Abstract
With human volunteers inoculated at two sites with Haemophilus ducreyi, outcomes for a subject were not independent. In a reinfection trial, 2 of 11 previous pustule formers and 6 of 10 previous resolvers resolved all sites of infection. There was no correlation between serum bactericidal or phagocytic activity and outcome in the trial. These data indicate that different hosts are differentially susceptible to disease progression versus resolution in the model.
Human inoculation experiments with many infectious agents have contributed to our understanding of transmission, pathogenesis, natural history, treatment, and vaccine development (12, 25, 31). After inoculation, some subjects may develop disease while others are asymptomatic or recover without treatment. Reinfection experiments usually have addressed whether experimental or natural infection with a pathogen affords protection against subsequent experimental challenge (5, 14, 17, 24, 33). Reinfection trials generally have not addressed the issue of differences in host susceptibility to disease.
To study Haemophilus ducreyi pathogenesis in humans, we developed an experimental model of infection in human volunteers (40). In the model, subjects are inoculated at multiple sites with strain 35000HP (HP, human passaged) via puncture wounds made in the skin of the upper arm by an allergy-testing device (6, 39). Within 24 h of inoculation, papules develop. These spontaneously resolve or progress to pustules in resemblance to the initial stages of natural chancroid. Lesion outcomes for an individual subject inoculated at multiple sites with identical suspensions of 35000HP sometimes differ in that a pustule may develop at one site while another site resolves (6, 39). Due to the fact that outcomes at different sites are not always the same, we initially used site as the unit of measurement for the calculation of papule and pustule formation rates. These analyses show a significant effect of the estimated delivered dose (EDD) on papule and pustule formation rates (4, 10). Although there is no effect of gender on papule formation, men are twice as likely to form pustules as women (consistent with the high male-to-female ratio in natural disease) (10).
Throughout experimental infection, H. ducreyi colocalizes with collagen and fibrin and professional phagocytes (7, 8). Fibrin and collagen deposition occur as part of the normal process of wound repair and provide a matrix for the infiltrating polymorphonuclear leukocytes (PMNs) and macrophages (7). The presence of fibrin suggests that serum transudates into the wounds. Interestingly, an isogenic mutant that lacks DsrA, an outer membrane protein which has several functions (including serum resistance) (13, 15), forms papules that do not progress to pustule formation (11). In pustules, the parent 35000HP strain is surrounded but not taken up by PMNs or macrophages. Thus, serum resistance and evasion of phagocytosis are virulence determinants in the model.
In the model, some subjects form at least one pustule and other subjects resolve all papules. Some individuals might be prone to resolution because their serum kills the organism or promotes opsonophagocytosis or because their phagocytes take up and kill the organism effectively. Conversely, some subjects might be prone to pustule formation because they lack effective clearance mechanisms.
In this study, we retrospectively analyzed our data with naïve volunteers to see whether sites behaved independently within each subject. We reasoned that if sites behaved similarly, host effects were likely to play a role in outcomes. If sites behaved dissimilarly, different outcomes could be due to factors unrelated to immunity such as variations in inoculum delivery. We also tested the hypothesis that there is a host effect on outcome by conducting a gender-matched reinfection trial of previous resolvers and pustule formers.
Analysis of site independence.
Of 116 volunteers who participated in human challenge trials once (prior to 1 January 2001) (38), 90 were included in the analysis of site independence. The 90 volunteers were infected at two sites each with strain 35000HP and achieved definite clinical outcomes (pustule formation or resolution) at both sites prior to antibiotic treatment. Results were divided into three dosage categories with approximately equal numbers of subjects. Within each category the expected number of people with 0, 1, and 2 pustules was calculated on the basis of the observed pustule formation rate and the assumption of independence. The observed and expected values were compared to determine whether this assumption was reasonable. If the sites behaved independently, approximately 43 (48%) of the subjects should have formed one pustule, but only 25 (28%) did (Table 1). Subjects for whom both sites resolved or formed pustules (n = 65) also exceeded the expected number (n = 37). These results suggested that there was a host effect on the outcomes for the sites.
TABLE 1.
Outcome of infection in subjects inoculated at two sites with strain 35000HP and the number expected if sites were independent
| No. of pustulesa | Total no. of subjects (expected no. of subjects) infected with an EDD (in CFU) of:
|
Total no. of subjects (expected no. of subjects) | ||
|---|---|---|---|---|
| 0-39 | 40-59 | 60-120 | ||
| 0 | 9 (6) | 6 (3.6) | 12 (8.5) | 27 (18.1) |
| 1 | 5 (11) | 10 (14.9) | 10 (17) | 25 (42.9) |
| 2 | 8 (5) | 18 (15.5) | 12 (8.5) | 38 (29) |
| Total | 22 | 34 | 34 | 90 |
Number of pustules formed at two inoculated sites.
