Abstract
Some patients with Plasmodium falciparum infections develop cerebral malaria, acute respiratory distress, and shock and ultimately die even though drug therapy has eliminated the parasite from the blood, suggesting that a systemic inflammatory response contributes to malarial pathogenesis. Plasmodium berghei-infected mice are a well-recognized model of severe malaria (experimental severe malaria [ESM]), and infected mice exhibit a systemic inflammatory response. Because platelets are proposed to contribute to ESM and other systemic inflammatory responses, we determined whether platelet adherence contributes to experimental malarial pathogenesis. Indeed, a significant (P < 0.005) increase in the number of rolling and adherent platelets was observed by intravital microscopy in brain venules of P. berghei-infected mice compared with the number in uninfected controls. P-selectin- or ICAM-1-deficient mice exhibit increased survival after P. berghei infection. We observed a significant (P < 0.0001) reduction in the morbidity of mice injected with anti-CD41 (αIIb or gpIIb) monoclonal antibody on day 1 of P. berghei infection compared with the morbidity of infected controls injected with rat immunoglobulin G. Additionally, platelet rolling and adhesion in brain venules were reduced in P. berghei mice lacking either P-selectin or ICAM-1 or when the platelets were coated with anti-CD41 monoclonal antibody. Unlike other inflammatory conditions, we did not detect platelet-leukocyte interactions during P. berghei malaria. Because (i) leukocyte adhesion is not markedly altered in the absence of P-selectin or ICAM-1 and (ii) CD41 is not an adhesion molecule for parasitized erythrocytes, these findings support the hypothesis that inhibition of platelet adhesion to the brain microvasculature protects against development of malarial pathogenesis.
An uncontrolled systemic inflammatory response elicited by infection and/or a dysregulated immune response is recognized as a major cause of human disease. The organ damage caused by autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosis, vasculitis, and inflammatory bowel disease, is mediated by the immune system. In industrialized nations, ischemia-reperfusion injury results in an inflammatory response in vascular diseases with high levels of morbidity and mortality, such as angina pectoris, myocardial infarction, and stroke. In ischemia-reperfusion injury, evidence obtained from animal models indicates that platelets collaborate with the inflammatory response to mediate organ damage (28, 33). The protective effect of CD41/CD61 (gpIIb/IIIa or integrin αIIb/β3) antagonists against coronary artery disease (Abciximab, Tirofiban, and Eptifibatide) indicates that platelet interaction with the immune system may also be important in the pathogenesis of human disease (36).
Malaria is the second leading cause of morbidity and mortality due to a single infectious agent (14). Patients with severe malaria caused by Plasmodium falciparum, the most virulent of the four species of Plasmodium that infect humans, present with obtundation, posturing, and seizures, indicating that they have cerebral malaria. Patients also exhibit respiratory distress with lactic acidosis, anemia, and occasionally nephritis (23). Patients with severe falciparum malaria have high levels of proinflammatory cytokines (gamma interferon [IFN-γ] and tumor necrosis factor alpha [TNF-α]) in their sera. These cytokines are believed to activate endothelial cells, thereby increasing the expression of cell adhesion molecules, such as P-selectin, and ICAM-1 (23). Parasitized erythrocytes sequester via cell adhesion molecules in capillaries and venules (areas of low oxygen tension away from the spleen and liver, which are immune and blood filtration organs) (18). Most investigators hypothesize that sequestration obstructs blood flow in the brain, which decreases the flow of oxygen, glucose, and other nutrients to the brain and increases the accumulation of waste products, which ultimately leads to the clinical manifestations of cerebral malaria (30).
An alternate hypothesis is that an inflammatory response to P. falciparum activates leukocytes to adhere to the activated endothelium via P-selectin and ICAM-1. Toxic factors (such as NO) are released, which damage the integrity of the endothelial barrier and result in vascular leakage, poor tissue perfusion, and tissue edema (30). Because (i) patients with severe falciparum malaria have thrombocytopenia, (ii) platelet accumulation in the brains of children who die from severe falciparum malaria is significantly greater than platelet accumulation in the brains of patients who die from other coma complications, and (iii) platelets are postulated to function in the pathogenesis of experimental severe malaria (ESM) and other inflammatory diseases (10, 26, 31), we determined whether platelet adhesion is required for ESM pathogenesis.
Specifically, we investigated (i) whether platelets adhere to the brain microvasculature under flow, (ii) which endothelial cell adhesion molecules bind platelets during malaria, and (iii) whether inhibition of platelet adhesion protects against malarial pathogenesis. For these studies, we used Plasmodium berghei-infected mice, which are a well-recognized model of severe human malaria (8, 27). Extrapolation of results obtained with the mouse model of cerebral malaria must be done with caution because there are many differences between the pathogenesis of P. berghei-infected mice and the pathogenesis of P. falciparum-infected humans. We selected P. berghei-infected mice instead of Plasmodium yoelii-infected mice (a second model of cerebral malaria) because P. berghei-infected mice exhibit (i) a marked inflammatory response, (ii) profound thrombocytopenia, and (iii) obtundation, which P. yoelii-infected mice do not (6, 12, 13, 21). Like P. falciparum-parasitized erythrocytes, both P. yoelii- and P. berghei-parasitized erythrocytes sequester by adhering to brain microvasculature (15, 21). P. berghei-infected mice also exhibit respiratory distress with lactic acidosis, anemia, and nephritis, indicating that these mice also exhibit other clinical manifestations of severe falciparum malaria (37).
We report here that platelets adhere to brain microvasculature under flow in P. berghei-infected mice by using P-selectin, ICAM-1, and CD41, with the maximal adherence coinciding with the development of cerebral malaria. Inhibition of platelet adherence via P-selectin, ICAM-1, or CD41 protects against the development of severe P. berghei malaria.
MATERIALS AND METHODS
Treatment and infection of mice.
Female C57BL/6 mice, as well as P-selectin-deficient (Psel−/−) and ICAM-1-deficient (ICAM-1−/−) mice with a C57BL/6 background, were purchased when they were 4 to 5 weeks old from Jackson Laboratories (Bar Harbor, Maine). The animals were housed at the Louisiana State University Health Sciences Center Animal Care Facility, an Association for Assessment of Laboratory Animal Care-approved facility, in autoclaved microisolator cages contained within HEPA-filtered vent racks. Sterilized food and water were provided ad libitum. Experimental mice were infected when they were between 6 and 12 weeks old by intraperitoneal injection of 1 × 106 erythrocytes parasitized with lactate dehydrogenase virus-free P. bergehi ANKA as described previously (19). Parasitemia was determined by counting the number of parasitized erythrocytes in 200 and 1,000 erythrocytes in Giemsa-stained thin blood films. In each experiment, age- and sex-matched groups containing between four and eight C57BL/6 mice were used. All procedures were approved by the Animal Resources Advisory Committee of the Louisiana State University Health Sciences Center.
