Abstract
Interleukin (IL)-9 is a pleiotropic cytokine secreted by T helper (Th)2 cells and has been proposed as a candidate gene for asthma and allergy. We have used mice genetically deficient in IL-9 to determine the role of this cytokine in the pathophysiologic features of the allergic pulmonary response–airway hyperreactivity (AHR) and eosinophilia. We have demonstrated that IL-9 is not required for the development of a robust Th2 response to allergen in sensitized mice. IL-9 knockout mice developed a similar degree of eosinophilic inflammation and AHR to their wild-type littermates. Goblet cell hyperplasia and immunoglobulin (Ig) E production were also unaffected by the lack of IL-9. Moreover, levels of bronchoalveolar lavage (BAL) IL-4, IL-5, and IL-13 were comparable between wild-type and knockout mice. These findings indicate that IL-9 is not obligatory for the development of eosinophilia and AHR, and imply that other Th2 cytokines can act in a compensatory fashion.
Keywords: Th2 cytokines, asthma, airway hyperreactivity, eosinophilia, mucus
Introduction
The pathophysiological symptoms of allergic asthma are thought to occur as a result of an inappropriate immune response to innocuous proteins or allergens. This immune response to allergen is characterized by eosinophilic inflammation of the airways, airway hyperreactivity (AHR)*, and antigen-specific IgE production. There is growing evidence that the predominant cell type involved in the regulation of this pathophysiology is the Th2 cell, dominated by expression of IL-4, IL-5, and IL-13. The contribution of individual cytokines to the development of particular facets of the allergic response has been the subject of intense investigation. In particular IL-4 and IL-13 are involved in the polarization of effector T cells to those of the Th2 phenotype. IL-4 is thought to have an important, but not obligatory, role in the regulation of eosinophilia, AHR and mucus production (1). In contrast, blockade of IL-13 signaling results in an attenuation of these features (2). Moreover, IL-13 is able to induce AHR and eosinophilia when directly instilled into the airways of naive mice or if expressed transgenically in the lung (2, 3). IL-5 appears to be more specifically involved in the recruitment and survival of eosinophils, and therefore plays an important part in the development of pulmonary eosinophilia.
IL-9 is another Th2-derived cytokine thought to play an important role in the development of asthma. Evidence from both murine and human studies shows the IL-9 gene to be located within an area of the chromosome associated with susceptibility to AHR (4, 5). Moreover, IL-9 protein expression is strongly associated with the degree of airway responsiveness and the asthmatic-like phenotype (6). This has led to the proposition that IL-9 is a susceptibility or risk factor for asthma. The biological effects of IL-9 are pleiotropic, as it acts as a growth factor for mouse T cells (7), a maturation factor for B cells (8) and a proliferation factor for mast cells and hematopoietic progenitors (9). In allergy, the expression of IL-9 and its receptor is increased and this increase correlates to changes in lung function (10). Eosinophils also synthesize and secrete IL-9 (11) and evidence suggests that IL-9 may potentiate eosinophil function in vivo via interactions with IL-5. IL-9 has been found to increase eosinophil survival, as well as IL-5–mediated differentiation and maturation (12, 13). In another in vitro study, IL-9 was found to stimulate mucin production in respiratory epithelial cells (14).
Functional evidence for the involvement of IL-9 in the development of airway inflammation and bronchial hyperresponsiveness comes from the phenotype of transgenic mice constructed using a lung specific promoter to overexpress IL-9 in pulmonary epithelial cells (15). Mice exhibited a striking eosinophil and lymphocyte rich pulmonary inflammation, lung mast cell hyperplasia, epithelial cell hypertrophy, and subepithelial collagen deposition. Although mice showed normal baseline airway resistance their response to inhaled methacholine was markedly increased compared with nontransgenic littermates. Moreover, in a separate study IL-9 transgenic mice were found to display significantly enhanced eosinophilic airway inflammation, AHR and serum IgE in response to pulmonary allergen challenge (16).
Studies where IL-9 is transgenically expressed associate IL-9 with phenomena pathognomic of allergic airway disease, however the direct effect of IL-9 deficiency has not yet been investigated. Mice deficient in the expression of IL-9 were described recently (17). Interestingly, these mice did not show the multifocal effects seen in IL-9 transgenic mice. Indeed, IL-9 was not required for T cell development or differentiation, but IL-9 was necessary for both mast cell and goblet cell hyperplasia in a Th2-driven pulmonary granuloma model. In the current study, we have used these deficient mice to determine the role of IL-9 in the development of pulmonary inflammation and AHR after allergen challenge.