Reinfection trial.
To test the hypothesis that certain individuals (resolvers) are resistant and that others (pustule formers) are susceptible to disease progression, we conducted a reinfection trial. Subjects were eligible for the trial if they had participated previously in either a parent or a mutant-parent strain trial and had been followed until they achieved a definite clinical end point. Resolvers were defined as subjects who had been challenged with EDDs of strain 35000HP that were likely to cause pustule formation (≥40 CFU) at two or more sites and had not developed pustules at any of those sites (or at any of three sites inoculated with a virulent mutant, if applicable). A pustule former was defined as a subject who had developed a pustule at one or more sites inoculated with any dose of the parent or a mutant strain.
Informed consent was obtained from the subjects for participation and for human immunodeficiency virus serology in accordance with the human experimentation guidelines of the U.S. Department of Health and Human Services and the Institutional Review Board of Indiana University—Purdue University at Indianapolis. The experimental challenge protocol, the preparation and inoculation of the bacteria, the calculation of the EDD, the clinical observations, and the treatment with antibiotics were done exactly as described previously (6, 39, 40). Each subject was inoculated on the upper arm with three doses of live strain 35000HP bacteria and one dose of heat-killed bacteria. The clinical end point was the development of a painful pustule, resolution of infection at all sites, or 14 days of infection. Determination of the end point required agreement between two study investigators. When the end point was achieved, up to three sites with active disease (if present) were subjected to biopsies performed with a punch forceps. Each pustule was minced in 1 ml of RPMI 1640 for flow cytometry studies that have been reported previously (20, 36) or are unpublished. To conserve the eukaryotic cell yield, only 10 μl of the tissue suspension was grown in cultures. Sites where disease had resolved clinically were not subjected to biopsies, because resolved sites contain no inflammatory infiltrate and are culture negative (39).
We hypothesized that previous pustule formers would have a 20% chance of resolving infection at all three sites inoculated with the parent strain and that resolvers would have an 80% chance of resolving at all three sites. A total of 10 subjects in each group were required to have 80% power to detect this difference (using a one-tailed Fisher's exact test) at a 5% significance level (a 20% resolution rate versus an 80% resolution rate). Due to the gender effect on pustule formation, the subjects were gender matched. There is no effect of ethnicity or age on pustule formation (4, 10), and we did not match these variables.
A total of 22 volunteers enrolled in the study. One male resolver failed to appear for scheduled challenges twice and was excluded from the analysis. The resolver group consisted of one man and nine women (mean age ± standard deviation [SD], 33 ± 10 years) who had been infected with the parent strain (n = 4) or in mutant-parent strain trials (n = 6) (Table 2). The pustule formers consisted of two men and nine women (mean age ± SD, 42 ± 10 years) who had participated in parent strain (n = 3) or mutant-parent strain (n = 8) trials (Table 2). The resolvers had been challenged initially with inoculations at 26 sites with 68.2 ± 21.4 (mean ± SD) CFU of strain 35000HP or a virulent isogenic mutant of 35000HP and had formed no pustules. The pustule formers had been challenged initially with inoculations at 37 sites with 52.9 ± 20.6 CFU of strain 35000HP or a virulent mutant derived from 35000HP and had formed 23 pustules. The mean interval from the first to the second challenge was 638.7 ± 467 days in the pustule group and 604.4 ± 558.8 days in the resolver group.
TABLE 2.
