Abstract
Infection with hepatitis C virus (HCV) is a leading cause of chronic liver disease worldwide. Little is known about how this virus is able to persist or whether this persistence might be because of its ability to alter the early innate immune response. The major HCV envelope protein E2 has been shown to bind to CD81. Thus, HCV binding to natural killer (NK) cells could result in the cross-linking of CD81. To explore this possibility, we investigated whether cross-linking CD81 on NK cells could alter NK cell function. CD81 cross-linking by monoclonal antibody (mAb) specific for CD81 or by immobilized E2 have been shown to result in costimulatory signals for human T cells. In this study, we show that CD81 cross-linking via immobilized E2 or mAbs specific for CD81 inhibits not only non major histocompatibility complex–restricted cytotoxicity mediated by NK cells but also interferon (IFN)-γ production by NK cells after exposure to interleukin (IL)-2, IL-12, IL-15, or CD16 cross-linking. These results show that CD81 cross-linking mediates completely different signals in NK cells versus T cells. Importantly, these results suggest that one mechanism whereby HCV can alter host defenses and innate immunity is via the early inhibition of IFN-γ production by NK cells.
Keywords: NK cells, cytokines, virus, hepatitis C virus
Introduction
Hepatitis C virus (HCV)*, a positive-strand RNA virus, is the principal causative agent of parenterally transmitted and community-acquired nonA, nonB hepatitis (1). HCV is a major health problem, and it is estimated that HCV affects 170 million people worldwide and >10% of the population in some countries (2). Infection with HCV results in subclinical chronic hepatitis in ∼85% of infected individuals. These individuals carry an increased risk of developing various liver diseases including cirrhosis and hepatocellular carcinoma (3).
The exact mechanism(s) whereby HCV establishes and maintains its persistence and the subsequent liver damage is not well understood. It is known that both cellular and humoral immune responses are present during acute and chronic infection (4). A number of possibilities have been proposed to explain why people infected with HCV fail to resolve this virus despite generating both cellular and humoral immune responses. One potential possibility is the mutability of the RNA of this virus that could lead to quasispecies that evade immune selection/recognition (5, 6). Another possibility is that HCV alters or inhibits selective but crucial immune functions. HCV-encoded proteins such as envelope protein E2, the nonstructural protein 5A, and core protein have been shown to have immunosuppressive potentials (7–9). In this regard, the innate immune response, which is believed to play a crucial role in the early host response to viruses and other pathogens, could be an important target for HCV. Inhibition of the innate immune response could allow HCV a growth advantage that could not be completely resolved or controlled when the adaptive immune response finally develops.
NK cells play an important role in the early innate host defense against a number of pathogens. The role of NK cells in host defense against various viral infections has been extensively investigated in humans and in different animal models (10–15). The ability to quickly produce large amounts of IFN-γ is believed to be a key function of NK cells in the innate immune response. The early production of IFN-γ by NK cells enable them to skew the adaptive immune response to a Th1 inflammatory response. Recently, it has been shown that IFN-γ–mediated clearance of hepatitis B virus (HBV) can occur in the liver of infected chimpanzees before the accumulation of T cells and that this could be a consequence of NK cell activation (16). The possibility that HCV might be able to alter NK cell functions and thus the innate immune response has not been fully explored.
In this study, we have investigated whether cross-linking CD81 on NK cells can alter NK cell function. The major HCV envelope protein E2 has been shown to bind to CD81 (17). Thus, HCV binding to NK cells could result in the cross-linking of CD81. CD81 cross-linking by immobilized E2 or mAb specific for CD81 have been shown to result in costimulatory signals for human T cells (18–20). In contrast, in this study, we show that CD81 cross-linking via immobilized E2 or mAb specific for CD81 can inhibit IFN-γ production by NK cells after exposure to IL-2, IL-12, IL-15, or CD16 cross-linking. These results show for the first time that CD81 cross-linking mediates different signals in NK cells than in T cells. More importantly, these results suggest that one mechanism whereby HCV can alter host defenses and innate immunity is via the early inhibition of NK cell production of IFN-γ.
Materials and Methods
Reagents and Antibodies.
