Abstract
Primary biliary cirrhosis (PBC) is characterized by an intense biliary inflammatory CD4+ and CD8+ T cell response. Very limited information on autoantigen-specific cytotoxic T lymphocyte (CTL) responses is available compared with autoreactive CD4+ T cell responses. Using peripheral blood mononuclear cells (PBMCs) from PBC, we identified an HLA-A2–restricted CTL epitope of the E2 component of pyruvate dehydrogenase (PDC-E2), the immunodominant mitochondrial autoantigen. This peptide, amino acids 159–167 of PDC-E2, induces specific MHC class I–restricted CD8+ CTL lines from 10/12 HLA-A2+ PBC patients, but not controls, after in vitro stimulation with antigen-pulsed dendritic cells (DCs). PDC-E2–specific CTLs could also be generated by pulsing DCs with full-length recombinant PDC-E2 protein. Furthermore, using soluble PDC-E2 complexed with either PDC-E2–specific human monoclonal antibody or affinity-purified autoantibodies against PDC-E2, the generation of PDC-E2–specific CTLs, occurred at 100-fold and 10-fold less concentration, respectively, compared with soluble antigen alone. Collectively, these data demonstrate that autoantibody, helper, and CTL epitopes all contain a shared peptide sequence. The finding that autoantigen–immune complexes can not only cross-present but also that presentation of the autoantigen is of a higher relative efficiency, for the first time defines a unique role for autoantibodies in the pathogenesis of an autoimmune disease.
Keywords: autoimmunity, cytotoxic T cells, cholangitis, epitopes, cross-priming
Introduction
While a large body of data has accumulated on the precise identification of the autoAgs that serve as targets for humoral immune responses and significant data exists on the nature of the autoAg-specific CD4+ Th cell responses in primary biliary cirrhosis (PBC)*, information on the nature of autoAg-specific CTL responses is relatively unknown (1, 2). Characterization of the CD8+ CTL response in PBC is important as such cells are likely involved in the lysis of biliary epithelial cells (BECs), the major target involved in the pathogenesis of this disease. Identification of the autoAg peptide, that is the target of MHC class I–restricted CTLs in PBC, will provide an important initial step not only in determining the mechanism by which tolerance to the self-peptide is abrogated, but also in facilitating the potential for therapeutic strategies.
Several sequence patterns or motifs for peptides that bind to particular MHC molecules or groups of MHC molecules have been defined by analysis of peptides presented by MHC class I molecules (3, 4). Amongst the best-studied motif is that of the MHC class I HLA-A2 molecule which is prevalent in 50% of the Caucasian population in the Western Hemisphere (5, 6). In fact, motif prediction analysis of HLA-A2 has led to the identification of the dominant epitopes of the human myelin basic proteins (7) and glutamic acid decarboxylase (8), the key autoAgs in multiple sclerosis and insulin-dependent diabetes mellitus, respectively.
Our present efforts focused on determining whether an HLA-A2–restricted CTL epitope of the E2 component of pyruvate dehydrogenase (PDC-E2), the immunodominant autoAg of PBC, could be identified. We report herein the successful use of this predictive approach to identify epitopes for autoreactive CTLs in PBC. Specifically, peptide epitopes, identified by scanning the PDC-E2 sequences for the presence of potential HLA-A2–binding motifs, were synthesized and tested in vitro for their ability to induce HLA-A2–restricted peptide-specific responses. In addition to the traditional cytotoxicity assay for CTLs, we also used a single-cell based intracellular cytokine staining assay (9) in efforts to better characterize the autoreactive CTLs. Using this strategy, we identified one HLA-A2–restricted peptide, amino acids 159–167 of PDC-E2, which is capable of inducing PDC-E2–specific CD8+ CTL lines in vitro in the majority of HLA-A2+ PBC patients but not from any HLA-A2+ control donors.
We also hypothesized that PDC-E2–specific autoAbs, detected exclusively in PBC patients, may capture PDC-E2 Ags released from dying cells to form PDC-E2 immune complexes (ICs), which may facilitate the uptake of PDC-E2 Ag through Fcγ receptors (FcγRs) on professional APCs. The fact that HLA-A2–expressing APCs when incubated with the recombinant PDC-E2 (rPDC-E2) protein could lead to the generation of CD8+ CTL specific for the PDC-E2 peptide in association with the HLA-A2 molecule suggests that such a peptide is in fact naturally processed from exogenous Ag and then presented. Of interest also was the finding that when APCs were loaded with PDC-E2-ICs, not only was a PDC-E2 peptide-specific CD8+ CTL line induced, but the efficiency of generating specific CD8+ CTL lines was 100-fold more efficient based on the net molar concentration of the immunodominant PDC-E2 peptide required. Our findings thus support the thesis that autoAbs can facilitate the priming of CD8+ T cell responses against soluble autoAg and that cross-priming may play an important role in the induction of autoimmune response in PBC with autoAb playing a contributory key role.
Materials and Methods
Patients.
12 HLA-A2+ patients with PBC and eight control patients were studied. Controls included four HLA-A2+ patients with other chronic liver diseases (two alcoholic hepatitis, one granulomatous hepatitis, and one α-antitrypsin deficiency) and four HLA-A2+ healthy individuals. The HLA-A2 haplotype of the subjects was determined by using A2-specific mAbs MA2.1 and BB7.2 (10). In addition, PBMCs from non-HLA-A2 patients were also collected for subsequent use as a source of control target cells.
Cells.
PBMCs from each subject were purified from venous blood using standard Ficoll-Histopaque gradient centrifugation techniques. The isolated cells were either used immediately or cryopreserved for later use. Epstein-Barr virus-transformed B lymphoblastoid cell lines (BCLs) were established from each donor and maintained in RPMI 1640 (GIBCO BRL) supplemented with 10% heat-inactivated FCS, penicillin (50 u/ml), and streptomycin (50 μg/ml; FCS medium).
Synthetic Peptides and Recombinant Protein.
A panel of 12 peptides derived from the amino acid sequence of PDC-E2 (11) were selected using the software of the Bioinformatics and Molecular Analysis Section (developed by K. Parker, National Institutes of Health, Washington, D.C.), which ranks 9-mer peptides based on a predicted half-time dissociation coefficient for HLA class I molecules (12). A previously defined HLA-A2–restricted CTL epitope, amino acids 18–27 of the Hepatitis B virus core protein (HBc), was used as a negative control (5). All peptides were synthesized with a free NH2 and a free COOH terminus, and their purity confirmed by HPLC. Lyophilized peptides were reconstituted at 10 mg/ml in DMSO and diluted in FCS medium before use. pET-PDC-E2 encoding PDC-E2 amino acids 1–561 was constructed by inserting the corresponding gene into pET vector (Promega). rPDC-E2 was expressed in Escherichia coli BL21 (Invitrogen) transformed with pET-PDC-E2 and purified.
Generation of Peptide-induced CTL Lines.
Immature dendritic cells (DCs) were generated from PBMCs of HLA-A2 positive donors as described previously (13) and used as APCs. In brief, PBMCs were resuspended in FCS medium, seeded in a 250-ml culture flask, and incubated at 37°C for 1.5 h. After removing nonadherent cells in the medium, the adherent cells were fed with FCS medium supplemented with GM-CSF (1,000 U/ml; Peprotech) and IL-4 (1,000 U/ml; Peprotech) and cultured at 37°C. On day 7, cells were harvested and incubated overnight in FCS medium containing either a mixture of the 12 peptides (10 μM of each peptide) or a single peptide (10 μM). The cells were then washed and γ-irradiated (5,000 rad). Cryopreserved PBMCs were thawed, washed twice with HBSS, resuspended in FCS medium, and seeded in 24-well plates at 2 × 106 cells per well along with 2 × 105 peptide-pulsed APCs. Cells were cultured at 37°C for a total of 12–14 d with appropriate feeding of the cultures with media as required. On day 3 recombinant human IL-2 (Peprotech) was added to each well at a final concentration of 10 U/ml.
Generation of CTL Lines by Exogenous Ags and ICs.
