Abstract
Receptor-stimulated generation of reactive oxygen species (ROS) has been shown to regulate signal transduction, and previous studies have suggested that T cell receptor (TCR) signals may involve or be sensitive to ROS. In this study, we have shown for the first time that TCR cross-linking induced rapid (within 15 min) generation of both hydrogen peroxide and superoxide anion, as defined with oxidation-sensitive dyes, selective pharmacologic antioxidants, and overexpression of specific antioxidant enzymes. Furthermore, the data suggest the novel observation that superoxide anion and hydrogen peroxide are produced separately by distinct TCR-stimulated pathways. Unexpectedly, TCR-stimulated activation of the Fas ligand (FasL) promoter and subsequent cell death was dependent upon superoxide anion, but independent of hydrogen peroxide, while nuclear factor of activated T cells (NFAT) activation or interleukin 2 transcription was independent of all ROS. Anti-CD3 induced phosphorylation of extracellular signal–regulated kinase (ERK)1/2 required hydrogen peroxide generation but was unaffected by superoxide anion. Thus, antigen receptor signaling induces generation of discrete species of oxidants that selectively regulate two distinct redox sensitive pathways, a proapoptotic (FasL) and a proliferative pathway (ERK).
Keywords: T lymphocytes; reactive oxygen species; signal transduction; genes, reporter; oxidoreductases
Introduction
Reactive oxygen species (ROS)* are generated by all mammalian cells as by-products of metabolism or apoptotic signals and by some cells in host defense response to noxious stimuli. In general, production of ROS is associated with deleterious effects in pathophysiologic conditions, including inflammatory responses, apoptosis, or ischemia/reperfusion (1). In a growing number of systems, however, generation of ROS is useful and even required by physiologic systems. Receptor systems as diverse as epidermal growth factor (EGF), CD40, and angiotensin II induce generation of intracellular ROS upon ligand binding and the ROS were necessary for appropriate signal transduction, kinase activation, and biologic responses associated with receptor signaling (2–4).
While there is obvious diversity in the immediate signal transduction pathways linked to receptors such as EGF (a receptor tyrosine kinase) or angiotensin II (a G protein–coupled receptor), data suggest that diverse receptors stimulate ROS generation via activation of an intracellular NADH/NADPH oxidase homologous to that expressed in inflammatory cells (5, 6). Activation of the oxidase, as in the respiratory burst of macrophages and neutrophils, leads to generation of superoxide anion in response to receptor stimulation. However, in these systems, the ROS produced are not cytotoxic, but are required for mitogen-activated protein (MAP) kinase family member activation, gene expression, and/or cell proliferation (3, 4, 7, 8). Although receptor-stimulated generation of superoxide anion is necessary, it is not sufficient. Signal transduction seems to require dismutation of superoxide to hydrogen peroxide, as overexpression of antioxidant enzymes such as catalase and thioredoxin peroxidase (TPx) could inhibit receptor-stimulated MAP kinase activation and downstream effects (3, 7–9).
In T lymphocytes, data does indicate that receptor stimulation can induce ROS generation although there is no evidence of expression of an NADH/NADPH oxidase (10). Studies investigating T cell activation and apoptosis have suggested that T cell mitogens (11, 12), lectins (ConA; reference 13), anti-CD3 (11, 14), and superantigens (mouse mammary tumor virus [15], Staphylococcal enterotoxin B, and Staphylococcal enterotoxin A [16]) stimulate ROS generation. Furthermore, by using antioxidants, regulatory roles have been ascribed for ROS in T cell proliferation and death. Further investigation indicated that ROS production was selectively required for expression of functional Fas ligand (FasL) on the surface of anti-CD3–stimulated T cells in models of activation-induced cell death (AICD; reference 11).
Thus, this study examines the kinetics and signaling role(s) of anti-CD3–stimulated ROS generation in mature T cells. The data suggest that TCR stimulation induces rapid production of discrete species of oxidants; namely superoxide anion and hydrogen peroxide, via separate pathways that can be differentiated using specific antioxidant enzymes and pharmacologic inhibitors. Beyond this notable observation, superoxide and hydrogen peroxide also regulate distinct TCR-activated signaling pathways. Activation of ERK was dependent upon TCR-induced hydrogen peroxide generation, while FasL promoter activation required superoxide anion production. Thus, antigen receptor–stimulated ROS generation in T cells serves to regulate a pro-apoptotic pathway (FasL) and a proliferative pathway (ERK) that are critical for T cell function and survival.
Materials and Methods
Chemicals.
Dihydroethidium (DHE), dichlorodihydrofluorescein diacetate (DCFDA), and 1,2-bis (2-aminophenoxyl) ethane-N,N,N′,N′–tetraacetic acid-acetoxymethyl ester (BAPTA-AM) were obtained from Molecular Probes. Manganese (III) tetrakis 4-benzoic acid) porphyrin (MnTBaP), and ebselen were from Calbiochem. Butylated hydroxyanisole (BHA), diphenylene iodonium (DPI), PMA, ionomycin, and all other chemicals were from Sigma-Aldrich. All cell culture supplies were all supplied by Life Technologies. 2C11 and OKT3 were prepared in house from hybridoma supernatants by Protein G affinity purification. Antibodies to phosphorylated p44/42 ERK and pan-specific anti-ERK1/2 were obtained from Cell Signaling. Antibodies to manganese superoxide dismutase (MnSOD; reference 17) and TPx II (9) have been described previously, while anti-copper/zinc superoxide dismutase (CuZnSOD) was supplied by UBI, and anti-catalase was obtained from Calbiochem. The luciferase assay system was from Promega.
Plasmids.
Mammalian expression vectors for green fluorescent protein (GFP; EGFP-N1) and red fluorescent protein (RFP; RFP-N1) were supplied by CLONTECH Laboratories, Inc. Full-length cDNA encoding human CuZnSOD (from American Type Culture Collection), bovine catalase, and the cDNA for the chimeric murine CD8-Igγ2a protein (provided by Dr. Michael Reth, University of Freiburg, Freiburg, Germany) were subcloned into pcDNA3.1 (Invitrogen). Mammalian expression vectors for TPx II (9) and MnSOD (17) have been described previously, while those for BCl-xL and N17 Rac1 were supplied by Charles Zacharchuk (National Cancer Institute, National Institutes of Health, Bethesda, MD) and J. Silvio Gutkind (National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health, Bethesda, MD), respectively. Luciferase reporter plasmids containing the minimal FasL promoter and a 16-mer fragment of the FasL promoter containing the egr-2/3 binding site (18) were a gift from Jon Ashwell (National Cancer Institute, National Institutes of Health, Bethesda, MD). Those containing the minimal IL-2 promoter and a multimerized nuclear factor of activated T cells (NFAT) binding site (19) were obtained from Charles Zacharchuk (National Cancer Institute, National Institutes of Health, Bethesda, MD).
Cells.
The murine T cell hybridoma, 9C127, was a gift from Dr. David Scott (American Red Cross, Rockville, MD) and was maintained in RPMI 1640 supplemented with 10% FBS, antibiotics, and 50 μM 2-mercaptoethanol (complete medium). Human peripheral T cell blasts were prepared as described previously (11). Briefly, human PBMCs were isolated by Ficoll separation and cultured for 2 d with PMA (10 ng/ml) and Ionomycin (1 μg/ml) in complete medium. Cells were washed and grown for at least 24 h in complete medium supplemented with 10 U/ml rhIL-2 (Roche Molecular Biochemicals). Complete medium with rhIL-2 will be referred to as complete medium in studies using human T blasts.
Determination of Anti-CD3–induced ROS Generation.
9C127 murine T cell hybridoma or human T blasts (10 × 106cells/ml) were precoated with anti-CD3 (2C11 for mouse and OKT3 for human) at 10 μg/ml for 30 min on ice. Cells were washed, resuspended in ice cold complete medium (CM), and maintained on ice at 10 × 106 cells/ml. At 15-min intervals, aliquots of cells were diluted 1:10 into tubes maintained at 37°C containing complete medium with 5 μg/ml rabbit anti–hamster antibody (to x-link 2C11) or goat anti–mouse (to x-link OKT3). The oxidation sensitive dyes DHE (2 μM) or DCFDA (2 μM) were added separately to tubes 15 min before harvest. Incubation was terminated by 10-fold dilution with ice cold FACS® buffer and the cells were washed before FACS® analysis. ROS generation was determined by the increase in DHE or DCFDA fluorescence upon anti-CD3 stimulation. Stimulated increase in dye oxidation was calculated as the percentage increase in mean channel fluorescence of anti-CD3–stimulated cells over unstimulated cells for each time point using the following equation: = ([MCF(stim) − MCF(unstim)]/MCF(unstim)) × 100. Neither cells prebound with control antibodies (anti-DNP; BD PharMingen), nor cells exposed to anti-CD3 or secondary cross-linking antibody alone showed evidence of oxidation of either DCFDA or DHE.