Listeria monocytogenes (strain 506; American Type Culture Collection) was used in experiments, described below, to stimulate PBMCs obtained from healthy volunteers. Bacteria were grown and prepared as described previously (18). The FITC- or PE-conjugated mouse mAbs against human CD3, CD4, CD8, TCR-α/β, TCR-γ/δ, CD19, CD14, CD16, CD56, and isotype-matched control antibodies (Caltag) were used for flow cytometric analysis. In some experiments, FITC-conjugated goat anti–mouse IgG, obtained from Becton Dickinson, was used as the secondary antibody. Purified mouse mAb against human CD81 (clone JS-81) was purchased from BD PharMingen. HCV envelope protein E2 and its specific anti-E2 mAb (291A2) were provided by Chiron Corp. and have been described previously (17). The antibody cocktails and other reagents required for purifying human NK cells, CD4+, or CD8+ T cells were obtained from StemCell Technologies.
Generation/Purification of TCR-γδ+ T Cells and NK Cells.
TCR-γδ+ T cells were expanded from PBMCs using Listeria monocytogenes as described previously (18). Lymphocyte cultures shown to contain high numbers of TCR-γδ+ T cells, as verified by flow cytometry, were then used for purifying TCR-γδ+ T cells by negative selection using a magnetic column system developed by StemCell Technologies, Inc. Briefly, ∼5 × 107 lymphocytes were suspended in 1 ml of PBS, pH 7.4, supplemented with 2% FCS. This suspension was then incubated with 100 μl of an antibody cocktail containing antibodies specific for monocytes, B cells, and T cells bearing CD4 or CD8. This suspension was incubated on ice for 30 min. After an additional incubation with 60 μl of magnetic colloid on ice for 30 min, the cell suspension was loaded onto a magnetic column for separation by gravity. The antibody-labeled cells were retained in the column, whereas the unlabeled cells were collected in the flow-through. For purification of NK cells from freshly obtained PBMCs, the same negative selection method was used, except that an antibody cocktail was made specifically to enrich human NK cells. Cells obtained using the above strategies contained highly purified TCR-γδ+ T cells (>95% TCR-γδ+) or NK cells (>95% CD56+, CD3−, TCR-αβ−).
Immobilized E2 and Antibody and Cell Stimulation.
The effects of CD81 cross-linking on the activation of T cells versus NK cells were investigated using a microtiter culture system. Microtiter plates (EIA/RIA plate; Corning Inc.) were coated in sodium bicarbonate buffer, pH 8.2, with one of the following: (i) isotype-matched control antibody; (ii) anti-CD3 antibody (0.5 μg/ml) or anti-CD16 (0.5 μg/ml); (iii) anti-CD81 mAb (5 μg/ml) or immobilized E2; or (iv) a mixture of anti-CD3 plus anti-CD81 or immobilized E2, or anti-CD16 plus anti-CD81 or immobilized E2. The plates were incubated overnight at 4°C, and washed three times with PBS. For immobilizing E2 onto microtiter wells, wells were first coated with anti-E2 mAb (5 μg/ml) as described previously. These wells were then washed and exposed to recombinant HCV E2 protein (1 μg/ml). These wells were then incubated at 37°C for 2 h and then washed with PBS. To determine the effect of CD81 cross-linking on the cytokine production, 105 T cells or NK cells in a total volume of 200 μl per well were cultured in wells coated as described previously and in the presence of 100 U/ml of IL-2. After 18 h, supernatants were collected and assessed for IFN-γ levels by ELISA.
Detection of IFN-γ.
The IFN-γ levels present in culture supernatants were assessed by ELISA as described previously (18, 20). Both capture mAb and biotinylated detection mAb for human IFN-γ were purchased from Becton Dickinson and BD PharMingen, and used at the concentrations recommended by the manufacturer. Various concentrations of recombinant IFN-γ, ranging from 50,000 to 50 pg/ml, were included in the assay as the standard. Avidin-conjugated peroxidase and the chromogenic substrate O-phenylenediamine dihydrochloride were obtained from Sigma-Aldrich. Spectrophotometer analysis was performed at 405 nm wavelength on an EMAX spectrophotometer (Molecular Devices) using Softmax Pro software (Molecular Devices). The levels of IFN-γ were expressed as the means ± SD of duplicate samples.
Flow Cytometric Analysis.
The phenotypic characterization of lymphocyte preparations was determined using standard one- or two-color flow cytometric analysis using a FACScan™ flow cytometer (Becton Dickinson). Briefly, aliquots of cells were stained with a panel of FITC- or PE-conjugated mAb specific to various lymphocyte surface proteins for 30 min at 4°C. After washing with PBS supplemented with 2% FCS, the cells were fixed with 0.5 ml paraformaldehyde in PBS, and analyzed by the flow cytometer. Isotype-matched antibodies were included in all flow cytometric analysis.
Cytotoxicity Assay.
