Abstract
T cell receptor interactions with peptide/major histocompatibility complex (pMHC) ligands control the selection of T cells in the thymus as well as their homeostasis in peripheral lymphoid organs. Here we show that pMHC contact modulates the expression of CD5 by naive CD4 T cells in a process that requires the continued expression of p56lck. Reduced CD5 levels in T cells deprived of pMHC contact are predictive of elevated Ca2+ responses to subsequent TCR engagement by anti-CD3 or nominal antigen. Adaptation to peripheral pMHC contact may be important for regulating naive CD4 T cell responsiveness.
Keywords: T cell receptor repertoire, T cell responsiveness, CD5, MHC contact, p56lck
Introduction
The fate of immature CD4+CD8+ double positive thymocytes is controlled by TCR interactions with self-peptide/MHC (pMHC)* in the thymus. Cells with overtly self-reactive TCRs are eliminated (negative selection; references 1 and 2) and a fraction of moderately self-reactive cells differentiate to the mature CD4+ or CD8+ single positive (SP) stage (positive selection; references 2 and 3). The latter undergo a series of preprogrammed events during which their responsiveness to selecting pMHC ligands is thought to change (4–7). This involves the regulated expression of CD5, a putative negative regulator of lymphocyte receptor signaling (8–15), and the tyrosine phosphatase 1 (16) which associates inducibly with an immunoreceptor tyrosine-based inhibitory motif in the cytoplasmic domain of CD5 (10, 11). Thymocytes (8), mature T cells (12), and B1 B cells (9) have all been shown to be hyperresponsive to TCR/BCR signaling in CD5-deficient mice. Although T cells develop in normal numbers in CD5-deficient mice (17, 18) the TCR repertoire selected in the thymus is affected both by the absence (8, 12, 18) and the overexpression of CD5 (14).
High level CD5 expression is an early indicator of TCR engagement at the double positive stage and associated with entry into the selection process (2, 14, 15, 19–21). The precise level of CD5 expressed by TCR transgenic thymocytes is determined by the nature of TCR/pMHC interactions driving thymocyte differentiation to the single positive stage, with interactions deemed to be ‘stronger’ driving higher levels of expression (14, 15, 20). Reminiscent of adaptation events in sensory receptors (22, 23), pMHC encounter has been shown to alter thymocyte responsiveness to TCR-mediated signals (15). CD5 levels were inversely correlated with thymocyte responsiveness in these experiments (15). These studies have led to proposals that the developmentally regulated expression of CD5 is significant for setting TCR response thresholds appropriate for the spectrum of pMHC complexes encountered by developing thymocytes and the establishment of tolerance via the fine-tuning of thymocyte responsiveness to self-pMHC complexes (14, 15). It has been assumed that CD5 levels and activation thresholds are set in the thymus ‘once and for all’ and subsequently maintained by naive peripheral T cells in the absence of TCR engagement (20). In this view, sensory adaptation would be limited to pMHC complexes encountered during thymic selection. However, TCR/pMHC interactions continue in the periphery, where they promote the long-term survival (24–29) and/or homeostasis of naive T lymphocytes (30–35). This role of pMHC in the maintenance of the naive peripheral T cell repertoire, together with evidence that not all thymic and peripheral pMHC complexes are the same (36) and data that LN T cells express more diverse CD5 levels than single positive thymocytes (unpublished data and see below) led us to explore an alternative hypothesis, namely that CD5 levels and T cell responsiveness may remain adaptable to signals received in the periphery. Here we show that CD5 expression by naive CD4 T cells is regulated by MHC contact. Interestingly, reduced CD5 levels in T cells deprived of MHC contact are associated with elevated Ca2+ responses to subsequent TCR engagement. Hence, naive CD4 T cells appear to undergo a process akin to sensory adaptation. In addition, we identify p56lck as a critical component of the mechanism by which CD5 expression is adjusted to reflect TCR/pMHC contact in CD4 T cells.
Materials and Methods
Cell Culture
Cells from the superficial inguinal, brachial, and cervical LN of AND TCR transgenic (37) recombinase activating gene (Rag)1o/o (38) mice were depleted of MHC class II (2G9, AF6–120.1, or 14–4-4S), CD11b (M1/70), CD11c (HL3), and CD16 (2.4G2) expressing cells using biotinylated antibodies (BD PharMingen) and SA-coated immunomagnetic beads (Dynal) and maintained either in suspension or in reaggregate cultures established as described previously (19) from thymic stromal cells (prepared by trypsinization of deoxyguanosine-treated fetal thymi and centrifugation over 55% Percoll; Amersham Pharmacia Biotech), bone marrow (BM)–derived APCs (obtained by culture in GM-CSF–containing medium as described previously; reference 39), and LN cells at a ratio of 3:1:10 on Nucleopore filters (0.8 μm; Costar) floating on IMDM (10% heat inactivated FCS, 2 × 10−5 M 2-ME, 50 μg/ml gentamycin).
Flow Cytometry and Cell Sorting
Cells were counted and stained with CD4-PE (Caltag), -PerCP or -allophycocyanin, CD5-FITC or -PE, TCR Vβ3-FITC or Vα11-FITC, CD45RB-FITC, CD44-FITC, or CD69-FITC (BD PharMingen) and analyzed on a FACScalibur™ (Becton Dickinson) as described previously (19).
For Ca2+ assays, cultured cells were first incubated for 20 min at room temperature in PBS, 2mM Ca2+, 2.5 μM Fluo-4 a.m. (Molecular Probes), and CD4-allophycocyanin (BD PharMingen). This was followed (where indicated) by incubation on ice with the specified dilution of KT3 (anti-CD3ε; reference 40) supernatant titrated to be saturating at 1/56. Samples were analyzed on a FACScalibur™ (Becton Dickinson) in a 37°C sample jacket immediately after addition of goat anti–rat Ig (20 μg/ml final concentration; Dako). Alternatively, Fluo-4–loaded AND T cells were mixed 1:1 with B10.BR (H-2k) BM–derived APCs which where indicated had been pulsed for 2 h with 10 μg/ml of the cognate pigeon cytochrome-C peptide 81–104 or the variant peptides K99R or K99A (41). Samples were acquired for 1–2 min, pelleted for 10 s (13,000 rpm) in a microfuge to provide T cell/APC contact, and moved back to the flow cytometer. For Ca2+ assays ex vivo, LN cells were stained with anti-CD44 (IM7; BD PharMingen) followed by anti–rat IgG-Cy5 (Caltag), and, after blocking with rat serum, CD4-PerCP, and CD5-PE (BD PharMingen). The cells were then loaded with Fluo-4 as above and analyzed on a FACScalibur™ (Becton Dickinson) in a 37°C sample jacket immediately after addition of goat anti–rat Ig (20 μg/ml final concentration; Dako). Ca2+ data were analyzed using FCSPress software (Ray Hicks, FCSPress, Cambridge, UK).