Flow cytometry.
Flow cytometry was performed as described previously (38). Briefly, Fc-block (Pharmingen, San Diego, Calif.) was added to 15 μl of blood to minimize nonspecific binding of monoclonal antibodies (MAbs). After 10 min of incubation, biotin-conjugated anti-P-selectin (Pharmingen) was added, and the cell suspension was incubated on ice with the antibody for 30 min. After the cells were washed, fluorescein isothiocyanate-conjugated anti-CD41, phycoerythrin-conjugated anti-CD45 (a panleukocyte marker), and streptavidin-APC (Pharmingen) were added to the cell suspension, and the mixture was incubated for 30 min. Cells were washed and resuspended in 0.5 ml of phosphate-buffered saline, and propidium iodide (Sigma Chemical Co., St. Louis, Mo.) was added 5 min before acquisition to allow exclusion of dead cells from the analysis. For quantification of platelets in blood, only the anti-CD41 MAb and counting beads were added, as described elsewhere (3). Flow cytometry data for the cell suspension were acquired with a FACSCalibur (Becton Dickinson) by using the CellQuest program and were analyzed by using the Attractors program (Becton Dickinson).
Assessment of rolling and adhesion in pial microvessels.
Assessment of platelet rolling and adhesion by intravital microscopy was performed as described previously (28). Briefly, mice were anesthetized by subcutaneous injection of 150 mg of ketamine per kg and 7.5 mg of xylazine per kg, and the jugular vein of each mouse was cannulated with polyethylene tubing (PE-10) to administer rhodamine 6G-labeled platelets and sodium pentobarbital for euthanasia. The pial vessels of the brain were exposed by making a circular opening in the skull with a hand-held surgical drill. The mouse was then placed on a Nikon upright fluorescence microscope with an intensified camera (Hammamatsu Photonics, Hamamatsu City, Japan). The body temperature of the mouse was maintained between 36 and 37°C by using a heating pad with a rectal thermometer, and the exposed brain was moistened with phosphate-buffered saline at 37°C. Five nonoverlapping regions of the brain microvasculature (magnification, ×10) were recorded on videotape for off-line analysis for each mouse, and each region was recorded for at least 5 min (approximately 25 min for each mouse). Arterioles with diameters between 20 and 40 μm were analyzed, as were small postcapillary venules (diameter, 10 to 17 μm) and large postcapillary venules (diameter, 30 and 50 μm). Rolling platelets were defined as cells moving at a velocity that was significantly less than the centerline velocity in the microvessel, whereas adherent platelets did not move in a 30-s observation period. In the experiments to determine the role of CD41 in platelet adhesion, fluorescently labeled platelets were incubated with 0.1 mg of either anti-CD41 MAb or rat immunoglobulin G (IgG) for 30 min, washed, and then injected into recipients.
Statistical analysis.
Analysis of variance with the Statview 5.0 program (SAS Institute) was performed to compare all measurements, and the level of significance used was a P value of <0.05. The mean and standard deviation for the level of parasitemia are reported below because they represent the average for a single measurement, whereas the mean of the mean numbers of rolling and adherent platelets per vessel and standard error are reported because they represent a mean of means. Kaplan-Meier survival curves of groups of mice were generated with Statview, and differences in survival were assessed by the Logrank test, in which a P value of <0.05 was considered significant.
RESULTS
P. berghei infection of mice is a very reproducible model of cerebral malaria, and virtually all infected mice develop petechiae, cerebral edema, and lactic acidosis and become moribund on day 6 or 7 of infection (27). Mice exhibit neurological symptoms on day 6, manifested as a loss of the righting and gripping reflexes and lethargy. Day 12 of infection is considered by most investigators to be the last time that the mice develop cerebral malaria, so our experiments assessing the pathogenic mechanisms of cerebral malaria were terminated at this time. Mice that exhibit minimal complications of cerebral malaria and survive beyond day 12 of infection do not mount a protective immune response to clear parasites from the circulation and succumb after day 20 of infection from anemia secondary to hyperparasitemia.
Platelet and endothelial cell interactions during P. berghei malaria.
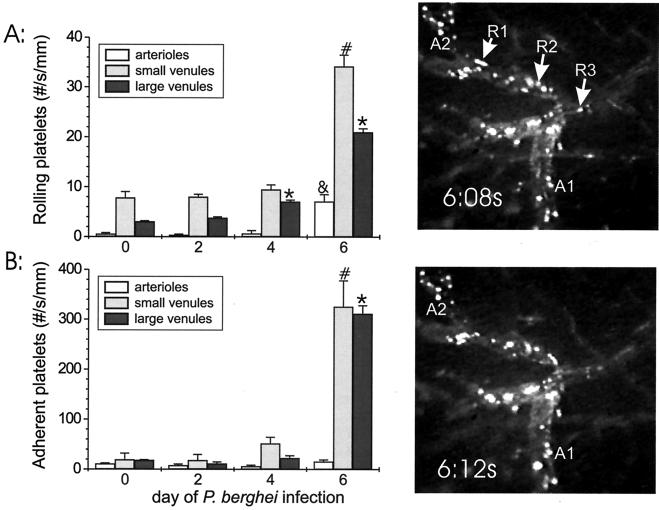
To determine whether platelets adhere in vivo when there is blood flow to the brain microvasculature of mice with severe P. berghei malaria, fluorescently labeled platelets were injected via a carotid catheter, and platelet rolling and adhesion in the brain microcirculation were assessed by intravital fluorescence microscopy during the course of P. berghei malaria. The numbers of rolling platelets in the brain microvasculature of mice on day 6 of P. berghei infection were greater than the numbers of rolling platelets in the arterioles and small and large postcapillary venules of uninfected controls (12.7-fold greater for arterioles [P = 0.0005], 4.4-fold greater for small venules [P < 0.0001], and 6.9-fold greater for large venules [P < 0.0001]) (Fig. 1). The number of adherent platelets at each time point during infection was always greater than the number of rolling cells (range, 2.2- to 22.6-fold greater). The numbers of adherent platelets were also greater in the small and large postcapillary venules but not in the arterioles (16.9-fold greater for small venules [P < 0.0001], 17.0-fold greater for large venules [P < 0.0001], and 1.4-fold greater for arterioles [P = 0.66]) (Fig. 1). The actual number of platelets binding in each large venule was greater than the number binding in each small venule, but when the data were normalized to the diameter, the small venules had about the same number of adherent platelets as the large venules. The levels of parasitemia in the four mice in each group on days 2, 4, and 6 were 0.4% ± 0.8%, 3.9% ± 2.1%, and 13.8% ± 2.7%, respectively, indicating that the mice were appropriately infected.