A PDC-E2–specific human mAb was harvested from the culture supernatant of a specific cell line, purified by affinity chromatography, and concentrated (14). An isotype-matched phosphatidyl-serine specific control human mAb was prepared in the same way. L-α-Phosphatidyl-L-serine was purchased from Sigma-Aldrich. Soluble ICs of rPDC-E2 and the human mAb was prepared by mixing rPDC-E2 with affinity purified PDC-E2–specific Ab at a molar ratio of 1:2 for 1 h. Phosphatidyl-serine ICs were prepared in the same way and used throughout as a control. To prepare F(ab)′2 fragment of the anti–PDC-E2 human mAb, a PDC-E2–specific human mAb was treated with pepsin and F(ab)′2 fragment were further purified. In addition, sera from PBC patients were affinity purified against rPDC-E2 as described previously (15). In parallel, control Abs were purified from normal sera. APCs were incubated with soluble rPDC-E2 alone or rPDC-E2 mixed with either mAb, F(ab)′2 fragment of mAb, or Abs purified from sera at different concentrations for 48 h, γ-irradiated, washed, and incubated with PBMCs to induce specific CTLs as described above.
Intracellular Cytokine Analysis Using Flow Cytometry.
Uncultured PBMCs or peptide-induced CTL lines were incubated in fresh FCS medium with peptide (10 μM) and brefeldin A (10 μg/ml; Sigma-Aldrich) for 4 h at 37°C. Cells were harvested and treated first with FACS® lysing solution and then with FACS® permeabilizing solution (BD Biosciences). The processed cells were resuspended in 50 μl of FACS® buffer (0.5% BSA and 0.05% sodium azide in PBS) and stained with PE-labeled anti-CD8 (Caltag), TC-labeled anti-CD4 (Caltag), and FITC-labeled Abs against one of the following cytokines: IFN-γ; TNF-α; or IL-2 (BD Biosciences). After incubation at room temperature in the dark for 30 min, the cells were washed twice with FACS® buffer, fixed with 1% paraformaldehyde in PBS, and analyzed on a FACScan™ flow cytometer. The acquired data were analyzed with CELLQuest™ software (BD Biosciences).
Analysis of Costimulatory Molecule Expression on Immature DCs.
Immature DCs were prepared for use as APCs as above. The cells were incubated with either PDC-E2 ICs or control phosphatidyl serine ICs for 48 h as described previously. Thence the cells were harvested, resuspended in 50 μl of FACS® buffer, and stained with FITC-labeled anti-CD11c, CD40, or CD80 and PE-labeled anti-CD1a, CD11c, or CD86 together with a cocktail of TC-labeled anti-CD3, CD14, CD19, and CD56 (Caltag or BD Bioscience). After incubation in the dark for 30 min at room temperature, the cells were washed twice with FACS® buffer, fixed with 1% paraformaldehyde in PBS, and analyzed on a FACScan™ flow cytometer. The acquired data were analyzed with CELLQuest™.
Vaccinia Vector.
VVrPDC-E2, a recombinant vaccinia virus expressing amino acids 1–414 of the PDC-E2 protein, was constructed as described by inserting the corresponding coding sequence into the plasmid pMJ601 (16). Stocks of the recombinant virus were prepared. Wild-type vaccinia virus prepared similarly was used as a negative control.
Cytotoxicity Assay.
Autologous BCLs were used as target cells. The cells were pulsed with peptides (10 μM) at 37°C overnight. Alternatively, the BCLs were infected with VVrPDC-E2 or wild-type vaccinia virus at a multiplicity of infectivity of 10:1 at room temperature for 1 h, washed once, incubated overnight at 37°C, washed, and then used as target cells. To assess the Ag-specific cytolytic activity of the CTL lines, a fluorescence-based cytotoxicity assay was performed with DELFIA europium (Eu) 2,2′:6′,2′′–terpyridine–6,6′′–dicarboxylic acid (TDA) cytotoxicity assay reagents (Wallac). In brief, the previously prepared Ag-pulsed target cells were washed, labeled with TDA, washed again, and resuspended in FCS medium; 5 × 103 target cells in a volume of 0.1 ml were plated into each well of 96-well round bottomed plates, followed by the addition of varying numbers of effector cells in 0.1 ml of medium. After a 4-h incubation at 37°C, the plates were centrifugated and a 20-μl portion of the supernatant from each well was collected and dispensed into a separate 96-well microtiter plate which contained 200 μl of DELFIA Eu solution in each well. The Eu forms a stable complex with released TDA in the mixture and generates fluorescence. The fluorescence of EuTDA was measured by a time-resolved fluorometer (1420 VECTOR; Wallac Oy). Percentage cytotoxicity was calculated by the formula: 100 × (release in assay − spontaneous release)/(maximum release − spontaneous release). Maximum release was determined by the lysis of 5 × 103 labeled target cells in triplicate wells with DELFIA lysis buffer (Wallac Oy). Spontaneous release was measured by incubating 5 × 103 target cells in triplicate wells in the absence of effector cells. In all instances, spontaneous release was <15% of maximum release. Results are reported herein as the mean of triplicate values.
Phenotypic Analysis of the CTL Line.
In efforts to confirm that the CTL activity being measured was a function of CD8+ T cells, effector cells generated as described above were incubated with either CD4 or CD8 mAb-coated magnetic Dynabeads (Dynal). The cells bound to the beads were removed and the depleted cell population was assessed in triplicate for cytotoxicity against appropriate peptide-pulsed autologous target cells. An aliquot of the CD4- or CD8-depleted cells were analyzed for viability and for the purity of depletion. Viability was always >80% and the depletion >95%.
Inhibition of CTL Activity by Abs.
For determination of inhibition, a mAb against HLA class I (W6/32), HLA class II DR (TAL.1B5; Dako), or a control mAb (mouse IgG2; Caltag) was added respectively to the peptide-loaded target cells at a predetermined optimally titered dilution and incubated for 30 min at 4°C. After incubation, the target cells were mixed with effector cells for the EuTDA release assay.
Statistical Analysis.
Values were statistically analyzed using the Fisher's exact test.
Results
Identification of an Autoreactive CTL Epitope on PDC-E2.
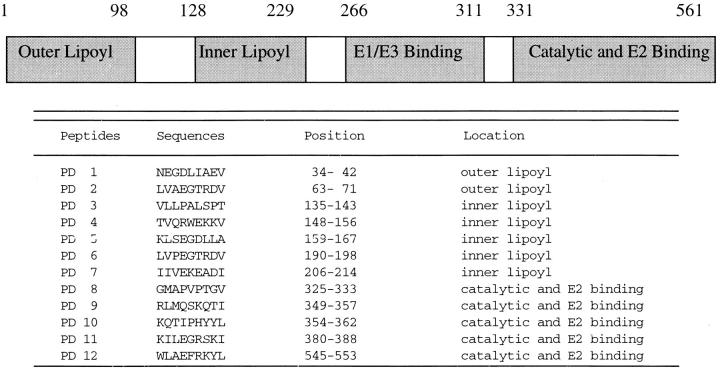
Potential HLA-A2–restricted CTL epitopes encoded by PDC-E2 were determined by scanning the amino acid sequence of the PDC-E2 protein for the presence of 9-mer peptides containing the HLA-A2–binding motif. This analysis resulted in the identification of 12 sequences that showed motifs similar to known CTL epitopes and these are shown in Table 1. PBMCs from four HLA-A2+ PBC patients were cocultured with autologous APCs pulsed with a mixture of all 12 peptides for 12 d to expand peptide-specific CD8+ T cells in vitro. After incubation, the cells were divided into aliquots and restimulated with individual peptides in the presence of brefeldin A, followed by intracellular staining for IFN-γ. Only one of the 12 candidate peptides, PD5, induced a significant frequency of IFN-γ–producing T cells after in vitro culture of PBMCs derived from each of the four patients (data not shown). Flow cytometric analysis demonstrated that all of the IFN-γ–producing cells expressed CD8. These results suggest that PD5-specific CD8+ T cells exist in the PBMC cultures after stimulation with the peptide pool. Of interest was the finding that the PD5 peptide spans residues amino acids 159–167 of PDC-E2, which is located within the inner lipoyl domain of PDC-E2.
Table I.
Computer Algorithm Predicted HLA-A2–restricted Epitopes on PDC-E2
PDC-E2 Peptide (159–167)-specific CD8+ CTL T Cells: Disease and Peptide Specificity.