To control for potential variations in dye uptake or de-esterification of DCFDA in cells stimulated with anti-CD3, the assays were also performed with oxidized forms of DCFDA; dichlorofluorescein or fluorescein diacetate (Molecular Probes). There was no difference in unstimulated versus anti-CD3–stimulated cells in the staining by these two dyes. The oxidized form of dihydroethidium (ethidium bromide) is relatively membrane impermeant in live cells, thus was not tested.
In experiments using pharmacologic inhibitors, the cells were preincubated with drugs while being precoated with anti-CD3 at 4°C. Cells were maintained in the presence of the appropriate concentration of drug throughout the subsequent incubations.
Overexpression of Antioxidants and ROS Generation.
9C127 T cell hybridoma or human T blasts (40 × 106/ml) were transiently transfected by electroporation (Gene Pulser; Bio-Rad Laboratories). Expression plasmids for BCl-xL, catalase, CuZnSOD, N17Rac1, MnSOD, TPx, or an empty vector (Vector Laboratories) were cotransfected with vectors encoding either GFP or RFP in a 2:1 ratio to ensure that any cell expressing GFP or RFP would also express the protein of interest. After 16 h incubation, transfected cells were harvested, counted, and assayed for anti-CD3–stimulated ROS production as described above. GFP fluorescence was used as a marker to gate on transfected cells in experiments using DHE while RFP fluorescence was used to gate on transfected cells in experiments with DCFDA. As shown in Fig. 3 C, expression of fluorescent proteins did not affect dye uptake.
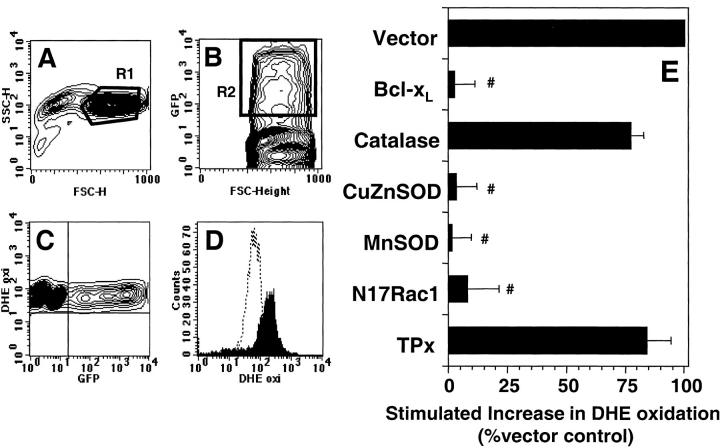
Figure 3.
Effect of protein overexpression on anti-CD3–stimulated DHE oxidation in 9C127 cells. 9C127 cells were transfected with the indicated vectors at a 2:1 ratio with one encoding GFP as a transfection marker. After 16 h incubation, anti-CD3–induced DHE oxidation was measured as described in Materials and Methods. (A) “Normal sized” cells as defined by R1 in the FSC/SSC plot were analyzed. (B) Representative GFP staining profile. GFP expressing, productively transfected cells were defined by R2. (C) Relationship of GFP fluorescence to basal DHE staining indicating that expression of GFP did not affect dye uptake. (D) Representative DHE profile of “normal sized,” GFP+ cells with unstimulated cells (dashed lines) and anti-CD3–stimulated cells (filled profile). (E) Effect of protein overexpression on anti-CD3–induced DHE oxidation in 9C127 cells. DHE oxidation was determined after 60 min anti-CD3 stimulation in “normal sized,” GFP+ cells and was normalized to Vector control. The data represent the average of at least four separate experiments (± SEM). #Significantly different from that measured in Vector transfected samples (P < 0.05).
Western Blot Determination of Protein Overexpression.
Overexpression of proteins was measured by Western blot analysis essentially as described previously (20). Cells were transiently transfected with expression plasmids as described above except a plasmid expressing a chimeric murine CD8-Igγ2a protein (21) was used as a transfection marker. After incubation for 16 h to allow protein overexpression, viable cells were enriched by Ficoll separation. CD8 positive, productively transfected cells were purified by magnetic selection using anti-CD8 linked to magnetic beads (Polysciences). Previous data have indicated that this method leads to >95% CD8+ cells. Purified cells were immediately pelleted and processed for Western blot. Cell pellets were suspended in lysis buffer (20 mM Tris HCl, pH 7.5; 1% Triton X-100; 50 mM NaCl; 5 mM EDTA; 10 μg/ml trypsin inhibitor; 10 μg/ml leupeptin; 2 μg/ml aprotinin; 1 mM phenylmethanesulphonyl fluoride; and 10 μg/ml pepstatin), and supernatants stored at –80°C. Equal amounts of protein (50 μg/sample) were separated by SDS-PAGE and blotted onto PVDF membranes, which were blocked with PBS plus 1% BSA. Primary antibodies were incubated with blots (50 mM Tris HCl, pH 7.5; 150 mM NaCl; 0.1% BSA; 0.05% Tween-20) for 2 h at 25°C, all using 1 μg/ml antibody. Blots were washed in the same buffer and incubated with the appropriate horseradish persoxidase (HRP)-conjugated secondary antibody and bands visualized by enhanced chemoluminescence (ECL; Pierce Chemical Co.).
Luciferase Reporter Gene Expression.
9C127 murine T cell hybridoma or human T blasts were transfected as above except that expression plasmids encoding BCl-xL, catalase, CuZnSOD, MnSOD, TPx, or an empty vector (Vector Laboratories) were cotransfected with luciferase reporter vectors (pGL3; Promega) containing the indicated promoters or cDNA. The transfected cells were cultured for 16 h in complete medium, counted, and stimulated in plates precoated with anti-CD3 for 6 h. The cells were then harvested, lysed, and supernatants assayed for luciferase activity as per manufacturers instructions using a plate chemiluminometer (Dynatech). Fold increase in luciferase activity was used as a measure to assess the effect of overexpressed proteins on FasL promoter activity and cotransfected β-galactosidase (assayed with Galacto-Light Plus kit; Tropix) was used to normalize transfection efficiency.
Determination of Cell Death and Apoptosis.
Apoptosis/cell death was induced in 9C127 T cells by incubation on anti-CD3 (2C11) coated plates (5 μg/ml). Anti-CD3–stimulated apoptosis was assessed after 16 h by staining cells with propidium iodide (PI; 25 μg/ml), RNase, and saponin (0.2%) for 60 min at room temperature and then quantitating hypodiploid cells by flow cytometry. Cell death was measured in GFP-transfected cells that had been cultured for 16 h after transfection to allow protein expression and then incubated a further 24 h on immobilized anti-CD3 or in the absence of stimulation. Harvested cells were resuspended in FACS® buffer containing 5 μg/ml PI and analysis of GFP+ and PI− cells was performed by flow cytometry. GFP+ cells were defined as those cells with fluorescence in the top 2 deciles on a log plot.
Immunohistochemical Staining for Activated ERK.
9C127 murine T cell hybridoma or human T blasts were transfected as above using GFP as a transfection marker. After 16 h incubation, cells were stimulated with anti-CD3 as for ROS generation. However, instead of pulsing with oxidation sensitive dyes, cells were fixed with 4% paraformaldehyde for 30 min on ice and then washed and incubated for 20 min on ice in ice-cold 80% MeOH. The cells were then washed and cryopreserved at –80°C for later use. Cryopreservation did not appear to affect cells as measured by GFP staining of cells. The cryopreserved cells were thawed and immunochemically stained for MAP kinase activation essentially as described previously (22). Cells were washed with wash buffer (PBS containing 1% BSA and 0.1% Triton X-100) and blocked with 2% nonfat dry milk at room temperature for 30 min. After a wash, primary antibody to phospho-ERK or pan-ERK was added and incubated on ice for 2 h. The cells were then washed, further stained with biotinylated secondary antibody (Southern Biotechnology Associates, Inc.) and streptavidin-allophycocyanin (APC; BD PharMingen), and APC staining in GFP+ cells was determined by FACS® analysis using a FACSCalibur™ flow cytometer (Becton Dickinson). Staining and wash conditions were developed such that a nonspecific rabbit anti-DNP antibody (provided by Dr. David Segal, National Cancer Institute, National Institutes of Health, Bethesda, MD) exhibited minimal staining over unstained control cells. Specificity of staining was determined using a phosphorylated ERK protein supplied by manufacturers to competitively block specific staining, and by using the MEK1 inhibitor PD98059 (Calbiochem) to block anti-CD3–induced ERK activation.