Response to inoculation with live H. ducreyi
| Subject | Gender | Group | Initial infection source or reference | No. of days infected | No. of papules | Final outcome for papule
|
||
|---|---|---|---|---|---|---|---|---|
| No. of papules | No. of pustules | No. resolved | ||||||
| Pustule formers | ||||||||
| 134RR | F | 1 | 16 | 14 | 3 | 0 | 2 | 1 |
| 189RR | M | 1 | 20 | 9 | 3 | 0 | 2 | 1 |
| 172RR | F | 2 | Unpublished study | 6 | 3 | 0 | 2 | 1 |
| 164RR | M | 2 | 42 | 9 | 3 | 0 | 1 | 2 |
| 187RR | F | 3 | 9 | 14 | 3 | 3 | 0 | 0 |
| 149RR | F | 3 | 7 | 7 | 3 | 0 | 2 | 1 |
| 92RR | F | 4 | 50 | 6 | 3 | 0 | 0 | 3 |
| 99RR | F | 5 | 3 | 12 | 3 | 1 | 2 | 0 |
| 176RR | F | 5 | 49 | 8 | 3 | 0 | 0 | 3 |
| 210RR | F | 6 | Unpublished study | 6 | 3 | 0 | 2 | 1 |
| 211RR | F | 6 | Unpublished study | 6 | 2 | 1 | 1 | 0 |
| Resolvers | ||||||||
| 154RR | M | 1 | Unpublished study | 9 | 3 | 0 | 1 | 2 |
| 166RR | F | 1 | 11 | 7 | 3 | 0 | 0 | 3 |
| 171RR | F | 2 | Unpublished study | 5 | 3 | 0 | 0 | 3 |
| 159RR | F | 3 | 11 | 6 | 2 | 0 | 0 | 2 |
| 203RR | F | 3 | 20 | 7 | 3 | 0 | 0 | 3 |
| 200RR | F | 4 | 20 | 6 | 1 | 0 | 0 | 1 |
| CS31RR | F | 5 | 5 | 6 | 3 | 0 | 3 | 0 |
| 100RR | F | 5 | 50 | 6 | 3 | 0 | 3 | 0 |
| 213RR | F | 6 | Unpublished study | 6 | 3 | 0 | 2 | 1 |
| 216RR | F | 6 | Unpublished study | 3 | 3 | 0 | 0 | 3 |
We infected one or two pairs of gender-matched subjects in six groups (Table 2). Due to scheduling conflicts, volunteer 216RR was infected with the same EDD as the other members of group 6 at 1 month after the other members of group 6. Each subject was inoculated at three sites with live strain 35000HP bacteria. The EDD for the second challenge was 60.6 ± 22.7 CFU for the pustule group and 60.9 ± 24.1 CFU for the resolver group. Papules developed at 32 of 33 sites inoculated with live bacteria in the pustule group and at 27 of 30 sites in the resolver group (P = 0.34). Of 11 subjects in the pustule group, 2 (18%; 95% confidence interval [CI], 2.3 to 51.8%) resolved all sites of infection, 8 formed at least one pustule, and 1 (187RR) had three papules at the 14-day end point. In contrast, 6 of 10 (60%; 95% CI, 26.2 to 87.8%) subjects in the resolver group resolved all sites of infection and 4 formed at least one pustule. The proportion of subjects who achieved the primary end point (resolution of disease) in the resolver group versus that in the pustule group approached statistical significance (P = 0.064 [one-tailed Fisher's exact test]). Adjusting for the effect of infecting subjects in groups, the difference between the resolvers and pustule formers was statistically significant (P = 0.029 [one-tailed Mantel-Haenszel test]). In secondary analyses, the pustule formation rates for inoculated sites were 30% (95% CI, 4.8 to 55.2%) for the resolver group and 42.4% (95% CI, 25.4 to 59.4%) for the pustule group (P = 0.42). The numbers of sites with no clinical evidence of infection at the end point were 14 of 33 in the pustule group and 21 of 30 in the resolver group (P = 0.083 [Wilcoxon rank sum test]).
Of 21 pustule biopsy cultures grown for the trial, 13 yielded growth of H. ducreyi. According to the results of flow cytometry, the samples that did not yield positive cultures contained CD4 cells, in consistency with the presence of inflammatory infiltrate in experimental lesions (20, 36) (data not shown).
One subject (187RR) had three papules at the end point. Biopsies were performed for two of the papules and the heat-killed control site, and each specimen was cut into halves. One half was cultured semiquantitatively (39, 40). The other half was fixed in 4% paraformaldehyde for histologic examination and immunohistochemical studies for the T-cell antigen CD3 (8, 30). These specimens and uninfected upper-arm skin provided by another donor (30) were coded and examined by a dermatopathologist who was unaware of the code. Both papules were culture negative. However, both papules contained a perivascular mononuclear infiltrate composed predominantly of CD3 cells and were histologically identical to papules of experimental chancroid (30) (data not shown). The heat-killed control site was histologically similar to uninfected upper-arm skin (data not shown).
Taken together, the data indicate that gender-matched pustule formers and resolvers were equally susceptible to infection in that they had similar papule formation rates. However, the subjects tended to segregate towards their initial outcomes when rechallenged, indicating that some hosts are prone to resolution while others are prone to pustule formation. We had assumed there would be an underlying pustule formation rate of approximately 70% at sites in the pustule former group, but the observed rate was only 42%. Almost all (87%) of the reinfection trial participants were women, who have lower pustule formation rates than men (10), which may have limited our power to detect a difference between the two groups. Nevertheless, several analyses of the data approached or achieved statistical significance.