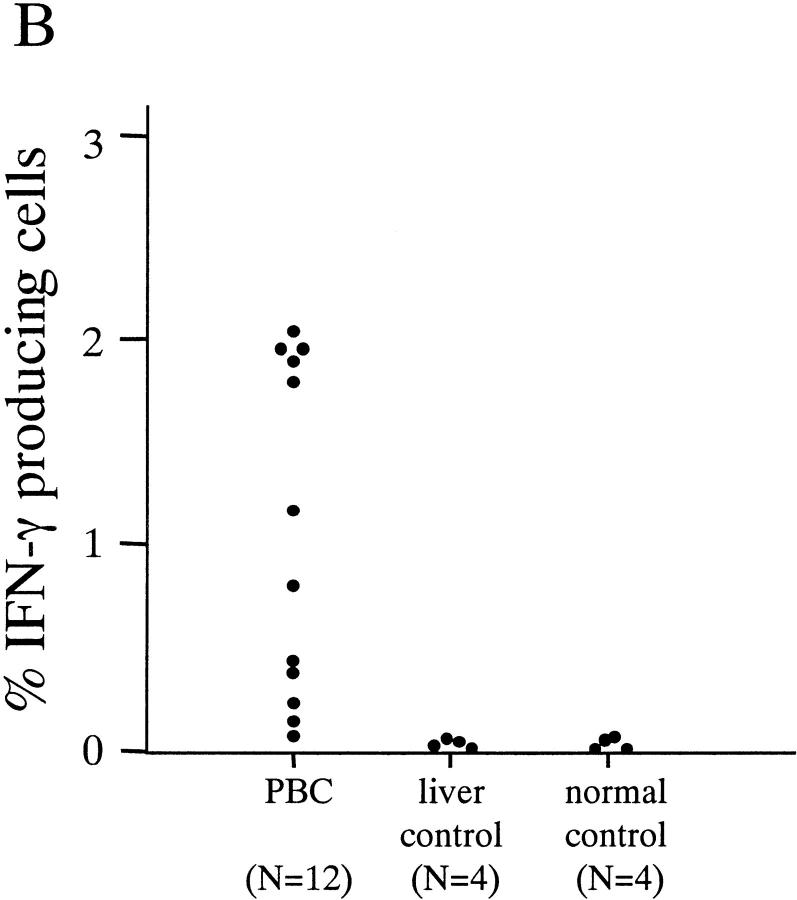
In an effort to examine the disease specificity of the CD8+ T cells against the identified PDC-E2 epitope (159–167) peptide, PBMCs from all 20 HLA-A2+ donors (12 PBC patients, PBC 1 to PBC 12; four patients with other chronic liver diseases CL1 to CL4, and four healthy donors H1 to H4) were cultured with autologous APCs loaded with the PD5 peptide. On day 12 the cells were restimulated with PD5 or a control peptide in the presence of brefeldin A, followed by intracellular IFN-γ staining. A significant number of IFN-γ–producing cells were detected after restimulation with PD5, but not with the control peptide, in 10 out of 12 (83%) PBC patients but none of the eight control individuals (P < 0.0007). Representative data from two PBC patients (PBC3 and PBC10) and two control individuals (CL1 and H1) are shown in Fig. 1 A, and the results of all 20 individuals tested are summarized in Fig. 1 B. The fact that PD5-specific CD8+ T cells can be expanded from the majority (10/12) of PBC patients but none of the control individuals strongly suggests that a CD8+ T cell response against the autoreactive epitope on PDC-E2 is associated with PBC.
Figure 1.
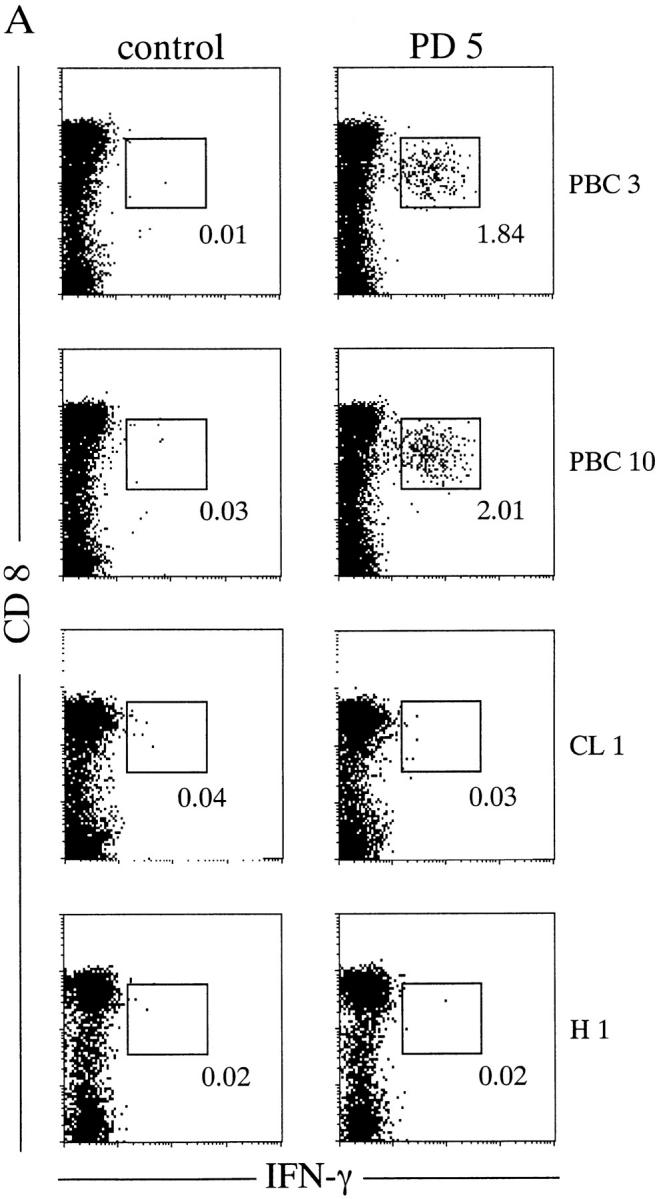
PD5-specific CD8+ T cells from in vitro–cultured PBMCs. PBMCs from HLA-A2+ donors were cocultured with PD5-loaded APCs (autologous immature DCs) for 12 d, then restimulated with PD5 or a control peptide in the presence of brefeldin A, followed by intracellular staining for IFN-γ. (A) IFN-γ staining of samples from two PBC patients, PBC3 and PBC10, and two control donors, CL1 (alcoholic liver disease) and H1 (a healthy donor). Displayed in the dot plots are cells gated for lymphocyte population by forward-scattering and side-scattering and the CD4− population. The cells within the box are considered IFN-γ+. The number next to the box is the percentage of IFN-γ+ cells in the CD8+ T cell population. (B) Frequency of PD5-specific CD8+ T cells in PD5-stimulated PBMC cultures derived from all 20 donors. The cut off value for a positive response was determined as 0.150%, or 3 SD above the mean percentage of IFN-γ producing cells in all 20 samples restimulated with the control peptide. A significant number of IFN-γ–producing cells were detected after restimulation with PD5 in 10/12 (83%) PBC patients but in 0/8 control individuals (P < 0.0007).
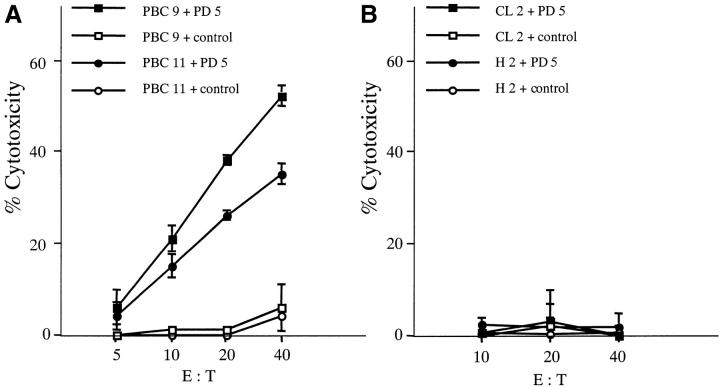
To determine if the IFN-γ synthesizing CD8+ T cell response corresponded with PD5 peptide specific cytotoxic activity, the PD5-stimulated PBMC cultures were assessed in 14 HLA-A2+ individuals: eight patients with PBC (PBC1, PBC2, PBC3, PBC4, PBC5, PBC9, PBC10, and PBC11); three patients with other chronic liver diseases (CL1, CL2, and CL3); and three healthy controls (H1, H2, and H3). The PBMCs were stimulated and expanded with PD5-loaded autologous APCs for 12–14 d and used as effector cells in a conventional CTL assay against autologous BCLs loaded with the PD5 peptide or a control HLA-A2–specific hepatitis B peptide. Targets loaded with PD5, but not with the control peptide, were lysed by the effector cells derived from each of the eight PBC patients. In contrast, PBMCs derived from the control individuals did not show significant cytotoxicity toward the PD5-loaded target cells. A representative CTL assay for two PBC patients (PBC9 and PBC11) is shown in Fig. 2 A along with two control individuals (CL2 and H2) in Fig. 2 B. These results indicate that PD5-specific CTL lines, inducible from PBC patients but not from other chronic liver diseases or healthy individuals, were indeed cytolytic for the targets presenting the PD5 epitope.