The standard 4-h chromium-51 release assay was used to assess the potential effect of CD81 cross-linking on the cytolytic activities of NK cells. Human K-562 tumor cells and Burkitt lymphoma cells (Daudi) were used as target cells for freshly isolated NK cells and IL-2–activated NK cells, respectively. Briefly, effector cells at varying concentrations were incubated 30 min at 37°C in microtiter wells that had been precoated with either immobilized E2 or anti-CD81. 51Cr-labeled target cells (104) were then added to each well. After an additional 4-h incubation, aliquots of culture supernatants were harvested and the percentage specific lysis determined. In some experiments immobilized anti-CD56 antibody was also included as an additional control for assessing NK cell–mediated cytotoxicity. Spontaneous releases for both target cells never exceed 15%.
Results
Cross-Linking of CD81 Inhibits IFN-γ Productions by IL-2–, IL-12–, or IL-15–stimulated NK Cells.
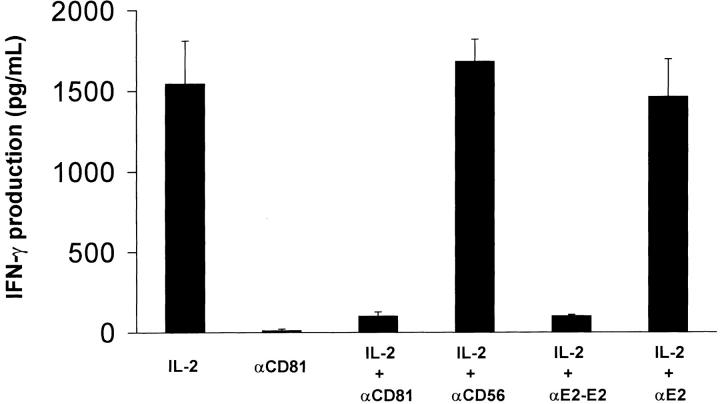
NK cells produce IFN-γ after exposure to a number of different cytokines (15). In initial experiments we investigated whether cross-linking CD81 on NK cells would alter IFN-γ production after exposure to IL-2. NK cells were placed in microtiter wells containing different immobilized antibodies or E2 and then immediately exposed to varying concentrations of IL-2. Results from a representative experiment are presented in Fig. 1 . The ability of NK cells to produce IFN-γ in response to IL-2 was dramatically inhibited upon CD81 cross-linking by either immobilized E2 or anti-CD81 antibody. No inhibition of IL-2–induced IFN-γ production was observed when NK cells were exposed to microtiter wells coated with an isotype-matched control antibody (IgG1) or anti-E2. Additionally, no inhibition was observed when NK cells were exposed to immobilized antibody specific for CD56, indicating that the inhibition was unique for CD81 cross-linking.
Figure 1.
Cross-linking of CD81 significantly inhibits IL-2–mediated IFN-γ production by NK cells. Purified NK cells (105 cells per well) were cultured in microtiter plates that were precoated with anti-CD81 (5 μg/ml), anti-CD56 (5 μg/ml), immobilized E2 (anti-E2 (5 μg/ml)/E2 (1 μg/ml) complex), or anti-E2 (5 μg/ml). Each culture contained 200 μl of medium with or without supplemented IL-2 (100 U/ml). Supernatants were collected after 18 h and assessed for IFN-γ levels by ELISA. The amounts of IFN-γ are presented as mean (pg/ml) ± SD of duplicate samples. Data are representative of five independent experiments using different donors.
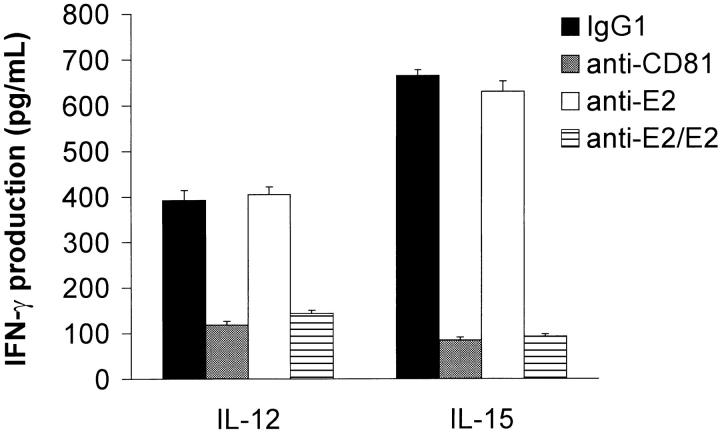
IL-12 and IL-15 are two cytokines believed to play a crucial role in skewing the T cell response to a Th1 cytokine profile via the stimulation of IFN-γ production by NK cells (21, 22). Thus, the effect of CD81 cross-linking on IL-12– versus IL-15–mediated activation of NK cells was also investigated. Results from a representative experiment are shown in Fig. 2 . Cross-linking of CD81 by either immobilized E2 or anti-CD81 resulted in a significant reduction in IFN-γ production by IL-12– or IL-15–activated NK cells.