For the isolation of CD5low and CD5high naive-phenotype CD4 T cells, suspensions of superficial inguinal, brachial, and cervical LNs of specific pathogen free C57BL/10 (H2b) or Balb/c (H2d) mice were depleted of CD8- and B220-expressing cells with biotinylated antibodies and SA-coated immunomagnetic beads (Dynal), stained with CD4-tricolor (Caltag), CD45RB-FITC or CD44-FITC (BD PharMingen), sorted on a FACSVantage™ (Becton Dickinson), labeled with 1 μg/ml CFSE (Molecular Probes) for 10 min at room temperature, washed twice in PBS 0.1% BSA and injected intraveneously (1–4 × 106 cells per mouse) into sublethally (450 rad) irradiated syngeneic or untreated Rag1o/o Aβo/o β2mo/o mice (38, 42, 43), referred to below as Rag1o/o MHCo/o.
Western Blot Analysis
Cell lysates were separated (5 × 105–106 T cell equivalents per lane) on 12% SDS polyacrylamide gels and blotted onto PVDF membranes (NEN Life Science Products). Blots were blocked (1% BSA in PBS, 0.1% Tween 20), probed with the anti-phosphotyrosine mAb 4G10 (1 μg/ml, 0.1% BSA; Upstate Biotechnology) followed by alkaline phosphatase-conjugated goat anti–mouse IgG and enhanced chemoluminescence detection (Western Star) on MR-1 film (Kodak). Blots were stripped (200 mM glycine, 1% Tween-20, 0.1% SDS, pH 2.2) and reprobed with anti-CD3 ζ (Santa Cruz Biotechnology, Inc.).
Results
Maintenance of CD5 Expression Levels on Naive Peripheral CD4 T Cells Requires pMHC Contact
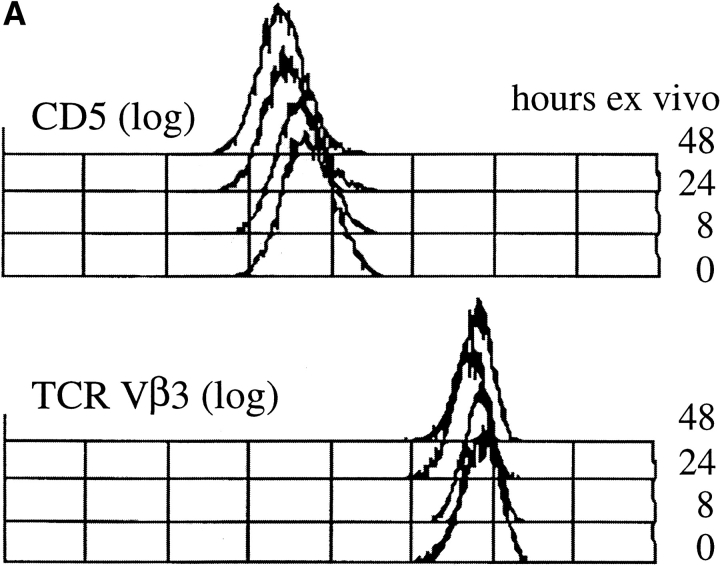
APC-depleted CD4 LN T cells from unprimed AND TCR transgenic mice (37) deficient in Rag1 (38) were employed as a near homogeneous population of naive peripheral T cells with uniform TCR specificity (37, 41). While CD5 expression increased dramatically when AND LN cells were cultured under conditions of TCR engagement (data not shown), CD5 levels declined to 50% or less of the ex vivo levels when these cells were cultured for 24 to 48 h in the absence of TCR stimulation. This downregulation was selective for CD5 since the expression of CD4 and TCR remained unchanged or increased slightly during the culture period (Fig. 1 A). CD5 downregulation was accompanied by a reduction of protein tyrosine phosphorylation relative to the level in resting T cells freshly isolated ex vivo, including p21 phospho-ζ (33, 44; Fig. 1 B).
Figure 1.
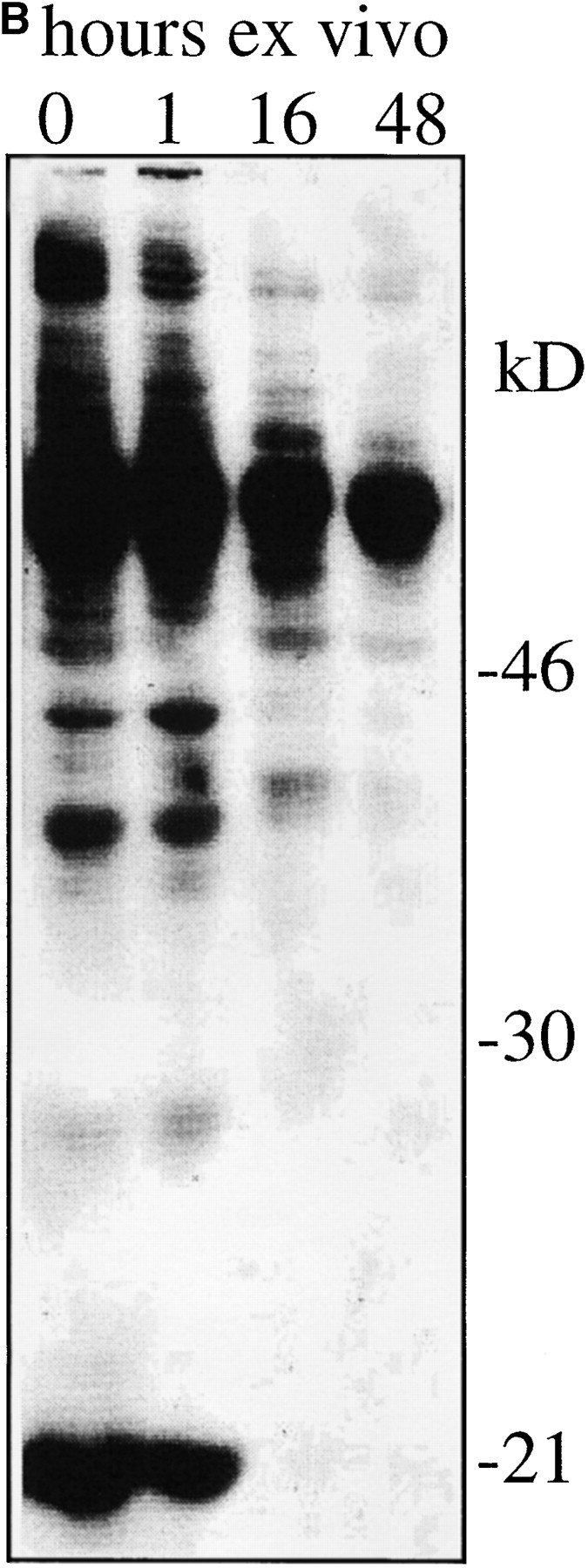
Loss of CD5 expression levels and basal tyrosine phosphorylation from naive CD4 LN cells maintained in vitro without TCR stimulation. Suspension cultures of APC-depleted Rag1o/o AND TCR transgenic LN cells (see Materials and Methods) were analyzed (A) for expression of TCR Vβ3 (the AND β chain) and CD5 by flow cytometry (histogram overlays are on a log scale and gated on CD4+ cells) and (B) for tyrosine phosphorylation status by Western blot analysis of total cell lysates with anti phosphotyrosine mAb. Note the decline of CD5 and tyrosine phosphorylation levels over time.