Statistics.
Tests for statistical significance were done using a paired, two-tailed Student's t test. Data were considered significantly different if P < 0.05.
Results
TCR-stimulated ROS Production.
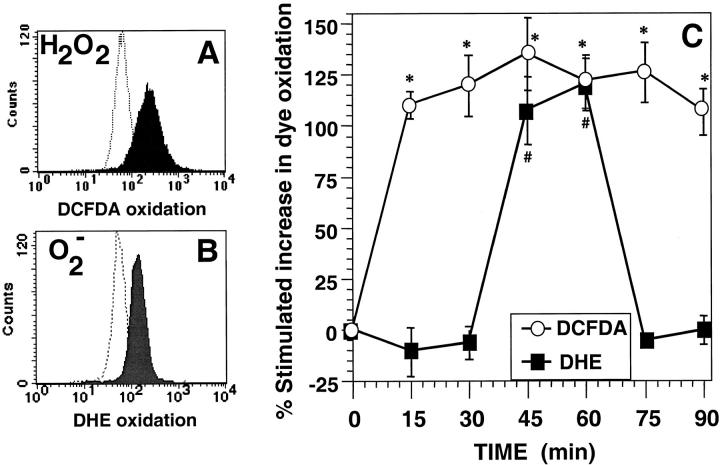
The kinetics of anti-CD3–stimulated ROS production in the murine T cell hybridoma 9C127 were measured using the cell permeant, oxidation sensitive dyes DCFDA or DHE. These dyes are nonfluorescent until oxidized by ROS and an increase in fluorescence of DCFDA (Fig. 1 A) indicates oxidation by peroxides, peroxynitrite, and/or hydroxyl radical (23), while DHE is selectively oxidized by superoxide anion (24) to the fluorescent product ethidium bromide (Fig. 1 B). Thus, DCFDA oxidation was measured as early as 15 min after TCR activation and was maintained throughout the time points monitored (Fig. 1 C). When these experiments were performed using preoxidized forms of DCFDA (fluorescein diacetate or dichlorofluorescein), anti-CD3 stimulation induced no alterations in fluorescence (data not shown) indicating that the observed changes in DCFDA signal were not due to altered uptake, sequestration, or de-esterification of the dye. Using DHE as a selective probe for superoxide anion, anti-CD3–stimulated oxidation of DHE was observed only at 45 and 60 min (Fig. 1 C). Thus, the data are consistent with anti-CD3–stimulated intracellular generation of distinct species of oxidants with different kinetics.
Figure 1.
Anti-CD3–stimulated generation of ROS in 9C127 murine T cell hybridoma. (A) Representative FACS® profile for anti-CD3–induced DCFDA oxidation at 15 min with unstimulated cells (dashed lines) and anti-CD3–stimulated cells (filled profile). (B) Representative FACS® profile for anti-CD3 stimulated DHE oxidation at 60 min with unstimulated cells (dashed lines) and anti-CD3–stimulated cells (filled profile). (C) Kinetics of DCFDA/DHE oxidation in 9C127 cells. 9C127 cells were stimulated with anti-CD3 as described in Materials and Methods and the oxidation of DCFDA (○) and DHE (▪) was determined by FACS® analysis. The data are expressed as the percent stimulated increase in mean channel fluorescence of DCFDA/DHE over unstimulated controls and represent the average of at least five separate experiments (± SEM). *Anti-CD3–stimulated DCFDA oxidation is significantly different from unstimulated controls (P < 0.05). #Anti-CD3–stimulated DHE oxidation is significantly different from unstimulated controls (P < 0.05).
Selective Antioxidants Regulate TCR-stimulated ROS Generation.
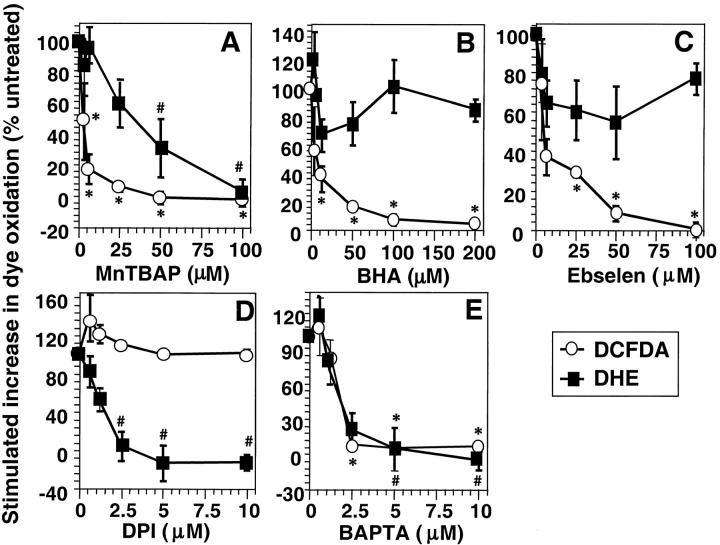
To further characterize the ROS generated upon TCR stimulation in 9C127 cells, antioxidants were used as scavengers of selective species of oxidants. In all experiments DCFDA oxidation was measured 15 min after anti-CD3 cross-linking, while DHE oxidation was determined at 60 min. The SOD mimetic MnTBaP, which has been shown to possess both SOD and peroxidase activity in vitro (25), inhibited oxidation of both DCFDA and DHE in a dose-dependent manner (Fig. 2 A). In contrast, the hydroxyl radical scavenger BHA (Fig. 2 B), and the glutathione peroxidase (GPx) mimetic ebselen (Fig. 2 C) only inhibited DCFDA oxidation while having a limited effect on TCR-stimulated DHE oxidation. The data suggest that hydrogen peroxide or some derivative thereof (as detected by DCFDA oxidation) is rapidly produced after TCR activation and that the early hydrogen peroxide generation is not required for the later generation of superoxide anion (as measured by DHE oxidation).
Figure 2.
Effect of ROS scavengers and chemical inhibitors of ROS. Anti-CD3–induced ROS generation was determined as described in Materials and Methods. DCFDA oxidation (○) was determined after 15 min and DHE oxidation (▪) after 60 min stimulation with anti-CD3 in the presence or absence of titrated concentrations of (A) MnTBaP, (B) ebselen, (C) BHA, (D) diphenylene iodonium (DPI), or (E) BAPTA-AM. The data are normalized to anti-CD3–induced DCFDA/DHE oxidation in the absence of drug and are expressed as percentage change in anti-CD3–stimulated fluorescence compared with cells exposed to drug alone. The data represent the average of at least three experiments (± SEM). *Anti-CD3–stimulated DCFDA oxidation is significantly different from that in the absence of drug (P < 0.05). #Anti-CD3–stimulated DHE oxidation is significantly different from that in the absence of drug (P < 0.05).
Receptor-stimulated generation of superoxide anion was previously shown to be sensitive to DPI, an inhibitor of many flavoprotein dehydrogenases. Anti-CD3–stimulated DHE oxidation was also blocked by DPI in a dose-dependent manner (Fig. 2 D). Nevertheless, DCFDA oxidation was completely insensitive to even high concentrations of DPI, suggesting that superoxide anion generation was not necessary to produce the oxidants that oxidize DCFDA. Oxidation of both dyes, however, was dependent upon TCR-stimulated calcium flux, as the intracellular calcium chelator, BAPTA-AM potently inhibited anti-CD3–induced increases in both DHE and DCFDA fluorescence (Fig. 2 E). In separate experiments, it was found that BAPTA-AM does not scavenge either hydrogen peroxide or superoxide anion when added exogenously to cells (not shown).
Overexpression of Specific Antioxidant Enzymes.
As pharmacologic antioxidants, such as MnTBaP, may not target specific ROS (25), overexpression of specific antioxidant enzymes was used to define TCR-stimulated production of ROS in 9C127 cells. Overexpression of MnSOD (mitochondrial) and CuZnSOD (cytosolic) were used to specifically scavenge superoxide anion. Similarly, catalase (peroxisomal) and thioredoxin peroxidase II (TPx; cytosolic) were used to specifically target hydrogen peroxide. Cells were cotransfected with vectors encoding the antioxidant enzymes and with one expressing GFP which was used as a transfection marker. Using flow cytometric sorting for GFP+ cells followed by Western blot analysis, it was found that high levels of the desired protein were achieved in cells expressing the marker protein (GFP; not shown). Thus, viable transfected cells were defined by both forward light scatter (FSC) versus side scatter (SSC) (R1; Fig. 3 A) and GFP fluorescence (R2; Fig. 3 B), and DHE oxidation was measured in these cells following anti-CD3 stimulation for 60 min (Fig. 3 D).