FIG. 1.
Rolling (A) and adhesion (B) of platelets obtained from an uninfected C57BL/6 source mouse in the brain microvasculature during the course of P. berghei malaria in C57BL/6 recipient mice. On each graph the y axis indicates the number of rolling or adherent cells in arterioles, small postcapillary venules, and large postcapillary venules, and the x axis indicates the day of infection. Significant (P ≤ 0.0005) differences between leukocyte rolling or adhesion in arterioles, small postcapillary venules, and large postcapillary venules during the course of P. berghei infection and leukocyte rolling or adhesion in uninfected C57BL/6 mice are indicated by an ampersand, asterisks, and number signs, respectively. Five vessels were analyzed in four recipient mice at each time point. R1, R2, and R3, rolling cells; A1 and A2, adherent cells.
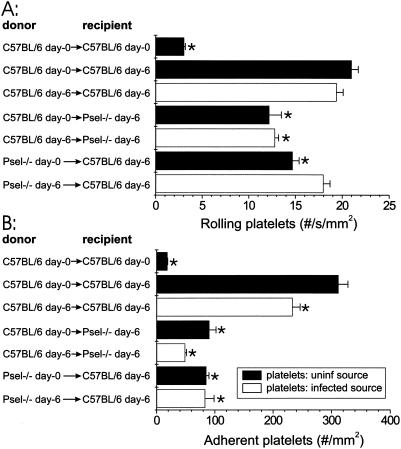
To determine whether platelet rolling or adherence was decreased if platelets were obtained from an infected source mouse, we assessed by using intravital microscopy rolling and adhesion of platelets obtained from infected source mice in the brain microvasculature on day 6 of P. berghei infection. The levels of platelet rolling in venules of P. berghei-infected recipients were similar when platelets were obtained from an uninfected source and when platelets were obtained from an infected source (P = 0.25 for a comparison of day 0 C57BL/6 donor platelets in day 6 C57BL/6 recipient mice with day 6 C57BL/6 donor platelets in day 6 C57BL/6 recipient mice) (Fig. 2) but were significantly greater than the levels in uninfected recipients (6.3-fold greater [P < 0.0001] for a comparison of day 6 C57BL/6 donor platelets in day 6 C57BL/6 recipient mice with day 0 C57BL/6 donor platelets in day 0 recipient C57BL/6 mice). The adherence of platelets obtained from an infected C57BL/6 source mouse in venules of P. berghei-infected mice was markedly greater than the adherence of these platelets in venules of uninfected C57BL/6 recipients (12.7-fold greater [P < 0.0001] for a comparison of day 6 C57BL/6 donor platelets in day 6 C57BL/6 recipient mice with day 0 C57BL/6 donor platelets in day 0 C57BL/6 recipient mice). However, the number of adherent platelets in P. berghei-infected recipients was modestly lower when platelets from infected source mice were used than when platelets from uninfected mice were used (75% lower [P = 0.15] for a comparison of day 6 C57BL/6 donor platelets in day 6 C57BL/6 recipient mice with day 0 C57BL/6 donor platelets in day 6 C57BL/6 recipient mice).
FIG. 2.
Rolling (A) and adhesion (B) of platelets obtained from an uninfected donor (uninf source) or a day 6 infection donor (infected source) in the brain microvasculature in selected recipient mice during the course of P. berghei malaria. The donor mouse for platelets and the recipient mice are indicated on the y axis, and the average number of rolling or adherent cells is plotted on the x axis. The numbers of recipients are indicated in Table 1. Asterisks indicate significant differences (P ≤ 0.0005) between donor platelet rolling or adhesion in the large postcapillary venules in recipient mice and platelet rolling or adhesion in infected C57BL/6 recipients that received platelets from uninfected donor mice. Psel−/−, P-selectin deficient.
P-selectin, ICAM-1, and CD41 are platelet adhesion molecules in P. berghei malaria.
To determine the role of platelet P-selectin in platelet rolling and adhesion to an activated endothelium during P. berghei malaria, fluorescently labeled P-selectin-deficient platelets from P-selectin-deficient source mice (either infected or uninfected) were injected into P. berghei-infected C57BL/6 recipients. The adhesion of P-selectin-deficient platelets to brain venules of infected C57BL/6 mice was less than the adhesion of platelets from C57BL/6 control mice (0.27-fold less [P < 0.0001] for a comparison of day 0 P-selectin-deficient platelets in day 6 C57BL/6 recipient mice with day 0 C57BL/6 donor platelets in day 6 C57BL/6 recipient mice and 0.27-fold less [P < 0.0001] for a comparison of day 6 P-selectin-deficient platelets in day 6 C57BL/6 recipient mice with day 0 C57BL/6 donor platelets in day 6 C57BL/6 recipient mice) (Fig. 2). In fact, the reduction in adhesion was so profound that the number of adherent P-selectin-deficient platelets in P. berghei-infected C57BL/6 mice was only slightly greater than the number of platelets from uninfected mice injected into uninfected recipients. There were also reductions in platelet rolling in infected recipients when P-selectin-deficient platelets were used, but the magnitude of the change was less pronounced than the magnitude of the effect on adhesion.
To determine the role of endothelial P-selectin in platelet rolling and adhesion to activated endothelium during P. berghei malaria, fluorescently labeled P-selectin-containing platelets from C57BL/6 source mice (either infected or uninfected) were injected into P. berghei-infected P-selectin-deficient recipients. The adhesion of normal platelets to brain venules of P-selectin-deficient mice infected with P. berghei was less than the adhesion of platelets to brain venules of infected C57BL/6 controls (0.29-fold less [P = 0.03] for a comparison of day 0 C57BL/6 donor platelets in day 6 P-selectin-deficient recipient mice with day 0 C57BL/6 donor platelets in day 6 C57BL/6 recipient mice and 0.16-fold less [P < 0.0001] for a comparison of day 6 C57BL/6 donor platelets in day 6 P-selectin-deficient mice with day 0 C57BL/6 donor platelets in day 6 C57BL/6 recipient mice) (Fig. 2). The levels of parasitemia were >0.5% on day 4 and >5% on day 6 of P. berghei infection, indicating that the mice were adequately infected (Table 1).
TABLE 1.
