.23 | 0.00 ± 0.00 | |
| Neu/H-2d | 0.35 ± 0.11 | 0.00 ± 0.00 | |
| Neu/H-2q plus IL-12 |
None | 0.65 ± 0.12 | 0.04 ± 0.03 |
| Neu/H-2q | 3.37 ± 0.95b | 0.18 ± 0.11 | |
| Neuneg/H-2q | 2.86 ± 0.64b | 0.10 ± 0.05 | |
| Neu/H-2d | 1.81 ± 0.32 | 0.11 ± 0.00 | |
Mice received two courses of vaccination.
P < 0.05 versus no restimulation (Student's t test).
Role of IFN-γ
In IFN-γ knockout Balb-neuT mice the combined administration of Neu/H-2q cells plus IL-12 was totally ineffective (Fig. 7) . This fading of the protection points to the crucial role of IFN-γ in the prevention observed. Along with its numerous immunomodulatory activities, IFN-γ may also directly modulate tumor cell phenotype and the in vivo behavior of tumor cells. Our results suggest that induced IFN-γ can affect the behavior of Neu/H-2d cells by inhibiting their angiogenic phenotype and the HER-2/neu–mediated signaling pathways leading to the neoplastic behavior (11). In vitro, IFN-γ inhibited the proliferation of Neu/H-2d cells, downmodulated their membrane expression of p185neu (which was even reduced by 80% by the combination of IFN-γ and TNF-α) and the production of the proangiogenic protease MMP-9, while it strongly upmodulated the production of antiangiogenic cytokines such as IP-10 and MIG (Fig. 8) .
Figure 7.
Lack of inhibition of mammary carcinogenesis in IFN-γ knockout Balb-neuT female mice. Transgenic mice received the indicated treatments. Tumor multiplicity was calculated as the cumulative number of incident tumors/total number of mice and is shown as mean ± SEM. Groups of 7–8 mice.
Figure 8.
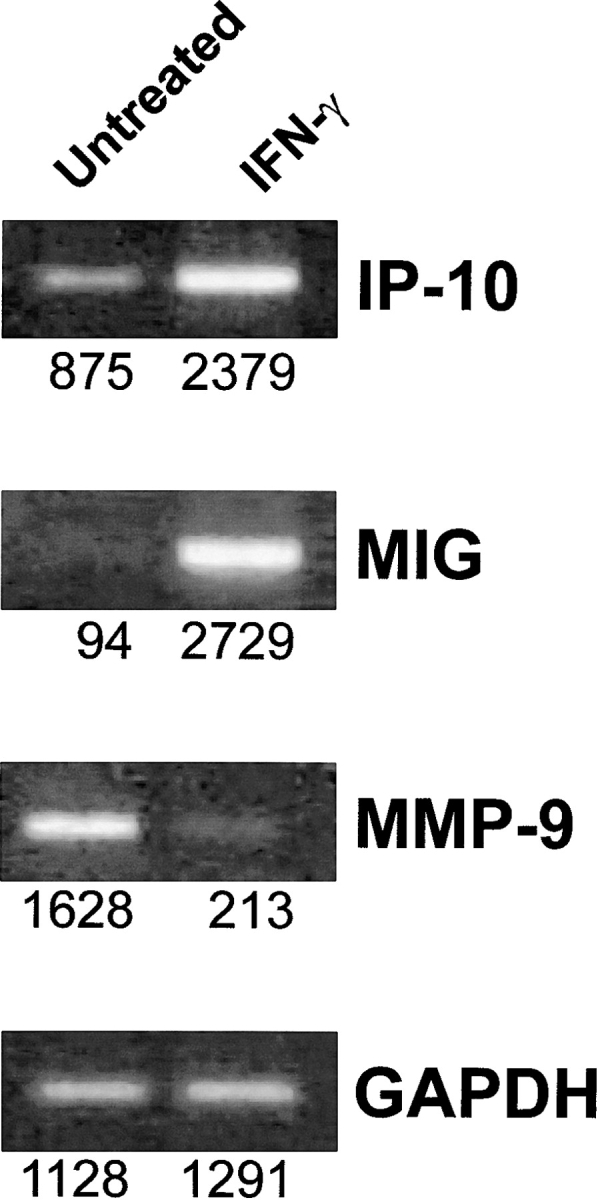
mRNA expression of angiogenic and antiangiogenic molecules determined by reverse transcriptase PCR in Neu/H-2d mammary carcinoma cells treated in vitro with IFN-γ. GAPDH, 20 cycles; MIG, 35 cycles; IP-10, 28 cycles; MMP-9, 28 cycles. Densitometric analysis was performed and values reported under each band.