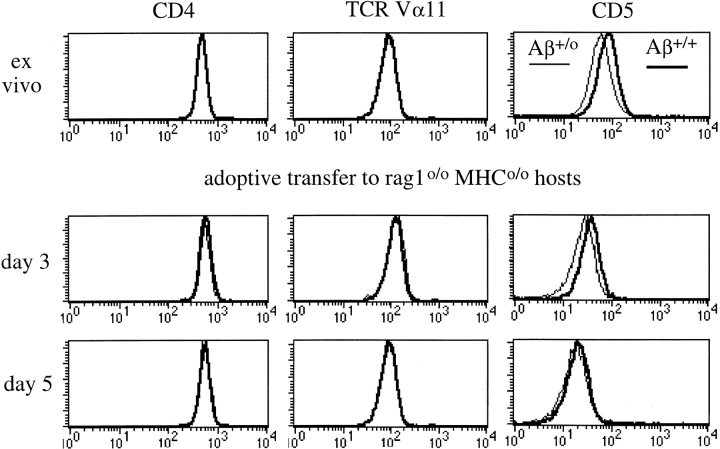
These data suggest that CD5 expression by peripheral CD4 T cells requires active maintenance, but they do not distinguish between a requirement for MHC contact or other factors (such as lymphokines; references 45 and 46) encountered in vivo. To discriminate between these possibilities we generated CD4 T cells that had been selected on different doses of the same pMHC ligands and followed their CD5 levels after transfer into deficient hosts. CD5 levels on Rag1o/o AND TCR transgenic CD4 cells reflected MHC class II dosage and was higher in Aβ+/+ mice than in Aβ+/o mice (mean fluorescence intensity 119 for Aβ+/+ versus 96 for Aβ+/o; n = 8; see Fig. 2 ex vivo). To ask whether CD5 levels are stable in vivo in the absence of MHC class II we adoptively transferred Rag1o/o AND CD4 T cells from Aβ+/+ and Aβ+/o donors into Rag1o/o MHCo/o hosts. 3 d after transfer, AND TCR transgenic CD4 cells showed reduced CD5 expression, but CD5 levels on cells from Aβ+/+ and Aβ+/o donors were still distinguishable (Fig. 2, d 3). 5 d after transfer CD5 expression by both populations was four to fivefold lower than on the day of transfer (compare Fig. 2 ex vivo and d 5). CD5 downregulation was selective (levels of CD4 and the transgenic TCR were not reduced) and occurred in the absence of early apoptotic markers as there was no increase in reactive oxygen species or loss of mitochondrial membrane potential (reference 47; data not shown). These data demonstrate that (i) CD5 expression declines on transfer of Rag1o/o AND TCR transgenic CD4 cells into MHCo/o Rag1o/o hosts and that (ii) the differences in CD5 levels found between Rag1o/o AND TCR transgenic CD4 cells selected on one or two doses of Ab (i.e., in Aβ+/o and Aβ+/+ mice, respectively) disappear on transfer into MHCo/o Rag1o/o hosts. We conclude that CD5 expression levels in naive peripheral CD4 T cells reflect continued MHC contact.
Figure 2.
Maintenance of CD5 expression levels on naive peripheral CD4 T cells requires MHC contact. 107 APC-depleted Rag1o/o AND TCR transgenic LN cells from Aβ+/+ (bold) or Aβ+/o (thin line) donors were transferred intravenously to Rag1o/o MHCo/o hosts and host LN cells were analyzed for CD4, TCR Vα11 (the AND α chain), and CD5 by flow cytometry at the indicated times (histogram overlays are gated on CD4+ cells).
Continued Expression of p56lck Is Required for the Maintenance of CD5 Levels by Peripheral CD4 T Cells
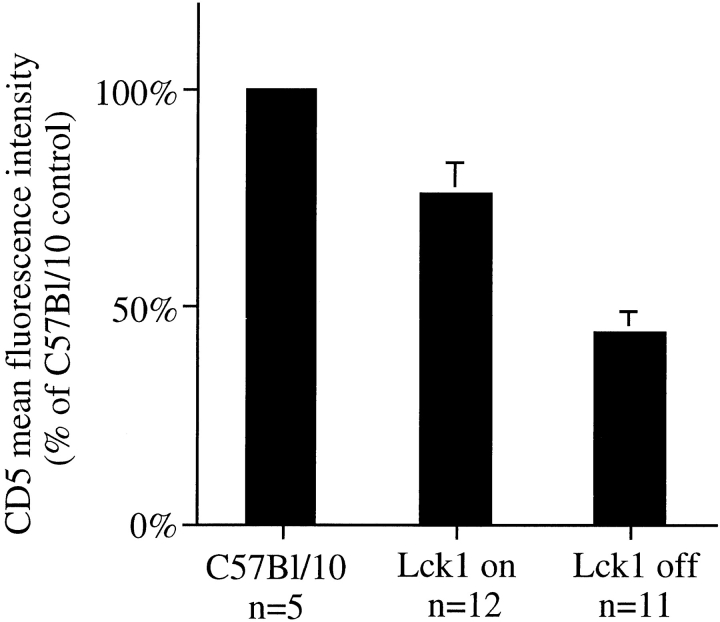
To begin to elucidate the mechanisms that maintain CD5 in peripheral CD4 T cells we made use of a recently described mouse model, Lck1/rtTA-C/lckneg, that allows the regulated expression of p56lck by reconstituting lck-deficient mice with a p56lck transgene under the control of the tetracyclin response element responsive to the reverse tetracyclin transactivator (48). lck expression is controlled by administration of the tetracycline derivative doxycycline. In the presence of doxycycline, lck expression in Lck1/rtTA-C/lckneg mice is higher than wild-type in the thymus but lower in peripheral T cells. Withdrawal of the drug results in the loss of lck expression. The resulting lck-deficient T cells show impaired homeostatic proliferation but normal long-term survival and, surprisingly, normal levels of basal CD3 ζ phosphorylation (48). We asked whether loss of lck expression was accompanied by a decrease of CD5 levels on CD4 T cells and found a decrease to ∼50% of the levels in wild type mice, compared with ∼80% in doxycycline-fed mice (Fig. 3) . We conclude that, in contrast to basal ζ chain phosphorylation (48), the maintenance of CD5 levels on peripheral CD4 T cells requires the continued expression of lck.
Figure 3.
Continued expression of lck is required for the maintenance of CD5 levels on naive peripheral CD4 T cells. CD5 expression by CD4 T cells from C57Bl/10 (wild-type), Lck1/rtTA-C/lckneg mice given doxycyclin, and Lck1/rtTA-C/lckneg mice taken off doxycyclin 12 wk earlier were analyzed by flow cytometry.