Figure 2.
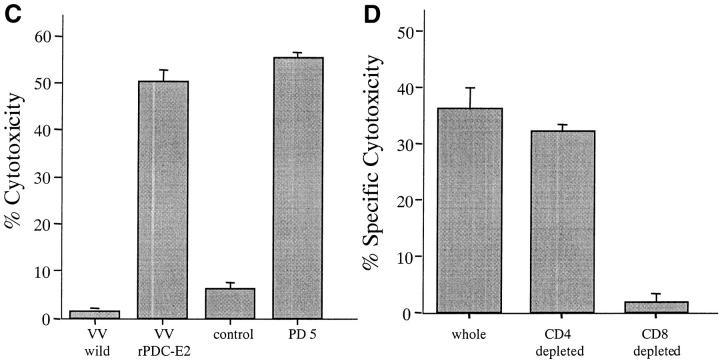
Cytotoxicity of PD5-induced CTL lines. PD5-specific CTL lines, induced by culturing PBMCs with PD5-loaded APCs for 12–14 d, were tested for their cytotoxicity against PD5 or control peptide-loaded autologous BCL targets at different effector/target (E/T) ratios. HBc18–27, an HLA-A2-restricted irrelevant epitope, was used as control. Displayed are mean specific lysis of triplicate cultures. (A) PBC patients PBC9 and PBC11. (B) Control donors CL2 (granulomatous liver disease) and H2 (healthy donor). (C) Cytotoxic activity of a PD5-induced CTL line against target cells presenting endogenously processed PDC-E2 Ag. The CTL line from patient PBC1 was tested for cytotoxicity against autologous BCL targets infected with a PDC-E2–expressing vaccinia vector (VVrPDC-E2), wild-type vaccinia virus (VVwild) alone, loaded with PD5 or a control peptide HBc18–27. Displayed are mean specific lysis of triplicate testing at an E/T ratio of 40:1. (D) Phenotype analysis of PD5-induced CTLs. CD4+ cell or CD8+ cells were depleted respectively from a CTL line derived from patient PBC2 using anti-CD4 or anti-CD8–coated magnetic beads before testing for cytotoxic activity with PD5 or control peptide-loaded autologous BCL targets. Unfractionated cells were also tested in parallel. Specific cytotoxicity was calculated by subtracting the cytotoxicity of the effector cells against control peptide-pulsed BCLs from that against PD5 peptide-pulsed BCLs. Displayed are mean specific lysis of triplicate testing at an E/T ratio of 40:1 according to the cell counts before depletion.
To examine whether the PD5 epitope is in fact naturally processed, presented, and serves as a target for the autoreactive CTLs, PD5 peptide-specific CTL lines from six PBC patients (PBC1, PBC2, PBC3, PBC4, PBC5, and PBC11) were tested for CTL activity using autologous BCL targets infected with VVrPDC-E2, a vaccinia vector carrying the coding sequence for 74% of the NH2 terminus of the PDC-E2 gene. Wild-type vaccinia virus-infected target cells were used as a control. PD5-induced CTL lines from all six patients lysed target cells infected with VVrPDC-E2 as well as BCLs loaded with PD5 peptide as expected, but did not lyse, to any appreciable extent, targets infected with the wild-type vaccinia virus or target cells loaded with the control peptide. Representative data from a PBC patient (PBC1) are shown in Fig. 2 C. These findings indicate that the endogenously synthesized PDC-E2 protein is processed to generate the PD5 peptide, which is presented and recognized by the peptide PD5-induced CTL lines from PBC patients.
The CD8+ phenotype of PD5-induced CTLs from six PBC patients (PBC2, PBC3, PBC4, PB5, PBC10, and PBC11) was also confirmed by a cytotoxicity assay after depletion of different T cell subsets. Depletion of the CD4 or CD8 fraction was performed after the in vitro priming and expansion of the CTLs and before the cytotoxic assay. In all patients tested, the CTL activity was abrogated by depletion of CD8+ cells, but not by depletion of CD4+ cells, consistent with the view that CTL activity was mediated by CD8+ T cells, which likely synthesize IFN-γ when restimulated with the specific peptide. Representative data from a PBC patient (PBC2) are shown in Fig. 3 D.
Figure 3.
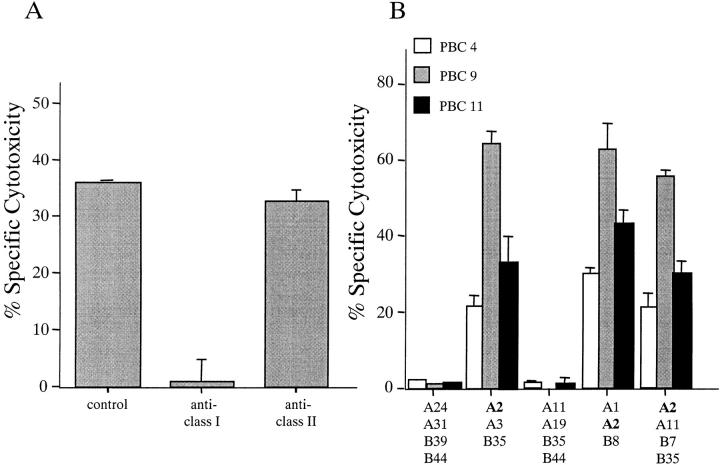
(A) Inhibition of CTL activity by HLA class I and HLA class II (DR) mAbs. A CTL line derived from patient PBC5 was tested for cytotoxicity against PD5 or control peptide-loaded autologous BCL targets in the presence of predetermined optimal concentrations of Abs against different HLA molecules. Ab against HLA class I (W6/32), HLA class II DR (TAL.1B5; Dako) or a control Ab (mouse IgG2a; Caltag) were added respectively to the peptide-loaded targets and incubated for 30 min at 4°C. After the incubation the target cells were mixed with effector cells for EuTDA release assay. The CTL assays were performed at an E/T ratio of 40:1. Specific cytotoxicity was calculated by subtracting the cytotoxicity of the effector cells against control peptide-pulsed BCLs from that against PD5 peptide-pulsed BCLs. Displayed are mean specific lysis of triplicate testing. (B) HLA restriction of the PD5 epitope. CTL lines derived from three PBC patients were tested for cytotoxicity against PD5 or control peptide-loaded allogeneic BCLs derived from five individuals with distinct combinations of HLA haplotypes. The CTL assays were performed at an E/T ratio of 40:1. Specific cytotoxicity was calculated by subtracting the cytotoxicity of the effector cells against control peptide-pulsed BCLs from that against PD5 peptide-pulsed BCLs. Displayed are mean specific lysis of triplicate testing.
HLA Restriction of the PD5 Epitope.
In efforts to confirm that class I restriction is involved in the presentation of the PD5 peptide to CD8+ CTLs, the cytotoxic activity of PD5-specific CTL lines from four patients were tested in the presence of Abs against class I or II HLA molecules. The CTL activity was inhibited by anti-HLA class I Ab but not by anti-HLA class II (DR) Ab in all cases tested. Representative data from a PBC patient (PBC5) are shown in Fig. 3 A. Then we examined the ability of CTL lines derived from PBC patients to lyse PD5-loaded allogeneic BCLs derived from five individuals, each with a distinct combination of MHC class I haplotypes. Three PBC patients (PBC4, PBC9, and PBC11), all positive for HLA-A2, were tested. As shown in Fig. 3 B, the presence of the HLA-A2 allele alone is sufficient for a specific BCL line to be lysed by all three CTL lines. Collectively, these results confirm that the epitope PD5 was restricted by the MHC class I–encoded HLA-A2 molecule.
Cytokine Profile of the PD5-specific CD8+ T Cells.
PD5-specific CTL lines derived from four PBC patients (PBC9, PBC10, PBC11, and PBC12) were restimulated with PD5 or control peptide in the presence of brefeldin A, followed by intracellular staining for the cytokines IFN-γ, TNF-α, and IL-2. Significant numbers of IFN-γ–producing CD8+ cells were detected in all four patients. However, no detectable level of cells producing TNF-α or IL-2 was observed in any of the patients studied (data not shown). The failure to detect TNF-α and IL-2 was not secondary to kinetics (data not shown).
Generation of Autoreactive CTLs using Exogenous Ag and Ag–Ab Complexes.