Figure 2.
Cross-linking of CD81 significantly inhibits IL-12– or IL-15–mediated IFN-γ production by NK cells. Purified NK cells (105 cells per well) were cultured in microtiter plates that were precoated with control IgG1 5 μg/ml antibody, 5 μg/ml anti-CD81, 5 μg/ml anti-E2, or immobilized E2 (5 μg/ml anti-E2/1 μg/ml E2 complex). Each culture contained 200 μl of medium supplemented with either IL-12 (500 U/ml) or IL-15 (5 U/ml). Supernatants were collected after 18 h and assessed for IFN-γ levels by ELISA. The amounts of IFN-γ are presented as mean (pg/ml) ± SD of duplicate samples. Data are representative of three independent experiments using different donors.
Differential Effects of CD81 Cross-Linking on NK Cell Activation versus T Cell Activation.
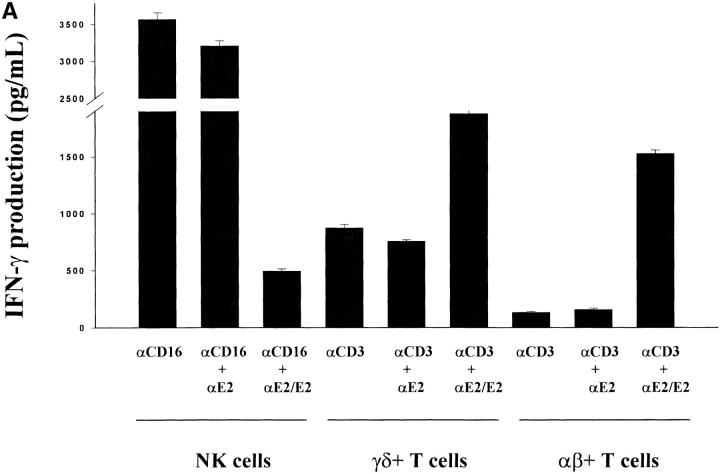
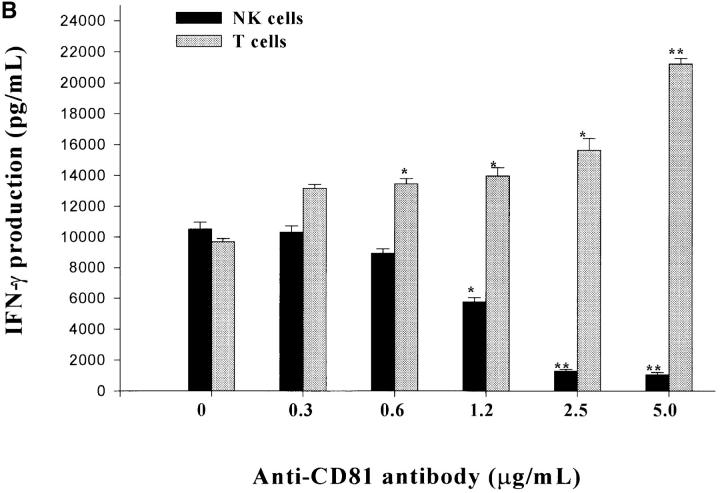
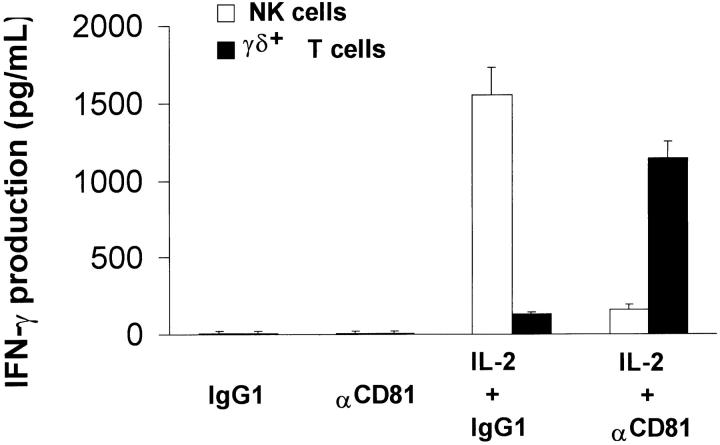
CD16 is a low-affinity IgG receptor (FcγRIII) that is expressed primarily on NK cells (23). Cross-linking of CD16 on NK cells has been shown to activate NK cells with regards to enhanced cytokine production and cytolytic activity (24). To further investigate the inhibitory actions of CD81 cross-linking on NK cell functions, we investigated whether CD81 cross-linking also inhibited NK cell activation via CD16 cross-linking. Since the cross-linking of CD81 on T cells results in an enhanced proliferative response and an enhancement in cytokine production after CD3 cross-linking (18–20), NK cells and T cells appear to differ significantly with regards to the consequences of CD81 cross-linking. To verify and further explore this interesting difference, we assessed T cells and NK cells in parallel for the effects of CD81 cross-linking following the cross-linking of CD3 on T cells versus CD16 on NK cells. To address this question, NK cells, TCR-γδ+ T cells, and TCR-αβ+ T cells were exposed to varying combinations of immobilized E2 or anti-CD81 and assessed for IFN-γ production at 18 h of culture. As shown in Fig. 3 A and B the simultaneous cross-linking of CD81 and CD3 resulted in an enhanced production of IFN-γ by TCR-αβ+ and TCR-γδ+ T cells. These results are identical to previously published findings (18–20). In striking contrast, when CD81 was cross-linked along with CD16 cross-linking (Fig. 3 A) or IL-2 stimulation (Fig. 3 B) a significant inhibition of IFN-γ production by NK cells was observed. As seen in Fig. 4 , these differences were not due to potential differences in the stimuli used for activation of NK cells versus T cells since identical results were also observed when both TCR-γδ+ T cells and NK cells were assessed for IFN-γ production after IL-2 stimulation. Cross-linking CD81 on T cells or NK cells with either immobilized E2 or mAb specific for CD81 yielded equivalent results. These results show that instead of providing costimulatory signals, as seen with T cells, CD81 cross-linking on NK cells induces negative regulatory signals that inhibit or downregulate NK cell activation.