MHC Haplotype-specific Modulation of CD5 Levels
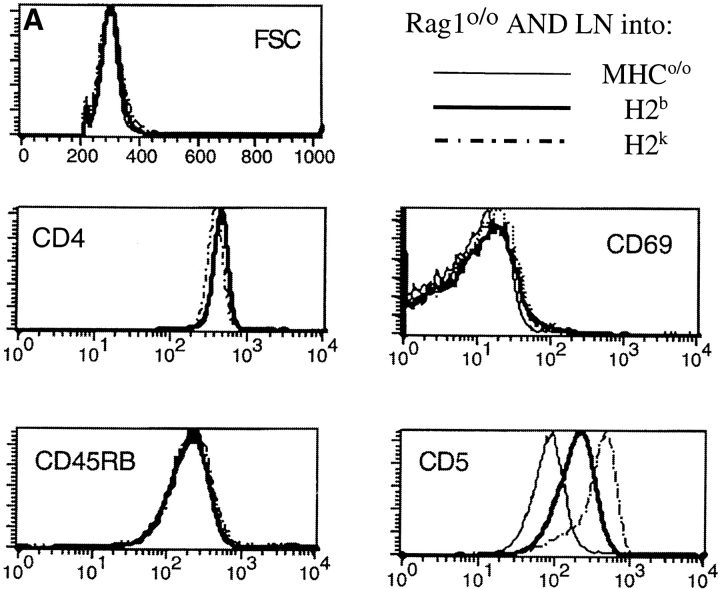
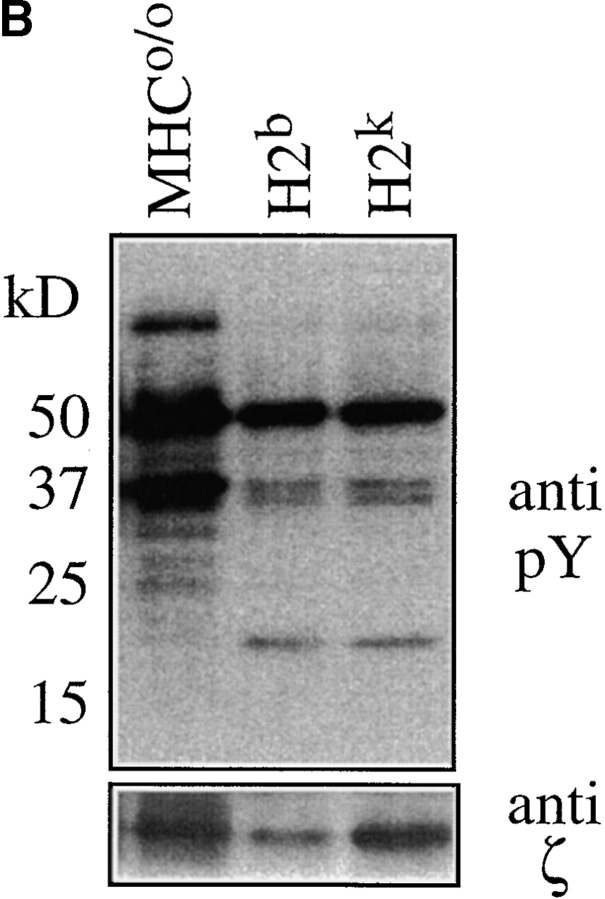
The data so far demonstrate that MHC contact and lck are required to sustain CD5 levels on naive peripheral CD4 T cells but they do not distinguish between TCR and coreceptor involvement in the regulation of CD5 expression. To approach this issue we made use of the fact that AND TCR transgenic T cells are positively selected on Ab as well as Ek, the latter being considered to provide ‘stronger’ positive selection (41). We adopted an in vitro 3-D reaggregate culture system (49) which allowed us to maintain naive AND CD4 T cells in an environment manipulated to either lack MHC expression or to express either of the two selecting MHC haplotypes, H-2b or H-2k. Rag1o/o H2bxk AND TCR transgenic naive CD4 LN cells were placed in reaggregate culture with a combination of thymic stroma cells (depleted of endogenous lymphoid cells by treatment with deoxyguanosine) and BM-derived APCs (39) prepared from MHCo/o, H2b (C57BL/10), or H2k (B10.BR) mice. 3 d later, although most cells maintained their initial phenotypic characteristics, CD5 levels varied according to MHC exposure. The lowest levels were evident on CD4 cells deprived of MHC interactions, intermediate levels on cells maintained in an H-2b expressing environment, and the highest levels on cells maintained in the presence of H-2k (Fig. 4) (neither H2b thymic stroma nor H2b BM-derived APCs on their own were able to sustain CD5 expression; data not shown). Basal ζ chain phosphorylation was lost in the absence of MHC, but maintained by either of the selecting MHC haplotypes (Fig. 4). These data show that CD5 expression by naive peripheral T cells is subject to haplotype-specific regulation. As described previously for the regulation of CD5 levels during thymocyte development (19, 20), this result suggests an involvement of specific TCR recognition over and above coreceptor engagement in the regulation of CD5 expression by peripheral T cells.
Figure 4.
Modulation of CD5 levels by environmental MHC expression and haplotype. APC-depleted Rag1o/o H2bxk AND TCR transgenic LN cells were placed in 3-D reaggregate culture with a combination of thymic stroma and BM-derived APCs (reference 39) prepared from MHCo mice (thin line) or from mice expressing one of two selecting MHC haplotypes, H2b (C57BL/10, bold line), or H2k (B10.BR, dashed line). 3 d later cell recovery was between 60 and 80% of input for all MHC haplotypes (data not shown) and cells were analyzed (A) for forward scatter, CD4, CD45RB, CD69, and CD5 expression by flow cytometry (histogram overlays are gated on CD4 cells; the brighter CD5 staining in this and subsequent figures is due to the use of PE-conjugated mAb instead of the FITC conjugates used in previous figures) and (B) for tyrosine phosphorylation status by Western blot analysis of total cell lysates with anti-phosphotyrosine mAb. The blot was stripped and reprobed for CD3 ζ protein.
Tuning CD4 T Cell Responsiveness by Environmental MHC Class II Expression
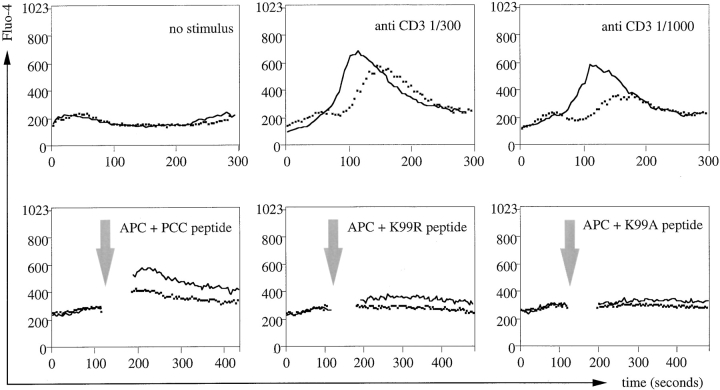
As CD5 is implicated as a modifier of lymphocyte receptor signaling (see Introduction) we were interested in comparing the functional properties of naive T cells expressing the same TCR but different levels of CD5 as the result of environmental MHC exposure. To this end, Rag1o/o AND LN CD4 T cells were placed in an environment either devoid of MHC expression or expressing H-2k, using the 3-D reaggregate system described in Fig. 4. After 3 d the cells were recovered and loaded with the Ca2+ indicator dye Fluo-4 to compare their responsiveness to TCR mediated stimuli. These were provided either by subsaturating concentrations of anti-CD3 followed by cross-linking (Fig. 5 top, cross-linking antibody added at 0 s) or by contact with peptide-loaded BM-derived APCs (Fig. 5, bottom). CD4 T cells that had been deprived of MHC contact (and as a result displayed reduced CD5 levels, see Fig. 4) responded more vigorously than cells from the same donor that had been maintained in an MHC+ environment.
Figure 5.
