Abstract
Mature dendritic cells (DCs) are believed to induce T cell immunity, whereas immature DCs induce T cell tolerance. Here we describe that injections of DCs matured with tumor necrosis factor (TNF)-α (TNF/DCs) induce antigen-specific protection from experimental autoimmune encephalomyelitis (EAE) in mice. Maturation by TNF-α induced high levels of major histocompatibility complex class II and costimulatory molecules on DCs, but they remained weak producers of proinflammatory cytokines. One injection of such TNF/DCs pulsed with auto-antigenic peptide ameliorated the disease score of EAE. This could not be observed with immature DCs or DCs matured with lipopolysaccharide (LPS) plus anti-CD40. Three consecutive injections of peptide-pulsed TNF/DCs derived from wild-type led to the induction of peptide-specific predominantly interleukin (IL)-10–producing CD4+ T cells and complete protection from EAE. Blocking of IL-10 in vivo could only partially restore the susceptibility to EAE, suggesting an important but not exclusive role of IL-10 for EAE prevention. Notably, the protection was peptide specific, as TNF/DCs pulsed with unrelated peptide could not prevent EAE. In conclusion, this study describes that stimulation by TNF-α results in incompletely matured DCs (semi-mature DCs) which induce peptide-specific IL-10–producing T cells in vivo and prevent EAE.
Keywords: dendritic cells, EAE, tolerance, IL-10, TNF
Introduction
Dendritic cell (DC)* development has been divided into the two major stages immature and mature. Immature DCs reside in peripheral tissues as sentinels of the immune system responding to inflammatory stimuli (e.g., TNF-α) or microbial products (e.g., LPS). Such signals induce maturation and migration of DCs into secondary lymphoid organs where they now appear as mature DCs expressing high surface levels of MHC II and costimulatory molecules enabling efficient T cell priming (1). Further DC-T cell cross-talk (e.g., via CD40-CD154 interaction) leads to full DC maturation, cytokine production, and Th1 or Th2 polarizations (2). However, these stimuli were also shown to have different qualities in stimulating DC maturation and cytokine production (3, 4), which finally might contribute to differential levels of T cell activation, Th1/2 polarization, and even T cell tolerance.
Immature DCs residing in peripheral tissues such as epidermal Langerhans cells are poor T cell stimulators because they express only moderate levels of MHC II (5) and no or very low levels of costimulatory molecules such as B7–1, B7–2, intercellular adhesion molecule (ICAM)-1, and CD40 (6). When we generated such MHC IIlow/B7neg/low immature DCs from murine bone marrow (BM) and applied them for antigen presentation to allogeneic T cells, they induced T cell anergy (7, 8). Human immature DCs generated from peripheral blood monocytes were shown to induce IL-10 producing regulatory T cells in vitro (9) and in vivo (10). Thus, the interaction of T cells with immature DCs is believed to cause T cell tolerance by inducing T cell anergy or IL-10 producing regulatory T cells, while mature DCs induce T cell immunity.
More recently this view has been challenged by the observation that DCs could produce IL-10 when treated with maturation stimuli (11–13) and thereby being tolerogenic. In vivo, IL-10–producing DCs have been shown to suppress experimental autoimmune encephalomyelitis (EAE; references 14 and 15) or mediate tolerance to intranasally administered antigens (16). However, most of these IL-10–mediated effects could also be due to bystander suppression and may therefore not be antigen specific.
Here we investigate the tolerogenicity of DCs that are matured with TNF-α in the murine EAE model. Such TNF/DCs produce low amounts of inflammatory cytokines and no IL-12 p70 and IL-10. However, after repetitive injections, they induce IL-10–producing T cells and antigen specifically prevent EAE. We further show that the capacity of TNF/DCs to prevent EAE is partially IL-10 dependent and provide data that this cannot be achieved by immature DCs or DCs matured with LPS plus anti-CD40.
Materials and Methods
Generation of Different BM-DCs.
DCs were generated from BM cells derived from C57BL/6 or IL-10−/− mice (backcrossed on C57BL/6; reference 17). Cultures were supplemented with high doses of GM-CSF (200 U/ml; TEBU/PeproTech) or the 10% supernatant of a GM-CSF producing cell line as described (18). For DC maturation, cultures were pulsed for 4 h with TNF-α (500 U/ml; TEBU/PeproTech) or with LPS (1 μg/ml; Sigma-Aldrich; E. coli, 0127:B8) with or without anti-CD40 (clone 3/23 or HM40, 5 μg/ml; BD PharMingen) together with or without 10 μM MOG peptide (see below) before intravenous injection into the tail vein of mice. To generate immature DCs, BM cells were cultured continuously in the presence of 20 ng/ml IL-10 (BD PharMingen; reference 19). DC cultures used for cytokine detection by ELISA were performed in bacterial quality 24-well plates (BD Falcon) to avoid DC activation by cell transfer.