Antibody Response
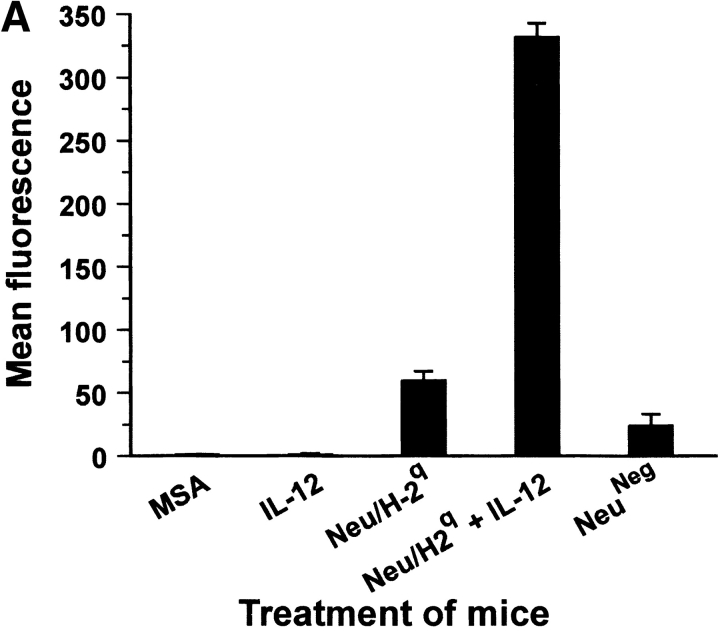
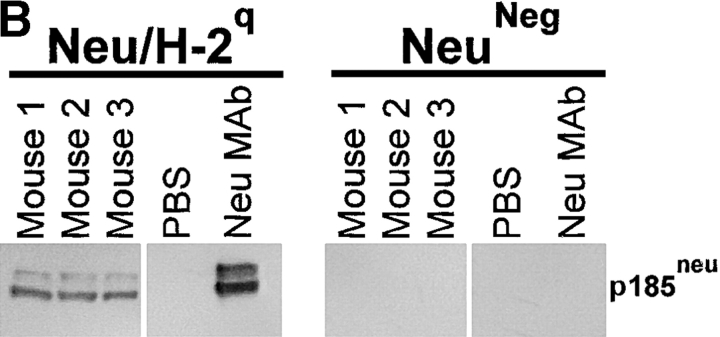
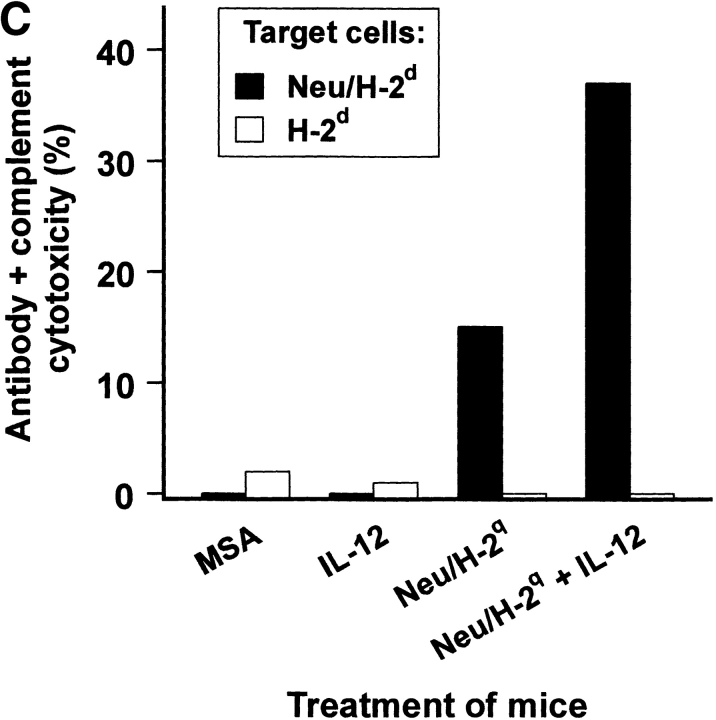
High-titer antibodies binding Neu/H-2q cells were detected in sera from mice vaccinated with allogeneic Neu/H-2q cells (Fig. 9 A). Staining of NeuNeg/H-2q cells was much lower than Neu/H-2q cells (mean fluorescence intensity 61 ± 9 vs. 332 ± 11), thus suggesting that sera of H-2d mice vaccinated with Neu/H-2q plus IL-12 contained p185neu-reactive antibodies along with alloreactive anti–H-2q antibodies. Neither untreated control mice nor mice treated with IL-12 alone produced antibody against Neu/H-2q cells (Fig. 9 A). To circumvent the issue of alloreactivity we performed a Western blot analysis (Fig. 9 B), which showed that antibodies of mice vaccinated with Neu/H-2q plus IL-12 specifically recognized p185neu (Fig. 9 B). Only sera from mice receiving cell vaccines resulted cytotoxic against syngeneic Neu/H-2d tumor cells, whereas no lysis was observed against syngeneic p185neu-negative H-2d lymphocytes (Fig. 9 C). The lysis was stronger when Neu/H-2q cell vaccine was combined with IL-12 administration.
Figure 9.