Figure 3.
Differential effects of CD81 crosslinking on the activation of NK cells versus TCR-γδ+ or TCR-αβ+ T cells. (A) Purified NK cells, TCR-γδ+ T cells, or TCR-αβ+ T cells were cultured in wells (105 per well) precoated with following antibodies: (i) 0.5 μg/ml anti-CD16 (NK cells) or anti-CD3 (T cells); (ii) anti-CD16 plus anti-E2 (5 μg/ml) for NK cells or anti-CD3 plus anti-E2 for T cells; or (iii) anti-CD16 plus immobilized E2 (1 μg/ml anti-E2/E2) for NK cells, or anti-CD3 plus immobilized E2 (anti-E2/E2) for T cells. TCR-γδ+ T cells were derived from bacteria-expanded cultures, as described in Materials and Methods. TCR-αβ+ T cells were derived from anti–CD3- or ConA-activated PBL. Each culture contained 200 μl of medium supplemented with IL-2 (100 U/ml). Supernatants were collected after 18 h and assessed for the IFN-γ levels by ELISA. IFN-γ levels are presented as mean (pg/ml) ± SD of duplicate samples. Data are representative of two independent experiments using different cell preparations. (B) Purified NK cells or TCR-γδ+ T cells were cultured in wells precoated with varying concentrations of antibody specific for CD81. For NK cells, IL-2 (100 U/ml) was added to each well. For T cells, wells were precoated with varying concentrations of anti-CD81 along with a constant amount of anti-CD3 (0.5 μg/ml). Culture supernatants were collected after 18 h and assessed for IFN-γ levels by ELISA. IFN-γ levels are presented as mean (pg/ml) ± SD of duplicate samples. Data presented is derived from one of two independent experiments. (*P < 0.05; **P < 0.01; Student's t test).
Figure 4.
CD81 inhibits IL2 activation of NK cells and TCR-γδ+ T cells. Purified NK cells or TCR-γδ T cells were cultured in wells (105 cells per well) either untreated or precoated with control IgG1 antibody (5 μg/ml) or anti-CD81 (5 μg/ml). Each culture contained 200 μl of medium with or without supplemented IL-2 (100 U/ml). Supernatants were collected after 18 h and assessed for IFN-γ levels by ELISA. The levels of IFN-γ are presented as mean (pg/ml) ± SD of duplicate samples. Data are representative of three independent experiments using different donors.
Cross-Linking of CD81 Inhibits Cytolytic Activity of NK Cells.