FACS® Analysis, Cell Sorting.
BM-DCs were stained with 5 μg/ml pure CD40, CD80, CD86 mAb (BD PharMingen), or undiluted N418 (CD11c), NLDC-145 (CD205), MHC II (M5/114), and YN1/1.7.4 (CD54) hybridoma supernatants followed by a 10 μg/ml goat anti–rat IgG and IgM-FITC (Southern Biotechnology Associates, Inc.) or anti–hamster Ig-FITC (BD PharMingen), and counterstained with CD11c-PE (all BD PharMingen), for 30 min on ice before they were analyzed with a FACScan™ (Becton Dickinson).
Before cell sorting with magnetic beads, spleen cells were treated with 0.83% wt/vol ammoniumchloride in water for 5 min at 37°C to lyse erythrocytes. To release DCs from spleen connective tissue the single cell suspensions were treated with 1 mg/ml Collagenase III (Worthington), 100 U/ml DNase I (Sigma-Aldrich), for 30 min, and 20 mM EDTA (Sigma-Aldrich) for 5 min as described (20). For enrichment of CD4+ T cells, positive selection with magnetic CD4-Dynabeads was followed by a Detachbeads procedure, both according to the company manuals (Dynal). The purity was generally >90%. To enrich CD11c+ DCs we used CD11c-conjugated magnetic beads (MACS; Miltenyi Biotech) according to the manufacturer's instructions. There the enrichment was generally 40–50%.
ELISA.
Cytokines produced from spleen cells were detected in culture supernatants at the indicated times by using the ELISA kits for IL-12 p70, IL-12 p40, IL-10, IL-2, IL-4, and IFN-γ (BD PharMingen). Supernatants from spleen cell restimulations were taken after 24, 48, 72, and 96 h and frozen or tested immediately. For detection of IL-10, IL-12 p40, and IL-12 p70 produced by DCs, cells were cultured for 24 h with the indicated stimuli or 50 ng/ml phorbol-myristate-acetate (PMA; Sigma-Aldrich) plus 500 ng/ml Ionomycin (Sigma-Aldrich) before supernatants were analyzed.
RNase Protection Assay.
Production of cytokine RNA was induced in DCs for 4, 7, and 24 h by the indicated stimuli and detected by an RPA kit (BD PharMingen) following the manufacturer's instructions.
EAE Induction, Treatment by DCs, and Other Factors.
MOG peptide 35–55 (Sigma Genosys) was used in C57Bl/6 mice to induce EAE. Briefly, C57BL/6 mice were injected subcutaneously with 50 μg MOG peptide (or additional 50 μg OVA 323–339 peptide in one experiment) in 50 μl PBS emulsified in 50 μl CFA that was further enriched with 10 mg/ml M. tuberculosis (H37Ra, Difco/BD PharMingen). In addition 200 ng Pertussis toxin (List/Quadratech) were injected intraperitoneally at day 0 and 2. The incidence of EAE induced in our mice was throughout 90%, however the day of EAE onset varied between day 8–11 or day 14–17 depending on the batch of MOG peptide. Therefore in each experiment an untreated control group was included. Paralysis was evaluated according to the following score: 1 = full tail, 2 = hind limbs, 3 = complete back, 4 = fore limbs (i.e., complete paralysis, such mice were killed). DCs were injected (2–2.5 × 106, intravenously) 4 h after stimulation and peptide pulse (see above) once or repeatedly as indicated in the figure legends. The rat anti–mouse IL-10R mAb (provided by M. Goldman, University of Brussels, Brussels, Belgium) and an isotype-matched control mAb were prepared as a concentrate in a cell factory (Integra Biosciences) and injected at 0.5 mg equivalents per mouse intraperitoneally at the same days as the DCs (day −7, −5, −3) before EAE induction (day 0) and additionally at day −1 and day 1.
Results
Stimulation with TNF-α Induces DC Maturation but Poor Cytokine Production.