Materials and Methods
Mice
Mice deficient in IL-9 were generated as described previously (17). Mice homozygous for the disrupted IL-9 gene were bred onto a BALB/cJ background backcrossing for six generations. IL-9−/− mice and their wild-type (WT) littermates were transferred to Imperial College animal facility at 6–8 wk of age (25–30 g) and housed for at least 1 wk before use.
Induction of Allergic Airway Disease
Groups of IL-9–deficient mice and their WT littermates were sensitized using OVA (Sigma-Aldrich), at a concentration of 0.01 mg/mouse in 0.2 ml alum (Au-Gel-S; Serva Electrophoresis) intraperitoneally on days 0 and 12. Control mice received the same volume of PBS in alum. All groups of mice were challenged daily with 5% OVA (aerosolized for 20 min) via the airways between days 19 and 24. Mice were killed by exsanguination under terminal anesthesia at 24 h after OVA administration and the following parameters analyzed: AHR, inflammation, IgE, and Th2 cytokine production.
AHR
Airway responsiveness was measured in mice 24 h after the final OVA challenge by recording respiratory pressure curves by whole body plethysmography (Buxco Technologies) in response to inhaled methacholine (Sigma-Aldrich; UK at concentrations of 3–100 mg/ml for 1 min, as described previously; reference 18).
Cell Recovery
Airway Lumen.
Bronchoalveolar lavage (BAL) was performed as described previously (19). Briefly, the airways of the mice were lavaged three times with 0.4 ml of PBS via a tracheal cannula. Bronchoalveolar lavage (BAL) fluid was centrifuged (700 × g, 5 min at 4°C), cells were counted then pelleted onto glass slides by cytocentrifugation (5 × 104 cells per slide). Differential cell counts (original magnification: 40×; total area 0.5 mm2 per area randomly selected) were performed on Giemsa (Shandon) stained cytospins. All differential counts were performed blind and in a randomized order at the end of the study.
Lung Parenchyma.
To disaggregate the cells from the lung tissue, one lobe (100 mg) of lung was incubated (37°C) for 1 h in digest reagent: 75 U/ml collagenase (Type D, 50 U/ml DNase; Boehringer Mannheim; Type 1, 100 U penicillin and 100 mg/ml streptomycin; Boehringer Mannheim, GIBCO BRL, and Life Technologies) in 100 ml RPMI 1640 and FCS. The recovered cells were filtered through a 70 μm nylon sieve (Falcon, Marathon Lab Supplies) washed twice and resuspended in 1 ml RPMI 1640/FCS. Cytocentrifuge preparations were stained and counted as for BAL. A proportion of cells were analyzed for T1/ST2 expression by FACS® as described previously (20).
Histology.
Lung sections from the different experimental groups of mice were prepared and analyzed as described previously (21). Briefly, lungs were fixed in 10% neutral buffered formalin, paraffin embedded, and sections (4 μm) were stained with H&E according to standard protocols. A semiquantitative scoring system was used to grade the size of lung infiltrates, where +5 signified a large (>3 cells deep) widespread infiltrate around the majority of vessels and bronchioles, and +1 signifies a small number of inflammatory foci. Goblet cells were counted on Periodic Acid-Schiff (PAS)-stained lung sections using an arbitrary scoring system, as described previously (2, 17). PAS-stained goblet cells in airway epithelium were measured double blind using a numerical scoring system (0 = <5% goblet cells; 1 = 5–25%; 2 = 25–50%; 3 = 50–75%; 4 = >75%). The sum of the airway scores from each lung was divided by the number of airways examined (20–30 per mouse), and expressed as mucus score in arbitrary units.
Cytokine Analysis
Cytokines were analyzed in BAL samples and lung tissue homogenates. Lung tissue (100 mg) was homogenized in 2 ml HBSS, centrifuged (1,900 rpm, 10 min) and the supernatant collected. Paired antibodies for murine IFN-γ and IL-4 (BD PharMingen) IL-5 (Endogen), and eotaxin (R&D Systems) were used in standardized sandwich ELISAs according to the manufacturer's protocol. Kits to measure IL-13 were purchased from R&D Systems.
IgE
Levels of total IgE were measured in serum by ELISA using paired antibodies according to the manufacturer's instructions (BD PharMingen).
Levels of anti-OVA IgE were measured in serum by ELISA as described previously (21), and antibody titers were then related to pooled standards generated in the laboratory and were assigned the arbitrary values U/ml.
Data Analysis
Data are expressed as mean ± SEM. Statistical significance was accepted when P < 0.05 using the Mann Whitney U Test.
Results and Discussion
IL-9 Deficiency Has No Effects on the Induction AHR.