Stimulation via anti-CD3 cross-linking induced a similar level of DHE oxidation in vector transfected cells as in nonelectroporated cells (not shown). However, upon overexpression of MnSOD or CuZnSOD, anti-CD3–induced DHE oxidation was completely abrogated while catalase or TPx had no effect (Fig. 3 E). This supports the hypothesis that superoxide anion is required for DHE oxidation and also reinforces the antioxidant data in that scavengers of hydrogen peroxide did not affect anti-CD3–stimulated superoxide generation.
In an effort to further elucidate potential sources of TCR-induced ROS generation, BCl-xL was overexpressed, as BCl-2 family members have been shown to regulate mitochondrially derived ROS under basal conditions and in apoptotic cells (26). In addition, dominant negative Rac1 (N17 Rac1) overexpression has been shown to inhibit receptor-stimulated ROS production and has been proposed to act via inhibition of an NADPH oxidase (5). Overexpression of both proteins exerted a potent inhibitory effect on anti-CD3–induced DHE oxidation (Fig. 3 E).
TCR-stimulated ROS Production in Human T Blasts.
To extend these observations to primary T cells, anti-CD3–induced ROS generation was also measured in activated peripheral human T blasts. Kinetics of anti-CD3 stimulated oxidant production demonstrated DCFDA oxidation as early as 15 min and, as in 9C127 cells, was sustained for all time points assayed (Fig. 4 A). In contrast to the results using the T cell hybridoma, anti-CD3–induced DHE oxidation was also observed after 15 min stimulation and this was maintained throughout the assay. Thus, anti-CD3 stimulation of primary activated human T cell blasts also resulted in rapid oxidation of both DHE and DCFDA, albeit at slightly different kinetics than in the murine hybridoma.
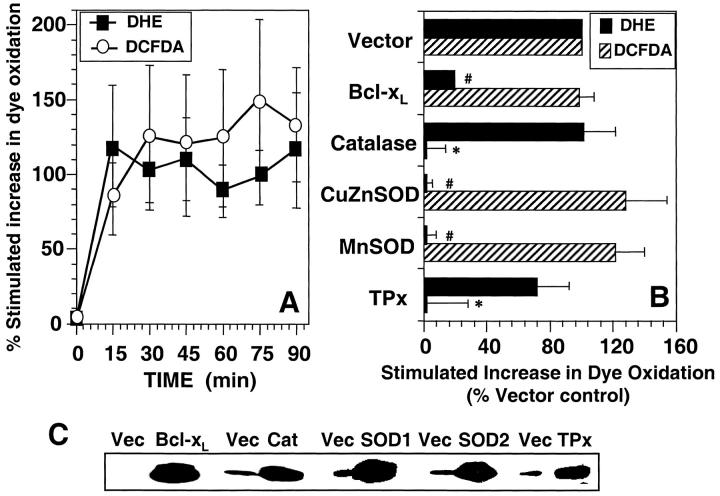
Figure 4.
Anti-CD3–induced ROS generation in human T blasts. (A). Kinetics of anti-CD3–stimulated DCFDA/DHE oxidation human T blasts. Human T blasts were stimulated with anti-CD3 as described in Materials and Methods and the oxidation of DCFDA (○) and DHE (▪) was determined by FACS® analysis. The data are expressed as the percent stimulated increase in mean channel fluorescence of DCFDA/DHE over unstimulated controls and represent the average of at least five separate experiments (± SEM). (B) Effect of protein overexpression on DCFDA/DHE oxidation in human T blasts. Human T blasts were transfected with empty vector (Vector) or expression vectors encoding BCl-xL, catalase, CuZnSOD, MnSOD, or TPx at a 2:1 ratio with one encoding GFP or RFP as a transfection marker. After 16 h incubation, anti-CD3–induced DCFDA (hatched bars) or DHE (closed bars) oxidation was measured as described in Materials and Methods and the legend to Fig. 3. DHE oxidation was measured in GFP+ cells while DCFDA oxidation was measured in RFP-transfected cells, both after 15 min anti-CD3 stimulation and was normalized to Vector control. The data represent the average of at least four separate experiments (± SEM). *Significantly different from that in Vector transfected samples (P < 0.05). #Significantly different from that in Vector transfected samples (P < 0.05). (C) Western blot confirmation of protein overexpression. Human T blasts were transiently transfected with the indicated mammalian expression vectors in twofold excess over a marker plasmid expressing a chimeric CD8-Igγ2a protein. After 16 h incubation, viable CD8+ cells were purified by magnetic bead selection. Protein overexpression was determined in positively selected cells by Western blot as described in Materials and Methods.
Overexpression of antioxidant enzymes in human T blasts was also used to characterize the species of oxidants generated upon anti-CD3 stimulation. Overexpression of MnSOD and CuZnSOD both completely blocked anti-CD3–induced DHE oxidation, while catalase and TPx had little or no inhibitory effect (Fig. 5 B). These data are consistent with that observed in 9C127 cells and are supported by the observation that BCl-xL also completely inhibited TCR-stimulated DHE oxidation. Western blot analysis of transfected cells indicated that there was significant overexpression of proteins in productively transfected cells, which were purified based upon expression of the transfection marker (Fig. 4 C).
Figure 5.
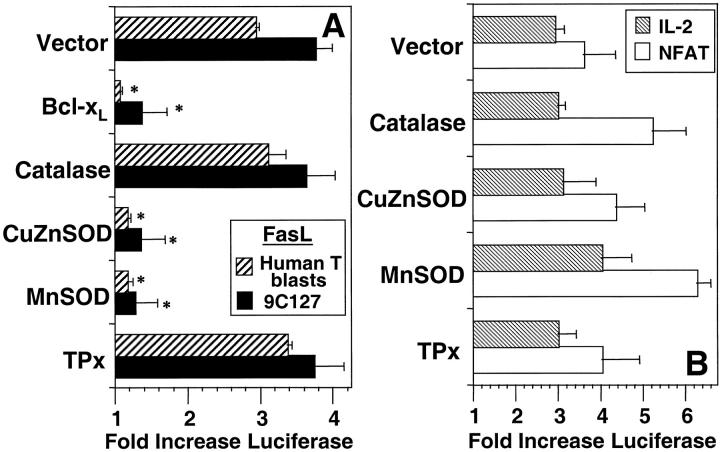
Effect of protein overexpression on anti-CD3–induced reporter plasmid activation. (A) 9C127 cells (solid bars) or human T blasts (hatched bars) were transfected with empty vector (Vector) or expression vectors encoding BCl-xL, catalase, CuZnSOD, MnSOD, or TPx at a 2:1 ratio with one containing a 0.5 kb portion of the FasL promoter inserted into a luciferase reporter vector. (B) 9C127 cells were transfected with the same vectors at a 2:1 ratio with luciferase reporter vectors containing the minimal IL-2 promoter (hatched bars) and a multimerized NFAT binding site (open bars). Anti-CD3–stimulated luciferase activity in cell lysates was measured by chemoluminescence as described in Materials and Methods. Data are expressed as fold induction of luciferase activity over unstimulated and represents the average of at least four experiments. *Significantly different from Vector transfected controls (P < 0.05).
The effects of antioxidant enzyme overexpression on anti-CD3–induced DCFDA oxidation was also determined in the human T blasts. As the emission spectrum of GFP and DCFDA overlap, RFP was used as a cotransfection marker, and DCFDA oxidation was measured in RFP+ cells in a manner analogous to that described with GFP and DHE (Fig. 4 B). As anticipated, overexpression of catalase or TPx completely blocked anti-CD3–induced DCFDA oxidation. Increased levels of CuZnSOD or MnSOD, which would convert any superoxide to hydrogen peroxide, did not significantly affect DCFDA oxidation. Bcl-xL overexpression did not affect TCR-stimulated DCFDA oxidation, further supporting the hypothesis that TCR-stimulated superoxide production was not necessary for the parallel generation of hydrogen peroxide or hydroxyl radical. Furthermore, incubation of human T blasts with DPI (10 μM) also inhibited DHE oxidation (7.2 ± 3.1% of that observed at 15 min in the absence of drug) while having little effect on DCFDA oxidation (89.7 ± 5.4% of that observed at 15 min in the absence of drug). Thus, as in the murine T cell hybridoma, TCR stimulation of human T blasts induces rapid production of both superoxide anion and hydrogen peroxide apparently via distinct pathways.
Requirement for ROS in FasL Gene Expression.