Tuning of naive CD4 T cell responsiveness by MHC contact. APC-depleted Rag1o/o Hk AND TCR transgenic LN cells were maintained in 3-D reaggregate culture as in Fig. 4 either in an MHCo/o (black line) or H2k (B10.BR) environment (dashed line). After 3 d cells were loaded with the Ca2+ indicator Fluo-4 and stimulated with subsaturating concentrations of anti-CD3 followed by the addition of cross-linking antibody at 0 s (top) or BM-derived APCs pulsed with the cognate pigeon cytochrome-C peptide 81–104 or the variant peptides K99R or K99A (bottom, the arrow indicates centrifugation of the sample to provide T cell/APC contact, see Materials and Methods).
Polyclonal CD5low Naive CD4 T Cells Are Hyperresponsive In Vitro but Less Effective at MHC-dependant Homeostatic Expansion than their CD5high Counterparts
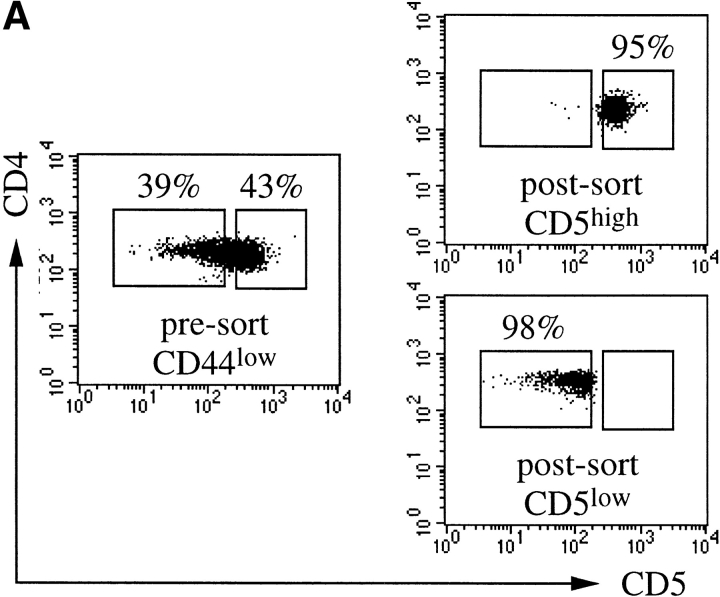
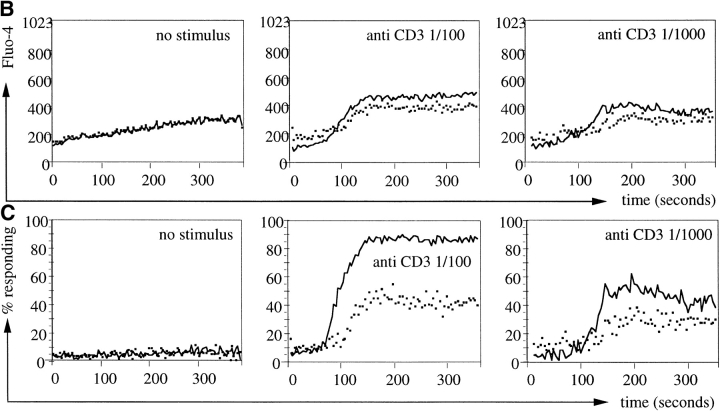
To ask whether CD5 expression levels are predictive of CD4 T cell responsiveness also in vivo, we separated polyclonal LN cells isolated from wild-type mice into CD4+CD44lowCD5low and CD4+CD44lowCD5high populations (Fig. 6 A) and compared their responsiveness to anti-CD3 followed by cross-linking (as in Fig. 5, top). The CD4+CD44lowCD5low population responded to CD3 ligation slightly but consistently better than CD4+CD44lowCD5high cells as reflected in a larger differential between resting and activated Ca2+ levels in CD5low cells (Fig. 6 B) and in a higher calculated frequency of responding cells (Fig. 6 C).
Figure 6.
Polyclonal CD5low CD4 LN cells are hyperresponsive to CD3 ligation. Freshly isolated polyclonal LN cells were stained for CD4, CD5, and CD44 and separated into CD4+CD44lowCD5low and CD4+CD44lowCD5high populations by cell sorting (A). LN cells were loaded with Fluo-4, and incubated with subsaturating concentrations of anti-CD3. Ca2+ responses were recorded immediately after cross-linking. Response traces by CD4+CD44low CD5low (black line) and CD4+CD44low CD5high (dashed line) cells are overlayed for comparison. Note the larger differential between resting and activated Ca2+ levels in CD5low cells (B) and the higher frequency of responding cells within the in CD5low population (C).
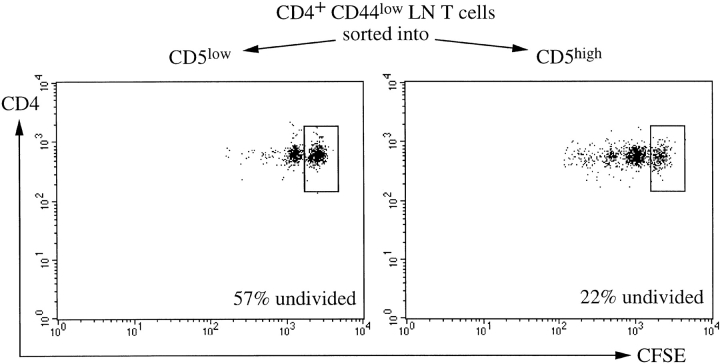
Hence, as shown above for AND T cells deprived of pMHC interactions, CD5 expression is predictive of responsiveness to anti-CD3 also in polyclonal naive CD4 T cells. We performed adoptive transfer experiments to investigate the role of MHC interactions in the regulation of CD5 expression in polyclonal CD4 T cells and to compare the ability of the CD44lowCD5low and CD44lowCD5high subsets to proliferate in sublethally irradiated syngeneic hosts or Rag-1o/o MHCo/o mice. As a test for their ability to engage in productive interactions with syngeneic pMHC ligands 30–32, 35) sorted CD4+ CD44low CD5high and CD5low populations of C57Bl/10 or Balb/c LN cells were labeled with CFSE and transferred into sublethally (450 rad) irradiated syngeneic hosts. 9–12 d after transfer LN and spleens were recovered and donor cells identified as CFSE+ CD4+. Both populations had maintained their initial CD5 levels (Table I). Analysis of cell division, quantitated as the dilution of CFSE, showed that only a minority of 20–25% of CD5high cells remained undivided whereas in excess of 50% of CD5low cells had failed to divide (Fig. 7 , Table I). The observation that CD5low cells proliferate poorly to syngeneic pMHC contact despite their elevated responsiveness to CD3-mediated receptor ligation (Figs. 5 and 6) might at first seem counterintuitive but is in fact predicted if, as suggested by the data in Figs. 2 and 4, low CD5 levels reflect poor self-pMHC interactions (see Discussion). We controlled for the possibility that the different rates of division after transfer into syngeneic hosts were due to underlying differences in the proliferative response of CD5high and CD5low naive CD4 T cells to MHC-independent factors in highly lymphopenic hosts (45, 46) by transfer of CD5high and CD5low naive CD4 T cells into Rag-1o/o MHCo/o mice. In this setting both populations responded with comparable rates of proliferation and CD5 expression by both populations was reduced two to threefold (Table I). Hence, deprivation from pMHC interactions results in reduced CD5 expression in polyclonal naive CD4 T cells. Consistent with a role for pMHC interactions in regulating CD5 expression by naive polyclonal CD4 T cells, the CD5low fraction of the naive CD4 T cell repertoire has limited ability to interact productively with syngeneic pMHC as measured by homeostatic proliferation in syngeneic hosts.