Antibody production by Balb-neuT mice. (A) Cytofluorometric analysis of serum binding to Neu/H-2q cells. Each bar represents the mean ± SEM of sera (diluted 1:65) from three mice at the fifteenth week of age bled after the second course of the indicated treatment. (B) Neu/H-2q and Neuneg/H-2q cell extracts were immunoprecipitated by sera from three mice treated with Neu/H-2q cells plus IL-12, PBS as negative control, and anti–rat p185neu monoclonal antibody 7.16.4 as positive control (Neu mAb). Western blot analysis was performed using the antineu polyclonal antibody C18. (C) Complement dependent cytotoxicity against syngeneic Neu/H-2d cells and Balb-neuT lymphocytes, which do not express p185neu (H-2d). The percentage of viable lymphocytes was determined after a 30-min incubation with sera of mice bled after the second course of treatment (diluted 1:10) followed by a 30-min incubation with rabbit low-tox complement (diluted 1:10). Results are expressed as 100 − % viable lymphocytes.
A much lower antibody response was displayed by IFN-γ knockout Balb-neuT mice treated with Neu/H-2q cells plus IL-12. Cytofluorometric analysis of serum binding to Neu/H-2q cells showed a mean fluorescence activity of 124 ± 44 for IFN-γ knockout Balb-neuT mice versus 396 ± 39 for Balb-neuT mice (P < 0.01).