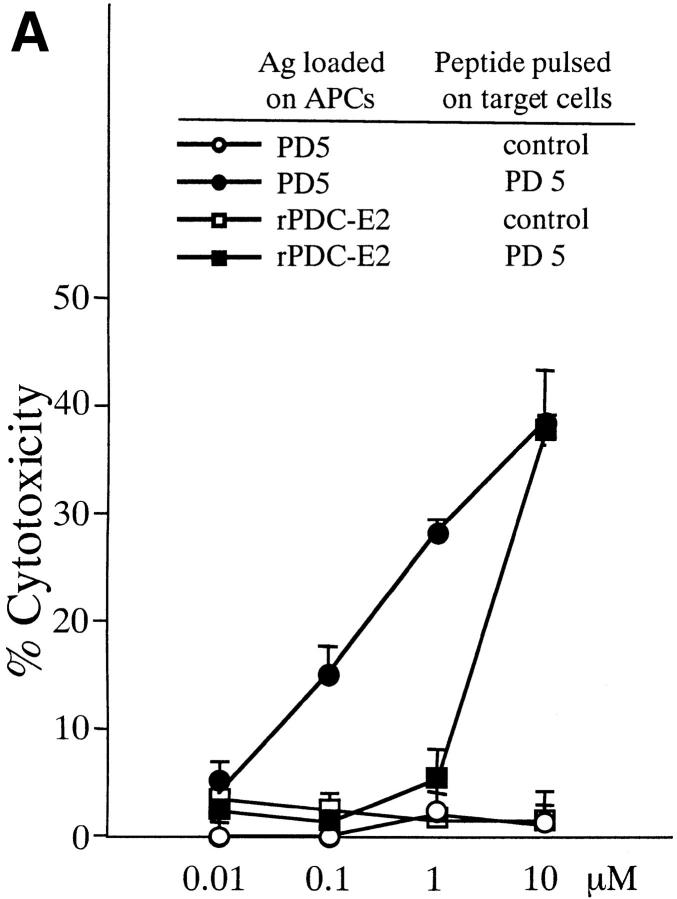
To determine if exogenous PDC-E2 protein can induce CTL lines specific for the MHC class I–restricted epitope PD5, PBMCs from three PBC patients (PBC9, PBC10, and PBC11) were cocultured with APCs loaded with rPDC-E2 protein or the PD5 peptide at various concentrations. CTL assays were performed against autologous BCLs targets loaded with either the PD5 peptide or a control peptide. CTL lines with PD5-specific cytotoxicity were consistently generated from each of the three PBC patients when PBMCs were stimulated with APCs loaded with PD5 peptide at concentrations >0.1 μM. However, when rPDC-E2 protein was used instead, PD5-specific CTL lines could only be generated when the PDC-E2 protein was added at the highest concentration (10 μM). Representative results of patient PBC10 is shown in Fig. 4 A. These data suggest that APCs exogenously pulsed with the soluble PDC-E2 protein, can still process and present the peptide by the MHC-class I molecule but at a relatively inefficient level.
Figure 4.
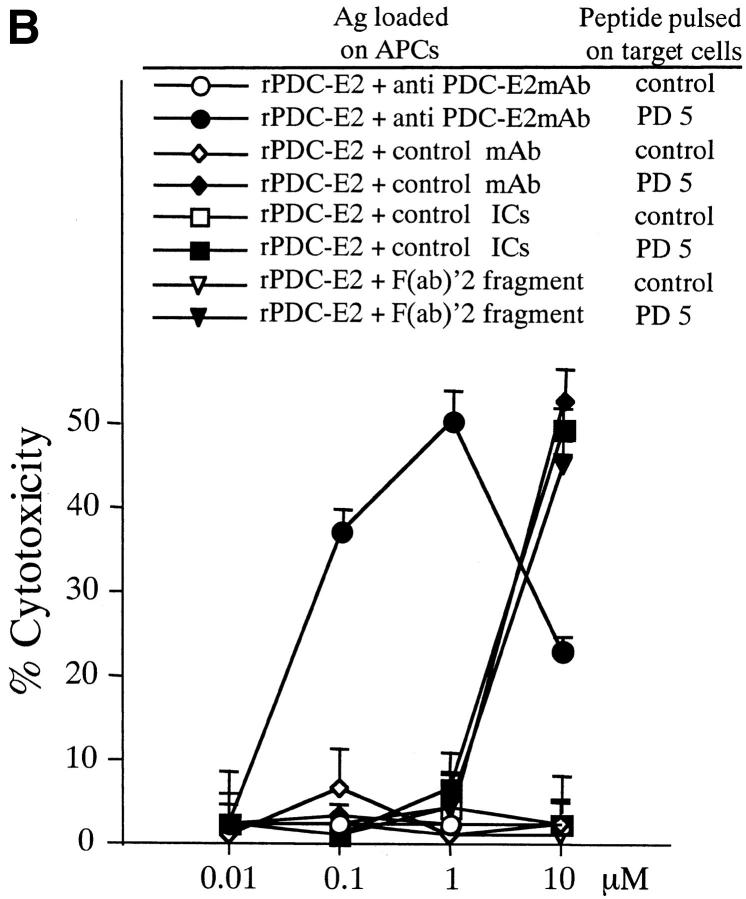
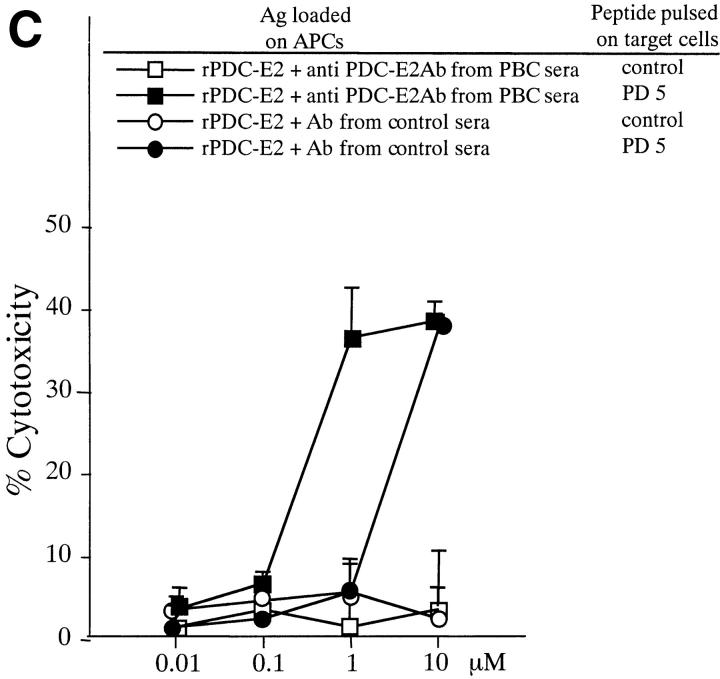
Induction of PD5-specific CTL lines with peptide, exogenous rPDC-E2 protein, or rPDC-E2 complexed with specific Abs. The cytotoxicity of CTL lines generated with the different Ags was tested against autologous BCL targets loaded with PD5 or control peptide at an E/T ratio of 40. (A) PBMCs from patient PBC10 were cocultured for 12 d with APCs loaded with rPDC-E2 protein or PD5 peptide at serial concentrations as indicated. (B) PBMCs from patient PBC9 were cocultured for 12 d with APCs loaded with serial concentrations of rPDC-E2 protein (as indicated) mixed with either human anti–PDC-E2 mAb, a control mAb, control ICs, or F(ab)′2 fragment of the human anti–PDC-E2 mAb. (C) PBMCs from patient PBC11 were cocultured for 12 d with APCs loaded with serial concentrations of rPDC-E2 protein (as indicated) mixed with affinity purified autoAbs from PBC sera or control Abs.