Of 135 naïve subjects who have been challenged to date with an EDD of strain 35000HP of ≥40 CFU, 30.4% (95% CI, 22.8 to 38.8%) are resolvers and 69.6% (95% CI, 61.1 to 77.2%) are pustule formers. Our investigators previously reported a reinfection experiment in which all seven subjects in a pustule former group developed pustules when rechallenged (5). The previous study was done before we were aware of the gender effect on pustule formation, and the participants included five men and two women (5). Taken together with the present trial, our cumulative experience is that 11% (95% CI, 1.4 to 34.7%) of 18 pustule formers and 60% (95% CI, 26.2 to 87.8%) of 10 resolvers resolve all sites of infection in a second challenge. Our cumulative data supporting a host effect on site outcome has implications for the design and analysis of trials done in the model. For example, in mutant-parent strain trials each subject is inoculated with parent and mutant strains, which controls for the host effect (38). However, analyses of these trials will also need to account for the correlation among sites for each subject.
Serum resistance assays.
To examine whether the difference in outcome between the pustule formers and the resolvers was due to differences in serum bactericidal activity, we determined the survival of strain 35000HP in their sera. Serum was obtained at the end point of or within two weeks of participation in the trial, processed to preserve complement activity, frozen, and stored. The survival of strain 35000HP was determined in a bactericidal assay, using 50% serum exactly as described previously (19). Results were expressed as percent survival [(CFU in wells containing serum with active complement/CFU in wells with heat-inactivated serum) × 100]. The serum-susceptible Escherichia coli K-12 strain HB101 was included in each assay to ensure that each serum contained active or inactive complement. Three independent experiments were performed to obtain a mean percent survival value for each subject.
The subject data were analyzed in groups according to clinical outcomes. For the group of subjects who did not resolve twice (n = 9), the mean percent survival ± SD of strain 35000HP was 57.4 ± 25.2%. For the group who formed pustules once and resolved once (n = 6), the mean ± SD was 74.0 ± 32.3%. For the double-resolution group (n = 6), the mean ± SD was 69.0 ± 15.1%. Thus, there was no correlation between serum bactericidal activity and outcome in the trial.
Phagocytosis assays.
To examine whether the difference in outcome between double-pustule formers and double resolvers was due to the ability of their PMNs and macrophages to take up the organism, we recalled subjects from each of these groups to donate blood. Only four double resolvers agreed to participate, and they were matched with four double-pustule formers. Blood was obtained from each subject once, and each blood sample was assigned a letter so that the source of the specimen was not known to the person performing the assays.
PMNs and monocytes were isolated using 1-Step Polymorphs (Accurate Chemical & Scientific Corporation) according to the manufacturer's instructions. PMNs (5 × 105) were seeded onto serum-coated glass coverslips, and monocytes were seeded onto glass coverslips. The monocytes (2 × 105) were cultured for 8 days in RPMI 1640 (Gibco) supplemented with 10% heat-inactivated human AB serum (Sigma) to allow differentiation into macrophages.
Phagocytosis assays were performed with strain 35000HP/pRB157K, a gift of Eric Hansen, which expresses green fluorescent protein (GFP). Positive and negative controls for the assays included GFP-expressing Yersinia pseudotuberculosis YPIII derivatives (23) that were the gifts of Joan Mecsas. Y. pseudotuberculosis strain 582 contained the virulence plasmid pYV that expresses several antiphagocytic Yop proteins, while strain 756 is cured of pYV and is readily phagocytosed. Assays were done at a multiplicity of infection in the range of 1:1 to 10:1. For these assays, 35000HP/pRB157K was grown in brain heart infusion broth containing 0.1% starch as described previously (19). The Y. pseudotuberculosis strains were grown in 2× YT medium containing 20 mM sodium oxalate and 20 mM MgCl2 supplemented with ampicillin (100 μg/ml) or chloramphenicol (20 μg/ml), as described previously (23). Bacteria were either not opsonized or opsonized with 100% autologous complement-replete serum, centrifuged onto the phagocytes at 10°C to synchronize uptake, and then incubated with the phagocytes for 30 min at 35°C. The slides were washed, fixed overnight at 4°C, and then stained with fluorescently labeled antibodies (Abs) for visualization by confocal microscopy (7, 8). Phagocytic cells were labeled with anti-CD45 monoclonal Ab (BD Biosciences) followed by a tetramethyl rhodamine-conjugated secondary Ab (Jackson ImmunoResearch). Because H. ducreyi is small and difficult to visualize, extracellular H. ducreyi bacteria were also labeled with polyclonal anti-H. ducreyi antiserum followed by an indodicarbocyanine (Cy5)-conjugated secondary Ab. Intracellular H. ducreyi fluoresced with GFP, while extracellular H. ducreyi fluoresced with Cy5 and GFP. Images were taken of three to five independent fields, and a mean value of 150 PMNs or macrophages was scored for the presence or absence of intracellular bacteria to obtain a mean percentage of phagocytes that took up bacteria for each subject. The subject data were analyzed in groups according to clinical outcomes.