Levels of parasitemia in the source and recipient mice and numbers of recipient micea
| Source (recipient) | Parasitemia (%) in source (recipient) on:
|
No. of recipients for intravital microscopy | |
|---|---|---|---|
| Day 4 | Day 6 | ||
| C57BL/6, day 0 (P-selectin deficient, day 6) | NA (3.7 ± 0.5)b | (18.2 ± 3.5) | 4 |
| C57BL/6, day 6 (P-selectin deficient, day 6) | 2.2 ± 0.3 (2.6 ± 0.8) | 16.6 ± 1.0 (8.9 ± 1.1) | 3 |
| C57BL/6, day 0 (C57BL/6, day 6) | NA (3.0 ± 0.7) | (13.8 ± 2.7) | 4 |
| C57BL/6, day 6 (C57BL/6, day 6) | 2.2 (2.2 ± 0.4) | 15.2 (9.4 ± 2.5) | 2 |
| P-selectin deficient day 0 (C57BL/6, day 6) | NA (3.9 ± 0.5) | (10.5 ± 0.8) | 3 |
| P-selectin deficient, day 6 (C57BL/6, day 6) | 1.7 ± 0.5 (2.5 ± 0.6) | 8.4 ± 0.7 (12.5 ± 1.6) | 4 |
See Fig. 2.
NA, not applicable.
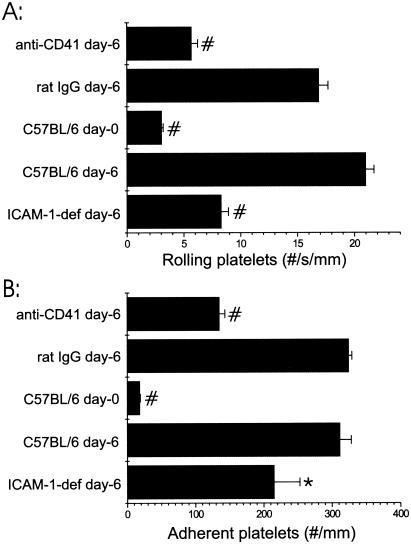
Because (i) ICAM-1-deficient mice are protected from P. berghei malaria, (ii) protection of ICAM-1-deficient mice is not due to leukocyte adherence to the brain microvasculature, and (iii) platelets adhere via fibrinogen to ICAM-1 during ischemia-reperfusion injury (7), we assessed the levels of platelet rolling and adhesion by using intravital microscopy in ICAM-1-deficient mice infected with P. berghei (29). The levels of platelet rolling and adhesion in the brain microvasculature in ICAM-1-deficient mice on day 6 of P. berghei infection were less than the levels of platelet rolling and adhesion in infected C57BL/6 control mice (39% less [P < 0.0001] and 68% less [P < 0.01], respectively) (Fig. 3). The levels of parasitemia were 1.7% ± 0.4% on day 4 and 9.0% ± 4.0% on day 6 of P. berghei infection, indicating that the recipient mice were adequately infected.
FIG. 3.
Rolling (A) and adhesion (B) of platelets obtained from an uninfected donor mouse in the large postcapillary brain venules of selected recipient mice that were either not infected or infected with P. berghei (day 6). The groups of recipient mice are indicated on the y axis, and the average numbers of rolling or adherent cells are plotted on the x axis. anti-CD41 day-6 indicates platelets incubated with anti-CD41 MAb prior to injection (n = 4), and rat IgG day-6 indicates the control for the MAb (n = 3). C57BL/6 day-0 and C57BL/6 day-6 indicate uninfected and infected C57BL/6 mice, respectively; the results shown in Fig. 2 are repeated for comparison to ICAM-1-deficient recipients (ICAM-1-def). Five ICAM-1-deficient mice were analyzed. The asterisk (P < 0.05) and the number signs (P < 0.0005) indicate significant differences in platelet rolling or adhesion in the recipient mice compared with platelet rolling or adhesion in infected C57BL/6 recipients.
Because CD41 is an important platelet adhesion molecule and because blockade by anti-CD41 MAb limits damage during myocardial infarction, we determined whether CD41 contributes to platelet adhesion during malaria by assessing rolling and adhesion of platelets coated with anti-41 MAb in infected C57BL/6 recipients. Platelets coated with anti-CD41 exhibited decreased rolling and adhesion in P. berghei-infected mice compared with the rolling and adhesion of rat IgG-treated platelets (0.34- and 0.42-fold reductions [P < 0.0001 and P < 0.0001] for rolling and adhesion, respectively) (Fig. 3). The levels of parasitemia were 1.7% ± 0.7% on day 4 and 7.9% ± 3.6% on day 6 of P. berghei infection for mice receiving anti-CD41 MAb-treated platelets and 1.8% ± 0.3% on day 4 and 8.2% ± 2.2% on day 6 of P. berghei infection for control recipients receiving rat IgG-treated platelets, indicating that both groups of recipient mice were adequately infected.
Platelets do not interact directly with leukocytes via P-selectin during the course of P. berghei malaria.
To determine whether platelet adherence to the endothelium during P. berghei malaria accounts for malarial thrombocytopenia, we compared the numbers of platelets in the blood by flow cytometry on days 0, 4, and 6 of infection in P-selectin-deficient mice (which exhibit minimal platelet adherence) to numbers of platelets in the blood in C57BL/6 controls. The numbers of platelets in the peripheral blood of both groups of mice declined markedly during the course of P. berghei malaria (Table 2). Both thrombocytopenia and parasitemia were similar in P-selectin-deficient mice and C57BL/6 mice on days 4 and 6 of P. berghei malaria (Table 2).
TABLE 2.
Numbers of platelets in blood as assessed by flow cytometry (CD41+ cells) and percentages of parasitemia during the course of P. berghei malaria in P-selectin-deficient mice and intact controlsa
| Day of P. berghei infection | Platelet count (no. of cells/μl)
|
Parasitemia (%)
|
||
|---|---|---|---|---|
| P-selectin-deficient mice | C57BL/6 mice | P-selectin-deficient mice | C57BL/6 mice | |
| 0 | 254,894 ± 51,192 | 479,846 ± 96,815 | NA | NA |
| 4 | 86,229 ± 35,985 | 76,354 ± 42,305 | 3.0 ± 0.9 | 3.0 ± 1.5 |
| 6 | 9,875 ± 3,200 | 8,809 ± 1,027 | 12.2 ± 3.7 | 11.0 ± 3.0 |
The data are the results for three separate experiments performed with 15 P-selectin-deficient mice and 13 C57BL/6 mice on day 0 because several of the controls succumbed on day 6; the values for day 6 for the C57BL/6 group therefore are the values for seven mice. The values are means ± standard deviations. NA, not applicable.