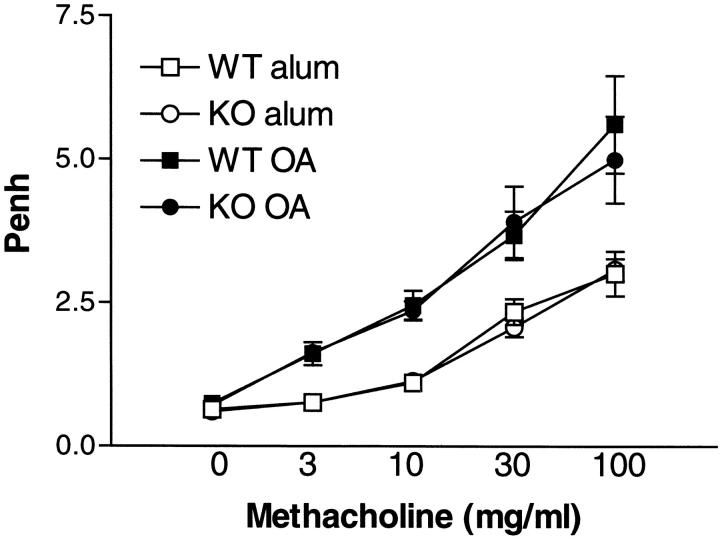
AHR is a cardinal feature of the pulmonary allergic response and has been linked with the development of robust Th2 responses. To determine whether deficiency in IL-9 has a direct effect on development of airway dysfunction, AHR was measured by whole body plethysmography 24 h after the final, serial OVA challenge in knockout (KO) and WT mice. Fig. 1 shows that increasing doses of methacholine elicited a similar response in IL-9–deficient mice compared with their WT littermates. There was no difference in the response to methacholine in unsensitized (alum/PBS) wild–type mice or IL-9 KO over the dose range studied. However, both OVA-sensitized WT and IL-9–deficient mice showed significantly enhanced response to methacholine after allergen challenge, as compared with the unsensitized control groups.
Figure 1.
Effect of IL-9 deficiency on AHR. AHR was measured 24 h after the final OVA challenge using a Buxco system where mice were exposed to increasing concentrations of methacholine (3–100 mg/ml). Results are shown for Penh after allergen challenge in WT mice and IL-9 KO mice either sensitized to alum/PBS or OVA/alum. Values are expressed as mean ± SEM; n = 9–13 per group in two separate experiments.
Previous studies have proposed IL-9 as a candidate gene for asthma on the basis of qualitative trait locus analysis (5). Bronchial hyporesponsiveness in C57Bl/6 mice was found to be associated with greatly reduced IL-9 levels, in comparison with higher IL-9 levels in hyperresponsive DBA/2 mice. Moreover, transgenic mice overexpressing IL-9 under the control of a lung specific promoter show increased bronchial hyperresponsiveness to inhaled methacholine. These studies led the authors to propose a role for IL-9 in the development of AHR. However, we have been unable to determine any effect on the development of AHR after allergen challenge of IL-9–deficient mice, indicating that IL-9 is not essential for the development of AHR. There are multiple cytokines that are thought to play key roles in the regulation of Th2-directed pathophysiology during an allergic response. In particular, neutralization studies with inhibitors of IL-13 in vivo have shown that this cytokine is absolutely required for AHR and mucus production (22) and have a role in the regulation of eosinophilia (2). In contrast, IL-4 is thought to be important for the regulation of eosinophilia, AHR, and mucus production, but is not obligatory for these processes (23). It is likely that IL-4, IL-9, and IL-13 cooperate to promote the development of AHR, however only IL-13 can compensate for a lack in IL-4 or IL-9.
Development of Pulmonary Inflammation in the Absence of IL-9.