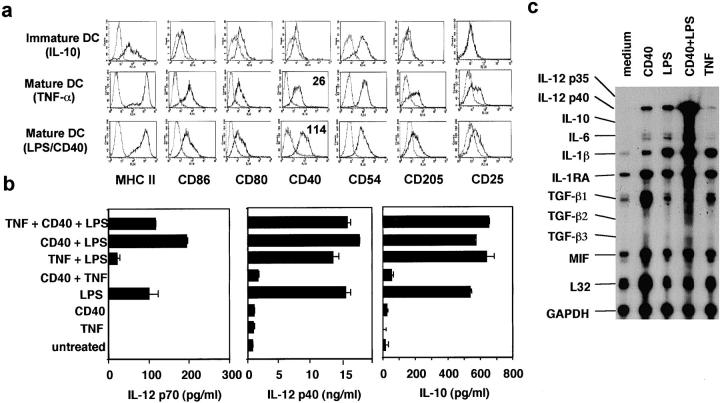
To investigate possible differences between typical DC stimuli, we treated DCs generated from murine BM for 24 h with TNF-α, LPS, or LPS plus anti-CD40. Untreated BM-DCs typically consist of a heterogeneous mixture of immature and spontaneously matured DCs (18) and were not used for further analysis. For comparison we generated specifically immature DCs by adding IL-10 throughout the BM-DC culture (19). Immature DCs expressed no maturation markers (CD205, CD25) and low levels of MHC II and the costimulatory molecules CD80, CD86, and CD40 (Fig. 1 a). In contrast, on mature DCs all of these markers were upregulated, but comparably high after stimulation with TNF-α, LPS, or LPS plus anti-CD40 (Fig. 1 a). Except the expression of CD40 was slightly higher on DCs stimulated with LPS plus anti-CD40 (Fig. 1 a).
Figure 1.
Surface phenotype and cytokine production of DCs matured with TNF-α. (a) DCs were generated from C57BL/6 mice until day 8 in the presence of IL-10 to inhibit their maturation, or in the absence of IL-10 and additional stimulation with TNF-α or anti-CD40 plus LPS overnight. FACS® analysis shows the expression of the indicated markers (straight line) or isotype controls (dotted line) of CD11c+ cells within a life gate. Numbers within histograms represent the mean fluorescence of the marker with subtracted isotype values. (b) DCs were cultured in a 24-well plate until day 8. Then, cells were stimulated without transfer for 24 h with TNF-α, anti-CD40, LPS, or their combinations, before supernatants were tested for their cytokine content by ELISA. (c) DCs were stimulated at day 8 as indicated and harvested after 24 h. The levels of mRNA expression were detected by RNase protection assay. The data for (a) is representative of more than six, and the data for b and c are each representative of three independent experiments with similar results.
However, the production of cytokines varied remarkably between the differentially stimulated DC. IL-12 p70 and IL-10 production were completely dependent on LPS stimulation, and could not be detected after treatment with TNF-α alone (Fig. 1 b). Basic levels of IL-12 p40 were produced by all types of DCs but strongly augmented by LPS. In addition, mRNA for IL-10 was not detectable in TNF/DCs by reverse transcription (RT)-PCR after 6 and 16 h (not shown) or by RPA after 4 and 7 h (not shown) or 24 h (Fig. 1 c). The mRNA levels for IL-1β and IL-6 were low in DC cultures matured with TNF-α or LPS alone but highly increased in cultures stimulated with LPS plus anti-CD40 (Fig. 1 c).
This shows that the quality of maturation stimuli for DCs varies predominantly on the level of cytokine production but not on the expression of surface markers. Therefore we compared the cytokineweak TNF/DCs (semi-mature or incompletely matured), cytokinestrong LPS/CD40/DCs (fully mature), and immature DCs for their capacity to influence EAE after auto-antigenic MOG peptide pulse.
Peptide-specific Prevention of EAE by Repetitive Injections of TNF/DCs.