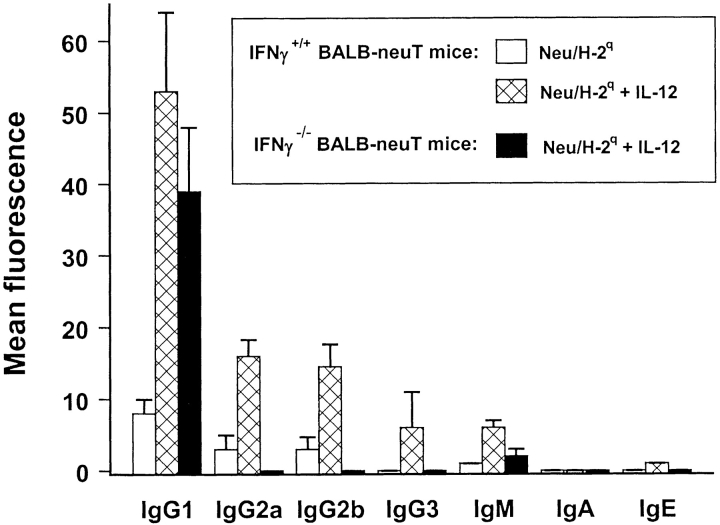
Analysis of Ig subclasses in sera from Balb-neuT transgenic mice treated with the cell vaccine or with the vaccine plus IL-12 is shown in Fig. 10 . The combined treatment with Neu/H-2q cells plus IL-12 significantly (P < 0.05) increased the level of IgG1, IgG2a, and IgG2b. By contrast, in IFN-γ knockout BALB-neuT mice receiving the vaccine plus IL-12 the treatment failed to increase the level of all Ig subclasses except IgG1.
Figure 10.
Subclasses of antibodies induced in Balb-neuT mice and in IFN-γ knockout Balb-neuT mice. Cytofluorometric analysis of serum binding to Neu/H-2q cells with secondary anti–mouse isotype antibodies is reported. Each bar represents the mean ± SEM of sera (diluted 1:65) from three mice at the fifteenth week of age bled after the second course of the indicated treatment.
Discussion
Combination of an allogeneic cell vaccine with systemic IL-12 prevented the onset of mammary carcinoma in tumor-prone Balb-neuT mice. Their lifespan was more than doubled, while the quality of their life was not impaired. The protection afforded was much more effective than those previously obtained in the same mice with the exogenous cytokine alone (2) or DNA vaccination (12). It was even stronger than that elicited by cytokines (2), DNA, or protein vaccines (5–7) in other lines of HER-2/neu transgenic mice which develop fewer tumors with a longer latency.
The treatment combined different immunological stimuli, namely p185neu, allogeneic class I MHC glycoproteins, and IL-12. Their separate evaluation showed that all were required. Balb-neuT mice receiving allogeneic Neu/H-2q cells or IL-12 alone displayed a slower carcinogenesis, but all eventually succumbed to carcinoma. Only mice receiving the combination were protected from carcinoma for up to 1 y.
To analyze the contribution of cell-bound determinants we replaced Neu/H-2q cells in the vaccine either with Neuneg/H-2q cells, a p185neu loss variant, or with Neu/H-2d cells, expressing p185neu on a syngeneic background. The various cell lines derive from independent transgenic carcinomas (11, 12), therefore the possibility that other factors may contribute to their different vaccine effect cannot be ruled out. The markedly higher protection afforded by IL-12 in combination with Neu/H-2q cells, as opposed to the poor resistance of mice vaccinated with IL-12 and Neuneg/H-2q cells, underlines the crucial importance of p185neu recognition. Similarly, the weight of allogeneic MHC signal was evident when the effective protection from carcinogenesis of mice receiving IL-12 and allogeneic Neu/H-2q cells was compared with the scarce efficacy of treatments with IL-12 and syngeneic Neu/H-2d cells. Allorecognition may provide a favorable cytokine microenvironment, but also induce a rapid destruction of the vaccine and facilitate the cross-presentation of p185neu by host APCs (17).
Tumor prevention appeared to rest on the activation of multiple immune mechanisms. The dissection of their in vivo role was made difficult by the complexity of the reaction elicited and the duration of treatments, however distinct immune responses were conspicuously present or absent in association with protection from tumor onset.
IL-12 alone significantly delays tumor progression through its effects on cell-mediated immunity and angiogenesis (18, 19). Tumors arising in Balb-neuT mice receiving IL-12 alone were infiltrated by dendritic cells, NK cells, and CD8+ lymphocytes. The local release of IFN-γ induced the expression of tertiary antiangiogenic mediators such as IP-10 and MIG, and the microvessel density was decreased. Similar findings have been obtained in other experimental models where IL-12 was employed for cancer prevention (1, 2).