As previous results suggested that ROS were necessary for surface expression of functional FasL (11), the potential role(s) of superoxide anion and/or peroxide in FasL expression were evaluated using a luciferase reporter plasmid driven by the FasL promoter. Anti-CD3 stimulation of 9C127 cells transfected with the FasL promoter luciferase construct induced a fourfold induction of reporter gene activity (Fig. 5 A). Overexpression of either MnSOD or CuZnSOD potently inhibited activation of the FasL promoter, as did the other protein that selectively inhibited DHE oxidation, BCl-xL (Fig. 5 A). Conversely, neither catalase nor TPx had an inhibitory effect on anti-CD3–mediated activation of the FasL promoter. The same profile of inhibition was obtained using the FasL promoter construct in the human T blasts (Fig. 5 A).
Multiple reports have implicated NFAT transactivation in either directly (27–29) or indirectly (18, 30) regulating FasL transcription. In addition, previous results indicated that anti-CD3–induced IL-2 secretion could be pharmacologically distinguished from FasL transcription in a murine T cell hybridoma (11). Thus, the effects of the antioxidant enzymes were measured using reporter constructs linked to the IL-2 promoter and one containing consensus binding sites for the transcription factor, NFAT. In the 9C127 cells, there was no effect of overexpression of any of the antioxidant enzymes on anti-CD3–stimulated transcription driven by the IL-2 promoter or NFAT (Fig. 5 B). In human T blasts, anti-CD3–induced surface expression of the early activation marker, CD69, was also not inhibited in cells transfected with the antioxidant enzymes (not shown). Thus, TCR-stimulated generation of superoxide anion selectively regulates gene and surface protein expression in T cells. Furthermore, regulation of FasL expression by superoxide anion does not appear to be through effects on NFAT.
Role of Superoxide Anion in Anti-CD3–induced Cell Death.
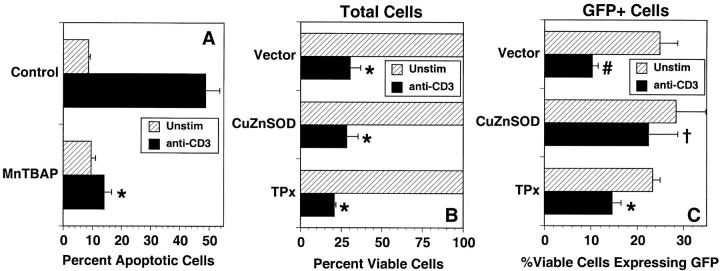
To test the significance of this selective role of ROS, the functional role of superoxide anion in anti-CD3–stimulated apoptosis and cell death was determined. Previous studies using antioxidants had suggested that ROS generation was required for anti-CD3–stimulated apoptosis of mature T cells (11). Incubation with the SOD mimetic, MnTBaP, at a concentration (50 μM) that blocked DHE oxidation, almost completely blocked anti-CD3–stimulated apoptosis of 9C127 cells (Fig. 6 A). This suggests that superoxide anion is necessary for AICD, but as MnTBaP also has peroxidase activity (Fig. 2), additional experiments in cells overexpressing CuZnSOD were performed to solidify a role for superoxide. 9C127 cells were cotransfected with CuZnSOD and GFP as above and after 16 h incubation to allow protein expression the cells were stimulated for 24 h on immobilized anti-CD3. As loss of membrane integrity (cell death) will result in loss of GFP within the cell, the expression of GFP was used as an indicator of whether overexpression of a cotransfected protein would lead to a survival advantage. In vector-transfected cells, anti-CD3 stimulation led to ∼70% death of total cells (Fig. 6 B) or 60% of those cells detected as GFP+ (Fig. 6 C). The same data were obtained when an irrelevant protein (β-galactosidase) was overexpressed (not shown). In cells transfected with CuZnSOD, anti-CD3–induced death of the total cell population was similar to that in the vector transfected samples. However, the stimulated death of those cells productively transfected with CuZnSOD (GFP+ cells) was significantly inhibited (only 21% cell death; Fig. 6 C), suggesting that cells overexpressing CuZnSOD were selectively resistant to anti-CD3–induced cell death. These data suggest that the inhibition of FasL expression by SOD overexpression is also reflected by an inhibition of anti-CD3–induced cell death. Overexpression of TPx also induced a slight decrease in the death of GFP+ cells (Fig. 6 C), although it was not significantly different from the death of vector transfected cells.
Figure 6.
Functional role for superoxide in anti-CD3–stimulated cell death. (A) 9C127 cells were unstimulated (hatched bars) or incubated on immobilized anti-CD3 (solid bars) for 16 h in the presence or absence of the SOD mimetic MnTBaP. Cells were harvested and apoptosis (hypodiploid cells) was measured after staining with propidium iodide by flow cytometry as described in Materials and Methods. The data are expressed as the percent apoptotic cells and represent the average of three separate experiments ± SEM. *Significantly different from untreated controls (P < 0.002). (B and C) 9C127 cells were transfected with empty vector (Vector) or an expression vector encoding CuZnSOD or TPx at a 2:1 ratio with one encoding GFP as a transfection marker. After 16 h culture, cells were incubated for 24 h further on immobilized anti-CD3 (solid bars) or in the absence of stimulation (hatched bars). The percent total viable (PI−) cells (B) and those expressing GFP (C) were quantitated by flow cytometry. Anti-CD3–stimulated death of productively transfected (GFP+) viable cells for vector transfected cells was 58.7 ± 6.3%, while that in CuZnSOD-transfected cells was 21.1 ± 7.4% (significantly different from vector control; P < 0.05) and in TPx overexpressing cells it was 39.7 ± 4.3% (not significantly different from vector control; P > 0.2). The data represent the average of three separate experiments ± SEM. *Significantly different from unstimulated controls (P < 0.01); #Significantly different from unstimulated controls (P < 0.05); †Not significantly different from unstimulated controls (P > 0.1).
Regulation of ERK Activation by ROS Generation.
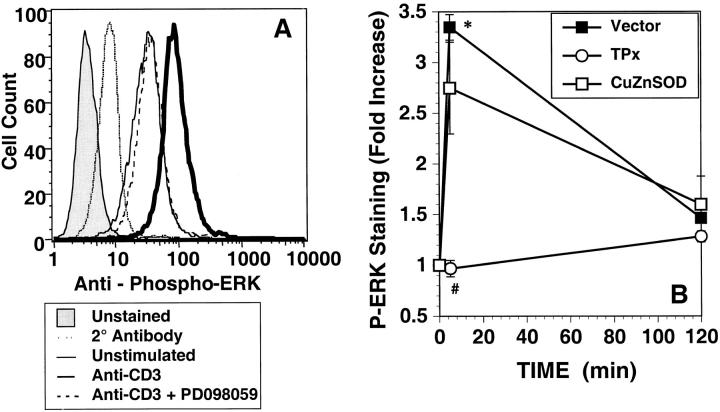
Further analysis of the requirement for ROS in anti-CD3–stimulated signal transduction focused upon the MAP kinase family, which have been shown to be sensitive to both exogenous and endogenously generated ROS (31, 32). To measure MAP kinase activation in the transiently transfected cell systems, a protocol for immunohistochemical staining of cells with an antibody specific for phosphorylated ERK1/2 (22) was adapted to lymphocytes transfected with GFP. Antibody specificity was demonstrated by competition of binding with a phosphorylated ERK protein supplied by the manufacturers, and by incubation with the MEK1/2 inhibitor PD98059 (Fig. 7 A) during anti-CD3 stimulation. Both approaches completely blocked anti-CD3–induced increases in staining and total ERK, as visualized with a pan-specific anti-ERK antibody, was unchanged under all conditions analyzed (not shown). In vector-transfected 9C127 cells, anti-CD3 induced ERK phosphorylation peaked at 5 min, and decreased to background after 2 h (Fig. 7 B). Cells transfected with TPx displayed no anti-CD3–induced ERK activation, while phospho-ERK staining was slightly decreased in cells overexpressing CuZnSOD. Similar inhibition of anti-CD3–induced phospho-ERK staining in TPx overexpressing cells was also observed in human T blasts (not shown). Thus, anti-CD3–stimulated ERK phosphorylation is selectively sensitive to hydrogen peroxide generation.
Figure 7.