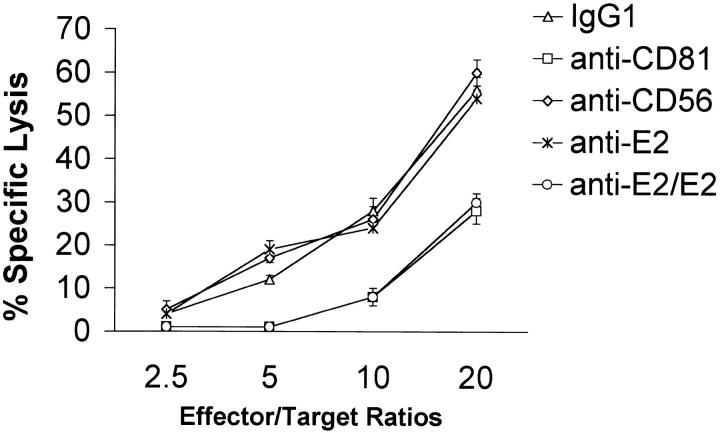
To determine whether cross-linking of CD81 can modulate NK cell–mediated cytotoxicity, freshly isolated and purified NK cells were incubated for 30 min in microtiter wells precoated with one of the following: (i) isotype matched control IgG1; (ii) anti-CD81; (iii) anti-CD56; (iv) anti-E2; or (v) immobilized E2. Non-MHC–restricted cytotoxic activity was then assessed against K562 tumor cells. A representative experiment is presented in Fig. 5 . No alteration in NK cell cytotoxic activity was observed when CD56 was cross-linked on NK cells or when NK cells were exposed to immobilized control IgG1 or mAb to E2. However, the cross-linking of CD81 on NK cells via either immobilized E2 or anti-CD81 resulted in a significant reduction in the cytolytic activity. Identical results were also observed when assessing IL-2–activated NK cells (data not presented).
Figure 5.
CD81 cross-linking inhibits the cytotoxic activities of fresh NK cells. The standard 4-h chromium release assay was used to assess NK cell–mediated cytotoxicity. Varying numbers of freshly isolated NK cells were cultured in microtiter wells precoated control IgG1, anti-CD81, or anti-CD56 antibody at 1 μg/ml of each antibody, 5 μg/ml anti-E2, or immobilized E2 (1 μg/ml anti-E2/E2) for 30 min before addition of 51Cr-labeled K-562 (104 cells per well). Effector:target cell ratios of 20, 10, 5, and 2.5 were used in each experiment. Spontaneous releases for K-562 were always below 10%. Specific lysis is presented as mean percentage ± SD of duplicate samples. Data are representative of six independent experiments using different cell preparations.
Discussion
NK cell production of IFN-γ and/or the recognition of virally infected cells are believed to play important roles in the innate immune response to virus infections. These functions are an important aspect of the immediate response of the host to virus and are believed to control the initial virus infection so that levels of virus are not too high or overwhelming for when the adaptive immune response develops. The production of IFN-γ by NK cells not only directly inhibits virus replication but more importantly, can also skew the Th cell response to a Th1 profile (15, 21). The importance of NK cells in host defense to different viruses has been extensively studied using animal models where NK cell depletion or deficiency results in a significant increase in the sensitivity of the animal to a number of viruses (15). In humans, low NK cell function has been linked with increased human susceptibility to HSV, Epstein-Barr virus, human cytomegalovirus, and HIV (10, 12, 14, 15). Additionally, NK cell function has also been shown to be decreased significantly in chronic HCV patients, compared with noninfected individuals, even though the numbers of circulating NK cells were comparable between these two groups of individuals (25). The possibility that HCV might alter NK cell function is also suggested because the exposure of PBMCs from healthy individuals to virus containing serum obtained from HCV-infected individuals resulted in the inhibition of NK-mediated cytotoxicity (25). Our results show that the major HCV envelope protein E2 can inhibit, via cross-linking CD81, NK cell production of IFN-γ and the nonMHC-restricted cytotoxic activity of these cells. Pileri and colleagues (17) have previously shown that HCV particles can bind human CD81 and that this binding takes place with high affinity and through the larger of the two extracellular loops of this protein. Thus, there is a distinct possibility that HCV could interact with NK cells via E2:CD81 interactions that could result in CD81 cross-linking, resulting in alterations in NK cell functions. This could have a potentially important impact on the initial host response, the innate immune response, to HCV infection and on the kinetics and magnitude of the T cell and B cell immune responses that later develop. Insufficient production of IFN-γ by NK cells in response to IL-12 and IL-15 activation could alter the development of Th1 responses and favor the development of enhanced Th2 responses. Interestingly, an imbalance between Th1 versus Th2 responses has recently been described in chronic HCV infected individuals (26, 27). Specifically, the defect in the production of Th1 cytokines with an enhanced Th2 response has been proposed to be responsible for the persistent virus and disease in chronic HCV infections. Thus, alterations in NK cell function could set up a situation where the virus might have an initial growth advantage that could then lead to the development of a chronic and persistent infection that could not be completely cleared by the T cell and B cell responses. Consistent with the above possibility are the recent findings that IFN-γ–mediated clearance of HBV can occur in the liver of infected chimpanzees before the accumulation of T cells (16). It was proposed that NK cell–derived IFN-γ might be responsible for this early noncytopathic anti-HBV activity.