To determine whether circulating platelets activated during malaria express P-selectin and adhere to leukocytes, we analyzed P-selectin expression on platelets and leukocyte-platelet conjugate formation during P. berghei malaria by flow cytometry. Few cells assessed by flow cytometry were positive for both CD45+ and CD41+ (leukocyte-platelet conjugates), and the numbers of leukocyte-platelet conjugates per microliter of blood were similar in uninfected mice and in infected mice on days 4 and 6 of P. berghei infection (39 ± 4, 28 ± 6, and 32 ± 6 conjugates/μl, respectively). The percentages of CD41+ cells (platelets) labeled with P-selectin increased modestly on day 6 of P. berghei malaria (on days 0, 4, and 6 of infection the percentages were 1.5% ± 0.3%, 1.3% ± 0.3%, and 5.7% ± 0.9%, respectively).
Treatment with anti-CD41 MAb protects against severe P. berghei malaria.
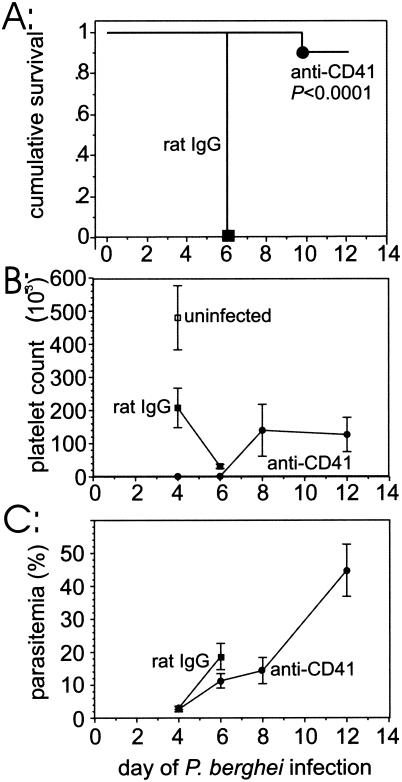
To determine whether treatment with anti-CD41 MAb protects against severe P. berghei malaria, we infected two groups of mice with P. berghei and compared the survival of mice inoculated intraperitoneally on day 1 of infection with anti-CD41 to the survival of controls that received rat IgG. The mice that received anti-CD41 MAb (n = 11) exhibited significantly (P < 0.0001) increased survival after P. berghei infection compared with the survival of the rat IgG-treated controls (n = 14). In fact, only one of the anti-CD41 MAb-treated mice died (day 10), whereas all anti-rat IgG-treated controls became moribund on day 6 of P. berghei infection (Fig. 4A). Two mice in the group that received anti-CD41 MAb were excluded because they died the day after injection of the MAb. The anti-CD41 MAb treatment on day 1 effectively blocked the platelet αIIb protein because only a relatively few CD41+ cells (1,067 ± 277 cells) were detected by flow cytometry in this group of mice on day 4 of infection compared with the number of such cells in rat IgG-treated controls (207,849 ± 59,606 cells) (Fig. 4B). It is unlikely that the dose of anti-CD41 MAb used depleted platelets because anti-platelet serum resulted in accelerated mortality of P. berghei-infected mice (data not shown), whereas anti-CD41 MAb protected the mice. The parasitemia on days 4 and 6 in the group of mice that received anti-CD41 MAb-injected and were infected with P. berghei was sufficient to cause mortality in untreated mice, indicating that these mice were adequately infected (Fig. 4C).
FIG. 4.
(A) Kaplan-Meier survival plots for C57BL/6 mice infected with P. berghei and injected intraperitoneally with anti-CD41 MAb or rat IgG. (B) Average numbers of CD41+ cells detected by flow cytometry during the course of P. berghei infection. (C) Average levels of P. berghei parasitemia in the two groups of mice.
DISCUSSION
Several independent lines of evidence indicate that an immune response to P. berghei is required for pathogenesis of experimental malaria. Results obtained with knockout mice lacking selected cell types or with mice depleted by MAb injection indicated that CD4+ αβ T cells, CD8+ αβ T cells, γδ T cells, macrophages, and neutrophils are all required for development of cerebral malaria in P. berghei-infected mice (4, 11, 35, 39, 40). Knockout mice lacking selected type 1 proinflammatory cytokines or their receptors (interleukin-2 [IL-2], IFN-γ, IFN-γR, and TNFR2), as well as mice treated with neutralizing anti-cytokine MAbs (TNF-α and IFN-γ), do not develop cerebral malaria, whereas knockout mice lacking type 2 or anti-inflammatory cytokines (IL-4 and IL-10) do develop cerebral malaria after infection with P. berghei (5, 40). In addition, treatment with IL-10 also protects mice from experimental P. berghei malaria (22). Eling's group has reported that anti-TNF-α MAb does not protect against P. berghei NK56 malaria (16). Whether this reflects parasite strain differences between ANKA and NK56 or contradicts previous results remains to be determined. Finally, we and other workers have observed that expression of the cell adhesion molecules ICAM-1, P-selectin, and VCAM-1 increases significantly on vascular endothelium of the brain and several other organs during the course of P. berghei infection and that these molecules are required for malarial pathogenesis (7).
The most widely accepted interpretation of the results described above is that leukocytes adhere to the cell adhesion molecules P-selectin and ICAM-1 during P. berghei malaria to mediate tissue damage. P-selectin plays a major role in leukocyte adhesion in ischemia-reperfusion injury in the intestine to the microvasculature (28), and activated platelets play a key role in mediating leukocyte adhesion to the activated endothelium. However, recent intravital microscopic studies of brain microvasculature indicated that leukocyte rolling and adhesion are not markedly attenuated during P. berghei malaria in the absence of either ICAM-1 or P-selectin (2, 24). Thus, the mechanism of leukocyte rolling and adhesion during P. berghei malaria in the brain must be distinct from our current understanding of this process obtained from other organ beds and from other models of inflammation. Using flow cytometry, we did not detect formation of platelet-leukocyte conjugates during severe P. berghei malaria that was greater than that observed in uninfected controls, suggesting that leukocytes are not coated with platelets prior to adhesion to the microvasculature. However, the conjugate formation may be too rapid for detection by flow cytometry. It is still theoretically possible that leukocytes roll on platelets adhering to endothelial cells during malaria, but this process must use ligands other than P-selectin, which makes it a much less likely scenario.