Anti-CD3–stimulated ERK phosphorylation. (A) Anti-CD3–induced phosphorylation of ERK was measured after 5 min stimulation in GFP+ 9C127 cells. Stimulation was performed in the presence (dashed line) or absence (heavy solid line) of the MEK1 inhibitor PD098059 by immunohistochemical staining using a phospho-specific antibody for ERK1/2 as described in Materials and Methods. Staining was quantitated by flow cytometry after stain with biotin goat anti–rabbit and streptavidin-APC. Profiles of unstimulated cells (solid line) and anti-CD3–stimulated cells stained with secondary antibody alone (dotted line) or unstained (shaded profile) are also shown. Staining with pan-anti-ERK1/2 was not altered in any sample (not shown). (B) 9C127 cells were transfected with empty vector (Vector) (▪) or expression vectors encoding CuZnSOD (□) or TPx (○) at a 2:1 ratio with one encoding GFP as a transfection marker. The data represent the anti-CD3–stimulated fold increase in mean APC fluorescence in GFP+ cells and are representative of at least three separate experiments. *Significantly different from unstimulated controls (P < 0.05). #Significantly different from Vector-transfected samples (P < 0.05).
Discussion
In this study, antigen receptor signaling of mature T cells has been shown to stimulate discrete generation of both superoxide anion and hydrogen peroxide by pathways distinguishable using pharmacologic and molecular tools. To our knowledge, this is the first demonstration of such a dichotomy in receptor-stimulated ROS production. In addition to this novel observation, the results suggest that the two species of oxidants produced after TCR stimulation regulate the activation of distinct signal transduction pathways. Thus, the data suggest that superoxide anion targets a pro-death signal in mature T cells (FasL expression), while hydrogen peroxide production regulates what has been typically characterized as a pro-life or proliferative signal (ERK activation).
Previous data had suggested that T cell activation and TCR signal transduction may involve ROS generation (11), but did not address the potential role(s), the species of oxidant, nor the kinetics of generation. Data from the present study support the hypothesis that TCR signaling rapidly activates 2 distinct pathways of ROS generation. Hydrogen peroxide production in the 9C127 cells measured via DCFDA oxidation preceded superoxide anion generation, suggesting that TCR-induced hydrogen peroxide was not derived from dismutation of superoxide anion. Furthermore, the selective ability of BCl-xL and DPI to inhibit superoxide generation without effect on DCFDA oxidation supports the hypothesis of discrete pathways for TCR-stimulated generation of superoxide and hydrogen peroxide. Similarly, early generation of hydrogen peroxide was not required for superoxide generation, as increased levels of catalase or TPx did not greatly affect DHE oxidation, enhancing the distinction between the two signaling pathways.
A recent report has suggested that TNF signaling can induce two distinguishable pathways of oxidant generation (33), where one is sensitive to overexpression of N17 Rac1 and is antiapoptotic and the other is potentially derived from mitochondria and regulates a proapoptotic pathway. In another study, mitochondrially derived ROS generation has been suggested to play a regulatory role in cell function and signaling and affect activation of a proapoptotic MAP kinase, JNK1 (34). In the current study, the effects of BCl-xL overexpression suggest a potential role for mitochondria in superoxide generation, but much more experiments are required to address this hypothesis.
PDGF, angiotensin II, or IL-1 have been proposed to induce generation of superoxide anion, which was inhibited by overexpression of a DN-Rac1 (N17 Rac1) or incubation with DPI (3, 35). These and other reports have led to the hypothesis that an NADPH/NADH oxidase is a source of receptor initiated ROS production (6, 36). N17 Rac1 and DPI also potently inhibited TCR-stimulated superoxide generation in the current study, but it is not clear that T lymphocytes express an oxidase capable of this production. We are currently investigating whether a form of this oxidase is expressed in T cells as has been shown in B lymphocytes (37).
Given that superoxide can be and is rapidly converted to hydrogen peroxide in many cell systems, it was surprising that TCR signals activated a separate pathway that directly leads to hydrogen peroxide production. The mechanism by which TCR stimulation induces hydrogen peroxide production in the absence of superoxide is unclear, but subcellular localization or perhaps the prerequisite of some intracellular signaling event may control the generation of distinct ROS. This separation in time and/or space may limit or change the redox sensitive targets for these discrete reactive oxygen species (i.e. phosphatases, GTP-binding proteins, or protein kinases [PKC, JNK, src]; references 38–40).
MAP kinase activation is a common target of exogenous or endogenously generated ROS (31, 32). Indeed, Hunt and colleagues suggested that mitogen-induced ERK2 activation was redox sensitive in T cells (41). The current study suggests that hydrogen peroxide regulates ERK phosphorylation, and we are currently pursuing the mechanism by which this occurs.
Exogenous oxidants and redox active substances (chemotherapeutic agents, MAP kinase activation, H2O2) can regulate FasL gene expression in the absence of TCR stimulation (42–44). These effects were mapped to sites both within and outside of the –511 bp region that has been proposed to regulate antigen-stimulated FasL expression (18). Thus, although TCR-induced ROS generation may also affect FasL transcription at upstream sites, the current data suggest that superoxide anion is required for TCR-mediated control of FasL expression within the minimal promoter. The minimal promoter contains a nuclear factor (NF)-κB site (45, 46) and 2 potential AP-1 sites (42, 47) that may be redox sensitive. Faris et al. have suggested that active MEKK1-induced binding of a jun/ATF-2 heterodimer at –330 bp (42), and it has been suggested that JNK-mediated jun activation is important for FasL expression (48). We are currently assessing if these transcription factors/enzymes are targets of TCR-stimulated superoxide anion production.
It does not appear that activation of NFAT, which has been proposed to be a key regulator of FasL transcription (27, 29), is modulated by superoxide anion. One means by which NFAT has been proposed to regulate FasL expression indirectly, is through control of TCR-induced expression of the egr family of transcription factors (18, 30). The egr-family of transcription factors have been proposed to directly transactivate FasL expression (18, 49) and reports suggest that at least egr-1 activation is redox sensitive (50). However, in 9C127 cells, transcriptional activation driven by the 16 mer egr-2/3 binding site of the FasL promoter (FLRE) (at –214 to –207) identified by Mittlestadt et al. (18) was not affected by overexpression of antioxidant enzymes (data not shown).
While receptor-stimulated generation of superoxide anion has precedence, utilization of superoxide for regulation of TCR-mediated FasL expression was unexpected. In systems of receptor-stimulated generation of superoxide anion, in general it has been shown that hydrogen peroxide, derived from dismutation of the superoxide anion and sensitive to catalase or peroxidase, were the relevant oxidant species that regulated signaling (8). Although the immediate target regulated by superoxide is unknown, the biochemical effects of superoxide are often mediated through effects on iron-sulfur (4Fe-4S) clusters (51). Examples of this are the superoxide-mediated inactivation of aconitase (52) or the regulation of the iron response binding protein (IBP; reference 53). Superoxide also reacts avidly with nitric oxide (NO) to form peroxynitrite. We have previously demonstrated a role for NO in anti-CD3–induced death of mature T cells (20). In addition, anti-CD3 stimulated formation of nitrotyrosine, which may be formed upon peroxynitrite production (20, 54). Thus, an important mechanism for superoxide may be based on its rapid reaction with NO.
Recent studies from Marrack and colleagues have suggested that superantigen-induced death of lymphocytes in vivo requires generation of ROS and appears to be independent of Fas/FasL interactions (16). It may be that superantigen stimulates similar signaling mechanisms to induce ROS generation at greater levels than anti-CD3 and that this level of oxidants directly induces cell death. Our results suggest that antioxidants can also regulate anti-CD3–induced cell death, perhaps via an alternative mechanism. Therefore, depending upon the stimulus, receptor stimulated ROS generation can regulate early signal transduction steps and/or sensitivity to death.