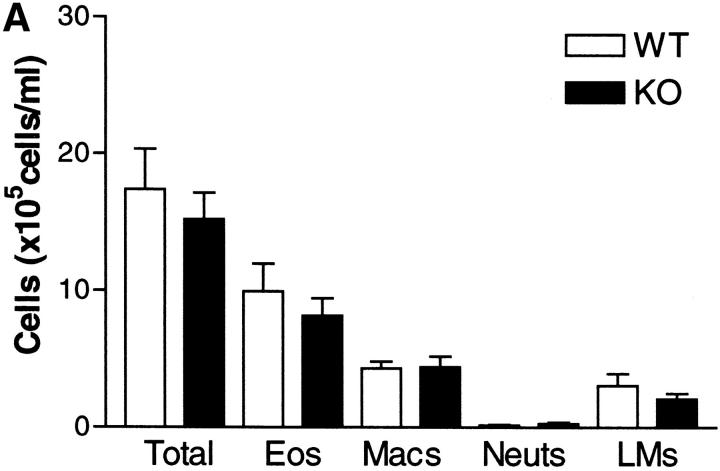
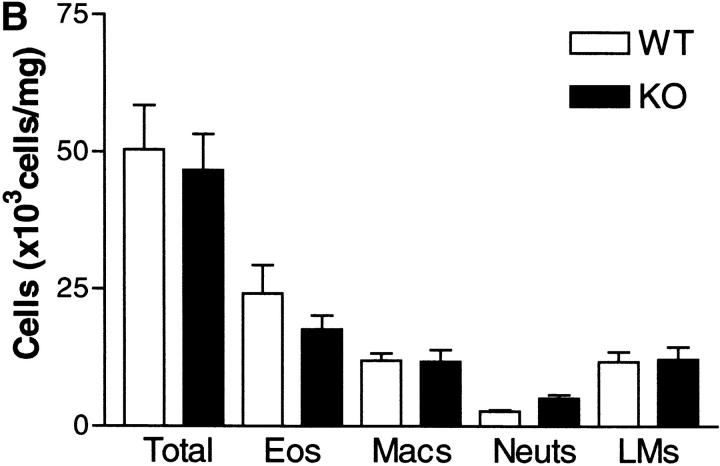
Pulmonary inflammation is one of the characteristic hallmarks of the allergic response to allergen. Leukocytic infiltrates are found within the airway lumen, as observed in BAL, and in the lung interstitium as observed in histological sections. The predominant leukocyte is the eosinophil in conjunction with lymphocytes, primarily of the Th2 phenotype. We determined the extent of inflammation in both compartments of the lung in sensitized WT and IL-9 KO mice after serial OVA challenge. Cell recruitment to BAL and lung was comparable between WT KO mice (Fig. 2 A and B). Differential counts revealed that infiltrates in both strains of mice were composed of mainly eosinophils and lymphocytes. However, there was no difference in either the total leukocyte infiltration of each compartment or in numbers of any leukocyte subset between IL-9–deficient and WT mice.
Figure 2.
Differential cell counts in BAL (A) and lung tissue digest (B) from WT mice (white bars) and IL-9 KO mice (black bars). Mice were killed 24 h after the final OVA challenge. BAL and lung tissue digest cells were isolated as described in Materials and Methods. Values are expressed as mean ± SEM; n = 11–14 per group in two separate experiments.
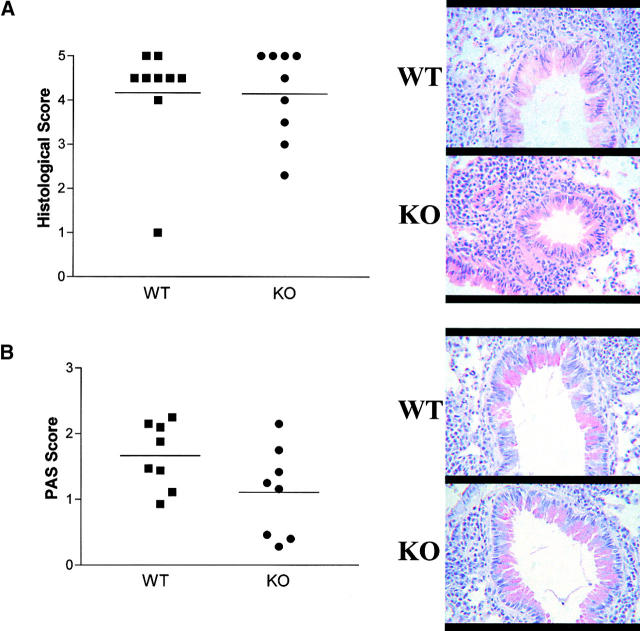
The extent and anatomical location of leukocyte infiltrates was determined in H&E-stained sections taken from mice 24 h after the final allergen challenge using a semiquantitative scoring system (20). Challenge with OVA induced a widespread peribronchiolar and perivascular inflammation, which was primarily eosinophilic. However, there was no appreciable difference in the degree of tissue inflammation observed between experimental groups after allergen challenge, with WT and IL-9 KO mice showing similar scores (WT: 4.2 ± 0.4; KO: 4.1 ± 0.3) (Fig. 3 A). Previous studies with transgenic mice have shown that overexpression of IL-9 induces eosinophilia (15, 16, 24). However, development of eosinophilic pulmonary granulomas in response to Schistosoma mansoni eggs is normal in IL-9 KO mice (17). Indeed, this latter study argues against an obligatory role for IL-9 in development of granuloma formation and eosinophilia in this model.
Figure 3.
OVA-induced lung inflammation in IL-9 KO and WT mice. An assessment of pulmonary inflammation was made by determining the extent of inflammatory infiltrates in sections stained with H&E (A) or PAS (B). Stained sections were scored as described in Materials and Methods, and scores for individual WT (squares) and KO (circles) mice are represented. Bars depict means of groups, n = 8–9 per group. Representative photomicrographs of H&E- and PAS-stained lung sections from WT (i) and KO (ii) OVA-challenged mice are shown.