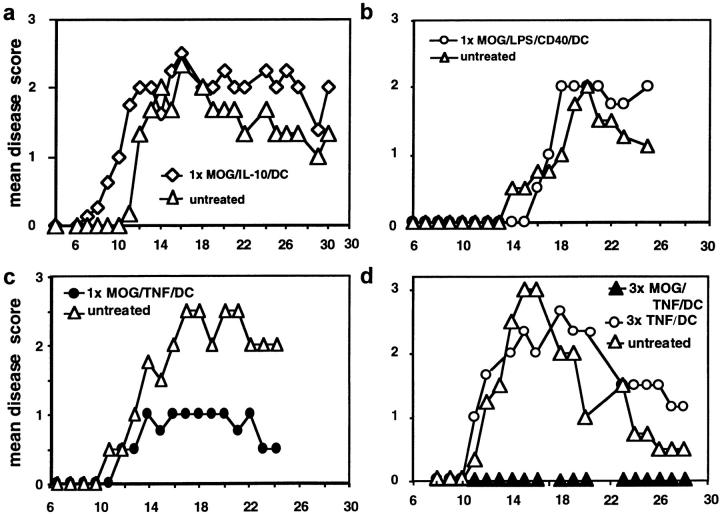
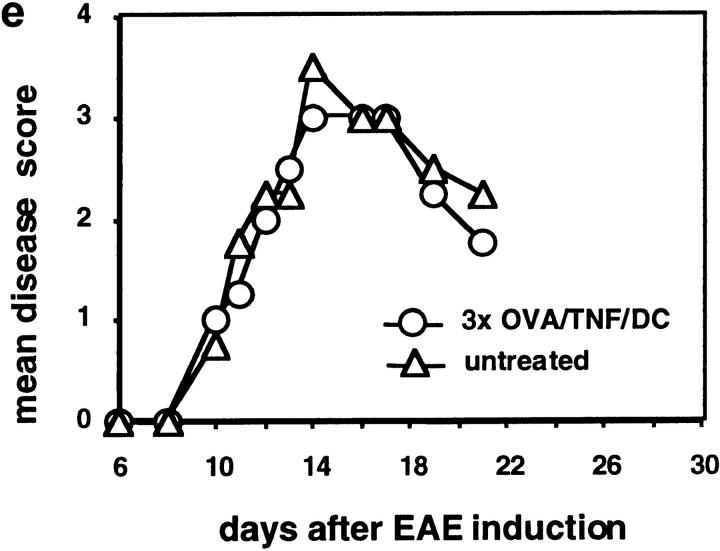
Immature DCs, generated by various methods, have been shown to induce T cell anergy or regulatory T cells and that they can act in tolerogenic fashion in mice (8, 21, 22), rat (14), and human (10). Therefore, we generated immature DCs by the continuous presence of IL-10 (19), pulsed them with the auto-antigenic MOG peptide 35–55, and injected them intravenously into mice 3 d before EAE induction. However, the EAE score was not influenced by these immature DC (Fig. 2 a) or MOG-pulsed DCs matured with LPS plus anti-CD40 (Fig. 2 b). Also three injections of MOG/LPS/CD40/DCs did not prevent EAE (not shown). Surprisingly, a single injection of MOG-pulsed TNF/DCs given 3, 5 (not shown), or 7 d before EAE induction ameliorated the course of the disease (Fig. 2 c), and three injections led to complete prevention of the disease. This prevention from EAE was dependent on the loading of TNF/DCs with MOG peptide and was not observed by unloaded TNF/DCs (Fig. 2 d) or when TNF/DCs were pulsed with irrelevant OVA peptide.
Figure 2.
Repetitive injections of TNF/DCs protect mice from EAE DCs were simultaneously pulsed with MOG peptide and treated with TNF-α for 4 h (MOG/TNF/DC), washed in PBS, and 2.5 × 106 cells were injected once (1×) or three times (3×) intravenously into 3–4 C57BL/6 mice per group. Control mice were left without DC injections (untreated). 3 d after the last/only DC injection EAE was induced and the mice observed for paralysis. (a) Injection of immature DCs, generated in the presence of IL-10, pulsed with MOG peptide, and injected 3 d before EAE induction, were not protective. (b) DCs matured with LPS plus anti-CD40 and pulsed with MOG peptide and injected before EAE induction were not protective. (c) Single injections of MOG pulsed and TNF-α matured DCs, given 7 d before EAE induction, could ameliorate the disease. (d) Three injections of MOG-pulsed TNF/DC but not unpulsed TNF/DC given at days −7, −5, and −3 before EAE induction completely protected mice from EAE. (e) OVA/TNF/DCs could not protect from EAE. As a peptide specificity control, three injections of OVA-pulsed TNF/DC were given at days −7, −5, and −3 before EAE induction or mice were left untreated. OVA peptide was additionally emulsified together with the MOG peptide in CFA at day 0 in this experiment. The data for a–d are each representative of three independent experiments with similar results.
TNF/DCs Induce Antigen-specific IL-10–producing CD4+ T Cells.