A stronger inflammatory response was evident in mice receiving IL-12 and Neu/H-2q allogeneic cells. Local production of MIP-2 and the expression of endothelial adhesion molecules correlated with the recruitment of granulocytes which may damage tumor vessels and mediate antibody-dependent cytotoxicity (20).
The combined treatment, but not IL-12 alone, elicited a marked specific humoral immune response. Anti-p185neu antibodies in the sera of treated mice may impair carcinogenesis by inducing a functional block of p185neu receptor function, downregulating its expression on the cell membrane (21), and impeding its ability to form the homo or heterodimers that spontaneously transduce proliferative signals to the cells (21, 22). The reduced number of p185neu-positive cells, the intracytoplasmic confinement of p185neu, and the diminished nuclear positivity to anti-PCNA monoclonal antibody in the mammary glands of mice treated with Neu/H-2q cells plus IL-12 after the second course point to a direct inhibitory activity by anti-p185neu antibody. The mammary glands of treated mice displayed fewer and fewer side buds after each course. This is a critical issue in tumor prevention since progression leading to the formation of invasive lobular carcinomas originates from side buds proliferating cells (PCNA-positive) that highly express p185neu (9, 16).
Activation of CD4+ and CD8+ cells by the combined treatment was associated with cytokine release within the mammary gland and in the spleen. Moreover, infiltrating lymphocytes were present in the stroma surrounding neoplastic cells and penetrated the basal membrane to interact with p185neu-positive neoplastic epithelial cells. This type of tumor infiltration by lymphocytes was not observed in Balb-neuT receiving IL-12 alone (2, 23) or Neu/H-2q cells alone. In human carcinomas and melanoma, the presence of a reactive infiltrate intermingled with tumor cells, as opposed to the presence of leukocytes at the periphery of tumor nests, often correlates with a more favorable prognosis (24, 25).
In vitro no evidence of a major involvement of specific CTL activity was found. Even though we cannot rule out the possibility that CTL activity could contribute to the preventive effect, data reported here show that in vitro cytotoxicity and Winn-type tumor neutralization assay were not predictive of treatment efficacy. The absence of a measurable CTL activity in lymphoid organs does not exclude direct killing of HER-2/neu positive mammary cells at the tissue level, but on the whole, it is unclear if vaccination elicited T cells against HER-2/neu.
A major contribution of T cells, whether alloreactive or recognizing HER-2/neu, was the copious production of IFN-γ. The high IFN-γ secretion by CD8+ T cells from mice receiving Neu/H-2q allogeneic cells plus IL-12 and the loss of efficacy of this combined treatment in IFN-γ knockout Balb-neuT mice point to a central role of IFN-γ in the inhibition of carcinogenesis. Along with its numerous activities, IFN-γ directly modulates tumor cell phenotype and immunogenicity (26, 27). It is also the crucial downstream mediator elicited by IL-12 (20) that induces the expression of tertiary antiangiogenic mediators such as IP-10 and MIG, and decreases the density of tumor microvessels (19). Our in vitro data (28) suggest that IL-12–induced IFN-γ modulates the behavior of Neu/H-2d cells by inhibiting their angiogenic phenotype and the HER-2/neu–mediated signaling leading to the neoplastic behavior (11). IFN-γ is also required for the complete activation of effector T cells (29) and is crucial for the induction of C-fixing IgG2a and IgG2b subclasses (30, 31).
In principle IFN-γ might also favor tumor growth through the downmodulation of tumor antigens (32), however, this effect is probably irrelevant in HER-2/neu carcinogenesis, because antigen p185neu expression is required for tumor growth (11), and the major consequence of its downmodulation would be the loss of tumorigenicity, rather than the enhancement of tumor growth resulting from reduced tumor recognition by T cells. Other contradictory effects of IFN-γ in vivo, such as the enhancement of metastatic spread (33), are exerted only on late stages of tumor progression, thus do not play a role in the early steps of mammary carcinogenesis which were prevented by the combined treatment used here.