Table I.
Cell Division and CD5 Expression after Adoptive Transfer into Sublethally Irradiated Syngeneic or Rag-1o/o MHCo/o Hosts
| Transferredpopulation | Host | Percentageundividedmean ± SD | CD5expressionmean ± SD |
|---|---|---|---|
| n | n | ||
| CD5low | syngeneic | 54.3 ± 9.8 (14) | 333 ± 74 (13) |
| CD5high | syngeneic | 25.3 ± 10.8 (11) | 1131± 165 (10) |
| CD5low | Rag1o/o MHCo/o | 25.5 ± 9.5 (3) | 140 ± 27 (3) |
| CD5high | Rag1o/o MHCo/o | 23.1 ± 7.7 (3) | 429 ± 116 (3) |
Polyclonal LN cells were separated into CD4+CD44lowCD5low and CD4+CD44lowCD5high populations, labeled with CFSE and 1–4 × 106 cells of each population were adoptively transferred either into 450 rad irradiated (i.e., moderately lymphopenic) syngeneic, or into Rag-1o/o MHCo/o (i.e., highly lymphopenic) hosts. Cell division and CD5 levels were recorded 9–12 d later. Most CD4+CD44lowCD5low as well as CD4+CD44lowCD5high cells undergo one or two cell divisions in Rag-1o/o MHCo/o hosts, presumably driven by factors present in the highly lymphopenic environment.
Figure 7.
Polyclonal CD5low CD4 LN cells show impaired MHC-dependent homeostatic expansion relative to their CD5high counterparts. Polyclonal LN cells were separated into CD4+CD44lowCD5low and CD4+CD44low CD5high populations by cell sorting as in Fig. 6 A. After labeling with CFSE 1–4 × 106 cells of each population were adoptively transferred into sublethally irradiated syngeneic hosts. Proliferation was recorded as reduced CFSE fluorescence intensity 9 d later. Displays are gated on CD4+ CFSE+ cells. The percentage of undivided cells is indicated.
Discussion
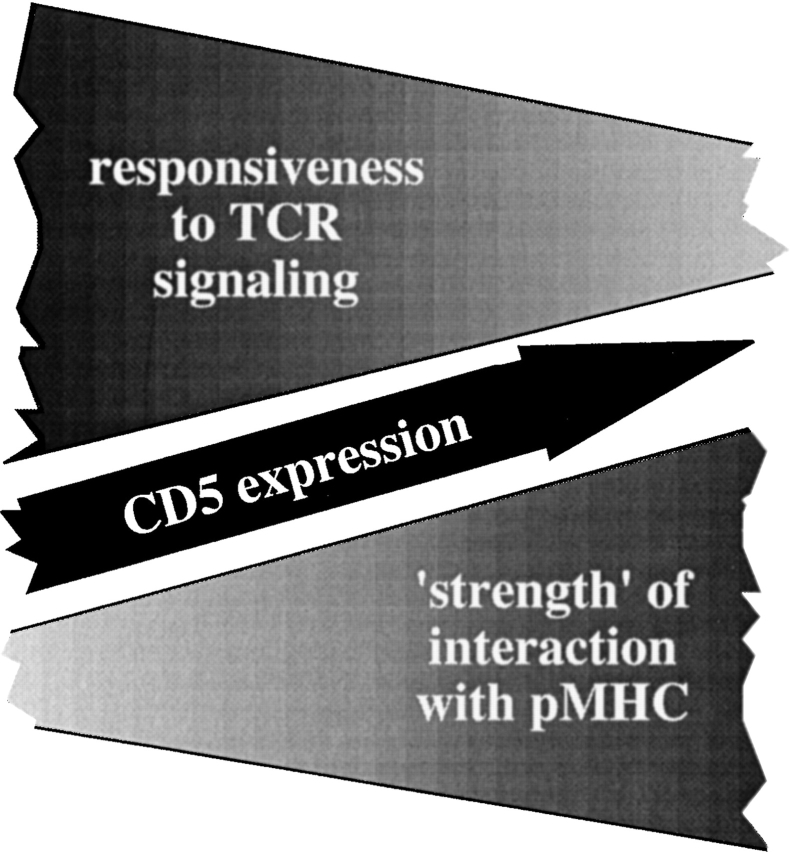
The data presented here show that CD5 levels on naive peripheral CD4 T cells are maintained by an active process which requires the availability of pMHC ligands on the part of the environment and the continued expression of p56lck on the part of the T cell. Reduced CD5 levels are predictive of elevated Ca2+ responses to TCR engagement in TCR transgenic CD4 T cells experimentally deprived of MHC contact as well as in unmanipulated polyclonal naive CD4 T cell populations. Despite this elevated responsiveness to TCR engagement by the surrogate ligand anti-CD3, naive polyclonal CD5low CD4 T cells proliferate poorly when their interaction with self-pMHC is tested by transfer into sublethally irradiated syngeneic hosts. As homeostatic proliferation in this experimental setting is promoted by pMHC recognition (30–32, 35) and we have shown that naive CD4 T cells reduce CD5 expression when experimentally deprived of MHC contact, the data are consistent with a model where CD5 levels reflect productive interactions with self-pMHC. Within a polyclonal repertoire, some naive CD4 T cells will engage self pMHC ligands more avidly than others. According to the model presented in Fig. 8 , the former become (or remain) CD5high and display dampened Ca2+ responses to anti-CD3 while the latter become (or remain) CD5low and display elevated Ca2+ responses.
Figure 8.
A model for sensory adaptation in naive CD4 T cells, illustrating the possible relationship between TCR engagement, CD5 expression, and responsiveness to TCR ligation. According to this model, naive CD4 T cells that interact more strongly than others with self-pMHC express higher levels of CD5. Their responsiveness to subsequent TCR ligation is dampened. The boundaries of the model remain to be defined. To the left, persistent lack of pMHC interactions may result in the loss of the cell from the naive peripheral T cell pool. To the right, excessive chronic stimulation may be accompanied by the loss of the naive T cell phenotype (reference 52).
Gene targeting and overexpression studies implicate CD5 as a negative regulator of lymphocyte receptor signaling (see Introduction) but CD5 levels may not be the only factor that modulates the responsiveness of naive CD4 T cells deprived of or subjected to pMHC interactions. For example, as a result of pMHC contact in vivo naive T cells have partially phosphorylated CD3 ζ chains (33, 50) which can affect T cell activation (51), and this basal level of phosphorylation is reduced on MHC deprivation (references 33 and 50; this study). The dissociation between reduced CD5 levels and maintained CD3 ζ phosphorylation in conditionally lck-deficient CD4 T cells does not clarify this relationship since lck deficiency itself impairs T cell activation (48). The cytoplasmic domain of CD5 has been shown to interact not only with tyrosine phosphatase 1 (via an immunoreceptor tyrosine-based inhibitory motif around tyrosine residue 378) but also the protein kinases p56lck and p59fyn as well as phosphatidylinositol (PI)3-K (via an imperfect immunoreceptor tyrosine-based activation motif around tyrosine residues 429 and 441; references 10, 11, 13, and 16), indicating the potential for a more complex role of CD5 in modulating lymphocyte receptor signaling. Regardless of the mechanistic involvement of CD5, our results suggest that pMHC contact in peripheral lymphoid organs, in addition to its role in T cell homeostasis, can fine-tune T cell responsiveness (Fig. 8).