A second explanation for the protection against malarial pathogenesis in ICAM-1- or P-selectin-deficient mice despite only a minimal decline in leukocyte adhesion to the brain microvasculature is that parasitized erythrocytes use these cell adhesion molecules to sequester and occlude blood flow (17, 18). However, blockade of CD41, which is a platelet adhesion molecule rather than a parasite-binding molecule, also protects against the development of severe experimental malaria. In addition, ESM was associated with profound thrombocytopenia and decreased cerebral platelet trapping in uPA−/− and uPAR−/− mice protects these mice from ESM (31). Each of these cell adhesion molecules contributes to platelet adhesion to inflamed endothelium. Thus, a third explanation is that P-selectin, ICAM-1, and CD41 mediate the adhesion of platelets during P. berghei malaria, which in turn is critical for the pathogenic process (29). Our intravital microscopic studies indicated that there is a marked increase in the number of platelets rolling on and adhering to the brain microvasculature in P. berghei-infected mice compared with the number in uninfected controls. The numbers of rolling and adherent platelets in uninflamed pial vessels are similar to the numbers observed in the small intestine (28). In addition, the numbers of rolling and adherent platelets in inflamed pial vessels after P. berghei malaria are similar to the numbers observed in the small intestine after ischemia-reperfusion injury (28). The observed increase in platelet rolling and adhesion confirms the results of immunohistochemical and electron microscopy studies and extends them by demonstrating that platelet adhesion is not a postmortem artifact but occurs in vivo and under flow. Platelets with P-selectin stored in granules obtained from P. berghei-infected mice showed a minor decrease in adhesion compared with the adhesion of platelets from uninfected controls. We propose that the platelets in an infected mouse are exposed to proinflammatory stimuli and that the most responsive platelets bind to the endothelium via P-selectin, whereas the platelets remaining in the circulation are less responsive. However, many circulating fluorescently labeled platelets from mice on day 6 of P. berghei infection adhere to the brain microvasculature of infected recipients, indicating that these platelets are still capable of responding and adhering. Why these circulating platelets are not activated to express P-selectin and adhere in the source mice infected with P. berghei remains to be determined.
The molecules involved in platelet adhesion to the brain microvasculature during P. berghei malaria have not been determined previously. Our finding that platelet rolling and adhesion are significantly (P < 0.05) reduced during P. berghei malaria in P-selectin- or ICAM-1-deficient mice, as well as in mice treated with anti-CD41 MAb, indicates that all three of the cell adhesion molecules are required for platelet adhesion in vivo. A lack of endothelial ICAM-1 had the greatest effect on platelet rolling rather than adhesion, whereas a lack of P-selectin or inhibition of CD41 had the greatest effect on adhesion. Few circulating CD41+ platelets exhibited increased expression of P-selectin during P. berghei malaria, indicating that once P-selectin is expressed on the surface, these platelets are destined to adhere to the endothelium. The platelet activation and degranulation processes are likely to occur on cells tethered or adherent to the endothelium. Given the multistep molecular nature of platelet adhesion to the brain microvasculature, our data indicate that P-selectin and ICAM-1 are important in tethering the platelets to the endothelium and P-selectin and gpIIb/IIIa enable the platelets to adhere firmly (9, 29, 34).
Both platelets and endothelial cells can express P-selectin under inflammatory conditions. Our observation that the number of platelets rolling and adhering in P-selectin-deficient mice with P. berghei malaria is markedly less than the number of platelets rolling and adhering in infected controls with P-selectin intact indicates that P-selectin on the vascular endothelium is required for platelet rolling and adherence during malaria. In addition, our finding that platelets from P-selectin-deficient mice also exhibit markedly reduced rolling and adherence in P. berghei-infected C57BL/6 mice compared with the rolling and adherence of control platelets with P-selectin intact indicates that platelet P-selectin is also required for maximal adhesion of platelets to the endothelium during malaria. Thus, P-selectin on both platelets and endothelial cells is required for optimal rolling and adhesion of platelets in the brain microvasculature during P. berghei malaria.
Our results indicate that platelet adhesion is primarily localized to the small and large postcapillary venules. One explanation for this is that the high shear wall stresses in arterioles exert forces too great for platelets to adhere. Given that leukocyte adhesion is not affected by shear stress in the arterioles, a more likely explanation involves the differences in cell adhesion molecule expression (1). Our results contrast with those of Massberg et al., who observed that the greatest number of platelets adhered to arterioles during ischemia-reperfusion injury in the intestine and proposed that platelet activation depends upon the shear stress exerted on the platelets (28). Thus, different mechanisms of platelet activation may occur during cerebral malaria and ischemia-reperfusion injury in the gut.
The marked adherence of platelets during severe malaria suggests that the mechanism of thrombocytopenia observed in experimental malaria and human malaria may be due to widespread adherence of platelets in venules throughout the body. Other investigators observed thrombocytopenia during experimental malaria and severe P. falciparum malaria in humans (20); the thrombocytopenia was interpreted as increased destruction of platelets during malaria (20), but it may have reflected the massive adherence of platelets in several vascular organ beds during malaria. However, increased destruction of platelets is still the primary explanation for malarial thrombocytopenia because we observed similar levels of thrombocytopenia in P-selectin-deficient mice with P. berghei malaria and controls, and platelets did not adhere to the microvasculature in P-selectin-deficient mice infected with P. berghei.
The role of platelets in the pathogenesis of severe P. berghei malaria is controversial; in one report the authors indicated that depletion of platelets protects against severe malaria, and in a second report the authors reached the opposite conclusion (25, 32). Our results showing that blockade of platelet adhesion molecules protects against severe P. berghei malaria supports the conclusion that platelets contribute to malarial pathogenesis. However, the mechanisms may be totally different because our data indicate that platelet-depleted mice exhibit accelerated mortality rather than the delayed mortality observed in anti-CD41 MAb-treated mice (unpublished data). We propose that rendering the mice thrombocytopenic exacerbates malarial pathogenesis by preventing the coagulation system from repairing the endothelial barrier and that inhibiting platelet adhesion protects against disseminated intravascular coagulation. The large protective effect of anti-CD41 MAb treatment compared with the level of protection against the development of malarial pathogenesis in P-selectin- and ICAM-1-deficient-mice suggests that CD41 has other roles in addition to a role in adhesion.