To examine whether the internalization and presentation of exogenous protein Ag can be enhanced by formation of Ag–Ab complexes, experiments were performed using soluble rPDC-E2 protein complexed with either a human mAb against PDC-E2, a control mAb, or control ICs, then added to the APCs at various concentrations. When the anti–PDC-E2 mAb was added to form the Ag–Ab complex with rPDC-E2 protein, PD5-specific CTL lines were generated at 100-fold lower concentration than rPDC-E2 mixed with the control mAbs as well as control ICs, indicating the internalization and presentation of exogenous PDC-E2 protein was greatly enhanced by the formation of specific Ag–Ab complex. Representative results with patient PBC10 are shown in Fig. 4 B. The highly efficient PDC-E2 presentation was not due to FcγR engagement per se, since induction of CTL activity with the soluble rPDC-E2 was not enhanced by the presence of the irrelevant mAb as well as irrelevant ICs. The level of expression of select costimulatory molecules, including CD40, CD80, and CD86 by APCs incubated with the ICs were also examined in efforts to determine whether increased expression could be responsible for the enhanced generation of CTLs. APCs were thus incubated with PDC-E2 ICs or with control phosphatidyl serine ICs for the same period of time as used for pulsing of the APCs with Ag. Results of such studies failed to demonstrate any significant changes of the level of expression in those molecules between control ICs or PDC-E2 ICs and between pre- and post-ICs incubation. While these data do not completely rule out a role for the contribution of such costimulatory molecules and/or additional costimulatory molecules not studied, at face value the data do not support such a role.
Next, to assess the effect of Ag aggregation on the internalization and presentation of exogenous protein Ag, experiments were performed using soluble rPDC-E2 protein alone or soluble rPDC-E2 complexed with an F(ab)′2 fragment of anti–PDC-E2 human mAb, added to the APCs at serial concentrations. When the F(ab)′2 fragment of anti–PDC-E2 mAb was added with rPDC-E2 protein, the concentration required to induce PD5-specific CTL lines was similar to that of rPDC-E2 alone, suggesting that increased cross-presentation of ICs was not due to Ag aggregation. Representative results with patient PBC9 are shown in Fig. 4 B.
Generation of Autoreactive CTLs Using Exogenous Ag Mixed with AutoAbs Purified from PBC Sera.
PDC-E2–specific autoAbs are detected exclusively in PBC patients. To examine whether the efficient internalization and presentation of exogenous PDC-E2 could be mediated with autoAbs in PBC sera, experiments were performed using soluble rPDC-E2 protein complexed with affinity purified autoAbs from PBC sera. When the autoAbs from PBC was added to form the Ag–Ab complex with rPDC-E2 protein, PD5-specific CTL lines were generated at 10-fold lower concentration than rPDC-E2 mixed with Abs from control sera, indicating that autoAbs in PBC have a potential to enhance internalization and presentation of exogenous PDC-E2 protein by the formation of specific Ag–Ab complex. Representative results with patient PBC11 are shown in Fig. 4 C.
Discussion
In disease states which involve tissue destruction and immune-mediated cell lysis, the identification and characterization of autoreactive T lymphocyte responses, and the nature of the peptide and the MHC encoded restricting element, is an important step in defining the role of these cells in disease pathogenesis. Previous work has focused on the characterization of CD4+ T cell lines and CD4+ T cell clones specific for PDC-E2 (17, 18). However, little work has been performed to define the CD8+ CTL response in PBC. Immunohistochemical studies reveal that the portal infiltrates in PBC are predominantly CD3+ T cells containing both CD8+ and CD4+ subsets bearing the 2β-TCR. The CD4/CD8 ratio ranges from 2–2.5:1 (19, 20). CD8+ T cells are most abundant during early stages of disease and as the disease progresses, a higher proportion of CD4+ T cells are observed (21, 22). In this paper, we identified PD5 as an HLA-A2–restricted epitope on PDC-E2, the major target protein of autoreactive Ab in PBC. PD5 peptide is capable of inducing peptide-specific CTL lines after 12–14 d of in vitro stimulation with PBMCs from the majority (83%) of PBC patients but not from control patients, suggesting that the PD5-specific CTLs are primed in vivo in PBC patients. The PD5-specific CTL lines generated from the patients' PBMCs display CTL effector function, including Ag-specific cytolytic activity and IFN-γ production. Therefore, it is reasonable to assume that the autoAg-specific CTLs in vivo are actively involved in the inflammatory responses and cytolytic destruction of BECs. The PD5-specific CD8+ T cells in the peripheral blood of patients cannot be detected directly with ex vivo intracellular cytokine assay (data not shown), suggesting that the frequency of the autoAg-specific CD8+ T cells in circulation is very low. Clearly, additional studies are needed using PD5-bearing tetramer reagents and cell populations within the lymph nodes and the liver to determine if the PD5-specific CD8+ CTL are selectively homing to the liver.
PDC-E2–specific autoreactive CTLs appear primed in vivo only in PBC patients. These data prompted us to hypothesize that a previously described nonconventional mechanism of Ag presentation, “cross-priming” or “cross-presentation,” is involved in the maintenance and/or amplification of CTL response against PDC-E2, with PDC-E2-specific autoAb playing a key role. Professional APCs have been shown capable of intracellular processing of exogenous Ag using proteosomes to generate peptides presented by MHC class I (23, 24), the exogenous Ag being presented by the same APCs to both CD4+ and CD8+ T cells (25). This is important for the induction of immunity to pathogens that avoid professional APCs (26). The precise nature of the APCs that are able to take up, process, and present exogenous Ags in association with MHC class I molecules remains to be defined. However, in vitro studies suggest that DCs (27–29), macrophages (30), or B cells (31) might be involved. Cross-priming is generally inefficient, but the efficiency is much higher with macropinocytosis (32) and phagocytosis of the particle (33, 34). Phagocytosis of apoptotic cells also results in efficient MHC class I–restricted Ag presentation in macrophages and/or DCs (29, 35, 36). DCs in murine models have been shown to mediate internalization of Ag–Ig complexes (ICs), and promote efficient MHC class I as well as class II–restricted Ag presentation (37). In terms of MHC class II presentation, FcγRs, which bind ICs represent a privileged Ag internalization route for efficient MHC class II–restricted Ag presentation in DCs (38). In murine systems, it has been suggested that DCs are capable of taking up ICs, and presenting the appropriate processed antigenic peptide to CD4+ T cells, which in turn activate DCs, and convert them into DCs capable of priming CD8+ T cells in vivo (37).
In this study, a PDC-E2–specific CD8+ CTL line has been induced from PBMCs by stimulating them with soluble Ag, suggesting the potential for cross-priming. Further, rPDC-E2-ICs could be internalized, processed, and presented by APCs much more efficiently than soluble protein alone in the induction of specific CTLs in vitro. PDC-E2-ICs are present only in PBC patients in vivo because PDC-E2–specific autoAbs are detected exclusively in PBC patients. In transgenic mice, cross-presentation can remove autoreactive CD8+ T cells and may tolerize the CD8+ compartment to self-Ags (27). Since the cross-presentation pathway is involved in both cross-tolerance and cross-priming, the mechanism by which Ags gain access to this pathway is likely to affect both aspects. What determines whether tolerance or immunity is induced is unclear, but CD4+ T cell help appears to be important (28, 39), which is in accordance with the evidence that PDC-E2–specific Th cell responses have been observed in most PBC patients. Another important factor might be the dose of Ag presented (40). One additional explanation for the selective induction of the PDC-E2–specific CTLs in PBC is that, in addition to the role of autoAbs in forming ICs, autoreactive B cells specific for PDC-E2 might play a role as APCs in the generation of T cell–mediated immune responses. In particular, activated B lymphocytes are highly efficient APCs for the specific Ag that their surface Igs bind; B cells are required as APCs to generate pathogenic autoimmune T cell responses in NOD mice (41, 42).
IgA is transcytosed and can be transported into BECs. In fact, PDC-E2 specific IgA has been detected in the bile of PBC patients (43). We have shown previously that antigenic materials reactive with antimitochondrial Abs can be detected in BECs of PBC patients but not in control individuals (44), suggesting that the autoAg PDC-E2 is either expressed at a higher level or presented in a more antigenic form in BECs of PBC patients. Both scenarios may render this protein more accessible for binding with autoreactive IgA inside the cells; endogenously synthesized PDC-E2 could be bound with autoAb in the cytosol, losing its capability to enter the mitochondria, resulting in the accumulation within the cytosol, which in turn could lead to processing via the MHC class I presentation pathway and recognized by the PDC-E2 specific CD8+ CTL. In fact, intracellular neutralization of influenza virus by specific IgA has been suggested using a Madine-Darby canine kidney cell line (45). We have also shown the colocalization of PDC-E2 with IgA from PBC patients within the same Madin-Darby canine kidney cell line (46). Moreover, when the cells die, the autoAb bound to the dominant B cell epitope may cover the overlapping or adjacent CD4+ and CD8+ epitopes, protecting them from degradation by proteases, and the Ab-peptide complex may be internalized efficiently by APCs. Once being taken up by APCs, the CD4+ epitope or CD8+ epitope on the peptide is revealed, processed, and presented by MHC class I and class II molecules, respectively. These peptides will then activate autoreactive CD4+ and CD8+ T cells to amplify the overall level of autoimmunity, including expansion of autoreactive effector T cells to mediate continued damage on BECs.