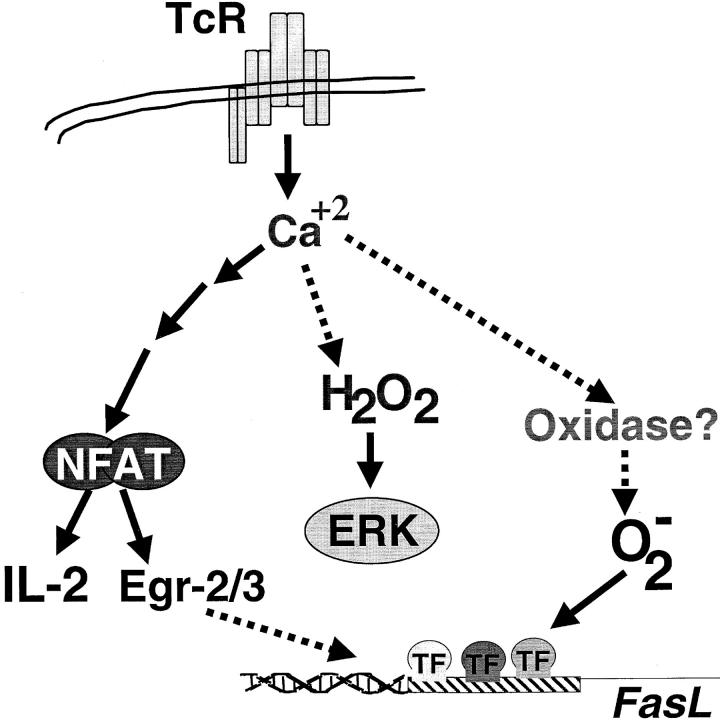
Thus, in this study, stimulation via the TCR was shown to induce generation of ROS that served to regulate signal transduction in T lymphocytes. The results suggest that TCR utilizes distinct biochemical pathways to yield production of two discrete species of oxidants, superoxide anion and hydrogen peroxide (Fig. 8) . These ROS, in turn, selectively regulate independent signaling pathways that can affect cell death decisions (FasL) or proliferative responses (ERK). Interestingly, it has recently been suggested that exposure to mild oxidative stress can enhance TCR-stimulated activation of MAP kinases and IL-2 transcription (55), and that synovial fluid T cells isolated from arthritic joints display redox-dependent alterations in signaling pathways (56). This suggests that there are redox sensitive pathways in T cells that may be altered by exposure to exogenous oxidative stress, as may be encountered in disease states such as AIDS or arthritis. The results of this study may suggest that T cells generate their own endogenous oxidative stress to regulate similar pathways.
Figure 8.
Proposed scheme for TCR-stimulated ROS generation. TCR signaling induces both hydrogen peroxide (H2O2) and superoxide (O2 − in a calcium dependent manner in pathway(s) independent of NFAT activation. Hydrogen peroxide production, from an unknown source, regulates ERK activation. Superoxide anion may be derived from an NADH/NADPH oxidase and is required for FasL promoter activation.
Acknowledgments
This maunscript is dedicated to the memory of Dr. William R. Mancini. The authors wish to thank Jon Ashwell, Silvio Gutkind, and Charles Zacharchuk for providing expression and luciferase reporter plasmids. We thank Yoichi Osawa, Pierre Henkart, and Jon Ashwell for critical reading of the manuscript.
This project was supported by a Scientist Development Grant (#0030033N) from the American Heart Association and by funds from the American Red Cross.
Footnotes
Abbreviations used in this paper: BHA, butylated hydroxyanisole; CuZnSOD, copper/zinc superoxide dismutase; DCFDA, dichlorodihydrofluorescein diacetate; DHE, dihydroethidium; DPI, diphenylene iodonium; ERK, extracellular signal–regulated kinase; GFP, green fluorescent protein; MAP, mitogen-activated protein; MnSOD, manganese superoxide dismutase; NFAT, nuclear factor of activated T cells; RFP, red fluorescent protein; ROS, reactive oxygen species; TPx, thioredoxin peroxidase.
References
- 1.Davies, K.J. 1995. Oxidative stress: the paradox of aerobic life. Biochem. Soc. Symp. 61:1–31. [DOI] [PubMed] [Google Scholar]
- 2.Bae, Y.S., S.W. Kang, M.S. Seo, I.C. Baines, E. Tekle, P.B. Chock, and S.G. Rhee. 1997. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272:217–221. [PubMed] [Google Scholar]
- 3.Ushio-Fukai, M., R.W. Alexander, M. Akers, and K.K. Griendling. 1998. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J. Biol. Chem. 273:15022–15029. [DOI] [PubMed] [Google Scholar]
- 4.Lee, J.R., and G.A. Koretzky. 1998. Production of reactive oxygen intermediates following CD40 ligation correlates with c-Jun N-terminal kinase activation and IL-6 secretion in murine B lymphocytes. Eur. J. Immunol. 28:4188–4197. [DOI] [PubMed] [Google Scholar]
- 5.Griendling, K.K., D. Sorescu, and M. Ushio-Fukai. 2000. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 86:494–501. [DOI] [PubMed] [Google Scholar]
- 6.Suh, Y.A., R.S. Arnold, B. Lassegue, J. Shi, X. Xu, D. Sorescu, A.B. Chung, K.K. Griendling, and J.D. Lambeth. 1999. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 401:79–82. [DOI] [PubMed] [Google Scholar]
- 7.Sundaresan, M., Z.X. Yu, V.J. Ferrans, K. Irani, and T. Finkel. 1995. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 270:296–299. [DOI] [PubMed] [Google Scholar]
- 8.Arnold, R.S., J. Shi, E. Murad, A.M. Whalen, C.Q. Sun, R. Polavarapu, S. Parthasarathy, J.A. Petros, and J.D. Lambeth. 2001. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc. Natl. Acad. Sci. USA. 98:5550–5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang, S.W., H.Z. Chae, M.S. Seo, K. Kim, I.C. Baines, and S.G. Rhee. 1998. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J. Biol. Chem. 273:6297–6302. [DOI] [PubMed] [Google Scholar]
- 10.Babior, B.M. 1992. The respiratory burst oxidase. Adv. Enzymol. 65:49–88. [DOI] [PubMed] [Google Scholar]
- 11.Williams, M.S., and P.A. Henkart. 1996. The role of reactive oxygen intermediates in TcR-induced death of T cell blasts and hybridomas. J. Immunol. 157:2395–2402. [PubMed] [Google Scholar]
- 12.Sekkat, C., J. Dornand, and M. Gerber. 1988. Oxidative phenomena are implicated in human T-cell stimulation. Immunology. 63:431–437. [PMC free article] [PubMed] [Google Scholar]
- 13.Pani, G., R. Colavitti, S. Borrello, and T. Galeotti. 2000. Endogenous oxygen radicals modulate protein tyrosine phosphorylation and JNK-1 activation in lectin-stimulated thymocytes. Biochem. J. 347:173–181. [PMC free article] [PubMed] [Google Scholar]
- 14.Los, M., H. Schenk, K. Hexel, P.A. Baeuerle, W. Droge, and K. Schulze-Osthoff. 1995. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J. 14:3731–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber, G.F., S. Abromson-Leeman, and H. Cantor. 1995. A signaling pathway coupled to T cell receptor ligation by MMTV superantigen leading to transient activation and programmed cell death. Immunity. 2:363–372. [DOI] [PubMed] [Google Scholar]
- 16.Hildeman, D.A., T. Mitchell, T.K. Teague, P. Henson, B.J. Day, J. Kappler, and P.C. Marrack. 1999. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 10:735–744. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, H.J., T. Yan, T.D. Oberley, and L.W. Oberley. 1999. Comparison of effects of two polymorphic variants of manganese superoxide dismutase on human breast MCF-7 cancer cell phenotype. Cancer Res. 59:6276–6283. [PubMed] [Google Scholar]
- 18.Mittelstadt, P.R., and J.D. Ashwell. 1998. Cyclosporin A-sensitive transcription factor Egr-3 regulates Fas ligand expression. Mol. Cell Biol. 18:3744–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrak, D., S.A. Memon, M.J. Birrer, J.D. Ashwell, and C.M. Zacharchuk. 1994. Dominant negative mutant of c-Jun inhibits NF-AT transcriptional activity and prevents IL-2 gene transcription. J. Immunol. 153:2046–2051. [PubMed] [Google Scholar]
- 20.Williams, M.S., S. Noguchi, P.A. Henkart, and Y. Osawa. 1998. Nitric oxide synthase plays a signaling role in TCR-triggered apoptotic death. J. Immunol. 161:6526–6531. [PubMed] [Google Scholar]
- 21.Yao, X.R., H. Flaswinkel, M. Reth, and D.W. Scott. 1995. Immunoreceptor tyrosine-based activation motif is required to signal pathways of receptor-mediated growth arrest and apoptosis in murine B lymphoma cells. J. Immunol. 155:652–661. [PubMed] [Google Scholar]
- 22.Poynter, M.E., Y.M. Janssen-Heininger, S. Buder-Hoffmann, D.J. Taatjes, and B.T. Mossman. 1999. Measurement of oxidant-induced signal transduction proteins using cell imaging. Free Radic. Biol. Med. 27:1164–72. [DOI] [PubMed] [Google Scholar]
- 23.Crow, J.P. 1997. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide. 1:145–157. [DOI] [PubMed] [Google Scholar]
- 24.Rothe, G., and G. Valet. 1990. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2',7'-dichlorofluorescin. J. Leukoc. Biol. 47:440–448. [PubMed] [Google Scholar]
- 25.Day, B.J., I. Fridovich, and J.D. Crapo. 1997. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch. Biochem. Biophys. 347:256–262. [DOI] [PubMed] [Google Scholar]