Although respiratory mucus protects the lower airways from dehydration and damage, excessive secretion from hyperplastic goblet cells contributes to the morbidity and mortality of many respiratory diseases, including asthma (25). Quantification of goblet cells stained with PAS revealed similar mucus scores in IL-9 KO mice compared with WT mice (Fig. 3 B). Transgenic expression of IL-9 in the lung led to the hypothesis that IL-9 was involved in the generation of mucus producing cells (15, 26). Moreover, goblet cell hyperplasia was found to be severely impaired in IL-9–deficient animals after induction with synchronous primary granulomas (17). Although we found a slight decrease in the degree of goblet cell hyperplasia developing in the absence of IL-9, the effect did not reach statistical significance, unlike during the primary granuloma model, and was evidently not enough to affect development of AHR. This difference is probably due to the different complexities of the immunological stimulus used. The primary granuloma model relies on one intravenous challenge of antigen, whereas the OVA model is induced by two peripheral adjuvant/antigen sensitizations followed by multiple pulmonary challenges with soluble antigen. Indeed, secondary challenge in the pulmonary granulomatous response produces an equivalent induction of goblet cells, even in the absence of IL-9 (17).
IL-13 has been shown to play an important role in goblet cell hyperplasia, since IL-13 neutralization ameliorates allergy-induced mucus production (2, 22), and direct instillation of IL-13 to the airway promotes an increase in mucus containing epithelial cells. The roles of IL-9 and IL-13 in regulating mucus cell hyperplasia are likely to be interrelated. These cytokines can independently regulate mucin gene expression when directly instilled into the lung or added to pulmonary epithelial cells in culture (24, 26). It is conceivable that during a primary response IL-9 is dominant, but IL-13 can compensate after multiple challenges. Our data indicate that IL-9 contributes to mucus production, but IL-13 is probably able to compensate for a deficiency in IL-9.
Previous studies determining the role of IL-9 in pulmonary inflammation have reported changes in mast cell numbers in conjunction with the changes in goblet cell hyperplasia. A decrease in mastocytosis during primary granuloma formation was observed in the absence of IL-9 (17). Mast cells do not feature prominently in the lungs after allergen challenge in this particular model, and we could not detect any difference in numbers of mast cells in lungs of IL-9 KO mice after challenge (data not shown).
Since IL-9 is a Th2-derived cytokine thought to be involved in the promotion of Th2 responses, we determined whether the recruitment of Th2 cells to the lung was affected by a lack of IL-9. This was accomplished by FACS® staining of cells isolated from digested lung tissue using an antibody specific for the Th2 marker, T1/ST2 (20). The percentage of T1/ST2 positive CD4 T cells in alum control mice was low in both types of mice (WT: 0.9 ± 0.2; KO: 0.6 ± 0.2). Allergen challenge of sensitized mice significantly increased the proportion of CD4 cells positive for T1/ST2, and levels were comparable between WT and IL-9 KO mice (WT: 11.9 ± 1.1; KO: 8.9 ± 1.5). These results indicate that the generation of Th2 cells and their recruitment to the lung is unaffected by the absence of IL-9.
Effect of IL-9 Deficiency on the Development of Humoral Immune Responses.
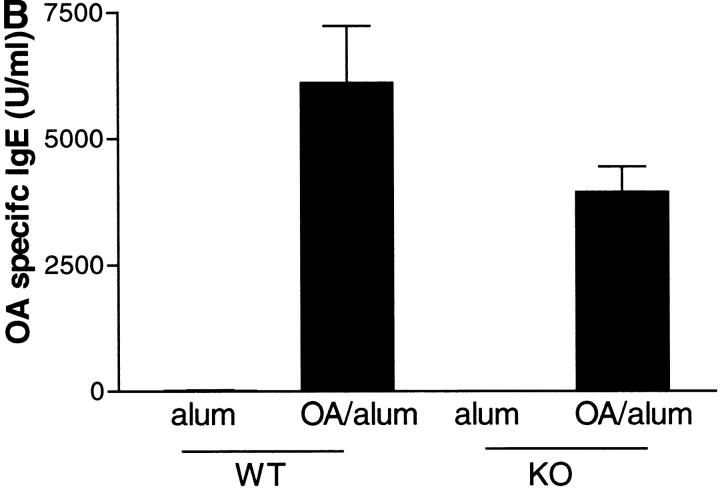
Elevated levels of IgE have been reported to be important in the development of an allergic pulmonary response, and evidence suggests that IL-9 can alter the in vitro differentiation of B cells and enhance Ig production (27, 8). We measured total IgE and OVA-specific IgE in sera from KO and WT mice after allergen challenge. Both groups of mice showed significantly enhanced production of total and antigen specific IgE after allergen challenge (Fig. 4) . IL-9 deficiency had no significant effect on the production of total IgE. Levels of OVA-specific IgE were slightly lower in the absence of IL-9, indicating that the antigen-specific Ig response was perhaps less efficient.