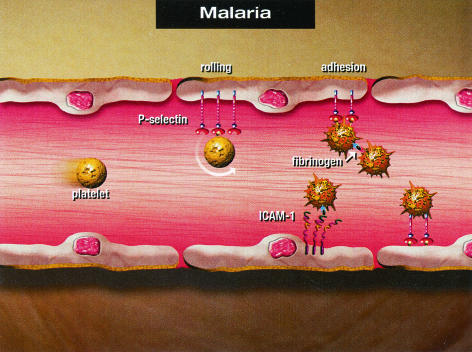
In conclusion, our data indicate that marked platelet rolling on and adherence to venules (but not arterioles) occur during P. berghei malaria. Platelet rolling and adherence during P. berghei malaria are significantly attenuated in the absence of P-selectin, in the absence of ICAM-1, and by blockade of gpIIb/IIIa by an MAb (P = 0.005, P = 0.04, and P <0.0001, respectively). The possible molecular mechanisms for platelet adhesion to the pial microvasculature during cerebral malaria are summarized in Fig. 5. The endothelium of pial microvessels is activated to increase expression of ICAM-1 and P-selectin among other cell adhesion molecules. Platelets roll on endothelial P-selectin as the first step in adherence and become activated to express P-selectin on their surfaces. Whether the magnitude of the P-selectin-P-selectin ligand interaction is sufficient to mediate platelet adhesion as shown in Fig. 5 remains to be determined. Platelet-platelet interactions are likely mediated via gpIIb/IIIa on an activated platelet binding to fibrinogen, and then a second platelet also adheres via gpIIb/IIIa to fibrinogen. Firm adhesion of platelets via endothelial ICAM-1, fibrinogen, and platelet gpIIb/IIIa is likely to be important. Alternatively, platelet LFA-1 may bind to ICAM-1 to mediate platelet adhesion (7). It is likely that several molecular adhesion events simultaneously facilitate platelet adhesion to an inflamed microvessel.
FIG. 5.
Summary of the proposed molecular mechanisms of platelet adhesion to the pial microvasculature during cerebral malaria.
As illustrated by case reports, patients with P. falciparum infection can develop cerebral malaria, acute respiratory distress, and shock and can die even though antimalarial therapy has killed the parasites in the circulation. We believe that these findings indicate that these patients develop a systemic inflammatory syndrome. Based on the results described above for experimental malaria and the accumulating evidence linking the inflammatory and coagulation systems (e.g., septic shock and activated protein C), we speculate that the coagulation cascade is also activated in patients with severe falciparum malaria, resulting in cerebral malaria and acute respiratory distress. Future studies may reveal that inhibition of platelet adhesion and/or activation is an effective therapy for some patients with severe falciparum malaria.
Acknowledgments
This research was supported by NIH grants KO8 AI01438 (to W.L.C.) and RO1 AI40667 (to H.C.V.D.H.).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bienvenu, K., and D. N. Granger. 1993. Molecular determinants of shear rate-dependent leukocyte adhesion in postcapillary venules. Am. J. Physiol. 264:H1504-H1508. [DOI] [PubMed] [Google Scholar]
- 2.Chang, W. L., J. Li, G. Sun, H. L. Chen, R. D. Specian, S. M. Berney, D. N. Granger, and H. C. van der Heyde. 2003. P-selectin contributes to severe experimental malaria but is not required for leukocyte adhesion to brain microvasculature. Infect. Immun. 71:1911-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, W. L., H. C. van der Heyde, and B. S. Klein. 1998. Flow cytometric quantitation of yeast: a novel technique for use in animal model work and in vitro immunologic assays. J. Immunol. Methods 211:51-63. [DOI] [PubMed] [Google Scholar]
- 4.Curfs, J. H., C. C. Hermsen, P. Kremsner, S. Neifer, J. H. Meuwissen, N. Van Rooyen, and W. M. Eling. 1993. Tumour necrosis factor-alpha and macrophages in Plasmodium berghei-induced cerebral malaria. Parasitology 107:125-134. [DOI] [PubMed] [Google Scholar]
- 5.de Kossodo, S., and G. E. Grau. 1993. Profiles of cytokine production in relation with susceptibility to cerebral malaria. J. Immunol. 151:4811-4820. [PubMed] [Google Scholar]
- 6.Eling, W. M., and P. G. Kremsner. 1994. Cytokines in malaria, pathology and protection. Biotherapy 7:211-221. [DOI] [PubMed] [Google Scholar]
- 7.Favre, N., C. Da Laperousaz, B. Ryffel, N. A. Weiss, B. A. Imhof, W. Rudin, R. Lucas, and P. F. Piguet. 1999. Role of ICAM-1 (CD54) in the development of murine cerebral malaria. Microbes Infect. 1:961-968. [DOI] [PubMed] [Google Scholar]
- 8.Finley, R. W., L. J. Mackey, and P. H. Lambert. 1982. Virulent P. berghei malaria: prolonged survival and decreased cerebral pathology in cell-dependent nude mice. J. Immunol. 129:2213-2218. [PubMed] [Google Scholar]
- 9.Frenette, P. S., R. C. Johnson, R. O. Hynes, and D. D. Wagner. 1995. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc. Natl. Acad. Sci. USA 92:7450-7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grau, G. E., C. D. Mackenzie, R. A. Carr, M. Redard, G. Pizzolato, C. Allasia, C. Cataldo, T. E. Taylor, and M. E. Molyneux. 2003. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J. Infect. Dis. 187:461-466. [DOI] [PubMed] [Google Scholar]
- 11.Grau, G. E., P. F. Piguet, H. D. Engers, J. A. Louis, P. Vassalli, and P. H. Lambert. 1986. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J. Immunol. 137:2348-2354. [PubMed] [Google Scholar]
- 12.Grau, G. E., P. F. Piguet, D. Gretener, C. Vesin, and P. H. Lambert 1988. Immunopathology of thrombocytopenia in experimental malaria. Immunology 65:501-506. [PMC free article] [PubMed] [Google Scholar]
- 13.Grau, G. E., P. F. Piguet, and P. H. Lambert. 1992. Immunopathology of malaria: role of cytokine production and adhesion molecules. Mem. Inst. Oswaldo Cruz Rio de J. 87(Suppl. 5):95-100. [DOI] [PubMed] [Google Scholar]
- 14.Greenwood, B., and T. Mutabingwa. 2002. Malaria in 2002. Nature 415:670-672. [DOI] [PubMed] [Google Scholar]
- 15.Hearn, J., N. Rayment, D. N. Landon, D. R. Katz, and J. B. de Souza. 2000. Immunopathology of cerebral malaria: morphological evidence of parasite sequestration in murine brain microvasculature. Infect. Immun. 68:5364-5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermsen, C. C., J. V. Crommert, H. Fredix, R. W. Sauerwein, and W. M. Eling. 1997. Circulating tumour necrosis factor alpha is not involved in the development of cerebral malaria in Plasmodium berghei-infected C57Bl mice. Parasite Immunol. 19:571-577. [DOI] [PubMed] [Google Scholar]
- 17.Ho, M., M. J. Hickey, A. G. Murray, G. Andonegui, and P. Kubes. 2000. Visualization of Plasmodium falciparum-endothelium interactions in human microvasculature: mimicry of leukocyte recruitment. J. Exp. Med. 192:1205-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho, M., T. Schollaardt, X. Niu, S. Looareesuwan, K. D. Patel, and P. Kubes. 1998. Characterization of Plasmodium falciparum-infected erythrocyte and P-selectin interaction under flow conditions. Blood 91:4803-4809. [PubMed] [Google Scholar]
- 19.Hoffmann, E. J., W. P. Weidanz, and C. A. Long. 1984. Susceptibility of CXB recombinant inbred mice to murine plasmodia. Infect. Immun. 43:981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horstmann, R. D., M. Dietrich, U. Bienzle, and H. Rasche. 1981. Malaria-induced thrombocytopenia. Blut 42:157-164. [DOI] [PubMed] [Google Scholar]
- 21.Kaul, D. K., R. L. Nagel, J. F. Llena, and H. L. Shear. 1994. Cerebral malaria in mice: demonstration of cytoadherence of infected red blood cells and microrheologic correlates. Am. J. Trop. Med. Hyg. 50:512-521. [DOI] [PubMed] [Google Scholar]
- 22.Kossodo, S., C. Monso, P. Juillard, T. Velu, M. Goldman, and G. E. Grau. 1997. Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology 91:536-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krogstad, D. J. 1999. Malaria, p. 736-766. In R. L. Guerrant, D. H. Walker, and P. F. Weller (ed.), Tropical infectious diseases. Principles, pathogens, and practice. Churchill Livingstone, Philadelphia, Pa.