In summary the lack of efficacy of the combined treatment in IFN-γ–deficient HER-2/neu transgenic mice demonstrated that IFN-γ is an essential component of the immune response leading to mammary carcinoma prevention. The breeding of Balb-neuT mice selectively deficient for other immune functions will allow to better appraise the weight of immune responses that were either strongly induced by the combined treatment (e.g., humoral immunity), or of uncertain value in determining the protective effect (e.g., CTL activity).
In conclusion each component of the combined treatment played a role, and a complete protection was obtained when all components were included to induce a wide spectrum of immune responses. Their concerted action stopped a devastating cancer-prone condition affecting all the ten mammary glands, and more than doubled the lifespan of Balb-neuT mice. This impressive result suggests that a similar approach might prevent tumors in persons with a high risk of cancer.
Acknowledgments
We thank Mrs. Gabriella Madrigali for her invaluable help.
This work was supported by grants from the Italian Association for Cancer Research, the Italian Ministry for University and Scientific and Technological Research, the Universities of Bologna and Torino, and by the U.S. Department of Army, grant DAMD17-98-1-8030. The information contained in this paper does not necessarily reflect the position or the policy of the U.S. government, and no official endorsement should be inferred. A. Astolfi and C. Ricci are holders of Ph.D. fellowships; I. Rossi is holder of a fellowship from University of Bologna.
Footnotes
Abbreviations used in this paper: IP, IFN-γ–inducible protein; MIG, monokine induced by IFN-γ; MIP, macrophage inflammatory protein; MitC, mitomycin C; MMP, matrix metalloproteinase; MSA, mouse serum albumin; PCNA, proliferating cell nuclear antigen; TEB, terminal ductal end bud.
References
- 1.Noguchi, Y., A. Jungbluth, E.C. Richards, and L.J. Old. 1996. Effect of interleukin 12 on tumor induction by 3-methylcholanthrene. Proc. Natl. Acad. Sci. USA. 93:11798–11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boggio, K., G. Nicoletti, E. Di Carlo, F. Cavallo, L. Landuzzi, C. Melani, M. Giovarelli, I. Rossi, P. Nanni, C. De Giovanni, et al. 1998. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J. Exp. Med. 188:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granziero, L., S. Krajewski, P. Farness, L. Yuan, M.K. Courtney, M.R. Jackson, P.A. Peterson, and A. Vitiello. 1999. Adoptive immunotherapy prevents prostate cancer in a transgenic animal model. Eur. J. Immunol. 29:1127–1138. [DOI] [PubMed] [Google Scholar]
- 4.Cefai, D., B.W. Morrison, A. Sckell, L. Favre, M. Balli, M. Leunig, and C.D. Gimmi. 1999. Targeting HER-2/neu for active-specific immunotherapy in a mouse model of spontaneous breast cancer. Int. J. Cancer. 83:393–400. [DOI] [PubMed] [Google Scholar]
- 5.Amici, A., F.M. Venanzi, and A. Concetti. 1998. Genetic immunization against neu/erbB2 transgenic breast cancer. Cancer Immunol. Immunother. 47:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reilly, R.T., M.B. Gottlieb, A.M. Ercolini, J.P. Machiels, C.E. Kane, F.I. Okoye, W.J. Muller, K.H. Dixon, and E.M. Jaffee. 2000. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 60:3569–3576. [PubMed] [Google Scholar]