Figure 4.
Effect of IL-9 deficiency on serum IgE levels. Mice were killed 24 h after the final OVA challenge, bled via cardiac puncture, serum collected, and analyzed by ELISA for total IgE (A) and OVA-specific IgE (B). Data are expressed as mean ± SEM; n = 4–13 per group.
Effect of IL-9 on the Production of Inflammatory Cytokines.
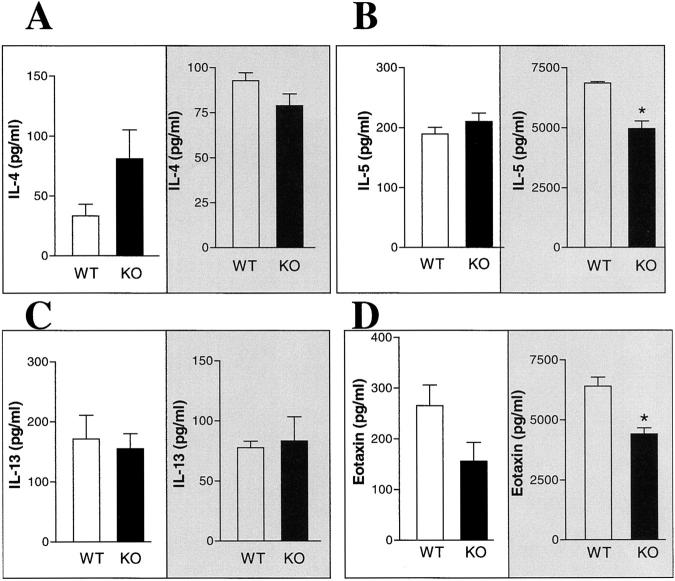
The immunologic response to pulmonary allergen challenge is characterized by the secretion of Th2 cytokines (28). In particular high levels of IL-4, IL-5, and IL-13 and low levels of IFN-γ are associated with a Th2-directed response to allergen in the lung. We found elevated levels of BAL IL-4, IL-5, and IL-13 in both WT and KO mice after allergen challenge (Fig. 5) . Although mice deficient in IL-9 seemed to produce more IL-4 in the BAL compared with WT mice this was not significant (Fig. 5 A). Interestingly, similar findings were documented after infection of IL-9 KO mice with the parasitic nematode worm Nippostrongylus brasiliensis. Increased IL-4 production was detected in restimulated cells from the mesenteric lymph node of IL-9–deficient mice (17). This reinforces the idea that the network of Th2 cytokines is delicately balanced during an immune response and that disruption of this balance by a deficiency in one cytokine results in an increase in one or more of the other cytokines. Levels of IFN-γ remained low in both IL-9 deficient and WT mice after allergen challenge (data not shown).
Figure 5.
Effect of IL-9 deficiency on cytokine production. IL-4 (A), IL-5 (B), IL-13 (C), and eotaxin (D) levels were measured in BAL fluid (left) and lung homogenate (right shaded) by ELISA in samples from WT mice (white bars) and IL-9 KO mice (black bars) killed 24 h after the final OVA challenge. Data are expressed as mean ± SEM; n = 14 per group for BAL cytokines; n = five per group for lung cytokines and * P < 0.05 in comparison to WT group using a Mann Whitney U test.
IL-9 has been demonstrated previously to induce chemokine expression in cultured lung epithelial cell lines or when expressed transgenically in the lung (24). We determined expression of the proeosinophilic chemokine eotaxin in BAL and lung samples from allergen-challenged WT and IL-9 KO mice. IL-9 KO mice produced less eotaxin in the lavage and lung tissue in comparison with WT mice, although this effect was only significant in the lung tissue (Mann Whitney U test, P = 0.008) (Fig. 5 D). However, other studies have shown that eotaxin production is also regulated by other Th2 cytokines such as IL-13 and/or IL-4 (29, 30). Our data suggest that although IL-9 may be involved in eosinophil recruitment by regulating chemokines such as eotaxin, IL-9 expression is not obligatory and other Th2 cytokines such as IL-4 or IL-13 can compensate for IL-9 deficiency.