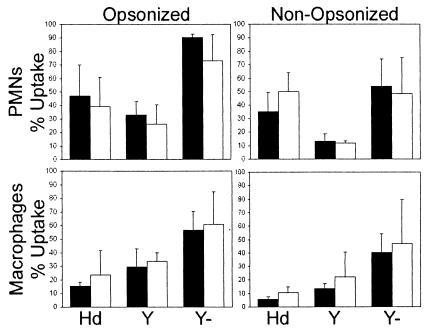
Under opsonic and nonopsonic conditions, there were no significant differences in the percentages of phagocytic cells from the double-pustule formers and double resolvers that were associated with each bacterial strain (data not shown). There were also no significant differences in the ability of PMNs or macrophages from the two groups to take up strains 35000HP/pRB157K and Y. pseudotuberculosis 582 or 756 (Fig. 1). For macrophages, 35000HP/pRB157K was taken up to an extent that was more similar to that of the antiphagocytic Y. pseudotuberculosis 582 than to that of the pYV-cured Y. pseudotuberculosis 756 (Fig. 1). For PMNs, 35000HP/pRB157K had an intermediate phenotype relative to the Y. pseudotuberculosis strains. Our results confirm that strain 35000HP has antiphagocytic properties for primary cells, as has been shown for macrophage-like cell lines (1, 48). However, there was no gross correlation between phagocytic activity in vitro and outcome in the trial.
FIG. 1.
Percentages of PMNs and macrophages isolated from double-pustule formers (black bars) and double resolvers (open bars) that took up bacteria under opsonized and nonopsonized conditions. Results are expressed as the mean and SD for each group. Hd, strain 35000HP/pRB157K; Y, YPIII strain 582; Y−, YPIII strain 756, which was cured of plasmid pYV.
Possible mechanisms for differences in host susceptibility.
The fact that the outcome of infection was not reflected by these in vitro assays suggests that the overall environment of the infection may be an important determinant of host susceptibility. The cutaneous immune response to H. ducreyi is unusual for an organism that induces abscess formation in that only 30% of the recruited cells are neutrophils after two days of infection (20, 29). In pustules, the PMNs coalesce to form an epidermal abscess, macrophages form a collar below the abscess, and T cells and macrophages coalesce into poorly formed granulomas below the collar (30, 38). The combination of an abscess and granulomas is unique compared with other skin pathogen infection outcomes (26, 41, 45, 47). The cutaneous response to H. ducreyi has some features of type 1 immunity, which usually facilitates phagocytosis (30, 37, 38). However, why phagocytic failure occurs in some subjects and clearance occurs in others is not clear.
Work done over the past two decades shows that T cells modulate the effectiveness of the PMN response in a murine model of intraabdominal infection (29). Athymic and cyclophosphamide-treated mice are less prone to abscess formation than control mice; these effects are reversed when T-cell-deficient mice are reconstituted with T cells from normal mice (28, 34). Abscess formation is blocked by interference with the T-cell costimulatory pathway with CTLA4Ig or by administration of interleukin-2 (IL-2) (43, 44), suggesting that immune suppression mediated by regulatory T cells promotes phagocytic failure and abscess formation (35).