CD81 is a member of the tetraspan superfamily (TM4SF) of proteins (28). Results from a number of studies suggest that TM4SF proteins are components of large molecular complexes. These complexes or molecular networks and associated proteins have been suggested to be surface organizers or facilitators that connect different molecules of the cell surface and therefore couple different functions (29). Cross-linking of different TM4SF proteins via different mAbs has been shown to regulate a number of functions including adhesion, activation, proliferation, differentiation, and motility. CD81 has been shown to be associated with other members of the TM4SF, the integrin α4β1, HLA-DR, CD4, CD8, CD21, and CD19 (28, 29). However, what molecules might be associated with CD81 on NK cells is not known. Cross-linking of CD81 has been shown to result in costimulatory signals for both T cells and B cells (18–19, 30). Recently, we have found that immobilized E2 can act like mAb specific for CD81 with regards to providing costimulatory signals for human T cells (18–20). The molecular mechanism(s) involved in CD81 signaling of T cells and B cells is not known. In this study, we show that cross-linking CD81 on NK cells results in inhibition of cytokine production and nonMHC-restricted cytotoxic activity. Thus, cross-linking of CD81 on NK cells results in a completely different signaling cascade and instead of giving a costimulatory signal, as with T cells, it gives an inhibitory signal. Whether this is due to CD81 being associated with NK cell inhibitory receptors is currently under investigation. Regardless of the mechanism, these results indicate that NK cells differ significantly from T cells or B cells with regards to CD81 cross-linking. The biological significance of the differential effects of CD81 cross-linking on the activation of T cells versus NK cells in the course of HCV infection remains to be determined. However, this difference could have important biological consequences for how HCV might regulate the innate and adaptive immune responses.
Acknowledgments
We would like to thank Dr. Nicholas M. Valiante for his valuable advice on this manuscript and more importantly, on the work presented in this manuscript. We also thank Dr. Sergio Abrignani for providing E2 and mAb to E2.
Footnotes
Abbreviations used in this paper: HBV, hepatitis B virus; HCV, hepatitis C virus; TM4SF, tetraspan superfamily.
References
- 1.Houghton, M. 1996. Hepatitis C viruses. Virology. B.N. Fields, D.M. Knipe, and P.M. Howley, editors. Lippincott-Raven, Philadelphia. 1035–1058 pp.
- 2.Cohen, J. 1999. The scientific challenge of hepatitis C virus. Science. 285:26–30. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 1997. Hepatitis C viruses. Wkly. Epidemiol. Rec. 72:65–72. 9115857 [Google Scholar]
- 4.Lechner, F., D.K.H. Wong, P. Rod Dunbar, R. Chapman, R.T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B.B.D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner, A.J., H.M. Geysen, C. Christopherson, J.E. Hall, T.J. Mason, G. Sarraco, F. Bonino, K. Crowford, C.D. Marion, K.A. Crowford, et al. 1992. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc. Natl. Acad. Sci. USA. 89:3468–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar, U., J. Monardino, and H.C. Thomas. 1994. Hypervariable region of hepatitis C virus envelope glycoprotein (E2/NS1) in an agammaglobulinemic patient. Gastroenterology. 106:1072–1075. [DOI] [PubMed] [Google Scholar]
- 7.Gale, M.J., M.J. Korth, N.M. Tang, S.L.Tan, D.A. Hopkins, T.E. Dever, S.J. Polyak, D.R. Gretck, and M.G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology. 230:217–227. [DOI] [PubMed] [Google Scholar]
- 8.Taylor, D.R., S.T. Shi, P.R. Romano, G.N. Barber, and M.M. Lai. 1999. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 285:107–110. [DOI] [PubMed] [Google Scholar]
- 9.Large, M.K., D. Kittlesen, and Y.S. Hahn. 1999. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J. Immunol. 162:931–938. [PubMed] [Google Scholar]