Receptor editing, receptor revision, and hypermutation play important roles in B cell biology at the price of risking the loss of receptor expression due to nonproductive secondary rearrangements or the introduction of premature stop codons (52, 53). This provides a clear rationale for the dependence of B cell survival on the continued expression of a functional BCR (54, 55). In mature T lineage cells alterations of receptor expression occur rarely (56, 57) and are of uncertain significance (52). Nevertheless, long-term survival and homeostasis of naive peripheral T cells is conditional to continued feedback through the TCR (24–35). It is attractive to think that this requirement may be more than evolutionary baggage carried by T cells on behalf of their B cell relatives. If, as our results indicate, pMHC contact continually modulates naive peripheral T cell responsiveness, a requirement for such contact could contribute to the maintenance of tolerance as suggested earlier on theoretical grounds (58). Such a scenario would extend the control of T cell responsiveness from thymic to peripheral pMHC.
Acknowledgments
We thank Drs. S. Elliot and A. Sardini for their valuable suggestions.
This work was supported by Medical Research Council UK (to K. Smith, B. Seddon, R. Zamoyska, A.G. Fisher, and M. Merkenschlager).
Footnotes
Abbreviations used in this paper: BM, bone marrow; pMHC, peptide/MHC; RAG, recombinase activating gene.
References
- 1.Kappler, J.W., N. Roehm, and P. Marrack. 1987. T cell tolerance by clonal elimination in the thymus. Cell. 49:273–280. [DOI] [PubMed] [Google Scholar]
- 2.Kisielow, P., and H. von Boehmer. 1995. Development and selection of T cells: facts and puzzles. Adv. Immunol. 58:87–209. [DOI] [PubMed] [Google Scholar]
- 3.Jameson, S.C., K.A. Hogquist, and M.J. Bevan. 1995. Positive selection of thymocytes. Annu. Rev. Immunol. 13:93–126. [DOI] [PubMed] [Google Scholar]
- 4.Sebzda, E., T.M. Kündig, C.T. Thomson, K. Aoki, S.Y. Mak, J.P. Mayer, T. Zamborelli, S.G. Nathenson, and P.S. Ohashi. 1996. Mature T cell reactivity altered by peptide agonist that induces positive selection. J. Exp. Med. 183:1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davey, G.M., S.L. Schober, B.T. Endrizzi, A.K. Dutcher, S.C. Jameson, and K.A. Hogquist. 1998. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J. Exp. Med. 188:1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas, B., I. Stefanova, K. Yasutomo, N. Dautigny, and R.N. Germain. 1999. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 10:367–376. [DOI] [PubMed] [Google Scholar]
- 7.Germain, R.N., and I. Stefanova. 1999. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu. Rev. Immunol. 17:467–522. [DOI] [PubMed] [Google Scholar]
- 8.Tarakhovsky, A., S.B. Kanner, J. Hombach, J.A. Ledbetter, W. Muller, N. Killeen, and K. Rajewsky. 1995. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 269:535–537. [DOI] [PubMed] [Google Scholar]
- 9.Bikah, G., J. Carey, J.R. Ciallella, A. Tarakhovsky, and S. Bondada. 1996. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science. 274:1906–1909. [DOI] [PubMed] [Google Scholar]
- 10.Sen, G., G. Bikah, C. Venkataraman, and S. Bondada. 1999. Negative regulation of antigen receptor-mediated signaling by constitutive asociation of CD5 with the SHP-1 protein tyrosine phosphatase in B-1 B cells. Eur. J. Immunol. 29:3319–3328. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Villar, J.J., G.S. Whitney, M.A. Bowen, D.H. Hewgill, A.A. Aruffo, and S.B. Kanner. 1999. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol. Cell. Biol. 19:2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pena-Rossi, C., L.A. Zuckerman, J. Strong, J. Kwan, W. Ferris, S. Chan, A. Tarakhovsky, A.D. Beyers, and N. Killeen. 1999. Negative regulation of CD4 lineage development and responses by CD5. J. Immunol. 163:6494–6501. [PubMed] [Google Scholar]
- 13.Gary-Gouy, H., P. Bruhns, C. Schmitt, A. Dalloul, M. Daeron, and G. Bismuth. 2000. The pseudo-immunoreceptor tyrosine-based activation motif of CD5 mediates its inhibitory action on B-cell receptor signaling. J. Biol. Chem. 275:548–556. [DOI] [PubMed] [Google Scholar]
- 14.Azzam, H.S., J.B. DeJarnette, K. Huang, R. Emmons, C.S. Park, C.L. Sommers, D. El-Khoury, E.W. Shores, and P.E. Love. 2001. Fine tuning of TCR signaling by CD5. J. Immunol. 166:5464–5472. [DOI] [PubMed] [Google Scholar]
- 15.Wong, P., G.M. Barton, K.A. Forbush, and A.Y. Rudensky. 2001. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J. Exp. Med. 193:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plas, D.R., R. Johnson, J.T. Pingel, R.J. Matthews, M. Dalton, G. Roy, A.C. Chan, and M.L. Thomas. 1996. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 272:1173–1176. [DOI] [PubMed] [Google Scholar]
- 17.Tarakhovsky, A., W. Muller, and K. Rajewsky. 1994. Lymphocyte populations and immune responses in CD5-deficient mice. Eur. J. Immunol. 24:1678–1684. [DOI] [PubMed] [Google Scholar]
- 18.Chan, S., C. Waltzinger, A. Tarakhovsky, C. Benoist, and D. Mathis. 1999. An influence of CD5 on the selection of CD4-lineage T cells. Eur. J. Immunol. 29:2916–2922. [DOI] [PubMed] [Google Scholar]
- 19.Merkenschlager, M., D. Graf, M. Lovatt, U. Bommhardt, R. Zamoyska, and A.G. Fisher. 1997. How many thymocytes audition for selection? J. Exp. Med. 186:1149–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azzam, H.S., A. Grinberg, K. Lui, H. Shen, E.W. Shores, and P.E. Love. 1998. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp Med. 188:2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhandoola, A., R. Cibotti, J.A. Punt, L. Grange, A.J. Adams, S.O. Sharrow, and A. Singer. 1999. Positive selection as a developmental progression initiated by αβTCR signals that fix TCR specificity prior to lineage commitment. Immunity. 10:301–311. [DOI] [PubMed] [Google Scholar]
- 22.McNaughton, P.A. 1990. The light response of vertebrate photoreceptors. Physiol. Rev. 70:847–883. [DOI] [PubMed] [Google Scholar]
- 23.Martin, J.H. 1991. Coding and processing of sensory information. Principles of Neural Science. 3rd edition. E.R. Kandel, J.H. Schwartz, and T.M. Jessel, editors. Prentice-Hall International Ltd., London, UK. 329–340.