An emerging literature suggests that pathogens interact with antigen-presenting cells to induce the development of regulatory T cells that suppress immune responses, facilitating organism survival in some cases and limiting tissue damage in others (22). Suppression of the immune response by regulatory T cells appears to be mediated by IL-10, transforming growth factor β (TGF-β), and possibly other unknown mechanisms that involve direct cell contact. Interestingly, approximately half of the T-cell lines isolated from experimental chancroid have characteristics of regulatory T cells in that they produce both gamma interferon and IL-10 but no IL-4 in response to the presence of H. ducreyi (18). Although volunteers who have a previous history of keloid formation were excluded from the human challenge trials, 22 of 139 (14.7%) subjects developed hypertrophic scars at sites that were infected and at which biopsies were performed. This rate is much higher than the incidence in the general population (2), considering the ethnic and racial composition of the volunteers. Hypertrophic scar formation is associated with excessive TGF-β production (27, 46). We postulate that H. ducreyi causes a tolerizing response that promotes phagocytic failure in pustule formers and a proinflammatory response that promotes clearance in resolvers. Women are more prone to autoimmune diseases or loss of self-tolerance than men (21, 32). If H. ducreyi induces tolerizing responses less frequently in women, women should be less susceptible to pustule formation than men (10).
In summary, our data show that some hosts are prone to form pustules and that others are prone to resolve experimental H. ducreyi infection. Future studies will examine whether H. ducreyi interaction with antigen-presenting cells in vivo leads to excessive production of IL-10 and TGF-β, which in turn leads to accumulation of regulatory T cells at sites of infection, and whether tolerizing responses occur more frequently in men than in women and in pustule formers than in resolvers.
Acknowledgments
This work was supported by grants AI31494 and AI27863 from the National Institute of Allergy and Infectious Diseases. The human challenge trials were also supported by grant MO1RR00750 to the General Clinical Research Center at Indiana University.
Confocal microscopy was performed at the Indiana Center for Biological Microscopy. We thank Martha Greenwald and Abby Olusanya for their assistance in the clinical trial, Mike Apicella and Mark Simons for their advice on the phagocytosis assays, Barbara van der Pol, Byron Batteiger, Ray Johnson, Brad Allen, and Eric Hansen for reviewing the manuscript, and the volunteers who participated in the trial.
Editor: F. C. Fang
REFERENCES
- 1.Ahmed, H. J., C. Johansson, L. A. Svensson, K. Ahlman, M. Verdrengh, and T. Lagergard. 2002. In vitro and in vivo interactions of Haemophilus ducreyi with host phagocytes. Infect. Immun. 70:899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhady, S. M. A., and K. Sivanantharajah. 1969. Keloids in various races. Plast. Reconstr. Surg. 44:564-566. [DOI] [PubMed] [Google Scholar]
- 3.Al-Tawfiq, J. A., M. E. Bauer, K. R. Fortney, B. P. Katz, A. F. Hood, M. Ketterer, M. A. Apicella, and S. M. Spinola. 2000. A pilus-deficient mutant of Haemophilus ducreyi is virulent in the human model of experimental infection. J. Infect. Dis. 181:1176-1179. [DOI] [PubMed] [Google Scholar]
- 4.Al-Tawfiq, J. A., J. Harezlak, B. P. Katz, and S. M. Spinola. 2000. Cumulative experience with Haemophilus ducreyi in the human model of experimental infection. Sex. Transm. Dis. 27:111-114. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tawfiq, J. A., K. L. Palmer, C.-Y. Chen, J. C. Haley, B. P. Katz, A. F. Hood, and S. M. Spinola. 1999. Experimental infection of human volunteers with Haemophilus ducreyi does not confer protection against subsequent challenge. J. Infect. Dis. 179:1283-1287. [DOI] [PubMed] [Google Scholar]
- 6.Al-Tawfiq, J. A., A. C. Thornton, B. P. Katz, K. R. Fortney, K. D. Todd, A. F. Hood, and S. M. Spinola. 1998. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J. Infect. Dis. 178:1684-1687. [DOI] [PubMed] [Google Scholar]