- 24.Li, J., W. L. Chang, G. Sun, H. L. Chen, R. D. Specian, S. M. Berney, D. Kimpel, D. N. Granger, and H. C. van der Heyde. 2003. Intercellular adhesion molecule 1 is important for the development of severe experimental malaria but is not required for leukocyte adhesion in the brain. J. Investig. Med. 51:128-140. [DOI] [PubMed] [Google Scholar]
- 25.Lou, J., Y. R. Donati, P. Juillard, C. Giroud, C. Vesin, N. Mili, and G. E. Grau. 1997. Platelets play an important role in TNF-induced microvascular endothelial cell pathology. Am. J. Pathol. 151:1397-1405. [PMC free article] [PubMed] [Google Scholar]
- 26.Lou, J., R. Lucas, and G. E. Grau. 2001. Pathogenesis of cerebral malaria: recent experimental data and possible applications for humans. Clin. Microbiol. Rev. 14:810-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackey, L. J., A. Hochmann, C. H. June, C. E. Contreras, and P. H. Lambert. 1980. Immunopathological aspects of Plasmodium berghei infection in five strains of mice. II. Immunopathology of cerebral and other tissue lesions during the infection. Clin. Exp. Immunol. 42:412-420. [PMC free article] [PubMed] [Google Scholar]
- 28.Massberg, S., G. Enders, R. Leiderer, S. Eisenmenger, D. Vestweber, F. Krombach, and K. Messmer. 1998. Platelet-endothelial cell interactions during ischemia/reperfusion: the role of P-selectin. Blood 92:507-515. [PubMed] [Google Scholar]
- 29.Massberg, S., G. Enders, F. C. Matos, L. I. Tomic, R. Leiderer, S. Eisenmenger, K. Messmer, and F. Krombach. 1999. Fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia-reperfusion in vivo. Blood 94:3829-3838. [PubMed] [Google Scholar]
- 30.Miller, L. H., M. F. Good, and G. Milon. 1994. Malaria pathogenesis. Science 264:1878-1883. [DOI] [PubMed] [Google Scholar]
- 31.Piguet, P. F., C. Da Laperrousaz, C. Vesin, F. Tacchini-Cottier, G. Senaldi, and G. E. Grau. 2000. Delayed mortality and attenuated thrombocytopenia associated with severe malaria in urokinase- and urokinase receptor-deficient mice. Infect. Immun. 68:3822-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polack, B., F. Delolme, and F. Peyron. 1997. Protective role of platelets in chronic (Balb/C) and acute (CBA/J) murine malaria. Haemostasis 27:278-285. [DOI] [PubMed] [Google Scholar]
- 33.Ritter, L. S., J. A. Orozco, B. M. Coull, P. F. McDonagh, and W. I. Rosenblum. 2000. Leukocyte accumulation and hemodynamic changes in the cerebral microcirculation during early reperfusion after stroke. Stroke 31:1153-1161. [DOI] [PubMed] [Google Scholar]
- 34.Salter, J. W., C. F. Krieglstein, A. C. Issekutz, and D. N. Granger. 2001. Platelets modulate ischemia/reperfusion-induced leukocyte recruitment in the mesenteric circulation. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G1432-G1439. [DOI] [PubMed] [Google Scholar]
- 35.Senaldi, G., C. Vesin, R. Chang, G. E. Grau, and P. F. Piguet. 1994. Role of polymorphonuclear neutrophil leukocytes and their integrin CD11a (LFA-1) in the pathogenesis of severe murine malaria. Infect. Immun. 62:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theroux, P., S. Kouz, L. Roy, M. L. Knudtson, J. G. Diodati, J. F. Marquis, J. Nasmith, A. Y. Fung, J. R. Boudreault, F. Delage, R. Dupuis, C. Kells, M. Bokslag, B. Steiner, and H. J. Rapold. 1996. Platelet membrane receptor glycoprotein IIb/IIIa antagonism in unstable angina. The Canadian Lamifiban Study. Circulation 94:899-905. [DOI] [PubMed] [Google Scholar]
- 37.van der Heyde, H. C., P. Bauer, G. Sun, W. L. Chang, L. Yin, J. Fuseler, and D. N. Granger. 2001. Assessing vascular permeability during experimental cerebral malaria by a radiolabeled monoclonal antibody technique. Infect. Immun. 69:3460-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Heyde, H. C., D. D. Manning, and W. P. Weidanz. 1993. Role of CD4+ T cells in the expansion of the CD4-, CD8-gamma delta T cell subset in the spleens of mice during blood-stage malaria. J. Immunol. 151:6311-6317. [PubMed] [Google Scholar]
- 39.Yanez, D. M., J. Batchelder, H. C. van der Heyde, D. D. Manning, and W. P. Weidanz. 1999. Gamma delta T-cell function in pathogenesis of cerebral malaria in mice infected with Plasmodium berghei ANKA. Infect. Immun. 67:446-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanez, D. M., D. D. Manning, A. J. Cooley, W. P. Weidanz, and H. C. van der Heyde. 1996. Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J. Immunol. 157:1620-1624. [PubMed] [Google Scholar]