In previous studies, we identified both the major B cell epitope as well as a class II–restricted T cell epitope for PDC-E2. Interestingly, both epitopes were located within the inner lipoyl domain with significant overlap (1, 47). It is striking to find that the newly identified immunodominant MHC class I–restricted CD8+ epitope, PD5 159–167, overlaps with the B cell epitope, and partially overlaps with the CD4+ epitope 163–176. The overlapping of the immunodominant T cell and B cell epitopes has also been observed in myelin basic protein (7, 48), the proteolipid protein in multiple sclerosis (49–52), and glutamic acid decarboxylase 65 in insulin-dependent diabetes mellitus (8, 53, 54), suggesting that this is a common theme for autoimmune diseases. It is well recognized that B cells, CD4+ Th cells, and CD8+ CTLs are functionally interrelated, as indicated by the fact that CD4+ Th cells provide important support for both Ab response and for the generation and maintenance of the CTL response. However, by conventional mechanisms of Ag presentation there is no requirement for the target epitopes of these different subsets of lymphocytes to be closely connected physically. Actually, targets for CD4+ cell and CD8+ cells are usually generated through different pathways and are not even derived from the same polypeptide molecule. CD4+ T cells recognize epitopes that are derived from exogenous proteins taken up by APCs and processed in the endocytic vesicles and are presented by MHC class II. On the other hand, CD8+ T cells recognize epitopes that are derived from endogenously synthesized proteins processed by proteosomes and are presented by MHC class I. In this work and our previous study, we have examined the full-length PDC-E2 protein of 561 amino acid residues for the B cell, CD4+ T cell, and CD8+ T cell epitopes in a systematic way. However, the only epitope for each category we have identified is localized within a short segment of amino acid residues. Efficient uptake of the PDC-E2 Ag coupled with specific autoAbs (ICs), and cross-presentation, if substantiated by further studies, may explain the unusual clustering of B cell and T cell epitopes on the autoAg and reveals a novel functional association between the humoral and cellular arms of autoimmunity. Although this mechanism does not address the question of what factor(s) initiate the autoimmune cascade that leads to the development of PBC, it may play an important role in the amplification of autoimmunity and progression of the disease.
Acknowledgments
Supported by National Institutes of Health Grant DK 39588.
Footnotes
Abbreviations used in this paper: BCL, Epstein-Barr virus-transformed B lymphoblastoid cell line; BEC, biliary epithelial cell; DC, dendritic cell; Eu, europium; FcγR, Fcγ receptor; HBc, Hepatitis B virus core protein; IC, immune complex; PBC, primary biliary cirrhosis; PDC-E2, E2 component of pyruvate dehydrogenase; rPDC-E2, recombinant PDC-E2; TDA, 2,2′:6′,2′′–terpyridine–6,6′′–dicarboxylic acid.
References
- 1.Coppel, R.L., and M.E. Gershwin. 1995. Primary biliary cirrhosis: the molecule and the mimic. Immunol. Rev. 144:17–49. [DOI] [PubMed] [Google Scholar]
- 2.Van de Water, J., S. Shimoda, Y. Niho, R. Coppel, A. Ansari, and M.E. Gershwin. 1997. The role of T cells in primary biliary cirrhosis. Seminars in Liver Disease. 17:105–113. [DOI] [PubMed] [Google Scholar]
- 3.Sidney, J., S. Southwood, M.F. del Guercio, H.M. Grey, R.W. Chesnut, R.T. Kubo, and A. Sette. 1996. Specificity and degeneracy in peptide binding to HLA-B7-like class I molecules. J. Immunol. 157:3480–3490. [PubMed] [Google Scholar]
- 4.Sidney, J., H.M. Grey, S. Southwood, E. Celis, P.A. Wentworth, M.F. del Guercio, R.T. Kubo, R.W. Chesnut, and A. Sette. 1996. Definition of an HLA-A3-like supermotif demonstrates the overlapping peptide-binding repertoires of common HLA molecules. Hum. Immunol. 45:79–93. [DOI] [PubMed] [Google Scholar]
- 5.Nayersina, R., P. Fowler, S. Guilhot, G. Missale, A. Cerny, H.J. Schlicht, A. Vitiello, R. Chesnut, J.L. Person, A.G. Redeker, et al. 1993. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J. Immunol. 150:4659–4671. [PubMed] [Google Scholar]
- 6.Cerny, A., J.G. McHutchison, C. Pasquinelli, M.E. Brown, M.A. Brothers, B. Grabscheid, P. Fowler, M. Houghton, and F.V. Chisari. 1995. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J. Clin. Invest. 95:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuchida, T., K.C. Parker, R.V. Turner, H.F. McFarland, J.E. Coligan, and W.E. Biddison. 1994. Autoreactive CD8+ T-cell responses to human myelin protein-derived peptides. Proc. Natl. Acad. Sci. USA. 91:10859–10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panina-Bordignon, P., R. Lang, P.M. van Endert, E. Benazzi, A.M. Felix, R.M. Pastore, G.A. Spinas, and F. Sinigaglia. 1995. Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J. Exp. Med. 181:1923–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kern, F., I.P. Surel, C. Brock, B. Freistedt, H. Radtke, A. Scheffold, R. Blasczyk, P. Reinke, J. Schneider-Mergener, A. Radbruch, et al. 1998. T-cell epitope mapping by flow cytometry. Nat. Med. 4:975–978. [DOI] [PubMed] [Google Scholar]
- 10.He, X.S., B. Rehermann, F.X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T.L. Wright, M.M. Davis, and H.B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD+ T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. USA. 96:5692–5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppel, R.L., L.J. McNeilage, C.D. Surh, J. Van de Water, T.W. Spithill, S. Whittingham, and M.E. Gershwin. 1988. Primary structure of the human M2 mitochondrial autoantigen of primary biliary cirrhosis: dihydrolipoamide acetyltransferase. Proc. Natl. Acad. Sci. USA. 85:7317–7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker, K.C., M.A. Bednarek, and J.E. Coligan. 1994. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 152:163–175. [PubMed] [Google Scholar]
- 13.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cha, S., P.S. Leung, J. Van de Water, K. Tsuneyama, R.E. Joplin, A.A. Ansari, Y. Nakanuma, P.J. Schatz, S. Cwirla, L.E. Fabris, et al. 1996. Random phage mimotopes recognized by monoclonal antibodies against the pyruvate dehydrogenase complex-E2 (PDC-E2). Proc. Natl. Acad. Sci. USA. 93:10949–10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long, S.A., C. Quan, J. Van de Water, M.H. Nantz, M.J. Kurth, D. Barsky, M.E. Colvin, K.S. Lam, R.L. Coppel, A. Ansari, and M.E. Gershwin. 2001. Immunoreactivity of organic mimeotopes of the E2 component of pyruvate dehydrogenase: connecting xenobiotics with primary biliary cirrhosis. J. Immunol. 167:2956–2963. [DOI] [PubMed] [Google Scholar]
- 16.Chakrabarti, S., K. Brechling, and B. Moss. 1985. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol. Cell. Biol. 5:3403–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimoda, S., J. Van de Water, A. Ansari, M. Nakamura, H. Ishibashi, R.L. Coppel, J. Lake, E.B. Keeffe, T.E. Roche, and M.E. Gershwin. 1998. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J. Clin. Invest. 102:1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van de Water, J., A. Ansari, T. Prindiville, R. Coppel, N. Ricalton, B.L. Kotzin, S. Liu, T.E. Roche, S.M. Krams, S. Munoz, and M.E. Gershwin. 1995. Heterogeneity of autoreactive T cell clones specific for the E2 component of the pyruvate dehydrogenase complex in primary biliary cirrhosis. J. Exp. Med. 181:723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colucci, G., F. Schaffner, and F. Paronetto. 1986. In situ characterization of the cell-surface antigens of the mononuclear cell infiltrate and bile duct epithelium in primary biliary cirrhosis. Clin. Immunol. & Immunopath. 41:35–42. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto, E., K.D. Lindor, H.A. Homburger, E.R. Dickson, A.J. Czaja, R.H. Wiesner, and J. Ludwig. 1993. Immunohistochemical characterization of hepatic lymphocytes in primary biliary cirrhosis in comparison with primary sclerosing cholangitis and autoimmune chronic active hepatitis. Mayo Clinic Proc. 68:1049–1055. [DOI] [PubMed] [Google Scholar]