Our data indicate that IL-9 does not play an critical role in the development of allergic eosinophilia and AHR. We have demonstrated that Th2 cells are able to migrate to the lungs and elicit Th2-mediated responses to allergen, even in the absence of IL-9. This study demonstrates the complexity of the Th2 cytokine response and its role in the development of pathophysiological features of the allergic response. Our results do not reflect the multifocal effect seen when IL-9 is transgenically overexpressed. However, our results indicate that studies investigating the relationships between key Th2 cytokines will be invaluable in addressing the contribution of individual cytokines to pathophysiologic phenomena characteristic of the asthmatic response, and questions the value of designing novel therapeutic strategies targeting single cytokines.
Acknowledgments
The authors would like to thank Lorraine Lawrence for technical assistance with histology.
This work was supported by The Wellcome Trust (ref #057704).
Footnotes
Abbreviations used in this paper: AHR, airway hyperreactivity; BAL, bronchoalveolar lavage; KO, knockout; PAS, Periodic Acid-Schiff; WT, wild-type.
References
- 1.Corry, D.B. 1999. IL-13 in allergy: home at last. Curr. Opin. Immunol. 11:610–614. [DOI] [PubMed] [Google Scholar]
- 2.Grunig, G., M. Warnock, A.E. Wakil, R. Venkayya, F. Brombacher, D.M. Rennick, D. Sheppard, M. Mohrs, D.D. Donaldson, R.M. Locksley, and D.B. Corry. 1998. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu, Z., R.J. Homer, Z. Wang, Q. Chen, G.P. Geba, J. Wang, Y. Zhang, and J.A. Elias. 1999. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Invest. 103:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postma, D.S., E.R. Bleecker, P.J. Amelung, K.J. Holroyd, J. Xu, C.I. Panhuysen, D.A. Meyers, and R.C. Levitt. 1995. Genetic susceptibility to asthma—bronchial hyperresponsiveness coinherited with a major gene for atopy. N. Engl. J. Med. 333:894–900. [DOI] [PubMed] [Google Scholar]
- 5.Nicolaides, N.C., K.J. Holroyd, S.L. Ewart, S.M. Eleff, M.B. Kiser, C.R. Dragwa, C.D. Sullivan, L. Grasso, L.Y. Zhang, C.J. Messler, et al. 1997. Interleukin 9: a candidate gene for asthma. Proc. Natl. Acad. Sci. USA. 94:13175–13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demoulin, J.B., and J.C. Renauld. 1998. Interleukin 9 and its receptor: an overview of structure and function. Int. Rev. Immunol. 16:345–364. [DOI] [PubMed] [Google Scholar]
- 7.Van Snick, J., A. Goethals, J.C. Renauld, E. Van Roost, C. Uyttenhove, M.R. Rubira, R.L. Moritz, and R.J. Simpson. 1989. Cloning and characterization of a cDNA for a new mouse T cell growth factor (P40). J. Exp. Med. 169:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vink, A., G. Warnier, F. Brombacher, and J.C. Renauld. 1999. Interleukin 9-induced in vivo expansion of the B-1 lymphocyte population. J. Exp. Med. 189:1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourette, R.P., J. Royet, G. Mouchiroud, E. Schmitt, and J.P. Blanchet. 1992. Murine interleukin 9 stimulates the proliferation of mouse erythroid progenitor cells and favours the erythroid differentiation of multipotent FDCP-mix cells. Exp. Hematol. 20:868–873. [PubMed] [Google Scholar]
- 10.Shimbara, A., P. Christodoulopoulos, A. Soussi-Gounni, R. Olivenstein, Y. Nakamura, R.C. Levitt, N.C. Nicolaides, K.J. Holroyd, A. Tsicopoulos, J.J. Lafitte, et al. 2000. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J. Allergy Clin. Immunol. 105:108–115. [DOI] [PubMed] [Google Scholar]
- 11.Gounni, A.S., E. Nutku, L. Koussih, F. Aris, J. Louahed, R.C. Levitt, N.C. Nicolaides, and Q. Hamid. 2000. IL-9 expression by human eosinophils: regulation by IL-1β and TNF-α. J. Allergy Clin. Immunol. 106:460–466. [DOI] [PubMed] [Google Scholar]
- 12.Gounni, A.S., B. Gregory, E. Nutku, F. Aris, K. Latifa, E. Minshall, J. North, J. Tavernier, R. Levit, N. Nicolaides, et al. 2000. Interleukin-9 enhances interleukin-5 receptor expression, differentiation, and survival of human eosinophils. Blood. 96:2163–2171. [PubMed] [Google Scholar]