- 10.Orange, J.S., and C.A. Biron. 1996. Characterization of early IL-12, IFN-α/β, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J. Immunol. 156:4746–4756. [PubMed] [Google Scholar]
- 11.Santoli, D., G. Trinchieri, and H. Koprowski. 1978. Cell-mediated cytotoxicity against virus-infected target cells in humans. II. Interferon induction and activation of natural killer cells. J. Immunol. 121:532–538. [PubMed] [Google Scholar]
- 12.Ching, C., and C. Lopez. 1979. Natural killing of herpes simplex virus type 1-infected target cells: normal human responses and influence of antiviral antibody. Infect. Immun. 26:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godeny, E.K., and C. Gauntt. 1986. Involvement of natural killer cells in coxsackievirus B3-induced murine myocarditis. J. Immunol. 137:1695–1702. [PubMed] [Google Scholar]
- 14.Merino, F., W. Henle, and P. Ramirez-Duque. 1986. Chronic active Epstein-Barr virus infection in patients with Chediak-Higashi syndrome. J. Clin. Immunol. 6:299–305. [DOI] [PubMed] [Google Scholar]
- 15.Biron, C.A., K.B. Nguyen, G.C. Pien, L.P. Cousens, and T.P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189–220. [DOI] [PubMed] [Google Scholar]
- 16.Guidotti, L.G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F.V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science. 284:825–829. [DOI] [PubMed] [Google Scholar]
- 17.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A.J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science. 282:938–941. [DOI] [PubMed] [Google Scholar]
- 18.Tseng, C.-T.K., E. Miskovsky, and G.R. Klimpel. 2001. Crosslinking CD81 results in activation of TCRγδ+ T cells. Cell. Immunol. 207:19–27. [DOI] [PubMed] [Google Scholar]
- 19.Wack, A., E. Elisabetta, C.-T.K. Tseng, S. Nuti, G.R. Klimpel, and S. Abrignani. 2001. Binding of the hepatitis C virus envelope protein E2 to CD81 provides a co-stimulatory signal for human T cells. Eur. J. Immunol. 31:166–175. [DOI] [PubMed] [Google Scholar]
- 20.Tseng, C.-H.K., E. Miskovsky, M. Houghton, and G.R. Klimpel. 2001. Characterization of liver TCRγδ+ T cells obtained from individuals chronically infected with hepatitis C virus (HCV). Evidence for these T cells playing a role in the liver pathology associated with HCV infections. Hepatology. 33:1312–1320. [DOI] [PubMed] [Google Scholar]
- 21.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251–276. [DOI] [PubMed] [Google Scholar]
- 22.Waldmann, T.A., and Y. Togaya. 1999. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and the host response to intracellular pathogens. Annu. Rev. Immunol. 17:19–49. [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri, G. 1989. Biology of natural killer cells. Adv. Immunol. 47:187–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anegon, I., M.C. Cuturi, G. Trinchieri, and B. Perussia. 1988. Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells. J. Exp. Med. 167:452–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corado, J., F. Toro, H. Rivera, N.E. Bianco, L. Deibis, and J.B. deSanctis. 1997. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin. Exp. Immunol. 109:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarih, M., N. Bouchrit, and A. Benslimane. 2000. Different cytokine profiles of peripheral blood mononuclear cells from patients with persistent and self-limited hepatitis C virus infection. Immunol. Letters. 74:117–120. [DOI] [PubMed] [Google Scholar]
- 27.Valiante, N.M., A. D'Andrea, S. Crotta, S. Nuti, A. Wack, and S. Abrignani. 2000. Life, activation, and death of intrahepatic lymphocytes in chronic hepatitis C. Immunol. Rev. 174:77–89. [DOI] [PubMed] [Google Scholar]
- 28.Levy, S., S.C. Scott, and H.T. Maecker. 1998. CD81 (TAPA-1): A molecule involved in signal transduction and cell adhesion in the immune system. Annu. Rev. Immunol. 16:89–109. [DOI] [PubMed] [Google Scholar]
- 29.Maecker, H.T., S.C. Todd, and S. Levy. 1997. The tetraspanin superfamily: molecular facilitators. FASEB J. 11:428–442. [PubMed] [Google Scholar]
- 30.Fearon, D.T., and R.H. Carter. 1995. The CD19/CR2/TAPA-1 complex of B lymphocytes. Annu. Rev. Immunol. 13:127–149. [DOI] [PubMed] [Google Scholar]