- 21.Bjorkland, A., R. Festin, I. Mendel-Hartvig, A. Nyberg, L. Loof, and T.H. Totterman. 1991. Blood and liver-infiltrating lymphocytes in primary biliary cirrhosis: increase in activated T and natural killer cells and recruitment of primed memory T cells. Hepatology. 13:1106–1111. [DOI] [PubMed] [Google Scholar]
- 22.Krams, S.M., J. Van de Water, R.L. Coppel, C. Esquivel, J. Roberts, A. Ansari, and M.E. Gershwin. 1990. Analysis of hepatic T lymphocyte and immunoglobulin deposits in patients with primary biliary cirrhosis. Hepatology. 12:306–313. [DOI] [PubMed] [Google Scholar]
- 23.Rock, K.L., S. Gamble, and L. Rothstein. 1990. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 249:918–921. [DOI] [PubMed] [Google Scholar]
- 24.Rock, K.L., L. Rothstein, S. Gamble, and C. Fleischacker. 1993. Characterization of antigen-presenting cells that present exogenous antigens in association with class I MHC molecules. J. Immunol. 150:438–446. [PubMed] [Google Scholar]
- 25.Shen, Z., G. Reznikoff, G. Dranoff, and K.L. Rock. 1997. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 158:2723–2730. [PubMed] [Google Scholar]
- 26.Sigal, L.I., S. Crotty, R. Andino, and K.L. Rock. 1999. Cytotoxic T cell immunity to virus-infected nonmaematopoietic cells requires presentation of exogenous antigen. Nature. 398:77–80. [DOI] [PubMed] [Google Scholar]
- 27.Kurts, C., H. Kosaka, F.R. Carbone, J.F. Miller, and W.R. Heath. 1997. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 186:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurts, C., F.R. Carbone, M. Barnden, E. Blanas, J. Allison, W.R. Heath, and J.F. Miller. 1997. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J. Exp. Med. 186:2057–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albert, M.L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 392:86–89. [DOI] [PubMed] [Google Scholar]
- 30.Kovacsovics-Bankowski, M., K. Clark, B. Benacerraf, and K.L. Rock. 1993. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc. Natl. Acad. Sci. USA. 90:4942–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ke, Y., and J.A. Kapp. 1996. Exogenous antigens gain access to the major histocompatibility complex class I processing pathway in B cells by receptor-mediated uptake. J. Exp. Med. 184:1179–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norbury, C.C., B.J. Chambers, A.R. Prescott, H.G. Ljunggren, and C. Watts. 1997. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur. J. Immunol. 27:280–288. [DOI] [PubMed] [Google Scholar]
- 33.Kovacsovics-Bankowski, M., and K.L. Rock. 1995. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 267:243–246. [DOI] [PubMed] [Google Scholar]
- 34.Reis e Sousa, C., and R.N. Germain. 1995. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J. Exp. Med. 182:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albert, M.L., S.F. Pearce, L.M. Francisco, B. Sauter, P. Roy, R.L. Silverstein, and N. Bhardwaj. 1998. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188:1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellone, M., G. Iezzi, P. Rovere, G. Galati, A. Ronchetti, M.P. Protti, J. Davoust, C. Rugarli, and A.A. Manfredi. 1997. Processing of engulfed apoptotic bodies yields T cell epitopes. J. Immunol. 159:5391–5399. [PubMed] [Google Scholar]
- 37.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanzavecchia, A. 1996. Mechanisms of antigen uptake for presentation. Curr. Opin. Immunol. 8:348–354. [DOI] [PubMed] [Google Scholar]
- 39.Bennett, S.R., F.R. Carbone, F. Karamalis, J.F. Miller, and W.R. Heath. 1997. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurts, C., R.M. Sutherland, G. Davey, M. Li, A.M. Lew, E. Blanas, F.R. Carbone, J.F. Miller, and W.R. Heath. 1999. CD8 T cell ignorance or tolerance to islet antigens depends on antigen dose. Proc. Natl. Acad. Sci. USA. 96:12703–12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falcone, M., J. Lee, G. Patstone, B. Yeung, and N. Sarvetnick. 1998. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J. Immunol. 161:1163–1168. [PubMed] [Google Scholar]
- 42.Serreze, D.V., S.A. Fleming, H.D. Chapman, S.D. Richard, E.H. Leiter, and R.M. Tisch. 1998. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Immunol. 161:3912–3918. [PubMed] [Google Scholar]
- 43.Nishio, A., J. Van de Water, P.S. Leung, R. Joplin, J.M. Neuberger, J. Lake, A. Bjorkland, T.H. Totterman, M. Peters, H.J. Worman, et al. 1997. Comparative studies of antimitochondrial autoantibodies in sera and bile in primary biliary cirrhosis. Hepatology. 25:1085–1089. [DOI] [PubMed] [Google Scholar]
- 44.Van de Water, J., J. Turchany, P.S. Leung, J. Lake, S. Munoz, C.D. Surh, R. Coppel, A. Ansari, Y. Nakanuma, and M.E. Gershwin. 1993. Molecular mimicry in primary biliary cirrhosis. Evidence for biliary epithelial expression of a molecule cross-reactive with pyruvate dehydrogenase complex-E2. J. Clin. Invest. 91:2653–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazanec, M.B., C.L. Coudret, and D.R. Fletcher. 1995. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 69:1339–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malmborg, A.C., D.B. Shultz, F. Luton, K.E. Mostov, E. Richly, P.S. Leung, G.D. Benson, A.A. Ansari, R.L. Coppel, M.E. Gershwin, and J. Van de Water. 1998. Penetration and co-localization in MDCK cell mitochondria of IgA derived from patients with primary biliary cirrhosis. J. Autoimmun. 11:573–580. [DOI] [PubMed] [Google Scholar]
- 47.Shimoda, S., M. Nakamura, H. Ishibashi, K. Hayashida, and Y. Niho. 1995. HLA DRB4 0101-restricted immunodominant T cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune diseases. J. Exp. Med. 181:1835–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wucherpfennig, K.W., I. Catz, S. Hausmann, J.L. Strominger, L. Steinman, and K.G. Warren. 1997. Recognition of the immunodominant myelin basic protein peptide by autoantibodies and HLA-DR2-restricted T cell clones from multiple sclerosis patients. Identity of key contact residues in the B-cell and T-cell epitopes. J. Clin. Invest. 100:1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelfrey, C.M., J.L. Trotter, L.R. Tranquill, and H.F. McFarland. 1993. Identification of a novel T cell epitope of human proteolipid protein (residues 40-60) recognized by proliferative and cytolytic CD4+ T cells from multiple sclerosis patients. J. Neuroimmunol. 46:33–42. [DOI] [PubMed] [Google Scholar]
- 50.Pelfrey, C.M., L.R. Tranquill, A.B. Vogt, and H.F. McFarland. 1996. T cell response to two immunodominant proteolipid protein (PLP) peptides in multiple sclerosis patients and healthy controls. Mult. Scler. 1:270–278. [DOI] [PubMed] [Google Scholar]
- 51.Honma, K., K.C. Parker, K.G. Becker, H.F. McFarland, J.E. Coligan, and W.E. Biddison. 1997. Identification of an epitope derived from human proteolipid protein that can induce autoreactive CD8+ cytotoxic T lymphocytes restricted by HLA-A3: evidence for cross-reactivity with an environmental microorganism. J. Neuroimmunol. 73:7–14. [DOI] [PubMed] [Google Scholar]
- 52.Dressel, A., J.L. Chin, A. Sette, R. Gausling, P. Hollsberg, and D.A. Hafler. 1997. Autoantigen recognition by human CD8 T cell clones: enhanced agonist response induced by altered peptide ligands. J. Immunol. 159:4943–4951. [PubMed] [Google Scholar]
- 53.Wicker, L.S., S.L. Chen, G.T. Nepom, J.F. Elliott, D.C. Freed, A. Bansal, S. Zheng, A. Herman, A. Lernmark, D.M. Zaller, et al. 1996. Naturally processed T cell epitopes from human glutamic acid decarboxylase identified using mice transgenic for the type 1 diabetes-associated human MHC class II allele, DRB1*0401. J. Clin. Invest. 98:2597–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reijonen, H., J.F. Elliott, P. van Endert, and G. Nepom. 1999. Differential presentation of glutamic acid decarboxylase 65 (GAD65) T cell epitopes among HLA-DRB1*0401-positive individuals. J. Immunol. 163:1674–1681. [PubMed] [Google Scholar]