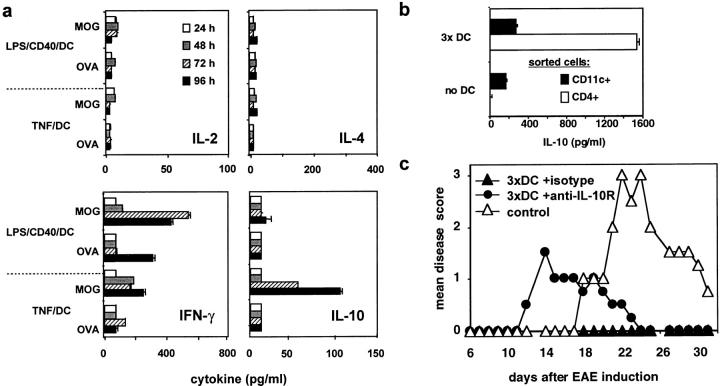
To assess the mechanism of complete EAE suppression in mice that received three injections with MOG/TNF/DCs or MOG/LPS/CD40/DCs their spleens were removed at day 0 and restimulated with MOG peptide or irrelevant OVA peptide, and the culture supernatants were analyzed after 24, 48, 72, and 96 h for their cytokine content. The predominant cytokine that could be detected after three MOG/TNF/DCs injections was IL-10, whereas only little IFN-γ and no IL-2 or IL-4 were present (Fig. 3 a). Preliminary data indicate that also TGF-β1 is absent (not shown). In contrast, three injections of MOG/LPS/CD40/DCs induced predominantly IFN-γ but no IL-2, IL-4, or IL-10, indicative for the induction of a Th1 response, which could explain why such DCs are not protective in the Th1-mediated EAE.
Figure 3.
Repetitive injections of TNF/DCs induce peptide-specific CD4+ T cells producing IL-10 which is partially involved in EAE protection. (a) TNF/DCs induce IL-10–producing cells from restimulated spleen cells. C57BL/6 mice received three intravenous injections of MOG-pulsed TNF/DCs or LPS/CD40/DCs at days −7, −5, and −3. Spleen cells from these mice were restimulated at day 0 with 10 μM MOG peptide or 10 μM unrelated OVA peptide. Cell supernatants were taken after 24, 48, 72, and 96 h and tested for their cytokine content by ELISA. (b) The IL-10 production from spleen cells induced by DC injections is derived from CD4+CD3+ T cells, but not CD11c+ DCs. C57BL/6 mice received three injections of MOG pulsed TNF/DC at days −7, −5, and −3. Spleen cells from these mice were sorted for CD4+ and CD11c+ cells at day 0 and then stimulated for 24 h with PMA plus Ionomycin before supernatants were tested by ELISA for their IL-10 content. From the CD4+ enriched cells 97% coexpressed CD3, and the contamination CD11c+ cells within the CD4+ cells was 0.8–1.4%. (c) The protection from EAE partially depends on IL-10. C57BL/6 mice (3–4 per group) were injected three times with MOG/TNF/DCs at days −7, −5, and −3 (3×) and EAE was induced at day 0. Mice were injected intraperitoneally with anti–IL-10R mAb or isotype mAb at the same days as TNF/DCs and additionally at day −1 and day 1. Control mice remained without pretreatment by DCs and were not injected with mAb. The data for (a) is representative of three, and the data for b and c are each representative of two independent experiments with similar results.
Although TNF/DCs were not found to produce sizable amounts of IL-10 after in vitro stimulation (Fig. 1, b and c), we analyzed whether the IL-10 produced from the spleen cell suspensions was secreted by the DCs we injected, or endogenous DCs, or T cells. Mice were injected three times with TNF/DCs and at day 0 their spleen cells were sorted for CD4+ and CD11c+ cells and stimulated with PMA plus Ionomycin. The sorted CD11c+ DCs from untreated and TNF/DC injected mice produced small amounts of IL-10 in the same range (Fig. 3 b). However, much higher levels of IL-10 were secreted by sorted CD4+CD3+ T cells after injection of TNF/DCs (Fig. 3 b). T cells of mice that were not injected with DCs did not secrete IL-10. To test whether a potential IL-10 production by the TNF/DCs could anyhow influence EAE prevention, we also injected MOG-pulsed TNF/DCs derived from IL-10−/− mice. However, wild-type and IL-10−/− TNF/DCs were equally potent to completely prevent EAE symptoms (not shown).
Together, our data indicate that the major source of IL-10 after repetitive TNF/DC injections and spleen cell restimulation with MOG peptide are CD4+CD3+ T cells.
EAE Prevention Is Partially Dependent on IL-10.
As IL-10 was the major cytokine detected after repetitive TNF/DC injections, we tested whether IL-10 blockade in vivo could influence the protection from EAE. Therefore, we injected mice treated with our standard TNF/DC injections and EAE protocol with anti–IL-10R mAb or isotype control mAb. In animals receiving anti–IL-10R, but not with isotype control mAb, the protection from EAE was partially reverted (Fig. 3 c). Together these data imply that TNF/DCs induce IL-10 producing CD4+ T cells in vivo. When this IL-10 production is blocked by anti–IL-10R the protection is reverted and the mice develop a mild form of EAE. This partial effect of anti–IL-10R might be indicative for other regulatory mechanisms.