- 26.Esposti, M.D., I. Hatzinisiriou, H. McLennan, and S. Ralph. 1999. Bcl-2 and mitochondrial oxygen radicals. New approaches with reactive oxygen species-sensitive probes. J. Biol. Chem. 274:29831–29837. [DOI] [PubMed] [Google Scholar]
- 27.Latinis, K.M., L.L. Carr, E.J. Peterson, L.A. Norian, S.L. Eliason, and G.A. Koretzky. 1997. Regulation of CD95 (Fas) ligand expression by TCR-mediated signaling events. J. Immunol. 158:4602–4611. [PubMed] [Google Scholar]
- 28.Li-Weber, M., O. Laur, and P.H. Krammer. 1999. Novel Egr/NF-AT composite sites mediate activation of the CD95 (APO-1/Fas) ligand promoter in response to T cell stimulation. Eur. J. Immunol. 29:3017–3027. [DOI] [PubMed] [Google Scholar]
- 29.Dzialo-Hatton, R., J. Milbrandt, R.D. Hockett, Jr., and C.T. Weaver. 2001. Differential expression of Fas ligand in Th1 and Th2 cells is regulated by early growth response gene and NF-AT family members. J. Immunol. 166:4534–4542. [DOI] [PubMed] [Google Scholar]
- 30.Rengarajan, J., P.R. Mittelstadt, H.W. Mages, A.J. Gerth, R.A. Kroczek, J.D. Ashwell, and L.H. Glimcher. 2000. Sequential involvement of NFAT and Egr transcription factors in FasL regulation. Immunity. 12:293–300. [DOI] [PubMed] [Google Scholar]
- 31.Guyton, K.Z., Y. Liu, M. Gorospe, Q. Xu, and N.J. Holbrook. 1996. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J. Biol. Chem. 271:4138–4142. [DOI] [PubMed] [Google Scholar]
- 32.Mukhin, Y.V., M.N. Garnovskaya, G. Collinsworth, J.S. Grewal, D. Pendergrass, T. Nagai, S. Pinckney, E.L. Greene, and J.R. Raymond. 2000. 5-Hydroxytryptamine1A receptor/Gibetagamma stimulates mitogen-activated protein kinase via NAD(P)H oxidase and reactive oxygen species upstream of src in chinese hamster ovary fibroblasts. Biochem. J. 347:61–67. [PMC free article] [PubMed] [Google Scholar]
- 33.Deshpande, S.S., P. Angkeow, J. Huang, M. Ozaki, and K. Irani. 2000. Rac1 inhibits TNF-alpha-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASEB J. 14:1705–1714. [DOI] [PubMed] [Google Scholar]
- 34.Nemoto, S., K. Takeda, Z.X. Yu, V.J. Ferrans, and T. Finkel. 2000. Role for mitochondrial oxidants as regulators of cellular metabolism. Mol. Cell Biol. 20:7311–7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundaresan, M., Z.X. Yu, V.J. Ferrans, D.J. Sulciner, J.S. Gutkind, K. Irani, P.J. Goldschmidt-Clermont, and T. Finkel. 1996. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem. J. 318:379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambeth, J.D., G. Cheng, R.S. Arnold, and W.A. Edens. 2000. Novel homologs of gp91phox. Trends Biochem. Sci. 25:459–461. [DOI] [PubMed] [Google Scholar]
- 37.Condino-Neto, A., and P.E. Newburger. 1998. NADPH oxidase activity and cytochrome b558 content of human Epstein-Barr-virus-transformed B lymphocytes correlate with expression of genes encoding components of the oxidase system. Arch. Biochem. Biophys. 360:158–164. [DOI] [PubMed] [Google Scholar]
- 38.Krejsa, C.M., and G.L. Schieven. 1998. Impact of oxidative stress on signal transduction control by phosphotyrosine phosphatases. Environ. Health Perspect. 106:1179–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lander, H.M. 1997. An essential role for free radicals and derived species in signal transduction. FASEB J. 11:118–124. [PubMed] [Google Scholar]
- 40.Adler, V., Z. Yin, S.Y. Fuchs, M. Benezra, L. Rosario, K.D. Tew, M.R. Pincus, M. Sardana, C.J. Henderson, C.R. Wolf, R.J. Davis, and Z. Ronai. 1999. Regulation of JNK signaling by GSTp. EMBO J. 18:1321–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldstone, S.D., and N.H. Hunt. 1997. Redox regulation of the mitogen-activated protein kinase pathway during lymphocyte activation. Biochim. Biophys. Acta. 1355:353–360. [DOI] [PubMed] [Google Scholar]
- 42.Faris, M., K.M. Latinis, S.J. Kempiak, G.A. Koretzky, and A. Nel. 1998. Stress-induced Fas ligand expression in T cells is mediated through a MEK kinase 1-regulated response element in the Fas ligand promoter. Mol. Cell Biol. 18:5414–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauer, M.K.A., M. Vogt, M. Los, J. Siegel, S. Wesselborg, and K. Schulze-Osthoff. 1998. Role of reactive oxygen intermediates in activation-induced CD95 (APO-1/Fas) ligand expression. J. Biol. Chem. 273:8048–8055. [DOI] [PubMed] [Google Scholar]
- 44.Hug, H., S. Strand, A. Grambihler, J. Galle, V. Hack, W. Stremmel, P.H. Krammer, and P.R. Galle. 1997. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J. Biol. Chem. 272:28191–28193. [DOI] [PubMed] [Google Scholar]
- 45.Kasibhatla, S., L. Genestier, and D.R. Green. 1999. Regulation of fas-ligand expression during activation-induced cell death in T lymphocytes via nuclear factor kappaB. J. Biol. Chem. 274:987–992. [DOI] [PubMed] [Google Scholar]
- 46.Matsui, K., A. Fine, B. Zhu, A. Marshak-Rothstein, and S.T. Ju. 1998. Identification of two NF-kappa B sites in mouse CD95 ligand (Fas ligand) promoter: functional analysis in T cell hybridoma. J. Immunol. 161:3469–3473. [PubMed] [Google Scholar]
- 47.Eichhorst, S.T., M. Muller, M. Li-Weber, H. Schulze-Bergkamen, P. Angel, and P.H. Krammer. 2000. A novel AP-1 element in the CD95 ligand promoter is required for induction of apoptosis in hepatocellular carcinoma cells upon treatment with anticancer drugs. Mol. Cell Biol. 20:7826–7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, J., J.X. Gao, K. Salojin, Q. Shao, M. Grattan, C. Meagher, D.W. Laird, and T.L. Delovitch. 2000. Regulation of fas ligand expression during activation-induced cell death in T cells by p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase. J. Exp. Med. 191:1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mittelstadt, P.R., and J.D. Ashwell. 1999. Role of Egr-2 in Up-regulation of Fas ligand in normal T Cells and Aberrant Double-negative lpr and gld T Cells. J. Biol. Chem. 274:3222–3227. [DOI] [PubMed] [Google Scholar]
- 50.Ohba, M., M. Shibanuma, T. Kuroki, and K. Nose. 1994. Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J. Cell Biol. 126:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rouault, T.A., and R.D. Klausner. 1996. Iron-sulfur clusters as biosensors of oxidants and iron. Trends Biochem. Sci. 21:174–177. [PubMed] [Google Scholar]
- 52.Gardner, P.R., and I. Fridovich. 1992. Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J. Biol. Chem. 267:8757–8763. [PubMed] [Google Scholar]
- 53.Cairo, G., E. Castrusini, G. Minotti, and A. Bernelli-Zazzera. 1996. Superoxide and hydrogen peroxide-dependent inhibition of iron regulatory protein activity: a protective stratagem against oxidative injury. FASEB J. 10:1326–1335. [DOI] [PubMed] [Google Scholar]
- 54.Brito, C., M. Naviliat, A.C. Tiscornia, F. Vuillier, G. Gualco, G. Dighiero, R. Radi, and A.M. Cayota. 1999. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J. Immunol. 162:3356–3366. [PubMed] [Google Scholar]
- 55.Hehner, S.P., R. Breitkreutz, G. Shubinsky, H. Unsoeld, K. Schulze-Osthoff, M.L. Schmitz, and W. Droge. 2000. Enhancement of T cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J. Immunol. 165:4319–4328. [DOI] [PubMed] [Google Scholar]
- 56.Gringhuis, S.I., A. Leow, E.A. Papendrecht-Van Der Voort, P.H. Remans, F.C. Breedveld, and C.L. Verweij. 2000. Displacement of linker for activation of T cells from the plasma membrane due to redox balance alterations results in hyporesponsiveness of synovial fluid T lymphocytes in rheumatoid arthritis. J. Immunol. 164:2170–2179. [DOI] [PubMed] [Google Scholar]