- 7.Esserman, L.J., T. Lopez, R. Montes, L.N. Bald, B.M. Fendly, and M.J. Campbell. 1999. Vaccination with the extracellular domain of p185neu prevents mammary tumor development in neu transgenic mice. Cancer Immunol. Immunother. 47:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurt, R.A., R. Whitaker, A. Baher, S. Seung, and W.J. Urba. 2000. Spontaneous mammary carcinomas fail to induce an immune response in syngeneic FVBN202 neu transgenic mice. Int. J. Cancer. 87:688–694. [PubMed] [Google Scholar]
- 9.Di Carlo, E., M.G. Diodoro, K. Boggio, A. Modesti, M. Modesti, P. Nanni, G. Forni, and P. Musiani. 1999. Analysis of mammary carcinoma onset and progression in HER-2/neu oncogene transgenic mice reveals a lobular origin. Lab. Invest. 79:1261–1269. [PubMed] [Google Scholar]
- 10.Guy, C.T., M.A. Webster, M. Schaller, T.J. Parsons, R.D. Cardiff, and W.J. Muller. 1992. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. USA. 89:10578–10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanni, P., S.M. Pupa, G. Nicoletti, C. De Giovanni, L. Landuzzi, I. Rossi, A. Astolfi, C. Ricci, R. De Vecchi, A.M. Invernizzi, et al. 2000. p185(neu) protein is required for tumor and anchorage-independent growth, not for cell proliferation of transgenic mammary carcinoma. Int. J. Cancer. 87:186–194. [PubMed] [Google Scholar]
- 12.Rovero, S., A. Amici, E.D. Carlo, R. Bei, P. Nanni, E. Quaglino, P. Porcedda, K. Boggio, A. Smorlesi, P.L. Lollini, et al. 2000. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J. Immunol. 165:5133–5142. [DOI] [PubMed] [Google Scholar]
- 13.Ricci, C., L. Landuzzi, I. Rossi, C. De Giovanni, G. Nicoletti, A. Astolfi, S. Pupa, S. Menard, K. Scotlandi, P. Nanni, and P.L. Lollini. 2000. Expression of HER/erbB family of receptor tyrosine kinases and induction of differentiation by glial growth factor 2 in human rhabdomyosarcoma cells. Int. J. Cancer. 87:29–36. [PubMed] [Google Scholar]
- 14.Harvey, M.B., K.J. Leco, M.Y. Arcellana-Panlilio, X. Zhang, D.R. Edwards, and G.A. Schultz. 1995. Proteinase expression in early mouse embryos is regulated by leukaemia inhibitory factor and epidermal growth factor. Development. 121:1005–1014. [DOI] [PubMed] [Google Scholar]
- 15.Allione, A., M. Consalvo, P. Nanni, P.L. Lollini, F. Cavallo, M. Giovarelli, M. Forni, A. Gulino, M.P. Colombo, P. Dellabona, et al. 1994. Immunizing and curative potential of replicating and nonreplicating murine mammary adenocarcinoma cells engineered with interleukin (IL)-2, IL-4, IL-6, IL-7, IL-10, tumor necrosis factor α, granulocyte-macrophage colony-stimulating factor, and γ-interferon gene or admixed with conventional adjuvants. Cancer Res. 54:6022–6026. [PubMed] [Google Scholar]
- 16.Muller, W.J., C.L. Arteaga, S.K. Muthuswamy, P.M. Siegel, M.A. Webster, R.D. Cardiff, K.S. Meise, F. Li, S.A. Halter, and R.J. Coffey. 1996. Synergistic interaction of the Neu proto-oncogene product and transforming growth factor α in the mammary epithelium of transgenic mice. Mol. Cell Biol. 16:5726–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauter, B., M.L. Albert, L. Francisco, M. Larsson, S. Somersan, and N. Bhardwaj. 2000. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251–276. [DOI] [PubMed] [Google Scholar]
- 19.Voest, E.E., B.M. Kenyon, M.S. O'Reilly, G. Truitt, R.J. D'Amato, and J. Folkman. 1995. Inhibition of angiogenesis in vivo by interleukin 12. J. Natl. Cancer Inst. 87:581–586. [DOI] [PubMed] [Google Scholar]
- 20.Cavallo, F., E. Di Carlo, M. Butera, R. Verrua, M.P. Colombo, P. Musiani, and G. Forni. 1999. Immune events associated with the cure of established tumors and spontaneous metastases by local and systemic interleukin 12. Cancer Res. 59:414–421. [PubMed] [Google Scholar]