Discussion
Increasing evidence indicates that DCs are not only decisive for T cell priming, but are also key players to maintain self-tolerance in vivo. The general view is that immature DCs are tolerogenic while mature DCs are immunogenic. This is based on experiments in mice where DC maturation was inhibited by certain substances or protocols and such immature DCs induced T cell anergy in vitro (8, 19) and influenced allogeneic transplantations (7, 23). The tolerogenicity by these immature DCs was attributed to the absence or low level expression of costimulatory molecules. However, more recently it has been clearly shown that costimulation is definitely required for the induction of T cell anergy (24) as well as for the generation of regulatory T cells (25). Thus, DCs expressing higher amounts of MHC and costimulatory molecules must not necessarily be immunogenic. We found evidence that the quality of the DC maturation stimulus regulating their cytokine production and the repetitive mode of application might also be decisive features for tolerogenicity of DCs.
The maturation signals delivered through TNF-α, anti-CD40, or LPS differ remarkably in their quality to stimulate the cytokine production by DCs (3, 4), and the effects of a certain cytokine might be further modulated when different receptor types exist for this cytokine, as shown for a tolerogenic role of TNF-α when acting through its TNF-receptor-2 (26). Here, DCs matured with TNF-α protected from EAE, but not DCs matured with LPS plus anti-CD40. This tolerogenic capacity of TNF/DCs was correlated with a low proinflammatory cytokine production when compared with LPS/CD40/DCs. In how far this is relevant for the EAE protection in our system, needs further investigation. Taken together, TNF-α seems to induce an incomplete DC maturation with respect to their cytokine production which correlates with a rather tolerogenic type of DC.
More recent work described DC types that can produce IL-10 and thereby control autoimmunity, as shown for the prevention of EAE (14, 15) or induction of mucosal tolerance (16). For the latter it has been shown that the IL-10 producing DC expressed a mature surface marker profile and that they induced IL-10 producing regulatory T cells. These murine data seem different from data derived from human immature monocyte-derived DCs which constitutively express IL-10 mRNA (27) and low amounts of IL-10 protein which could be further induced by stimulation with anti-CD40 or LPS (13). Although in other studies such immature monocyte-derived DCs were not investigated for IL-10 production, they, similarly to the murine data, induced IL-10 producing T cells (9, 10). It is of note that the DCs in their so-called immature state already express substantial amounts of MHC II and costimulatory molecules, and thereby resemble more the incompletely matured/semi-mature TNF/DCs rather than the immature IL-10/DCs that we used in this study. Together the requirements for the induction of IL-10 producing regulatory T cells by DC in vitro and in vivo seem to be a certain grade of DC maturity and their capacity to produce IL-10. However, when we generated TNF/DCs from IL-10−/− mice they induced IL-10 production by T cells and prevented EAE (not shown).
While a single injection of TNF/DCs ameliorated EAE, three injections were required for full protection, indicating that repetition of antigen presentation is important. Repetitive stimulation seems to be required to enrich or expand regulatory T cells in vitro (9), but a single injection can already be effective in vivo (10), indicating that the maturation state of DCs is more important and repetition might solely enhance the effects revealed by a single injection.
Although the induction of suppressive activities by regulatory T cells seem to need antigen-specific stimulation, their effects mediated through IL-10, TGF-β, or cell–cell contacts can act also through bystander suppression (28, 29). For the prevention of EAE in other studies (14, 15) or induction of mucosal tolerance (16) by IL-10 producing DCs, no controls with unrelated peptides were included to show antigen specificity. Here, we found that TNF/DCs induce peptide-specific prevention from EAE, which cannot be due to bystander suppression since OVA-pulsed TNF/DCs were unable to influence EAE. The immunological discrimination between foreign- and self-antigens, dangerous and harmless influences in vivo is especially important on the level of DCs to maintain self-tolerance. Therefore, the maturation stage of TNF/DC presenting antigens which induce IL-10–producing T cells, possibly represent a tolerance mechanism also relevant in immune-physiology.