- 13.Louahed, J., Y. Zhou, W.L. Maloy, P.U. Rani, C. Weiss, Y. Tomer, A. Vink, J. Renauld, J. Van Snick, N.C. Nicolaides, et al. 2001. Interleukin 9 promotes influx and local maturation of eosinophils. Blood. 97:1035–1042. [DOI] [PubMed] [Google Scholar]
- 14.Longphre, M., D. Li, M. Gallup, E. Drori, C.L. Ordonez, T. Redman, S. Wenzel, D.E. Bice, J.V. Fahy, and C. Basbaum. 1999. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J. Clin. Invest. 104:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Temann, U.A., G.P. Geba, J.A. Rankin, and R.A. Flavell. 1998. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J. Exp. Med. 188:1307–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLane, M.P., A. Haczku, M. Van de Rijn, C. Weiss, V. Ferrante, D. MacDonald, J.C. Renauld, N.C. Nicolaides, K.J. Holroyd, and R.C. Levitt. 1998. Interleukin-9 promotes allergen-induced eosinophilic inflammation and airway hyperresponsiveness in transgenic mice. Am. J. Respir. Cell. Mol. Biol. 19:713–720. [DOI] [PubMed] [Google Scholar]
- 17.Townsend, J.M., G.P. Fallon, J.D. Matthews, P. Smith, E.H. Jolin, and N.A. McKenzie. 2000. IL-9 deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 13:573–583. [DOI] [PubMed] [Google Scholar]
- 18.Hamelmann, E., J. Schwarze, K. Takeda, A. Oshiba, G.L. Larsen, C.G. Irvin, and E.W. Gelfand. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156:766–775. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalo, J.-A., C.M. Lloyd, L. Kremer, E. Finger, C. Martinez-A., M.H. Siegelman, M.I. Cybulsky, and J.-C. Gutierrez-Ramos. 1996. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines and adhesion receptors. J. Clin. Invest. 98:2332–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coyle, A.J., C. Lloyd, J. Tian, T. Nguyen, C. Erikkson, L. Wang, P. Ottoson, P. Persson, T. Delaney, S. Lehar, et al. 1999. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J. Exp. Med. 190:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd, C.M., J.A. Gonzalo, T. Nguyen, T. Delaney, J. Tian, H. Oettgen, A.J. Coyle, and J.C. Gutierrez-Ramos. 2001. Resolution of bronchial hyperresponsiveness and pulmonary inflammation is associated with IL-3 and tissue leukocyte apoptosis. J. Immunol. 166:2033–2040. [DOI] [PubMed] [Google Scholar]
- 22.Wills-Karp, M., J. Luyimbazi, X. Xu, B. Schofield, T.Y. Neben, C.L. Karp, and D.D. Donaldson. 1998. Interleukin-13: central mediator of allergic asthma. Science. 282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 23.Corry, D.B., H.G. Folkesson, M.L. Warnock, D.J. Erle, M.A. Matthay, J.P. Wiener Kronish, and R.M. Locksley. 1996. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J. Exp. Med. 183:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong, Q., J. Louahed, A. Vink, C.D. Sullivan, C.J. Messler, Y. Zhou, A. Haczku, F. Huaux, M. Arras, K.J. Holroyd, et al. 1999. IL-9 induces chemokine expression in lung epithelial cells and baseline airway eosinophilia in transgenic mice. Eur. J. Immunol. 29:2130–2139. [DOI] [PubMed] [Google Scholar]
- 25.Kim, W.D. 1997. Lung mucus: a clinician's view. Eur. Respir. J. 10:1914–1917. [DOI] [PubMed] [Google Scholar]
- 26.Louahed, J., M. Toda, J. Jen, Q. Hamid, J.C. Renauld, R.C. Levitt, and N.C. Nicolaides. 2000. Interleukin-9 upregulates mucus expression in the airways. Am. J. Respir. Cell. Mol. Biol. 22:649–656. [DOI] [PubMed] [Google Scholar]
- 27.Petit-Frere, C., B. Dugas, P. Braquet, and J.M. Mencia-Huerta. 1993. Interleukin-9 potentiates the interleukin-4-induced IgE and IgG1 release from murine B lymphocytes. Immunology. 79:146–151. [PMC free article] [PubMed] [Google Scholar]
- 28.Renauld, J.C. 2001. New insights into the role of cytokines in asthma. J. Clin. Pathol. 54:577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, L., Y. Xia, A. Nguyen, Y.H. Lai, L. Feng, T.R. Mosmann, and D. Lo. 1999. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J. Immunol. 162:2477–2487. [PubMed] [Google Scholar]
- 30.Mattes, J., M. Yang, A. Siqueira, K. Clark, J. MacKenzie, A.N. McKenzie, D.C. Webb, K.I. Matthaei, and P.S. Foster. 2001. IL-13 induces airways hyperreactivity independently of the IL-4rα chain in the allergic lung. J. Immunol. 167:1683–1692. [DOI] [PubMed] [Google Scholar]