- 7.Bauer, M. E., M. P. Goheen, C. A. Townsend, and S. M. Spinola. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer, M. E., and S. M. Spinola. 2000. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect. Immun. 68:2309-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bong, C. T. H., K. R. Fortney, B. P. Katz, A. F. Hood, L. R. San Mateo, T. H. Kawula, and S. M. Spinola. 2002. A superoxide dismutase C mutant of Haemophilus ducreyi is virulent in human volunteers. Infect. Immun. 70:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bong, C. T. H., J. Harezlak, B. P. Katz, and S. M. Spinola. 2002. Men are more susceptible to pustule formation than women in the experimental model of Haemophilus ducreyi infection. Sex. Transm. Dis. 29:114-118. [DOI] [PubMed] [Google Scholar]
- 11.Bong, C. T. H., R. E. Throm, K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2001. A DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun. 69:1488-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen, M. S., and J. G. Cannon. 1999. Human experimentation with Neisseria gonorrhoeae: progress and goals. J. Infect. Dis. 179:S375-S379. [DOI] [PubMed] [Google Scholar]
- 13.Cole, L. E., T. H. Kawula, K. L. Toffer, and C. Elkins. 2002. The Haemophilus ducreyi serum resistance antigen DsrA confers attachment to human keratinocytes. Infect. Immun. 70:6158-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnenberg, M. S., C. O. Tacket, G. Losonsky, G. Frankel, J. P. Nataro, G. Dougan, and M. M. Levine. 1998. Effect of prior experimental human enteropathogenic Escherichia coli infection on illness following homologous and heterologous rechallenge. Infect. Immun. 66:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkins, C., K. J. Morrow, and B. Olsen. 2000. Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect. Immun. 68:1608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortney, K. R., R. S. Young, M. E. Bauer, B. P. Katz, A. F. Hood, R. S. Munson, Jr., and S. M. Spinola. 2000. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman, D. O. 1991. Experimental infection of human subjects with Strongyloides species. Rev. Infect. Dis. 13:1221-1226. [DOI] [PubMed] [Google Scholar]
- 18.Gelfanova, V., T. L. Humphreys, and S. M. Spinola. 2001. Characterization of Haemophilus ducreyi-specific T-cell lines from lesions of experimentally infected human subjects. Infect. Immun. 69:4224-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiltke, T. J., M. E. Bauer, J. Klesney-Tait, E. J. Hansen, R. S. Munson, Jr., and S. M. Spinola. 1999. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb. Pathog. 26:93-102. [DOI] [PubMed] [Google Scholar]
- 20.Humphreys, T. L., C. T. Schnizlein-Bick, B. P. Katz, L. A. Baldridge, A. F. Hood, R. A. Hromas, and S. M. Spinola. 2002. Evolution of the cutaneous immune response to experimental Haemophilus ducreyi infection and its relevance to HIV-1 acquisition. J. Immunol. 169:6316-6323. [DOI] [PubMed] [Google Scholar]
- 21.Jansson, L., and R. Holmdahl. 1998. Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm. Res. 47:290-301. [DOI] [PubMed] [Google Scholar]
- 22.McGuirk, P., and K. H. G. Mills. 2002. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 23:450-455. [DOI] [PubMed] [Google Scholar]
- 23.Mecsas, J., B. Raupach, and S. Falkow. 1998. The Yersinia Yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol. Microbiol. 28:1269-1281. [DOI] [PubMed] [Google Scholar]
- 24.Melby, P. C. 1991. Experimental leishmaniasis in humans: review. Rev. Infect. Dis. 13:1009-1017. [DOI] [PubMed] [Google Scholar]
- 25.Miller, F. G., and C. Grady. 2001. The ethical challenge of infection-inducing challenge experiments. Clin. Infect. Dis. 33:1028-1033. [DOI] [PubMed] [Google Scholar]
- 26.Modlin, R. L., J. Melancon-Kaplan, S. M. M. Young, C. Pirmez, H. Kino, J. Convit, T. H. Rea, and B. R. Bloom. 1988. Learning from lesions: patterns of tissue inflammation in leprosy. Proc. Natl. Acad. Sci. USA 85:1213-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niessen, F. B., M. P. Andriessen, J. Schalkwijk, L. Visser, and W. Timens. 2001. Keratinocyte-derived growth factors play a role in the formation of hypertrophic scars. J. Pathol. 194:207-216. [DOI] [PubMed] [Google Scholar]
- 28.Nulsen, M. F., J. J. Finlay-Jones, and P. J. McDonald. 1986. T-lymphocyte involvement in abscess formation in nonimmune mice. Infect. Immun. 52:633-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onderdonk, A. B., R. L. Cisneros, J. H. Crabb, R. W. Finberg, and D. L. Kasper. 1989. Intraperitoneal host cellular responses and in vivo killing of Bacteroides fragilis in a bacterial containment chamber. Infect. Immun. 57:3030-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer, K. L., C. T. Schnizlein-Bick, A. Orazi, K. John, C.-Y. Chen, A. F. Hood, and S. M. Spinola. 1998. The immune response to Haemophilus ducreyi resembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J. Infect. Dis. 178:1688-1697. [DOI] [PubMed] [Google Scholar]