The in vivo counterparts that most closely resemble our in vitro generated TNF/DCs are the “veiled cells” of the lymphatics, which migrate to the peripheral lymph nodes transporting tissue antigens (30) or apoptotic cells (31) and there finally represent a distinguishable subset of skin derived DC (32). In all these reports both veiled cells and skin-derived DCs express a remarkably mature morphology (veiled!) and surface phenotype (MHC IIhighCD80+ CD86+CD40+). As both types of veiled cells and skin-derived DCs are detectable under healthy steady-state conditions, they might actively induce tolerance to peripheral tissue antigens in the draining lymph nodes and spleen (33, 34). The signals which induce the homeostatic DC migration are unclear. TNF-α might be a likely candidate to be involved in tolerogenic homeostasis. Besides its potential tolerogenic role (26), it also promotes DC migration (35), induces antigen processing (36), and upregulates CC chemokine receptor (CCR)7 to guide DCs into the T cell areas of the draining lymph nodes (37). Thus, TNF-α would fulfill the prerequisites for promoting steady-state migration and tolerogenic antigen presentation in the secondary lymphoid organs.
In summary, we show that the stimulation of DCs by TNF-α, but not LPS plus anti-CD40, induces incomplete DC maturation (semi-maturation). Repetitive injections of such TNF/DCs are able to induce peptide-specific IL-10 producing CD4+ T cells in vivo and to prevent EAE in mice. Generation of such peptide-specific TNF/DCs may also lead to the development of strategies against human autoimmune diseases.
Acknowledgments
We thank Alexander Steinkasserer and Armin Bender for critical reading of the manuscript, and Michel Goldmann and Heiner Körner for providing reagents and mice.
This work was supported by the ELAN-Fonds of the University of Erlangen (AZ 98.07.42.1), the Deutsche Forschungsgemeinschaft (SFB 263), and the Else Kröner-Fresenius-Foundation.
Footnotes
Abbreviations used in this paper: BM, bone marrow; DC, dendritic cell; EAE, experimental autoimmune encephalomyelitis.
References
- 1.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245–252. [DOI] [PubMed] [Google Scholar]
- 2.Moser, M., and K.M. Rock. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199–205. [DOI] [PubMed] [Google Scholar]
- 3.Granucci, F., C. Vizzardelli, E. Virzi, M. Rescigno, and P. Ricciardi-Castagnoli. 2001. Transcriptional reprogramming of dendritic cells by differentiation stimuli. Eur. J. Immunol. 31:2539–2546. [DOI] [PubMed] [Google Scholar]
- 4.Morelli, A.E., A.F. Zahorchak, A.T. Larregina, B.L. Colvin, A.J. Logar, T. Takayama, L.D. Falo, and A.W. Thomson. 2001. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 98:1512–1523. [DOI] [PubMed] [Google Scholar]
- 5.Mommaas, A.M., A.A. Mulder, C.J. Out, G. Girolomoni, H.K. Koerten, B.J. Vermeer, and F. Koning. 1995. Distribution of HLA class II molecules in epidermal Langerhans cells in situ. Eur. J. Immunol. 25:520–525. [DOI] [PubMed] [Google Scholar]
- 6.Inaba, K., P.M. Witmer, M. Inaba, K.S. Hathcock, H. Sakuta, M. Azuma, H. Yagita, K. Okumura, P.S. Linsley, S. Ikehara, et al. 1994. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J. Exp. Med. 180:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutz, M.B., N.A. Kukutsch, M. Menges, S. Röbner, and G. Schuler. 2000. Culture of bone marrow cells in GM-CSF plus high doses of lipopolysaccharide generates exclusively immature dendritic cells which induce alloantigen-specific CD4 T cell anergy in vitro. Eur. J. Immunol. 30:1048–1052. [DOI] [PubMed] [Google Scholar]
- 8.Lutz, M.B., R.M. Suri, M. Niimi, A.L.J. Ogilvie, N.A. Kukutsch, S. Röbner, G. Schuler, and J.M. Austyn. 2000. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation-resistant and prolong allograft survival in vivo. Eur. J. Immunol. 30:1813–1822. [DOI] [PubMed] [Google Scholar]
- 9.Jonuleit, H., E. Schmitt, G. Schuler, J. Knop, and A.H. Enk. 2000. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhodapkar, M.V., R.M. Steinman, J. Krasovsky, C. Munz, and N. Bhardwaj. 2001. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Saint-Vis, B., I. Fugier-Vivier, C. Massacrier, C. Gaillard, B. Vanbervliet, S. Ait-Yahia, J. Banchereau, Y.J. Liu, S. Lebecque, and C. Caux. 1998. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J. Immunol. 160:1666–1676. [PubMed] [Google Scholar]
- 12.Iwasaki, A., and B.L. Kelsall. 1999. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corinti, S., C. Albanesi, A. la Sala, S. Pastore, and G. Girolomoni. 2001. Regulatory activity of autocrine IL-10 on dendritic cell functions. J. Immunol. 166:4312–4318. [DOI] [PubMed] [Google Scholar]