- 21.Katsumata, M., T. Okudaira, A. Samanta, D.P. Clark, J.A. Drebin, P. Jolicoeur, and M.I. Greene. 1995. Prevention of breast tumour development in vivo by downregulation of the p185neu receptor. Nat. Med. 1:644–648. [DOI] [PubMed] [Google Scholar]
- 22.Klapper, L.N., N. Vaisman, E. Hurwitz, R. Pinkas-Kramarski, Y. Yarden, and M. Sela. 1997. A subclass of tumor-inhibitory monoclonal antibodies to ErbB-2/HER2 blocks crosstalk with growth factor receptors. Oncogene. 14:2099–2109. [DOI] [PubMed] [Google Scholar]
- 23.Boggio, K., E. Di Carlo, S. Rovero, F. Cavallo, E. Quaglino, P.L. Lollini, P. Nanni, G. Nicoletti, S. Wolf, P. Musiani, and G. Forni. 2000. Ability of systemic interleukin-12 to hamper progressive stages of mammary carcinogenesis in HER2/neu transgenic mice. Cancer Res. 60:359–364. [PubMed] [Google Scholar]
- 24.Yakirevich, E., O.B. Izhak, G. Rennert, Z.G. Kovacs, and M.B. Resnick. 1999. Cytotoxic phenotype of tumor infiltrating lymphocytes in medullary carcinoma of the breast. Mod. Pathol. 12:1050–1056. [PubMed] [Google Scholar]
- 25.Clemente, C.G., M.C. Mihm, Jr., R. Bufalino, S. Zurrida, P. Collini, and N. Cascinelli. 1996. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 77:1303–1310. [DOI] [PubMed] [Google Scholar]
- 26.Kacha, A.K., F. Fallarino, M.A. Markiewicz, and T.F. Gajewski. 2000. Spontaneous rejection of poorly immunogenic P1.HTR tumors by Stat6-deficient mice. J. Immunol. 165:6024–6028. [DOI] [PubMed] [Google Scholar]
- 27.Shankaran, V., H. Ikeda, A.T. Bruce, J.M. White, P.E. Swanson, L.J. Old, and R.D. Schreiber. 2001. IFN-γ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 410:1107–1111. [DOI] [PubMed] [Google Scholar]
- 28.Cavallo, F., E. Quaglino, L. Cifaldi, E. Di Carlo, A. Andre, P. Bernabei, P. Musiani, G. Forni, and R.A. Calogero. 2001. Interleukin 12-activated lymphocytes influence tumor genetic programs. Cancer Res. 61:3518–3523. [PubMed] [Google Scholar]
- 29.Landolfo, S., F. Cofano, M. Giovarelli, M. Prat, G. Cavallo, and G. Forni. 1985. Inhibition of interferon-γ may suppress allograft reactivity by T lymphocytes in vitro and in vivo. Science. 229:176–179. [DOI] [PubMed] [Google Scholar]
- 30.Germann, T., M. Bongartz, H. Dlugonska, H. Hess, E. Schmitt, L. Kolbe, E. Kolsch, F.J. Podlaski, M.K. Gately, and E. Rude. 1995. Interleukin-12 profoundly up-regulates the synthesis of antigen- specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur. J. Immunol. 25:823–829. [DOI] [PubMed] [Google Scholar]
- 31.Rodolfo, M., C. Melani, C. Zilocchi, B. Cappetti, E. Luison, I. Arioli, M. Parenza, S. Canevari, and M.P. Colombo. 1998. IgG2a induced by interleukin (IL) 12-producing tumor cell vaccines but not IgG1 induced by IL-4 vaccine is associated with the eradication of experimental metastases. Cancer Res. 58:5812–5817. [PubMed] [Google Scholar]
- 32.Beatty, G.L., and Y. Paterson. 2000. IFN-γ can promote tumor evasion of the immune system in vivo by down-regulating cellular levels of an endogenous tumor antigen. J. Immunol. 165:5502–5508. [DOI] [PubMed] [Google Scholar]
- 33.Lollini, P.-L., M.C. Bosco, F. Cavallo, C. De Giovanni, M. Giovarelli, L. Landuzzi, P. Musiani, A. Modesti, G. Nicoletti, G. Palmieri, et al.1993. Inhibition of tumor growth and enhancement of metastasis after transfection of the γ-interferon gene. Int. J. Cancer. 55:320–329. [DOI] [PubMed] [Google Scholar]