- 31.Rosenbaum, J. R., and K. A. Sepkowitz. 2002. Infectious disease experimentation involving human volunteers. Clin. Infect. Dis. 34:963-971. [DOI] [PubMed] [Google Scholar]
- 32.Sarvetnick, N., and H. S. Fox. 1990. Interferon-gamma and the sexual dimorphism of autoimmunity. Mol. Biol. Med. 7:323-331. [PubMed] [Google Scholar]
- 33.Schmidt, K. A., H. J. Schneider, J. A. Lindstrom, J. W. Boslego, R. A. Warren, L. VanDeVerg, C. D. Deal, J. B. McClain, and J. M. Griffiss. 2001. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex. Transm. Dis. 28:555-564. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro, M. E., D. L. Kasper, D. F. Zaleznik, S. Spriggs, A. B. Onderdonk, and R. W. Finberg. 1986. Cellular control of abscess formation: role of T cells in the regulation of abscesses formed in response to Bacteroides fragilis. J. Immunol. 137:341-346. [PubMed] [Google Scholar]
- 35.Shevach, E. M. 2002. CD4+CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389-400. [DOI] [PubMed] [Google Scholar]
- 36.Solar, D., T. L. Humphreys, S. M. Spinola, and J. J. Campbell. 2003. CCR4 versus CCR10 in human cutaneous Th lymphocyte trafficking. Blood 101:1677-1683. [DOI] [PubMed] [Google Scholar]
- 37.Spellburg, B., and J. E. Edwards, Jr. 2001. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 32:76-102. [DOI] [PubMed] [Google Scholar]
- 38.Spinola, S. M., M. E. Bauer, and R. S. Munson, Jr. 2002. Immunopathogenesis of Haemophilus ducreyi infection (chancroid). Infect. Immun. 70:1667-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spinola, S. M., A. Orazi, J. N. Arno, K. Fortney, P. Kotylo, C.-Y. Chen, A. A. Campagnari, and A. F. Hood. 1996. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J. Infect. Dis. 173:394-402. [DOI] [PubMed] [Google Scholar]
- 40.Spinola, S. M., L. M. Wild, M. A. Apicella, A. A. Gaspari, and A. A. Campagnari. 1994. Experimental human infection with Haemophilus ducreyi. J. Infect. Dis. 169:1146-1150. [DOI] [PubMed] [Google Scholar]
- 41.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 42.Throm, R. E., and S. M. Spinola. 2001. Transcription of candidate virulence genes of Haemophilus ducreyi during infection of human volunteers. Infect. Immun. 69:1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzianabos, A. O., A. Chandraker, W. Kalka-Moll, F. Stingele, V. M. Dong, R. W. Finberg, R. Peach, and M. H. Sayegh. 2000. Bacterial pathogens induce abscess formation by CD4+ T-cell activation via the CD28-B7-2 costimulatory pathway. Infect. Immun. 68:6650-6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzianabos, A. O., P. R. Russell, A. B. Onderdonk, F. C. Gibson III, C. Cywes, M. Chan, R. W. Finberg, and D. L. Kasper. 1999. IL-2 mediates protection against abscess formation in an experimental model of sepsis. J. Immunol. 163:893-897. [PubMed] [Google Scholar]
- 45.Van Voorhis, W., L. Barrett, D. Koelle, J. Nasio, F. Plummer, and S. Lukehart. 1996. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J. Infect. Dis. 173:491-495. [DOI] [PubMed] [Google Scholar]
- 46.Varga, J., and S. A. Jimenez. 1995. Modulation of collagen gene expressions: its relation to fibrosis in systemic sclerosis and other disorders. Ann. Intern. Med. 122:60-62. [DOI] [PubMed] [Google Scholar]
- 47.Wannamaker, L. W. 1970. Differences between streptococcal infections of the throat and of the skin. N. Engl. J. Med. 282:23-31. [DOI] [PubMed] [Google Scholar]
- 48.Wood, G. E., S. M. Dutro, and P. A. Totten. 2001. Haemophilus ducreyi inhibits phagocytosis by U-937 cells, a human macrophage-like cell line. Infect. Immun. 69:4726-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young, R. S., M. J. Filiatrault, K. R. Fortney, A. F. Hood, B. P. Katz, R. S. Munson, Jr., A. A. Campagnari, and S. M. Spinola. 2001. Haemophilus ducreyi lipooligosaccharide mutant defective in expression of β-1,4-glucosyltransferase is virulent in humans. Infect. Immun. 69:4810-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young, R. S., K. Fortney, J. C. Haley, A. F. Hood, A. A. Campagnari, J. Wang, J. A. Bozue, R. S. Munson, Jr., and S. M. Spinola. 1999. Expression of sialylated or paragloboside-like lipooligosaccharides are not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect. Immun. 67:6335-6340. [DOI] [PMC free article] [PubMed] [Google Scholar]