- 14.Yang, J.S., L.Y. Xu, Y.M. Huang, P.H. Van Der Meide, H. Link, and B.G. Xiao. 2000. Adherent dendritic cells expressing high levels of interleukin-10 and low levels of interleukin-12 induce antigen-specific tolerance to experimental autoimmune encephalomyelitis. Immunology. 101:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legge, K.L., B. Min, J.J. Bell, J.C. Caprio, L. Li, R.K. Gregg, and H. Zaghouani. 2000. Coupling of peripheral tolerance to endogenous interleukin 10 promotes effective modulation of myelin-activated T cells and ameliorates experimental allergic encephalomyelitis. J. Exp. Med. 191:2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbari, O., R.H. DeKruyff, and D.T. Umetsu. 2001. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2:725–731. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 75:263–274. [DOI] [PubMed] [Google Scholar]
- 18.Lutz, M.B., N. Kukutsch, A.L. Ogilvie, S. Rößner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 223:77–92. [DOI] [PubMed] [Google Scholar]
- 19.Steinbrink, K., M. Wolfl, H. Jonuleit, J. Knop, and A.H. Enk. 1997. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 159:4772–4780. [PubMed] [Google Scholar]
- 20.Vremec, D., and K. Shortman. 1997. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J. Immunol. 159:565–573. [PubMed] [Google Scholar]
- 21.Lu, L., D. McCaslin, T.E. Starzl, and A.W. Thomson. 1995. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7-1dim, B7-2-) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. 60:1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enk, A.H., J. Saloga, D. Becker, M. Mohammadzadeh, and J. Knop. 1994. Induction of hapten-specific tolerance by interleukin 10 in vivo. J. Exp. Med. 179:1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu, F., Y. Li, S. Qian, L. Lu, F. Chambers, T.E. Starzl, J.J. Fung, and A.W. Thomson. 1996. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86−) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 62:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenwald, R.J., V.A. Boussiotis, R.B. Lorsbach, A.K. Abbas, and A.H. Sharpe. 2001. CTLA-4 regulates induction of anergy in vivo. Immunity. 14:145–155. [DOI] [PubMed] [Google Scholar]
- 25.Salomon, B., D.J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J.A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12:431–440. [DOI] [PubMed] [Google Scholar]
- 26.Kassiotis, G., and G. Kollias. 2001. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J. Exp. Med. 193:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Saint Vis, B., V.I. Fugier, C. Massacrier, C. Gaillard, B. Vanbervliet, Y.S. Ait, J. Banchereau, Y.J. Liu, S. Lebecque, and C. Caux. 1998. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J. Immunol. 160:1666–1676. [PubMed] [Google Scholar]
- 28.Maloy, K.J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816–822. [DOI] [PubMed] [Google Scholar]
- 29.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of cd4+cd25+ t cells with regulatory properties from human blood. J. Exp. Med. 193:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishima, Y. 1966. Melanosomes in phagocytic vacuoles in Langerhans cells. J. Cell Biol. 30:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang, F.P., N. Platt, M. Wykes, J.R. Major, T.J. Powell, C.D. Jenkins, and G.G. MacPherson. 2000. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruedl, C., P. Koebel, M. Bachmann, M. Hess, and K. Karjalainen. 2000. Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes. J. Immunol. 165:4910–4916. [DOI] [PubMed] [Google Scholar]
- 33.Steinman, R.M., S. Turley, I. Mellman, and K. Inaba. 2000. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 191:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawiger, D., K. Inaba, Y. Dorsett, M. Guo, K. Mahnke, M. Rivera, J.V. Ravetch, R.M. Steinman, and M.C. Nussenzweig. 2001. Dendritic cells induce peripheral t cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoitzner, P., M. Zanella, U. Ortner, M. Lukas, A. Tagwerker, K. Janke, M.B. Lutz, G. Schuler, B. Echtenacher, B. Ryffel, F. Koch, and N. Romani. 1999. Migration of Langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNF-alpha and IL-1beta. J. Leukoc. Biol. 66:462–470. [PubMed] [Google Scholar]
- 36.Fiebiger, E., P. Meraner, E. Weber, I.F. Fang, G. Stingl, H. Ploegh, and D. Maurer. 2001. Cytokines regulate proteolysis in major histocompatibility complex class II–dependent antigen presentation by dendritic cells. J. Exp. Med. 193:881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Förster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 99:23–33. [DOI] [PubMed] [Google Scholar]