- 24.Takeda, S., H.R. Rodewald, H. Arakawa, H. Bluethmann, and T. Shimizu. 1996. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 5:217–228. [DOI] [PubMed] [Google Scholar]
- 25.Rooke, R., C. Waltzinger, C. Benoist, and D. Mathis. 1997. Targeted complementation of MHC class II deficiency by intrathymic delivery of recombinant adenoviruses. Immunity. 7:123–134. [DOI] [PubMed] [Google Scholar]
- 26.Kirberg, J., A. Berns, and H. von Boehmer. 1997. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J. Exp. Med. 186:1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanchot, C., F.A. Lemonnier, B. Perarnau, A.A. Freitas, and B. Rocha. 1997. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 276:2057–2062. [DOI] [PubMed] [Google Scholar]
- 28.Viret, C., F.S. Wong, and C.A. Janeway. 1999. Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity. 10:559–568. [DOI] [PubMed] [Google Scholar]
- 29.Pestano, G.A., Y. Zhou, L.A. Trimble, J. Daley, G.F. Weber, and H. Cantor. 1999. Inactivation of misselected CD8 T cells by CD8 gene methylation and cell death. Science. 284:1187–1191. [DOI] [PubMed] [Google Scholar]
- 30.Bender, J., T. Mitchell, J. Kappler, and P. Marrack. 1999. CD4+ T cell division in irradiated mice requires peptides distinct from those responsible for thymic selection. J. Exp. Med. 190:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ernst, B., D.S. Lee, J.M. Chang, J. Sprent, and C.D. Surh. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 11:173–181. [DOI] [PubMed] [Google Scholar]
- 32.Goldrath, A.W., and M.J. Bevan. 1999. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 11:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorfman, J.R., I. Stefanova, K. Yasutomo, and R.N. Germain. 2000. CD4+ T cell survival is not directly linked to self-MHC-induced TCR signaling. Nat. Immunol. 1:329–335. [DOI] [PubMed] [Google Scholar]
- 34.Clarke, S.A., and A.Y. Rudensky. 2000. Survival and homeostatic proliferation of naive peripheral CD4+ T cells in the absence of self peptide:MHC complexes. J. Immunol. 165:2458–2464. [DOI] [PubMed] [Google Scholar]
- 35.Marrack, P., J. Bender, D. Hildeman, M. Jordan, T. Mitchell, M. Murakami, A. Sakamoto, B.C. Schaefer, B. Swanson, and J. Kappler. 2000. Homeostasis of αβ TCR+ T cells. Nat. Immunol. 1:107–111. [DOI] [PubMed] [Google Scholar]
- 36.Marrack, P., L. Ignatowicz, J.W. Kappler, J. Boymel, and J.H. Freed. 1993. Comparison of peptides bound to spleen and thymus class II. J. Exp. Med. 178:2173–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaye, J., M.L. Hsu, M.E. Sauron, S.C. Jameson, N.R. Gascoigne, and S.M. Hedrick. 1989. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 341:746–749. [DOI] [PubMed] [Google Scholar]
- 38.Spanopoulou, E., P. Cortes, C. Shih, C.M. Huang, D.P. Silver, P. Svec, and D. Baltimore. 1995. Localization, interaction, and RNA binding properties of the V(D)J recombination-activating proteins RAG1 and RAG2. Immunity. 3:715–726. [DOI] [PubMed] [Google Scholar]
- 39.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R.M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomonari, K. 1988. A rat antibody against a structure functionally related to the mouse T-cell receptor/T3 complex. Immunogenetics. 28:455–458. [DOI] [PubMed] [Google Scholar]
- 41.Kaye, J., G. Kersh, I. Engel, and S.M. Hedrick. 1991. Structure and specificity of the T cell antigen receptor. Semin. Immunol. 3:269–281. [PubMed] [Google Scholar]
- 42.Koller, B.H., P. Marrack, J.W. Kappler, and O. Smithies. 1990. Normal development of mice deficient in β2M, MHC class I proteins, and CD8+ T cells. Science. 248:1227–1230. [DOI] [PubMed] [Google Scholar]
- 43.Cosgrove, D., D. Gray, A. Dierich, J. Kaufman, M. Lemeur, C. Benoist, and D. Mathis. 1991. Mice lacking MHC class II molecules. Cell. 66:1051–1066. [DOI] [PubMed] [Google Scholar]
- 44.van Oers, N.S., N. Killeen, and A. Weiss. 1994. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR ζ in murine thymocytes and lymph node T cells. Immunity. 1:675–685 [DOI] [PubMed] [Google Scholar]
- 45.Ku, C.C., M. Murakami, A. Sakamoto, J. Kappler, and P. Marrack. 2000. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Scienc e. 288:675–678 [DOI] [PubMed] [Google Scholar]
- 46.Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. [DOI] [PubMed] [Google Scholar]
- 47.Zamzami, N., P. Marchetti. M. Castedo, C. Zanin, J.L. Vayssiere, P.X. Petit, and G. Kroemer. 1995. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. 181:1661–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seddon, B., G. Legnam, P. Tomlinson, and R. Zamoyska. 2000. Long-term survival but impaired homeostatic proliferation of naive T cells in the absence of p56lck. Science. 290:127–131. [DOI] [PubMed] [Google Scholar]
- 49.Ernst, B., C.D. Surh, and J. Sprent. 1995. Thymic selection and cell division. J. Exp. Med. 182:961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witherden, D., N. van Oers, C. Waltzinger, A. Weiss, C. Benoist, and D. Mathis. 2000. Tetracycline-controllable selection of CD4+ T cells: half-life and survival signals in the absence of major histocompatibility complex class II molecules. J. Exp. Med. 191:355–364. [DOI] [PubMed] [Google Scholar]
- 51.Kersh, E.N., G.J. Kersh, and P.M. Allen. 1999. Partially phosphorylated T cell receptor ζ molecules can inhibit T cell activation. J. Exp. Med. 190:1627–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fink, P.J., and C.J. McMahan. 2000. Lymphocytes rearrange, edit and revise their antigen receptors to be useful yet safe. Immunol. Today. 21:561–566. [DOI] [PubMed] [Google Scholar]
- 53.Nemazee, D., and M. Weigert. 2000. Revising B cell receptors. J. Exp. Med. 191:1813–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam, K.P., R. Kuhn, and K. Rajewsky. 1997. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 90:1073–1083. [DOI] [PubMed] [Google Scholar]
- 55.Neuberger, M.S. 1997. Antigen receptor signaling gives lymphocytes a long life. Cell. 90:971–973. [DOI] [PubMed] [Google Scholar]
- 56.Zheng, B., W. Xue, and G. Kelsoe. 1994. Locus-specific somatic hypermutation in germinal centre T cells. Nature. 372:556–559. [DOI] [PubMed] [Google Scholar]
- 57.McMahan, C.J., and P.J. Fink. 1998. RAG reexpression and DNA recombination at T cell receptor loci in peripheral CD4+ T cells. Immunity. 9:637–647. [DOI] [PubMed] [Google Scholar]
- 58.Grossman, Z., and W.E. Paul. 2000. Self-tolerance: context dependent tuning of T cell antigen recognition. Semin. Immunol. 12:197–203. [DOI] [PubMed] [Google Scholar]